Abstract
Background:
Clinicians often express concerns about poor sensitivity of blood cultures in neonates resulting from inadequate inoculant volumes. Our objective was to determine the inoculant volume sent for neonatal sepsis evaluations and identify areas of improvement.
Methods:
Single-center prospective observational study of infants undergoing sepsis evaluation. Blood volume was determined by clinician-documentation over 21 months, and additionally by weighing culture bottles during 12 months. Adequate volume was defined as ≥1 mL total inoculant per evaluation. For first-time evaluations, local guidelines recommend sending an aerobic-anaerobic pair with 1 mL inoculant in each.
Results:
There were 987 evaluations in 788 infants. Clinicians reported ≥1 mL total inoculant in 96.9% of evaluations. Among 544 evaluations where bottles were weighed, 93.4% had ≥1 mL total inoculant. Very low birth weight infants undergoing evaluations >7 days after birth had the highest proportion of inadequate inoculants (14.4%). Only 3/544 evaluations and 26/1,011 bottles had total inoculant <0.5 mL. Ninety evaluations had <1 mL in both aerobic and anaerobic bottles despite a total inoculant volume that allowed inoculation of ≥1 mL in one of the bottles.
Conclusions:
Obtaining recommended inoculant volumes is feasible in the majority of neonates. Measuring inoculant volumes can focus improvement efforts and improve test reliability.
INTRODUCTION
Blood culture is a common test among infants admitted to the neonatal intensive care unit (NICU), and is important in diagnosing bacteremia and in ensuring accurate antibiotic therapy.(1, 2) Reliability of blood culture results depends on the inoculant volume with 1 mL blood volume recommended as adequate inoculant for neonates.(3–6) However, insufficient inoculant volumes have been reported in both adult and pediatric settings.(7–11) Given these reports, neonatal clinicians express concern about the reliability of blood culture tests resulting in frequent diagnosis of ‘culture-negative sepsis’,(12–14) and prolonged use of empiric antibiotics in neonatal units.(15–17) Four studies have objectively measured inoculant volumes specifically in neonates and report rates of inadequate inoculants varying from 33% to over 90% of all cultures sent.(7, 9, 10, 18) Potential reasons for inadequate inoculant volume include technical difficulty obtaining blood,(10, 18) concerns regarding the hemodynamic impact of acute volume loss, contribution of iatrogenic blood loss to neonatal anemia,(19, 20) and the differing recommendations for optimal inoculant volume.(20) These challenges have made clinicians question whether consistently obtaining adequate inoculant volumes is feasible,(10) and are deterrents to accepting blood culture results when negative.(7, 13, 14, 21)
As part of local antibiotic stewardship efforts, our center has developed written guidelines, provided clinician education and mandated the use of real-time documentation for blood culture procedures. The objective of this study was to determine the frequency with which our center was obtaining ≥1 mL inoculant for blood culture tests, and identify clinical scenarios or technical issues associated with inadequate inoculant volumes.
MATERIALS AND METHODS
Study Design:
Prospective observational single-center study.
Setting and Population:
Pennsylvania Hospital NICU is a 50-bed Level 3, high-risk perinatal center. All neonates admitted to the NICU with orders placed for blood culture between 12/01/2017 and 8/31/2019 were included in the study. This study was approved with waiver of consent by the Institutional Review Board at the University of Pennsylvania.
Clinicians at our center identified reliable blood culture results as a key component of antibiotic stewardship. Consistent documentation of the blood culture procedure was required to allow the information to be incorporated into decision-making regarding antibiotic use and clinical care. We created a standardized format for the blood culture procedure note in the electronic medical record. Because the clinician obtaining the blood sample is often different from the nurse inoculating the culture bottle, we chose to weigh the bottles to accurately determine inoculant volume. Education about optimal technique for obtaining blood cultures was provided to clinicians at study initiation. Interim results from the weighing procedure were provided to clinicians at several timepoints during the study. Supplemental Figure S1 (online) provides a timeline for specific study interventions along with monthly blood culture numbers and monthly percent positive cultures (excluding commensal contaminants and repeat positive cultures).
Blood culture guidelines:
Center guidelines recommend that 2 mL total blood be obtained for blood culture tests sent in an initial sepsis evaluation, with 1 mL inoculated into a single pediatric aerobic (BD Bactec™ Peds Plus/F) and 1 mL into a single anaerobic (BD Bactec™ Lytic/10 Anaerobic/F) bottle. For confirmed infection, 1 mL blood inoculated into an appropriate culture bottle to ensure resolution of bacteremia is sufficient. Blood is obtained after aseptic preparation by venous or arterial phlebotomy or by withdrawing blood from a central catheter. If difficulties arise with obtaining blood for initial evaluation, guidelines recommend that a minimum of 1 mL be cultured, ideally in an aerobic bottle. Therefore, the minimum inoculant considered adequate in this study was 1 mL, in either a single bottle or divided between two culture bottles.
Inoculant measurement:
As part of routine clinical care, clinicians obtaining the specimen are required to write a procedure note in the electronic medical record system (Epic Systems Corporation, Verona, WI) documenting the following in real-time: (a) total volume obtained, (b) number and type of bottles submitted for culture, (c) blood source (phlebotomy or catheter), and (d) the number of phlebotomy attempts, if applicable. Data from these procedure notes was abstracted from the medical records Monday through Friday. Clinicians were contacted to obtain missing or incomplete data.
From 8/30/2018 to 8/31/2019, blood culture bottles were additionally weighed before and after inoculation to objectively determine inoculant volume. Culture bottles were pre-weighed (to obtain accurate tare weight) by research staff in batches of 10–15 and stored on-site in the NICU. When a blood culture test was ordered, the NICU charge nurse recorded the pre-inoculation weight written on the culture bottle, provided the bottle to the clinical team, and re-weighed the bottle (with cap) immediately after inoculation. All weights were obtained with a Sartorius precision balance (margin of error, ±10−4 gram) (Sartorius AG, Göttingen, Germany). Inoculant volume was calculated by dividing the difference in pre- and post-inoculation bottle weights by 1.0581, the density of blood.(22)
Data sources and definitions:
Patient data were abstracted from the electronic medical records. Culture-confirmed infection was defined as growth of a pathogen from at least one blood culture bottle. Micrococcus, Propionibacterium, Corynebacterium, and Bacillus species were considered contaminants. Coagulase-negative staphylococci were considered pathogens if treated by the clinical team with an appropriate course of antibiotics.
Analysis:
We defined total inoculant volume as the blood volume obtained per sepsis evaluation, which may be inoculated into a pair of aerobic and anaerobic bottles or a single bottle. We calculated the proportion of evaluations with adequate inoculant by clinician report and by weight. We also report inoculant volume per bottle for evaluations completed during the 12 months when bottles were weighed. Inoculant volume assessed by weight was retained at 10−4 precision for all calculations and rounded to 10−2 only for presentation. Birth weight and chronological age influences frequency of blood culture orders(23) and presence of central lines, which can in turn affect the source of inoculant collection. Therefore, we analyzed blood culture procedures in three clinical categories: Very low birth weight (VLBW, <1,500 grams) infants ≤7 days after birth; VLBW infants >7 days after birth; and infants with birth weight ≥1,500 grams. All analyses were done in SAS 9.4 (Cary, NC).
RESULTS
Study characteristics:
During the study period, 987 blood culture orders were placed for 788 infants (Figure 1). Overall, 961/987 (97.4%) culture orders were associated with a clinician-reported volume: 856 (89.1%) were documented in the clinician’s procedure note and 105 (10.9%) were obtained by contacting the clinician. During the 12-month period in which inoculant volume was also assessed by weighing culture bottles, weights were obtained for bottles from 544/559 (97.3%) evaluations. These 544 evaluations included 1,011 culture bottles: 467 aerobic-anaerobic pairs, 72 aerobic bottles only, and 5 anaerobic bottles only.
Figure 1:
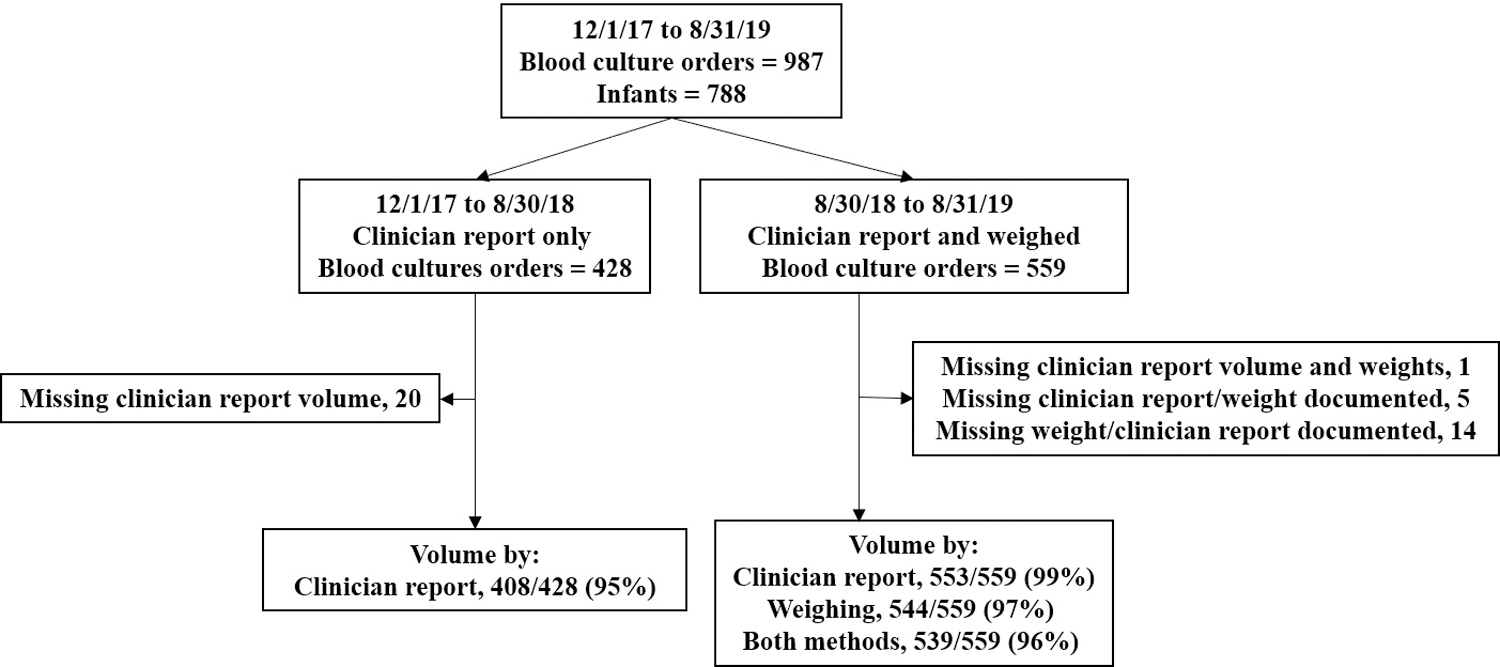
In this flow diagram, we describe the study design and inoculant volume determination methods during the study period.
Of the 987 sepsis evaluations, 703 (71.2%) were performed ≤24 hours after birth and 817 (82.8%) were performed ≤7 days after birth (Table 1). VLBW infants comprised 12.4% of the study infants, but contributed 27.1% of the culture orders. Sixty infants were evaluated for sepsis >7 days after birth and 85.0% of them were VLBW.
Table 1.
Study patient characteristics
| Patient characteristics | All infants n = 788 |
VLBW, BW <1500g n = 98 |
BW ≥1500g n = 690 |
|---|---|---|---|
| Gestational age at birth, mean (SD) | 36.5 (4.5) | 27.5 (2.7) | 37.7 (3.0) |
| <33 weeks, n (%) | 149 (18.9) | 96 (98.0) | 53 (7.7) |
| Birth weight (grams), mean (SD) | 2765 (967) | 982 (320) | 3018 (733) |
| Race/ethnicity, n (%) | |||
| White/non-Hispanic | 372 (47.2) | 34 (34.7) | 338 (49.0) |
| Black/non-Hispanic | 251 (31.9) | 45 (45.9) | 206 (29.9) |
| Hispanic | 84 (10.7) | 11 (11.2) | 73 (10.6) |
| Asian | 57 (7.2) | 4 (4.1) | 53 (7.7) |
| Other/unknown | 24 (3.0) | 4 (4.1) | 20 (2.9) |
| Male sex, n (%) | 415 (52.7) | 46 (46.9) | 369 (53.5) |
| Mode of delivery, Cesarean section, n (%) | 310 (39.3) | 58 (59.2) | 252 (36.5) |
| Length of hospital stay (days), median (IQR) | 5 (3–19.5) | 68.5 (38–100) | 4 (2–13) |
| Blood culture characteristics | |||
| Total number of cultures contributed to study, n | 987 | 267 | 720 |
| Age at first blood culture (hours), median (IQR) | 1.4 (0.9–3.5) | 1.4 (1.0–45.0) | 1.4 (0.9–3.2) |
| Total number of culture orders per infant, median (IQR) | 1 (1–1) | 2 (1–3) | 1 (1–1) |
| Infants with at least one culture at >7 days of age, n (%) | 60 (7.6) | 51 (52.0) | 9 (1.3) |
Abbreviations: VLBW – very low birth weight, BW – birth weight, SD – standard deviation, IQR – interquartile range
Blood culture procedure and results:
Clinicians documented ≥1 mL inoculant in 931/961 (96.9%) sepsis evaluations (Table 2). Distribution of inoculant source based on birth weight and chronological age of the infant at the time of evaluation are shown in Supplemental Figure S2 (online). In cultures obtained ≤7 days after birth, 82.2% in VLBW infants were drawn from a central line compared to 7.8% from larger infants. Insufficient volumes were more frequently reported in VLBW infants >7 days after birth. Blood cultures from 61 evaluations in 32 infants grew an organism. Seven were deemed as contaminants by clinical team and managed without antibiotics (Supplemental Table S3 (online)). Counting the first positive culture from each episode, 24 patients had 29 episodes of bacteremia. Pathogens were most frequently isolated from VLBW infants >7 days after birth. Consistent with center policy, follow-up evaluations performed to confirm resolution of bacteremia mostly consisted of a single culture bottle.
Table 2.
Details of blood cultures and clinician-reported documentation
| Total n = 987 |
Birth weight >1500 gramsa n = 720 |
VLBW & culture obtained ≤7 days n = 107 |
VLBW & culture obtained >7 days n = 160 |
p-value | |
|---|---|---|---|---|---|
| Clinician-reported total volume per order, n (%)b | |||||
| 2 mL documented and 2 bottles sent | 807 (81.8) | 617 (85.7) | 86 (80.4) | 104 (65.0) | <0.001 |
| 1 mL documented and 1 bottle sent | 124 (12.6) | 66 (9.2) | 15 (14.0) | 43 (26.9) | <0.001 |
| Documented <1 mL per bottle sent | 30 (3.0) | 18 (2.5) | 1 (0.9) | 11 (6.9) | 0.006 |
| Missing documentation | 26 (2.6) | 19 (2.6) | 5 (4.7) | 2 (1.3) | 0.24 |
| Site for specimen draw, n (%) | |||||
| Phlebotomy | 787 (79.7) | 664 (92.2) | 18 (16.8) | 105 (65.6) | <0.001 |
| Peripheral arterial line | 8 (0.8) | 2 (0.3) | 1 (0.9) | 5 (3.1) | |
| Umbilical line | 145 (14.7) | 53 (7.4) | 85 (79.4) | 7 (4.4) | |
| Peripherally inserted central catheter | 47 (4.8) | 1 (0.1) | 3 (2.8) | 43 (26.9) | |
| Bottles sent, n (%) | |||||
| Aerobic-Anaerobic pair | 822 (83.3) | 625 (86.8) | 88 (82.2) | 109 (68.1) | <0.001 |
| Aerobic/Anaerobic alone | 135 (13.7) | 70 (9.7) | 16 (15.0) | 49 (30.6) | |
| Missing documentation | 30 (3.0) | 25 (3.5) | 3 (2.8) | 2 (1.3) | |
| More than 2 attempts at phlebotomy, n (%) | 98/787 (12.5) | 83/664 (12.5) | 4/18 (22.2) | 11/105 (10.5) | 0.38 |
| Missing documentation | 20/787 (2.5) | 20/664 (3.0) | 0 | 0 | |
| Orders growing any organism, n (%) | 61 (6.2) | 10 (1.4) | 3 (2.8) | 48 (30.0) | <0.001 |
| Contaminants | 7/61 (11.5) | 5/10 (50.0) | 1/3 (33.3) | 1/48 (2.1) | <0.001 |
| CoNS (not treated as contaminant) | 10/54 (18.5) | 1/5 (9.3) | 1/2 (50.0) | 8/47 (17.0) | 0.13 |
Abbreviations: VLBW – very low birth weight, CoNS – Coagulase negative staphylococci
All blood culture orders for infants with birth weight >1500 grams were drawn at ≤7 days after birth except 10 orders (among 9 infants) drawn >7 days.
Assessment of inoculate volume adequacy based on documented note, e.g. 2 mL and 2 bottles sent, the assumption was that each bottle contained ≥1 mL; if 1.8 mL sent in 2 bottles, then categorized as <1 mL per bottle.
Inoculant volume by weighing:
Among the 544 evaluations in which bottles were weighed, 508 (93.4%) had ≥1 mL total inoculant volume and 418 (76.8%) had ≥1 mL in at least one bottle (Table 3). Even when the two bottles sent in an evaluation separately had inoculant volume of <1 mL (n=126), the total inoculant was often ≥1 mL in 71.4%. Figure 2 shows the distribution of total inoculant volume between the bottles in each of the 544 blood culture sets. Inaccurate distribution resulted in <1 mL inoculant in both bottles in 90/544 evaluations, despite the total inoculant volume being ≥1 mL. Of 1,011 bottles, 427 separate bottles had <1 mL inoculant: 296 had inoculant volume 0.8 to <1 mL, 105 had 0.5 to <0.8 mL, and 26 had <0.5 mL.
Table 3.
Blood inoculant volume from weighing bottles
| Total | Birth weight >1500 grams |
VLBW & culture obtained ≤7 days | VLBW & culture obtained >7 days | p-value | |
|---|---|---|---|---|---|
| Blood culture evaluationa, n | 544 | 380 | 60 | 104 | |
| Total volume (mL) per evaluation, median (IQR) | 2.0 (1.8–2.3) | 2.1 (1.9–2.3) | 2.0 (1.7–2.1) | 1.8 (1.2–2.1) | <0.001 |
| ≥1 mL per evaluation, n (%) | 508 (93.4) | 362 (95.3) | 57 (95.0) | 89 (85.6) | 0.002 |
| <1 to 0.5 mL per evaluation, n (%) | 33 (6.1) | 17 (4.5) | 2 (3.3) | 14 (13.5) | 0.002 |
| <0.5 mL per evaluation, n (%) | 3 (0.5) | 1 (0.3) | 1 (1.7) | 1 (1.0) | 0.32 |
| At least one bottle with ≥1 mL, n (%) | 418 (76.8) | 307 (80.8) | 43 (71.7) | 68 (65.4) | 0.003 |
| Aerobic culture bottlesa, n | 539 | 378 | 59 | 102 | |
| Volume (mL) in aerobic bottle, median (IQR) | 1.0 (0.9–1.2) | 1.1 (0.9–1.2) | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | 0.03 |
| ≥1 mL in aerobic bottle, n (%) | 304 (56.4) | 224 (59.3) | 28 (47.5) | 52 (51.0) | 0.11 |
| <1 to 0.5 mL in aerobic bottleb, n (%) | 225 (41.7) | 147 (38.9) | 30 (50.9) | 48 (47.1) | 0.11 |
| <0.5 mL in aerobic bottle, n (%) | 10 (1.9) | 7 (1.9) | 1 (1.7) | 2 (2.0) | 0.99 |
| Anaerobic culture bottlesa, n | 472 | 348 | 53 | 71 | |
| Volume (mL) in anaerobic bottle, median (IQR) | 1.0 (0.9–1.2) | 1.1 (1.0–1.2) | 1.0 (0.9–1.2) | 1.0 (0.9–1.1) | 0.004 |
| ≥1 mL in anaerobic bottle, n (%) | 280 (59.3) | 220 (63.2) | 28 (52.8) | 32 (45.1) | 0.01 |
| <1 to 0.5 mL in anaerobic bottleb, n (%) | 176 (37.3) | 117 (33.6) | 25 (47.2) | 34 (47.9) | 0.02 |
| <0.5 mL in anaerobic bottle, n (%) | 16 (3.4) | 11 (3.2) | 0 (0) | 5 (7.0) | 0.09 |
Abbreviations: VLBW – very low birth weight, IQR – interquartile range
Blood culture evaluation includes all culture bottles sent per evaluation and may include one bottle or a pair of bottles. Inoculant volume per bottle is shown separately.
When inoculant volume was <1 to 0.5 mL, 156/225 (69%) aerobic bottles and 140/176 (80%) anaerobic bottles had volume of 0.8 to <1 mL.
Figure 2:
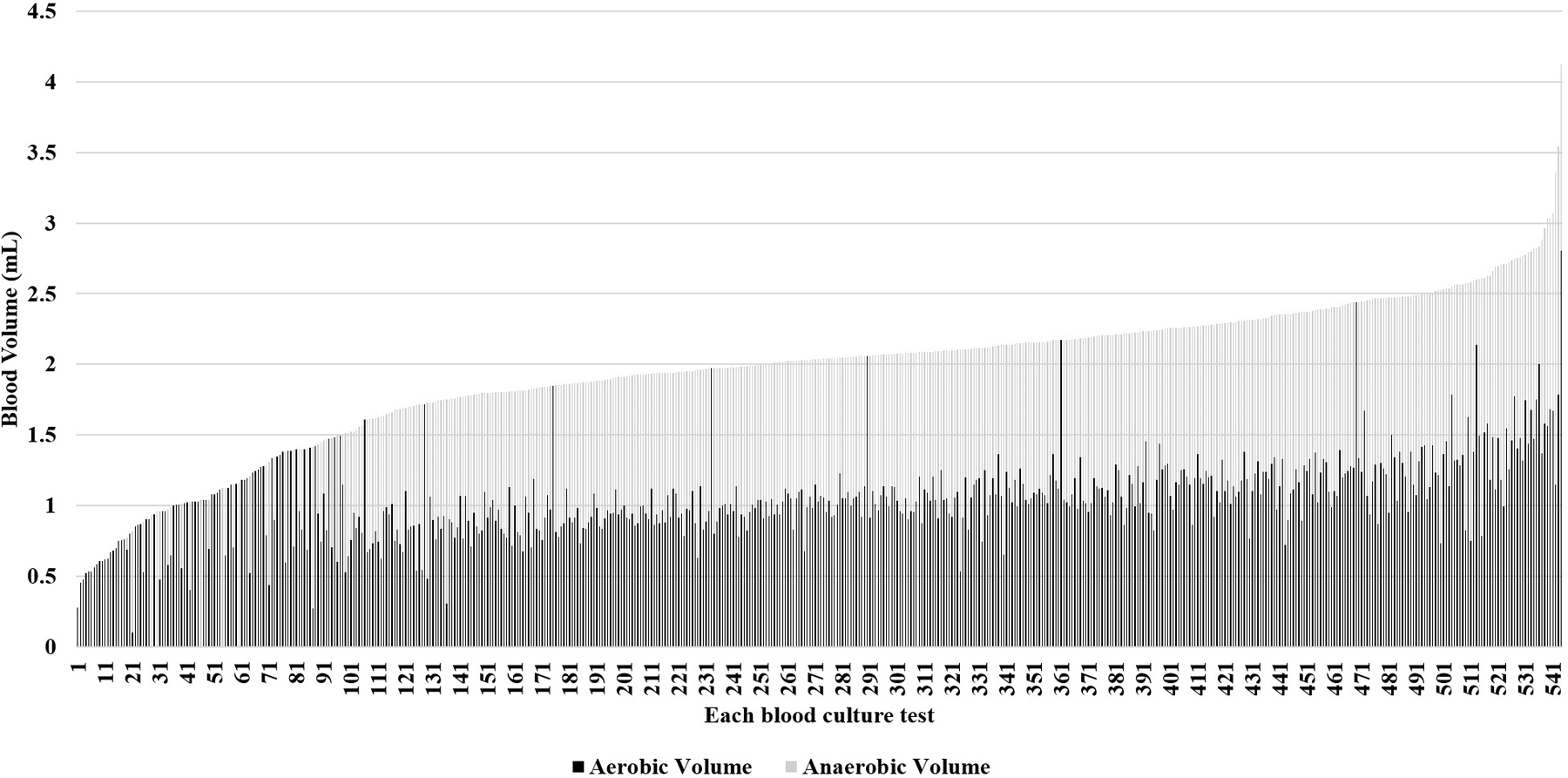
In this stacked bar graph, each of the 544 blood culture evaluations in which bottles were weighed are shown. The x-axis numbers each evaluation and is arranged by total inoculant volume, from lowest volume at 1 to highest at 544. The y-axis shows the blood volume in mL by weight. Total height of each bar corresponds to the total inoculant volume for that evaluation. Some bars consist of a light portion and a dark portion. The height of the dark portion corresponds to the inoculant volume in the aerobic bottle and the height of the light portion corresponds to the inoculant volume in the anaerobic bottle. For evaluations in which only one type of bottle was sent, the bar color corresponds with the type of bottle.
Pathogen isolation in the 36 evaluations with total inoculant <1 mL was high (6/36), compared to evaluations with total inoculant ≥1 mL (29/508, p=0.009). Aligned with the results of the clinician-reported volumes, VLBW infants evaluated >7 days after birth had lower median volume of inoculant and were more likely to have inoculant volumes between 0.5 to <1 mL (13.5%) compared to VLBW infants evaluated ≤7 days after birth (3.3%) or compared to non-VLBW infants (4.5%) (p=0.002).
Inoculant volume from procedure note versus by weight:
There were 539 evaluations for which both clinician-reported inoculant volume and bottle weight measurement were available. In 351/539 (65.1%), the weighed volume was greater than or equal to the clinician-reported volume. In 166/539 (30.8%), the weighed volume was less than the clinician-reported volume by 0.1–0.4 mL, and in 22/539 (4.1%), the weighed volume was less than the clinician-reported volume by ≥0.5 mL. Figure 3 shows the distribution of inoculant difference (reported minus weighed inoculant volume) across increasing documented inoculant volume. When documented inoculant volume was <1 mL (n=5), weighed inoculant volume was even lower. As documented inoculant volume increased, median difference between documented and weighed inoculant decreased.
Figure 3:
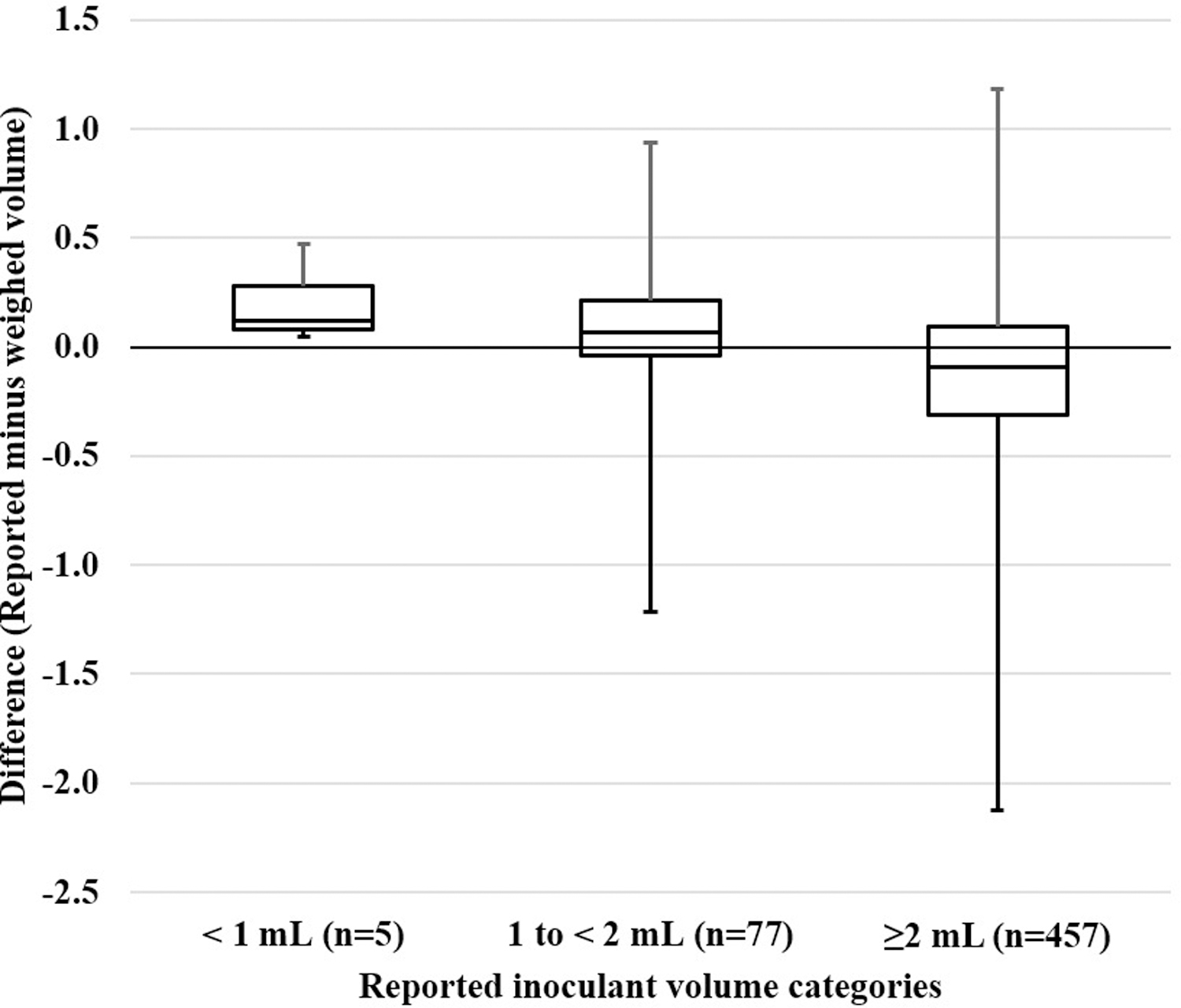
This box and whisker plot shows the median distribution of difference between reported and weighed inoculant volume across three categories of reported inoculant volume (<1 mL, 1 to <2 mL and 2 or more mL). Median difference greater than zero between reported and weight inoculant when <2 mL was documented suggests reported volumes were more frequently more than weighed volumes. In contrast, among the majority of the cultures (n=457) where 2 or more mL were reported, weighed volume was higher or the same as the reported inoculant volume, giving a median difference less than zero.
During the 12 months of bottle weight measurement, only 3 infants had a total inoculant <0.5 mL. Clinicians in each case reported that the volumes were <1 mL and documented difficulty obtaining phlebotomy specimens. In each case, the cultures were sterile. The attending physician discontinued antibiotics after 48 hours in 2 cases – one was a well-appearing term infant evaluated for maternal risk factors and the second was a 29-week infant on continuous positive airway pressure. The third case involved an extremely preterm infant evaluated at 39 days of life due to acute clinical decompensation, and the attending physician opted to administer 7 days of empiric antibiotics.
DISCUSSION
In this single center study, we investigated the oft-heard assertion that the technical challenges of obtaining neonatal blood culture results in inadequate sampling for this critical laboratory test. Using a combined approach of standard local guidance, clinician-reported documentation and weight measurement, we found that ≥1 mL total blood inoculant was cultured in 93.4% of neonatal sepsis evaluations at our center. We observed good compliance with local guidelines for number of culture bottles per sepsis evaluation, for target volume, and for recording details of the evaluation in the medical record in real-time. Total blood volume cultured per evaluation and volume per bottle was rarely below 0.5 mL. However, we identified issues with dividing blood inoculants accurately between culture bottles. We also identified the highest incidence of <1 mL inoculant being sent per evaluation among VLBW infants undergoing sepsis evaluation >7 days after birth, though 86.4% of these evaluations also had ≥1 mL and only one infant had inoculant <0.5 mL.
Compared to prior reports assessing neonatal blood culture volume, we found a higher proportion of sepsis evaluations to have adequate total inoculant volume. One study reported ≤0.5 mL inoculant in 55% of aerobic and 58% of anaerobic bottles.(9) Another reported that 40% of neonatal culture bottles contained <0.5 mL inoculant.(18) Connell et al. measured blood volume in the context of an educational intervention for improved blood culture collection technique.(7) They defined ≥0.5 mL as adequate neonatal specimen. Prior to education, 62/95 (65.3%), and after education, 31/38 (81.6%), were deemed adequate. More recently, in a quality improvement study, Singh et al. reported an improvement in adequate blood inoculant volume, defined as >0.8 mL, from <4% to 75% of all cultures.(10) In contrast, we found that 529/539 (98.1%) aerobic and 456/472 (96.6%) anaerobic bottles that were weighed to determine inoculant volume contained ≥0.5 mL. We speculate that local center policy targeting 1 mL blood per culture bottle and two blood culture bottles per evaluation provides a greater margin of error by ‘underfilling’ and may account for the higher proportion of adequate specimens we found.
Recommendations for optimal inoculant volume in newborns vary.(20, 24) In the 2019 American Academy of Pediatrics report on early-onset sepsis management, 1 mL inoculant in a pediatric blood culture bottle is the recommended minimum with consideration for sending an additional anaerobic bottle.(25) The Infectious Disease Society of America recommends a weight-based inoculant volume adapted from work by Kellogg et al.: 2 mL blood for infants with weight ≤1,000 grams, 4 mL for infants 1,100–2,000 grams, and 6 mL for infants >2,000 grams, all inoculated into 1–2 pediatric aerobic bottles.(26) This is estimated to obtain a maximum of 4.5% of the total blood volume of an infant, a percent loss that was not associated with hemodynamic changes on continuous cardiorespiratory monitoring in that study. In contrast, the Clinical and Laboratory Standards Institute guideline recommends not exceeding 1% of total blood volume, which suggests that ~0.5 mL inoculant is sufficient for an infant weighing 500 grams.(24) The utility of anaerobic culture for neonates is controversial and the lack of a pediatric-specific anaerobic culture bottle is a disadvantage.(27) Our center uses an aerobic-anaerobic pair due to the following potential benefits: to aid in determination of contaminant species, potential for selective growth of some facultative species in anaerobic condition, and identification of obligate anaerobes.(28–32) Most pathogens in this study were facultative anaerobes and most isolates grew in both culture bottles. One early-onset infection with an obligate anaerobe, Bacteroides fragilis, was detected in the current study.
Inoculant volume is related to pathogen isolation, with lower volume resulting in decreased detection. Yet, pathogens were isolated in a significantly higher proportion of evaluations with <1 mL total inoculant compared to inoculant volume >1 mL (6/36 vs. 29/508, p=0.009). The apparent contradiction between lower inoculant volume and higher pathogen isolation has been reported by others and can be eliminated when adjusting for severity of illness.(3, 18) The heightened efficiency of late-onset evaluations in the NICU compared to early-onset evaluations in clinical practice is aligned with this preferential selection of higher-risk infants who are evaluated for late-onset evaluations.(33) In this study, we did not have data to conduct an adjusted analysis to account for severity of illness. However, 41.6% of evaluations with <1 mL inoculant and five of the six positive cases in this group occurred in VLBW infants evaluated >7 days after birth. In contrast to early-onset sepsis evaluation, which may occur in a well-appearing infant evaluated for maternal risk factors, late-onset sepsis evaluations among VLBW infants were all performed due to acute clinical instability. This enriches the chance of true bacteremia with a higher bacterial load that may counter the effect of a smaller inoculant volume on overall diagnostic yield.
We identified areas of improvement in this study. Our local policy states, “If insufficient volume is drawn, inoculate the aerobic culture bottle with the required amount and then inoculate the anaerobic culture bottle with the remaining volume of blood.” However, inoculant volume per bottle occasionally remained below 1 mL for both bottles (Figure 2). For example, 2 mL total inoculant was occasionally distributed as 0.8 mL in one bottle and 1.2 mL in the second, when ideally, 1 mL would be distributed in each. Imprecision in transferring small volumes is perhaps not unexpected. Vacuum in the culture bottle exceeds the volume desired for inoculation and contributes to the challenge of regulating flow and ensuring appropriate volume transfer. Use of a 3 mL or small volume syringe when inoculating may aid in precise volume deliverance. Although most pediatric blood culture bottles are designed for 1–3 mL blood inoculation, it remains unclear if increasing the dilution of blood:broth by inoculating <1 mL in a single bottle significantly impacts the likelihood of detecting bacteremia if the total inoculant volume is ≥1 mL.(34) In one prospective neonatal study, investigators obtained 2 mL blood, inoculated 1 mL into an aerobic bottle, and divided the remaining 1 mL as 0.5 mL inoculants into an aerobic and anaerobic pair. A higher detection rate was found with the aerobic-anaerobic pair, which may suggest that with ≥0.5 mL of inoculant volume, the impact of greater volume per bottle may not be substantial.(32)
Differences in adequacy of inoculant volume was associated with the clinical scenario of VLBW infants being evaluated after >7 days after birth. In contrast, the majority of blood cultures, in preterm and term infants, were obtained for early-onset sepsis evaluations and over 95% contained adequate inoculant volumes. The absence of a newly placed central line for obtaining specimen after the first week of life and the greater frequency of acute clinical instability in VLBW infants triggering the evaluation may make obtaining adequate inoculant volumes more challenging. However, even in this group inoculant volumes were below 0.5 mL in only one infant. Building a dedicated team of skilled clinicians, akin to what many units have for peripherally inserted central line placement, may be one way of minimizing attempts at phlebotomy and ensuring adequate inoculant volumes. Preterm infants requiring intensive care suffer high rates of iatrogenic blood loss in the NICU.(35) Balancing the tension between iatrogenic blood removal and adequate inoculant volume remains challenging. Better risk assessment to limit blood culture tests to infants at highest risk of infection is an obvious first step. In early-onset sepsis evaluations, risk assessment approaches to improve the efficiency of sepsis evaluation among term and preterm infants can guide efforts to diagnostic stewardship,(6, 25) and use of cord blood can alleviate the phlebotomy demands.(36)
Our study has limitations. This is a single-center study, and the clinicians were not blinded to the study, although the clinicians drawing the specimens, inoculating the specimens, and weighing the bottles were separate individuals. Our results may not be generalizable to centers with different patient populations or clinician experience. We do not have data assigning pathogen recovery to bottle type, and thus we cannot estimate the relative efficiency of aerobic and anaerobic culture.
Many microbiological facilities have incorporated automated weighing of blood culture bottles for adult patients, providing feedback that can both improve technique and inform decision-making.(8) These techniques are not available for neonatal specimens, but we found that documenting culture volume, at a minimum, reinforces standards and informs antibiotic use. We also found ongoing education and intermittent auditing at a unit-level to be useful tools in maintaining optimal techniques when obtaining blood cultures. Our results demonstrate that contrary to commonly expressed concern for inadequate inoculant volume in neonatal blood culture bottles and consequent lack of reliance on negative blood culture results, adequate inoculant volume could be obtained in most NICU patients undergoing sepsis evaluations.
Supplementary Material
Article Impact:
What is the key message of your article?
Current recommendations for adequate inoculant volume for blood cultures are met in a majority of neonates.
Areas of improvement include preterm late-onset sepsis evaluations and distribution techniques during inoculation.
What does it add to the existing literature?
Clinicians express concern about the unreliability of neonatal blood cultures because of inadequate inoculant volume.
We investigated over 900 evaluations and found >90% of evaluations have ≥1 mL inoculant.
What is the impact?
Monitoring adequacy of blood culture technique can identify areas of improvement and may allay concerns about blood culture reliability.
ACKNOWLEDGEMENTS
Elizabeth Quigley, MSN, Ann Schwoebel, MSN, and all advanced care clinicians and nursing staff of the Intensive Care Nursery for their support in measuring culture bottles and documenting volume of inoculants. We would also like to acknowledge Tracy Healy for her guidance in weighing blood culture bottles.
Statement of financial support:
This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health grant (K23HD088753).
Footnotes
Disclosure statement: The authors have no financial relationships or conflicts of interest to disclose.
Category of study: Clinical Research Article
Social media summary: Adequacy of neonatal blood culture
Statement of patient consent: This study was approved with waiver of informed consent by the Institutional Review Board at the University of Pennsylvania.
REFERNCES
- 1.Schelonka RL, Chai MK, Yoder BA, et al. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996; 129:275–8 [DOI] [PubMed] [Google Scholar]
- 2.Flannery DD, Ross RK, Mukhopadhyay S, et al. Temporal trends and center variation in early antibiotic use among premature infants. JAMA network open. 2018;1(1):e180164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouza E, Sousa D, Rodríguez-Créixems M, et al. Is the volume of blood cultured still a significant factor in the diagnosis of bloodstream infections? J Clin Microbiol. 2007;45(9):2765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cockerill FR III, Wilson JW, Vetter EA, et al. Optimal testing parameters for blood cultures. Clinical infectious diseases. 2004;38(12):1724–30. [DOI] [PubMed] [Google Scholar]
- 5.Hazen KC, Polage CR. Using Data to Optimize Blood Bottle Fill Volumes and Pathogen Detection: Making Blood Cultures Great Again. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020;70(2):269. [DOI] [PubMed] [Google Scholar]
- 6.Puopolo KM, Benitz WE, Zaoutis TE, Committee on Infectious Diseases. Management of neonates born at≥ 35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142(6):e20182894. [DOI] [PubMed] [Google Scholar]
- 7.Connell TG, Rele M, Cowley D, et al. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics. 2007;119(5):891–6. [DOI] [PubMed] [Google Scholar]
- 8.Khare R, Kothari T, Castagnaro J, et al. Active monitoring and feedback to improve blood culture fill volumes and positivity across a large integrated health system. Clinical Infectious Diseases. 2020;70(2):262–8. [DOI] [PubMed] [Google Scholar]
- 9.Neal PR, Kleiman MB, Reynolds JK, et al. Volume of blood submitted for culture from neonates. J Clin Microbiol. 1986;24(3):353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh MP, Kumar BV, Kiran, et al. The practice of blood volume submitted for culture in a neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2020;105(6):600. [DOI] [PubMed] [Google Scholar]
- 11.van Ingen J, Hilt N, Bosboom R. Education of phlebotomy teams improves blood volume in blood culture bottles. J Clin Microbiol. 2013;51(3):1020–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piantino JH, Schreiber MD, Alexander K, et al. Culture negative sepsis and systemic inflammatory response syndrome in neonates. NeoReviews. 2013;14(6):e294–305. [Google Scholar]
- 13.Simonsen KA, Anderson-Berry AL, Delair SF, et al. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30(11):937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantey JB, Baird SD. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics. 2017;140(4). [DOI] [PubMed] [Google Scholar]
- 16.Puopolo KM, Mukhopadhyay S, Hansen NI, et al. Identification of extremely premature infants at low risk for early-onset sepsis. Pediatrics. 2017;140(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulman J, Dimand RJ, Lee HC, et al. Neonatal intensive care unit antibiotic use. Pediatrics. 2015;135(5):826–33. [DOI] [PubMed] [Google Scholar]
- 18.Jawaheer G, Neal TJ, Shaw NJ. Blood culture volume and detection of coagulase negative staphylococcal septicaemia in neonates. Archives of Disease in Childhood-Fetal and Neonatal Edition. 1997;76(1):F57–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellogg JA, Ferrentino FL, Goodstein MH, et al. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J. 1997;16(4):381–5. [DOI] [PubMed] [Google Scholar]
- 20.Lancaster DP, Friedman DF, Chiotos K, et al. Blood volume required for detection of low levels and ultralow levels of organisms responsible for neonatal bacteremia by use of Bactec Peds Plus/F, Plus Aerobic/F medium, and the BD Bactec FX system: an in vitro study. J Clin Microbiol. 2015;53(11):3609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzialla C, Manzoni P, Achille C, et al. New diagnostic possibilities for neonatal sepsis. Am J Perinatol. 2018;35(06):575–7. [DOI] [PubMed] [Google Scholar]
- 22.Trudnowski RJ, Rico RC. Specific gravity of blood and plasma at 4 and 37 C. Clin Chem. 1974;20(5):615–6. [PubMed] [Google Scholar]
- 23.Cantey JB, Wozniak PS, Sánchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J. 2015;34(3):267–72. [DOI] [PubMed] [Google Scholar]
- 24.Bard JD, TeKippe EM. Diagnosis of bloodstream infections in children. J Clin Microbiol. 2016;54(6):1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puopolo KM, Benitz WE, Zaoutis TE. Management of Neonates Born at ≤34 6/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics. 2018;142(6):e20182896. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg JA, Manzella JP, Bankert DA. Frequency of low-level bacteremia in children from birth to fifteen years of age. J Clin Microbiol. 2000;38(6):2181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukhopadhyay S, Puopolo KM. Relevance of Neonatal Anaerobic Blood Cultures: New Information for an Old Question. Journal of the Pediatric Infectious Diseases Society. 2018;7(3):e126. [DOI] [PubMed] [Google Scholar]
- 28.Messbarger N, Neemann K. Role of anaerobic blood cultures in neonatal bacteremia. Journal of the Pediatric Infectious Diseases Society. 2018;7(3):e65–9. [DOI] [PubMed] [Google Scholar]
- 29.Shoji K, Komuro H, Watanabe Y, et al. The utility of anaerobic blood culture in detecting facultative anaerobic bacteremia in children. Diagn Microbiol Infect Dis. 2013;76(4):409–12. [DOI] [PubMed] [Google Scholar]
- 30.Zaidi AK, Knaut AL, Mirrett S, et al. Value of routine anaerobic blood cultures for pediatric patients. J Pediatr. 1995;127(2):263–8. [DOI] [PubMed] [Google Scholar]
- 31.Riley JA, Heiter BJ, Bourbeau PP. Comparison of recovery of blood culture isolates from two BacT/ALERT FAN aerobic blood culture bottles with recovery from one FAN aerobic bottle and one FAN anaerobic bottle. J Clin Microbiol. 2003;41(1):213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaacobi N, Bar-Meir M, Shchors I, et al. A prospective controlled trial of the optimal volume for neonatal blood cultures. Pediatr Infect Dis J. 2015;34(4):351–4. [DOI] [PubMed] [Google Scholar]
- 33.Schulman J, Benitz WE, Profit J, et al. Newborn Antibiotic Exposures and Association With Proven Bloodstream Infection. Pediatrics. 2019;144(5):e20191105. [DOI] [PubMed] [Google Scholar]
- 34.Kennaugh JK, Gregory WW, Powell KR, et al. The effect of dilution during culture on detection of low concentrations of bacteria in blood. Pediatr Infect Dis J. 1984;3(4):317–8. [DOI] [PubMed] [Google Scholar]
- 35.Counsilman CE, Heeger LE, Tan R, et al. Iatrogenic blood loss in extreme preterm infants due to frequent laboratory tests and procedures. The Journal of Maternal-Fetal & Neonatal Medicine. 2019:1–6. [DOI] [PubMed]
- 36.Baer VL, Lambert DK, Carroll PD, et al. Using umbilical cord blood for the initial blood tests of VLBW neonates results in higher hemoglobin and fewer RBC transfusions. Journal of Perinatology. 2013;33(5):363–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


