Abstract
Purpose
This study investigated the association between dietary patterns, total mortality, and cancer mortality in the United States.
Methods
We identified the four major dietary patterns at baseline from 13,466 participants of the NHANES III cohort using principal component analysis (PCA). Dietary patterns were categorized into ‘prudent’ (fruits and vegetables), ‘western’ (red meat, sweets, pastries, oils), ‘traditional’ (red meat, legumes, potatoes, bread), and ‘fish and alcohol’. We estimated hazard ratios for total mortality, and cancer mortality using Cox regression models.
Results
A total of 4,963 deaths were documented after a mean follow-up of 19.59 years. Higher adherence to the ‘prudent’ pattern was associated with the lowest risk of total mortality (5th vs. 1st quintile HR 0.90, 95% CI 0.82–0.98), with evidence that all-cause mortality decreased as consumption of the pattern increased. No evidence was found that the ‘prudent’ pattern reduced cancer mortality. The ‘western’ and the ‘traditional’ patterns were associated with up to 22% and 16% increased risk for total mortality (5th vs. 1st quintile HR 1.22, 95% CI 1.11–1.34; and 5th vs. 1st quintile HR 1.16, 95% CI 1.06–1.27, respectively), and up to 33% and 15% increased risk for cancer mortality (5th vs. 1st quintile HR 1.33, 95% CI 1.10–1.62; and 5th vs. 1st quintile HR 1.15, 95% CI 1.06–1.24, respectively). The associations between adherence to the ‘fish and alcohol’ pattern and total mortality, and cancer mortality were not statistically significant.
Conclusion
Higher adherence to the ‘prudent’ diet decreased the risk of all-cause mortality but did not affect cancer mortality. Greater adherence to the ‘western’ and ‘traditional’ diet increased the risk of total mortality and mortality due to cancer.
Keywords: Dietary patterns, United States, Total mortality, Cancer mortality
Introduction
In the last decades, efforts have been made to quantify the burden of disease attributable to specific dietary factors [1–3]. The study of diet and disease associations has traditionally originated from the consumption of single nutrients or foods. Dietary patterns describe diet quality and explain the total variability in food intake [4, 5]. Additionally, dietary patterns account for complex interactions that occur among foods and nutrients [6]. Therefore, the study of dietary patterns provides a clearer view of food and nutrient consumption and could better predict long-term outcomes such as mortality due to dietary exposure [7].
Several studies evaluated the effect of dietary patterns and mortality in other countries [8–11]. In the United States, studies have shown the association between adherence to specific dietary patterns and chronic diseases, including obesity [12, 13], cardiovascular disease, and diabetes [14]. Moreover, only a few studies have investigated the relationship between specific dietary patterns and total mortality [15–20]. Most of these studies have focused on particular race and ethnicities [17], older adults [15, 20], or participants with specific health conditions, such as chronic kidney disease [19]. Therefore, evidence of the association between adherence to dietary patterns and total mortality in the United States in the general population is still limited.
This study evaluates the association between dietary patterns, total mortality, and cancer mortality in the NHANES III adult cohort in the U.S. We hypothesize that dietary patterns characterized by consumption of nutrient-rich foods are associated with lower total mortality, and mortality due to cancer in the United States. Conversely, we hypothesize that dietary patterns that reflect poor nutrition are associated with higher total mortality and mortality due to cancer. We used data from 13,466 adults from the National Health and Nutrition Examination Survey III (NHANES III). We identified the predominant dietary patterns in relationship with total mortality and mortality due to cancer.
Materials and methods
Study population
The study used data from the NHANES III, a nationally representative sample of the civilian, non-institutionalized U.S. population conducted between 1988 and 1994 by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The NHANES study protocol obtained ethical approval from the CDC/NCHS Ethics Review Board and received informed consent from all participants. The current study included 13,466 participants 18 to 90 years old, with complete data on mortality status, diet, and relevant covariates. The examination component involved examinations by a health professional, including physicians, dentists, and health technicians. Detailed information on the standardized protocols used in NHANES III has been previously published [21].
Assessment of dietary exposure
During the interviews, dietary records providing detailed information about the foods and beverages were collected using computer-assisted software. We followed the recommendation from the NHANES III dietary methodology committee to use the 24-h recall as the principal methodology to provide detailed quantitative food and nutrient intake assessment, and the food frequency questionnaire (FFQ) to supplement data from the 24-h recall to provide typical or qualitative data [22, 23]. Therefore, the percentage of daily calories and macronutrients was calculated based on the information gathered during the 24-h food recall interview, and the usual diet assessment was based on the FFQ that included 60 food items and beverages. This was calculated in standardized portion sizes and nutrient intakes as grams per day. Participants without complete dietary data and those that reported excessively high or low values for total food or energy intake (less than 600 kcal/day in or more than 4,200 kcal/day) were excluded based on previously defined cut-offs [24–26]. Food groups were classified based on their nutrient profiles based according to the USDA Nutrient Lists from Standard Reference Legacy [27]. The food groups and items are shown in Table 1.
Table 1.
Food groups and food items
| Food groups | Food items |
|---|---|
| Dairy | Chocolate milk, milk, yogurt, beverages made with milk and creams, cheese, and cheese dishes |
| Red meat | Beef and pork |
| Poultry | Poultry |
| Processed meat | Processed meat, entrails |
| Fish | Fish and seafood |
| Eggs | Eggs and egg products |
| Vegetables | Soups and dishes with vegetables, all types of vegetables |
| Fruit | Fruits and fruit juices |
| Legumes | Bean including kidney, pinto and black beans, lentils, chickpeas, and rice |
| Potatoes | All types of potatoes, including sweet potatoes and yams |
| Nuts and seeds | Nuts and seeds |
| Cereal | Bran, fiber, cold and hot cereals, box cereals |
| Bread | bread, tortillas, and past |
| Sweets and pastries | pastries, chocolate, candy |
| Caffeinated beverages | Tea and coffee |
| Carbonated drinks | Regular and diet sodas, colas, drinks with vitamin C, beer |
| Alcohol beverages | wine, champagne, hard liquors |
| Oils | Butter, Margarine, vegetable oils and salad dressings |
Assessment of covariates
The sociodemographic and lifestyle characteristics information was collected during the face-to-face household interview. The participants’ characteristics included in the analysis were: age (years at the time of the recruitment); sex (male or female); educational attainment (years of schooling completed); and current smoking status (yes, no, or missed to answer). The medical history of chronic diseases, including ischemic heart disease, stroke, hypertension, diabetes, was also collected during the household interview. The variables were dichotomized as yes or no. The questions participants were asked were: “Has a doctor ever told you that you had a heart attack?”; “Has a doctor ever told you that you had a stroke?”; “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?”; “Have you ever been told by a doctor that you have diabetes?”; “Has a doctor ever told you that you had skin cancer?” and “Has a doctor ever told you that you had any other cancer?”. The collection of anthropometric measurements during the physical examination included standing height and weight using standard protocols. We used these measurements to calculate body mass index (BMI) as kg/m2.
Assessment of the outcome
The NHANES III cohort study’s mortality information was identified through linkage to the National Death Index through 31 December 2015. The mortality data included the underlying cause of death. For total mortality, we included deaths from all causes. For cancer-specific mortality, we included deaths from malignant neoplasms coded from C00–C97 in the International Classification of Diseases, 10th Edition, Clinical Modification System codes (ICD, 2021) [21, 26, 28]. The follow-up time was determined based on the interval from the 24-h recall interview to the date of death or to 31 December 2015, for those that were censored.
Statistical analysis
We used principal component analysis (PCA) to assess the major dietary patterns in the NHANES III cohort. We tested the sample adequacy for PCA using the Kaiser–Meyer–Olkin (KMO) test. We identified the major dietary patterns based on the components with eigenvalues greater than one and a confirmatory scree test (Fig. 1). Then, we calculated scores for each of the dietary patterns. Food groups with absolute scoring coefficients > 0.3 were considered substantial contributors to a pattern. We evaluated the distribution of population characteristics by quintiles of dietary pattern scores and tested for trend analysis. We used 1-way ANOVA for quantitative variables and Chi-square tests for qualitative variables to identify significant differences across quintile of dietary pattern scores.
Fig. 1.
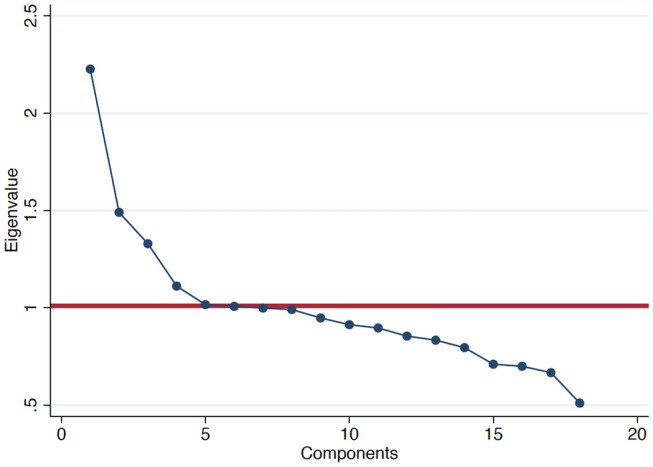
Scree plot used for identification of dietary patterns (components) by principal component analysis. Components with eigenvalues greater than one are considered major dietary pattern scores
After confirming the proportionality of hazards assumption, we used Cox regression models with the length of follow-up as the primary time variable to test the association between dietary pattern adherence and mortality due to all causes and mortality due to cancer. Hazard ratios (HR) with 95% confidence intervals (CI) across quintiles of dietary patterns were calculated using the lowest quintile of adherence as the reference category. In the multivariable models, potential confounders included as covariates were age, sex, total energy intake, smoking status, baseline BMI and previous history of chronic diseases (diabetes, hypertension, stroke, heart attack) and cancer. Also, tests of linear trend across successive quintiles of adherence to each pattern were conducted, treating the variable as continuous. We considered p values < 0.05 statistically significant. Sample weights were used to calculate population estimates to account for unequal probability of selection. All analyses were performed using STATA version 13.1.
Results
Dietary pattern assessment
We identified four major dietary patterns, explaining 33% of the total variance in the food groups’ consumption in the study population. The factor loadings are shown in Table 2. The first dietary pattern was named ‘prudent’ because it had a high score for vegetables, fruits, and a low score in meat and oils. The second dietary pattern was named ‘western’ since it was characterized by a high score for red meat, sweets, pastries, oils and low scores for vegetables and fruits. The third pattern was named ‘traditional’ because it had high scores in traditional foods, including red meat, eggs, legumes, potatoes, and bread. Finally, the fourth dietary pattern was labeled as ‘fish and alcohol’ due to the notably high score for alcoholic beverages compared to other foods, fish and seafood.
Table 2.
Factor loading matrix for the major factors identified by using food consumption data from NHANES IIIa
| ‘Prudent’ pattern | ‘Western’ pattern | ‘Traditional’ pattern | ‘Fish and alcohol’ pattern | |
|---|---|---|---|---|
| Dairy | 0.01 | 0.03 | 0.06 | −0.11 |
| Red meat | −0.21 | 0.41 | 0.36 | 0.14 |
| Poultry | 0.21 | −0.20 | −0.09 | 0.26 |
| Processed meat | 0.04 | 0.03 | 0.02 | 0.03 |
| Fish | 0.21 | −0.26 | 0.11 | 0.45 |
| Eggs | 0.21 | 0.29 | 0.06 | −0.18 |
| Vegetables | 0.43 | −0.30 | −0.02 | 0.07 |
| Fruit | 0.33 | −0.34 | 0.21 | −0.12 |
| Legumes | 0.25 | −0.22 | 0.53 | −0.01 |
| Potatoes | 0.33 | 0.22 | 0.39 | 0.02 |
| Nuts and seeds | 0.24 | 0.12 | 0.28 | −0.15 |
| Cereal | 0.21 | −0.15 | −0.25 | −0.43 |
| Bread | 0.35 | 0.17 | 0.30 | −0.22 |
| Sweets and pastries | 0.14 | 0.46 | 0.11 | 0.19 |
| Caffeinated beverages | 0.02 | 0.06 | −0.01 | −0.06 |
| Soda | −0.21 | 0.09 | 0.16 | 0.28 |
| Alcohol beverages | 0.05 | 0.15 | 0.18 | 0.42 |
| Oils | 0.27 | 0.37 | 0.21 | −0.03 |
aBolded numbers correspond to absolute values > 0.30 and are considered significant contributors to the pattern
‘Starch’ pattern explained 12% of the variance; the ‘refined sugars & high protein’ pattern explained 8%; the ‘traditional’ pattern 7%; and the ‘fish and alcohol’ pattern explained 6% of the variance
Demographic characteristics
The mean age of the 13,446 participants was 46.89 years. The mean follow-up of participants was 19.59 years, with a minimum and maximum follow-up of 0.10 and 27.2 years, respectively. During this period, 4,963 deaths were registered. The leading causes of death were cardiovascular disease (23.65%), cancer (21.89%), cerebrovascular disease (6.64%), and other reasons (47.8%). The mean age of death for the deceased participants was 64.10 years. In Table 3, the baseline characteristics of the participants are shown across the quintiles for the dietary patterns. On average, the participants with the highest adherence to the ‘prudent’ dietary pattern were more likely to be men, older, had more years of education, and were less likely to be current smokers. They were also less likely to have a previous history of stroke and cancer; they were leaner despite having a higher calorie intake; and they consumed higher amounts of fiber, carbohydrates, and protein and consumed a lower amount of fats and alcohol.
Table 3.
Baseline characteristics and nutrient consumption of the study population by quintiles of dietary patterns (n = 13,465)
| ‘Prudent’ pattern | ‘Western’ pattern | ‘Traditional’ pattern | ‘Fish and alcohol’ pattern | |||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | |
| n | 2,694 | 2,693 | 2,694 | 2,693 | 2,694 | 2,693 | 2,694 | 2,693 |
| % | ||||||||
| Sex, Male | 8.04 | 8.73* | 8.55 | 9.92* | 11.08* | 7.06 | 8.25 | 8.31 |
| Current smoker | 5.57* | 3.97 | 3.01 | 6.92* | 2.97 | 5.86* | 3.49 | 5.76* |
| Ischemic Heart Disease | 0.81 | 0.99 | 1.02 | 0.65 | 0.61 | 1.41 | 1.31 | 0.51 |
| Stroke | 0.52* | 0.43 | 0.47 | 0.78 | 0.83* | 0.37 | 1.07 | 0.25 |
| Hypertension | 5.40 | 5.25 | 6.59 | 4.09* | 6.23* | 4.60 | 5.23 | 4.85 |
| Diabetes | 9.77 | 9.26 | 9.57 | 9.37 | 9.28* | 8.13 | 8.49 | 8.79 |
| Cancer | 1.67* | 1.07 | 1.25 | 1.48* | 0.52 | 2.70 | 1.93 | 1.62 |
| Mean ± Standard deviation | ||||||||
| Age, years | 42.45 ± 18.16 | 49.l5 ± 20.35* | 50.27 ± 18.74 | 41.83 ± 18.92* | 56.44 ± 20.28* | 41.56 ± 17.31 | 55.24 ± 21.64* | 43.09 ± 16.95 |
| BMI, kg/m2 | 26.83 ± 5.62* | 25.22 ± 5.11 | 26.56 ± 5.09 | 26.97 ± 5.52* | 26.64 ± 5.23* | 25.74 ± 5.01 | 25.94 ± 4.92 | 26.17 ± 5.49 |
| Education, years | 11.34 ± 3.32* | 10.91 ± 3.97 | 11.06 ± 4.30 | 10.38 ± 2.80* | 9.02 ± 4.24 | 12.15 ± 3.20* | 10.09 ± 3.92 | 12.42 ± 3.18* |
| Total energy intake (kcal) | 1886.67 ± 780.93 | 2087.31 ± 812.13* | 1823.65 ± 737.76 | 2222.53 ± 823.28* | 2111.32 ± 817.57* | 1900.55 ± 757.18 | 1902.83 ± 775.54 | 2051.16 ± 793.49* |
| kcal from fat | 34.06 ± 9.46* | 32.84 ± 9.02 | 30.03 ± 9.53 | 35.46 ± 8.61* | 32.69 ± 8.98 | 32.70 ± 9.27 | 31.95 ± 8.98 | 33.77 ± 9.56* |
| kcal from saturated fat | 11.27 ± 3.99* | 10.61 ± 3.90 | 9.60 ± 3.92 | 12.10 ± 3.82* | 10.76 ± 3.93 | 10.83 ± 3.90 | 11.17 ± 3.95 | 10.98 ± 3.93 |
| kcal from monounsaturated fat | 12.91 ± 4.02* | 12.34 ± 3.96 | 11.04 ± 4.10 | 13.58 ± 3.73* | 12.21 ± 3.87 | 12.28 ± 4.12 | 12.40 ± 3.89 | 12.67 ± 4.13* |
| kcal from polyunsaturated fat | 7.22 ± 3.68 | 7.22 ± 3.31 | 6.88 ± 3.57 | 7.06 ± 3.21* | 6.98 ± 3.41 | 7.06 ± 3.39 | 6.73 ± 3.11 | 7.47 ± 3.72* |
| kcal from protein | 15.12 ± 4.96 | 15.95 ± 4.98* | 16.74 ± 5.16* | 14.57 ± 4.61 | 16.04 ± 4.78* | 15.58 ± 4.56 | 14.67 ± 4.43 | 15.75 ± 5.23* |
| kcal from carbohydrates | 49.32 ± 11.64 | 50.82 ± 10.79* | 52.21 ± 11.32* | 48.30 ± 10.83 | 50.54 ± 10.93 | 50.35 ± 11.10 | 51.13 ± 10.79 | 47.41 ± 11.52 |
| kcal from alcohol | 1.50 ± 1.02* | 0.39 ± 0.2 | 1.02 ± 0.53 | 1.67 ± 0.83* | 0.73 ± 0.45 | 1.37 ± 0.24* | 2.25 ± 0.78 | 3.07 ± 0.89* |
| Dietary fiber (g) | 13.36 ± 8.95 | 18.68 ± 11.38* | 15.16 ± 10.89* | 14.32 ± 8.82 | 17.38 ± 12.42 | 16.94 ± 9.62 | 16.28 ± 11.02 | 15.20 ± 9.27 |
Q1: quintile 1, lowest consumption; Q5: quintile 5, highest consumption
*p < 0.05 based on analysis of variance or Chi-square test
The participants with the highest adherence to the ‘western’ dietary pattern were more likely to be male, younger, current smokers, have a higher BMI, and have fewer years of education. Additionally, they were more likely to report a history of hypertension and cancer. On average, they had a higher caloric intake, higher consumption of total fat and alcohol, and lower protein consumption, carbohydrates, and dietary fiber.
Those with the highest consumption of the ‘traditional’ and the ‘fish and alcohol’ dietary patterns were more likely to be men, younger, current smokers, and have more years of education. Nonetheless, the participants with the highest adherence to the ‘traditional’ diet were less likely to have a previous history of chronic diseases, such as stroke, hypertension, and diabetes at baseline, probably due to their age. Those with the consumption of the ‘traditional’ pattern consumed less protein and more alcohol. And those with the highest consumption of the ‘fish and alcohol’ pattern had higher calorie intake and consumed more fats, protein, and alcohol.
Dietary patterns and total mortality
There were 4,963 deaths due to all causes among the 13,446 participants from NHANES III cohort. The results of the study showed that the lowest risk of total mortality was found among those with the highest adherence to the ‘prudent’ dietary pattern compared to those with the lowest (5th vs. 1st quintile HR 0.86, 95% CI 0.78–0.94) in the age-adjusted model showing that the ‘prudent’ diet reduced the risk of total mortality over the observation period. In the fully adjusted model, the inverse association between adherence to the ‘prudent’ pattern became attenuated but remained significant (5th vs. 1st quintile HR 0.90, 95% CI 0.82–0.98). Furthermore, there was evidence of a significant trend in both models (p for trend 004; and p for trend 0.007, respectively). Conversely, those in the highest group of consumption of the ‘western’ (5th vs. 1st quintile HR 1.38, 95% CI 1.26–1.51) and the ‘traditional’ (5th vs. 1st quintile HR 1.32, 95% CI 1.21–1.44) dietary patterns had the highest risk of total mortality in the age-adjusted models. In the fully adjusted models, the positive associations between greater adherence to the ‘western’ (5th vs. 1st quintile HR 1.22, 95% CI 1.11–1.34) and the ‘traditional’ (5th vs. 1st quintile HR 1.16, 95% CI 1.06–1.27) dietary patterns and total mortality became attenuated; nonetheless, they remained significant and showed a positive trend. In this study, the association between the ‘fish and alcohol’ dietary pattern and total mortality in the age-adjusted and the fully adjusted models was marginally significant for those in the highest consumption group (5th vs. 1st quintile HR 0.94, 95% CI 0.87–1.01; and 5th vs. 1st quintile HR 0.96, 95% CI 0.89–1.03, respectively). The HR and 95% CI for total mortality according to baseline adherence to the four dietary patterns are shown in Table 4.
Table 4.
Hazard ratios for total mortality according to quintiles of adherence categories of dietary patterns in NHANES III
| Quintiles of dietary pattern consumption | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| ‘Prudent’ pattern | ||||||
| All-cause of death, N (%) | 825 (16.62) | 949 (19.12) | 1,006 (20.27) | 1,090 (21.96) | 1,093 (22.02) | |
| Person-years | 55,307.99 | 53,588.89 | 52,618.99 | 51,385.39 | 50,934.99 | |
| Age-adjusted model | 1 | 0.90 (0.82–0.99) | 0.90 (0.81–0.98) | 0.89 (0.81–0 .98) | 0.86 (0.78–0.94) | 0.004 |
| Fully adjusted model | 1 | 0.96 (0.88–1.06) | 0.94 (0.89–0.99) | 0.91 (0.83–0 .99) | 0.90 (0.82–0.98) | 0.007 |
| ‘Western’ pattern | ||||||
| All-cause of death, N (%) | 1,047 (21.10) | 1,069 (21.54) | 1,037 (20.89) | 961 (19.36) | 849 (17.11) | |
| Person-years | 52,029.39 | 51,462.89 | 52,213.09 | 53,409.99 | 54,720.89 | |
| Age-adjusted model | 1 | 1.11 (1.02–1.21) | 1.15 (1.06–1.25) | 1.21 (1.11–1.32) | 1.38 (1.26–1.51) | < 0.001 |
| Fully adjusted model | 1 | 1.07 (0.98–1.17) | 1.10 (1.01–1.19) | 1.12 (1.02–1.22) | 1.22 (1.11–1.34) | 0.020 |
| ‘Traditional’ pattern | ||||||
| All-cause of death, n (%) | 806 (16.24) | 783 (15.78) | 909 (18.32) | 1,028 (20.71) | 1,437 (28.95) | |
| Person-years | 55,049.09 | 55,459.09 | 54,048.29 | 52,586.49 | 46,693.29 | |
| Age-adjusted model | 1 | 1.03 (0.95 1.12) | 1.03 (0.95–1.13) | 1.15 (1.05–1.29) | 1.32 (1.21–1.44) | < 0.001 |
| Fully adjusted model | 1 | 0.99 (0.91–1.07) | 0.94 (0.86–1.02) | 1.04 (0.95–1.14) | 1.16 (1.06 1.27) | 0.011 |
| ‘Fish and alcohol’ pattern | ||||||
| All-cause of death, n (%) | 1,491(30.04) | 1,120 (22.57) | 886 (17.85) | 733 (14.77) | 733 (14.77) | |
| Person-years | 44,877.99 | 51,143.39 | 54,655.29 | 56,900.79 | 56,258.79 | |
| Age-adjusted model | 1 | 0.99 (0.92–1.06) | 0.95 (0.88–1.02) | 0.96 (0.89–1.03) | 0.94 (0.87–1.01) | 0.010 |
| Fully adjusted model | 1 | 0.92 (0.85–0.99) | 0.89 (0.78–1.01) | 0.93 (0.86–1.00) | 0.96 (0.89–1.03) | 0.037 |
Fully adjusted model: Adjusted for age, sex, energy intake, smoking status, BMI and chronic diseases (diabetes, stroke, hypertension, ischemic heart disease, and cancer)
Dietary patterns and mortality due to cancer
In this study, there were 1,077 cancer deaths over the study period. Participants with the highest consumption of the ‘western’ pattern had the highest risk of cancer mortality in the age-adjusted model (5th vs. 1st quintile HR 1.34, 95% CI 1.16–1.52). In the fully adjusted model, the positive association between the highest adherence to the ‘western’ pattern and cancer mortality remained significant after accounting for demographic characteristics and comorbidities (5th vs. 1st quintile HR 1.33, 95% CI 1.10–1.62). Similar results were found among those with the highest consumption to the ‘traditional’ pattern in age-adjusted model (5th vs. 1st quintile HR 1.17, 95% CI 1.06–1.28), and the fully adjusted model (5th vs. 1st quintile HR 1.15, 95% CI 1.06–1.24). The associations between adherence to the ‘prudent’ or the ‘fish and alcohol’ pattern and mortality due to cancer in the age-adjusted and fully adjusted models were not statistically significant. The HR and 95% CI for mortality due to cancer according to baseline adherence to the four dietary patterns are shown in Table 5.
Table 5.
Hazard ratios for mortality due to cancer according to quintiles of adherence categories of dietary patterns in NHANES III
| Quintiles of dietary pattern consumption | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
| ‘Prudent’ pattern | ||||||
| Death due to cancer, N (%) | 169 (15.69) | 213 (19.78) | 213 (19.78) | 241 (22.38) | 241 (22.38) | |
| Person-years | 55,307.99 | 53,588.89 | 52,618.99 | 51,385.39 | 50,934.99 | |
| Age-adjusted model | 1 | 1.03 (0.84–1.27) | 1.01 (0.82–1.24) | 1.00 (0.82–1.22) | 1.04 (0.85–1.27) | 0.854 |
| Fully adjusted model | 1 | 1.08 (0.88–1.32) | 1.05 (0.86–1.29) | 1.02 (0.84–1.25) | 1.07 (0.87–1.30) | 0.919 |
| ‘Western’ pattern | ||||||
| Death due to cancer, N (%) | 218 (20.24) | 213 (19.68) | 225 (20.89) | 241 (18.29) | 225 (20.89) | |
| Person-years | 52,029.39 | 51,462.89 | 52,213.09 | 53,409.99 | 54,720.89 | |
| Age-adjusted model | 1 | 1.05 (0.87–1.27) | 1.11 (1.08–1.15) | 1.16 (0.05–1.27) | 1.34 (1.16–1.52) | < 0.001 |
| Fully adjusted model | 1 | 1.06 (0.88–1.28) | 1.02 (0.84–1.23) | 1.17 (0.06–1.28) | 1.33 (1.10–1.62) | 0.789 |
| ‘Traditional’ pattern | ||||||
| Death due to cancer, n (%) | 196 (18.20) | 213 (15.41) | 211 (19.59) | 241 (21.73) | 270 (25.07) | |
| Person-years | 55,049.09 | 55,459.09 | 54,048.29 | 52,586.49 | 46,693.29 | |
| Age-adjusted model | 1 | 0.98 (0.81–1.19) | 0.99 (0.81–1.21) | 1.15 (1.05–1.20) | 1.17 (0.06–1.28) | 0.012 |
| Fully adjusted model | 1 | 1.01 (0.83–1.20) | 0.97 (0.80–1.18) | 1.14 (1.05–1.19) | 1.15 (1.06–1.24) | 0.829 |
| ‘Fish and alcohol’ pattern | ||||||
| Death due to cancer, n (%) | 301 (27.95) | 213 (20.71) | 201 (18.66) | 241 (15.32) | 187 (17.36) | |
| Person-years | 44,877.99 | 51,143.39 | 54,655.29 | 56,900.79 | 56,258.79 | |
| Age-adjusted model | 1 | 0.88 (0.74–1.05) | 0.88 (0.73–1.05) | 0.86 (0.72–1.00) | 0.89 (0.74 1.07) | 0.015 |
| Fully adjusted model | 1 | 0.86 (0.72–1.02) | 0.84 (0.68–1.00) | 0.80 (0.61–0.99) | 0.93 (0.80–1.08) | 0.627 |
Fully adjusted model: Adjusted for age, sex, energy intake, smoking status, BMI and chronic diseases (diabetes, stroke, hypertension, ischemic heart disease, and cancer)
Discussion
Most studies in the United States have focused on investigating associations between specific dietary factors and chronic diseases [12–14].The association between the adherence dietary patterns and total mortality and mortality due to cancer in the general population is still limited. Dietary patterns condense information about food consumption, reflect diet composition, and capture the overall effect of dietary exposures on health and disease. This study evaluated the association between dietary patterns, total mortality, and cancer mortality in the NHANES III adult cohort in the U.S. Four previously described dietary patterns were found in this study at baseline. The ‘prudent’ dietary pattern, with high intakes of vegetables, fish, fruits, and legumes [29–33]; the ‘western’ dietary pattern, rich in sweets and oils, and low in fruits and vegetables [29–33]; the ‘traditional’ dietary pattern, rich in bread, legumes, eggs; [34, 35]; and the dietary pattern characterized by high consumption of alcoholic beverages [36, 37]. On average, those with the highest consumption of the ‘prudent’ dietary pattern were more likely to adhere to healthier behaviors and less likely to report a previous history of chronic diseases and cancer, in contrast with the participants with the highest consumption of the other patterns. Previous studies support this evidence and demonstrate a correlation between dietary behaviors and lifestyle choices [38, 39].
Our findings confirmed the hypothesis that dietary patterns characterized by consuming nutrient-rich foods are associated with lower total mortality, and dietary patterns that reflect poor nutritional habits are associated with higher total mortality. Herein, the lowest risk of total mortality was among those with the highest adherence to the ‘prudent’ dietary pattern. The ‘prudent’ pattern is characterized by consuming nutrient-rich foods high in antioxidants and vitamins. Prudent diets contain a complex combination of antioxidant and prooxidant elements that can modify the human body’s oxidative status. The oxidative stress resulting from imbalances between reactive oxygen species and the antioxidant defense is a common factor in many pathological conditions [40]. Oxidative stress has been related to cardiovascular disease, cancer, and other chronic diseases that account for a significant portion of deaths in the United States [41].
In this study, participants with the highest consumption of the ‘western’ dietary pattern showed the highest risk of total mortality. The ‘western’ dietary pattern with high consumption of sweets and oils and low consumption of vegetables and fruits was associated with an increased risk of chronic diseases and cancer, probably due to micronutrient deficiencies that lead to several pathophysiological events increase the risk of chronic disease. Westernized diets are known to be deficient in magnesium, zinc, folate, and vitamins C, E, and K [42, 43]. Deficiencies in the micronutrients above were previously related to metabolic syndrome [44], cardiovascular disease [45, 46], and coronary heart disease [47, 48], which are principal causes of mortality in the U.S.
Our findings did not show an association between the ‘traditional’ or the ‘fish and alcohol’ pattern and total mortality. The ‘traditional’ dietary pattern has not been extensively studied in the United States, where traditional foods, inherited from the native communities, served as the foundation for the contemporary diet. Nonetheless, acculturation led to increasing consumption of a westernized diet in native communities, increasing chronic diseases [49]; therefore, more research is needed to understand the factors that influence the adherence to the ‘traditional’ diet and its effects on health and mortality. Alcohol consumption has been previously linked with increased mortality; nonetheless, the harmful effects depend on the level of alcohol consumption and the amount of time that individuals consume alcoholic beverages [50, 51]. Furthermore, these effects depend on individuals’ characteristics, such as their age and cardiovascular health [52, 53]. In line with these findings, our study showed that individuals with the highest adherence to the alcohol pattern were younger and less likely to have a previous history of chronic diseases and cancer. Moreover, the harmful effects of alcohol consumption, for those with the highest adherence to the ‘fish and alcohol’ dietary pattern, may have been compensated by the high intake of fish and seafood, which has been linked with positive health outcomes such as reduced cardiovascular risk [54], and all-cause mortality [55].
We partially confirmed our second hypothesis and provided evidence that dietary patterns that reflect lower quality nutrition are associated with higher cancer mortality. Our findings suggest that participants with the highest consumption of the ‘western’ and the ‘traditional’ dietary pattern had the highest risk of mortality due to cancer, probably due to the high consumption of fats. Carrol and colleagues reviewed experimental and epidemiological studies on the role of dietary fat and cancer. Their findings suggested that high‐fat diets increase cancer risk, and total dietary fat correlates with cancer mortality [56]. Similar findings were found across different types of cancers [57, 58]. This study found no association between adherence to the ‘prudent’ or the ‘fish and alcohol’ pattern and cancer mortality. Diets rich in fruits and vegetables are widely considered beneficial to health since they contain antioxidants, vitamins, and dietary fiber responsible for the benefits.
Nonetheless, the results of several studies have been inconsistent in the association between the consumption of fruits and vegetables and cancer risk. Additionally, a recent meta-analysis indicated that higher consumption of fruits and vegetables was not significantly associated with cancer mortality risk [59]. The evidence presented herein suggests that efforts to increase consumption of a ‘prudent’ dietary pattern may reduce the risk of total mortality, and the benefit for cancer mortality remains possible.
Strengths and limitations
This study’s key strengths include the long length of follow-up time and the large nationally representative sample of adults in the United States that were included. Furthermore, it is the first study investigating the role of adherence to dietary patterns and total mortality, and cancer mortality in the United States, to the best of our knowledge. Nonetheless, the study has limitations. First, the study used self-reported data that could be subject to recall bias due to the lack of medical records validation to confirm the medical diagnoses. Second, residual confounding may also be a limitation due to unmeasured socioeconomic variables. Finally, the study analyzed baseline dietary information and did not capture information about diet changes over time.
Conclusion
In this nationally representative cohort, adherence to the ‘western’ and the ‘traditional’ dietary pattern was strongly associated with an increased risk of total mortality and mortality due to cancer. The ‘prudent’ dietary pattern was associated with a decreased risk of total mortality, but not due to cancer. The evidence presented herein suggests that efforts to increase consumption of a ‘prudent’ dietary pattern may reduce the risk of total mortality, and the benefit for cancer mortality remains possible.
Author contributions
ME was responsible for the study design, statistical analysis, and writing the paper. DS was responsible for providing comments on the writing, methodology, and results. RC was responsible for supervising the work. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
As determined by the Institutional Review Board at the University of California, Merced, the use of public datasets with de-identified information does not constitute research with human subjects. There was no interaction with the participants, and no identifiable private information was used; therefore, human subjects’ approval was not required for this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pomerleau J, McKee M, Lobstein T, et al. The burden of disease attributable to nutrition in Europe. Public Health Nutr. 2003;6:453–461. doi: 10.1079/PHN2002456. [DOI] [PubMed] [Google Scholar]
- 2.Lock K, Pomerleau J, Causer L, et al. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ. 2005;83:100–108. [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization, 2009.
- 4.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Moeller SM, Reedy J, Millen AE, et al. Dietary patterns: challenges and opportunities in dietary patterns research. J Am Diet Assoc. 2007;107:1233–1239. doi: 10.1016/j.jada.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Tapsell LC, Neale EP, Satija A, et al. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. 2016;7:445–454. doi: 10.3945/an.115.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michels KB, Schulze MB. Can dietary patterns help us detect diet–disease associations? Nutr Res Rev. 2005;18:241–248. doi: 10.1079/NRR2005107. [DOI] [PubMed] [Google Scholar]
- 8.Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ. 2009 doi: 10.1136/bmj.b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckland G, Agudo A, Travier N, et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Br J Nutr. 2011;106:1581–1591. doi: 10.1017/S0007114511002078. [DOI] [PubMed] [Google Scholar]
- 10.Michels KB, Wolk A. A prospective study of variety of healthy foods and mortality in women. Int J Epidemiol. 2002;31:847–854. doi: 10.1093/ije/31.4.847. [DOI] [PubMed] [Google Scholar]
- 11.Huijbregts P, Feskens E, Räsänen L, et al. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and the Netherlands: longitudinal cohort study. BMJ. 1997;315:13–17. doi: 10.1136/bmj.315.7099.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aqeel MM, Guo J, Lin L, et al. Temporal dietary patterns are associated with obesity in US adults. J Nutr. 2020;150:3259–3268. doi: 10.1093/jn/nxaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeCroy MN, Nicastro HL, Truesdale KP, et al. Dietary patterns and associations with BMI in low-income, ethnic minority youth in the USA according to baseline data from four randomised controlled trials. Br J Nutr. 2020;126:1–11. doi: 10.1017/S0007114520003852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunner EJ, Mosdøl A, Witte DR, et al. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr. 2008;87:1414–1421. doi: 10.1093/ajcn/87.5.1414. [DOI] [PubMed] [Google Scholar]
- 15.Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104:615–635. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Reedy J, Krebs-Smith SM, Miller PE, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144:881–889. doi: 10.3945/jn.113.189407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinyemiju T, Moore JX, Pisu M, et al. A prospective study of dietary patterns and cancer mortality among Blacks and Whites in the REGARDS cohort. Int J Cancer. 2016;139:2221–2231. doi: 10.1002/ijc.30287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in adventist health study 2. JAMA Intern Med. 2013;173:1230–1238. doi: 10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly JT, Palmer SC, Wai SN, et al. Healthy dietary patterns and risk of mortality and ESRD in CKD: a meta-analysis of cohort studies. CJASN. 2017;12:272–279. doi: 10.2215/CJN.06190616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson AL, Harris TB, Tylavsky FA, et al. Dietary patterns and survival of older adults. J Am Diet Assoc. 2011;111:84–91. doi: 10.1016/j.jada.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1 1994; 1–407. [PubMed]
- 22.Briefel RR, Sempos CT. Dietary methodology workshop for the third National Health and Nutrition Examination Survey. March 1986. Vital Health Stat 4 1992; 1–108. [PubMed]
- 23.Woteki CE, Briefel R, Hitchcock D, et al. Selection of nutrition status indicators for field surveys: the NHANES III design. J Nutr. 1990;120:1440–1445. doi: 10.1093/jn/120.suppl_11.1440. [DOI] [PubMed] [Google Scholar]
- 24.Alonso A, Beunza JJ, Bes-Rastrollo M, et al. Vegetable protein and fiber from cereal are inversely associated with the risk of hypertension in a Spanish cohort. Arch Med Res. 2006;37:778–786. doi: 10.1016/j.arcmed.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Ivanovitch K, Klaewkla J, Chongsuwat R, et al. The intake of energy and selected nutrients by Thai urban sedentary workers: an evaluation of adherence to dietary recommendations. J Nutr Metab. 2014;2014:e145182. doi: 10.1155/2014/145182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamura T, Hashimoto Y, Miki A, et al. High brain natriuretic peptide is associated with sarcopenia in patients with type 2 diabetes: a cross-sectional study of KAMOGAWA-DM cohort study. Endocr J. 2019;66:369–377. doi: 10.1507/endocrj.EJ19-0024. [DOI] [PubMed] [Google Scholar]
- 27.USDA Nutrient Data Laboratory|Food and Nutrition Information Center|NAL|USDA, https://www.nal.usda.gov/fnic/usda-nutrient-data-laboratory. Accessed 17 Aug 2020.
- 28.ICD-10 Resources | CMS, https://www.cms.gov/Medicare/Coding/ICD10/ICD-10Resources. Accessed 8 March 2021.
- 29.van Dam RM, Rimm EB, Willett WC, et al. Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann Intern Med. 2002;136:201–209. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 30.Fung T, Hu FB, Fuchs C, et al. Major dietary patterns and the risk of colorectal cancer in women. Arch Intern Med. 2003;163:309–314. doi: 10.1001/archinte.163.3.309. [DOI] [PubMed] [Google Scholar]
- 31.Kerver JM, Yang EJ, Bianchi L, et al. Dietary patterns associated with risk factors for cardiovascular disease in healthy US adults. Am J Clin Nutr. 2003;78:1103–1110. doi: 10.1093/ajcn/78.6.1103. [DOI] [PubMed] [Google Scholar]
- 32.Varraso R, Fung TT, Hu FB, et al. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62:786–791. doi: 10.1136/thx.2006.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velie EM, Schairer C, Flood A, et al. Empirically derived dietary patterns and risk of postmenopausal breast cancer in a large prospective cohort study. Am J Clin Nutr. 2005;82:1308–1319. doi: 10.1093/ajcn/82.6.1308. [DOI] [PubMed] [Google Scholar]
- 35.Noel SE, Newby PK, Ordovas JM, et al. A traditional rice and beans pattern is associated with metabolic syndrome in puerto rican older adults. J Nutr. 2009;139:1360–1367. doi: 10.3945/jn.109.105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shikany J, Safford M, Newby PK, et al. Southern Dietary pattern is associated with hazard of acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terry P, Bergkvist L, Holmberg L, et al. No association between Fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:913–914. [PubMed] [Google Scholar]
- 38.Thiele S, Mensink GB, Beitz R. Determinants of diet quality. Public Health Nutr. 2004;7:29–37. doi: 10.1079/PHN2003516. [DOI] [PubMed] [Google Scholar]
- 39.Marques-Vidal P, Waeber G, Vollenweider P, et al. Sociodemographic and behavioural determinants of a healthy diet in Switzerland. ANM. 2015;67:87–95. doi: 10.1159/000437393. [DOI] [PubMed] [Google Scholar]
- 40.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 41.WHO reveals leading causes of death and disability worldwide: 2000–2019, https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019. Accessed 16 Feb 2021.
- 42.McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr. 2009;90:889–907. doi: 10.3945/ajcn.2009.27930. [DOI] [PubMed] [Google Scholar]
- 43.Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. PNAS. 2006;103:17589–17594. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ford ES, Li C, McGuire LC, et al. Intake of dietary magnesium and the prevalence of the metabolic syndrome among US Adults. Obesity. 2007;15:1139–1146. doi: 10.1038/oby.2007.628. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen FH, Milne DB, Klevay LM, et al. Dietary magnesium deficiency induces heart rhythm changes, impairs glucose tolerance, and decreases serum cholesterol in post menopausal women. J Am Coll Nutr. 2007;26:121–132. doi: 10.1080/07315724.2007.10719593. [DOI] [PubMed] [Google Scholar]
- 46.Hatzistavri LS, Sarafidis PA, Georgianos PI, et al. Oral magnesium supplementation reduces ambulatory blood pressure in patients with mild hypertension. Am J Hypertens. 2009;22:1070–1075. doi: 10.1038/ajh.2009.126. [DOI] [PubMed] [Google Scholar]
- 47.Beulens JWJ, Bots ML, Atsma F, et al. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203:489–493. doi: 10.1016/j.atherosclerosis.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Gast GCM, de Roos NM, Sluijs I, et al. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009;19:504–510. doi: 10.1016/j.numecd.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Colby S, McDonald L, Adkison G. Traditional native american foods: stories from northern plains elders. J Ecol Anthropol. 2012;15:65–73. [Google Scholar]
- 50.White IR, Altmann DR, Nanchahal K. Alcohol consumption and mortality: modelling risks for men and women at different ages. BMJ. 2002;325:191. doi: 10.1136/bmj.325.7357.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camargo CA, Hennekens CH, Gaziano JM, et al. Prospective study of moderate alcohol consumption and mortality in US male physicians. Arch Intern Med. 1997;157:79–85. doi: 10.1001/archinte.1997.00440220083011. [DOI] [PubMed] [Google Scholar]
- 52.Thun MJ, Peto R, Lopez AD, et al. Alcohol consumption and mortality among middle-aged and elderly US adults. New Engl J Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs CS, Stampfer MJ, Colditz GA, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–1250. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 54.Mizushima S, Moriguchi EH, Ishikawa P, et al. Fish intake and cardiovascular risk among middle-aged Japanese in Japan and Brazil. J Cardiovasc Risk. 1997;4:191–199. doi: 10.1097/00043798-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Takata Y, Zhang X, Li H, et al. Fish intake and risks of total and cause-specific mortality in 2 population-based cohort studies of 134,296 men and women. Am J Epidemiol. 2013;178:46–57. doi: 10.1093/aje/kws584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carroll KK, Braden LM, Bell JA, et al. Fat and cancer. Cancer. 1986;58:1818–1825. doi: 10.1002/1097-0142(19861015)58:8+<1818::AID-CNCR2820581406>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 57.Sasaki S, Horacsek M, Kesteloot H. An ecological study of the relationship between dietary fat intake and breast cancer mortality. Prev Med. 1993;22:187–202. doi: 10.1006/pmed.1993.1016. [DOI] [PubMed] [Google Scholar]
- 58.Rose DP, Boyar AP, Wynder EL. International comparisons of mortality rates for cancer of the breast, ovary, prostate, and colon, and per capita food consumption. Cancer. 1986;58:2363–2371. doi: 10.1002/1097-0142(19861201)58:11<2363::AID-CNCR2820581102>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014 doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.


