Abstract
In this work, graphitic carbon nitride-supported l-arginine (g-C3N4@l-arginine) nanocatalyst was synthesized and evaluated using FT-IR, EDX, XRD, TGA, and FESEM analyses. The performance of the prepared nanocatalyst was examined in the synthesis of 1,4-dihydropyridine, 4H-chromene, and 2,3-dihydro quinazoline derivatives. The novel g-C3N4@l-arginine nanocatalyst showed high thermal stability, easy separation from reaction media, the capability to be used in various multicomponent reactions, and acceptable reusability.
Subject terms: Medical research, Chemistry
Introduction
These days, most chemical processes are carried out in the presence of catalysts. Among various catalysts, supported catalysts have more application than other catalysts. Synthesis of this class of catalysts requires a support with high surface area for the adequate dispersion of primary catalyst. For this reason, using a suitable support is of high importance in the synthesis of such catalysts1–4.
Graphitic carbon nitride (g-C3N4) can be used as a metal-free catalyst or catalyst support due to its excellent properties and exceptional performance. g-C3N4 sheets are an important class of conjugated polymers for the synthesis of new heterogeneous catalysts due to their unique electronic band structure, excellent physical, chemical, and thermal stability, high abrasion resistance, high hardness, low density, versatile performance, low synthesis cost, and recyclability 5,6. On the other hand, amino acids are one of the most interesting catalyst substrates due to their unique structure 7. Arginine is one of the main and semi-essential amino acids in the body of living organisms with advantages such as nontoxicity, ability to easily bind to catalytic support, and low cost for the preparation of acidic catalysts. In addition, acidic catalysts play an important role in the synthesis of organic compounds. Heterocyclic compounds are one of the best candidates in organic synthesis and pharmaceutical chemistry. They are mainly used as medicines, chemicals, veterinary products, disinfectants, expanders, and antioxidants 8.
The use of inexpensive and non-toxic reagents as well as low waste production is of great importance in green chemistry reactions. Hence, multicomponent reactions (MCRs) are considered as a useful method for the synthesis of heterocyclic organic molecules. Significant advantages of MCRs are the elimination of intermediates, short reaction times, high reaction yield, and easy separation of products 9–12. Compounds such as 1,4-dihydropyridine, tetrahydro-4H-chromenes, and dihydroquinazolines, which have high medicinal activity, are synthesized by MCRs.
Dihydropyridines are divided into two classes; symmetrical and asymmetrical. The latter is synthesized by the reaction of an aldehyde, 2 mmol of two different β-keto esters, and a nitrogen donor such as ammonium acetate or ammonia. The product of the initial reaction is dihydropyridine that can later be converted to pyridine. These compounds are an important class of antihypertensive drugs, vasodilators, hypnotic, anti-tumor 13,14, anti-inflammatory15,16, anti-diabetic, anti-anxiety, anti-mutation, and are known as calcium channel blockers17.
Tetrahydro-4H-chromenes are an important class of heterocyclic compounds with simple structure and low side effects. They are synthesized by the single-step condensation of aldehydes with malononitrile and dimedone18. In addition, their derivatives have important activities such as anticancer, antiviral, anti-inflammatory, antibacterial, antifungal, antioxidant, and anticoagulant. They are also used as cognitive enhancers to treat Alzheimer's disease19,20.
Dihydroquinazolines are the building blocks of about 150 natural alkaloids which are prepared by the reaction between aldehydes, isotonic anhydride, and ammonium acetate. Moreover, they have a range of pharmaceutical and biological activities such as anti-inflammatory, antimalarial, antibacterial, anticancer, and antiviral activities 21,22. Hence, many efforts have been made to synthesize such high-yield compounds. There are various methods for the synthesis of these compounds using different catalysts such as MCM-41@Schiff base-Co (OAC)223, Yb (NPf2)324, MCM-41@serine@Cu(II) 25, titanium silicon oxide nanopowder26, Y(NO3)3.6H2O 27, etc. despite their numerous advantages, they have some limitations such as long reaction time, expensive reagents, and the possibility of their contamination in final products.
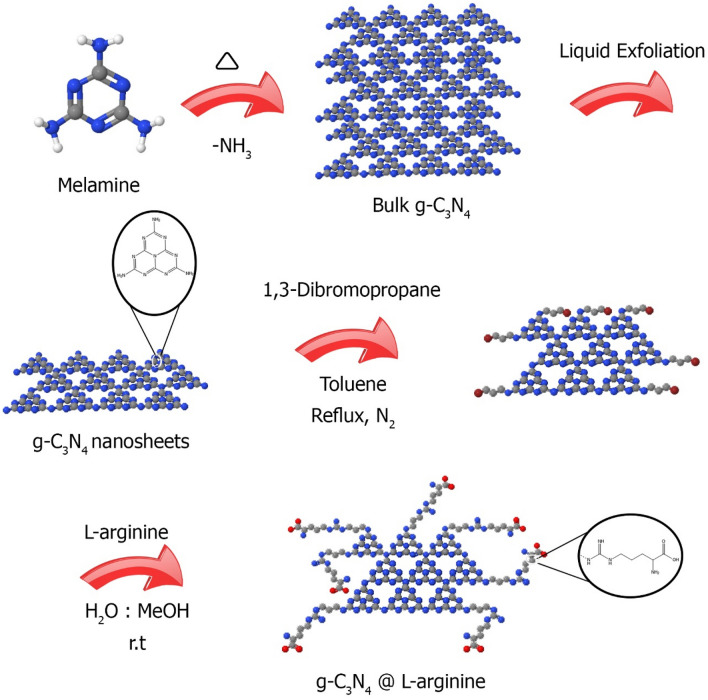
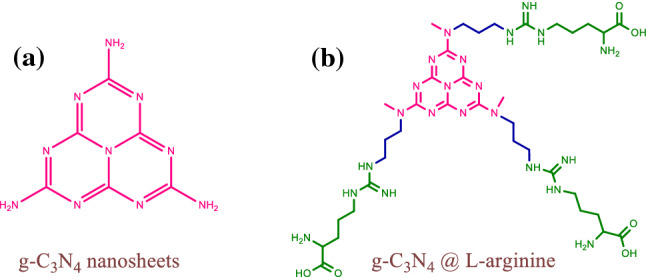
In this paper, new g-C3N4@l-arginine catalyst with the ability to perform various multi-combination reactions with high yield, short reaction time, recyclability, and easy separation from the reaction mixture is synthesized and examined (Fig. 1).
Figure 1.
Synthesis of g-C3N4@l-arginine.
Results and discussion
The g-C3N4@l-arginine synthesis process consists of three main steps, as shown in Fig. 1. The first step is the synthesis of nanosheet g-C3N4 from melamine, which melamine was polymerized to bulk g-C3N4, then nanosheet g-C3N4 is synthesized by liquid exfoliation and sonication. In the second step, g-C3N4 nanosheets were modified by 1,3-dibromopropane at 100 °C for 24 h under nitrogen atmosphere. Finally, the g-C3N4@l-arginine was obtained via the reaction between l-arginine and modified g-C3N4 nanosheets. In this work, various techniques such as FTIR, EDX, XRD, FESEM, and TGA have been used to identify and characterize the novel nanocatalyst.
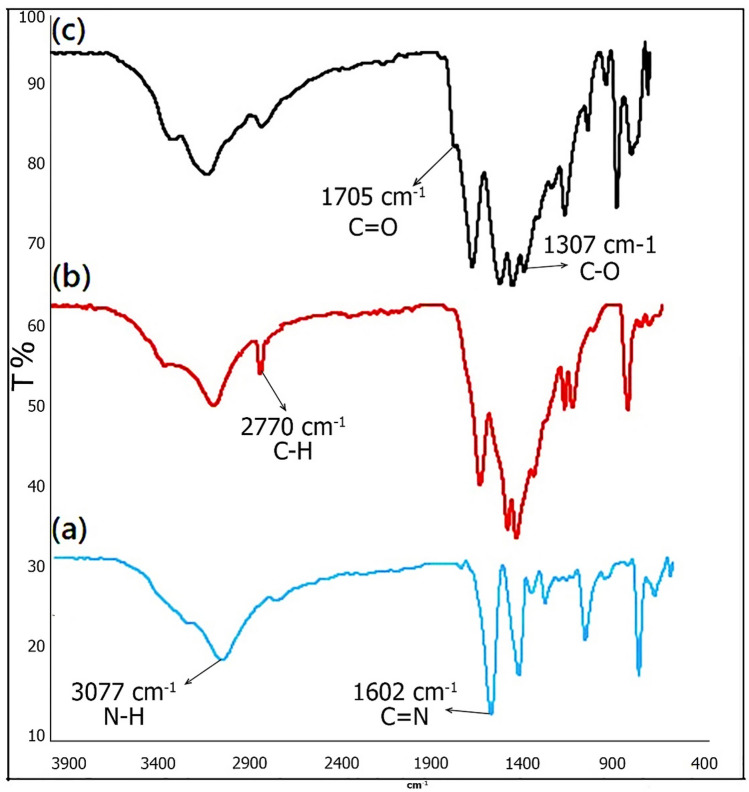
FTIR spectra of g-C3N4 nanosheets (Fig. 2a) and g-C3N4@l-arginine nanocatalyst (Fig. 2b) are shown in Fig. 2. The strong and broad peak in the range of 3000–3300 cm−1 is related to stretching vibration of N–H bonds, breadth peak can be assigned to N–H groups involved in H-bonding or the presence of O–H groups due to water adsorption by nanosheets g-C3N428,29. The stretching vibration peak of C=N can be observed at 1602 cm−1. The peaks at 1303 and 1082 cm−1 are attributed to the stretching vibration of C–N bonds formed between triazine and N–H groups, while the stretching vibration of C–N bonds in the ring is easily visible at 1448 and 1379 cm−1 29,30. In addition, the peak at 786 cm−1 is associated with the vibration of tri-s-triazine units 1 (Fig. 3a). Figure 3b shows that the g-C3N4 nanosheets has been modified with 1,3-dibromopropane; the peak at 3000–2800 cm−1 is related to C-H stretching vibration.
Figure 2.
Nanosheets g-C3N4, (a) and g-C3N4@l-arginine (b).
Figure 3.
FT-IR spectra of (a) nanosheets g-C3N4, (b) modified g-C3N4, (c) g-C3N4@l-arginine.
The spectrum of g-C3N4@l-arginine is presented in Fig. 3c in which the existence of l-arginine on the surface of g-C3N4 nanosheets can be confirmed based on the 1705 cm−1 and 1307 cm−1 peaks relating to the stretching vibration of C = O and C-O bonds, respectively. O–H and C-H bonds already existed in the structure of modified nanosheets g-C3N4.
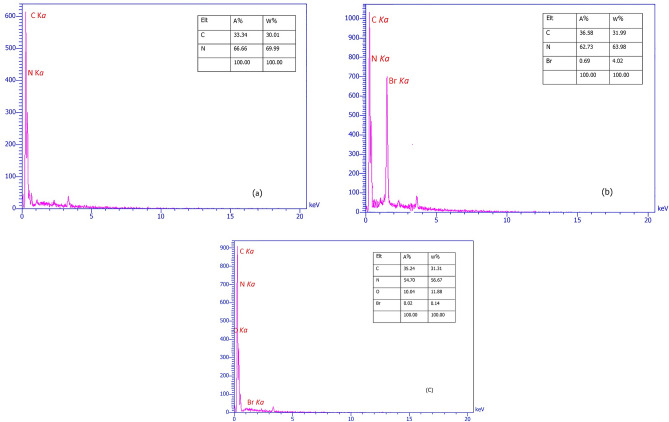
The presence of carbon and nitrogen elements in the structure of g-C3N4 nanosheets is visible in Fig. 4a. The presence of Br element in the structure proves that g-C3N4 nanosheets have been modified by 1,3-dibromopropane (Fig. 4b). Finally, the presence of carbon, nitrogen, and oxygen in the final structure (g-C3N4@l-arginine) confirmed the synthesis of g-C3N4@l-arginine nanocatalyst (Fig. 4c).
Figure 4.
EDX spectrum of (a) nanosheets g-C3N4, (b) modified g-C3N4, (c) g-C3N4@l-arginine.
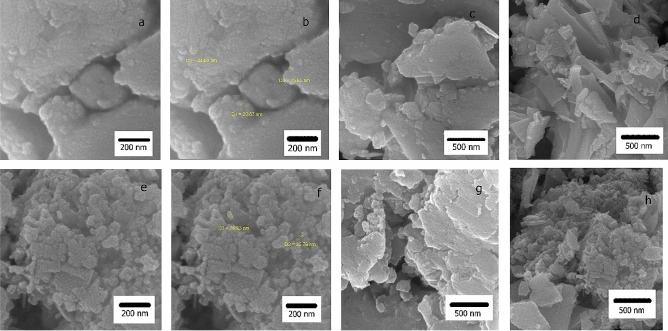
The morphology of g-C3N4 nanosheets and g-C3N4@l-arginine was investigated by FE-SEM. Figure 5a-d shows FE-SEM images of g-C3N4 nanosheets. As shown in Figs. 5a-c, the g-C3N4 nanosheets have a smooth and flat surface, while in Fig. 5d, g-C3N4 nanosheets are irregular and connected together. FE-SEM images of g-C3N4@l-arginine are shown in Fig. 5e-h. It can be seen from Fig. 5e-g that g-C3N4 nanosheets have a flake-like morphology with a relatively rough surface, mainly due to the presence of l-arginine on the surface of g-C3N4 nanosheets. In Fig. 5h, more irregular-shape g-C3N4 nanosheets with tiny particles on the surface are observed, which again confirms the deposition of l-arginine on the g-C3N4 nanosheets.
Figure 5.
FE-SEM image of nanosheets g-C3N4 (a, b, c, and d), and g-C3N4@l-arginine (e, f, g, h).
XRD patterns of g-C3N4 nanosheets and g-C3N4@l-arginine are shown in Figs. 6a and 4b, respectively. The diffraction peaks at 2θ = 27.69 and 15.96 (Fig. 6a) prove the successful synthesis of g-C3N4 nanosheets 1,7,28, while the diffraction peaks at 2θ = 6.07, 10.85, 12.21, 23.60, and 30.97 (Fig. 6b) correspond to l-arginine (JCPDS card no. 00–004-0180), confirming the presence of l-arginine on the surface of g-C3N4 nanosheets.
Figure 6.
XRD pattern of (a) nanosheets g-C3N4, (b) g-C3N4@l-arginine.
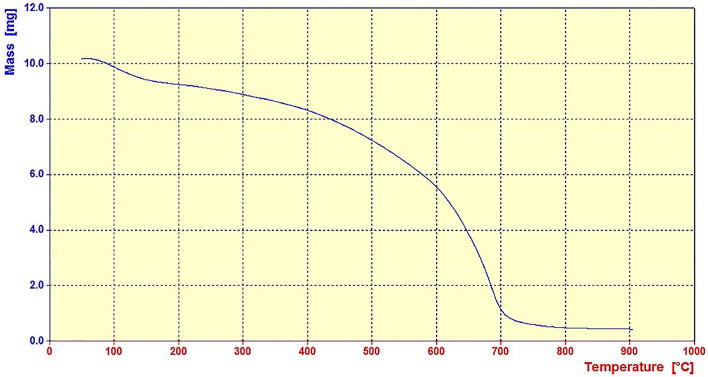
Figure 7 shows the thermal stability of the synthesized g-C3N4@l-arginine in the range of 50–800 °C. As can be seen, the weight ratio has gradually decreased by increasing the temperature from 100 to 200 °C, which is most likely related to the removal of water absorbed on the surface of g-C3N4@l-arginine. Then, another weight loss is observed in the range of 200 to 400 °C, which is attributed to the separation of l-arginine from the structure. Finally, there is another weight loss in the range of 400 to 700 °C due to the decomposition of g-C3N4 nanosheets 31.
Figure 7.
TGA analysis of g-C3N4@l-arginine.
Model reactions
The performance of the prepared g-C3N4@l-arginine nanocatalyst was evaluated for the synthesis of 1,4-dihydropyridine, 4H-chromene, and 2,3-dihydro quinazoline derivatives. For this purpose, various parameters such as reaction time, catalyst concentration, and the solvent were examined (Table 1). The reaction of 4-chlorobenzaldehyde (1 mmol), ethyl acetoacetate (1 mmol), dimedone (1 mmol), and ammonium acetate (1 mmol) for the synthesis of 1,4-dihydropyridine derivatives, the reaction of 4-chlorobenzaldehyde (1 mmol), dimedone (1 mmol), and malononitrile (1 mmol) for the synthesis of 4H-chromene derivatives, and the reaction of 4-chlorobenzaldehyde (1 mmol), isotonic anhydride (1 mmol), and ammonium acetate (1 mmol) for the synthesis of 2,3-dihydro quinazoline derivatives were considered as model reactions with and without g-C3N4@l-arginine nanocatalyst under different conditions. The reaction progress was monitored by Thin-layer chromatography (TLC). As can be seen in Table 1 (entries 1 and 2), no progress was observed for the model reactions without nanocatalyst. By introducing 1.00 mg of g-C3N4@l-arginine (Table 1, entry 3), however, the model reactions occurred easily. Then, the influence of other parameters including catalyst concentration, reaction time, and solvent were examined. As can be seen, time had not significant effect on the reaction progress, thus 15 min was considered as the optimum reaction time for all the model reactions (Table 1, entries 7, 8, and 9). Furthermore, the highest product yield was obtained using ethanol as solvent at 80 °C in the presence of 20.00 mg of g-C3N4@l-arginine (Table 1, entry 5).
Table 1.
Optimization of different parameters for model reactions 1 to 3.
| Entry | Catalyst | Catalyst loading (mg) | Solvent | Time (min) | Temperature (°C) | Yield (%) | ||
|---|---|---|---|---|---|---|---|---|
| Reaction (1)a | Reaction (2)b | Reaction (3)c | ||||||
| 1 | – | – | EtOH | 60 | r.t | – | – | – |
| 2 | – | – | EtOH | 60 | Reflux | – | – | – |
| 3 | g-C3N4@l-arginine | 1.00 | EtOH | 15 | Reflux | 53 | 60 | 57 |
| 4 | g-C3N4@l-arginine | 10.00 | EtOH | 15 | Reflux | 85 | 90 | 87 |
| 5 | g-C3N4@l-arginine | 20.00 | EtOH | 15 | Reflux | 94 | 97 | 96 |
| 6 | g-C3N4@l-arginine | 30.00 | EtOH | 15 | Reflux | 95 | 98 | 96 |
| 7 | g-C3N4@l-arginine | 20.00 | EtOH | 10 | Reflux | 89 | 97 | 93 |
| 8 | g-C3N4@l-arginine | 20.00 | EtOH | 20 | Reflux | 95 | 98 | 93 |
| 9 | g-C3N4@l-arginine | 20.00 | EtOH | 30 | Reflux | 96 | 98 | 94 |
| 10 | g-C3N4@l-arginine | 20.00 | CH2Cl2 | 15 | Reflux | 54 | 67 | 60 |
| 11 | g-C3N4@l-arginine | 20.00 | DMF | 15 | Reflux | 52 | 56 | 55 |
| 12 | g-C3N4@l-arginine | 20.00 | H2O | 15 | Reflux | 75 | 78 | 77 |
| 13 | g-C3N4@l-arginine | 20.00 | EtOH | 15 | r.t | 57 | 66 | 68 |
aReaction of 4-chlorobenzaldehyde (1 mmol), ethyl acetoacetate (1 mmol), dimedone (1 mmol), and ammonium acetate (1 mmol) for the synthesis of 1,4-dihydropyridine.
bReaction of 4-chlorobenzaldehyde (1 mmol), dimedone (1 mmol), and malononitrile (1 mmol) for the synthesis of 4H-chromene.
cReaction of 4-chlorobenzaldehyde (1 mmol), isotonic anhydride (1 mmol), and ammonium acetate (1 mmol) for the synthesis of 2,3-dihydroquinazoline.
In the following, various aldehydes were applied for the synthesis of 1,4-dihydropyridine, 4H-chromene, and 2,3-dihydro quinazoline derivatives under optimal reaction conditions. Based on model reactions that are provided in Tables 2, 3 and 4, a wide range of different derivatives of the desired multicomponent reactions were prepared with high yield.
Table 2.
Synthesis of 1,4-dihydropyridine derivatives using g-C3N4@l-arginine nanocatalyst.
| Entry | R | Product | Time (min) | Mp (°C) | Mp (°C, ref.) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | H | 5a | 10 | 217–219 | 218–220 17 | 95 |
| 2 | 4-Cl | 5b | 15 | 240–242 | 241–243 17 | 94 |
| 3 | 4-OH | 5c | 20 | 230–232 | 231–232 14 | 89 |
| 4 | 4-NO2 | 5d | 15 | 239–241 | 240–242 14 | 90 |
| 5 | 4-Me | 5e | 25 | 254–256 | 250 32 | 87 |
Reaction conditions: benzaldehyde (1 mmol), ethyl acetoacetate (1 mmol), dimedone (1 mmol), and ammonium acetate (1 mmol), g-C3N4@l-arginine (20 mg) and ethanol (7 mL) under reflux conditions.
Table 3.
Synthesis of 4H-chromene derivatives using g-C3N4@l-arginine nanocatalyst.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R | Product | Time (min) | Mp (°C) | Mp (°C, ref.) | Yield (%) |
| 1 | H | 9a | 7 | 228–229 | 225–228 33 | 95 |
| 2 | 4-Cl | 9b | 10 | 210–212 | 211–213 33 | 97 |
| 3 | 4-NO2 | 9c | 15 | 178–179 | 177–179 34 | 88 |
| 4 | 2,4-Cl | 9d | 10 | 118–120 | 115–117 24 | 95 |
| 5 | 4-OH | 9e | 20 | 206–208 | 208–210 24 | 93 |
| 6 | 4-Me | 9f. | 30 | 217–220 | 220–221 35 | 91 |
| 7 | 3-NO2 | 9g | 20 | 206–207 | 206–209 34 | 93 |
| 8 | 2-Cl | 9h | 15 | 213–214 | 211–213 35 | 96 |
| 9 | 4-CN | 9i | 25 | 184–187 | 184–186 35 | 89 |
| 10 | 4-OMe | 9j | 30 | 203–204 | 196–19833 | 87 |
Reaction conditions: Reaction of benzaldehyde (1 mmol), dimedone (1 mmol), and malononitrile (1 mmol) g-C3N4@l-arginine (20 mg) and ethanol (7 mL) under reflux conditions.
Table 4.
Synthesis of 2,3-dihydroquinazoline derivatives using g-C3N4@l-arginine nanocatalyst.
| Entry | R | Product | Time (min) | Mp (°C) | MP (°C , ref.) | Yield |
|---|---|---|---|---|---|---|
| 1 | H | 13a | 15 | 207–210 | 208–21021 | 90 |
| 2 | 4-Cl | 13b | 15 | 203–206 | 203–20636 | 96 |
| 3 | 3-NO2 | 13c | 30 | 192–193 | 190–19321 | 89 |
| 4 | 4-OH | 13d | 25 | 213–215 | 213–21536 | 87 |
| 5 | 2-Cl | 13e | 15 | 205–206 | 206–20822 | 95 |
| 6 | 4-Me | 13f. | 25 | 200–201 | 198–20122 | 86 |
| 7 | 4-NO2 | 13g | 20 | 201–202 | 200–20236 | 90 |
| 8 | 4-OMe | 13h | 25 | 180–182 | 178–18236 | 87 |
| 9 | 2,4-Cl | 13i | 35 | 163–166 | 165–16921 | 94 |
| 10 | 3-OH | 13j | 30 | 212–216 | 215–21736 | 89 |
Reaction conditions: benzaldehyde (1 mmol), isotonic anhydride (1 mmol), and ammonium acetate (1 mmol), C3N4@l-arginine (20 mg) and ethanol (7 mL) under reflux conditions.
Reusability of g-C3N4@l-arginine nanocatalyst
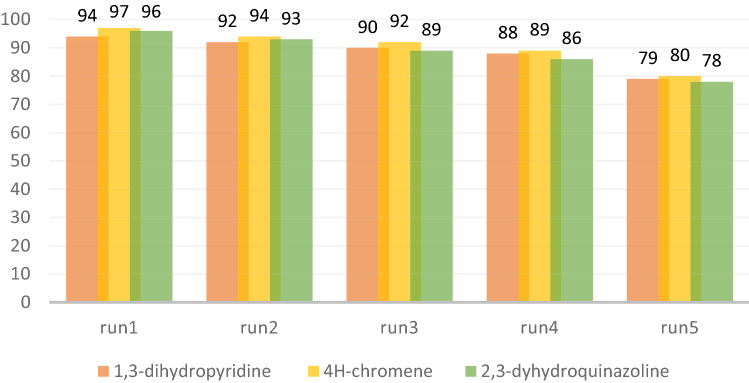
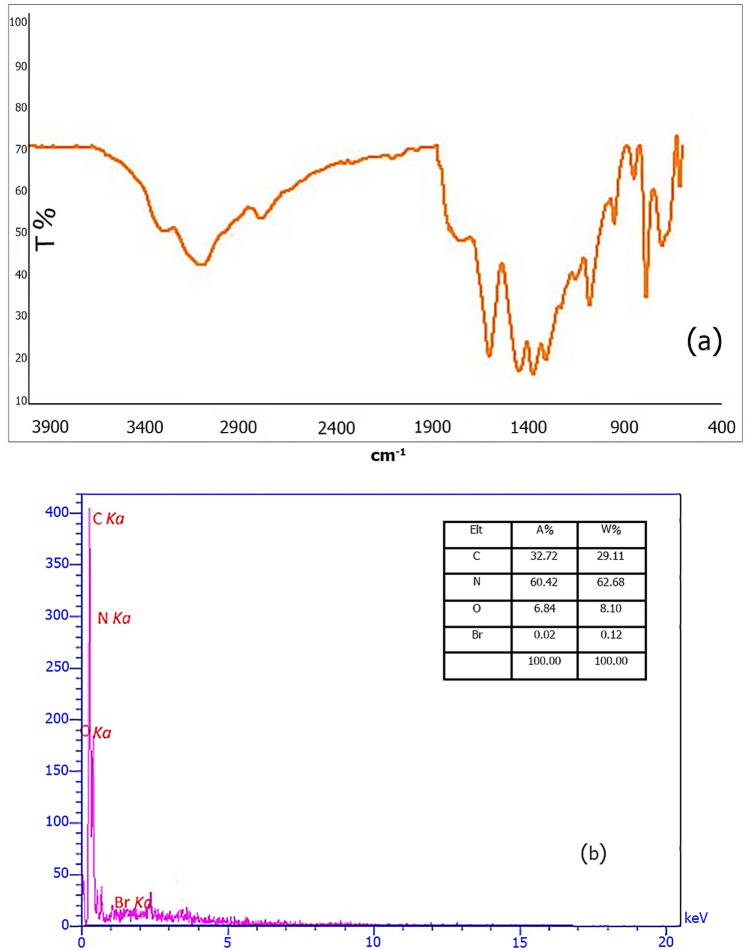
According to the importance of recovery and recyclability in green chemistry, in this section, the reusability of g-C3N4@l-arginine was examined for the synthesis of 1,3-dihydropyridine 5b, 4H-chromene 9b, and 2,3-dihydroquinazoline 13b products. For this purpose, after the first reaction (run 1), g-C3N4@l-arginine catalyst was separated from the reaction media, washed with ethanol, and dried in an oven at 70 °C. Then, the catalyst was reused for the next run. This was repeated for five times and the obtained yields were acceptable for catalytic reactions, and although performed reaction yields were decreased at each run bit by bit, in run 5th, the observed decrement was impressive in comparison with other runs (Fig. 8). EDX and FTIR spectra after the 5th run showed no significant changes in the primary structure of the g-C3N4@l-arginine nanocatalyst, as shown in Fig. 9.
Figure 8.
Examination of g-C3N4@l-arginine reusability in synthesis of 1, 3-dihydropyridine 5b, 4H-chromene 9b, and 2, 3-dihydroquinazoline 13b.
Figure 9.
EDX (b) and FT-IR spectra (a) of g-C3N4@l-arginine after the five-times recycling.
Mechanistic study of the prepared nanocatalyst in the synthesis of 1,4-dihydropyridine, 4H-chromene, and 2,3-dihydro quinazoline derivatives
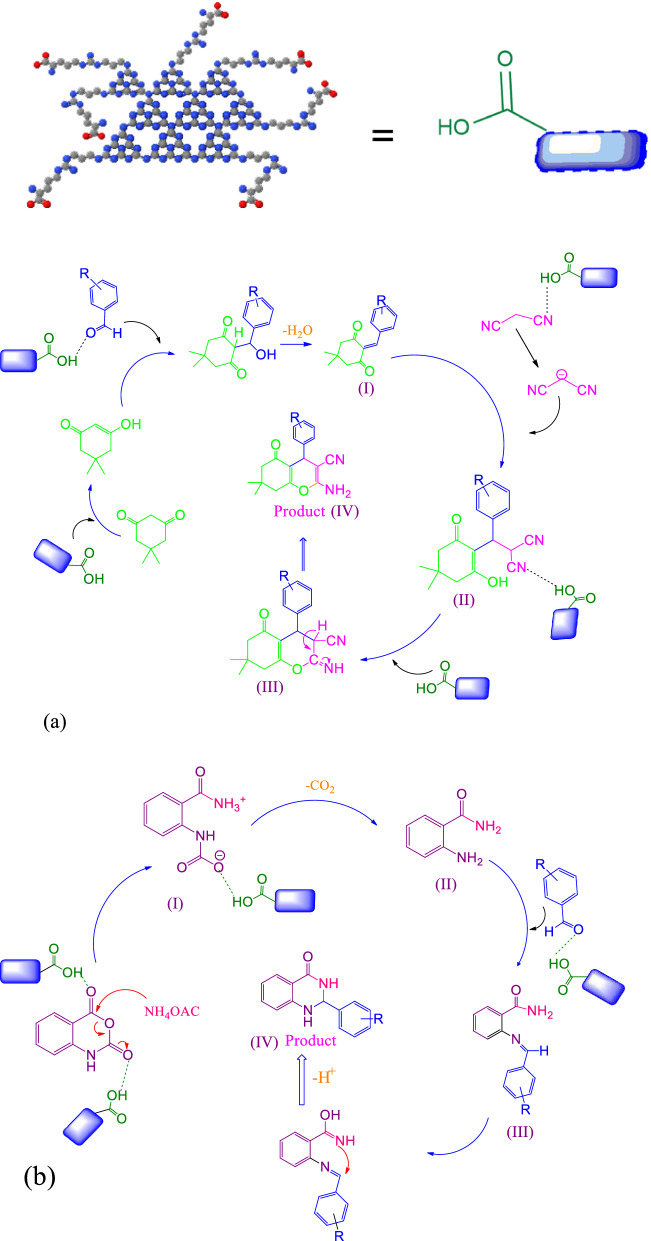
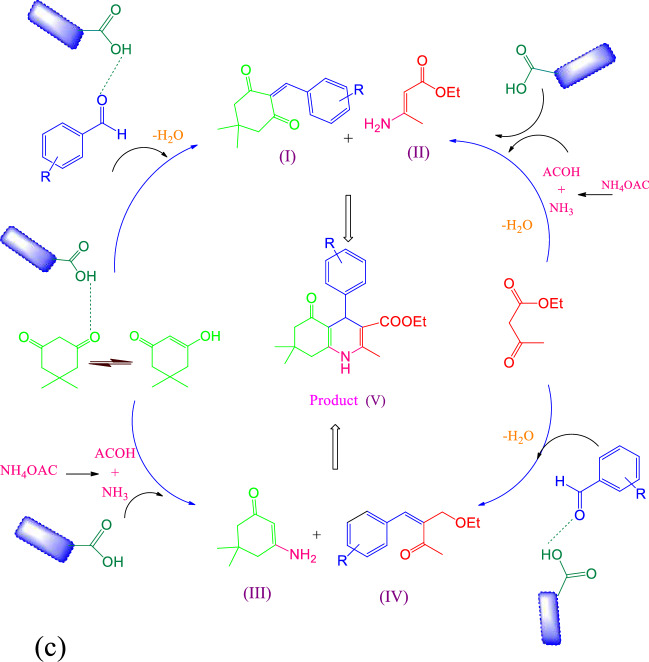
In Fig. 10, the suitable mechanism for the formation of 1,4-dihydropyridine, 2,3-dihydro quinazoline, and 4H-chromene derivatives are provided. In each reaction, the presence of g-C3N4@l-arginine can activate reactants and different intermediates. As can be seen in Fig. 10a, 1,4-dihydropyridine derivatives can be synthesized in two methods. In the first method, aldehyde and dimedone produce intermediate I in the presence of g-C3N4@l-arginine, and the intermediate II is formed from the reaction between ethyl acetoacetate and ammonium acetate. But in the second method, dimedone and ammonium acetate produce intermediate III in the presence of g-C3N4@l-arginine, and the reaction between ethyl acetoacetate and aldehyde forms intermediate IV. Both methods ultimately lead to the formation of product V.
Figure 10.
Proposed mechanism for synthesis of 4H-chromene derivatives (a), 2,3-dihydro quinazoline (b), and 1,4-dihydropyridine (c) by using g-C3N4@l-arginine.
A suggested mechanism for the formation of 2,3-dihydro quinazoline derivatives is shown in Fig. 10b. At first, isotonic anhydride reacts with ammonium acetate in the presence of g-C3N4@l-arginine and produces intermediate I, then aldehyde activates by g-C3N4@l-arginine and adds to intermediate II. Finally, after removing H, the desired product IV is synthesized.
Figure 10c presents a probable method for the synthesis of 4H-chromene derivatives in the presence of g-C3N4@l-arginine is. In this mechanism, intermediate I is produced from the reaction between aldehyde and dimedone. Then, addition of malononitrile leads to the formation of intermediate II. At last, product IV is obtained.
Catalytic activity of the synthesized nanocatalyst
Tables 5, 6 and 7 show the performance of g-C3N4@l-arginine in comparison with the catalysts reported in the literature for the synthesis of 1,4-dihydropyridine, 4H-chromene, and 2,3-dihydro quinazoline derivatives. For this purpose, various parameters such as catalyst concentration, reaction time, reaction temperature, and reaction yield were investigated. According to the data presented in each table, g-C3N4@l-arginine can be considered as a unique heterogonous nanocatalyst that can be used in a wide range of condensation reactions in addition to simple separation conditions of the reaction mixture. On the other hand, this nanocatalyst exceptionally showed higher synthesis yield at shorter reaction times.
Table 5.
Comparison of catalytic activity of g-C3N4@l-arginine with other reported catalysts for the synthesis of 4H-chromene derivatives.
| Entry | Catalyst | Solvent/temperature | Time (min) | Chromene yield (%) | Ref |
|---|---|---|---|---|---|
| 1 | Fe3O4@MCM-41@Zr-piperazine-MNPs | EtOH/H2O/75 °C | 40 | 74 | 37 |
| 2 | AIL@MNP | Solvent-free/90 °C | 25 | 89 | 38 |
| 3 | MCM-41@Schiff base-Co(OAC)2 | H2O/50 °C | 180 | 94 | 23 |
| 4 | Yb(NPf2)3 | EtOH/80 °C | 240 | 91 | 24 |
| 5 | g-C3N4@l-arginine | EtOH/reflux | 7 | 95 | This work |
Reaction conditions: benzaldehyde (1 mmol), dimedone (1 mmol), malononitrile (1.5 mmol), g-C3N4@l-arginine catalyst (20.00 mg), and ethanol (7 mL) under reflux.
Table 6.
Comparison of catalytic activity of g-C3N4@l-arginine with other reported catalysts for the synthesis of 1,4-dihydropyridine derivatives.
| Entry | Catalyst | Solvent/temperature | Time (min) | Hanztsch yield (%) | Ref |
|---|---|---|---|---|---|
| 1 | BNPs @ Si(CH2)3@NH SO3H | EtOH/reflux | 25 | 95 | 39 |
| 2 | Aluminized polyborate | Solvent-free/100 °C | 15 | 94 | 14 |
| 3 | Cell-Pr-NHSO3H | EtOH/reflux | 45 | 91 | 40 |
| 4 | MCM-41@serine@Cu(II) | EtOH/80 °C | 170 | 96 | 25 |
| 5 | g-C3N4@l-arginine | EtOH/reflux | 10 | 95 | This work |
Reaction conditions: Reaction of benzaldehyde (1 mmol), dimedone (1 mmol), and malononitrile (1 mmol) g-C3N4@l-arginine (20 mg) and ethanol (7 mL) under reflux conditions.
Table 7.
Comparison of catalytic activity of g-C3N4@l-arginine with other reported catalysts for the synthesis of 2,3-dihydro quinazoline derivatives.
| Entry | Catalyst | Solvent/temperature | Time (min) | Quinazoline yield (%) | Ref |
|---|---|---|---|---|---|
| 1 | Titanium silicon oxide nanopowder | H2O/100 °C | 120 | 94 | 26 |
| 2 | Wang-OSO3H | H2O/100 °C | 24 | 84 | 22 |
| 3 | Y(NO3)3.6H2O | CH3CN | 300 | 97 | 27 |
| 4 | Montmorillonite-KSF | Solvent-free/100 °C | 150 | 93 | 41 |
| 5 | g-C3N4@l-arginine | EtOH/reflux | 15 | 96 | This work |
Reaction condition: 4-chlorobenzaldehyde (1mmol), isotonic anhydride (1mmol), and ammonium acetate (1mmol), g-C3N4@l-arginine catalyst (20.00 mg), and ethanol (7 mL) under reflux.
Experimental
Reagents and apparatus
All chemicals were purchased from Merck and Sigma-Aldrich Co. Fourier Transform Infrared (FTIR) spectra were recorded on Tensor27. Nuclear Magnetic Resonance (NMR) data were acquired on a Varian-Inova 500 MHz. X-Ray Diffraction (XRD) patterns were obtained using Dron-8 diffractometer. Energy-dispersive X-ray (EDX) spectrum was recorded on Numerix DXP–X10P. Thermal gravimetric analysis (TGA) was performed using STA 504 instrument under argon atmosphere. Field Emission Scanning Electron Microscopy (FESEM) images were recorder with TESCAN-MIRA III.
Preparation of bulk g-C3N4 and g-C3N4 nanosheets
For the synthesis of bulk g-C3N4, the melamine was heated at 550 °C in a furnace at the heating rate of 2.5 °C min−1 in static air for 4 h. A yellow powder was obtained which was then grounded in a ball mill. For the synthesis of g-C3N4 nanosheets, bulk g-C3N4 (1.0 g) was first stirred in H2SO4 (20 mL) at 90 °C for 5 h. The solution was then diluted with ethanol (200 mL) and stirred again at room temperature for 2 h. The resulting product was dispersed in 100.0 mL water/isopropanol (1:1) solution and sonicated for 6 h. Finally, the formed suspension was centrifuged at 5000 rpm to separate g-C3N4 nanosheets.
Preparation of g-C3N4@l-arginine
g-C3N4 (1.0 g) nanosheets were dispersed in dry toluene (20.0 mL). Then, the reaction mixture was refluxed under N2 atmosphere for 24 h after addition of 1,3-dibromopropane (2.0 mL). Finally, the product was filtered and washed with ethyl acetate, and dried at room temperature. The resulting product was dissolved in a mixture of water and methanol (1:1) followed by the addition of l-arginine (1 mmol), K2CO3 (1.0 mmol), and NaI (1.0 mmol). The solution was stirred at room temperature for 24 h. the reaction mixture was then washed with water and methanol and dried at room temperature.
General procedure for the synthesis of 1,4-dihydropyridine derivatives
A mixture of aldehyde (1.0 mmol), ethyl acetoacetate (1.0 mmol), dimedone (1.0 mmol), ammonium acetate (1.0 mmol), g-C3N4@l-arginine (20.0 mg), and ethanol (2.0 mL) was added in a round bottom flask and refluxed at 70 °C. When the reaction was completed (monitored by TLC), the catalyst was separated by filtration and washed with ethanol, and then to purifying the product was used recrystallization.
General procedure for the synthesis of 2,3-dihydro quinazoline derivatives
In a round bottom flask, aldehyde (1.0 mmol), isotonic anhydride (1.0 mmol), ammonium acetate (2.0 mmol), and g-C3N4@l-arginine (20.0 mg) were added and refluxed in ethanol (2.0 mL) at 70 °C. After reaction completion (monitored by TLC), the catalyst was removed by filtration and washed with ethanol, and then to purifying the product was used recrystallization.
General procedure for the synthesis of 4H-chromene derivatives
In a round bottom flask was added aldehyde (1.0 mmol), dimedone (1.0 mmol), malononitrile (1.0 mmol), g-C3N4@l-arginine (20.0 mg), and ethanol (2.0 mL). The mixture was then refluxed at 70 °C until the reaction was completed (monitored by TLC). At last, catalyst separation by filtration and washed with ethanol, and then to purifying the product was used recrystallization.
Conclusions
In summary, heterogeneous g-C3N4@-arginine nanocatalyst was prepared and used for the synthesis of 1,4-dihydropyridine, 4H-chromene, and 2,3-dihydro quinazoline derivatives as important products in pharmacologically active compounds. The main advantages of this nanocatalyst is its reusability, simple separation from the reaction mixture, applicability for a broad range of high efficiency condensation reactions, and short reaction time. In addition, the use of an easy and convenient method for the preparation of the nanocatalyst is another advantage of this catalyst over other reported catalysts.
Selected spectral data
Ethyl 2,7,7-trimethyl-5-oxo-4-(4-hydroxylphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (5c)
FTIR (KBr, cm-1): 3270, 3194, 3071, 2957, 1678, 1645, 1481, 1377, 1214 cm-1. 1H NMR (500 MHz, DMSO): δ H (ppm) = 0.85(s, 3H, CH3), 1.0(s, 3H, CH3), 1.13(t, 3H, CH3), 1.9–2.41(m,4H, 2CH2), 2.25(s, 3H, CH3), 3.95–3.99(q, 2H, OCH2), 4.73(s, 1H, Ar–CH), 6.54(d, 2H, Ar–H), 6.93(d, 2H, Ar–H), 8.95(s, 1H, NH), 9.01(s,1H, OH).
Ethyl 1,4,7,8-tetrahydro-2,7,7-trimethyl-4-(4-nitrophenyl)-5(6H)-oxoquinoline-3-carboxylate (5d)
FTIR (KBr, cm-1): 3276, 3210, 3076, 2969, 2902, 1703, 1641, 1530, 1379 cm-1. 1H NMR (500 MHz, DMSO): δH (ppm) = 0.83(s, 3H, CH3), 1.01(s, 3H, CH3), 1.11(t, 3H, CH3), 1.96–2.46(m,4H, 2CH2), 2.31(s, 3H, CH3), 3.93–4.0(m, 2H, OCH2), 4.97(s, 1H, Ar–CH), 7.5–7.61 (m, 4H, Ar–H), 7.97(s, 1H, NH), 9.23(s,1H, OH).
2-amino-4-(4-nitrophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (9c)
FTIR(KBr, cm-1): 3403, 3312, 3170, 2969, 2881,2181, 1668, 1626, 1517, 1345, 856 cm-1.1 H NMR (500 MHz, DMSO): δ H (ppm) = 0.95(s, 3H, CH3), 1.04(s, 3H, CH3), 2.09–2.53(m, 4H, 2CH2), 4.36(s, 1H, CH), 7.1(s, 2H, NH2), 7.43–8.17(m, 4H, Ar–H).
2-phenyl-2, 3-dihydro-4(1H)-quinazolinone (13a)
FTIR (KBr, cm-1): 3300, 3176, 2981, 1651, 1610, 1507, 1440, 1385, 745 cm-1. 1H NMR (500 MHz, DMSO): δ H (ppm) = 5.75(s, 1H, CH), 6.67(t, 1H, Ar–H), 6.74(d, 1H, Ar–H), 7,1(s, 1H, NH), 7.23(t, 1H, Ar–H), 7.34(t, 1H, Ar–H), 7.38(t, 1H, Ar–H), 7.49(d, 1H, Ar–H), 7.60(d, 1H, Ar–H), 8.27(s, 1H, CONH).
2-(4-chloro-phenyl)-2, 3-dihydro-1H-quinazoline-4-one (13b)
FTIR (KBr, cm-1): 3305, 3184, 3062, 1654, 1606, 1431, 1090, 749 cm-1. 1H NMR (500 MHz, DMSO): δ H (ppm) = 5.77(s, 1H, CH), 6.68(t, 1H, Ar–H), 6.74(d, 1H, Ar–H), 7,1(s, 1H, NH), 7.24(t, 1H, Ar–H), 7.45(d, 1H, Ar–H), 7.50(d, 1H, Ar–H), 7.61(d, 1H, Ar–H), 8.27(s, 1H, CONH).
Supplementary Information
Acknowledgements
We are grateful for the financial support from The Research Council of Iran University of Science and Technology (IUST), Tehran, Iran.
Author contributions
All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-97360-x.
References
- 1.Rashidizadeh A, Ghafuri H, EsmailiZand HR, Goodarzi N. Graphitic carbon nitride nanosheets covalently functionalized with biocompatible vitamin B1: synthesis, characterization, and its superior performance for synthesis of quinoxalines. ACS Omega. 2019;4(7):12544–12554. doi: 10.1021/acsomega.9b01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahmati, M. & Ghafuri, H. Catalytic Strecker reaction: gC 3 N 4-anchored sulfonic acid organocatalyst for the synthesis of α-aminonitriles. Research on Chemical Intermediates, 1–14 (2021). https://www.x-mol.com/paperRedirect/1359656597111214080
- 3.Zhang Z, Liu K, Feng Z, Bao Y, Dong B. Hierarchical sheet-on-sheet ZnIn2S4/gC3N4 heterostructure with highly efficient photocatalytic H2 production based on photoinduced interfacial charge transfer. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep19221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar R, Barakat M, Alseroury F. Oxidized gC3N4/polyaniline nanofiber composite for the selective removal of hexavalent chromium. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-12850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong G, Zhang Y, Pan Q, Qiu J. A fantastic graphitic carbon nitride (g-C3N4) material: electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014;20:33–50. doi: 10.1016/j.jphotochemrev.2014.04.002. [DOI] [Google Scholar]
- 6.Wu Y, et al. Electrocatalytic performances of gC3N4-LaNiO3 composite as bi-functional catalysts for lithium-oxygen batteries. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han H, et al. Cu and boron doped carbon nitride for highly selective oxidation of toluene to benzaldehyde. Molecules. 2015;20:12686–12697. doi: 10.3390/molecules200712686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davarpanah J, Ghahremani M, Najafi O. Synthesis of 1, 4-dihydropyridine and polyhydroquinoline derivatives via Hantzsch reaction using nicotinic acid as a green and reusable catalyst. J. Mol. Struct. 2019;1177:525–535. doi: 10.1016/j.molstruc.2018.10.002. [DOI] [Google Scholar]
- 9.Kusampally U, Dhachapally N, Kola R, Kamatala CR. Zeolite anchored Zr-ZSM-5 as an eco-friendly, green, and reusable catalyst in Hantzsch synthesis of dihydropyridine derivatives. Mater. Chem. Phys. 2020;242:122497. doi: 10.1016/j.matchemphys.2019.122497. [DOI] [Google Scholar]
- 10.Alponti LH, Picinini M, Urquieta-Gonzalez EA, Corrêa AG. USY-zeolite catalyzed synthesis of 1,4-dihydropyridines under microwave irradiation: Structure and recycling of the catalyst. J. Mol. Struct. 2021;1227:129430. doi: 10.1016/j.molstruc.2020.129430. [DOI] [Google Scholar]
- 11.Das D. Multicomponent reactions in organic synthesis using copper-based nanocatalysts. ChemistrySelect. 2016;1:1959–1980. doi: 10.1002/slct.201600414. [DOI] [Google Scholar]
- 12.Domling A, Wang W, Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khazaei A, Moosavi-Zare AR, Mohammadi Z, Khakyzadeh V, Afsar J. Nano-TiO2 as an efficient catalyst for tandem Knoevenagel–Michael-cyclocondensation reaction of dimedone with aromatic aldehydes and ammonium acetate or aromatic amines under solvent-free conditions. J. Chin. Chem. Soc. 2016;63:165–170. doi: 10.1002/jccs.201500384. [DOI] [Google Scholar]
- 14.Aute D, Kshirsagar A, Uphade B, Gadhave A. Aluminized polyborate-catalysed green and efficient synthesis of polyhydroquinolines under solvent-free conditions. Res. Chem. Intermed. 2020;46:3491–3508. doi: 10.1007/s11164-020-04158-z. [DOI] [Google Scholar]
- 15.Zhang Q, Ma X-M, Wei H-X, Zhao X, Luo J. Covalently anchored tertiary amine functionalized ionic liquid on silica coated nano-Fe3O4 as a novel, efficient and magnetically recoverable catalyst for the unsymmetrical Hantzsch reaction and Knoevenagel condensation. RSC Adv. 2017;7:53861–53870. doi: 10.1039/C7RA10692K. [DOI] [Google Scholar]
- 16.Kiyani H, Ghorbani F. Boric acid-catalyzed multi-component reaction for efficient synthesis of 4H-isoxazol-5-ones in aqueous medium. Res. Chem. Intermed. 2015;41:2653–2664. doi: 10.1007/s11164-013-1411-x. [DOI] [Google Scholar]
- 17.Yü S-J, Wu S, Zhao X-M, Lü C-W. Green and efficient synthesis of acridine-1,8-diones and hexahydroquinolines via a KH2PO4 catalyzed Hantzsch-type reaction in aqueous ethanol. Res. Chem. Intermed. 2017;43:3121–3130. doi: 10.1007/s11164-016-2814-2. [DOI] [Google Scholar]
- 18.Zhang Q, Wei H, Li J, Zhao X, Luo J. One-pot synthesis of benzopyrans catalyzed by silica supported dual acidic ionic liquid under solvent-free conditions. Heterocycl. Commun. 2017;23:411–414. doi: 10.1515/hc-2017-0163. [DOI] [Google Scholar]
- 19.Lu J, Fu X, Zhang G, Wang C. β-Cyclodextrin as an efficient catalyst for the one-pot synthesis of tetrahydrobenzo [b] pyran derivatives in water. Res. Chem. Intermed. 2016;42:417–424. doi: 10.1007/s11164-015-2027-0. [DOI] [Google Scholar]
- 20.Malviya J, Kala S, Sharma L, Singh R. Efficient three-component one-pot synthesis of 4 H-Pyrans. Russ. J. Org. Chem. 2019;55:686–693. doi: 10.1134/S1070428019050178. [DOI] [Google Scholar]
- 21.Mirjalili BBF, Zaghaghi Z, Monfared A. Synthesis of 2,3-dihydroquinazolin-4 (1H)-ones in the presence of Fe3O4@ nano-cellulose–OPO3H as a bio-based magnetic nanocatalyst. J. Chin. Chem. Soc. 2020;67:197–201. doi: 10.1002/jccs.201900264. [DOI] [Google Scholar]
- 22.Rao AD, et al. Sulfonic acid functionalized Wang resin (Wang-OSO3H) as polymeric acidic catalyst for the eco-friendly synthesis of 2,3-dihydroquinazolin-4 (1H)-ones. Tetrahedron Lett. 2015;56:4714–4717. doi: 10.1080/00397911.2016.1213850. [DOI] [Google Scholar]
- 23.Pan S, et al. MCM-41@ Schiff base-Co (OAc) 2 as an efficient catalyst for the synthesis of pyran derivatives. Res. Chem. Intermed. 2020;46:1353–1371. doi: 10.1080/00397911.2016.1213850. [DOI] [Google Scholar]
- 24.Hong M, Cai C. Ytterbium(III) bis (perfluorooctanesulfonyl) imide catalyzed one-pot synthesis of tetrahydrobenzo [b] pyrans in fluorous biphase system. J. Chem. Res. 2010;34:568–570. doi: 10.3184/030823410X12863845344182. [DOI] [Google Scholar]
- 25.Tamoradi T, Ghadermazi M, Ghorbani-Choghamarani A. Synthesis of polyhydroquinoline, 2,3-dihydroquinazolin-4 (1H)-one, sulfide and sulfoxide derivatives catalyzed by new copper complex supported on MCM-41. Catal. Lett. 2018;148:857–872. doi: 10.1007/s10562-018-2311-x. [DOI] [Google Scholar]
- 26.Mekala R, et al. Efficient synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones catalyzed by titanium silicon oxide nanopowder in aqueous media. Synth. Commun. 2017;47:121–130. doi: 10.1080/00397911.2016.1254801. [DOI] [Google Scholar]
- 27.Khan AA, Mitra K, Mandal A, Baildya N, Mondal MA. Yttrium nitrate catalyzed synthesis, photophysical study, and TD-DFT calculation of 2,3-dihydroquinazolin-4 (1H)-ones. Heteroat. Chem. 2017;28:e21379. doi: 10.1002/hc.21379. [DOI] [Google Scholar]
- 28.Guo X, Duan J, Li C, Zhang Z, Wang W. Highly efficient Z-scheme gC3N4/ZnO photocatalysts constructed by co-melting-recrystallizing mixed precursors for wastewater treatment. J. Mater. Sci. 2020;55:2018–2031. doi: 10.1007/s10853-019-04097-0. [DOI] [Google Scholar]
- 29.Yang X, Tang B, Wu T, Cao X. g-C3N4/TiO2 composite photocatalyst and its application to asphalt for NO removal. J. Mater. Civ. Eng. 2019;31:04019141. doi: 10.1061/(ASCE)MT.1943-5533.0002763. [DOI] [Google Scholar]
- 30.Li X, et al. Synergistic effect of efficient adsorption g-C3N4/ZnO composite for photocatalytic property. J. Phys. Chem. Solids. 2014;75:441–446. doi: 10.1016/j.jpcs.2013.12.001. [DOI] [Google Scholar]
- 31.Rahmati M, Ghafuri H, Ghanbari N, Tajik Z. 1,4-butanesultone functionalized graphitic carbon nitride: efficient catalysts for the one-pot synthesis of 1,4-dihydropyridine and polyhydroquinoline derivative through Hantzsch reaction. Polycycl. Arom. Compd. 2020 doi: 10.1080/10406638.2020.1852583. [DOI] [Google Scholar]
- 32.Maleki A, Eskandarpour V, Rahimi J, Hamidi N. Cellulose matrix embedded copper decorated magnetic bionanocomposite as a green catalyst in the synthesis of dihydropyridines and polyhydroquinolines. Carbohyd. Polym. 2019;208:251–260. doi: 10.1016/j.carbpol.2018.12.069. [DOI] [PubMed] [Google Scholar]
- 33.Brahmachari G, Laskar S, Banerjee B. Eco-friendly, one-pot multicomponent synthesis of pyran annulated heterocyclic scaffolds at room temperature using ammonium or sodium formate as non-toxic catalyst. J. Heterocycl. Chem. 2014;51:E303–E308. doi: 10.1002/jhet.1974. [DOI] [Google Scholar]
- 34.Qareaghaj OH, Mashkouri S, Naimi-Jamal MR, Kaupp G. Ball milling for the quantitative and specific solvent-free Knoevenagel condensation+ Michael addition cascade in the synthesis of various 2-amino-4-aryl-3-cyano-4 H-chromenes without heating. RSC Adv. 2014;4:48191–48201. doi: 10.1039/C4RA06603K. [DOI] [Google Scholar]
- 35.Dekamin MG, Eslami M. Highly efficient organocatalytic synthesis of diverse and densely functionalized 2-amino-3-cyano-4 H-pyrans under mechanochemical ball milling. Green Chem. 2014;16:4914–4921. doi: 10.1039/C4GC00411F. [DOI] [Google Scholar]
- 36.Ghafuri H, Goodarzi N, Rashidizadeh A, Fard MAD. ompg-C 3 N 4/SO 3 H: an efficient and recyclable organocatalyst for the facile synthesis of 2, 3-dihydroquinazolin-4 (1 H)-ones. Res. Chem. Intermed. 2019;45:5027–5043. doi: 10.1007/s11164-019-03873-6. [DOI] [Google Scholar]
- 37.Pourhasan-Kisomi R, Shirini F, Golshekan M. Introduction of organic/inorganic Fe3O4@MCM-41@ Zr-piperazine magnetite nanocatalyst for the promotion of the synthesis of tetrahydro-4H-chromene and pyrano [2,3-d] pyrimidinone derivatives. Appl. Organomet. Chem. 2018;32:e4371. doi: 10.1002/aoc.4371. [DOI] [Google Scholar]
- 38.Zhang Q, Gao Y-H, Qin S-L, Wei H-X. Facile one-pot synthesis of amidoalkyl naphthols and benzopyrans using magnetic nanoparticle-supported acidic ionic liquid as a highly efficient and reusable catalyst. Catalysts. 2017;7:351. doi: 10.3390/catal7110351. [DOI] [Google Scholar]
- 39.Khodabakhshi, M. R., Kiamehr, M. & Karimian, R. Efficient one-pot synthesis of 1,4-dihydropyridine and polyhydroquinoline derivatives using sulfanilic acid-functionalized boehmite nano-particles as an organic-inorganic hybrid catalyst. Polycyclic Aromat. Compd. 1–15. 10.1080/10406638.2021.1884100 (2021).
- 40.Karhale S, Bhenki C, Rashinkar G, Helavi V. Covalently anchored sulfamic acid on cellulose as heterogeneous solid acid catalyst for the synthesis of structurally symmetrical and unsymmetrical 1, 4-dihydropyridine derivatives. New J. Chem. 2017;41:5133–5141. doi: 10.1039/C7NJ00685C. [DOI] [Google Scholar]
- 41.Tekale SU, Munde SB, Kauthale SS, Pawar RP. An efficient, convenient, and solvent-free synthesis of 2,3-dihydroquinazolin-4 (1 H)-ones using montmorillonite-KSF clay as a heterogeneous catalyst. Org. Prep. Proced. Int. 2018;50:314–322. doi: 10.1080/00304948.2018.1462058. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.