Abstract
Since the early descriptions of language function based on observations of patients with language deficits by Broca and Wernicke, neurosurgeons have been focused on characterizing the anatomic regions necessary for language perception and production, and preserving these structures during surgery to minimize patient deficits post operatively. In this supplementary issue on awake intraoperative mapping, we review language processing across multiple domains, highlighting key advances in direct electrical stimulation of different cortical and subcortical regions involved in naming, repetition, reading, writing, and syntax. We then discuss different intraoperative tasks for assessing the function of a given area and avoiding injury to critical, eloquent regions.
ABBREVIATIONS
- AF
arcuate fasciculus
- AFp
posterior segment of the arcuate fasciculus
- AN
auditory naming
- BPTC
basal posterotemporal cortex
- DES
direct electrical stimulation
- ECoG
electrocorticography
- FAT
frontal aslant tract
- fMRI
functional magnetic resonance imaging
- IFG
inferior frontal gyrus
- IFOF
inferior fronto-occipital fasciculus
- ILF
inferior longitudinal fasciculus
- ITG
inferior temporal gyrus
- MFG
middle frontal gyrus
- MTG
middle temporal gyrus
- NWR
nonword repetition
- PN
picture naming
- pSTG
posterior superior temporal gyrus
- SLF
superior longitudinal fasciculus
- SMG
supramarginal gyrus
- Spt
Sylvian-parietal-temporal
- STG
superior temporal gyrus
- TSA
transcortical sensory aphasia
- UF
uncinate fasciculus
- VWFA
visual word form area
- WM
white matter
- WR
word repetition
Preserving language function in resective brain surgery is a principal tenant of neurosurgery. The last several decades has witnessed a surge in publications investigating how networks in the human brain subserve the complexity of human language. Modern advances in functional imaging have rapidly broadened our understanding of language processing to encompass a multitude of key language functions. Intraoperative mapping, however, remains central in validating these findings and translating their clinical impact. For example, reliance on functional magnetic resonance imaging (fMRI) alone is inadequate for preoperative surgical planning. Although fMRI may be helpful for lateralization of language, it lacks sufficient spatial resolution and is unable to discriminate between redundant and compensatory network activity due to tumor progression. As detailed in the following review, the use of varied intraoperative language tasks affords increased sensitivity to essential language regions.
As part of a special issue on awake language mapping, we describe key direct electrical stimulation (DES) experiments and their respective intraoperative tasks for naming, reading, writing, repetition, and syntax. Supportive literature from functional imaging, transcranial magnetic stimulation, or electrocorticography (ECoG) will not be discussed in detail. We highlight key anatomic structures to consider for language preservation in surgical planning and we describe common postoperative aphasias and their rates of recovery.
DOMAINS OF LANGUAGE
See Tables 1 and 2 and Figures 1–7 for a summary of the tasks and anatomic regions studied by DES experiments.
Table 1.
Summary of Locations and Intraoperative Tasks Performed During DES
| Region | Task | Error | Selected references |
|---|---|---|---|
| Frontal lobe | |||
| Pars orbitalis | PN | Semantic paraphasia | 18 |
| Pars opercularis + triangularis | Picture description | Syntactic errors | 58 |
| Exner's | Writing | Letter omission, writing arrest, illegible script | 51,67 |
| Temporal lobe | |||
| AF | Word and pseudoword repetition | Phonological disorder, conduction aphasia | 29,33 |
| Naming | Anomia, phonemic paraphasia | 13,18 | |
| AFp | Irregular and pseudoword reading | Lexical and phonological alexia | 47 |
| Naming | Phonological paraphasia | 18 | |
| ILF | Regular word reading | Semantic paraphasia | 18 |
| ILFp | Reading | Global alexia | 47 |
| IFOF | Naming | Semantic paraphasia | 13,45 |
| Repetition | Semantic paraphasia | 29 | |
| UF | Naming | Semantic paraphasia | 18 |
| Anterior > posterior | AN > PN | Anomia | 11,68 |
| Mid fusiform gyrus | AN and PN | Anomia | 69 |
| Reading | Alexia | 45,46 | |
| pSTS | PN | Phonological paraphasia, neologisms, circumlocutions | 6 |
| aSTG | AN | Receptive aphasia | 7 |
| pSTG | Repetition | Conduction aphasia | 30-32 |
| STG | Irregular word reading | Lexical alexia | 41 |
| Writing | Agraphia | 53 | |
| MTG | AN and PN | Semantic paraphasia | 70 |
| Parietal lobe | |||
| AF | Word and pseudoword repetition | Conduction aphasia | 29 |
| Spt | Word and pseudoword repetition | Conduction aphasia | 32 |
| Writing pseudowords | Phonological agraphia | 53 | |
| SMG | PN | Semantic paraphasia/performance error | 6 |
| Repetition | Conduction aphasia | 32 | |
| Pseudoword reading | Phonological alexia | 41 | |
| Writing | Agraphia | 53 | |
| Angular | Writing | Agraphia | 52,53 |
| Insular lobe | |||
| IFOF | PN and AN | 18 | |
| AF | PN and AN | 18 | |
AF: arcuate fasciculus, AN: auditory naming, aSTG: anterior superior temporal gyrus, DES: direct electrical stimulation, IFOF: inferior frontal occipital fasciculus, ILFp: posterior inferior longitudinal fasciculus, MTG: middle temporal gyrus, PN: picture naming, pSTG: posterior superior temporal gyrus, pSTS: posterior superior temporal sulcus, SMG: supramarginal gyrus, Spt: sylvian-parietal-temporal, UF: uncinate fasciculus.
Table 2.
Summary of Brain Regions Implicated in Each Task With DES
| Region | Task | Error | Selected reference |
|---|---|---|---|
| Naming | |||
| Pars orbitalis | PN | Semantic paraphasia | 18 |
| Anterior > posterior temporal lobe | AN > PN | Anomia | 11,68 |
| pSTS | PN | Phonological paraphasia, neologisms, circumlocutions | 6 |
| aSTG | AN | Receptive aphasia | 7 |
| MTG | AN and PN | Semantic paraphasia | 70 |
| SMG | PN | Semantic paraphasia/performance error | 6 |
| Mid fusiform gyrus | AN and PN | Anomia | 69 |
| AF | Anomia, phonemic paraphasia | 13,18 | |
| SLF | Phonological paraphasia | 18 | |
| IFOF | Semantic paraphasia | 13,45 | |
| UF | Semantic paraphasia | 18 | |
| Repetition | |||
| pSTG | Conduction aphasia | 30-32 | |
| Spt | Word and pseudoword repetition | Conduction aphasia | 32 |
| SMG | Conduction aphasia | 32 | |
| AF | Word and pseudoword repetition | Phonological disorder, conduction aphasia | 29,33 |
| IFOF | Semantic paraphasia | 29 | |
| Reading | |||
| STG | Irregular word reading | Lexical alexia | 41 |
| Mid fusiform gyrus | Alexia | 45,46 | |
| SMG | Pseudoword reading | Phonological alexia | 41 |
| AFp | Irregular and pseudoword reading | Lexical and phonological alexia | 47 |
| ILF | Semantic paraphasia | 18 | |
| ILFp | Global alexia | 47 | |
| Writing | |||
| Exner's | Letter omission, writing arrest, illegible script | 51,67 | |
| SMG | Agraphia | 53 | |
| Angular | Agraphia | 52,53 | |
| Spt | Writing pseudowords | Phonological agraphia | 53 |
| STG | Agraphia | 53 | |
| Syntax | |||
| Pars opercularis + triangularis | Picture description | 58 | |
AFp: posterior segment of the arcuate fasciculus, AN: auditory naming, aSTG: anterior superior temporal gyrus, DES: direct electrical stimulation, IFOF: inferior frontal occipital fasciculus, ILFp: posterior inferior longitudinal fasciculus, MTG: middle temporal gyrus, PN: picture naming, pSTG: posterior superior temporal gyrus, pSTS: posterior superior temporal sulcus, SMG: supramarginal gyrus, Spt: sylvian-parietal-temporal, UF: uncinate fasciculus.
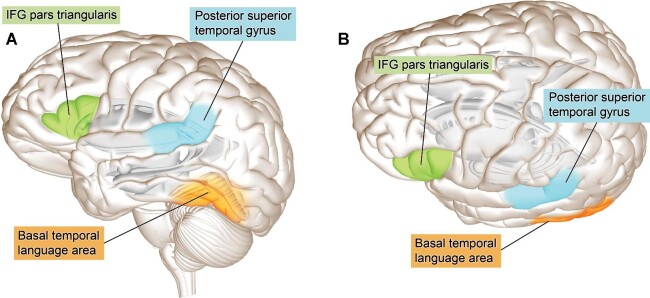
Figure 1.
Naming. A, Top down and B, lateral projections of the WM tracts and cortical regions involved in naming, typically tested with a PN task.
Figure 7.
Motor. A, Top down and B, lateral projections of the WM tracts and cortical regions involved in motor mapping.
Naming
Picture naming (PN) is the most widely used task in intraoperative language mapping.1,2 Patients are shown line drawings of common objects,3 and positive DES sites were preserved during tumor and epilepsy surgery of surgery.4
In one study, 58% of patients had at least one cortical site with stimulation-induced PN deficit.5 Subsequent studies have mapped naming errors to specific cortical regions for improving language mapping outcomes and understanding human language processing systems.
Corina et al aggregated PN errors into 6 subtypes: semantic paraphasias (tiger → “lion”), circumlocutions (belt → “used to keep pants up”), phonological paraphasias (deletions or substitutions of syllables), neologisms (made-up words), performance errors (slurred or stuttered responses), and no response errors.6 DES of the posterior supramarginal gyrus (SMG), a region that is within the dorsal production stream, evoked significantly more performance errors. In contrast, DES of the posterior middle temporal gyrus (MTG) and anterior SMG, both regions of the ventral lexical-semantic stream, resulted in semantic paraphasias, while DES of the posterior superior temporal sulcus resulted in phonological paraphasias, neologisms, and circumlocutions.
Naming can also occur with auditory inputs in the absence of visual stimuli. In auditory naming (AN), participants name an object upon hearing a description: eg, “A fruit that makes wine.” “Grape.” An increasing number of studies have shown that AN may be more sensitive to detecting naming impairments in naming during intraoperative mapping, and performance on AN tasks may be more correlated with real-world, postoperative word-finding difficulties.7-9
Both PN and AN have been associated with the dominant temporal lobe, with a third of anterior temporal lobectomy patients exhibiting reduced naming performance.9,10 Early DES experiments have supported differential localization of AN and PN. In a series of studies by Hamberger and colleagues, stimulation of the anterior temporal lobe resulted in preferential deficits in AN, whereas stimulation of the posterior temporal region resulted in equal AN and PN deficits.11 The authors found that perceptive AN errors clustered in the middle portion of the superior temporal gyrus (STG), whereas expressive sites largely fell outside of this region.7 Interestingly, removal of AN sites resulted in both AN and PN deficits despite preservation of PN sites in all patients. This strongly argues for the inclusion of AN tasks in intraoperative language mapping protocols, which historically has been less utilized compared to PN.8
Subcortical stimulation mapping of white matter (WM) tracts has identified several language tracts critical in naming, again in a manner consistent with the dual stream model of language processing (Figure 1). The dorsal stream subserved by the superior longitudinal fasciculus (SLF), while the ventral stream with 2 separate pathways: (1) the direct ventral pathway, which comprised the inferior fronto-occipital fascicle (IFOF) that connects the occipital lobe, parietal lobe, and the postero-temporal cortex with the frontal lobe, and (2) the indirect ventral pathway, which comprised the inferior longitudinal fasciculus (ILF; running below the IFOF) that connects the posterior occipito-temporal region and the temporal pole, with hypothesized relays to frontal regions via the uncinate fasciculus (UF).12 Stimulation of the arcuate fasciculus (AF) at the level of the superior and posterior rim of the superior insular sulcus has resulted in phonemic paraphasia, while stimulation of the IFOF within the roof of the temporal horn of the ventricle and the extreme capsule under the limen insulae specifically has elicited semantic paraphasia.13-17 Stimulation of ILF and UF has also been shown to cause semantic paraphasias.18
Figure 2.
Word/sentence repetition. A, Top down and B, lateral projections of the WM tracts and cortical regions involved in word/sentence repetition, typically tested with word and pseudoword repetition.
Figure 3.
Phonological reading. A, Top down and B, lateral projections of the WM tracts and cortical regions involved in phonological reading, typically tested by reading pseudowords.
Figure 4.
Lexically semantic reading. A, Top down and B, lateral projections of the WM tracts and cortical regions involved in lexical-semantic reading, typically tested by reading irregular words.
Figure 5.
Syntax. A, Frontal and B, lateral projections of the WM tracts and cortical regions involved in syntax, typically tested by sentence generation/completion tasks.
Figure 6.
Writing. A, Top down and B, lateral projections of the WM tracts and cortical regions involved in writing.
Repetition
Conduction aphasia, first described in 1874 by Wernicke, is a syndrome with pervasive phonological paraphasia and severe impairment in verbatim repetition with otherwise largely fluent natural speech production and perception.19-22 The classical Wernicke-Geschwind model of language posits damage to left hemisphere WM tracts, particularly the AF connecting inferior frontal cortex with posterior superior temporal cortex, to be the primary driver in verbal repetition.23
The specific WM tracts that subserve the dorsal pathway are the 3 bundles of the SLF24,25: the most medial direct pathway corresponding to the AF, which connects the middle and inferior temporal gyri with the precentral and inferior frontal gyri, and 2 more lateral indirect pathways—an anterior portion (SLFIII) connecting the ventral premotor cortex with the SMG, and the posterior portion (SLFII) connecting angular gyrus with the posterior temporal area. The AF and SLFIII join together in connecting the cortical regions recruited in word repetition (WR).26-28 Consistent with subcortical stimulation for naming, subcortical stimulation of the AF can induce phonological and articulatory disorders during WR. DES of IFOF either caused no deficits or produced semantic paraphasias related to the target word.29
Conduction aphasia likely can be caused by more than just damage to the AF. Anderson et al and Quigg and Fountain in 1999 published DES studies whereby stimulation of the posterior STG (pSTG) and SMG resulted in patients with decreased repetition of words and intact semantic knowledge.30-32 The use of pseudowords may be a confounding factor to these experiments, as repetition of known words may theoretically be compensated by lexical retrieval pathways in the ventral stream, raising the possibility that non-word repetition (NWR) tasks may be more sensitive to identifying effective AF function. Indeed, significantly more repetition errors were identified when using NWR than WR tasks intra-operatively.33
Recent studies have begun to shift the attention away from serial modular processing for verbal repetition to a more distributed language network beyond the AF and pSTG. This complexity was illustrated in a recent study by Leonard et al combining ECoG and DES during WR trials. With ECoG, a large peri-Sylvian network was activated by sensorimotor processing throughout all phases of speech perception and production, yet DES to the posterior temporal regions during perceptual phases of repetition did not cause difficulties in motor production of words.19 This combined ECoG and DES study suggests a parallel processing network for verbal repetition that is distributed across many peri-Sylvian and sensorimotor nodes as opposed to a serial, feedforward model of language processing for repetition.19
Reading
Contemporary dual-route models separate reading into 2 pathways: (1) the lexical-semantic pathway for orthographically irregular (ie, irregularly spelled) words and (2) the phonological pathway for unknown or pseudowords. In the lexical-semantic pathway, words are directly recognized as lexicon members and mapped onto verbal semantic representations, whereas in the phonological pathway, or sublexical pathway, words are read using grapheme to phoneme rules (ie, spelling-sound correspondences). Thus, patients with lexical dyslexia have difficulty reading irregular words but not pseudowords, whereas those with phonological dyslexia can read irregular words but not pseudowords.34-38
Ojemann found reading sites that were distinct from naming in temporal-parietal and frontal areas.39 Using PN and reading tasks, Roux et al found reading interference sites in the dominant supramarginal, angular, pSTG, dominant MTG, and dominant inferior frontal gyrus (IFG) and middle frontal gyrus (MFG) that were both distinct and more numerous than naming sites.40 Overall, significantly more reading interference sites were mapped compared to PN interference sites, with the majority (∼70%) of reading sites occurring adjacent to naming sites. Approximately 95% of speech arrest sites were commonly shared between PN and reading tasks, while only 65% of anomia sites during naming tasks resulted in reading deficits. This same study also found that although the overall distribution of naming and reading sites for bilingual patients were no different than monolingual patients, language-specific and reading-specific sites were distributed across temporo-parietal and frontal regions, suggesting a separate anatomic network for processing aspects of a second language.40
To find evidence of cortical sites subserving dorsal phonological and ventral lexical-semantic reading streams, Roux et al then asked patients undergoing awake language mapping to read frequent but irregular French words along with pronounceable pseudowords. Reading irregular words accessed phonological output lexicon and assembled phonological representations within the ventral stream, whereas pseudoword reading required sublexical orthography-to-phonology processing.41 Using this approach, the authors found that stimulation of inferior and anterior portion of left SMG resulted in pseudoword deficits, whereas stimulation of the left STG resulted in word reading deficits. These results provided early evidence that lexical and sublexical processes could be anatomically dissociated in a manner reminiscent of dual-stream theories of language.41
Dissociations between naming and reading have also been found with DES within the left basal posterotemporal cortex (BPTC), which includes the left middle and posterior fusiform gyrus and the posterior third of the inferior temporal gyrus (ITG).42-44 This region has been hypothesized to contain a visual word form area (VWFA), an area containing visual representation of words specifically dedicated to reading. In a small series of patients undergoing awake language mapping for tumor resection, Gil-Robles et al found that stimulation of the BPTC resulted in deficits of reading short sentences but not articulatory or naming difficulties.45 During stimulation, patients frequently reported difficulties with reading words and had to resort to “letter-by-letter” reading. Furthermore, postoperative language assessment of one patient who underwent surgical resection of the left BPTC showed acquired alexia and its characteristic letter-by-letter reading.46 Similarly, stimulation of the left ILF posterior to the VWFA resulted in reading impairment without naming disturbances, again consistent with the BPTC having a role in interfacing between vision and language. In contrast, stimulation of the left IFOF, directly superior to the ILF resulted in semantic paraphasias during PN but not reading errors.
In a separate series of experiments focused on subcortical WM tracts, Zemmoura and colleagues showed that resection of the posterior ILF—a tract demonstrated to feedforward visual input from the occipital lobe to the VWFA—induced global alexia, while stimulation of the anterior VWFA resulted in lexical dyslexia, perhaps supporting a posterior-anterior gradient in transforming information from visual inputs to words within the VWFA. Resection of the posterior segment of the arcuate fasciculus (AFp) caused impairments in both irregular and pseudoword reading, which the authors suggest are supportive of parallel feedback connections between phonological and lexical streams.47
Writing
Because writing subsumes many of the same processes of speech and other language tasks, deficits in writing alone are rare. Termed agraphia, they can exist in multiple forms, including phonological and lexical agraphia. Patients with phonological agraphia are unable to sound out words and thus have impairment in spelling unfamiliar or pseudowords (eg, flig), whereas those with lexical agraphia lose their orthographic memory, that is their ability to visualize words, particularly useful for words that are irregular or ambiguous (eg, breeze vs breze).
In the decades following the discovery of Broca's area as a premotor region rostral to the mouth region of the primary motor, an analogous region dedicated to writing located rostral to the hand area has been hypothesized. First proposed by the Austrian physiologist Sigmund Exner in 1881 as located in the foot of the second frontal convolution (F2), a writing center in F2 (or “Exner's area”) has remained controversial. The bulk of the evidence for an F2 writing center stems from the odd case report of patients who lost their ability to write in specific ways. Anderson and colleagues described a patient whose surgical lesion in Exner's area left her with severe alexia and agraphia specifically to letters but not to numbers.48 Shallice described a patient with severe deficits in writing non-sense words, yet retained the ability to repeat them.49 Penfield and Roberts found sites of writing arrest in and surrounding Exner's area, including a patient with transient writing impairments after removal of a lesion from F2 and F3 regions.1 However, DES experiments have failed to locate an area that produced isolated writing deficits without concomitant impairment in speech or rapid finger movements.50
Results from more recent DES experiments found evidence of writing centers in both the frontal and temporal/supramarginal cortices, supporting a dual-route model for writing similar to that of language and vision. Lubrano et al isolated cortical stimulation sites in IFG and MFG that resulted in writing deficits like letter omissions, writing arrest, or illegible script in an intraoperative task of transcribing dictated texts.51 In a study using DES and fMRI, Roux et al found a writing-specific center corresponding to Exner's, with stimulation of areas rostral to the primary motor hand area impairing handwriting without disturbing hand movements or oral language tasks. The location of frontal sites that resulted in impaired handwriting was more dorsal and posterior to sites involved in naming or speech, as hypothesized by Exner's.
DES experiments have also identified pure agraphia sites in the dominant posterior temporoparietal areas. A series of DES experiments for mapping the angular gyrus uncovered deficits reminiscent of Gerstmann syndrome (ie, agraphia, acalculia, finger agnosia, and left-right dissociation) and found areas that exclusively impaired writing.52 In a separate series of DES experiments for tumor resection, Roux et al found 62 cortical sites in the left temporoparietal lobes of 30 patients that resulted in writing deficits, with 27% of patients experiencing pure agraphia without deficits in sentence reading or naming.53 These errors included both semantic and phonological paraphasias and were predominantly found in the dominant STG, with phonological errors being more distributed than semantic ones. Interestingly, the authors also found sites that resulted in both written and spoken languages, suggesting overlap between language processing required for each task. In a subset of patients with pure dysgraphia symptoms, lexico-semantic errors during writing were mainly elicited by stimulation of the temporal cortex while phonological (sublexical) errors were associated around posterior MTG, STG, and SMG.53
Syntax
Syntactic processing is what allows us to combine words in a way that can ascribe the meaning of sentences. In contemporary language models, syntactic encoding takes the lexical items (“lemmas”) from the mental lexicon and arranges them in a grammatical order that utilizes the stored syntactic information of words which together determines their proper order and inflectional markings in a spoken sentence.54 For example, differences in verb agreement (“the daughters of the colonel who were killed” vs “the daughters of the colonel who was killed”) and thematic roles (“John loves Mary” vs “Mary loves John”) can dramatically alter our understanding of the information conveyed in each sentence.55 Patients with deficits in syntax suffer from agrammatism. Lesion studies have historically suggested Broca's area as the seat of language syntax.
Ojemann and Mateer found several sites resulting in syntactic errors through stimulation of frontal, temporal, and parietal areas.4 The authors stimulated the cortex while having patients read a sentence with a portion near the end of the sentence omitted, requiring the patient to complete it correctly with specific syntactic form. These grammatical reading errors were scattered around the peri-Sylvian networks. Moreover, some lesion-deficit studies have suggested that action naming (ie, verbs), which is more pivotal for correct syntactic structure than object naming (ie, nouns), is located in different regions of the brain. Corina et al also found support for this hypothesis, with more anterior regions in the dominant temporal lobe specific for object naming and more posterior sites specific for action naming.56,57
Chang et al applied DES while asking patients to describe a picture of transitive actions using a complete sentence, and also perform counting, repetition, and PN tasks as controls to ensure stimulation-induced errors were specific to syntax encoding.58 Each picture depicted a boy and a girl engaged in a simple transitive action (eg, push, pull, hug, kick, chase, wash, and kiss), with either the boy or the girl acting as the agent of the action.59
The authors reasoned that errors following stimulation to a particular brain region during sentence production but not repetition, counting, or naming tasks were specific for the syntactic encoding of sentence production. The authors found syntactic encoding errors at sites in the left IFG in 50% of patients that specifically did not result in speech arrest, automatic speech, or world level language function. This frequency of syntactic encoding errors (50%) is similar to the rate of anomia (33%) and speech arrest (58%) found during standard DES.5 Specifically, stimulation of the left pars triangularis and opercularis was more likely to create syntactic errors, perseverations (eg, previous trial action was used to describe the current trial's verb), and retracings (eg, complete words uttered more than once in a row), which may also represent interferences with syntactic encoding than stimulation in the left STG or MTG.
A key methodological discrepancy that may explain the distribution of DES sites between these 2 studies is the use of sentence completion in the Ojemman study and sentence generation from pictures in the Chang protocol. Tasks involving sentence comprehension presumably recruit an additional receptive language component from the larger, distributed language processing network compared to picture description. Future research will need to determine with discrepancies in methodologies can reconcile differences in our understanding of syntactic encoding.
Comprehension
Although language comprehension is essential to communication, remarkably little is understood compared to other language domains. Seen primarily in stroke patients, transcortical sensory aphasia (TSA) is characterized by impaired comprehension with intact repetition and fluent speech.60,61 Boatman et al investigated TSA during awake epilepsy surgery by incorporating single-step commands (eg, “move the green square”) to standard language tests. DES resulted in a pattern of deficits consistent with TSA in sites primarily in posterior temporal cortex (STG, MTG, ITG), temporal-occipital region, and inferior parietal lobe.62 Phonological processing, as assessed by syllable discrimination (ie, distinguishing “bi” vs “ba”), was intact in all TSA sites, and repetition of word and nonwords was not affected. Naming and reading was intact in a subset of TSA sites. These findings argue for a model of TSA that incorporates the inability for phonology to access intact lexical-semantic processing, and underscore comprehension as a distinct language process.
CORTICAL AND WM BOUNDARIES DURING SURGERY
The horizontally organized association fibers and the more vertically oriented cortical-subcortical tracts can be thought of as functional boundaries by surgeons when operating in different brain regions. While the location of these fibers is variable across individuals and the diffusor tensor imaging tractography does not show functional connectivity or eloquent areas, using these tracts when planning a surgical resection is useful and they should be used by surgeons to decide when to resume DES during tumor resections.
Frontal Lobe
The motor system, including the primary motor cortex and corona radiata/internal capsule/corticospinal tract, is critical when considering any resection in the frontal lobe. With regards to language, it is debatable whether the supplemental motor area should be mapped. Although stimulation can result in slowed speech or mutism, these deficits are usually temporary and surgeons should be mindful of prematurely aborting a surgical resection when encountering the frontal aslant tract (FAT), since the deficits invariably recover.63,64
During frontal lobe resections, most surgeons will employ counting, naming, or reading tasks when mapping the IFG to find a safe cortical entry window and then again to map the SLF and the IFOF once they reach the WM tracts beneath the cortical U fibers. SLF stimulation typically produces a phonetic error while IFOF stimulation typically produces a semantic error.65,66 Recent data support careful intraoperative mapping of the pars triangularis during reading of irregular words and mapping of the pars opercularis during reading of pseudowords. The use of picture description to test for syntax can be mapped across the IFG. Exner's area just rostral to primary hand cortex can be investigated intraoperatively for preservation of writing, including legibility, writing arrest, and letter omissions.51
Temporal Lobe
Cortical mapping of the temporal lobe with a PN or AN task is key to identify sites with the STG, MTG, or, less commonly, the ITG critical for speech production. For resections in the temporal lobe, surgeons typically plan resections with the following boundaries in mind: anteriorly the resection can be carried from the anterior extent of the middle cranial fossa. Posteriorly, the limit is usually made by a confluence of tracts, such as the vertical portion of the AF (phonological processing), superiorly and posteriorly the posterior portion of the SLF (articulation) and inferiorly the ILF (for global alexia) and AFp (for irregular and pseudoword reading). An important distinction here is the intraoperative use of regular, irregular (uncommon or irregularly spelled), and pseudo words as each may elicit differing neural processing streams. The deep limit is made up of the IFOF (semantic processing). AN and PN appear to be differentially distributed across the anterior-posterior axis of the temporal lobe, with increased AN sites represented in the anterior portion (<4 cm from temporal pole). If possible, preservation of the left midfusiform gyrus may lessen postoperative AN and PN deficits as this region may potentially be a hub of lexical and semantic information. Continued mapping posterior and lateral to the midfusiform gyrus may potentially identify the VWFA within the ITG during intraoperative reading tasks, with stimulation of the anterior portion potentially leading to lexical dyslexia and the posterior portion for global dyslexia.
Parietal Lobe
While other critical tracts, such as the corticospinal tract and optic tracts, are important to consider when undergoing parietal lobe resections, the language tracts should also be preserved when performing resections in this region. The deep limit to resections of the parietal lobe includes the SLF (articulation) and the AF (repetition and phonological processes). The junction of temporal and parietal lobe encompassing the supramarginal and angular gyri and posterior superior temporal cortex should be investigated with verbal repetition tasks in order to avoid removing the dorsal phonological pathway. NWR tasks may be higher yield in detecting repetition deficits to avoid compensation from ventral lexical-semantic mechanisms. Similarly, mapping with writing of pseudowords may be employed in areas near the phonological pathway, while writing of irregular words can be tested in the lexical-semantic pathway.
Insular Lobe
The SLF/AF complex forms the superior and posterior limits of insular resection. If interested in preserving the FAT, which may not be necessary,64 it can also be found superiorly and posteriorly. The deep and ventral limit is the IFOF running in the temporal stem lateral to the anterior perforating substance, which produces semantic errors, while stimulation of the AF at the level of the superior insular sulcus usually results in phonemic paraphasia. Finally, the posterior and deep border is formed by the posterior limb of the internal capsule.
CONCLUSIONS AND FUTURE DIRECTIONS
In this paper, we have reviewed the domains of language via findings from DES tasks for each particular domain, and how neurosurgeons can use this information to assist with resections in regions likely to contain language function. Although the processing of language is extremely complex, and appears to vary depending on modality, the majority of tumors near and in language regions can be safely resected with the utilization of DES to map the crucial cortical and subcortical areas. Future work is needed to better select the optimal intraoperative tasks for localization of function, particularly for complex language domains like sentence syntax and comprehension. Moving forward, neurosurgeons would be well served to stay current on cognitive neuroscience advances in language production and comprehension, as these advances have the potential to improve our knowledge of the functional networks that must be respected during surgery.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Anthony T Lee, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California.
Edward F Chang, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California.
References
- 1.Penfield W, Roberts L.. Speech and Brain Mechanisms. Princeton, NJ: Princeton University Press; 1959. [Google Scholar]
- 2.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71(3):316-326. [DOI] [PubMed] [Google Scholar]
- 3.Snodgrass JG, Vanderwart M.. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6(2):174-215. [DOI] [PubMed] [Google Scholar]
- 4.Ojemann G, Mateer C.. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science. 1979;205(4413):1401-1403. [DOI] [PubMed] [Google Scholar]
- 5.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18-27. [DOI] [PubMed] [Google Scholar]
- 6.Corina DP, Loudermilk BC, Detwiler L, Martin RF, Brinkley JF, Ojemann G. Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain Lang. 2010;115(2):101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamberger MJ, Seidel WT, Goodman RR, Perrine K, McKhann GM. Temporal lobe stimulation reveals anatomic distinction between auditory naming processes. Neurology. 2003;60(9):1478-1483. [DOI] [PubMed] [Google Scholar]
- 8.Hamberger MJ, Seidel WT, Mckhann GM, Perrine K, Goodman RR. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128(11):2742-2749. [DOI] [PubMed] [Google Scholar]
- 9.Emerton BC, Gansler DA, Sandberg EH, Jerram M. Functional anatomic dissociation of description and picture naming in the left temporal lobe. Brain Imaging Behav. 2014;8(4):570-578. [DOI] [PubMed] [Google Scholar]
- 10.Sherman EMS, Wiebe S, Fay‐McClymont TBet al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 2011;52(5):857-869. [DOI] [PubMed] [Google Scholar]
- 11.Hamberger MJ, Goodman RR, Perrine K, Tamny T. Anatomic dissociation of auditory and visual naming in the lateral temporal cortex. Neurology. 2001;56(1):56-61. [DOI] [PubMed] [Google Scholar]
- 12.Duffau H, Herbet G, Moritz-Gasser S. Toward a pluri-component, multimodal, and dynamic organization of the ventral semantic stream in humans: lessons from stimulation mapping in awake patients. Front Syst Neurosci. 2013;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benzagmout M, Gatignol P, Duffau H. Resection of World Health Organization grade II gliomas involving Broca's area: methodological and functional considerations. Neurosurgery. 2007;61(4):741-753; discussion 752. [DOI] [PubMed] [Google Scholar]
- 14.Duffau H.Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4(8):476-486. [DOI] [PubMed] [Google Scholar]
- 15.Plaza M, Gatignol P, Cohen H, Berger B, Duffau H. A discrete area within the left dorsolateral prefrontal cortex involved in visual-verbal incongruence judgment. Cereb Cortex. 2008;18(6):1253-1259. [DOI] [PubMed] [Google Scholar]
- 16.Duffau H.The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46(4):927-934. [DOI] [PubMed] [Google Scholar]
- 17.Duffau H, Moritz-Gasser S, Mandonnet E. A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 2014;131:1-10. [DOI] [PubMed] [Google Scholar]
- 18.Bello L, Gallucci M, Fava Met al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60(1):67-82; discussion 80. [DOI] [PubMed] [Google Scholar]
- 19.Leonard MK, Cai R, Babiak MC, Ren A, Chang EF. The peri-Sylvian cortical network underlying single word repetition revealed by electrocortical stimulation and direct neural recordings. Brain Lang. 2019;193:58-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchsbaum BR, Baldo J, Okada Ket al. Conduction aphasia, sensory-motor integration, and phonological short-term memory—an aggregate analysis of lesion and fMRI data. Brain Lang. 2011;119(3):119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damasio H, Damasio AR.. The anatomical basis of conduction aphasia. Brain. 1980;103(2):337-350. [DOI] [PubMed] [Google Scholar]
- 22.Goodglass H.Diagnosis of conduction aphasia. Conduction Aphasia. Psychology Press; 1992:49-60. [Google Scholar]
- 23.Geschwind N.Disconnexion syndromes in animals and man. I. Brain. 1965;88(2):237. [DOI] [PubMed] [Google Scholar]
- 24.Catani M, Jones DK. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8-16. [DOI] [PubMed] [Google Scholar]
- 25.Saur D, Kreher BW, Schnell Set al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105(46):18035-18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fridriksson J, Kjartansson O, Morgan PSet al. Impaired speech repetition and left parietal lobe damage. J Neurosci. 2010;30(33):11057-11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makris N, Kennedy DN, McInerney Set al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15(6):854-869. [DOI] [PubMed] [Google Scholar]
- 28.Martino J, De Witt Hamer PC, Berger MSet al. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2013;218(1):105-121. [DOI] [PubMed] [Google Scholar]
- 29.Moritz-Gasser S, Duffau H.. The anatomo-functional connectivity of word repetition: insights provided by awake brain tumor surgery. Front Hum Neurosci. 2013;7:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson JM, Gilmore R, Roper Set al. Conduction aphasia and the arcuate fasciculus: a reexamination of the Wernicke-Geschwind model. Brain Lang. 1999;70(1):1-12. [DOI] [PubMed] [Google Scholar]
- 31.Quigg M, Fountain NB.. Conduction aphasia elicited by stimulation of the left posterior superior temporal gyrus. J Neurol Neurosurg Psychiatry. 1999;66(3):393-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quigg M, Geldmacher DS, Elias WJ. Conduction aphasia as a function of the dominant posterior perisylvian cortex. Report of two cases. J Neurosurg. 2006;104(5):845-848. [DOI] [PubMed] [Google Scholar]
- 33.Sierpowska J, Gabarrós A, Fernandez-Coello Aet al. Words are not enough: nonword repetition as an indicator of arcuate fasciculus integrity during brain tumor resection. J Neurosurg. 2017;126(2):435-445. [DOI] [PubMed] [Google Scholar]
- 34.Beauvois MF, Dérouesné J.. Phonological alexia: three dissociations. J Neurol Neurosurg Psychiatry. 1979;42(12):1115-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funnell E.Phonological processes in reading: new evidence from acquired dyslexia. Br J Psychol. 1983;74(2):159-180. [DOI] [PubMed] [Google Scholar]
- 36.Damasio AR, Damasio H.. The anatomic basis of pure alexia. Neurology. 1983;33(12):1573. [DOI] [PubMed] [Google Scholar]
- 37.Warrington EK, Shallice T.. Word-form dyslexia. Brain. 1980;103(1):99-112. [DOI] [PubMed] [Google Scholar]
- 38.Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 2001;108(1):204-256. [DOI] [PubMed] [Google Scholar]
- 39.Ojemann G.Intraoperative investigations of the neurobiology of reading. In: Euler CV, Lundberg I, Llinás RR, eds. Basic Mechanisms in Cognition and Language with Special Reference to Phonological Problems in Dyslexia. Amsterdam, Netherlands: Elsevier Science Limited; 1998:288. [Google Scholar]
- 40.Roux FE, Lubrano V, Lauwers-Cances V, Trémoulet M, Mascott CR, Démonet JF. Intra-operative mapping of cortical areas involved in reading in mono- and bilingual patients. Brain. 2004;127(8):1796-1810. [DOI] [PubMed] [Google Scholar]
- 41.Roux FE, Durand JB, Jucla M, Réhault E, Reddy M, Démonet JF. Segregation of lexical and sub-lexical reading processes in the left perisylvian cortex. PLoS One. 2012;7(11):e50665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen L, Dehaene S, Naccache Let al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(2):291-307. [DOI] [PubMed] [Google Scholar]
- 43.Dehaene S, Le Clec’H G, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13(3):321-325. [DOI] [PubMed] [Google Scholar]
- 44.Dehaene S, Cohen L.. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15(6):254-262. [DOI] [PubMed] [Google Scholar]
- 45.Gil-Robles S, Carvallo A, Jimenez MMet al. Double dissociation between visual recognition and picture naming: a study of the visual language connectivity using tractography and brain stimulation. Neurosurgery. 2013;72(4):678-686. [DOI] [PubMed] [Google Scholar]
- 46.Hirshorn EA, Li Y, Ward MJ, Richardson RM, Fiez JA, Ghuman AS. Decoding and disrupting left midfusiform gyrus activity during word reading. Proc Natl Acad Sci USA. 2016;113(29):8162-8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zemmoura I, Herbet G, Moritz-Gasser S, Duffau H. New insights into the neural network mediating reading processes provided by cortico-subcortical electrical mapping. Hum Brain Mapp. 2015;36(6):2215-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson SW, Damasio AR, Damasio H. Troubled letters but not numbers: domain specific cognitive impairments following focal damage in frontal cortex. Brain. 1990;113(3):749-766. [DOI] [PubMed] [Google Scholar]
- 49.Shallice T.Phonological agraphia and the lexical route in writing. Brain. 1981;104(3):413-429. [DOI] [PubMed] [Google Scholar]
- 50.Lesser RP, Lueders H, Dinner DS, Hahn J, Cohen L. The location of speech and writing functions in the frontal language area. Results of extraoperative cortical stimulation. Brain. 1984;107(1):275-291. [DOI] [PubMed] [Google Scholar]
- 51.Lubrano V, Roux FE, Démonet JF. Writing-specific sites in frontal areas: a cortical stimulation study. J Neurosurg. 2004;101(5):787-798. [DOI] [PubMed] [Google Scholar]
- 52.Roux FE, Boetto S, Sacko O, Chollet F, Trémoulet M. Writing, calculating, and finger recognition in the region of the angular gyrus: a cortical stimulation study of Gerstmann syndrome. J Neurosurg. 2003;99(4):716-727. [DOI] [PubMed] [Google Scholar]
- 53.Roux FE, Durand JB, Réhault E, Planton S, Draper L, Démonet JF. The neural basis for writing from dictation in the temporoparietal cortex. Cortex. 2014;50:64-75. [DOI] [PubMed] [Google Scholar]
- 54.Indefrey P, Brown CM, Hellwig Fet al. A neural correlate of syntactic encoding during speech production. Proc Natl Acad Sci USA. 2001;98(10):5933-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaan E, Swaab TY.. The brain circuitry of syntactic comprehension. Trends Cogn Sci. 2002;6(8):350-356. [DOI] [PubMed] [Google Scholar]
- 56.Corina DP, Gibson EK, Martin R, Poliakov A, Brinkley J, Ojemann GA. Dissociation of action and object naming: evidence from cortical stimulation mapping. Hum Brain Mapp. 2005;24(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caterina Silveri M, Perri R, Cappa A. Grammatical class effects in brain-damaged patients: functional locus of noun and verb deficit. Brain Lang. 2003;85(1):49-66. [DOI] [PubMed] [Google Scholar]
- 58.Chang EF, Kurteff G, Wilson SM. Selective interference with syntactic encoding during sentence production by direct electrocortical stimulation of the inferior frontal gyrus. J Cogn Neurosci. 2018;30(3):411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson SM, Henry ML, Besbris Met al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(7):2069-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lichtheim L. On aphasia. Brain. 1885;7(4):433-484. [Google Scholar]
- 61.Goldstein K.Language and language disturbances; aphasic symptom complexes and their significance for medicine and theory of language. JAMA 1948;139(14):967-968. [Google Scholar]
- 62.Boatman D, Gordon B, Hart J, Selnes O, Miglioretti D, Lenz F. Transcortical sensory aphasia: revisited and revised. Brain. 2000;123(8):1634-1642. [DOI] [PubMed] [Google Scholar]
- 63.Fontaine D, Capelle L, Duffau H. Somatotopy of the supplementary motor area: evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery. 2002;50(2):297-303; discussion 303. [DOI] [PubMed] [Google Scholar]
- 64.Young JS, Morshed RA, Mansoori Z, Cha S, Berger MS. Disruption of frontal aslant tract is not associated with long-term postoperative language deficits. World Neurosurg. 2020;133:192-195. [DOI] [PubMed] [Google Scholar]
- 65.Duffau H, Capelle L, Sichez Net al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain. 2002;125(1):199-214. [DOI] [PubMed] [Google Scholar]
- 66.Duffau H.New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128(4):797-810. [DOI] [PubMed] [Google Scholar]
- 67.Roux FE, Dufor O, Giussani Cet al. The graphemic/motor frontal area Exner's area revisited. Ann Neurol. 2009;66(4):537-545. [DOI] [PubMed] [Google Scholar]
- 68.Malow BA, Blaxton TA, Sato Set al. Cortical stimulation elicits regional distinctions in auditory and visual naming. Epilepsia. 1996;37(3):245-252. [DOI] [PubMed] [Google Scholar]
- 69.Forseth KJ, Kadipasaoglu CM, Conner CR, Hickok G, Knight RT, Tandon N. A lexical semantic hub for heteromodal naming in middle fusiform gyrus. Brain. 2018;141(7):2112-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamberger MJ, Miozzo M, Schevon CAet al. Functional differences among stimulation-identified cortical naming sites in the temporal region. Epilepsy Behav. 2016;60:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]