Abstract
Objective.
The two approaches to symptom-cluster research include grouping symptoms and grouping patients. The objective of this systematic review was to examine the conceptual approaches and methodologies used in symptom-cluster research in patients with head and neck cancer.
Data sources.
Articles were retrieved from electronic databases (CINAHL, MEDLINE via Ovid, APA PsycINFO, Scopus, Embase, and Cochrane Central Register of Controlled Trials-CENTRAL), five grey literature portals, and Google Scholar.
Results.
Seventeen studies met the eligibility criteria. Eight studies grouped symptoms to identify symptom clusters, of which two used qualitative methods. The number of symptom clusters ranged from two to five and the number of symptoms in a cluster ranged from two to eleven. Nine studies grouped patients based on their experiences with multiple symptoms. Cluster analysis and factor analysis were most commonly used. Despite variable names and composition of symptom clusters, synthesis revealed three prominent symptom clusters– general, head and neck cancer-specific, and gastrointestinal. Being female and quality of life were significantly associated with high symptom group or cluster severity. Biological mechanisms were sparsely examined.
Conclusion.
Symptom cluster research in head and neck cancer is emerging. Consensus on nomenclature of a symptom cluster will facilitate deduction of core clinically relevant symptom clusters in head and neck cancer. Further research is required on understanding patients’ subjective experiences, identifying predictors and outcomes, and underlying mechanisms for symptom clusters.
Implications for Nursing Practice:
Identification of clinically relevant symptom clusters would enable targeted symptom assessment and management strategies, thus improving treatment efficiencies and patient outcomes.
Keywords: Symptom cluster, Head and neck cancer, Cluster analysis, Factor analysis, Systematic review
INTRODUCTION
Head and neck cancer (HNC) contributes significantly to global cancer burden with the latest estimates indicating over 0.9 million new cases and 0.4 million deaths worldwide.1 HNC is a broad term that includes squamous cell cancers of the oral cavity, larynx, pharynx, paranasal sinuses, nasal cavity, and salivary glands.2 Symptoms experienced by HNC patients are not only severe and debilitating, but also broad in scope.3 Patients typically experience local symptoms – mucositis, xerostomia, dysphagia, taste alterations and voice changes,4-7 general symptoms – fatigue, sleep problems, depression, peripheral neuropathy and neurocognitive changes, 4, 7-9 and gastrointestinal symptoms – nausea and vomiting.10, 11 These symptoms rarely present themselves in isolation, but as concurrent symptoms,12-14 leading to a high symptom burden,7, 9 psychosocial dysfunction,15 and poorer quality of life (QoL).12
Since Dodd et al. proposed that studying concurrent symptoms as a ‘symptom cluster’ (SC) could advance cancer symptom management due to their possible common underlying mechanisms,16 the science of symptom management evolved to move from focusing on a single symptom to exploring SCs.17 Kim et al.’s concept analysis of SC led to its generally accepted definition – “two or more symptoms that are related to each other and that occur together”.18 Symptoms within a cluster may share a common identifiable etiology, or reflect different underlying mechanisms, or influence one another’s presence or exacerbation.17, 19 Fuelled by growing evidence that an SC has a greater adverse impact on treatment outcomes, prognosis, functional status, and QoL than individual symptoms due to synergistic effects,8, 20-23 the complex interrelationships among multiple concurrent symptoms and their causal mechanisms remain an important area within cancer research. This is especially relevant given that treatment of SCs is of high clinical utility. Emerging evidence suggest that treatments for one symptom may ‘cross-over’ and reduce the severity of other symptoms included in a cluster.24 Williams19 presented several rationales for hypothesizing that a single treatment might address multiple symptoms in a cluster. For instance, if symptoms in a cluster share a common etiology, targeting treatment at the underlying etiology may relieve or decrease the severity of all symptoms in that cluster.19 The possibility of targeting a single intervention to manage multiple symptoms with a common mechanism holds significant potential for advancing symptom science and treatment in cancer.
While the methodological considerations of SCs are still evolving, two different conceptual approaches to SC research have been described – grouping symptoms to identify SCs (de novo), or grouping patients based on their experiences with a pre-specified SC (a priori).25-27 If the research intent is to derive SCs from a set of observed symptoms, it would require a variable-centred approach to analysis. In contrast, if the intent is to identify patient subgroups in which individuals are similar to each other within a cluster but different from each other between clusters, a person-centred approach to analysis is required.28
To date, various seminal reviews have provided evidence on various aspects of cancer SCs.19, 25,29-34 However, most of these reviews synthesised evidence for heterogeneous cancer populations, which do not reflect sub-population differences in symptom clustering. With the current focus on identifying tumor-specific SCs in addition to core SCs common to all cancer types,26, 27 SC reviews are emerging in cancer sub-populations. Thus far, sub-population specific reviews are limited to lung cancer,35 breast cancer,36 advanced or metastatic cancer,37, 38 and children/adolescents.39, 40 Similar efforts of evidence synthesis are required for HNC as well. Focusing on a specific cancer population – undergoing similar treatments and disease trajectories – may prove to be more useful in detecting more relevant SCs and subsequently aid in formulating personalized treatment plans. To date, no published review has explained approaches of SCs used in HNC population. In a pivotal work, Barsevick26 examined the evolution of concept of SC over the past decade and synthesized evidence till 2016. But this review did not feature any exclusive studies among HNC. A recent review aimed to discuss impact of oral SCs in the pathogenesis of radiation-related caries in HNC.41 This review was limited to oral and GI symptoms following HNC treatment and did not detail on SC approaches or predictors or outcomes. Therefore, the purpose of this systematic review was to comprehensively synthesize evidence on SCs in individuals with HNC. The specific review questions were:
How have SCs been examined in HNC in terms of conceptual approaches, methodologies, and symptom assessment?
What SCs have been identified empirically?
What predictors and outcomes of SCs have been examined?
What gaps need to be addressed by future SC research in HNC?
For the purpose of this review, conceptual approaches refer to the two approaches (grouping symptoms vs grouping patients). Methodologies refer to statistical methods, cut-off criteria, stability, and generalizability of SCs. Symptom assessment refers to assessment tools and other details of symptom measurement. Predictors refer to variables that have an influence on SC severity or subgroup membership while outcomes refer to variables which result from or are influenced by SCs or subgroup membership.
METHODS
This systematic review was guided by Cooper’s prescriptions for research syntheses,42 Garrard’s structured review method,43 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.44
Search Strategies
Multiple strategies45 were used for information retrieval. First, six databases including the Cumulative Index to Nursing and Allied Health Literature (CINAHL), MEDLINE via Ovid, APA PsycINFO, Scopus, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched during the period from 04 December 2020 through 10 December 2020. Keywords and search strings were identified in an iterative process, and study titles were sampled for relevance through a pilot scoping search. The iterative process helped identify potential subject headings in databases and keywords used in studies on SCs. Using these pilot findings, a high-recall search using multiple strategies was done to retrieve articles which otherwise could have been missed.45, 46 The search was conducted with no date limits and included articles of all study designs and all languages. Key search terms included head and neck cancer OR head and neck neoplasms OR squamous cell carcinoma of head and neck; AND symptom cluster OR symptom burden OR symptom distress OR symptom constellation OR co-occurring symptoms OR concurrent symptoms OR multiple symptoms. Search strings were database-specific, with use of field codes, proximity operators, nesting features, and wildcard symbols. Searches comprised a combination of subject terms selected from the controlled vocabulary or thesaurus (‘exploded' where appropriate) and free-text terms with title, abstract, and keyword variations for HNC and its subsites.46, 47 Information on the search strings is provided in the supplementary table (Table A).
In addition to database-searching, search was also done in Google Scholar. The first 800 records in Google Scholar were examined. Grey literature searches were completed in OpenGrey, ProQuest “Dissertations and Theses@CIC”, NIH Research Portfolio Online Reporting Tools (RePORT), Agency for Healthcare Research and Quality (AHRQ), and ClinicalTrials.gov to minimize publication bias. Ancestry and forward searching were performed on all articles that met the inclusion criteria. In addition, journal runs were conducted, wherein, online indices of five journals in areas of cancer symptom management (Journal of Pain and Symptom Management, Head and Neck, Cancer, Oral Oncology, and European Journal of Oncology Nursing) were searched using titles and abstracts. Expert guidance from a health sciences librarian enhanced the search and retrieval efforts.
Screening and Selection of studies
Eligibility criteria were developed to guide the selection of appropriate studies by two authors (AM and AZD). These criteria were decided based on the focus of the systematic review – quantitative (primary and secondary analyses of data sets) and qualitative studies that identified symptom clusters or patient subgroups in HNC. The inclusion criteria were: (a) studies referring to symptom clusters or their analogous terms mentioned in the search strategy and (b) study populations involving HNC. Exclusion criteria included (a) review articles (b) theoretical articles (c) conference abstracts (d) intervention trials not focused on symptom clusters (e) full text articles not available in English language (f) oncology SC studies that did not report sub-group analysis for HNC patients (g) articles not focusing on symptom clustering or patient subgroups (h) studies with the aim of validating a symptom measure and (i) dissertation which were available as published articles.
The titles and abstracts of studies obtained by the search strategies were screened against the inclusion/exclusion criteria. Those that met the inclusion criteria were deemed eligible for the full-text screening process. Ancestry searching of the eligible articles resulted in one additional article. Forward searching and journal runs resulted in redundant articles. Full text screening was done for 41 articles, out of which 18 articles were selected for final synthesis. Figure 1 provides a detailed flow chart of the article retrieval process, including the reasons for exclusion of articles.
FIG 1.

PRISMA flowchart illustrating article selection process. PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses.
Data Extraction and Synthesis
A data extraction form, developed per the review questions and guided by Garrard’s matrix method43 was used to extract data from each study. Using a spreadsheet, data from the included articles were extracted in descending chronological order on purpose, design, sample characteristics, clustering approach used, measures, statistical methods, predictors, outcomes, and findings. Deductive data extraction techniques were subsequently used to re-examine each study, and the standard format of extracted data was refined to include symptom dimensions, recall period, and internal consistency of measures. Due to reasons such as wide range of symptoms, mixture of factor and cluster analyses, and other methodological heterogeneity, a meta-analysis to provide a meaningful summary was not possible. A meta-synthesis could not be done because only two qualitative studies had examined SCs in HNC; both with varied sample characteristics and study aims. However, in addition to thorough narrative synthesis,43 a visual display of commonly occurring symptom pairs is included.
Critical Appraisal
AM and HL critically appraised the studies using the Joanna Briggs Institute’s (JBI) critical appraisal tool for risk of bias assessment48 and the Crowe Critical Appraisal Tool (CCAT).49 Critical appraisal of studies was challenging for seven articles. Two articles reported cross-sectional cluster analyses from primary prospective studies. Although the primary study design would have mandated the use of an appraisal tool specific for cohort studies, the cluster analyses findings did not align with the ‘exposure-outcome’ approach of the appraisal tool for cohort studies. Five articles were secondary analyses and did not report on key methodological aspects. These challenges may have resulted in an under-valuation of quality of findings on SCs, so we employed three solutions to avoid under-valuation of quality of findings on SCs: (a) choice of design-specific JBI tool was made based on the analytical cross-sectional cluster analyses and not on the primary prospective study; (b) for secondary analyses articles, the primary studies were also examined to determine adherence to appraisal tools; and (c) two tools were used to obtain a comprehensive view on study quality and possibility of bias in their design, conduct, and analysis. Because the purpose of the appraisal was to critically evaluate the extant literature and describe the results, poor study quality was not considered to be an exclusion criterion.
The JBI tools are specific to research designs and the number of items vary from 8 (tool for analytical cross-sectional research) to 10 (for qualitative research). Each item on the tools was answered against ‘Yes’, ‘No’, ‘Unclear’, or ‘Not Applicable’. The risk of bias was categorized as high if a study scored ≤ 49% on the JBI tool, moderate for a JBI score between 50–69%, and low for a score more than 70%. This categorization was based on previous use of the tools by other systematic review authors 41 and in consensus with the authors of this review. The CCAT is a valid and reliable tool designed to critically appraise the quality of varied research designs and has eight categories – preliminaries, introduction, design, sampling, data collection, ethical matters, results, and discussion.49, 50 Each category is assigned scores ranging from of 0 to 5 (highest category score). Using the CCAT Version 1.4, each study was given a score on these categories and the total score was obtained as a sum of all category scores (ranging from 0 to 40). The score was then converted to a percent and was reported in addition to each category score. Both the raters appraised the studies independently and described the study characteristics that supported their judgment for methodological quality; these descriptions were used for discussion when there were disagreements. Percentage agreement between the two raters was 94%. Agreement was defined as the proportion of items for which both raters gave the same score for both tools.
RESULTS
Study characteristics
Although the search was conducted with no date limits, the retrieved articles on HNC were published in or after 2012, thus indicating when the evidence on HNC SC first started to emerge. The studies were conducted in USA,6-9, 15, 51-57 China,12, 58 Taiwan,14 Indonesia,59 Canada,60 and multi-sites in USA and Canada.13, 61 Since two articles13, 61 reported secondary analyses from the same Phase 3 randomized controlled trial, this review synthesizes evidence from 17 studies (hence 18 articles and 17 studies; see Tables 1 and 2). Of the 17 studies, 13 studies (76%) examined a heterogeneous HNC sample, while two studies assessed patients with specific-site HNCs – oropharyngeal tumors55 and nasopharyngeal tumors.58 Two studies assessed SCs in general oncology population, with sub-group analysis for HNC.56, 60 Barring one,59 all articles reported relevant participant characteristics. Also, same research sites were used in multiple studies. For instance, five articles 6, 7, 9, 54, 55 reported findings from the University of Texas MD Anderson Cancer Center, while two others 8, 15 reported findings from the Vanderbilt-Ingram Cancer Center.
TABLE 1.
Identification of Symptom Clusters in Head and Neck Cancer (n = 8)
| Study (year); purpose | Sample | Design | Symptom measures; number of symptoms assessed/open-ended questions |
Statistical analysis methods for identifying SCs and cutoff criteria for cluster definition and symptom inclusion |
Identified symptom clusters and their constituent symptoms |
Patterns/predictors/ outcomes related to SCs |
|---|---|---|---|---|---|---|
| Li et al. 2020; * investigate SCs among HNC patients with ETT and determine independent associations between SCs and HRQoL | 203 HNC patients in China with ETT and ≥1 wk after surgery; 90.6% male; age (mean ± SD): 61.7 ± 8.5 | Analytical cross-sectional | MDASI-HN; 22 symptoms (13 general + 9 HNC specific symptoms); used only 21 symptoms because 1 symptom had a zero average score (problem with teeth or gums) | 3-step method: correlation analysis, partial correlation analysis, hierarchical cluster analysis (agglomerative with average-linkage between groups and Euclidean distance); cutoff criteria: not specified; mentioned inclusion of all symptoms with average nonzero scores ––––––––- |
- 7 symptoms found independent of SCs |
- Low positive associations between the pain and fatigue SCs (r = 0.460, P < .001), pain and tracheostomy-related SCs (r = 0.354, P < .001), and HNC-specific and tracheostomy-related SCs (r = 0.415, P < .001) - 88.7% of patients had high-severity pain SC, 73.9% had high-severity fatigue SCs, 68.0% had high-severity tracheostomy-related SCs, 50.2% had high-severity HNC-specific SCs, and 33.3% had high-severity digestive SCs - 12.3% of patients had no more than one high-severity SCs, and 44.9% showed 4-5 co-occurring high-severity SCs- Significant differences on all dimensions of HRQoL between the low-severity and high-severity SCs groups except digestive SCs (all P < .001 except for mental health component)- Independent associations between the pain SCs and the physical component dimension of QoL (b = 7.934, η2 = 0.090), and between the fatigue SCs and both the physical (b = 6.610, η2 = 0.118) and mental components of QoL (b = 4.690, η2 = 0.138) on covariance analyses |
| Kendall’s tau-b correlation, student’s t test, covariance analysis - Patients classified as high-severity SCs when they reported at least 2 symptoms together and at least 1 symptom in the cluster scored as moderate to severe | ||||||
| Sari et al, 2019; investigate the SC, self-care for symptom alleviation, and impact on QoL in HNC patients | 5 HNC patients in Indonesia without brain metastasis; 60% female; Age range 40-60 years | Qualitative | Semi-structured interview guide; 1 question on how the patients perceived the experiences of SC | Content analysis; cutoff criteria: NA |
|
- “After chemotherapy I experienced nausea and wanted to vomit. For 2 weeks, I felt the effects of chemotherapy, every day I feel both. I still feel nauseated even though I have taken medicine from the doctor and sometimes vomited.” (P 3: GI SC)- All patients expressed decreased QoL in physical, psychological, and social aspects - Symptom alleviation self-care performed by the participants included changes in diet/nutrition/lifestyle, mind/body/spiritual control, vitamins, herbal treatment, and prescribed treatment. |
| Chiang et al, 2018; evaluate SCs among patients with HNC and examine the pattern of SCs throughout the course of postoperative RT | 100 HNC patients in Taiwan pre-RT to 6 wk of postoperative RT, without concurrent chemotherapy; 90% male; age (mean ± SD): 54.8 ± 12.5 | Longitudinal; wk 1 to 6 after starting RT | MDASI-T; 13 general symptoms | HCA (agglomerative with Wald’s method, average linkage between groups); cutoff criteria: correlation of ≥0.68 was used to define a cluster |
|
-Both SC were stable from wk 1 to 6 of postoperative RT |
| Xiao W. et al, 2017; identify SCs experienced by NPC patients, and examine relationships among SC and patients’ sociodemographic and clinical characteristics, as well as patient outcomes, including symptom interference and QoL | 130 NPC patients in China undergoing RT, with or without chemotherapy; 72.3% male; age (mean ± SD): 43.22 ± 9.75 | Analytical cross-sectional | MDASI-HN-C; 22 symptoms (13 general + 9 HNC specific symptoms) | Factor analysis (principal axis factoring with oblimin rotation); cutoff criteria: factor loading of >0.3 used to define a cluster ––––––––- |
|
- The 4 SCs explained 47.25%, 8.79%, 47.65%, and 11.48% of the variance respectively - The intercorrelations of symptoms within clusters were significant and ranged from 0.190 to 0.823 - Among the 4 SCs, the nutrition impact type was the most severe, followed by the GI, general, and social interaction impact types - Weight loss correlated with severity of all 4 clusters (r ranging from 0.214 to 0.310, all P < .05) - Occupational status correlated with severity of 2 clusters: nutrition impact (t = 2.096; P < .05) and social interaction impact clusters (t = 2.641; P < .01) - Number of times RT had been received correlated with severity of social interaction impact SC (r = 0.336, P < .01). - Degree of symptom interference correlated with severity of all 4 clusters (r ranging from 0.427 to 0.666; all P < .01) - FACT-HN QoL total score correlated with severity of all 4 clusters (r ranging from −0.249 to −0.523; all P < .01) - Physical, emotional, and HNC-specific subscales of QoL correlated with severity of all 4 clusters (r ranging from −0.184 to −0.705; all P < .05) - Functional subscale of QoL correlated with severity of only the general SC (r = −0.228, P < .05) - Social/family subscale not correlated with severity of any of the 4 clusters |
| Pearson product-moment correlation, independent t test, ANOVA | ||||||
| Deng et al, 2016; describe symptom experiences related to head and neck lymphedema | 20 HNC patients in United States who were >3 months after HNC treatment and had undergone lymphedema therapy; 65% male; median age: 58.7 years, range 42-75 years | Qualitative descriptive | Semi-structured interview guide; 1 question: “What symptoms did you have with the lymphedema/tissue swelling?” | Content analysis per Hsieh and Shannon; cutoff criteria: NA |
|
- 65% of the participants stated symptoms related to SC 1 “The amount of numbness I had and combined with the swelling. It would be more intense, the numbness, because I if I moved my neck and my head very far it would stretch and hurt. So, I basically tried not to turn my head or uh lift it or lower it and, to kind of like keep the numbness from bothering me um.” (ID1003: numbness)- 60% of the participants stated symptoms related to SC 2 “I had like problems opening my mouth. So like, with the swelling and the problems opening my jaw, you know it was a lot of pressure in there.” (ID1013: problems opening mouth)- 55% of the participants stated symptoms related to SC 3 “I could detect a stiffness. I knew something was, it was stiff, it felt stiff and swollen. Stiff and swollen was the way it felt.” (ID1021: stiffness) - 50% expressed symptoms related to psychosocial SC “Every now and then I will look around. People looking at me because I mean you know it has gotten into almost a nervous habit. I just feel like I need to make sure there is nothing there.” (ID1013: Negative self-image, anxious feeling) |
| Rosenthal et al, 2014; characterize the pattern of acute local and systemic symptoms reported by HNC patients, evaluate symptom profiles experienced by selected patient subgroups, and identify factors associated with symptom severity | 149 HNC patients in United States who received RT or CCRT; 74% male; age (median ± SD): 59 ± 11.06 | Longitudinal (clustering of symptoms performed only at the end of treatment) | MDASI-HN; 22 symptoms (13 general + 9 HNC-specific symptoms) | HCA; cutoff criteria: not specified ––––––––– |
|
Patterns/predictors of SC not examined |
| Independent student t tests, multivariate mixed modeling | ||||||
| Xiao C. et al, 2013†; identify SC for HNC patients and to examine the generalizability of the identified symptom clusters over different time points and in different subgroups | 684 HNC patients in United States and Canada receiving concurrent chemoRT; 83% male; age (mean ± SD): 55.7 ± 8.84 | Secondary analysis of Phase III RCT (RTOG trial); RT-related symptoms assessed at three time points: end of I first cycle of chemotherapy, end of II cycle of chemotherapy, and 3 months after the start of RT | NCI-CTC 2.0; all adverse events with subjective component (specific number not reported) | EFA (with principal axis factoring), CFA (with CFI, RMSEA, and SRMR as model fit indices), correlations, variance components analysis, coefficients of congruence and alpha coefficients; cutoff criteria: (i) 3 or more symptoms that had eigenvalues equal to or greater than 0.40; (i) produced internal consistency with alpha coefficients equal to or more than 0.7; (iii) had the highest hyperplane count; (iv) obtained a parsimonious coverage; and (v) made clinical and theoretical sense as determined by expert review - 12 symptoms with more than 10% average prevalence across the 3 time points were chosen for analysis |
|
-Proportion of variance unique to HNC-specific SC was 44% and for the GI SC was 49% - Low correlation coefficient between the 2 clusters (0.28), demonstrating the unique interpretation of each cluster - Average congruence coefficients ranged from 87.96 to 99.91, with the majority above 96, demonstrating almost identical cluster structure matching - Average alpha coefficients varied from 0.60 to 0.79, with majority higher than 0.70, demonstrating adequate internal consistency - Both SC generalizable to subgroups defined by age, gender, race, education, marital status, history of tobacco use, treatments, primary sites, disease stages, and tube feedings, as well as to the 3 symptom assessment time points (end of wk 1, wk4, and wk 21) - Both SCs not generalizable or stable in patients aged 65 years or older, diagnosed with larynx cancer, or those with stage III cancer |
| Xiao C. et al, 2014†; examine demographic and clinical risk factors for the 2 previously identified SCs | Step-wise mixed-effect modeling (t test, χ2 test, correlation coefficients, univariate mixed-effect model, multivariate mixed-effect model) | - Demographic characteristics were more predictive of SCs than clinical characteristics - Race (nonwhite, β = 2.20, SE = 0.66, P = .0010) and education (>12 years, β = −1.88, SE = 0.53, P = .0005) independent predictors for HNC SC; Sex (female, β = −1.87, SE = 0.69, P = .006) and positive history of tobacco use (β = 1.87, SE = 0.67, P = .0057)) independent predictors for the GI SC - Effect of clinical characteristics significant in univariate analyses but not in multivariate analyses (after controlling for race, sex, and education) - Oropharyngeal cancer had more severe HNC SCs than those diagnosed with laryngeal cancer |
||||
| Howell et al., 2012; identify and validate common SCs in a large population-based cohort of ambulatory cancer subjects | 14,247 patients in Canada within the first 6 months following cancer diagnosis; HNC no. (%) = 439 (3%) | Physical symptoms and psychological distress data derived from the Symptom Management Reporting Database | ESAS; 9 general symptoms | EFA (using ML estimation and CF oblique rotation), CFA (model fit indices for EFA and CFA included CFI, SRMR, GFI, adjusted GFI, NFI, NNFI, CFI, and RMSEA); cutoff criteria: a priori defined a SC as having factor loading of at least >0.30, more than 2 symptoms, and meaningful interpretation of latent factors contributing to variables in a factor |
|
- The common three-factor cluster explained 43% of the variance in the full sample with a range of 44%-47% of the variance explained by the clusters in the cancer sub-populations |
ANOVA, analysis of variance; CCRT, concurrent chemoradiotherapy; CF, Crawford–Ferguson; CFA, confirmatory factor analysis; CFI, comparative fit index; EFA, exploratory factor analysis; ESAS, Edmonton Symptom Assessment System; ETT, endotracheal tube; FACT-H&N, functional assessment of cancer therapy-head & neck; GFI, goodness of fit; GI, gastrointestinal; HCA, hierarchical cluster analysis; HNC, head and neck cancer; HRQoL, health-related quality of life; MDASI-HN, MD Anderson Symptom Inventory–Head & Neck; MDASI-HN-C, MD Anderson Symptom Inventory–Head & Neck-Chinese version; MDASI-T, MD Anderson Symptom Inventory–Taiwanese version; ML, maximum likelihood; NA, not applicable; NCI-CTC 2.0, National Cancer Institute-Common Toxicity Criteria Version 2.0; NFI, normed Fit Index; NNFI, non-normed fit index; NPC, nasopharyngeal carcinoma; QoL, quality of life; RMSEA, root-mean-square error of approximation; RCT, randomized controlled trial; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group; SC, symptom cluster; SD, standard deviation; SE, standard error; SRMR, standardized root mean square residual.
Identified symptom clusters and then grouped patients based on identified SCs.
Secondary analyses of the same primary RCT.
TABLE 2.
Identification of Patient Subgroups in Head and Neck Cancer (n = 9)
| Study (year); purpose | Sample | Design | Symptom measures; total symptoms assessed |
Statistical analysis methods for subgroup identification and cutoff criteria for symptom inclusion–––––––Statistical analysis methods for associations |
Symptoms and processes used in subgroup identification |
Patterns/predictors/outcomes related to subgroups |
|---|---|---|---|---|---|---|
| Bai et al, 2020; examine associations between the gut microbiome and the PNS cluster pre- and post-RT | 13 HNC patients in United States pre-and 1 month post-IMRT; 84.6%male; Age (mean ± SD):60 ± 9.4 | Longitudinal; pre- and 1-month post IMRT | PRO-CTCAE; 5 symptoms | Mean value of total cluster score as cutoff point for high vs low clusters Cutoff criteria: not specified Spearman correlational analysis, Kruskal–Wallis test, Bray-Curtis distance, weighted UniFrac distance, Principal coordinates analysis, Linear mixed effect models, Linear discriminant analysis, Mann-Whitney U test, 16S rRNA sequencing-related analysis |
- A priori identified psychoneurological SC = pain, fatigue, sleep disturbance, depressive symptoms, cognitive dysfunction - Average score of the 5 symptoms computed as the total score of the PNS cluster for each patient - Mean value of total score used as the cutoff point to create high (above mean) vs low (below mean) PNS clusters |
- Patients divided into high and low PNS clusters -α-and β-diversity and taxa abundance showed potential associations with the PNS cluster - The high PNS cluster had a greater decrease in microbial evenness than the low PNS cluster from pre- to post-RT (β = −.039, z = −2.223, P = .026) - The high and low PNS clusters showed significant gut microbiome dissimilarities, controlling time point; those with high PNS clusters had higher abundances in phylum Bacteroidetes and 4 genera, and the low PNS cluster had higher abundances in family Acidaminococcaceae and 3 genera (weighted UniFrac distance, F = 2.117, P = .034, R2 = 0.081). - The longitudinal relative abundance analyses showed that a higher Bacteroidetes/Firmicutes ratio was significantly associated with higher PNS (continuous) score over time (P = .027) - Glycan metabolism (lipopolysaccharide biosynthesis) and vitamin metabolism (folate biosynthesis and lipoic acid metabolism) were different between the high and low PNS clusters pre- and post-RT (P ranging from .004 to .046) |
| Wulff-Burchfield et al, 2019; determine prevalence and impact of late systemic symptoms and assess the relationship between systemic symptoms and body image and QoL | 105* HNC patients in United States who completed treatment a minimum of 12 months prior and without evidence of recurrence; 74.3% male; age (mean ± SD):61.9 ± 9.6 | Cross-sectional | GSS, VHNSS 2.0; 13 symptoms | 2-step log-likelihood cluster analysis (using BIC) Cutoff criteria: not specified –––––––Spearman’s rank correlation |
- Used all the 11 symptoms from GSS = fatigue, fatigue limiting activity, joint pain/muscle aches, trouble staying asleep, trouble falling asleep, trouble with memory, sensation of cold, sensation of warmth, sweating, anxiety, feeling sad - Used only 2 systemic symptoms from VHNSS = weight loss, loss of appetite |
- Identified two unique patient groups: a low systemic symptom group (69.5%) and a high systemic symptom group (30.5%) - Low symptom group characterized by patients with none or very few moderate to severe systemic symptoms - High symptom group characterized by those with at least 2 moderate to severe systemic symptoms - 58.6% in the high systemic symptom group had five or more symptoms; most common moderate to severe symptoms were fatigue, difficulty staying asleep, sensation of cold, memory,joint pain/muscle aches - Prevalence of neuropsychiatric symptoms strikingly different between the 2 patient cluster groups; especially in lack of motivation and slowed movements - Poor global QoL reported by 20.7% of patients in the high systemic symptom cluster as opposed to 1.5% in the low systemic symptom patient cluster - Patients in the high systemic symptom cluster group had lower median QoL life in all domains except spiritual QoL, compared to the low systemic symptom cluster group |
| Castellanos et al, 2019; determine association of caregiving task burden and patient symptom burden with psychological distress among caregivers of HNC patients | 84 HNC patients in United States currently receiving or having completed curative intent therapy within the past year; 77.4% male; age (mean ± SD):60.6 ± 10.4 | Secondary analysis of a cross-sectional study to validate HNC Caregiving Task Inventory | VHNSS 2.0;50 symptoms from 13 domains | 2-step log–likelihood cluster analysis (using AIC) Cutoff criteria: not specified ––––––-Mann–Whitney tests, Logistic regression |
- 13 domains = nutrition, swallowing, xerostomia, mucositis, excess mucus, speech, hearing, taste change, smell, dental health, mucosal sensitivity, range of motion, pain - Clustering of all VHNSS items resulted in patient groups with different symptom burden |
- Identified 2 patient groups: mod-high symptom burden (51%) and low symptom burden (49%) - Substantial differences in median symptom scores between the patient groups (P < .001) except for symptoms related to jaw/trismus, hearing, neck/shoulder, and teeth - Patient symptom burden cluster not significantly associated the caregiver’s psychological distress cluster membership (OR = 2.48; 95% CI, 0.88–6.93; P =.085) |
| Rhoten et al, 2018; examine the relationship between trajectories of perceived neck function with depressive symptoms and social anxiety | 83 HNC patients in United States pre-RT to 18 months after RT; 72.3% male; age (mean ± SD):57.8 ± 11.3 | Secondary analysis of a longitudinal study | NDI, CES-D, LSAS; 10 symptoms from NDI |
GBTM (using AIC and BIC) Cutoff criteria: not specified –––––––Likelihood χ2 tests, linear mixed-level analysis, χ2, Kruskal-Wallis |
- Pain, personal care, lifting, reaching, headache, concentration, work, driving, sleeping, and recreation - Used all NDI items and generated clusters of patients with similar baseline and trajectories of NDI scores for 12-18 months after treatment |
- Identified 3 clusters of patients based on longitudinal patterns in neck disability: minimal (18.1%), moderate-mild (45.8%), and severe-moderate (36.1%) - Patients in the severe-moderate group experienced notable neck disability before treatment, and the median level increased during treatment and generally fluctuated around the initial level throughout the study period - The moderate group had minimal disability before treatment but experienced considerably higher levels of neck disability at the end of treatment and the limitations did not diminish as much posttreatment - The minimal group had minimal disability before treatment with some increasing levels at the end of treatment with diminishment to very few limitations by 18 weeks after treatment - Significant associations were found between membership in the NDI trajectories and membership in the longitudinal patterns of depressive symptoms (Cramer’s V, CeS-D: 0.55, P< .001)and social anxiety (Cramer’s V, LSAS Total: 0.35, P = .002) - 77% of the severe-to-moderate NDI group were members of depression cluster with the highest levels of depressive symptoms - 53.3% of the severe to moderate NDI group were members of social anxiety cluster with the highest levels of social anxiety - Lower median years of education (P = .010), female sex (P = .042), current or past medical problems (P = .018), and the need for a PEG tube (P = .007) significantly associated with severe-moderate symptom trajectory |
| Eraj et al, 2017; characterize the late symptom profile of older patients, identify factors associated with late symptom severity, and explore differences in long-term symptom burden | 79 OPC patients in United States who were ≥65 years old and >6 months since treatment completion; 82% male; age (median): 71 years | Analytical crosssectional | MDASI-HN; 22 symptoms† | HCA Cutoff criteria: not specified –––––––Univariate and multivariate regression analyses |
- Used all MDASI core and HN-specific symptoms† - Method of patient grouping: symptom free (all ratings 0), no more than mild (all ratings <5), no more than moderate (all ratings <7), and severe (at least one item with rating ≥7) |
- Identified 3 patient groups: Cluster A with none to moderate symptom burden (64%), Cluster B with moderate-severe symptom burden (33%), Cluster C with severe symptom burden (~2%) - Each cluster had subsets - Cluster A had a symptom free subset; a subset with no more than moderate ratings for fatigue, memory, drowsiness, and sadness; and a third subset with no more than moderate ratings for classic RT-related symptoms - Cluster B had heterogeneous distribution of several severely rated items; had a subset with moderate ratings for global symptoms, yet more broadly spread than cluster A; and a subset with severe ratings for RT-related symptoms. - Cluster C had essentially severe ratings for the majority of all 22 items - No variables associated with increased composite symptom scores - RT dose (P < .03) and T-category (P < .04) significantly associated with composite score of the top 5 symptoms on univariate analysis but not on multivariate analysis |
| Hanna et al, 2015; assess symptom severity and interference, explore symptoms in various HNC subgroups by anatomic site, and report disease factors correlating with symptom severity and interference | 748 treatment-naive HNC patients in United States; 68% male; age (median ± SD):59 ± 14.6 | Retrospective analysis of symptom data from hospital | MDASI; 12 symptoms and 6 interference items† |
2-step cluster analysis (using Kaufman and Rousseeuw’s interpretation of cluster structures, silhouette measure of cohesion and separation) Cutoff criteria: not specified –––––––Backward logistic regression |
- Used all items of MDASI core†,‡ and interference items† to identify patient groups with different overall symptom burden - Patient clusters were based on the percentages of patients reporting moderate to severe symptoms (rated as ≥5 on the MDASI’s scale of 0-10) versus mild symptoms (rated as <5) |
- Identified two patient clusters of overall symptom burden: high overall symptom burden (39%) and low overall symptom burden (61%) - Standardized effect size for symptom severity between the two patient clusters was 1.6, suggesting that a large effect size separated the patient groups - Predictors of a high symptom burden included T3 to T4 disease (OR [95% CI]: 2.2 [1.3-3.7]; P < .005), N1 to N3 disease (OR [95% CI]: 1.9 [1.1-3.7]; P = .023), having a mucosal tumor (OR [95% CI]: 2.0 [1.1-3.6]; P = .029), and being female (OR [95% CI]: 3.1 [1.8-5.4]; P < .001) (Nagelkerke R2 0.167 and correct classification rate of ~70%) |
| Shi et al, 2013; describe the heterogeneity of symptom burden, identify subgroups with distinct symptom-development trajectories, and compare GBTM’s ability to describe longitudinal symptom burden with that of LMM | 130 HNC patients in United States qualified to receive RT or chemoRT; 75.3% male; age: 46.9% were younger than 60 years | Longitudinal; before treatment to 10 wk after RT or chemoRT | MDASI-HN; 22 symptoms | Group-based trajectory modeling (using BIC, BLRT, and LMR LRT), Linear mixed-effect modeling Cutoff criteria: 5 most severe symptoms at the end of treatment were included –––––––Group-based trajectory modeling, linear mixed-effect modeling |
- Used only the 5 most severe symptoms at end of treatment = problems with taste, difficulty swallowing or chewing, problems with mucus, fatigue, and dry mouth - Used average score of these 5 symptoms to identify patient subgroups with distinct symptom-development trajectories over the course of therapy |
- Generated both 2-group and 4-group using GBTM; both models met the criteria for adequate model fit - Identified 2-group symptom trajectories: low (32 % of patients) and high (68 %), and 4-group symptom trajectories: low (28 %), medium (44 %), high (24 %), and stable (4 %). - All 2 models (the 2-group GBTM, 4-group GBTM, and LMM) exhibited significant linear and quadratic functions for symptom change over the course of chemoRT or RT - For 2-group model, the predictors of being assigned to the high-symptom group included having an ECOG performance status 1 (est = 2.97, P = .002) and receiving chemoRT (est = 1.23, P = .046). - For the 4-group model, predictors for being assigned to the medium group included being female (est = 2.06, P = .022), being at least 60 years old (est = 1.27, P = .048), and receiving chemoradiotherapy (est = 1.64, P = .027); the predictor for being assigned to the high group was having an ECOG performance status 1 (est = 2.73, P = .003) - LMM revealed symptom-change patterns similar to that produced by GBTM but was inferior in identifying risk factors for high symptom burden |
| Gunn et al, 2013; describe the pattern of symptoms experienced before beginning RT, explore potential differences in patterns of symptoms in various subgroups, and identify factors correlating with higher overall symptom burden | 270 HNC patients in United States before RT; 75.6% male; age (median ± SD):58.5 ± 11.9 | Longitudinal; cluster analysis involved only baseline data | MDASI-HN; 22 symptoms† | HCA (using Ward’s method on squared Euclidean distance) Cutoff criteria: not specified ––––––-Univariate and multivariate logistic regression |
- Used all 22 symptoms of MDASI-HN† to classify symptom burden - Decided beforehand to identify 2 groups (high and low), based on clinical utility |
- Identified 2 patient clusters of overall symptom burden: high-symptom burden (32.6%) and low-symptom burden (67.4%) - Average proportions of patients experiencing moderate- to severe-symptom levels across all MDASI–HN symptom items were 26.3% for the high-symptom burden group and 13.6% for the low-symptom burden group; corresponding percentages of patients with severe symptoms were 1.2% and 0.2% - Correlates of high-symptom burden included poor ECOG performance status (OR [95% CI]: 3.71 [1.96–7.03]; P < .01), T3 to T4 tumors (OR [95% CI]: 2.0 [1.09-3.65); P = .025), previous chemotherapy (OR [95% CI]: 2.31 [1.1-4.87]; P = .027), and previous surgery (OR [95% CI]: 2.47 [1.15-5.3]; P = .021) |
| Fodeh et al, 2013; explore whether selfreported symptoms and impairments in ADL form clusters based on the site of cancer | 111 patients in United States with advanced GI, HN, lung, and gynecological cancers within 100 days of diagnosis; age (mean ± SD):59.6 ± 12.27; HNC no. (%) = 20 (18%) | Secondary analysis of a cross-sectional study | SDS, personal competence component of ESDS; 19 items including symptoms and ADL | Cluster analysis (compared agglomerative, spectral, and K-means clustering algorithms; finally chose K-means clustering algorithm using cosine function) Cutoff criteria: included only 15 items (symptoms and impairments in ADL) that contributed to cluster formation and were not highly correlated with other variables |
- Used only 10 symptoms = nausea, appetite, insomnia, pain, bowel patterns, concentration, appearance, breathing, outlook, and cough; and - 5 ADL items: eating/feeding, dressing, walking, bathing, and toileting |
- Identified 5distinct clusters of patients based on symptoms and ADL - Patients in Cluster 4 were more likely to present with impairments in eating/feeding, dressing, bathing, toileting, and walking - 44% of patients in Cluster 4 had HNC |
ADL, activities of daily living; AIC, Akaike’s information criterion; BIC, Bayesian information criterion; BLRT, Bootstrap Likelihood Ratio Test; CES-D, Center for Epidemiological Studies-Depression; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ESDS, Enforced Social Dependency Scale; GBTM, Group-Based Trajectory Model; GI, gastrointestinal; GSS, General Symptom Survey; HCA, hierarchical cluster analysis; HNC, head and neck cancer; IMRT, intensity-modulated radiation therapy; LMM, linear mixed effect modeling; LMR LRT, Lo-Mendell-Rubin adjusted Likelihood Ratio Test; LSAS, Liebowitz Social Anxiety Scale; MDASI, MD Anderson Symptom Inventory; MDASI-HN, MD Anderson Symptom Inventory–Head & Neck; NDI, Neck Disability Index; NR, not reported; OPC, oropharyngeal cancer; OR, odds ratio; PEG, percutaneous endoscopic gastrostomy; PNS, psychoneurological symptoms; POMS-SF, Profile of Mood States-Short Form; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; rRNA, ribosomal ribonucleic acid; RT, radiation therapy; QoL, quality of life; SC, symptom cluster; SD, standard deviation; SDS, Symptom Distress Scale; VHNSS-2.0, Vanderbilt Head and Neck Symptom Survey–2.0.
Patients included in cluster analysis was 95.
MDASI 13 core symptoms: pain, fatigue, nausea and vomiting, disturbed sleep, distress, shortness of breath, difficulty remembering, lack of appetite, drowsiness, dry mouth, sadness, numbness/tingling; MDASI HN-specific 9 symptoms: mucus, difficulty swallowing, choking or coughing, difficulty with voice or speech, skin pain/burning/rash, constipation, problem with tasting food, mouth/throat sores, problems with teeth/gum; MDASI interference 6 items: general activity, mood, work, relations with others, walking, enjoyment of life
Nausea and vomiting combined as one item,
Four studies used a cross-sectional design to examine SCs,8, 12, 55, 58 while three used a longitudinal design.6, 14, 51 Two studies were primarily prospective, but performed cluster analysis only at pre-treatment 7 or post-treatment.54 Five articles reported secondary analyses of data from primary studies – two from a randomized controlled trial13, 61, two from cross-sectional studies,52, 56 and one from a longitudinal study.15 One study reported retrospective analyses of symptom data collected during scheduled hospital visits9 and another used data from a symptom management reporting database.60 Two studies utilized qualitative methods to identify SCs.57, 59
Participant characteristics
A total of 3,262 patients with HNC were enrolled in these 17 studies. The sample size ranged from 559 to 748.9 The mean age of HNC patients ranged from 43.2 years58 to 61.9 years.8 Male patients were predominant in the all the studies, except a qualitative study which had 60% females.59 The recruited patients were at different time-points on the treatment continuum, in alignment with respective study aims. They were treatment naive or pre-chemo/radiation therapy in three studies 6, 7, 9; currently receiving radiation therapy (RT) with or without chemotherapy in three studies 13, 54, 58; and post-treatment in four studies.8, 12, 55, 57 In three studies, patients were assessed before RT and were prospectively assessed till one to 18 months post-RT,14, 15, 51 Patients with mixed treatment patterns were included in three studies.52, 56, 60 Study and participant characteristics are summarized in Tables 1 and 2.
Study quality
Barring one study which was classified as having high risk of bias,59 all the other studies (94%) had a low risk of bias and scored equal to or more than 90% on CCAT. The average CCAT score was 37.2 (range 29-39). Overall, all the studies had clearly specified their sample and objectives and used standardized measures to assess symptoms and other variables of interest. However, only two studies mentioned processes of sample size estimation,12, 57 and issues arising due to confounding variables were not clear in studies employing factor analysis or cluster analysis. The results of the appraisal are shown in Table 3.
TABLE 3.
Critical Appraisal Scores of Articles per CCAT Categories and Risk of Bias per JBI Tool (n - 17)
| Study | Preliminaries (5) | Introduction (5) | Design (5) | Sampling (5) | Data collection (5) | Ethical matters (5) | Results (5) | Discussion (5) | TOTAL CCAT %* | JBI Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Li et al, 2020 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 98% | Low |
| Bai et al, 2020 | 5 | 5 | 4 | 4 | 4 | 5 | 5 | 5 | 93% | Low |
| Castellanos et al, 2019 | 5 | 5 | 4 | 4 | 4 | 5 | 4 | 5 | 90% | Low |
| Wulff-Burchfield et al, 2019 | 5 | 5 | 4 | 4 | 4 | 5 | 5 | 5 | 93% | Low |
| Sari et al, 2019 | 5 | 5 | 4 | 3 | 3 | 3 | 3 | 3 | 73% | High |
| Rhoten et al, 2018 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 95% | Low |
| Chiang et al, 2018 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 95% | Low |
| Xiao W. et al, 2017 | 5 | 5 | 4 | 4 | 4 | 5 | 5 | 5 | 93% | Low |
| Eraj et al, 2017 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 95% | Low |
| Deng et al, 2016 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 95% | Low |
| Hanna et al, 2015 | 5 | 5 | 5 | 3 | 4 | 5 | 5 | 5 | 93% | Low |
| Rosenthal et al, 2014 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 98% | Low |
| Xiao C. et al, 2014 † | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 98% | Low |
| Xiao C. et al, 2013 † | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 98% | Low |
| Gunn et al, 2013 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 98% | Low |
| Shi et al, 2013 | 5 | 5 | 4 | 3 | 4 | 5 | 5 | 5 | 90% | Low |
| Fodeh et al, 2013 | 5 | 5 | 4 | 4 | 4 | 5 | 5 | 5 | 93% | Low |
| Howell et al, 2012 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 5 | 95% | Low |
CCART, Crowe Critical Appraisal Tool; JBI, Joanna Briggs Institute; RCT, randomized controlled trial.
Total CCAT score divided by 40 and converted to percentage
Secondary analyses of the same primary RCT.
Conceptual approaches and methodologies
Of the 17 studies, eight studies grouped symptoms to identify SCs (de novo).12-14, 54, 57-60 Of these, one study identified SCs first and then grouped patients as ‘high severity’ or ‘low severity’ for each identified SC.12 Nine studies identified patient subgroups – one study based on an a priori specified SC 51; three studies based on a limited number of symptoms (ranging from five to 13 co-occurring symptoms) selected from a comprehensive list of assessed symptoms 6, 8, 56; the rest five studies using all the items in the symptom measure.7, 9, 15, 52, 55 Of these, one study presented the dendrogram of symptom cluster analysis as well, but did not describe the details of identified SCs.7 Studies using limited number of symptoms for analysis based their decision of symptom inclusion on severity scores,6 study aims,8 or correlations.56
Symptom clusters were identified using cluster analysis in three studies,12, 14, 54 and factor analysis in three studies.13, 58, 60 Of the studies using factor analyses, two studies used both exploratory and confirmatory factor analyses.13, 60 The two qualitative studies used content analysis to identify concurrent symptoms.57, 59 See Table 1.
Patient subgroups were identified using cluster analysis in six studies7-9, 52, 55, 56 and group-based trajectory modelling (GBTM) in two studies.6, 15 One study used the mean value of the total score to divide patients into high (above mean) vs low (below mean) psychoneurological symptom clusters.51 Two studies reported comparison of statistical methods – one study found that the K-means clustering formed the most cohesive and interpretable clusters,56 and the second study concluded that linear mixed-effects modeling revealed symptom-change patterns similar to that produced by GBTM but was inferior in identifying predictors.6 See Table 2.
Cut-off criteria for defining a cluster were mentioned in four studies.13, 14, 58, 60 These criteria included correlation ≥ 0.68, factor loading of > 0.3, or eigen values ≥ 0.40. Cut-off criteria for symptom inclusion in the analysis were mentioned in four studies, and these included severity, presence, correlations, or more than 10% prevalence.6, 12, 13, 56 Stability of SCs across time points was reported only in two studies – both reported relatively stable ‘HNC-specific’ SC and ‘GI.’ SC across chemotherapy or radiation therapy.13, 14 Generalizability of the identified SCs to subgroups was examined in one study, which found that the cluster structure was generalizable to all subgroups except age ≥ 65 years, larynx cancer, and Stage III cancer.13
Symptom assessment
Tables 1 & 2 describe the various symptom assessment tools used in the 17 studies. The two qualitative studies used semi-structured interview guides with one question on experiences of concurrent symptoms.57, 59 Among the 15 quantitative studies, majority (14, 93%) used multi-symptom measures to assess symptoms. Only one study used single-symptom assessment tools to assess symptoms of neck dysfunction, depressive symptoms, and social anxiety.15 Symptom clusters were derived from a list of multiple symptoms (either 9, 12, 13, or 22 symptoms). The M.D. Anderson Symptom Inventory (MDASI) was used in eight studies,6, 7, 9, 12, 14, 54, 55, 58 while the Vanderbilt Head and Neck Symptom Survey (VHNSS) was used in two studies.8, 52 Two studies used symptomatic toxicity criteria to assess symptoms. One study used the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) that captures symptomatic toxicity by self-report in patients on cancer clinical trials.51 The other study used the clinician-observed National Cancer Institute-Common Toxicity Criteria 2.0 and defined symptoms as RT-related adverse events having a subjective component.13 Other symptom assessment tools are described in Tables 1 and 2.
The most common symptom dimension assessed was symptom intensity (severity) – in ten studies.6, 7, 12-14, 51, 54, 55, 58, 60 symptom intensity was combined with other dimensions in four studies – impact / interference 8, 9, 52; frequency and impact / interference.15. One study assessed symptom frequency along with ADL.56 None of the studies considered symptom management strategies while examining symptom clusters. Also, only two articles6, 58 explicitly reported the recall period of symptom assessment, both assessing symptom severity in the previous 24 hours. Internal consistency of the symptom measure using Cronbach’s alpha for respective study samples was reported in only two studies.12, 58 Both used MDASI–Head & Neck instrument and the reported values for its subscales ranged from α = .78 to .91.
Symptom clusters in HNC
Analysis of the study findings revealed that patients with HNC had multiple symptoms occurring together. The number of identified SCs ranged from two to five and the number of symptoms in a cluster ranged from two to eleven. The review demonstrated that SCs and the cluster constituents varied with symptom measure and statistical methods. Even among those using the same tool, such as the MDASI, the SC names and composition varied, and same symptoms appeared in different clusters in different studies. For instance, in one study,14 researchers used cluster analysis on 13 general symptoms of the MDASI tool. They identified a cluster of symptoms such as pain + fatigue + drowsiness + lack of appetite + dry mouth + sleep disturbance + distress + sadness and named it ‘HNC-specific SC’. The reason for naming was that clustering of these symptoms may be explained due to RT-induced sequelae. In another study,58 researchers identified same clustering pattern using factor analysis on 13 general symptoms and nine HNC-specific symptoms of the MDASI-HN. But in this case, they named the cluster containing these symptoms as ‘General SC’ after examining the internal structure of the 13 general symptoms in line with the original MDASI development and linguistic validation studies. Again, another team of researchers54 named the cluster of pain + fatigue + drowsiness + lack of appetite as ‘Systemic SC’. Dry mouth which was in the ‘HNC-specific’ or ‘General’ SC in earlier studies appeared in the ‘Local’ SC in the latter study. Due to such naming inconsistencies and variations in SC composition, deducing common SCs in HNC population was challenging (See Table 1).
However, despite the differences in SC composition, the investigators could discern few patterns of co-occurring symptoms. A cluster of fatigue + pain + lack of appetite was reported in 50% of the studies14, 54, 58, 59 and a cluster of fatigue + pain + lack of appetite + sleep disturbance was reported in 38% of the studies.14, 58, 59 These symptoms formed a cluster with varying additional symptoms in different studies. Similarly, a cluster of dry mouth + difficulty swallowing/chewing + mouth/throat sores was reported in 25% of the studies.13, 54 These three symptoms co-occurred with taste disturbance or problems with mucus in different studies. A cluster of nausea + vomiting was reported in 50% of the studies.13, 14, 58, 59 Sadness + distress was reported in 38% of the studies.12, 14, 58 Two other studies reported anxiety co-occurring with depression60 or negative self-image and fear.57
Overall, three main SCs could be observed despite nomenclature variations – a) General / Systemic / Sickness SC; b) HNC-specific SC; and c) GI SC. The General / Systemic / Sickness SC was observed in 38% of the studies54, 58, 59 and pain + fatigue was reported in all of them. A fourth study reported pain + fatigue but named it ‘fatigue-sickness’ SC.60 The HNC-specific SC was observed in 63% of the studies, and dry mouth was reported in most of them. Most of the discrepancies in composition were seen in this cluster – symptoms related to functional impairments57; mixed-bag symptoms including general and treatment-specific local symptoms13, 14; and only local symptoms.12, 54 Additionally, a group of HNC-specific symptoms clustered in another study as well, but it was named as nutrition-impact SC.58 The GI SC was observed in 63% of the studies,12-14, 58, 59 and nausea was reported in all of them. The two qualitative studies used content analysis; one identified ‘Sickness’ SC and ‘GI’ SC,59 while the other reported ‘HNC-specific’ SC, and three other SCs associated with lymphedema.57 Since names of clusters and components within each cluster varied across studies and a meaningful synthesis was difficult, the investigators examined recurrent symptom-pairs in detail. Figures 2a and 2b describe these symptom-pairs involving fatigue and dry mouth. Table 1 describes all the identified SCs and symptoms within these clusters.
FIG 2.

(A) Fatigue-related symptom pairs with evidence. (B) Dry mouth-related symptom pairs with evidence.
Predictors and outcomes of symptom clusters
Predictors.
Only two studies examined factors associated with symptom clusters. One study61 reported that demographic characteristics were more predictive to SCs, whereas clinical characteristics, such as cancer site and treatment arms, were more significant for individual symptoms. This study found that patients who were white or had more than 12 years of education, were more likely to have severe HNC-specific SC; while patients who were female or never smoked were more likely to experience a more severe gastrointestinal SC. Another study58 reported that participants who lost more weight during treatment and had more symptom interference experienced more severe SCs. Occupational status and number of times RT had been received also were associated with specific SCs.
Factors predicting or associated with subgroup membership were examined in five studies.6, 7, 9, 15, 51 Overall, these characteristics included being female,6, 9, 15 years of education,15 age ≥ 60 years6, poor baseline performance status,6, 7 previous or current chemotherapy,6, 7 previous surgery,7 current or past medical problems,15 need for a percutaneous gastroenterostomy tube,15 larger tumor size – T3 to T4 disease,7, 9 lymph node involvement – N1 to N3 disease,9 having a mucosal tumor,9 and diversity in gut microbiome.51
Outcomes.
QoL was examined in four studies. These studies reported that QoL negatively correlated with SC severity,58 or significantly differed between the low-severity SC group and high-severity SC group,8,12 or was affected as expressed by patients.59 One study reported that membership in neck-disability trajectory was significantly associated with depression and social anxiety trajectories.15 Tables 1 and 2 describe the details of these associations.
DISCUSSION
To our knowledge, this review is the first to provide a summary of evidence regarding conceptual approaches and findings of SC research among patients with HNC. All the 17 studies were published within the last nine years, supporting an emerging interest in SCs in this population. Since no published reviews on SCs in HNC could be found, we compared our findings with similar reviews on other cancer sub-populations35-38 and cancer SC studies in general. This review revealed that two categories of studies were present: (a) studies that grouped symptoms to identify SCs (de novo) and (b) studies that grouped patients. Of the studies that examined patient subgroups, researchers used either a limited number of symptoms (decided a priori / severity-based / study-aim based) or all the items of the symptom measure. When comparing with existing cancer SC literature, we find certain similarities. Some studies in this review explicitly mentioned various cut-off criteria for defining a cluster and/or symptom inclusion. For specifying a group of symptoms as a cluster, SC experts have explained the importance of inter-correlations among symptoms within a cluster.17, 62 In other studies, researchers have reported correlations in the range of r = 0.29-0.79 among symptoms included in a cluster.63-66 Apart from correlations, a SC has also been specified using Cronbach alpha reliability coefficients,67 interfactor coefficients,67 cross-symptom pathways,64 and a combination of co-occurrence, severity, and correlations.66 As observed in this review, patient subgroups have been identified using similar three patterns of using symptoms: a priori identified symptom cluster68; few symptoms chosen out of a comprehensive list of symptoms, based on certain prevalence cut-off23, 69-71; and all the items of the symptom measure.72 The number of identified SCs and the number of symptoms within a cluster in this review were more similar to those found among patients with lung cancer.35 Difficulty in obtaining analogous results across studies due to disparities in methodology, sample characteristics, assessment tools, and analytical methods were reported in all other similar reviews.
Expert SC researchers have previously reported that cluster type and composition differ with the disease stage, instrument, timeframe, and statistical methods.26, 27, 31 Cut-off criteria used for cluster definition or symptom inclusion in analysis also dictate identified SCs.31 For instance, the issue of whether there should be a certain cut-off in symptom severity / prevalence or whether the presence or absence of the symptom is sufficient for SC analysis is still unresolved.27 Therefore, it is not surprising that the present review shows variation in identified SCs in HNC, even among studies that used the same tool for cluster or subgroup identification. In addition to these known causes, a specific reason for the inconsistencies could also be attributed, to an extent, to the heterogeneity in cancer sites within HNC cancer. Since symptoms vary with anatomic sites of HNC,73 SCs could be specific to HNC subtypes. This was evident when the SC structure identified for the full sample changed on generalizing for patients with larynx cancer.13 Therefore, it remains to be seen in future analyses if the identified SCs and their compositions are generalizable to all HNC sites. Finally, deduction of common SCs in HNC was also challenging due to inconsistencies in naming an identified SC and lack of consensus on cluster nomenclature.38, 74 Expert recommendations on criteria or guidelines on naming a SC would be helpful to make comparisons, meaningful conclusions, and subsequent translation in practice. For instance, standardized names like ‘generic’ SC, ‘disease-specific SC’, ‘treatment-specific SC’ etc. along with additional SCs with names based on the core symptom within that SC could be used.
Although it was difficult to draw general conclusions due to the variations in the identified clusters, there was a “core” of symptoms that consistently made up some of these clusters. General or systemic symptoms such as pain, fatigue, drowsiness, and lack of appetite may be attributed to cytokine release and inflammatory effects of cancer therapy,31 as previously demonstrated in other disease sites.75, 76 Similarly, connections between prevalent symptoms found in the HNC-specific SC such as mucositis, dysphagia, dysgeusia, and xerostomia have been reported in various studies5, 77-79 and can be attributed to acute side effects or long-term fibrotic changes caused by chemotherapy or RT.5, 78, 80, 81 Our review findings revealed that xerostomia commonly occurred with dysphagia, mucositis, and dysgeusia. Interestingly, while patient-reported dysphagia has been found to be significantly correlated with xerostomia,78, 79 dysgeusia is correlated with patient-reported xerostomia while eating, but not to xerostomia while not eating.82 This suggests a mechanistic explanation to the clustering of these symptoms and warrant further investigation. The GI cluster has been strongly supported in research on other cancer populations, and nausea and vomiting have been consistently grouped together regardless of cancer population, symptom measurement tools, or statistical methods in different studies.38, 67, 84, 85 Thus, although our review reveals inconsistencies, the fact that analogous symptoms clustered across studies could offer a reference for future symptom management in HNC patients. In order to address stability of these SCs over time, more longitudinal studies are necessary to delineate changes in SC structure over time.
The review draws together certain noteworthy findings and implications for future research. The co-occurrence of certain symptoms implies common underlying biological mechanisms. Considering that fatigue-related concurrent symptoms were prevalent among all the studies, biological mechanisms underlying fatigue and related symptoms need to be further examined. Recent data has demonstrated significant role of inflammation in fatigue in HNC86 and in the SC consisting of pain + fatigue + sleep disturbance.87 Significant differences in gut microbiome between the high and low psychoneurological SC subgroups indicate a significant direction for future research, as this may partially explain the commonality of the GI SC. Also, whether similar results would be obtained in oral microbiome studies needs to be seen. Future gut or oral microbiome studies need to understand function and cause–effect of microbes on SCs, with statistical control of potential confounders such as diet, antibiotic usage, age, socio economic status, exposure to environmental toxins etc..88 Using shotgun metagenomics and multiomics studies, particularly proteomics, metabolomics, and transcriptomics are important to advance this area as these provide additional information to guide identification of the pathways involved in clustering of symptoms. In addition, high stress levels throughout the cancer trajectory89 and subsequent chronic stress dysregulation leading to a cumulative biological burden, i.e. allostatic load90, 91 is associated with higher cancer-specific mortality.92 Stress is also associated with SC distress and interference.87 Considering all these findings, further examination of stress-related mechanisms including biomarkers is needed.
Clinical relevance of the statistically extracted SCs is another key area that needs further exploration in order to determine whether the statistically derived SCs are “really” clusters in the clinical sense.93 If so, this could lead to the development of guidelines and interventions for clinicians across settings. Various seminal articles on statistical methods in symptom cluster research exist.30, 94, 95 For instance, existing evidence suggest that cluster analysis should be used when the goal is to target interventions to clusters of individuals showing similar patterns of behaviors, and factor analysis should be used to target intervention strategies at behaviors that share the same underlying source.28, 96 Similar consensus and clear guidelines on statistical cut-off points vis-à-vis clinical relevance or plausibility need to be detailed. Clinical relevance can be elaborated by examining if the symptoms and their dimensions are important to the patient, the cluster occurs commonly, and some practical consequence occurs for both symptom management and patient outcomes.97
In this context of improving clinical relevance, qualitative and mixed methods studies could prove useful insights, as they would provide meaningful data on how patients view, prioritize, and evaluate SCs.26, 74 Additionally, qualitative and mixed methods studies would also help determine the most important symptom dimensions to include on an assessment instrument that will be used to identify SCs.27 This is especially relevant considering the importance of capturing impairments in ADL along with SCs for patients presenting with late-stage cancers at the time of diagnosis.56 The study found that majority of patients with late-stage HNC did not present with a cluster of troublesome physical symptoms, that would typically prompt further diagnostic testing to rule out cancer. Rather, they presented with a cluster of ADL impairments, which led them to seek additional diagnostic tests, which in turn led to a confirmation of cancer. The issue of which symptom dimensions to include needs resolution, along with if and when ADL should be included in a SC. ADL-related disability is highly prevalent in adults living with cancer, with basic ADLs such as personal hygiene, walking and transfers, and instrumental ADLs such as housework, shopping and transportation being the most frequently affected.98 Frailty and measures of independence in ADL have been found to be independent predictors of length of hospital stay and medical complications in HNC.99 Future SC research using symptoms integrated with ADL could offer clarity, validation, and guidance to this line of inquiry, and thus enhance clinical relevance.
Also, subjective judgements of researchers are used to guide decisions on choice of symptom measure, a priori SC for subgroup identification, or cut-off criteria for symptom inclusion. Since these decisions often reflect what is observed in clinical practice, explicit mention of these judgements in articles would help synthesis of evidence on clinical relevance of SCs. Next, SC research thus far has mostly focused on symptom as a subjective experience,16 however, one study in this review used clinician-observed toxicity data for SC identification. Comparison between clinician-observed SCs and patient-reported SCs in the same study sample may be illuminating. While patients are in the best position to report their symptom experiences, clinicians are in the best position to contextualize those experiences in terms of disease continuum.100 Clinician-observed symptoms are found to be more predictive of unfavorable clinical outcomes and patient-reported symptoms are associated more with their daily health status and QoL.100-102 However, significant correlations between observer-rated toxicities and objective measurements of salivary and swallowing dysfunction with patients’ quality of life have also been reported.83 Considering that discrepancies exist between the two reporting methods and that clinicians tend to underestimate symptom severity or distress,102 further studies using this collaborative reporting approach100 may be beneficial in three ways: help examine if discrepancies continue to exist even in SC identification; bring in the value of both symptom reporting approaches and help evaluate the linkages between signs and symptoms within a cluster; and validate SCs in patients who cannot self-report symptoms.27 However, while comparing the approaches, precision of cluster identification must be considered because toxicity assessments are usually documented at the time of patient visit while standardized patient-reported symptom assessments usually have a recall period of a week or 24 hours.
This review also validates the issues surrounding statistical methods for symptom and/or patient clusters.26, 74 Majority of the studies in the present review used either cluster analysis or factor analysis. Cluster analysis assigns similar cases to groups based on quantitative measures of similarity such the proximity (distance) of ratings, similar response patterns (correlations) across individuals, and underlying constructs are not a consideration.30 Factor analysis combines symptoms from datasets by assigning several symptoms into one group that is relatively independent in associations with other groups of symptoms. The underlying factors or components are based on the statistical strength within a correlational matrix.31 Implications and details of using these analytical methods are well-researched and documented.30, 31, 97, 103, 104 None of the studies that grouped patients used latent class analysis (LCA) or latent profile analysis, despite an emerging interest in these analytical methods in cancer.69, 105, 106 LCA has several advantages over cluster analysis in identifying patient subgroups. LCA is model-based and generates probabilities for group membership.107 It also allows examination of covariates and addresses methodological challenges that arise in subgroup analysis, including a high Type I error rate, low statistical power, and limitations in examining higher-order interactions.108 Also, only two studies in the review compared statistical methods. Further studies using latent class models with inclusion of covariates and examining concordance between statistical approaches would help decide on optimal analytical method in identifying clinically relevant SCs.
Lastly, the review highlights the scant information on predictors and outcomes of SCs or sub-group membership in HNC, making it difficult to generalize. Also, because symptoms change over the course of cancer treatment, patient groups may vary across time points and this inconsistent group membership across time may hinder further analysis on predictor identification.6 However, some promising areas emerge out of these findings. Association of female sex with cluster severity or high symptom subgroup was reported in four studies. Although the precise mechanism underlying the increased risk for female patients remains to be established, the fact that women are at increased risk of toxicities including nausea and vomiting following chemotherapy is well-recognized in cancer.109, 110 Similarly, positive correlation of weight loss with SC severity is of strong clinical significance. Weight loss has been associated with insomnia and fatigue in cancer111 and its association with SCs need to be further explored. Two studies found contrary information on years of education as a risk factor for SC severity.15, 61 It is generally observed that well-educated patients, who may be better informed about treatment-related symptoms, might find it easier to discuss symptoms with their physician. However, conflicting evidence also exist that suggest that educated persons are less likely to report problematic symptoms.112, 113 These preliminary findings reveal the need for further exploration of predictors using well-powered studies Also, it needs to be considered that symptoms change over the course of cancer treatment. Hence, patient groups may vary across time points and this inconsistent group membership across time may hinder further analysis on predictor identification.6 Among outcomes, in addition to QoL, other consequences of SC severity or subgroup membership including caregiver burden, productivity, and healthcare utilization need to be examined.
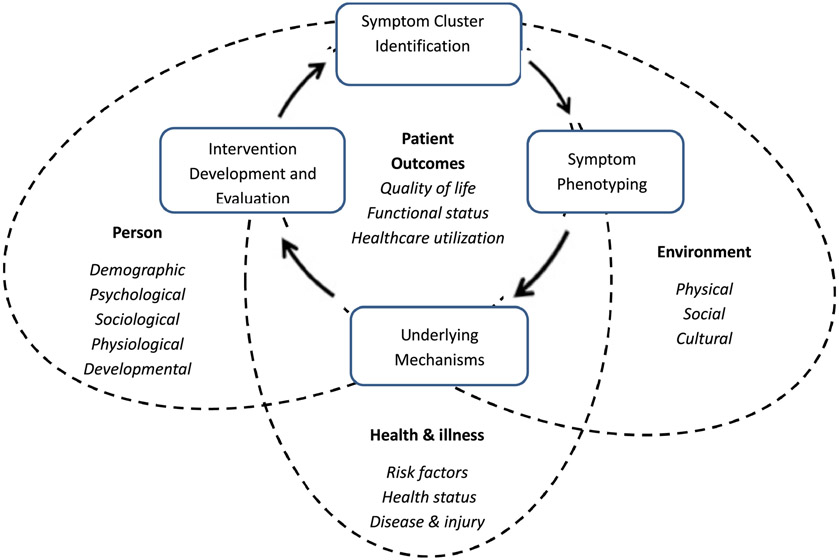
Conceptual model
Thus, translation of SC research in HNC to clinical practice requires a progression of rigorous activities – a) identifying clinically relevant SCs using quantitative, qualitative, or mixed methods; b) identifying high-risk patient subgroups based on these empirically-supported and clinically-relevant SCs; c) evaluation of predictors (context) of SCs and subgroup membership such as factors pertaining to nursing metaparadigm domains of person, environment, and health and illness; d) understanding mechanisms underlying the clustering of symptoms; and e) evaluation of outcomes of SCs and subgroup membership. This progression would enhance clinical utility of SC research and enable targeting high-risk groups for intervention development and evaluation.26 A conceptual model, not intended to be exhaustive, but to serve as an illustrative reminder of how findings from SC research in HNC could be clinically relevant is presented in Figure 3. The model is informed by the National Institutes of Health/National Institutes of Nursing Research Symptom Science Model114 and the Symptom Management Theory.16 The proposed model is modified to incorporate the elements described in the review, with patient at the center and all the elements lying within the context of the nursing metaparadigm domains.
FIG 3.
Conceptual model based on the NIH Symptom Science Model and Symptom Management Theory applied in symptom cluster research in head and neck cancers. NIH, National Institutes of Health.
Methodological rigor
Although the methodological rigor of the included studies was strong, certain factors could be addressed in future studies. An important confounder such as symptom management strategy was not accounted for in any of the studies. Symptom management strategies may change the natural course of symptoms, which may lead to different symptom cluster structures and also change the predictive effects of the risk factors for these clusters.61 An explicit mention of recall period used for symptom assessment and the Cronbach values of symptom measures for respective study samples will enhance clarity and validity of findings. Finally, most of the findings emerged from cross-sectional studies, but the power analysis was not described in majority of them. Addressing confounding variables is a critical step in quality appraisal, which is difficult to examine in studies using cluster and factor analyses. A solution to this issue could be modifying quality appraisal tools that adjust for these statistical approaches. Nevertheless, the findings of the included studies contribute to SC related data that are necessary for statistical assumptions in future HNC studies.
Limitations
This review has a few limitations. This review includes only studies available in English. A meta-analysis could not be performed due to methodological heterogeneity and resulting inconsistencies in predictors and outcomes. Although this review was current at the time of submission, it is possible that additional studies on the subject have been published since the completion of this analysis. Nevertheless, this is the first review to provide a detailed summary on the extant evidence on SCs in HNC, along with display of key co-occurring symptom pairs. Efforts to reduce potential publication bias were taken. The reviewers have also used two quality assessment tools to critically analyze the rigor of the included studies.
Conclusions
The current body of empirical evidence for SC in HNC give insights into significant findings and concerns. Guidelines on nomenclature of SCs are warranted. Future adequately powered studies are needed to identify clinically relevant SCs in order to elucidate the core SCs that could independently predict patient outcomes in HNC. Longitudinal studies are required to study the SC trajectories with appropriate adjustment for possible demographic and clinical confounders. Continuing work is needed on patients’ subjective experiences, predictors and outcomes, underlying mechanisms, and identification of high-risk patient groups (at risk for more severe symptom-clusters and/or worse outcomes) that can be targeted for intervention development and evaluation.
Supplementary Material
Review highlights.
This review on symptom clusters, first among patients with head and neck cancer, reports findings on commonly occurring symptom clusters and symptom pairs. The review draws together noteworthy findings on symptom clusters which are of high clinical utility due to their impact on prognosis and patient outcomes. The variability in naming a symptom cluster made deductions challenging; nevertheless, three prominent symptom clusters were identified – general, head and neck cancer-specific, and gastrointestinal. Being female and quality of life were significantly associated with high symptom group or cluster severity. A visual display illustrates symptom pairs and a conceptual diagram, informed by the NIH Symptom Science Model and Symptom Management Theory, illustrates implications of symptom-cluster research for clinical practice.
Acknowledgements
This article was supported by the National Institute of Nursing Research of the National Institutes of Health (grant number #K24NR015340). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge Information Services Liaison Librarian, Rebecca Raszewski, MS, AHIP, for her expertise and guidance regarding article search and retrieval strategies.
Footnotes
Conflict of interest
The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;0:1–41. [DOI] [PubMed] [Google Scholar]
- 2.Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. Harrison's principles of internal medicine, 19e. vol 1. Mcgraw-hill; 2015. [Google Scholar]
- 3.Chera BS, Eisbruch A, Murphy BA, et al. Recommended patient-reported core set of symptoms to measure in head and neck cancer treatment trials. J Natl Cancer Institute. 2014;106(7),dju127, 10.1093/jnci/dju127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townes TG, Navuluri S, Pytynia KB, et al. Assessing patient-reported symptom burden of long-term head and neck cancer survivors at annual surveillance in survivorship clinic. Article. Head Neck. 2020;42(8):1919–1927. 10.1002/hed.26119 [DOI] [PubMed] [Google Scholar]
- 5.Bressan V, Stevanin S, Bianchi M, Aleo G, Bagnasco A, Sasso L. The effects of swallowing disorders, dysgeusia, oral mucositis and xerostomia on nutritional status, oral intake and weight loss in head and neck cancer patients: a systematic review. Cancer Treat Rev. 2016;45:105–119. [DOI] [PubMed] [Google Scholar]
- 6.Shi Q, Mendoza TR, Gunn GB, Wang XS, Rosenthal DI, Cleeland CS. Using group-based trajectory modeling to examine heterogeneity of symptom burden in patients with head and neck cancer undergoing aggressive non-surgical therapy. Qual Life Res. 2013;22(9):2331–2339. 10.1007/s11136-013-0380-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunn GB, Mendoza TR, Fuller CD, et al. High symptom burden prior to radiation therapy for head and neck cancer: A patient-reported outcomes study. Article. Head Neck. 2013;35(10):1490–1498. 10.1002/hed.23181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wulff-Burchfield E, Dietrich MS, Ridner S, Murphy BA. Late systemic symptoms in head and neck cancer survivors. Support Care Cancer. August2019;27(8):2893–2902. 10.1007/s00520-018-4577-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna EY, Mendoza TR, Rosenthal DI, et al. The symptom burden of treatment-naive patients with head and neck cancer. Article. Cancer. 2015;121(5):766–773. 10.1002/cncr.29097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paiar F, Cristaudo A, Gonnelli A, et al. Radiation-induced nausea and vomiting in head and neck cancer: Is it something worth considering in the intensity modulated radiotherapy era?“A narrative review”. Head Neck. 2020;42(1):131–137. [DOI] [PubMed] [Google Scholar]
- 11.Stinson J, Chan K, Lee J, et al. Managing chemotherapy-induced nausea and vomiting in head and neck cancer patients receiving cisplatin chemotherapy with concurrent radiation. Ann Palliat Med. 2017;6(Suppl 1):S13–S20. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Li X, Mao C, et al. Symptom clusters in head and neck cancer patients with endotracheal tube: Which symptom clusters are independently associated with health-related quality of life? Eur J Oncol Nurs. 2020;48:N.PAG–N.PAG. 10.1016/j.ejon.2020.101819 [DOI] [PubMed] [Google Scholar]
- 13.Xiao C, Hanlon A, Zhang Q, et al. Symptom clusters in patients with head and neck cancer receiving concurrent chemoradiotherapy. Article. Oral Oncol. 2013;49(4):360–366. 10.1016/j.oraloncology.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang SH, Ho KY, Wang SY, Lin CC. Change in symptom clusters in head and neck cancer patients undergoing postoperative radiotherapy: A longitudinal study. Eur J Oncol Nurs. August2018;35:62–66. 10.1016/j.ejon.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 15.Rhoten BA, Murphy BA, Dietrich MS, Ridner SH. Depressive symptoms, social anxiety, and perceived neck function in patients with head and neck cancer. Article. Head Neck. 2018;40(7):1443–1452. 10.1002/hed.25129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–676. [DOI] [PubMed] [Google Scholar]
- 17.Aktas A, Walsh D, Rybicki L. Symptom clusters: myth or reality? Palliat Med. 2010;24(4):373–385. [DOI] [PubMed] [Google Scholar]
- 18.Kim H-J, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–282. [DOI] [PubMed] [Google Scholar]
- 19.Williams LA. Clinical management of symptom clusters. Seminars in oncology nursing. 2007;23(2):113–120. [DOI] [PubMed] [Google Scholar]
- 20.Given B, Given C, Azzouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nursing Res. 2001;50(4):222–232. [DOI] [PubMed] [Google Scholar]
- 21.Grisanzio KA, Goldstein-Piekarski AN, Wang MY, Ahmed APR, Samara Z, Williams LM. Transdiagnostic symptom clusters and associations with brain, behavior, and daily function in mood, anxiety, and trauma disorders. JAMA Psychiatry. 2018;75(2):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almutary H, Douglas C, Bonner A. Towards a symptom cluster model in chronic kidney disease: A structural equation approach. J Adv Nurs. 2017;73(10):2450–2461. [DOI] [PubMed] [Google Scholar]
- 23.Astrup GL, Hofsø K, Bjordal K, et al. Patient factors and quality of life outcomes differ among four subgroups of oncology patients based on symptom occurrence. Acta Oncol. 2017;56(3):462–470. [DOI] [PubMed] [Google Scholar]
- 24.Kwekkeboom KL. Cancer symptom cluster management. Semin Oncol Nurs. 2016;32(4):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miaskowski C, Aouizerat BE, Dodd M, Cooper B. Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J Natll Cancer Inst Monogr. 2007;2007(37):39–46. [DOI] [PubMed] [Google Scholar]
- 26.Barsevick A. Defining the symptom cluster: how far have we come? Semin Oncol Nurs. 2016;32(4):334–350. [DOI] [PubMed] [Google Scholar]
- 27.Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. J Natl Cancer Inst. 2017;109(4): djw253, 10.1093/jnci/djw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan CJ, Vuckovic KM, Finnegan L, et al. Acute Coronary Syndrome Symptom Clusters: Illustration of Results Using Multiple Statistical Methods. West J Nurs Res. July2019;41(7):1032–1055. 10.1177/0193945918822323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao C. The state of science in the study of cancer symptom clusters. Eur J Oncol Nurs. 2010;14(5):417–434. [DOI] [PubMed] [Google Scholar]
- 30.Skerman HM, Yates PM, Battistutta D. Multivariate methods to identify cancer-related symptom clusters. Res Nurs Health. 2009;32(3):345–360. [DOI] [PubMed] [Google Scholar]
- 31.Kirkova J, Aktas A, Walsh D, Davis MP. Cancer symptom clusters: clinical and research methodology. J Palliat Med. 2011;14(10):1149–1166. [DOI] [PubMed] [Google Scholar]
- 32.Aktas A. Cancer symptom clusters: current concepts and controversies. Curr Opin Support Palliat Care. 2013;7(1):38–44. [DOI] [PubMed] [Google Scholar]
- 33.Kelly DL, Dickinson K, Hsiao C-P, et al. Biological basis for the clustering of symptoms. Semin Oncol Nurs. 2016;32(4):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HJ, Barsevick AM, Fang CY, Miaskowski C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Article. Cancer Nurs. 2012;35(6):E1–E20. 10.1097/NCC.0b013e318233a811 [DOI] [PubMed] [Google Scholar]
- 35.Chen E, Nguyen J, Cramarossa G, et al. Symptom clusters in patients with lung cancer: a literature review. Expert Rev Pharmacoecon Outcomes Res. 2011;11(4):433–439. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen J, Cramarossa G, Bruner D, et al. A literature review of symptom clusters in patients with breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2011;11(5):533–539. [DOI] [PubMed] [Google Scholar]
- 37.Thavarajah N, Chen E, Zeng L, et al. Symptom clusters in patients with metastatic cancer: a literature review. Expert Rev Pharmacoecon Outcomes Res. 2012;12(5):597–604. [DOI] [PubMed] [Google Scholar]
- 38.Dong ST, Butow PN, Costa DS, Lovell MR, Agar M. Symptom clusters in patients with advanced cancer: a systematic review of observational studies. J Pain Symptom Manage. 2014;48(3):411–450. [DOI] [PubMed] [Google Scholar]
- 39.Rodgers C, Hooke MC, Ward J, Linder LA. Symptom clusters in children and adolescents with cancer. Semin Oncol Nurs. 2016;32(4):394–404. [DOI] [PubMed] [Google Scholar]
- 40.Erickson JM, MacPherson CF, Ameringer S, Baggott C, Linder L, Stegenga K. Symptoms and symptom clusters in adolescents receiving cancer treatment: a review of the literature. Int J Nurs Stud. 2013;50(6):847–869. [DOI] [PubMed] [Google Scholar]
- 41.Vasconcellos AFG, Palmier NR, Ribeiro ACP, et al. Impact of Clustering Oral Symptoms in the Pathogenesis of Radiation Caries: A Systematic Review. Caries Res. 2020;54(2):129–142. [DOI] [PubMed] [Google Scholar]
- 42.Cooper H. Research Synthesis and Meta-analysis: A Step-by-Step Approach. vol 2. Applied Social Research Methods Series. Sage Publications; 2015. [Google Scholar]
- 43.Garrard J. Health sciences literature review made easy. Jones & Bartlett Learning; 2016. [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG. Group PRISMA. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 45.Bates MJ. The design of browsing and berrypicking techniques for the online search interface. Online Rev. 1989;13(5):407–424. [Google Scholar]
- 46.Higgins JP, Thomas J, Chandler J, et al. Higgins JP, Thomas J, Chandler J, et al. , eds. Cochrane handbook for systematic reviews of interventions Version 6.1. John Wiley & Sons; 2020. www.training.cochrane.org/handbook [Google Scholar]
- 47.Salvador-Oliván JA, Marco-Cuenca G, Arquero-Avilés R. Errors in search strategies used in systematic reviews and their effects on information retrieval. J Med Libr Assoc. 2019/April// 2019;107(2):210–221. doi: 10.5195/jmla.2019.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aromataris E, Munn Z. Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. 2020. https://synthesismanual.jbi.global [Google Scholar]
- 49.Crowe M, Sheppard L. A general critical appraisal tool: an evaluation of construct validity. Int J Nurs Stud. 2011;48(12):1505–1516. [DOI] [PubMed] [Google Scholar]
- 50.Crowe M, Sheppard L, Campbell A. Comparison of the effects of using the Crowe Critical Appraisal Tool versus informal appraisal in assessing health research: a randomised trial. Int J Evid Based Healthc. 2011;9(4):444–449. [DOI] [PubMed] [Google Scholar]
- 51.Bai J, Bruner DW, Fedirko V, et al. Gut Microbiome Associated with the Psychoneurological Symptom Cluster in Patients with Head and Neck Cancers. Cancers. 2020;12(9):2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castellanos EH, Dietrich MS, Bond SM, et al. Impact of patient symptoms and caregiving tasks on psychological distress in caregivers for head and neck cancer (HNC). Psychooncology. 2019;28(3):511–517. [DOI] [PubMed] [Google Scholar]
- 53.Ridner SH, Dietrich MS, Niermann K, Cmelak A, Mannion K, Murphy B. A prospective study of the lymphedema and fibrosis continuum in patients with head and neck cancer. Lymphat Res Biol. 2016;14(4):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenthal DI, Mendoza TR, Fuller CD, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: A prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module. Article. Cancer. 2014;120(13):1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eraj SA, Jomaa MK, Rock CD, et al. Long-term patient reported outcomes following radiation therapy for oropharyngeal cancer: cross-sectional assessment of a prospective symptom survey in patients≥ 65 years old. Radiat Oncol. 2017;12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fodeh SJ, Lazenby M, Bai M, Ercolano E, Murphy T, McCorkle R. Functional impairments as symptoms in the symptom cluster analysis of patients newly diagnosed with advanced cancer. J Pain Symptom Manage. 2013;46(4):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng J, Ridner S, Rothman R, et al. Perceived Symptom Experience in Head and Neck Cancer Patients with Lymphedema. J Palliat Med. 2016;19(12):1267–1274. [DOI] [PubMed] [Google Scholar]
- 58.Xiao W, Chan CWH, Fan Y, et al. Symptom clusters in patients with nasopharyngeal carcinoma during radiotherapy. Eur J Oncol Nurs. June2017;28:7–13. [DOI] [PubMed] [Google Scholar]
- 59.Sari WI, Ismail S. The Experience of Symptom Cluster and Symptom Alleviation Self-Care in Patients with Head and Neck Cancer: A Qualitative Study. Jurnal Keperawatan Padjadjaran. 2019;7(1). 10.24198/jkp.v7i1.858 [DOI] [Google Scholar]
- 60.Howell D, Husain A, Seow H, et al. Symptom clusters in a population-based ambulatory cancer cohort validated using bootstrap methods. Eur J Cancer. 2012;48(16):3073–3081. [DOI] [PubMed] [Google Scholar]
- 61.Xiao C, Hanlon A, Zhang Q, et al. Risk factors for clinician-reported symptom clusters in patients with advanced head and neck cancer in a phase 3 randomized clinical trial: RTOG 0129. Clinical Trial, Phase III; Journal Article; Randomized Controlled Trial; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't. Cancer. 2014;120(6):848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dodd M, Miaskowski C, West C, Paul S, Lee K. The prevalence of symptom clsuters and comorbities in oncology outpatients. Oncol Nurs Forum. 2002;29(2) [Google Scholar]
- 63.Cheng KKF, Lee DTF. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Critical Reviews in Oncology/Hematology. 2011;78(2):127–137. doi: 10.1016/j.critrevonc.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 64.Ho S-Y, Rohan KJ, Parent J, Tager FA, McKinley PS. A Longitudinal Study of Depression, Fatigue, and Sleep Disturbances as a Symptom Cluster in Women With Breast Cancer. J Pain Symptom Manage. 2015;49(4):707–715. doi: 10.1016/j.jpainsymman.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan CWH, Richardson A, Richardson J. A study to assess the existence of the symptom cluster of breathlessness, fatigue and anxiety in patients with advanced lung cancer. Eur J Oncol Nurs. 2005;9(4):325–333. doi: 10.1016/j.ejon.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 66.Lin S, Chen Y, Yang L, Zhou J. Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs. 2013;22(9-10):1281–1290. [DOI] [PubMed] [Google Scholar]
- 67.Molassiotis A, Wengström Y, Kearney N. Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manage. 2010;39(5):847–858. [DOI] [PubMed] [Google Scholar]
- 68.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33. 10.1188/06.ONF.E79-E89 [DOI] [PubMed] [Google Scholar]
- 69.Miaskowski C, Dunn L, Ritchie C, et al. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage. 2015;50(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong HC, Kim YM, Min A. Symptom clusters in childhood cancer survivors in Korea: A latent class analysis. Eur J Cancer Care. 2020;29(6):e13322. [DOI] [PubMed] [Google Scholar]
- 71.Finnegan L, Campbell RT, Ferrans CE, Wilbur J, Wilkie DJ, Shaver J. Symptom cluster experience profiles in adult survivors of childhood cancers. J Pain Symptom Manage. 2009;38(2):258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dhingra LK, Lam K, Cheung W, et al. Variation in symptom distress in underserved Chinese American cancer patients. Cancer (0008543X). 2015;121(18):3352–3359. 10.1002/cncr.29497 [DOI] [PubMed] [Google Scholar]
- 73.Cunha Pinto RM, Bezerra Rodrigues A, Peres de Oliveira P. Multiple symptoms in people with head and neck neoplasia: a descriptive study. Online Brazilian Journal of Nursing. 2014;13(4):591–601. [Google Scholar]
- 74.Miaskowski C. Future directions in symptom cluster research. Semin Oncol Nurs. 2016;32(4):405–415. [DOI] [PubMed] [Google Scholar]
- 75.Wang XS, Williams LA, Krishnan S, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Article. Brain Behav Immun. 2012;26(5):699–705. doi: 10.1016/j.bbi.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24(6):968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong HM. Oral complications and management strategies for patients undergoing cancer therapy. ScientificWorldJournal. 2014;2014:581795. doi: 10.1155/2014/581795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moroney LB, Helios J, Ward EC, et al. Patterns of dysphagia and acute toxicities in patients with head and neck cancer undergoing helical IMRT±concurrent chemotherapy. Oral Oncol. 2017;64:1–8. [DOI] [PubMed] [Google Scholar]
- 79.Vainshtein JM, Samuels S, Tao Y, et al. Impact of xerostomia on dysphagia after chemotherapy-intensity-modulated radiotherapy for oropharyngeal cancer: Prospective longitudinal study. Head Neck. April2016;38Suppl 1:E1605–12. doi: 10.1002/hed.24286 [DOI] [PubMed] [Google Scholar]
- 80.Çakmak S, Nural N. Incidence of and risk factors for development of oral mucositis in outpatients undergoing cancer chemotherapy. Int J Nurs Pract. 2019;25(1):e12710. [DOI] [PubMed] [Google Scholar]
- 81.Cartmill B, Cornwell P, Ward E, et al. Emerging understanding of dosimetric factors impacting on dysphagia and nutrition following radiotherapy for oropharyngeal cancer. Head Neck. 2013;35(8):1211–1219. [DOI] [PubMed] [Google Scholar]
- 82.Sapir E, Tao Y, Feng F, et al. Predictors of Dysgeusia in Patients With Oropharyngeal Cancer Treated With Chemotherapy and Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. October12016;96(2):354–361. doi: 10.1016/j.ijrobp.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 83.Hunter KU, Schipper M, Feng FY, et al. Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat Oncol Biol Phys. March152013;85(4):935–40. doi: 10.1016/j.ijrobp.2012.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hird A, Wong J, Zhang L, et al. Exploration of symptoms clusters within cancer patients with brain metastases using the Spitzer Quality of Life Index. Support Care Cancer. 2010;18(3):335–342. [DOI] [PubMed] [Google Scholar]
- 85.Ward Sullivan C, Leutwyler H, Dunn LB, Miaskowski C. A review of the literature on symptom clusters in studies that included oncology patients receiving primary or adjuvant chemotherapy. J Clin Nurs. 2018;27(3-4):516–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao C, Beitler JJ, Higgins KA, et al. Associations among human papillomavirus, inflammation, and fatigue in patients with head and neck cancer. Cancer. 2018;124(15):3163–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwekkeboom KL, Tostrud L, Costanzo E, et al. The Role of Inflammation in the Pain, Fatigue, and Sleep Disturbance Symptom Cluster in Advanced Cancer. J Pain Symptom Manage. 2018;55(5):1286–1295. doi: 10.1016/j.jpainsymman.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maki KA, Diallo AF, Lockwood MB, Franks AT, Green SJ, Joseph PV. Considerations when designing a microbiome study: Implications for nursing science. Biol Res Nurs. 2019;21(2):125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Devins GM, Payne AYM, Lebel S, et al. The burden of stress in head and neck cancer. Psychooncology. 2013;22(3):668–676. doi: 10.1002/pon.3050 [DOI] [PubMed] [Google Scholar]
- 90.McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- 91.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacol. 2000;22:108–124 [DOI] [PubMed] [Google Scholar]
- 92.Mathew A, Doorenbos AZ, Li H, Jang MK, Park CG, Bronas UG. Allostatic Load in Cancer: A Systematic Review and Mini Meta-Analysis. Biol Res Nurs. 2021;23(3):341–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skerman HM, Yates PM, Battistutta D. Cancer-related symptom clusters for symptom management in outpatients after commencing adjuvant chemotherapy, at 6 months, and 12 months. Support Care Cancer. 2012;20(1):95–105. [DOI] [PubMed] [Google Scholar]
- 94.Tein J-Y, Coxe S, Cham H. Statistical power to detect the correct number of classes in latent profile analysis. Struct Equ Modeling. 2013;20(4):640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dalmaijer ES, Nord CL, Astle DE. Statistical power for cluster analysis. 2020arXiv preprint arXiv:2003.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hofstetter H, Dusseldorp E, van Empelen P, Paulussen TW. A primer on the use of cluster analysis or factor analysis to assess co-occurrence of risk behaviors. Prev Med. October2014;67:141–6. doi: 10.1016/j.ypmed.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 97.Skerman HM, Yates PM, Battistutta D. Identification of cancer-related symptom clusters: An empirical comparison of exploratory factor analysis methods. Article. J Pain Symptom Manage. 2012;44(1):10–22. doi: 10.1016/j.jpainsymman.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 98.Neo J, Fettes L, Gao W, Higginson IJ, Maddocks M. Disability in activities of daily living among adults with cancer: A systematic review and meta-analysis. Cancer treat rev. 2017;61:94–106. [DOI] [PubMed] [Google Scholar]
- 99.Goldstein DP, Sklar MC, de Almeida JR, et al. Frailty as a predictor of outcomes in patients undergoing head and neck cancer surgery. The Laryngoscope. 2020;130(5):E340–E345. [DOI] [PubMed] [Google Scholar]
- 100.Basch E, Bennett A, Pietanza MC. Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst. 2011;103(24):1808–1810. [DOI] [PubMed] [Google Scholar]
- 101.Chitapanarux I, Tharavichitkul E, Kamnerdsupaphon P, Pukanhapan N, Vongtama R. Randomized phase III trial of concurrent chemoradiotherapy vs accelerated hyperfractionation radiotherapy in locally advanced head and neck cancer. J Radiat Res. 2013;54(6):1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao C, Polomano R, Bruner DW. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer nurs. 2013;36(6):E1–E16. [DOI] [PubMed] [Google Scholar]
- 103.Chouikhi H, Charrad M, Ghazzali N. A comparison study of clustering validity indices. IEEE; 2015:1–4. [Google Scholar]
- 104.Cameron DH, Streiner DL, Summerfeldt LJ, Rowa K, McKinnon MC, McCabe RE. A comparison of cluster and factor analytic techniques for identifying symptom-based dimensions of obsessive-compulsive disorder. Psychiatry Res. 2019;278:86–96. [DOI] [PubMed] [Google Scholar]
- 105.Ferrat E, Audureau E, Paillaud E, et al. Four distinct health profiles in older patients with cancer: latent class analysis of the prospective ELCAPA cohort. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2016;71(12):1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reese JB, Blackford A, Sussman J, et al. Cancer patients’ function, symptoms and supportive care needs: a latent class analysis across cultures. Qual Life Res. 2015;24(1):135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. vol 718. John Wiley & Sons; 2009. [Google Scholar]
- 108.Lanza ST, Rhoades BL. Latent class analysis: an alternative perspective on subgroup analysis in prevention and treatment. Prev Sci. 2013;14(2):157–168. doi: 10.1007/s11121-011-0201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wagner AD, Grothey A, Andre T, et al. Sex and adverse events of adjuvant chemotherapy in colon cancer: an analysis of 34,640 patients in the ACCENT database. J Natl Cancer Inst. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mosa ASM, Hossain AM, Lavoie BJ, Yoo I. Patient-related risk factors for chemotherapy-induced nausea and vomiting: a systematic review. Front Pharmacol. 2020;11:329. 10.3389/fphar.2020.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiu C, Yang N, Tian G, Liu H. Weight loss during radiotherapy for nasopharyngeal carcinoma: a prospective study from northern China. Nutr Cancer. 2011;63(6):873–879. [DOI] [PubMed] [Google Scholar]
- 112.Movsas B, Scott C, Watkins-Bruner D. Pretreatment factors significantly influence quality of life in cancer patients: a Radiation Therapy Oncology Group (RTOG) analysis. International Journal of Radiation Oncology* Biology* Physics. 2006;65(3):830–835. [DOI] [PubMed] [Google Scholar]
- 113.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97(6):448–456. [DOI] [PubMed] [Google Scholar]
- 114.Cashion AK, Grady PA. The National Institutes of Health/National Institutes of Nursing Research intramural research program and the development of the National Institutes of Health symptom science model. Nurs Outlook. 2015;63(4):484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.