Abstract
Avoidance of sick individuals is vital to the preservation of one’s health and preventing transmission of communicable diseases. To do this successfully, one must identify social cues for sickness, which include sickness behaviors and chemosignals, and use this information to orchestrate social interactions. While many social species are highly capable with this process, the neural mechanisms that provide for social responses to sick individuals are only partially understood. To this end, we used a task in which experimental rats were allowed to investigate two conspecifics, one healthy and one sick. To imitate sickness, one conspecific received the viral mimic Polyinosinic:polycytidylic acid (Poly I:C) and the other saline. In a 5-minute social preference test, experimental male and female adult rats avoided Poly I:C treated adult conspecifics but did not adjust social interaction in response to Poly I:C treated juvenile conspecifics. Seeking a neural locus of this behavior, we inhibited the insular cortex, a region necessary for social behaviors directed toward conspecifics in distress. Insular cortex inactivation via administration of the GABAA agonist muscimol to experimental rats prior to social preference tests eliminated the preference to avoid sick adult conspecifics. These results suggest that some aspect of conspecific illness may be encoded in the insular cortex which is anatomically positioned to coordinate a situationally appropriate social response.
Keywords: Insular Cortex, sickness behavior, social preference, social avoidance, rat
1. Introduction
Limiting the spread of disease is important to group survival[1]. In social species, this requires identifying sick individuals and deciding whether or not to approach those individuals. Avoiding sick others is a natural and effective means of limiting the spread of communicable diseases through a population[2], thus increasing a group’s overall wellness. Across species, animals will avoid conspecifics that show signs of illnesses caused by viruses, bacteria or parasitic infections[3]. For example, male mice will pass up mating opportunities with sick females[4], underscoring the importance of illness recognition to social behavior. As such, it is of great interest to understand the process by which individuals recognize the signs of sickness in others and then use this information to avoid or limit interactions.
Acute infections cause a range of sickness behaviors, including changes in physical appearance[5], vocalizations[6] and the production of chemical signals[3] that are used by observers to determine whether to avoid individuals. Recognizing these cues from a distance, likely via the detection of odors produced by the sick individual, is an effective strategy to mitigate contact with sick rodents[3,7]. Interestingly, the decision to approach or avoid sick others is sensitive to the age of the sick conspecific[8]. When presented odorants from sick adults, rats avoid same-sex conspecifics, but when presented with odorants from sick juveniles, animals do not change their approach behavior. In similar interactions with stressed conspecifics, rats exhibit age specific avoidance of stressed adults but approach stressed juveniles[9]. It is also possible that sex differences would occur in response to interacting with sick others as group housing augments sickness responses in females whereas isolation increases sickness in males indicating that immune response itself is affected by the social environment[10]. In sum, the variance in behavior observed when faced with social stimuli indicating imminent stress or illness that can be attributed to age, sex or context provided evidence of a social-decision making process which involves the integration of a range of social stimuli to guide situationally suitable behaviors, but understanding the underlying circuitry triggering this avoidance is just beginning.
An important mechanism underlying sickness behaviors is the acute action of peripheral and central cytokines. In response to pathogens, peripheral and central cytokines are produced, for example interleukin 1-beta (IL-β), that decrease overall activity and feeding[11]. Moreover, sickness can lead to context dependent changes in sociality, increasing positively valenced sociality while decreasing negatively valenced social behavior[1,12]. Increases in IL-β lead to the production of odorants in adults that are then avoided by conspecifics[3]. Chemicals that signal sickness are first detected by the vomeronasal organ[13] which projects to the medial amygdala which contributes to the social avoidance of sick conspecifics[14]. Recent work has shown that the posteromedial nucleus of the cortical amygdala (COApm), a region with inputs from the vomeronasal organ and outputs to the medial amygdala, prevents males from mating with sick females[4]. However, while inactivation of the COApm prevents mating, it does not appear to alter overall sociability. Thus, changes in behavior towards a same-sex conspecific are likely mediated by another mechanism. As such, individuals observing sickness behavior in others must integrate context, visual, auditory and chemosignal cues and make a decision on whether or not to approach that individual.
Here we investigated the insular cortex, a region that is important to processing danger cues from conspecifics and corresponding avoidance behaviors[9]. Anatomically, the posterior insular cortex receives interoceptive and exteroceptive sensory inputs and interfaces with many brain regions known for risk assessment and social behavior[15–18]. Importantly, insula activity increases when people are presented with pictures of pain or distress[19,20] and it is thought to be central to the circuitry for detecting affect in others[15,21–24]. Regarding detection of sickness in rat, insular cortex receives monosynaptic input from the medial amygdala[25]. Therefore, we adapted the social preference test used in our prior studies[9,26] for a social choice test with healthy or sick conspecifics injected with the viral mimic Poly I:C. To investigate the role of the insular cortex, we conducted these tests following microinjection of the GABAA agonist muscimol into the insular cortex of focal rats to determine if insular activity was necessary for social preference. We hypothesized that 1) animals would avoid sick others in the social preference test, and 2) that the insular cortex would be necessary for this behavior.
2. Methods
2.1. Animals
Male and female Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA) at postnatal day 45 (PN 45) for test rats and adult conspecifics or PN 21 for juvenile conspecifics. Shipping on PN45 means that rats were likely undergoing puberty during shipping which can lead to reduced social behavior and an increased response to stressors[27,28]. All rats were, however, shipped at the same age and allowed the same amount of time to recover. Importantly, social preference behaviors among rats shipped as described is equivocal to social behaviors of rats breed and raised in our facility (N. Rieger & J. Christianson, unpublished observations). All rats were allowed at least one week to acclimate to the vivarium prior to testing. Rats were housed in isosexual groups of 2–3 per cage with food and water available ad libitum. The vivarium was maintained on a 12 h light/dark cycle and all behavioral testing occurred within the first 4 h of the light cycle. All procedures were conducted in accordance with the National Institute of Health’s Guide for the care and use of laboratory animals and were approved by the Boston College Institutional Animal Care and Use Committee.
2.2. Activation of sickness response
Conspecifics were randomly assigned to be injected i.p with either Poly I:C (Sigma-Aldrich, St. Louis MO; Product #P9582, Lot #118M4035V), a viral mimic which invokes an immune response, or saline 3 hours prior to social preference testing (Fig. 1). Poly I:C was dissolved in saline to a dose of 0.5 mg/kg, a dose which has been shown to cause short term fevers[29]. Poly I:C is sometimes contaminated by endotoxin[30,31], therefore it is possible that some of the behavior observed here was due to immune responses to bacteria. Rats temperatures were taken prior to injection via rectal thermometer and again after the completion of behavioral testing to validate the presence of fever.
Figure 1: Adult rats avoid conspecifics treated with Poly I:C.
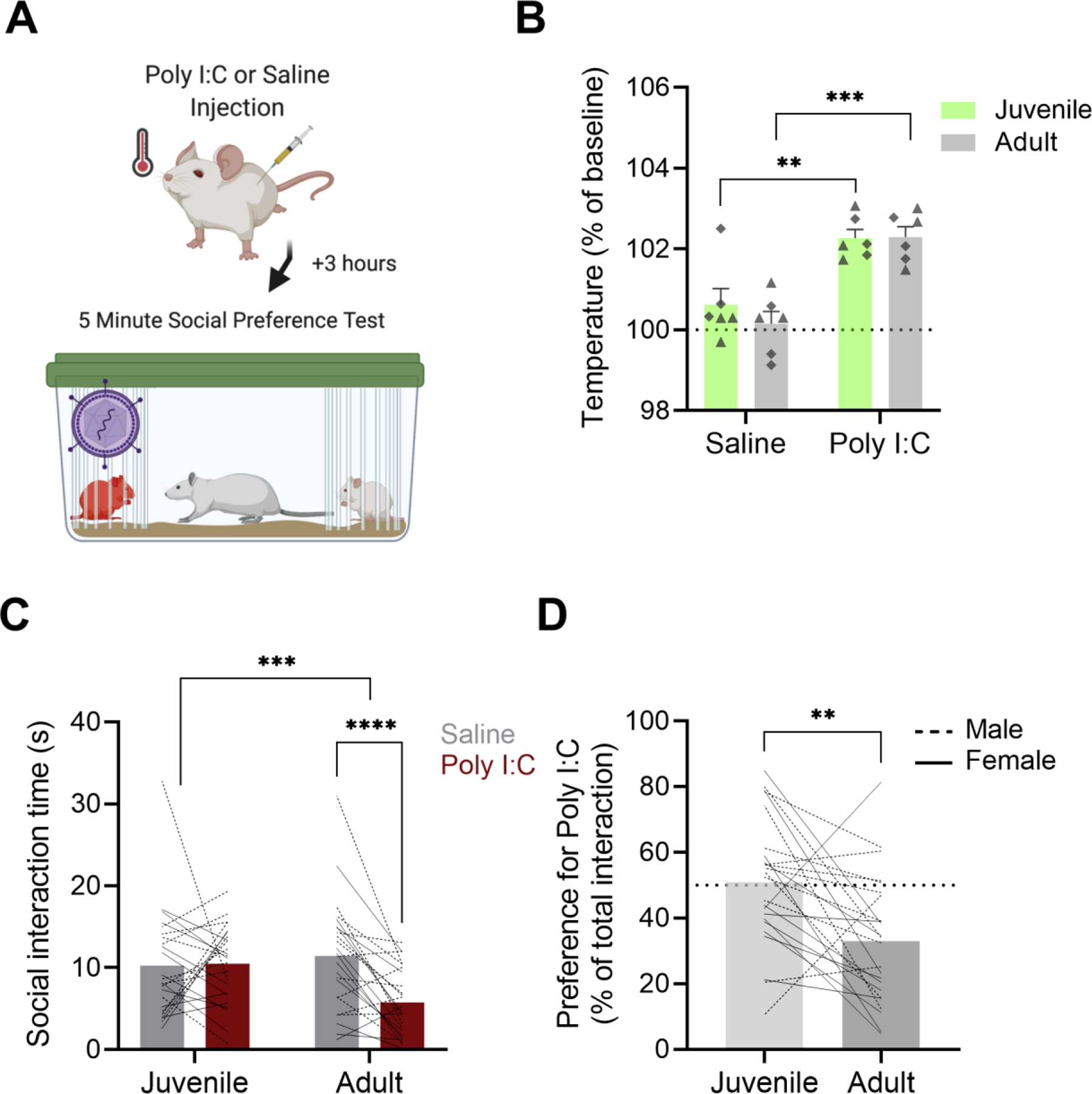
A. Conspecific adults (P50) or juveniles (P28) were injected with Poly I:C (0.5 mg/kg) or saline 3 h prior to social preference tests. Temperatures for all conspecifics were taken prior to injection and following the completion of testing. Social preference tests were 5 min and consisted of a focal rat interacting with a Poly I:C and saline injected same-sex conspecific. Created with BioRender.com B. Mean (+/− SEM with individual replicates) rectal temperature change of a separate cohort of adult and juvenile rats 2 h after i.p. saline or Poly I:C injections. Regardless of age, rats injected with Poly I:C showed a significant increase in temperature compared to baseline as well as compared to saline injected controls. Males are represented with triangles and females are represented by diamonds. C. Mean (with individual replicates) time spent interacting with juvenile or adult saline or Poly I:C treated rats in social preference tests. Rats spent equal time interacting with juvenile rats but adult rats spent more time investigating the saline treated rat compared to the Poly I:C conspecific. D. Mean (with individual replicates) preference for the Poly I:C rat (data in panel C expressed as the percent of time investigating the Poly I:C rat of the total investigation time). Adult rats avoided the sick conspecific. In C and D individual male replicates are represented with dashed lines while females are represented with solid lines. ** p < 0.01, *** p < 0.001, **** p < 0.0001.
2.3. Surgical implantation of indwelling cannula
While under isoflurane anesthesia (2–5% v/v in O2), rats were surgically implanted with bilateral guide cannula (26-gauge, Plastics One, Roanoke VA) in the posterior insular cortex (from bregma: AP: −1.8 mm, M/L: ± 6.5 mm, D/V: −6.8 mm from skull surface) that were affixed with stainless steel screws and acrylic dental cement. The posterior insular cortex is the site of multisensory inputs[15,18] and division determined to contribute to social affective preference previously[13]. Immediately following surgery, rats were injected with analgesic meloxicam (1 mg/kg, Eloxiject, Henry Schein), antibiotic penicillin (12,000 units, Combi-pen 48, Covetrus) and Ringer’s solution (10 mL, Covetrus). Rats were then returned to their homecage and allowed 7–14 days for recovery prior to behavioral testing.
Muscimol (Tocris) was first dissolved in saline and 100 ng/side was injected at a rate of 1.0 μL/minute with an additional one minute diffusion time after injection[9,32]. After behavioral testing concluded rats were overdosed with tribromoethanol (Sigma) and brains were immediately dissected and fresh frozen on dry ice. Brains were sectioned on a cryostat at 40 μm, slices were then mounted onto gel-subbed slides (Fisher) and stained with cresyl-violet to verify cannula placements by comparing microinjector tip location to the rat whole brain stereotaxic atlas. Rats were excluded from all analyses if their cannula placements were found to be outside the insula or if both of their cannulas were occluded prior to injection.
2.4. Social Preference Test
The social preference test allows for the observation of rat social decision making when presented with 2 same-sex conspecifics that differ on some experimental treatment. The method used here is exactly as described previously as the “social affective preference test” [9,33,34]with a variation that conspecifics were treated with either Poly I:C or saline. Social preference tests occurred in a long plastic arena (76.2 cm × 20.3 cm × 17.8 cm, Length × Width × Height) with shaved wood bedding. Conspecifics are placed at opposite ends of the testing chamber in enclosures (18 cm × 20 cm × 10 cm, Length × Width × Height) with horizontal bars placed 1 cm apart on one side to facilitate interactions. On day 1, experimental rats were moved to the testing room for 1 h and exposed to the testing arena for 10 minutes to habituate before being returned to their home cages. On day 2, rats were moved to the testing room 1 h before a 5 min exposure to a pair of unfamiliar and experimentally naive conspecifics to provide habituation to the social preference test. In experiment 1, on day 3 social preference tests were conducted with 1 h in the test room followed by a 5-minute exposure to 2 conspecifics, one of which received a saline injection (Control) and the other which received a Poly I:C injection (Sick) 3 h prior to testing. In Experiment 2, these tests were conducted on consecutive days for within-subject’s analysis of social preference under muscimol or saline as described below. Importantly, the conspecifics are always unfamiliar to the test rat and different conspecifics are used for each day. During the 5 min exposure to conspecifics, test rats were allowed to freely interact with the conspecifics and social investigation time was calculated as time spent sniffing or grabbing the conspecific as scored by observers blind to treatment.
2.5. Experimental Designs
To characterize the behavior of male and female rats toward sick juvenile or adult conspecifics, in experiment 1 (Fig. 1A) 12 male and 12 female rats underwent social preference tests first with juvenile conspecifics and then one week later with adult conspecifics following the same process of habituation and testing. All behavioral tests occurred with conspecifics of the same sex. Time spent interacting with the control and sick conspecifics was determined in a 5-minute social preference test and analyzed in a 2 (Sex of the test rat: male or female) × 2 (Age of the conspecific: adult or juvenile) by 2 (Sickness of the conspecific: Control or Poly I:C) analysis of variance with Sex as a between-subject’s factor and age and sickness as within-subject’s factors. Time spent investigating the sick conspecific was converted to a social preference score by dividing time spent investigating the sick rat divided by the sum time spent interacting with both the control and sick rats multiplied by 100.
To determine the role of the posterior insular cortex, in experiment 2 (Fig. 2A) a separate cohort of 12 male and 12 female rats were implanted with bilateral microinjection cannula guides with stylets and allowed to recover. Due to cannula damage and placements outside of the insula 1 male and 3 females were excluded from final analysis for a final sample size of 11 males and 9 females. Because test rats only showed avoidance of sick adults in experiment 1, we conducted social preference tests with insular inhibition with only same-sex adult conspecifics. Habituation to the arena and to the social preference test occurred on days 1 and 2 as described above. On day 3, adult conspecifics were administered saline or Poly I:C 3 hours prior to social preference tests and experimental rats received muscimol or saline in the insula 30 minutes prior to testing, a time previously shown to impair social preference for stressed conspecifics[9]. Assignment to saline or muscimol conditions was random and counterbalanced. After tests on day 3, rats were returned to their home cages and left undisturbed for 48h to ensure muscimol was cleared. On day 5, rats were given a second social preference test with saline and Poly I:C treated conspecifics exactly as on day 3 with the exception that the experimental rat received the opposite drug treatment allowing for a within-subjects comparison of social preference under muscimol. The resulting social interaction times were analyzed with a 2 (Sex of test rat: male vs female) by 2 (Sickness of conspecific rat: control vs Poly I:C) by 2 (Drug: muscimol or saline) with sex treated as a between-subjects factor and sickness and drug treated as within-subjects factors. Data were first screened for drug-treatment order effects, and none were evident.
Figure 2: Insula activity is required for rats to distinguish between healthy and sick conspecifics in social preference tests.
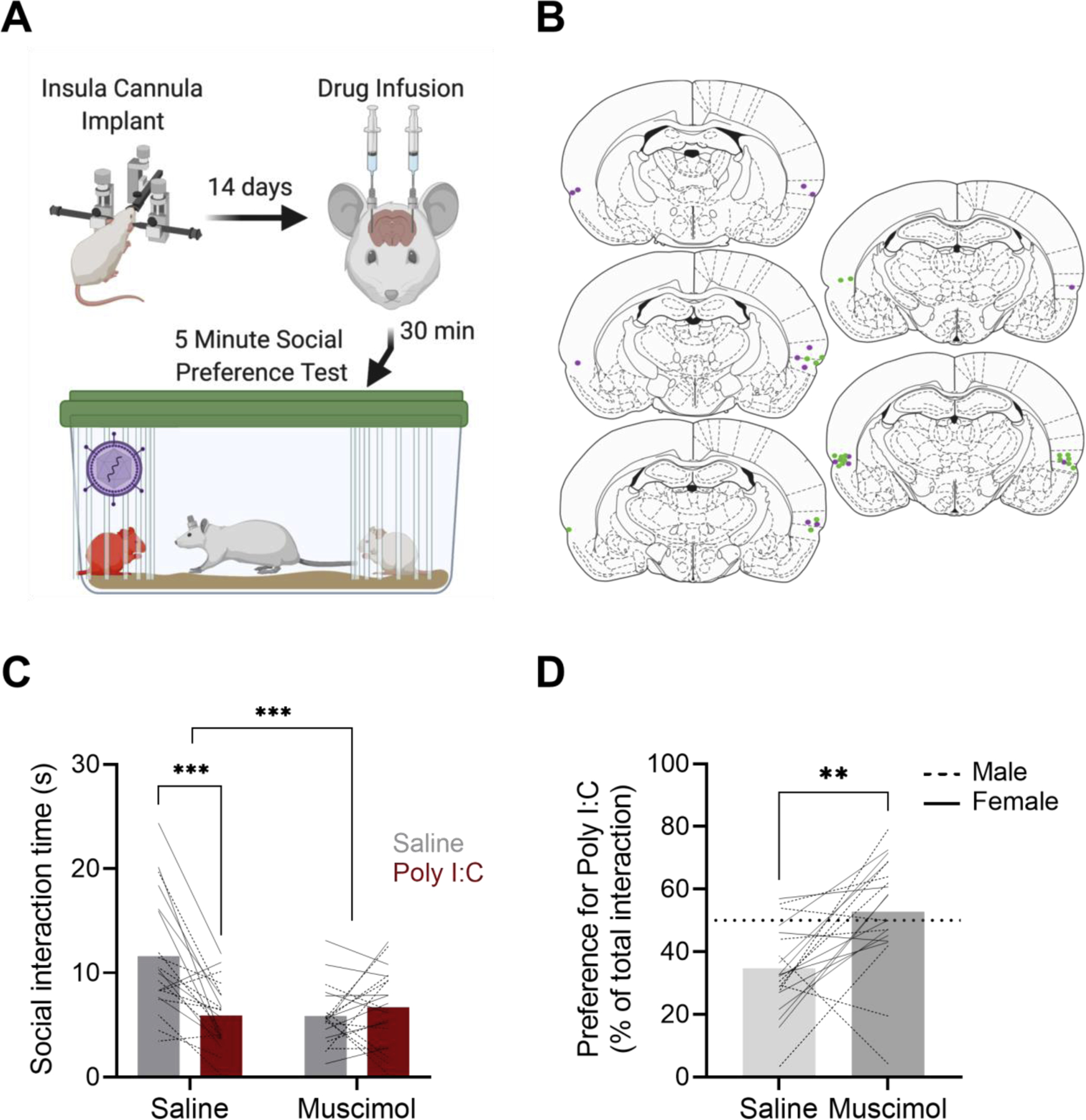
A. To determine the role of the insula cortex in distinguishing between sick and healthy conspecifics, focal animals were implanted with cannula in the insular cortex 2 weeks prior to social preference testing. On testing day rats were injected with saline or muscimol (100 ng/side) in a counterbalanced order 30 minutes prior to social preference testing with saline and Poly I:C treated adult conspecifics. Created with BioRender.com B. Representative locations of cannulas in the posterior insular cortex for rats included in analysis; male and female rats are represented in green and purple, respectively. C. Mean (with individual replicates) time spent interacting with adult saline or Poly I:C treated rats in social preference tests after saline or muscimol injections. After saline injections, focal adult rats avoided the sick conspecifics but this did not occur after muscimol injections. D. Mean (with individual replicates) preference for the Poly I:C rat (data in panel C expressed as the percent of time investigating the Poly I:C rat of the total investigation time). Adult rats avoided the sick conspecific after saline injections but spent equal time investigating after insular cortex muscimol injections. In C and D individual male replicates are represented with dashed lines while females are represented with solid lines. *** p < 0.001, ** p < 0.01, * p < 0.05.
2.6. Statistical analysis
All statistics were completed in Prism (Graphpad, version 9.1.2). Sample sizes were determined based on prior experience with social preference tests. In repeated measures experiments, treatment and test order were counterbalanced over 2 experimental days. Analysis of variance was used to determine differences between mean scores of behavioral interactions. In both experiments the design is a 3 factor ANOVA which includes rat sex (male or female) as an independent variable. All analyses were first conducted with the complete 3-way ANOVA to determine if there were significant main effects of sex, or interactions between sex and any of the other factors. In no cases did we find significant effects for sex. Therefore, data from male and female rats were combined for analysis with 2 factor ANOVAs. For clarity, individual replicates are indicated in all of the figures. Total sample size for each experiment is indicated on the figures. Main effects and interactions were deemed significant when p < 0.05 and all reported post hoc test p values are Sidak-adjusted, to maintain an experiment-wise risk of type I errors at a = 0.05.
3. Results
3.1. Poly I:C induced acute sickness.
To generate ‘healthy’ and ‘sick’ conspecific rats, rats were injected i.p. with either Poly I:C (0.5 mg/kg) or saline 3 h prior to social preference testing. To validate this treatment, a separate cohort of rats (n = 24) was tested for their fever response to saline or Poly I:C injections including an equal number of male and female adult and juvenile rats (n = 3 per group). rectal temperatures were taken before injection and 2 h later to confirm the onset of a fever prior to testing at a consistent time point. In our initial analysis we conducted a 3-way ANOVA with Sex (male vs female) Age (juvenile vs adult) and Treatment (saline vs Poly I:C) as factors. Because no main effect of sex F1,4 = 0.0083, p = 0.9317) or any interactions between sex and the other variables (Sex × Age × Treatment F1,4 =1.587, p = 0.2762, Sex × Treatment F1,4 = 0.2586, p = 0.639; Sex × Age F1,4 = 2.078, p = 0.2229) were present we combined male and female data and ran our final analyses as a 2 factor ANOVA with Treatment (Saline vs Poly I:C) and Age (Juvenile vs Adult) as between subjects factors. Using this analysis there was a significant effect of Poly I:C (ANOVA F1,10 = 69.31, p < 0.0001, Fig. 1B) but no interaction of Poly I:C with age (Treatment × Age F1,10 = 0.5040, p = 0.4940) indicating that both adult and juvenile rats produced significant fevers in response to Poly I:C injection (Temperature as °C: Adult saline: Baseline = 33.76 ± 0.22, 2 h = 33.81 ± 0.23; Adult Poly:IC: Baseline = 33.45 ± 0.24, 2 h = 34.22 ± 0.20; Juvenile Saline: Baseline = 32.3 ± 0.55, 2 h = 32.5 ± 0.52; Juvenile Poly:IC: Baseline = 33.13 ± 0.31, 2 h = 33.88 ± 0.29). These results were replicated in the cohort of animals that acted as conspecifics in the social preference test where final temperatures were taken after the conclusion of testing (data not shown), 4–5 hours post injection indicating that fevers were still present in animals that received Poly I:C. Qualitatively, rats that received Poly I:C showed piloerection and drooping eyelids prior to the start of social preference testing.
3.2. Experimental rats avoid Poly I:C treated adult but not juvenile conspecifics.
To determine if Poly I:C induced sickness affected interaction with sick conspecifics in male and female rats we used a social preference test. As above, we first analyzed this data as a 3-way ANOVA with Sex of the test rat (Male vs Female), Age of the conspecific (Juvenile vs Adult) and Sickness of the conspecific (Healthy vs Sick). Again, we found no main effects (F1, 22 = 3.702, p = 0.0674) or significant interactions with Sex (Sex × Age × Sickness F1, 22 = 0.0012, p = 0.9716; Sex × Sickness F1, 22 = 0.7586, p = 0.3932; Sex × Age F1, 22 = 0.0162, p = 0.8998) so we combined across sex and completed our final analyses as a 2 (Juvenile vs Adult) × 2 (Healthy vs Sick) ANOVA. Using this design, we found a significant Age × Sickness interaction (Age × Sickness F1, 23 = 16.17, p = 0.0005, Fig. 1C) such that rats spent significantly less time interacting with sick compared to healthy adult conspecifics but showed no preference in interactions with juvenile conspecifics (Sidak’s comparison, Adults: p <0.0001; Juveniles p = 0.9692, Fig. 1C). When analyzing percent preference for sick conspecifics we found a significantly reduced preference for sick adults compared to sick juveniles (paired t-test, t23 = 3.444, p = 0.0022, Fig. 1D). Taken together, these results indicate that sickness based avoidance of conspecifics is age dependent, such that rats will avoid sick adults but not sick juveniles.
3.3. Insular inactivation prevents avoidance of sick adults.
Because preference for stressed juveniles and unstressed adults in the social preference test is insula dependent we were interested in whether social preference induced by sickness would also be insula dependent. As such, we placed in-dwelling cannulas in the insula of male and female rats and tested them for social preference behavior with sick and healthy adult conspecifics following injections of either muscimol, to inactivate the insula, or saline in a within-subject’s design. As in the prior experiments we first analyzed these results in a 2 (Male vs Female) × 2 (Muscimol vs Saline) × 2 (Healthy vs Sick) 3-way ANOVA and again found no main effect (F1,17 = 3.841, p > 0.05) or interactions with sex (Sex × Treatment × Sickness F1,17 = 2.601, p = 0.1252; Sex × Treatment F1,17 = 0.4187, p = 0.5262; Sex × Sickness F1,17 = 1.511, p = 0.2357). Therefore, we combined sexes and completed our final analyses as a 2 (Muscimol vs Saline) × 2 (Healthy vs Sick) ANOVA. Using this design, we found a significant Muscimol × Sickness interaction (Treatment × Sickness: F1,19 = 16.74, p = 0.0006, Fig 2C) such that an initial preference to interact with healthy versus sick conspecifics was blocked by insular microinjection of muscimol (Sidak’s comparison, Saline, p = 0.0001; Muscimol, p = 0.7174). We also found that percent preference for sick adult conspecifics was significantly increased by muscimol treatment (paired t-test, t19 = 3.392, p = 0.0031, Fig 2D). These data indicate that the insula is required for the avoidance of sick conspecifics.
4. Discussion
In these studies, we hypothesized that rats would prefer to interact with healthy versus sick adult and juvenile conspecifics and that preference formation would be insula dependent. To do this, we modified the social affective preference test[9] so that we could quantify the time spent interacting with healthy or sick conspecifics. In social preference tests in which adult rats were given the choice to interact with a same age adult conspecific that had received Poly I:C 3 h earlier or a same age rat that received saline, male and female rats spent more time investigating the saline treated rat, indicating a decision to avoid the sick rat. Interestingly, if the experimental rat was given a choice to interact with healthy or sick juvenile rats, the time spent investigating the targets was equivocal. Thus, in this paradigm behavioral responses to sick conspecifics is dependent on the sick individual’s age. In our prior work, we observed that the age-dependent approach and avoidance responses to stressed rats activated and required the posterior insular cortex[9]. Here, when we conducted social preference tests with sick adult rats we found that inactivation of the insula with muscimol eliminated the experimental rat’s avoidance of sick adults. These findings expand the brain systems implicated in social responses to sick individuals to include the insular cortex and reinforce the importance of the insular cortex in the circuitry organizing behaviors related to social threat.
Determining that others are sick and potentially contagious with a pathogen and then avoiding them is important for social living animals to survive. Many outward cues can be used to convey sickness and thus drive avoidance. For example, sick animals show signs of illness such as piloerection, drooping eyes or hunching over that can be seen visually[5]. Sick animals also produce fewer vocalizations compared to healthy animals[6,35] and these reduced vocalizations could lead to reduced overall social interaction. However, because rats predominantly use odor cues in social interactions it seems most likely that in this experiment rats are discriminating between healthy and sick adult conspecifics via an odorant brought on by the activation of the immune system[3]. When exposed to healthy versus sick urine, rats will avoid the urine of males treated with lipopolysaccharide (LPS)[36]. It is likely that in the social preference test used here, in which rats can investigate each other directly, there is a mixture of all of these cues contributing to test rats’ avoidance of sick adults.
The presence of an age effect in social approach to sick rats is fascinating as experimental rats did not bias interaction towards sick juveniles. In terms of the biological mechanisms that may underlie the age dependent effects, we first consider the potential for differences in the quantity or quality of sickness signals emitted by juvenile rats compared to adults. In our prior work, we quantified the behaviors and ultrasonic vocalizations emitted by adult and juvenile rats in social preference tests where one conspecific had received footshock stress. Adult rats produced more 22kHz distress type ultrasonic vocalizations after stress while juvenile rats, which did not emit distress vocalizations, emitted fewer prosocial high-frequency vocalizations. Further, we observed differences in self grooming behavior after stress[9] which together suggest that the auditory and overt behavioral stimuli present for the focal experimental rat to integrate and appraise are differentially expressed by adult and juvenile rats experiencing stress. In social approach tests using only the urine obtained from donors treated with LPS, adult rats avoid urine from adult donors but do not avoid urine from pre-pubescent donors leading to the hypothesis that juveniles do not not produce the same sickness related odor cues as adults. Instead, pre-pubescent rats only produced aversive odors in response to LPS if they were also injected with estradiol or testosterone[8] indicating that the aversive odor cues brought on by sickness are tied to an interplay between sex-steroids and the immune system which may change during puberty.
While our fever data show that juvenile rats responded to Poly I:C a number of studies have shown that juvenile rats have a blunted overall response following LPS treatment including reduced production of cytokines[37] and reduced fos expression[38]. This blunted response in juveniles could cause them to produce fewer chemogenetic cues for test rats to pick up on leading them to not recognize the juveniles sick state. While this has previously been shown in LPS, a bacterial mimic, this is likely to be true of a viral mimic as well[39]. Another possibility is that juvenile sick rats emit signals that attract social interaction which may merit further investigation. Although more difficult to investigate directly with neurobiological methods, a second possibility is that the age of the sick individual alters the decision making of rats in response to aversive cues. Simply put, the experimental rat may detect sickness in the conspecific but decide that the juvenile does not pose a social threat. This could also help to explain why, even though both adult and juvenile conspecifics produced fevers and showed visible signs of illness, only adults were avoided. To investigate these processes will require identifying with more detail the neural responses evoked in observers of sick adult or juvenile rats.
A number of factors including familiarity[33], stress[9,40–42], fear state[43], or relief (from water deprivation)[44] can affect whether an individual is approached or avoided. In some ways, these cues are very similar to responses to sick conspecifics. The transmission of psychological stressors, pain and recognition of sickness in others have all been shown to be odorant driven and tied to increased cytokine levels[3,45,46] and, at least in adult rats, both stress[9] and sickness[8] drive avoidance via signals being detected by the vomernasal organ[13] and changes to subcortical regions such as the medial amygdala[14] which in turn projects to the insular cortex[25]. Thus, this trisynaptic tract may provide an anatomical substrate for chemosensory information relating to sickness to reach the insular cortex where it is integrated with other sensory information in order to drive social decision making[15,41,42].
Social information is conveyed to the insula via primary sensory afferents and the insula outputs this information to a number of subcortical structures involved in social avoidance including the bed nucleus of the stria terminalis, the central amygdala and the periaqueductal gray[15,18]. With this, the insula may be uniquely activated by social signals that convey distress, such as pain, sickness and fear[9,16,19,26,47,48]. Interestingly, when the insula was inactivated by muscimol, social approach towards healthy conspecifics was also reduced and thus it is possible that without insular function rats can detect sickness but are unable to assign it to the proper social source leading to reduced overall interactions. However, whether the constellation of stimuli presented by sick, stressed, or otherwise affected conspecifics activate the same neural circuitry beyond the insula remains unknown and is of great interest. Understanding this system could provide better insights into how the immune system, insula and social decision making network interact to make social decisions and to inform future treatments for aberrant social processing.
Recognizing and evaluating cues from others and using that information to choose who to approach or avoid is vital to successfully navigating social situations. Close social ties and interactions are necessary to create relationships, form cooperative partnerships and navigate many aspects of life across taxa. However, being able to recognize that another is sick and thus potentially dangerous changes the balance of these decisions, requiring social distancing in order to prevent harm. Recently, the global COVID-19 pandemic has heightened attention to detail about the behaviors and wellness of others providing renewed justification for understanding how humans and other animals accomplish this vital form of social cognition. Here, we identify posterior insular cortex as a new component of the neural systems mediating social distancing and suggest its role is to integrate overt indicators of sickness with internal and situational factors which ultimately inform the decision to approach or avoid others.
Highlights.
Male and Female rats avoid sick adult but not sick juvenile conspecifics
Insula cortex inactivation prevents avoidance of sick adults
Insular cortex integrates sickness cues in order to make social decisions
Acknowledgments
The authors wish to thank Nancy McGilloway and Todd Gaines, administrators of the Boston College Animal Care Facility, for outstanding animal husbandry, and Rahul Alturi and Bridget Brady for assistance with behavioral tests and scoring.
Funding
Funding for this work was provided by National Institutes of Health Grants MH119422.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hennessy MB, Deak T, Schiml PA, Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure?, Brain. Behav. Immun 37 (2014) 15–20. 10.1016/j.bbi.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stockmaier S, Bolnick DI, Page RA, Carter GG, Sickness effects on social interactions depend on the type of behaviour and relationship, J. Anim. Ecol 89 (2020) 1387–1394. 10.1111/1365-2656.13193. [DOI] [PubMed] [Google Scholar]
- [3].Arakawa H, Cruz S, Deak T, From models to mechanisms: odorant communication as a key determinant of social behavior in rodents during illness-associated states, Neurosci. Biobehav. Rev 35 (2011) 1916–1928. 10.1016/j.neubiorev.2011.03.007. [DOI] [PubMed] [Google Scholar]
- [4].Kwon J-T, Ryu C, Lee H, Sheffield A, Fan J, Cho DH, Bigler S, Sullivan HA, Choe HK, Wickersham IR, Heiman M, Choi GB, An amygdala circuit that suppresses social engagement, Nature. 593 (2021) 114–118. 10.1038/s41586-021-03413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kentner AC, Bilbo SD, Brown AS, Hsiao EY, McAllister AK, Meyer U, Pearce BD, Pletnikov MV, Yolken RH, Bauman MD, Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model, Neuropsychopharmacology. 44 (2019) 245–258. 10.1038/s41386-018-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kirsten TB, Galvão MC, Reis-Silva TM, Queiroz-Hazarbassanov N, Bernardi MM, Zinc Prevents Sickness Behavior Induced by Lipopolysaccharides after a Stress Challenge in Rats, PLOS ONE. 10 (2015) e0120263. 10.1371/journal.pone.0120263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR, In Sickness and in Health: The Co-Regulation of Inflammation and Social Behavior, Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 42 (2017) 242–253. 10.1038/npp.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arakawa H, Arakawa K, Deak T, Acute illness induces the release of aversive odor cues from adult, but not prepubertal, male rats and suppresses social investigation by conspecifics, Behav. Neurosci 123 (2009) 964–978. 10.1037/a0017114. [DOI] [PubMed] [Google Scholar]
- [9].Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, Christianson JP, Insular cortex mediates approach and avoidance responses to social affective stimuli, Nat. Neurosci 21 (2018) 404–414. 10.1038/s41593-018-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yee JR, Prendergast BJ, Sex-specific social regulation of inflammatory responses and sickness behaviors, Brain. Behav. Immun 24 (2010) 942–951. 10.1016/j.bbi.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dantzer R, Bluthé RM, Gheusi G, Cremona S, Layé S, Parnet P, Kelley KW, Molecular basis of sickness behavior, Ann. N. Y. Acad. Sci 856 (1998) 132–138. 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- [12].Moieni M, Eisenberger NI, Effects of inflammation on social processes and implications for health, Ann. N. Y. Acad. Sci 1428 (2018) 5–13. 10.1111/nyas.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boillat M, Challet L, Rossier D, Kan C, Carleton A, Rodriguez I, The Vomeronasal System Mediates Sick Conspecific Avoidance, Curr. Biol 25 (2015) 251–255. 10.1016/j.cub.2014.11.061. [DOI] [PubMed] [Google Scholar]
- [14].Arakawa H, Arakawa K, Deak T, Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor, Neuroscience. 171 (2010) 1141–1151. 10.1016/j.neuroscience.2010.10.013. [DOI] [PubMed] [Google Scholar]
- [15].Rogers-Carter MM, Christianson JP, An insular view of the social decision-making network, Neurosci. Biobehav. Rev 103 (2019) 119–132. 10.1016/j.neubiorev.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gogolla N, The insular cortex, Curr. Biol. CB 27 (2017) R580–R586. 10.1016/j.cub.2017.05.010. [DOI] [PubMed] [Google Scholar]
- [17].Gehrlach DA, Dolensek N, Klein AS, Chowdhury RR, Matthys A, Junghänel M, Gaitanos TN, Podgornik A, Black TD, Vaka NR, Conzelmann K-K, Gogolla N, Aversive state processing in the posterior insular cortex, Nat. Neurosci 22 (2019) 1424–1437. 10.1038/s41593-019-0469-1. [DOI] [PubMed] [Google Scholar]
- [18].Gehrlach DA, Weiand C, Gaitanos TN, Cho E, Klein AS, Hennrich AA, Conzelmann K-K, Gogolla N, A whole-brain connectivity map of mouse insular cortex, ELife. 9 (2020) e55585. 10.7554/eLife.55585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Decety J, Skelly LR, Kiehl KA, Brain response to empathy-eliciting scenarios involving pain in incarcerated individuals with psychopathy, JAMA Psychiatry. 70 (2013) 638–645. 10.1001/jamapsychiatry.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gu X, Hof PR, Friston KJ, Fan J, Anterior insular cortex and emotional awareness, J. Comp. Neurol 521 (2013) 3371–3388. 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Menon V, Uddin LQ, Saliency, switching, attention and control: a network model of insula function, Brain Struct. Funct 214 (2010) 655–667. 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nieuwenhuys R, The insular cortex: a review, Prog. Brain Res 195 (2012) 123–163. 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- [23].Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB, A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis, Brain Struct. Funct 214 (2010) 519–534. 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lu C, Yang T, Zhao H, Zhang M, Meng F, Fu H, Xie Y, Xu H, Insular Cortex is Critical for the Perception, Modulation, and Chronification of Pain, Neurosci. Bull 32 (2016) 191–201. 10.1007/s12264-016-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pardo-Bellver C, Cadiz-Moretti B, Novejarque A, Martinez-Garcia F, Lanuza E, Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice, Front. Neuroanat 0 (2012). 10.3389/fnana.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rogers-Carter MM, Djerdjaj A, Gribbons KB, Varela JA, Christianson JP, Insular Cortex Projections to Nucleus Accumbens Core Mediate Social Approach to Stressed Juvenile Rats, J. Neurosci. Off. J. Soc. Neurosci 39 (2019) 8717–8729. 10.1523/JNEUROSCI.0316-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Holder MK, Blaustein JD, Developmental time course and effects of immunostressors that alter hormone-responsive behavior on microglia in the peripubertal and adult female mouse brain, PloS One. 12 (2017) e0171381. 10.1371/journal.pone.0171381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laroche J, Gasbarro L, Herman JP, Blaustein JD, Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period, Endocrinology. 150 (2009) 2351–2358. 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fortier M-E, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN, The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism, Am. J. Physiol.-Regul. Integr. Comp. Physiol 287 (2004) R759–R766. 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- [30].Kowash HM, Potter HG, Edye ME, Prinssen EP, Bandinelli S, Neill JC, Hager R, Glazier JD, Poly(I:C) source, molecular weight and endotoxin contamination affect dam and prenatal outcomes, implications for models of maternal immune activation, Brain. Behav. Immun 82 (2019) 160–166. 10.1016/j.bbi.2019.08.006. [DOI] [PubMed] [Google Scholar]
- [31].Mueller FS, Richetto J, Hayes LN, Zambon A, Pollak DD, Sawa A, Meyer U, Weber-Stadlbauer U, Influence of poly(I:C) variability on thermoregulation, immune responses and pregnancy outcomes in mouse models of maternal immune activation, Brain. Behav. Immun 80 (2019) 406–418. 10.1016/j.bbi.2019.04.019. [DOI] [PubMed] [Google Scholar]
- [32].Foilb AR, Flyer-Adams JG, Maier SF, Christianson JP, Posterior insular cortex is necessary for conditioned inhibition of fear, Neurobiol. Learn. Mem 134Pt B (2016) 317–327. 10.1016/j.nlm.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rogers-Carter MM, Djerdjaj A, Culp AR, Elbaz JA, Christianson JP, Familiarity modulates social approach toward stressed conspecifics in female rats, PLOS ONE. 13 (2018) e0200971. 10.1371/journal.pone.0200971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rieger NS, Varela JA, Ng AJ, Granata L, Djerdjaj A, Brenhouse HC, Christianson JP, Insular cortex corticotropin-releasing factor integrates stress signaling with social decision making., BioRxiv. (2021) 2021.03.23.436680. 10.1101/2021.03.23.436680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nascimento AF, Alves GJ, Massoco CO, Teodorov E, Felicio LF, Bernardi MM, Lipopolysaccharide-Induced Sickness Behavior in Lactating Rats Decreases Ultrasonic Vocalizations and Exacerbates Immune System Activity in Male Offspring, Neuroimmunomodulation. 22 (2015) 213–221. 10.1159/000363350. [DOI] [PubMed] [Google Scholar]
- [36].Arakawa H, Arakawa K, Deak T, Sickness-related odor communication signals as determinants of social behavior in rat: a role for inflammatory processes, Horm. Behav 57 (2010) 330–341. 10.1016/j.yhbeh.2010.01.002. [DOI] [PubMed] [Google Scholar]
- [37].Doremus-Fitzwater TL, Gano A, Paniccia JE, Deak T, Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure, Physiol. Behav 148 (2015) 131–144. 10.1016/j.physbeh.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Girard-Joyal O, Faragher A, Bradley K, Kane L, Hrycyk L, Ismail N, Age and sex differences in c-Fos expression and serum corticosterone concentration following LPS treatment, Neuroscience. 305 (2015) 293–301. 10.1016/j.neuroscience.2015.06.035. [DOI] [PubMed] [Google Scholar]
- [39].Marsland P, Parrella A, Orlofsky M, Lovelock DF, Vore AS, Varlinskaya EI, Deak T, Neuroendocrine and neuroimmune responses in male and female rats: evidence for functional immaturity of the neuroimmune system during early adolescence, Eur. J. Neurosci. n/a (n.d.) 10.1111/ejn.15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sterley T-L, Baimoukhametova D, Füzesi T, Zurek AA, Daviu N, Rasiah NP, Rosenegger D, Bains JS, Social transmission and buffering of synaptic changes after stress, Nat. Neurosci 21 (2018) 393–403. 10.1038/s41593-017-0044-6. [DOI] [PubMed] [Google Scholar]
- [41].Sterley T-L, Bains JS, Social communication of affective states, Curr. Opin. Neurobiol 68 (2021) 44–51. 10.1016/j.conb.2020.12.007. [DOI] [PubMed] [Google Scholar]
- [42].Peen NF, Duque-Wilckens N, Trainor BC, Convergent neuroendocrine mechanisms of social buffering and stress contagion, Horm. Behav 129 (2021) 104933. 10.1016/j.yhbeh.2021.104933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ferretti V, Maltese F, Contarini G, Nigro M, Bonavia A, Huang H, Gigliucci V, Morelli G, Scheggia D, Managò F, Castellani G, Lefevre A, Cancedda L, Chini B, Grinevich V, Papaleo F, Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice, Curr. Biol 29 (2019) 1938–1953.e6. 10.1016/j.cub.2019.04.070. [DOI] [PubMed] [Google Scholar]
- [44].Scheggia D, Managò F, Maltese F, Bruni S, Nigro M, Dautan D, Latuske P, Contarini G, Gomez-Gonzalo M, Requie LM, Ferretti V, Castellani G, Mauro D, Bonavia A, Carmignoto G, Yizhar O, Papaleo F, Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice, Nat. Neurosci 23 (2020) 47–60. 10.1038/s41593-019-0551-8. [DOI] [PubMed] [Google Scholar]
- [45].Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE, Social transfer of pain in mice, Sci. Adv 2 (2016) e1600855. 10.1126/sciadv.1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Carnevali L, Montano N, Tobaldini E, Thayer JF, Sgoifo A, The contagion of social defeat stress: Insights from rodent studies, Neurosci. Biobehav. Rev 111 (2020) 12–18. 10.1016/j.neubiorev.2020.01.011. [DOI] [PubMed] [Google Scholar]
- [47].Marusak HA, Etkin A, Thomason ME, Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth, NeuroImage Clin. 8 (2015) 516–525. 10.1016/j.nicl.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rattel JA, Miedl SF, Franke LK, Grünberger LM, Blechert J, Kronbichler M, Spoormaker VI, Wilhelm FH, Peritraumatic Neural Processing and Intrusive Memories: The Role of Lifetime Adversity, Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4 (2019) 381–389. 10.1016/j.bpsc.2018.12.010. [DOI] [PubMed] [Google Scholar]


