Abstract Summary:
Isocitrate dehydrogenase 1 (IDH1) mutations that generate the oncometabolite 2-hydroxyglutarate (2-HG) from α-ketoglutarate (α-KG) have been identified in many types of tumors and are an important prognostic factor in gliomas. 2-hydroxyglutarate production can be determined by hyperpolarized carbon-13 magnetic resonance spectroscopy (HP-13C-MRS) using [1-13C]-α- KG as a probe, but peak contamination from naturally-occurring [5-13C]-α-KG overlaps with the [1-13C]-2-HG peak. Via a newly developed oxidative-Stetter reaction, [1-13C-5-12C]-α-KG was synthesized. α-KG metabolism was measured via HP-13C-MRS using [1-13C-5-12C]-α-KG as a probe. [1-13C-5-12C]-α-KG was synthesized in high yields, and successfully eliminated the signal from C5 of α-KG in the HP-13C-MRS spectra. In HCT116 IDH1 R132H cells, [1-13C-5-12C]-α-KG allowed for unimpeded detection of [1-13C]-2-HG. 12C-enrichment represents a novel method to circumvent spectral overlap, and [1-13C-5-12C]-α-KG shows promise as a probe to study IDH1 mutant tumors and α-KG metabolism.
Keywords: Hyperpolarized carbon-13 nuclear magnetic resonance spectroscopy, alpha-ketoglutarate, Isocitrate dehydrogenase 1, 2-hydroxyglutarate, Carbon-13 labeled metabolites
Graphical Abstract
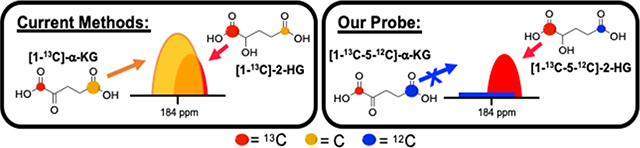
2-hydroxyglutarate production can be determined by HP-13C-MRS using [1-13C]-α- KG, but contamination from naturally-occurring [5-13C]-α-KG overlaps with the [1-13C]-2-HG peak. We synthesized [1-13C-5-12C]-α-KG via a newly developed oxidative-Stetter reaction and eliminated the signal from C5 of α-KG in the HP-13C-MRS spectra, thereby representing a novel method to circumvent spectral overlap.
Introduction:
Atypical metabolism of alpha-ketoglutarate (α-KG) has been linked to increased tumor cell differentiation,(1) amplified malignant progression,(1) as well as altered protein synthesis and catabolism.(2) α-KG is also the substrate for the mutant isocitrate dehydrogenase I (IDH1) enzyme. IDH1 is a cytosolic enzyme that catalyzes the oxidation of isocitrate to α-KG. Mutations in this enzyme, most commonly a heterozygous point mutation of arginine 132 to histidine (R132H), instead allow for the reduction of α-KG to 2-hydroxyglutarate (2-HG).(3) This gain-of-function mutation leads to a buildup of 2-HG in IDH1 mutant cells.(3) As pyruvate and α-KG are structurally similar, 2-HG can also be produced through non-canonical activity of lactate dehydrogenase (LDH), but these promiscuous reactions by LDH do not typically lead to high concentrations of 2-HG in human tissues.(4,5) As 2-HG acts as an inhibitor of α-KG-dependent dioxygenases,(6) the high concentrations of 2-HG in IDH1 mutant cells can have multiple downstream effects.(6)
Roughly 80% of grade II and III gliomas and secondary glioblastomas contain mutations in IDH1.(7) Similar mutations have been found in other tumor types, including acute myeloid leukemia,(8,9) chondrosarcoma,(10) and intrahepatic cholangiocarcinoma(11) as well as in colorectal and pancreatic adenocarcinomas.(12,13) As IDH1 R132H mutations correlate with hypermethylation,(14) increased radiosensitivity,(15,16) and a less aggressive phenotype,(15,17) IDH1 status has become important for patient stratification and tumor classification.(18,19)
In the absence of a gain of function mutation in IDH1, concentrations of 2-HG are typically below the levels detectable by magnetic resonance spectroscopy (MRS) methods, which allows for the high concentrations of 2-HG in IDH1 mutant cells to be a unique indicator of IDH1 status in vivo.(20) MRS is a technique that can be used to measure 2-HG production in both biopsy samples(21) and in patients.(21,22) While in vivo 1H MRS in principle offers a method for measuring 2-HG levels, overlapping resonances(23) and the general insensitivity of 1H MRS has made accurate quantitation difficult.(24)
In 2013, Chaumeil and colleagues developed a complementary approach, measuring the real-time metabolism of α-KG to 2-HG and glutamate by injecting [1-13C]-α-KG into rats bearing glioblastoma xenografts.(25) When mutant IDH1 is present, [1-13C]-2-HG is produced and can be measured via hyperpolarized carbon-13 MRS (HP-13C-MRS). HP-13C-MRS has become an important tool in the study of real-time metabolism in vivo, as hyperpolarization allows for an over 10,000-fold enhancement of MRI signal of 13C labeled molecules.(26) To perform HP-13C-MRS studies, a highly-concentrated solution of the desired metabolite is cooled by liquid helium, polarized using a super-conducting magnet, and subsequently rapidly dissolved in a pH neutralizing solution.(27) The hyperpolarized sample can then be injected either in vitro or in vivo, and metabolism of the sample can be followed by MRI.(27) Currently, HP-13C-MRS is being used to track lactate production from injected hyperpolarized [1-13C]-pyruvate to non-invasively diagnose cancer, image tumor location, and monitor response to therapy in patients with prostate cancer.(28,29) Additionally, HP-13C-MRS is being developed for use in various other tumor types.(29)
Unfortunately, when using [1-13C]-α-KG, the HP-13C-MRS signal from the naturally-present 13C at the C5 position of [1-13C]-α-KG (184.0 ppm) overlaps with C1 of [1-13C]-2-HG (183.9 ppm) produced by IDH1 mutant enzyme (Figure 1A).(25) This makes the detection of [1-13C]-2-HG and therefore characterization of IDH1 status difficult. We hypothesized that 12C enrichment of C5 of α-KG would eliminate the peak contamination from the naturally-occurring [5-13C]-α-KG and allow for unimpeded detection of [1-13C]-2-HG via HP-13C-MRS (Figure 1B). Consequently, we synthesized [1-13C-5-12C]-α-KG to test its utility as a probe for non-invasive in vivo characterization of IDH1 status and overall α-KG metabolism.
Figure 1: Peak contamination from the naturally abundant 13C of C5 of α-KG and synthesis of [1-13C-5-12C]-α-KG.
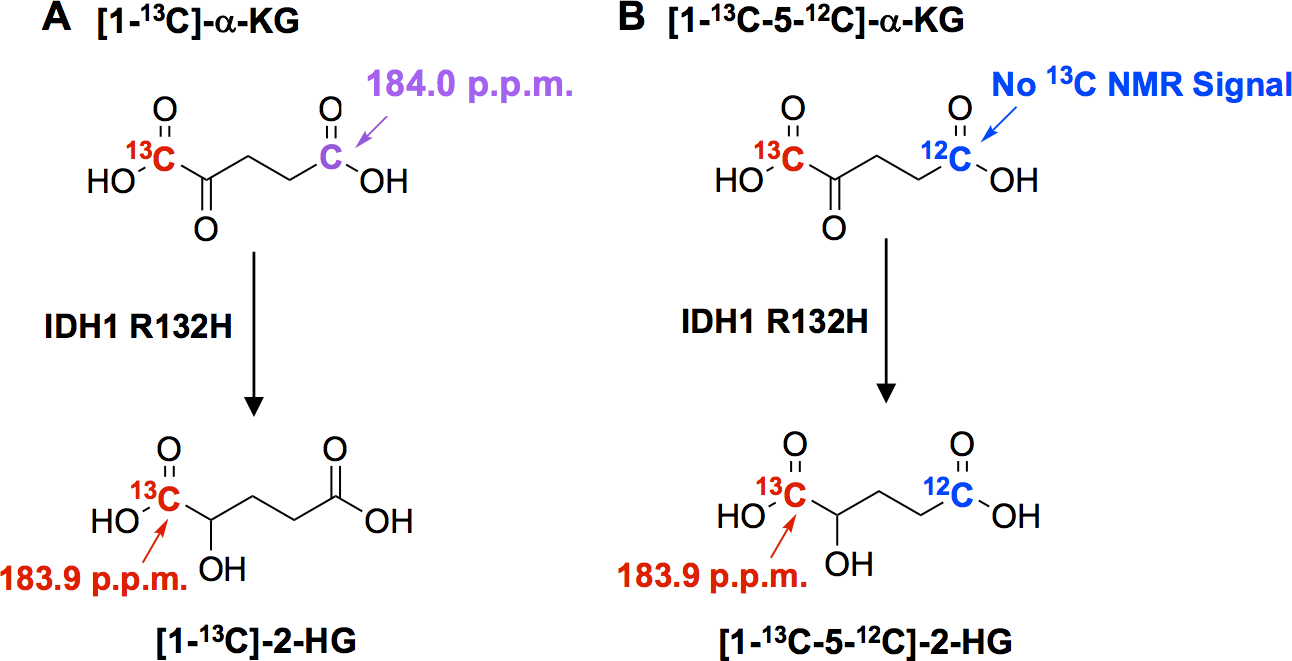
IDH1 R132H enzymes can catalyze the conversion of [1-13C]-α-KG (A) or [1-13C-5-12C]- α -KG (B) to [1-13C]-2-HG or [1-13C-5-12C]-2-HG respectively.
Experimental:
Chemicals and reagents
2-HG, α-KG, L-glutamate (Glu), [1-13C]-α-KG, N-acetyl-glutamine (NAG), ammonium acetate, ammonium hydroxide and UPLC/MS grade acetonitrile were purchased from Sigma-Aldrich (St. Louis, MO). Water was purified through a Milli-Q Integral 5 system supplied by EMD Millipore (Billerica, MA). See SI for further details on the synthesis of all substrates and 13C,12C-bis-labeled compounds.
Synthesis of [1-13C-5-12C]-alpha-ketoglutarate
Our synthesis of [1-13C-5-12C]-α-KG is outlined in Figure 2 and detailed in the SI. Briefly, we synthesized substrates 2-Bromo-1-morpholinoethan-1-one-1-13C (6) and Benzyl acrylate-1-12C (7) using standard methods. We then optimized a one-pot oxidation-Stetter reaction to form Benzyl 5-morpholino-4,5-dioxopentanoate-1-12C-5-13C (8) without detection of benzoin product (9). We converted (8) into the bis-isopropyl ester Diisopropyl 2-oxopentanedioate-5-12C-1-13C (10) and then de-esterified to form [1-13C-5-12C]-α-KG (11).
Figure 2: Background and synthesis of [1-13C-5-12C]-α-KG.
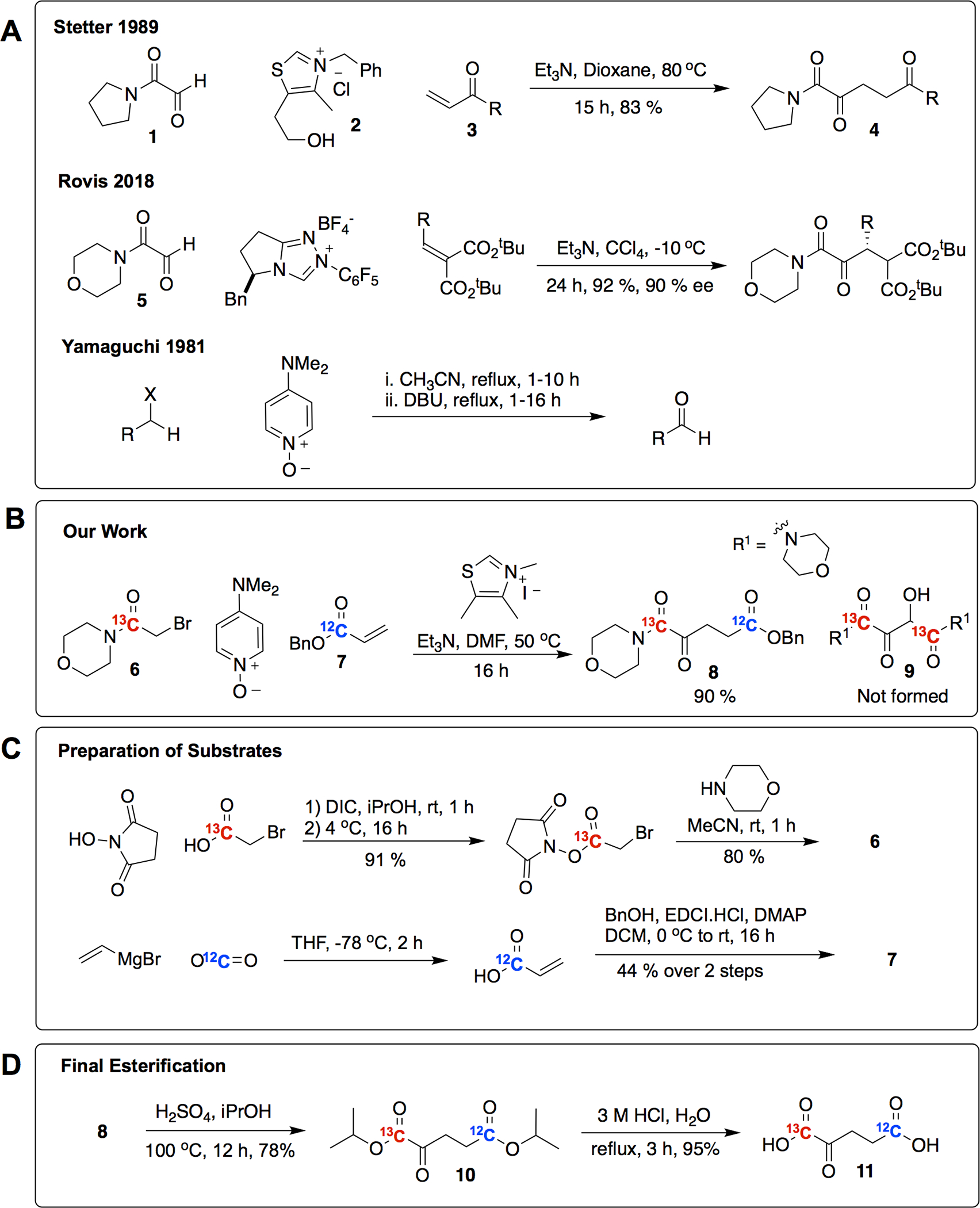
(A) Key aspects of the Stetter, Rovis and Yamaguchi reactions were used to create the one-pot oxidation-Stetter reaction (B) we developed to streamline the synthesis of [1-13C-5-12C]-α KG. (C) Standard synthetic methods were used to reach the precursors of our oxidation-Stetter reaction. (D) The final steps of the synthesis of [1-13C-5-12C]-α-KG.
Cell culture
HCT116 IDH1 R132H cells were purchased from Horizon Discovery (Cambridge, United Kingdom). Cells were cultured at 37 °C under 5% CO2 in RPMI medium supplemented with 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin.
Reagent preparation
All α-KG derivatives were dissolved in D2O: D-glycerol = 1:1 containing 17.3 mM of Ox063 at a concentration of 5.9 M. After three freeze and thaw cycles using liquid nitrogen followed by vortexing for 1 min, samples were stored at 4°C until use.
13C MRI of hyperpolarized 13C-labeled α-KG
For all hyperpolarization experiments, 35 μL of Sigma [1-13C]-α-KG or [1-13C-5-12C]-α-KG solution containing 2.5 mM of gadolinium chelate (ProHance, Bracco Diagnostics, Milano, Italy) was polarized at 3.35 T and 1.45–1.5 K in a Hypersense DNP polarizer (Oxford Instruments, Abingdon, UK) for 3–5 h according to the manufacturer’s instructions. The polarized samples were rapidly dissolved in 4.0 mL of alkaline buffer containing 25 mM Tris(hydroxymethyl)aminomethane, 50 mg/L ethylendiaminetetraacetic acid, and 37.5 mM NaOH, for the final dissolution buffer to be pH 7.4 after mixture with α-KG. For in vitro experiments, the hyperpolarized 13C-α-KG solution (1 mL) was injected into a glass vial (Wheaton Science Products, Millville, NJ) placed in a 3T scanner (MR Solutions, Guildford, UK) via a plastic tube using a 17-mm custom-build 13C solenoid leg coil placed inside of a saddle coil for 1H.
Preparation for HP-13C-MRS with enzymes
1.5 mL of a solution of 300 mM NaCl, 40 mM Tris–HCl pH 7.5, 20 mM MgCl2, and 0.06% bovine serum albumin were prepared in a glass vial. After shimming, 75 μL of 10 mM NADH and 500 μL of 5 kU/mL of LDH (porcine heart LDH, Sigma) in PBS were added, mixed and shimmed again immediately before measurement.
Preparation of cells for in vitro HP-13C-MRS
Cells harvested by trypsinization 24 hr after plating, washed with serum-free DMEM without pyruvate and resuspended in serum-free DMEM without pyruvate with the concentration of 5.0 × 107 cells/mL. After pre-warmed to 37 °C, 2 mL of cell suspension (1.0 × 108 cells total) were transferred to a glass vial immediately before measurement.
Results:
Initially, we synthesized [1-13C-5-12C]-α-KG following a procedure reported by Baldwin using 12C-benzylacrylate (6) as well as the 13C-labeled nitromethane.(30) We noted that several steps were low yielding and not scalable, so we sought a more robust synthetic route. Our final complete synthesis of [1-13C-5-12C]-α-KG, as outlined in Figure 2, was run on gram scale with an overall yield of 88% over 3 steps. The 13C spectrum of [1-13C-5-12C]-α-KG can be seen in Figure 3.
Figure 3:
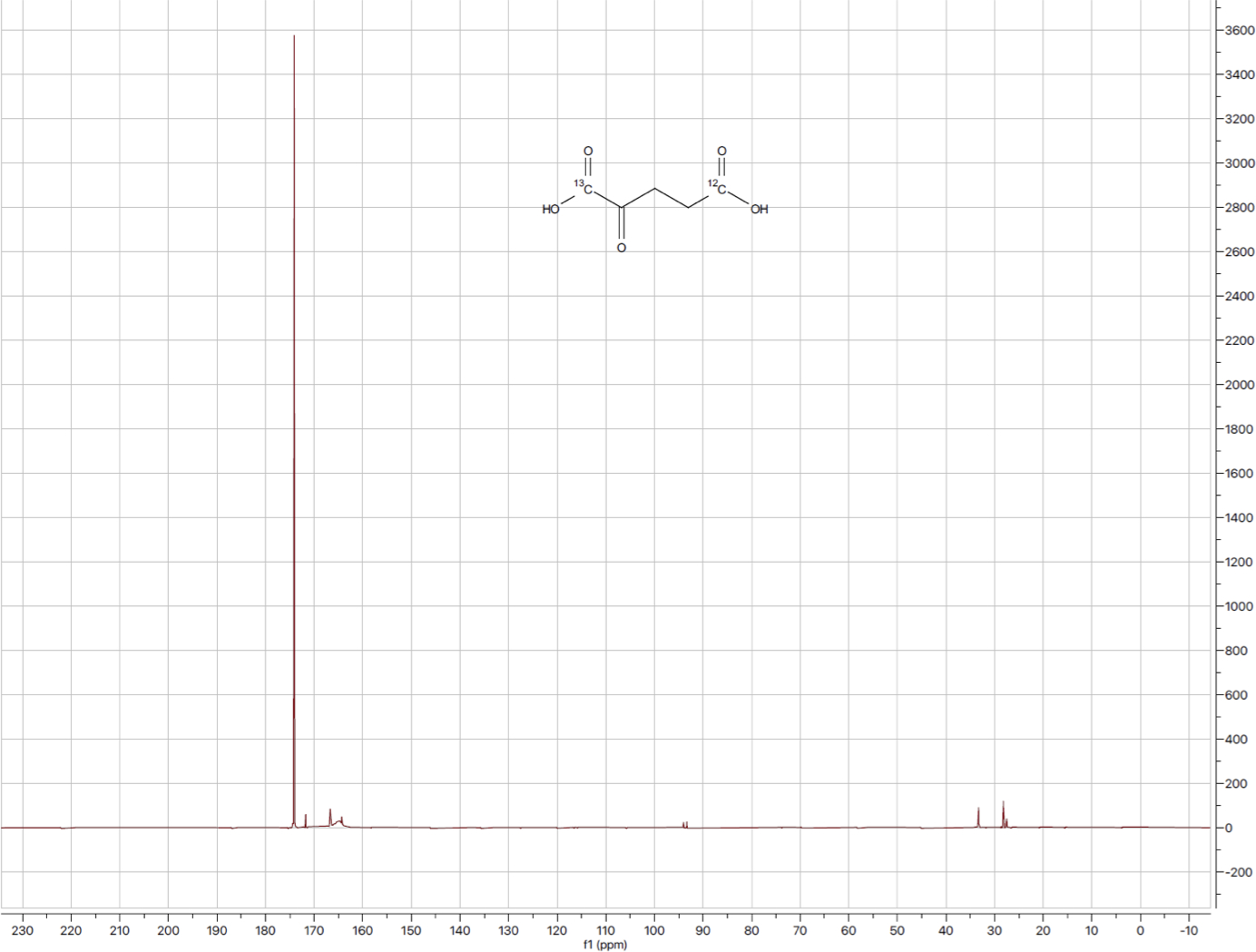
13C spectrum of [1-13C-5-12C]-α-KG
Building on previous work by Stetter,(31) Rovis,(32) and Yamaguchi,(33) we devised a synthetic approach that is both high-yielding and scalable. In the Stetter reaction, thiazolium salt (2) catalyzes the addition of a glyoxylpyrrolidide (1) to α,β-unsaturated carbonyl (3) to form α-ketoglutarate derivative (4) (Figure 2A). Rovis and colleagues further refined this reaction, demonstrating that glyoxylmorpholides (5) proceed with better yields than glyoxylpyrrolidides (1).(32) Glyoxylamides (e.g. 1 and 5) are air and water sensitive, and present challenges in synthesis and isolation.(32)
To circumvent these problems, we chose to incorporate the mild N-hydroxy-4-N,N-dimethylaminopyridine, in-situ oxidation of 1-13C-2-bromo-morpholino-acetamide (6) to the desired glyoxylamide (5) developed by Yamaguchi(33) that was used for the subsequent Stetter reaction (Figure 2B). This one-pot oxidation-Stetter reaction was found to efficiently prepare the 13C-12C-bislabeled products, forming 8 in 90% yield (Figure 2B). The lack of benzoin product (9) resulting from the self-condensation of glyoxyamides implies that the rate of oxidation of the bromo-acetamide (6) to the intermediate glyoxylamide (5) was slow, and when coupled to an efficient Michael addition, the resulting low concentrations of the glyoxylamide made self-condensation unfeasible. The coupling of a slow oxidation to an efficient Michael addition should be useful in other challenging Stetter reactions.
To determine whether [1-13C-5-12C]-α-KG would successfully eliminate the C5 peak in the HP-13C-MRS, signals from hyperpolarized [1-13C-]-α-KG and [1-13C-5-12C]-α-KG were compared. The hyperpolarized [1-13C-5-12C]-α-KG had a sufficient lifetime with a calculated T1 of 43.4 ± 0.3 seconds at 3 Tesla. When [1-13C-]-α-KG was hyperpolarized, a peak corresponding to C5 of α-KG ([5-13C]-α-KG) was clearly monitored at 184 ppm (Figure 4A), but there was no comparable peak at 184 ppm for hyperpolarized [1-13C-5-12C]-α-KG (Figure 4B). The disappearance of this signal suggests that 12C enrichment of the C5 position of α-KG successfully eliminated the signal from the naturally abundant 13C at this position, which is more clearly visible in Figure 4C. In both [1-13C-]-α-KG and [1-13C-5-12C]-α-KG spectra, C1-hydrate, and C2 peaks were identified at 182 and 208 ppm, respectively. Under hyperpolarization conditions, cyclization of both [1-13C]-α-KG and [1-13C-5-12C]-α-KG occurred, as seen by the peaks at 163 and 209 ppm in both phantom spectra, corresponding to C1 and C2 of the cyclized product respectively (Figures 4A–B). The [1-13C]-α-KG phantom spectra also included a peak at 175 ppm which corresponds to C5 of the cyclized product.
Figure 4: Elimination of C5 peak and detection of [1-13C]-2-HG by HP-13C-MRS.
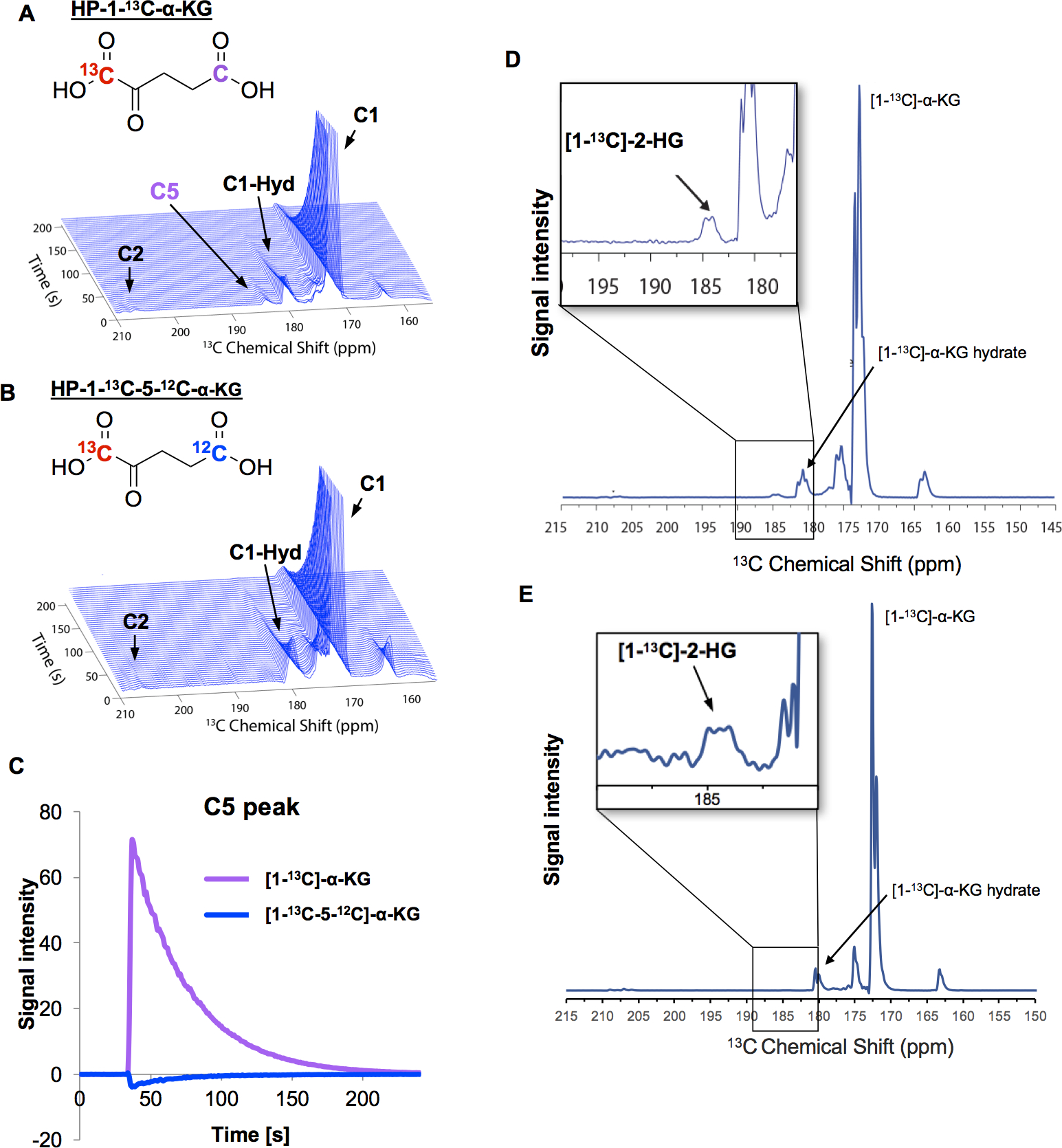
(A) Hyperpolarization of commercial [1-13C]-α-KG. (B) Hyperpolarization of the newly synthesized [1-13C-5-12C]-α-KG. (C) Time-dependent changes in peaks detected in C5 position of α-KG. Purple and Blue lines: changes in peak heights at the position of 184 ppm in after injection of [1-13C]-α-KG and [1-13C-5-12C]-α-KG, respectively. (D) Representative spectrum from purified LDH enzymes after injecting with [1-13C-5-12C]-α-KG. HP-13C-MRS was magnified at 185 ppm for better visualization of [1-13C]-2-HG. (E) Representative spectra from HCT116 IDH1 R132H cells after injecting with [1-13C-5-12C]-α-KG. HP-13C-MRS was magnified at 185 ppm for better visualization of [1-13C]-2-HG. N=2 biological replicates.
With the spectral signal corresponding to C5 of α-KG eliminated, we tested the utility of [1-13C-5-12C]-α-KG as a probe to measure α-KG metabolism. As lactate dehydrogenase can catalyze the conversion of α-KG to 2-HG via a non-canonical reaction,(4) we utilized purified lactate dehydrogenase as proof of principle for the conversion of [1-13C-5-12C]-α-KG. Using LDH enzymes supplemented with NADH, [1-13C-5-12C]-α-KG was rapidly metabolized to [1-13C]-2-HG, and a peak at 184 ppm could be clearly detected with HP-13C-MRS (Figure 4D).
After confirming that [1-13C-5-12C]-α-KG allows for detection of [1-13C]-2-HG using LDH enzymes, we tested this probe in cellulo. The metabolism of α-KG in HCT116 IDH1 R132H cells was monitored by adding hyperpolarized [1-13C-5-12C]-α-KG directly before the HP-13C-MRS measurements. In IDH1 R132H cells, [1-13C]-2-HG was detected at 184 ppm without interference from [5-13C]-α-KG (Figure 4E). A peak at 163 ppm was also seen, which is likely due to overlapping signals from C1 of the cyclic lactone form of [1-13C-5-12C]-α-KG and [1-13C]-bicarbonate formed during the decarboxylation of [1-13C-5-12C]-α-KG by α-ketoglutarate dehydrogenase. The unidentified metabolite at 175 ppm is consistent with previously published literature.(25,34)
Discussion:
These results demonstrate [1-13C-5-12C]-α-KG as a probe for non-invasive imaging of IDH1 status. We developed an oxidation-Stetter reaction to streamline the synthesis of labeled α-KG. This reaction was high yielding, and benzoin side-products were not formed. Our total synthetic route was easily scalable and allowed for production of [1-13C-5-12C]-α-KG on gram scale and in high yields.
By removing peak-contamination from the naturally-occurring [5-13C]-α-KG, the [1-13C]-2-HG produced in IDH1 mutant cells could be detected cleanly in cellulo using [1-13C-5-12C]-α- KG. Though [1-13C-5-12C]-α-KG allowed for the classification of IDH1 status via detection of [1-13C]-2-HG in cellulo, the low cell permeability of α-KG led to low concentrations of labeled [1-13C]-2-HG. In 2007, MacKenzie et al. demonstrated that esters of α-KG had more favorable permeability than α-KG, where introduction of α-ketoglutarate derivatives increased prolyl hydroxylase (PHD) activity and HIF1α levels in vitro.(35) Esterification of our probe might therefore increase intracellular concentrations of [1-13C]-2-HG and improve detection of the metabolite, as well as allow for tracking of overall α-KG metabolism in vivo.
With the successful use of hyperpolarized [1-13C-5-12C]-α-KG in tracking 2-HG production, we hypothesize that this probe can be a tool to study α-KG metabolism in a broad range of applications. Further study of these probes is warranted for transition into clinical use, but [1-13C-5-12C]-α-KG shows promise as a probe to study α-KG metabolism.
Supplementary Material
Figure S1: The stacked 1H-NMR plots of [1-13C-5-12C]-α-KG (upper) and the α-KG standard from Sigma Chemical (lower).
Acknowledgements:
This study was supported by the National Institutes of Health Intramural Research Program; National Cancer Institute R00CA222493 (to A.H.K.); and JSPS Research Fellowships for Japanese Biomedical and Behavioral Researchers at NIH (to N.M. and T.S.).
Abbreviations:
- α-KG
α-ketoglutarate
- 2-HG
2-hydroxyglutarate
- HP
Hyperpolarized
- IDH1
Isocitrate dehydrogenase
- LDH
Lactate dehydrogenase
- PHD
Prolyl hydroxylase
Footnotes
Conflicts of interest:
Work as presented in this manuscript has been filed under a pending patent application.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- 1.Morris JP, Yashinskie JJ, Koche R, Chandwani R, Tian S, Chen CC, Baslan T, Marinkovic ZS, Sanchez-Rivera FJ, Leach SD, Carmona-Fontaine C, Thompson CB, Finley LWS, Lowe SW. alpha-Ketoglutarate links p53 to cell fate during tumour suppression. Nature 2019;573(7775):595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu N, Yang M, Gaur U, Xu H, Yao Y, Li D. Alpha-Ketoglutarate: Physiological Functions and Applications. Biomol Ther (Seoul) 2016;24(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009;462(7274):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Intlekofer AM, Wang B, Liu H, Shah H, Carmona-Fontaine C, Rustenburg AS, Salah S, Gunner MR, Chodera JD, Cross JR, Thompson CB. L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat Chem Biol 2017;13(5):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng X, Emmett MJ, Lazar MA, Goldberg E, Rabinowitz JD. Lactate Dehydrogenase C Produces S-2-Hydroxyglutarate in Mouse Testis. ACS Chem Biol 2016;11(9):2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011;19(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 2009;27(25):4150–4154. [DOI] [PubMed] [Google Scholar]
- 8.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, Sasaki M, Jin S, Schenkein DP, Su SM, Dang L, Fantin VR, Mak TW. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 2010;207(2):339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrozek K, Margeson D, Holland KB, Whitman SP, Becker H, Schwind S, Metzeler KH, Powell BL, Carter TH, Kolitz JE, Wetzler M, Carroll AJ, Baer MR, Caligiuri MA, Larson RA, Bloomfield CD. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol 2010;28(14):2348–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, Pollock R, O’Donnell P, Grigoriadis A, Diss T, Eskandarpour M, Presneau N, Hogendoorn PC, Futreal A, Tirabosco R, Flanagan AM. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 2011;224(3):334–343. [DOI] [PubMed] [Google Scholar]
- 11.Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, Kwak EL, Clark JW, Ryan DP, Deshpande V, Dias-Santagata D, Ellisen LW, Zhu AX, Iafrate AJ. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012;17(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brody JR, Yabar CS, Zarei M, Bender J, Matrisian LM, Rahib L, Heartwell C, Mason K, Yeo CJ, Peiper SC, Jiang W, Varieur K, Madhavan S, Petricoin E 3rd, Fortuna D, Curtis M, Wang ZX, Pishvaian MJ, Winter JM. Identification of a novel metabolic-related mutation (IDH1) in metastatic pancreatic cancer. Cancer Biol Ther 2018;19(4):249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleeson FC, Kipp BR, Voss JS, Campion MB, Minot DM, Tu ZJ, Klee EW, Sciallis AP, Graham RP, Lazaridis KN, Henry MR, Levy MJ. Endoscopic ultrasound fine-needle aspiration cytology mutation profiling using targeted next-generation sequencing: personalized care for rectal cancer. Am J Clin Pathol 2015;143(6):879–888. [DOI] [PubMed] [Google Scholar]
- 14.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, Thompson CB, Kaufman A, Guryanova O, Levine R, Heguy A, Viale A, Morris LG, Huse JT, Mellinghoff IK, Chan TA. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012;483(7390):479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler J, Guttler A, Wichmann H, Rot S, Kappler M, Bache M, Vordermark D. IDH1(R132H) mutation causes a less aggressive phenotype and radiosensitizes human malignant glioma cells independent of the oxygenation status. Radiother Oncol 2015;116(3):381–387. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Chou AP, Chen W, Chen R, Deng Y, Phillips HS, Selfridge J, Zurayk M, Lou JJ, Everson RG, Wu KC, Faull KF, Cloughesy T, Liau LM, Lai A. Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro Oncol 2013;15(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 2010;75(17):1560–1566. [DOI] [PubMed] [Google Scholar]
- 18.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res 2012;18(20):5562–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalinina J, Carroll A, Wang L, Yu Q, Mancheno DE, Wu S, Liu F, Ahn J, He M, Mao H, Van Meir EG. Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J Mol Med (Berl) 2012;90(10):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen AG. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 2012;4(116):116ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi C, Ganji S, Hulsey K, Madan A, Kovacs Z, Dimitrov I, Zhang S, Pichumani K, Mendelsohn D, Mickey B, Malloy C, Bachoo R, Deberardinis R, Maher E. A comparative study of short- and long-TE (1)H MRS at 3 T for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR Biomed 2013;26(10):1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertolino N, Marchionni C, Ghielmetti F, Burns B, Finocchiaro G, Anghileri E, Bruzzone MG, Minati L. Accuracy of 2-hydroxyglutarate quantification by short-echo proton-MRS at 3 T: a phantom study. Phys Med 2014;30(6):702–707. [DOI] [PubMed] [Google Scholar]
- 24.Neuberger U, Kickingereder P, Helluy X, Fischer M, Bendszus M, Heiland S. Accuracy of 1H magnetic resonance spectroscopy for quantification of 2-hydroxyglutarate using linear combination and J-difference editing at 9.4T. Z Med Phys 2017;27(4):300–309. [DOI] [PubMed] [Google Scholar]
- 25.Chaumeil MM, Larson PE, Yoshihara HA, Danforth OM, Vigneron DB, Nelson SJ, Pieper RO, Phillips JJ, Ronen SM. Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nat Commun 2013;4:2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang ZJ, Ohliger MA, Larson PEZ, Gordon JW, Bok RA, Slater J, Villanueva-Meyer JE, Hess CP, Kurhanewicz J, Vigneron DB. Hyperpolarized (13)C MRI: State of the Art and Future Directions. Radiology 2019;291(2):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A 2003;100(18):10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci Transl Med 2013;5(198):198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, Bankson JA, Brindle K, Cunningham CH, Gallagher FA, Keshari KR, Kjaer A, Laustsen C, Mankoff DA, Merritt ME, Nelson SJ, Pauly JM, Lee P, Ronen S, Tyler DJ, Rajan SS, Spielman DM, Wald L, Zhang X, Malloy CR, Rizi R. Hyperpolarized (13)C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019;21(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin JE AR, Russell MA, Schofield CJ, Wood ME. Syntheses of [2–13C] and [1,2–13C] labelled a-ketoglutaric acid. J Labelled Compd Radiopharm 1989;27:1091–1099. [Google Scholar]
- 31.Stetter H, Skobel H. Addition von glyoxylsaureamiden an a,b-ungesättigte carbon-saure ester und ketone. Chem Ber. 1987;120:643–645. [Google Scholar]
- 32.Liu Q, Perreault S, Rovis T. Catalytic asymmetric intermolecular Stetter reaction of glyoxamides with alkylidenemalonates. J Am Chem Soc 2008;130(43):14066–14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukaiyama S IJ, Yamaguchi M. Dimethylaminopyridine N-oxide as an efficient oxidizing agent for alkyl halides. Bull Chem Soc Jpn 1981;54:2221–2222. [Google Scholar]
- 34.Chaumeil MM, Larson PE, Woods SM, Cai L, Eriksson P, Robinson AE, Lupo JM, Vigneron DB, Nelson SJ, Pieper RO, Phillips JJ, Ronen SM. Hyperpolarized [1–13C] glutamate: a metabolic imaging biomarker of IDH1 mutational status in glioma. Cancer Res 2014;74(16):4247–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, Watson DG, Gottlieb E. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol 2007;27(9):3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: The stacked 1H-NMR plots of [1-13C-5-12C]-α-KG (upper) and the α-KG standard from Sigma Chemical (lower).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


