Atrioventricular valve regurgitation is the most prevalent form of valve disease in developed countries. Transcatheter edge-to-edge repair (TEER) has emerged as a therapeutic option for the treatment of severe mitral regurgitation and tricuspid regurgitation and avoids the morbidity and mortality associated with open heart surgery [1, 2]. One challenge to successful application of this therapy is delivering the clip to a precise location within the constraints of the unique anatomy and resulting geometry of an individual patient. Three-dimensional echocardiography (3DE) is typically used to plan and execute TEER using catheter delivery systems designed to access a specific anatomic location (mitral valve, tricuspid valve) via a known path. These systems are then empirically validated and iteratively improved via large clinical trials and clinical practice. However, there has been little work on modeling the catheter path and approach to deliver the clip to the desired location in atypical or surgically altered anatomy. Modeling the trajectory of the clip delivery system could be especially beneficial for procedural planning in patients with congenital heart disease in whom there is greater anatomic variability.
In order to inform both patient selection and optimal catheter path for TEER, we created a realistically constrained virtual modeling system which we named the Valve Clip Simulator. The simulator can be applied within all major forms of 3D cardiac imaging datasets (3DE, computed tomography, and magnetic resonance imaging), and we have made this new functionality free and open-source as part of the SlicerHeart extension for 3D Slicer (www.slicer.org)[3–6]. We based the simulator on the commercially available system, but the framework can be easily customized for any system governed by similar controls.
We first demonstrate an overview of the system and the controls within a transparent CT-derived segmented model of an adolescent with a structurally normal heart (Figure 1A and 1C, Video 1), and demonstrate how planning can also be performed by volume rendering the CT image (Figure 1C, Video 2). We then demonstrate application within 3DE imaging in a patient with severe mitral valve prolapse and regurgitation (Figure 2, Video 3). We show how frequently utilized 2D views of the mitral valve (e.g. simultaneous mid-commissural and long-axis views) can be created post-hoc from 3DE within this modeling framework to facilitate planning within views familiar to practitioners. Finally, we demonstrate the planning of interventions from cardiac MRI-derived models in a patient with hypoplastic left heart syndrome at the Fontan stage, and a patient with double outlet right ventricle and interrupted inferior vena cava (IVC) with azygous continuation (Figure S1, Video 3). The typical femoral venous access site is not feasible in the setting of the interrupted IVC; as such, alternative access points are explored.
Figure 1.
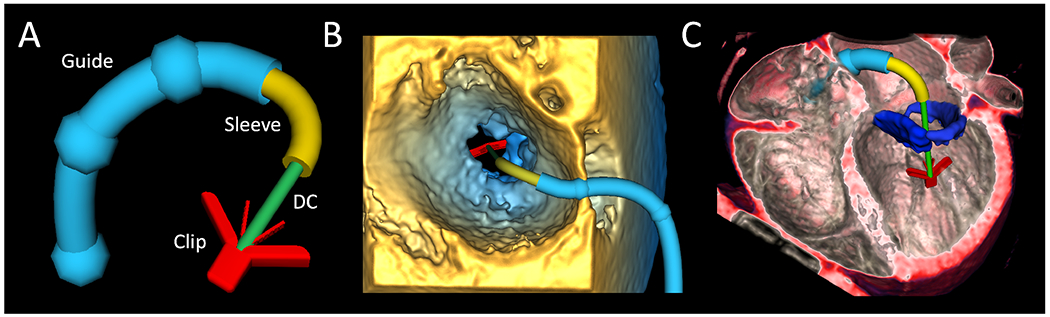
Valve Clip Simulator Employed in Multimodality Imaging. A. Virtual clip delivery system with components labeled; B. Virtual clip delivery system demonstrated in 3DE; C. Virtual clip delivery system demonstrated in volume rendered CT of structurally normal heart with segmented mitral valve shown in blue. 3DE = 3D echocardiogram, MRI = magnetic resonance imaging, CT = computed tomography, DC= Delivery Catheter
Figure 2.
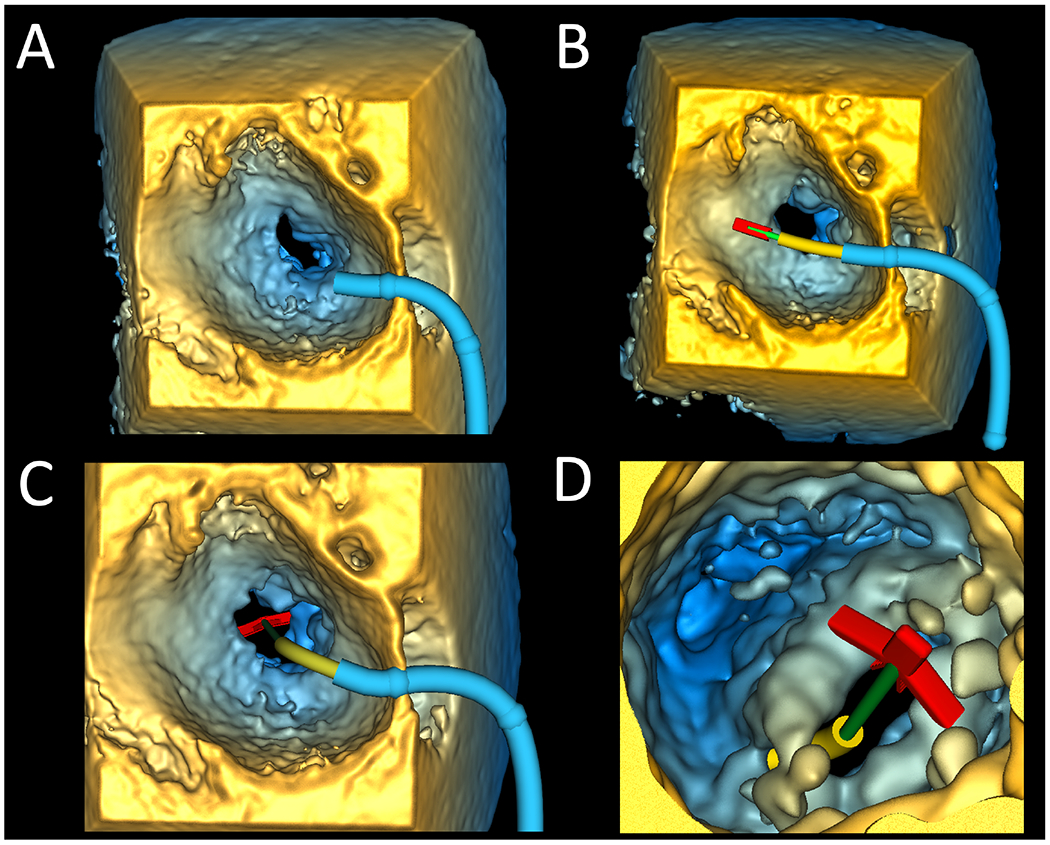
Valve Clip Simulator within Volume Rendered 3DE of a Patient with Mitral Valve Prolapse. A. Left atrial view of guide placement; B. Left atrial view of delivery system advanced through guide into left atrium; C. Left atrial view of delivery system advanced into position; D. Left ventricular view of clip opened and grippers raised. 3DE = 3D echocardiography
It is unlikely that an empirically validated, custom delivery system will be available for each of the diverse anatomies encountered in congenital heart disease. As such, existing commercial systems may need to be applied in anatomy they were never designed or validated for. We found that modeling of TEER allowed the pre-interventional team to simulate potential catheter approaches, as well as practice necessary device manipulation. This system, and those based upon it, may further be useful for designing new access strategies, as we demonstrate in patients with palliated congenital heart disease. Modeling may also be informative in more standard applications when anatomy is considerably distorted due to adverse cardiac remodeling related to chronic atrioventricular valve regurgitation. Furthermore, experience with TEER for tricuspid regurgitation is still limited, and the design of, and manipulation of the clip delivery system to target the more variable tricuspid valve anatomy remains an ongoing clinical challenge.
Our current modeling does have limitations. While we based the catheter behavior on validated mathematics and a clinical system, we did not disassemble and precisely reverse-engineer the system, nor does the model incorporate friction or the complex mechanics of the components of the system interacting with one another in a non-linear manner.[7] As such, further optimization and validation is needed.
In the future, we hope that we and others incorporate emerging delivery systems and more precise mechanistic behavior to this system. The 3D Slicer platform is available at http://www.slicer.org. Open-source code and documentation are available at https://github.com/SlicerHeart. This study was approved by the institutional review board at the Children’s Hospital of Philadelphia.
Supplementary Material
Video 1: Demonstration of Valve Clip Simulator in Segmented Model of CT Angiogram. A segmented model derived from a CT angiogram of an adolescent with a structurally normal heart is shown. The virtual clip modeling is demonstrated beginning with placement of the guide. The delivery system is passed through the guide into the left atrium. The clip is then advanced into the left ventricle to simulate deployment.
Video 2: Visualization of the Valve Clip Simulator integrated within a Volume Rendered CT Angiogram. Virtual clip modeling is demonstrated from different perspectives by cropping into the volume rendered CT angiogram of an adolescent with a structurally normal heart.
Video 3: Demonstration of Valve Clip Simulator Applied to Patient with Mitral Regurgitation in Volume Rendered 3D Echocardiogram. The guide is placed followed by catheter manipulation and advancing clip into the left ventricle. The 3D image with the Valve Clip Simulator is sliced to create familiar 2D image planes (mid-commissural view, long axis) utilized during clinical Mitraclip procedures.
Video 4: Demonstration of Valve Clip Simulator to Explore Approach to Access in Complex Congenital Heart Disease in Cardiac MRI-Derived models. The first simulation shows the Valve Clip Simulator applied to a patient with hypoplastic left heart syndrome at the Fontan stage. The importance of the baffle puncture site optimal clip is demonstrated. The second demonstration is in a patient with double-outlet right ventricle, interrupted inferior vena cava(IVC) with azygous continuation, and single ventricle physiology at the Fontan stage. The Fontan baffle incorporating hepatic flow is shown in green. The IVC to azygous continuation is shown in yellow. The first example demonstrates right internal jugular(IJ) access to deliver clip to the tricuspid valve (blue) via trans-baffle puncture. The second example demonstrates right internal jugular access to deliver clip to the mitral valve (orange) via trans-baffle puncture. The third example demonstrates transhepatic access to deliver clip to the tricuspid valve (blue) via trans-baffle puncture. The final example demonstrates of surgical access via right atrial appendage via thoracotomy to deliver clip to the tricuspid valve (blue).
Supplemental Figure S1. Demonstration of Valve Clip Simulator in MRI based model of patient with with Double-Outlet Right Ventricle, Interrupted Inferior Vena Cava(IVC) with Azygous continuation, and Single Ventricle Physiology at the Fontan Stage. The Fontan baffle incorporating hepatic flow is shown in green. The IVC to azygous continuation is shown in yellow. A. Demonstration of right internal jugular access to deliver clip to the tricuspid valve (blue) via trans-baffle puncture; B. Demonstration of right internal jugular access to deliver clip to the mitral valve (orange) via trans-baffle puncture; C. Demonstration of transhepatic access to deliver clip to the tricuspid valve (blue) via trans-baffle puncture; D. Demonstration of surgical access via right atrial appendage via thoracotomy to deliver clip to the tricuspid valve (blue). MRI = Magnetic Resonance Imaging
Funding:
This work was supported by NIH R01 HL153166, the Cora Topolewski Cardiac Research Fund at the Children’s Hospital of Philadelphia(CHOP), a CHOP Frontier Grant (Pediatric Valve Center), and CANARIE’s Research Software Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: C. Pinter is a contracted developer employed by Pixel Medical.
References
- [1].Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–11. [DOI] [PubMed] [Google Scholar]
- [2].Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307–18. [DOI] [PubMed] [Google Scholar]
- [3].Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scanlan AB, Nguyen AV, Ilina A, Lasso A, Cripe L, Jegatheeswaran A, et al. Comparison of 3D Echocardiogram-Derived 3D Printed Valve Models to Molded Models for Simulated Repair of Pediatric Atrioventricular Valves. Pediatr Cardiol. 2018;39:538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nam HH, Herz C, Lasso A, Drouin S, Posada A, Morray B, et al. Simulation of Transcatheter Atrial and Ventricular Septal Defect Device Closure Within Three-Dimensional Echocardiography-Derived Heart Models on Screen and in Virtual Reality. J Am Soc Echocardiogr. 2020;33:641–4 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nguyen AV, Lasso A, Nam HH, Faerber J, Aly AH, Pouch AM, et al. Dynamic Three-Dimensional Geometry of the Tricuspid Valve Annulus in Hypoplastic Left Heart Syndrome with a Fontan Circulation. J Am Soc Echocardiogr. 2019;32:655–66 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vrooijink GJ, Denasi A, Grandjean JG, Misra S. Model predictive control of a robotically actuated delivery sheath for beating heart compensation. Int J Rob Res. 2017;36:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Demonstration of Valve Clip Simulator in Segmented Model of CT Angiogram. A segmented model derived from a CT angiogram of an adolescent with a structurally normal heart is shown. The virtual clip modeling is demonstrated beginning with placement of the guide. The delivery system is passed through the guide into the left atrium. The clip is then advanced into the left ventricle to simulate deployment.
Video 2: Visualization of the Valve Clip Simulator integrated within a Volume Rendered CT Angiogram. Virtual clip modeling is demonstrated from different perspectives by cropping into the volume rendered CT angiogram of an adolescent with a structurally normal heart.
Video 3: Demonstration of Valve Clip Simulator Applied to Patient with Mitral Regurgitation in Volume Rendered 3D Echocardiogram. The guide is placed followed by catheter manipulation and advancing clip into the left ventricle. The 3D image with the Valve Clip Simulator is sliced to create familiar 2D image planes (mid-commissural view, long axis) utilized during clinical Mitraclip procedures.
Video 4: Demonstration of Valve Clip Simulator to Explore Approach to Access in Complex Congenital Heart Disease in Cardiac MRI-Derived models. The first simulation shows the Valve Clip Simulator applied to a patient with hypoplastic left heart syndrome at the Fontan stage. The importance of the baffle puncture site optimal clip is demonstrated. The second demonstration is in a patient with double-outlet right ventricle, interrupted inferior vena cava(IVC) with azygous continuation, and single ventricle physiology at the Fontan stage. The Fontan baffle incorporating hepatic flow is shown in green. The IVC to azygous continuation is shown in yellow. The first example demonstrates right internal jugular(IJ) access to deliver clip to the tricuspid valve (blue) via trans-baffle puncture. The second example demonstrates right internal jugular access to deliver clip to the mitral valve (orange) via trans-baffle puncture. The third example demonstrates transhepatic access to deliver clip to the tricuspid valve (blue) via trans-baffle puncture. The final example demonstrates of surgical access via right atrial appendage via thoracotomy to deliver clip to the tricuspid valve (blue).
Supplemental Figure S1. Demonstration of Valve Clip Simulator in MRI based model of patient with with Double-Outlet Right Ventricle, Interrupted Inferior Vena Cava(IVC) with Azygous continuation, and Single Ventricle Physiology at the Fontan Stage. The Fontan baffle incorporating hepatic flow is shown in green. The IVC to azygous continuation is shown in yellow. A. Demonstration of right internal jugular access to deliver clip to the tricuspid valve (blue) via trans-baffle puncture; B. Demonstration of right internal jugular access to deliver clip to the mitral valve (orange) via trans-baffle puncture; C. Demonstration of transhepatic access to deliver clip to the tricuspid valve (blue) via trans-baffle puncture; D. Demonstration of surgical access via right atrial appendage via thoracotomy to deliver clip to the tricuspid valve (blue). MRI = Magnetic Resonance Imaging


