Abstract
Advancements in hematopoietic cell transplantation (HCT) have led to increased survivorship rates in many childhood diseases. However, this growing group of long-term survivors face a myriad of late effects. There are currently limited guidelines for surveillance of gastrointestinal polyps for pediatric transplant patients. Here we describe five patients undergoing HCT with total body irradiation-based conditioning regimens for leukemia who developed symptomatic polyps a median of 4.5 (range, 0.75 – 5.75) years after HCT. Due to limited surveillance guidelines in children, we conclude that the development of new or progressive symptoms related to the gastrointestinal tract deserves prompt recognition and evaluation.
Keywords: gastrointestinal polyps, hematopoietic cell transplantation, total body irradiation, late effects, leukemia
INTRODUCTION
Improvements in supportive care, donor selection, and timing of hematopoietic cell transplantation (HCT) have increased rates of longevity in several childhood disorders leading to larger cohorts of transplant survivors. A report from the Bone Marrow Transplant Survivor Study found that in patients who survived for at least two years, non-relapse mortality was seen in 9–12% at 10 years after transplant1. However, for most patients who survive, risks for health-related complications associated with their prior treatments continue to exist. Screening for late effects and early detection can decrease late mortality and improve the quality of life for survivors. National consensus conferences have provided extensive guidelines for practitioners to follow, including timelines for testing based on prior disease risk factors and treatment2. While the gastrointestinal (GI) system is a frequent target of injury in the early period following HCT, with the exception of the hepatobiliary system, extensive guidelines for long-term surveillance of the GI system are limited3.
Colorectal polyps are found in 6.1% of children undergoing colonoscopy, with incidence increasing to 12% when the indications for the procedure include hematochezia4. Although most polyps in children are benign, some are associated with genetic disorders and pre-malignancy5,6. Given the role of inflammation and environmental factors leading to their potential development, it is not surprising that GI polyps have also been discovered in the post-HCT setting7,8. In one retrospective study, approximately 15% of gastric polyps were discovered in the transplant population (including solid organ transplants), of which 30% were in HCT recipients 9. However, there is limited data on their incidence, prevalence, and risk factors contributing to their development after HCT.
In this report, we present five cases of children who were treated for either acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML), and who each underwent HCT following relapse (n=4) or high-risk status in first complete remission (n=1). Each patient presented with symptomatic GI polyp formation after HCT using conditioning containing 12–14 Gy total body irradiation (TBI).
MATERIALS AND METHODS
The clinical information of five subjects transplanted for hematologic malignancies at Children’s Hospital of Wisconsin between 1980–2017 was retrospectively collected from medical records following an institutional review board–approved research application. Data collected included demographics, therapy prior to transplant, transplant regimen and side effects, and presentation of GI polyps.
RESULTS
Case Reports (see Table 1)
TABLE 1:
Characteristics of Five Patients with Identified GI Polyps
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Sex / Cancer Diagnosis / Age of Diagnosis | Female / AML / 10 months | Male / ALL / 2 years 11 months | Female / ALL / 9 years 8 months | Male / ALL / 1 year 9 months | Male / ALL / 15 years 11 months |
| Initial Cancer Therapy | CCG regimen C 2891: IT Ara-C, 6-thioguanine, Decadron Ara-C, daunomycin etoposide | POG Protocol 9605: prednisone, vincristine, L-asparaginase, IT methotrexate, Ara-C, and hydrocortisone and continuation therapy with 6-MP, methotrexate, vincristine, prednisone, and IT methotrexate | POG Protocol 9605: prednisone, vincristine, L-asparaginase, IT methotrexate, Ara-C, and hydrocortisone and continuation therapy with 6-MP, methotrexate, vincristine, prednisone, and IT methotrexate. | CCG regimen 1991: TBI, thiotepa, cyclophosphamide, methotrexate, and tacrolimus | COG AALL1131: Ara-C, vincristine, prednisone daunorubicin peg-asparaginase, IT methotrexate |
| Re-Induction | Twice weekly Ara-C and idarubicin | Vincristine, prednisone, L-asparaginase and daunomycin | Prednisone, doxorubicin, L-asparaginase, vincristine, dexamethasone, intrathecal Ara-C, methotrexate | Methotrexate, Ara-C, etoposide, cyclophosphamide, vincristine, mercaptopurine, dexamethasone, doxorubicin, and pegaspargase | N/A |
| HCT Conditioning | Cyclophosphamide, Ara-C, ATG, TBI 1200 cGy with 900 cGy cranial radiation | Ara-C, cyclophosphamide, TBI 1400 cGy | Ara-C, cyclophosphamide, TBI 1400 cGy, with 900 cGy cranial boost | Thiotepa, cyclophosphamide, TBI 1200 cGy | Thiotepa, cyclophosphamide, TBI 1200 cGy with testicular boost |
| Donor Source and HLA Matching | Mother - 5/10 HLA Matched CD34-selected peripheral blood stem cell infusion | Unrelated Donor – 8/8 HLA Matched partially T-cell depleted bone marrow transplant | Unrelated Donor – 7/8 HLA Matched partially T-cell depleted bone marrow transplant | Sister – 8/8 HLA Matched CD34-selected peripheral blood stem cell infusion | Unrelated Donor – 10/10 HLA Matched partially T-cell depleted bone marrow transplant |
| Post-Transplant Immune Suppression | None | Cyclosporine | Cyclosporine | Tacrolimus | Tacrolimus, sirolimus |
| Max Acute GVHD | None | Skin stage 2, GI stage 4 (Grade III overall) | Skin stage 1, GI stage 4 (Grade III overall) | GI stage 1 (Grade II overall) | Skin grade 1, GI stage 4 (Grade III overall) |
| Chronic GVHD | None | Chronic Skin GVHD | Chronic Skin GVHD | None | Chronic Skin & GI GVHD |
| Time from HCT to 1st Polyp Identification | 4.5 years | 4.5 years | 5.75 years | 3.25 years | 0.75 years |
| Family History | Father had stomach ulcers | Maternal grandfather had 29 colonic polyps | None | None | None |
CCG – Children’s Cancer Study Group; COG – Children’s Oncology Group; GI – gastrointestinal; GVHD – graft-versus-host disease; HCT – hematopoietic cell transplantation; HLA – human leukocyte antigen; IT – intrathecal; POG – Pediatric Oncology Group
Patient 1 was transplanted for relapsed AML. She developed Clostridioides difficile (C. difficile) diarrhea on the day of transplant and tested positive for stool adenovirus on day +28 until 8 months post-transplant, resolving with cidofovir treatment. During this time, she underwent an esophagogastroduodenoscopy (EGD) for further evaluation which was unremarkable. At 4.5 years after transplant, she developed hematochezia and underwent an EGD and colonoscopy. She was found to have two colorectal polyps (transverse colon and rectal) with final pathology demonstrating juvenile polyps (Figure 1), and one hyperplastic gastric polyp with erosion and inflammation. At 6.5 years after transplant, another juvenile polyp was discovered in her ascending colon. In retrospect, rectal bleeding had been intermittently observed in the preceding months.
FIGURE 1: Representative Colonic Polyps from Patient 1 (1A) and Patient 3 (1B).
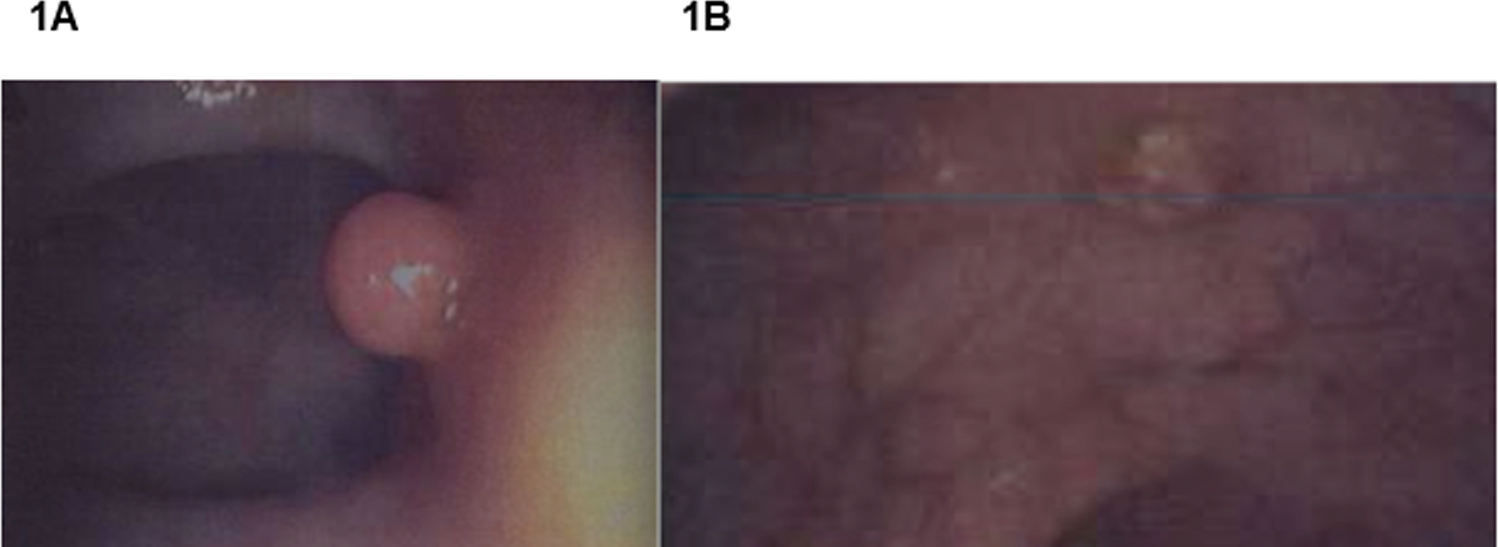
1A) A pedunculated juvenile polyp found in the ascending colon of Patient 1. 1B): A sessile polyp found in the ascending colon of Patient 3. Although these lesions are located in the same portion of the colon, the precancerous potential of the sessile polyp in Patient 3 is more concerning.
Patient 2 was transplanted for relapsed ALL. Six weeks after transplant, he presented with hematochezia and abdominal pain, and clinically diagnosed with severe (stage 4) acute gut GI graft versus host disease GVHD. He underwent a flexible sigmoidoscopy revealing friability of mucosa and necrosis in the ascending colon and histologic evidence of GVHD. After a flare of GI GVHD one month later, repeat sigmoidoscopy revealed an edematous ascending colon with pneumatosis, and ulceration in the area that previously appeared necrotic. Six months after transplant, he presented to the emergency room with diarrhea and vomiting due to rotavirus. At 4.5 years after transplant, he presented to a gastroenterologist for evaluation of recurrent, dull abdominal pain and poor weight gain. He underwent a colonoscopy which was significant for two sigmoid, one ascending, and one cecal polyp, with pathology consistent with juvenile polyps. He subsequently had six more polyps diagnosed within the next 7 years, located from the gastroesophageal junction to the cecum, with several types of histology present, including two adenomatous polyps. The adenomatous polyps were located in the sigmoid colon and cecum and were excised. This patient had a grandfather who had 29 colonic polyps of unknown pathology removed. Genetic testing was performed for hereditary juvenile polyposis (SMAD4 and BMPR1A) and was negative, although testing was presumably conducted on blood of donor origin.
Patient 3 was transplanted for relapsed ALL. She was C. difficile positive at the time of transplantation, and ultimately developed acute stage 2 skin GVHD. At two months post-transplant, due to severe abdominal pain concerning for acute gut GHVD, she underwent an EGD and flex sigmoidoscopy which revealed diffuse erythema and edema of the colonic mucosa of the hepatic flexure, with pathology consistent with GVHD. Histology revealed no viral inclusions, but at five months after transplant she presented with hepatitis and diarrhea due to adenovirus. After presenting with symptoms characteristic for chronic gastroesophageal reflux disease (GERD), she underwent an EGD at 5.75 years after transplant, which revealed four gastric polyps (hyperplastic histology). Four months later, two rectal and one sessile (precancerous) polyps were found in her ascending colon (Figure 1).
Patient 4 was transplanted for relapsed ALL. At two months post-transplant, he presented with fever and severe abdominal pain, and EGD showed pathology consistent with GVHD. Three months after HCT, he was diagnosed with GERD and developed recurrent vomiting between 3 to 12 months after transplant. He again presented with vomiting and hematochezia at 3.25 years after transplant. He underwent a colonoscopy which identified a juvenile colonic polyp, although the location of the polyp was unavailable.
Patient 5 was transplanted for high-risk ALL in first complete remission. He was C. difficile positive at day 0 and continued to be positive until two months after transplant. At one-month after transplant, after having abdominal pain concerning for gut GVHD, he underwent an EGD and a flexible sigmoidoscopy consistent with a pathologic diagnosis of stage 3 acute GVHD of the gut. He received eight infusions of mesenchymal stem cells to treat his GVHD, which resolved at four months after transplant. He had worsening abdominal pain and hematochezia five months after transplant that prompted repeat flexible sigmoidoscopy. It revealed multiple areas of erosion as well as adherent clots in the sigmoid colon and cecum with otherwise healthy-looking mucosa. With recurrent abdominal pain, he underwent an additional EGD and a flexible sigmoidoscopy that revealed mildly active chronic gastritis and a juvenile sigmoid polyp.
DISCUSSION
This report describes polyp formation as a potentially under-recognized secondary complication for childhood leukemia patients who undergo HCT. Currently, routine screening for asymptomatic polyps in the general population begins at 45 years of age unless there is a family history of colon cancer or polyps 10. Recently, the Children’s Oncology Group has updated their Survivorship Guidelines and now recommends colonoscopy and multitarget stool DNA test screening for all asymptomatic patients who received radiation ≥20 Gy to the abdomen, pelvis, lumbar/sacral/total spine, or TBI 11. However, this COG-recommended screening does not begin until 5 years after radiation exposure or by age 30, whichever occurs last. Notably, our patients presented with symptoms of polyps much earlier than when screening for asymptomatic patients would have occurred, at a median of 4.5 years after HCT. There is potential that they may have benefited from earlier detection and treatment of polyps, prior to the onset of their symptoms. In support of this concept of developing earlier screening programs, one prospective study evaluated colorectal polyp development in young adult survivors of cancer who received abdominal and/or pelvic radiation as part of their treatment. The goal of this study was to pre-emptively identify colorectal polyps during the preclinical phase of disease, when a screening program would be most useful. Survivors of childhood cancers were eligible to participate if they had received ≥25 Gy radiation to the abdomen, pelvis, or spine, or ≥12 Gy TBI. They were excluded if they had unexplained pelvic pain, blood in stool, history of polyps, recent endoscopic screening, or pertinent family history. Fifty-four patients having a median age of 45 (range, 36–49) years and with median interval of 19 (range, 10.6–43.5) years from radiation treatment were enrolled on this study. Twenty-four patients (44%) were identified who developed a total of 49 polyps. Fifty-three percent of these polyps were identified within or at the edge of radiation fields.12
While the direct etiology of GI polyp formation is unknown, one accepted thought is that polyps arise as a by-product of repair to damaged mucosa, typically induced by Helicobacter pylori or autoimmune-induced inflammation 13,14. Gastric polyps are also associated with proton-pump inhibitor (PPI) treatment, which is frequently used in the post-transplant setting, with polyps often described as being hyperplastic on histology 15. While none of our patients presented with Helicobacter pylori infections, many of them developed acute and/or chronic GVHD which is a form of autoimmunity, and all of them received at least 12 Gy of TBI, which is known to cause GI mucosal damage 16. One study described five long-term survivors of childhood cancers who developed non-familial polyposis an average of 24.8 years after chemotherapy and radiotherapy 17. While these patients did not undergo HCT, these authors, similar to others, also suggest that abdominal radiotherapy contributed to polyp formation.12
In addition to our patients all having received high-dose TBI, each patient we describe also presented with additional GI complications within two months of undergoing transplantation. For example, Patients 1, 3, and 5 were symptomatic with C. difficile infection early in their transplant courses, which could have initiated mucosal injury, and Patient 2 had pneumatosis intestinalis at 10 weeks after HCT. Additionally, all but one patient presented clinically with gut GVHD, which was severe in three, and histologically confirmed in all. Each of these patients also had recurrent histories of emesis, diarrhea, and/or GERD from time of transplantation until polyp identification. PPIs were used in all patients as standard of care during HCT, and chronic use was documented in Patient 5. Finally, due to the myeloablative nature of their transplants, all patients developed mucositis. While the duration and severity of this known transplant complication could not be further elicited from chart review, this mucosal inflammation could have been a contributing factor to polyp formation.
One major limitation of this retrospective study is that full details relating to histology, polyp location, and frequency of regular surveillance endoscopy are not fully available to us. We are also unable to describe the true incidence of GI polyps in this population. Our patients were identified because of GI symptoms requiring diagnostic procedures, and thus it is unclear how many asymptomatic patients also had polyps. Similarly, non-transplant clinicians who perform survivorship care may not be as familiar with TBI-related GI complications, and thus for mild symptoms, may not refer for EGD or colonoscopy. Over time, many pediatric patients are lost to follow-up from their primary transplant center due to transition to adult care teams, or receipt of survivorship care at their local institutions. Due to this lack of awareness and difficulty of follow-up, the true incidence of polyps may be higher than observed. Furthermore, as GI side effects are exceedingly common and diverse in the post-transplant setting, causation of this relatively rare side effect of polyp formation is not possible without having a much larger cohort of patients to study. Despite these limitations, as polyp formation is felt to arise in the environment of inflammation, it is not unreasonable to consider that TBI, GVHD, infection, and mucositis in the post-transplant setting could play an important role in its formation and pathogenesis. Finally, it is conceivable that pre-existing asymptomatic juvenile polyps could have been present, and thus these findings may not be directly related to the transplant procedure itself.
Although most polyps discovered in children are benign hamartomas (only 1.5% – 4.5% are of neoplastic potential), they can still cause worrisome symptoms such as hematochezia, leading to increased anxiety and decreased quality of life. There is also the propensity of these lesions to promote an increased risk of neoplasia in the surrounding abnormal mucosa 18. An evaluation by a gastroenterologist in the post-transplant period should be advised for any active pediatric HCT patient or long-term survivor with ongoing gastrointestinal complaints and/or hematochezia. Larger cohort studies are needed to determine the true incidence and prevalence of this complication. Comparably, while heart failure can be seen at a rate of 4.8% at 5 years after HCT, it is recommended to have routine echocardiograms in high-risk patient populations 19. Therefore, development of more robust and earlier screening algorithms based on prior GI toxicities and risk factors should be considered when designing future survivorship guidelines for patients who have undergone HCT.
Acknowledgements:
MST and RP received funding from the Midwest Athletes Against Childhood Cancer (MACC) Research Fund. RP has served on an advisory board for Orchard Therapeutics. This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001436.
We thank Cassandra Longsine for her help in manuscript preparation.
Footnotes
Compliance with Ethical Standards: All retrospective data collection was performed in accordance with the ethical standards of Children’s Hospital of Wisconsin IRB and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: Authors have no conflicts of interest to disclose that are relevant to this publication.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request within one year of publication.
REFERENCES
- 1.Baker KS, Ness KK, Weisdorf D, et al. Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Leukemia. 2010;24(12):2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majhail NS, Rizzo JD. Surviving the cure: long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013;48(9):1145–1151. [DOI] [PubMed] [Google Scholar]
- 3.Pulsipher MA, Skinner R, McDonald GB, et al. National Cancer Institute, National Heart, Lung and Blood Institute/Pediatric Blood and Marrow Transplantation Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: the need for pediatric-specific long-term follow-up guidelines. Biol Blood Marrow Transplant. 2012;18(3):334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakkar K, Alsarraj A, Fong E, Holub JL, Gilger MA, El Serag HB. Prevalence of colorectal polyps in pediatric colonoscopy. Digestive diseases and sciences. 2012;57(4):1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma C, Giardiello FM, Montgomery EA. Upper tract juvenile polyps in juvenile polyposis patients: dysplasia and malignancy are associated with foveolar, intestinal, and pyloric differentiation. The American journal of surgical pathology. 2014;38(12):1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adolph VR, Bernabe K. Polyps in children. Clinics in colon and rectal surgery. 2008;21(4):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda I, Tsuchida YA, Toyoshima F, et al. Occurrence of colon tumors in a 16-year-old Japanese boy after hematopoietic stem cell transplantation for Diamond Blackfan anemia at age of 4: a case report. International journal of clinical and experimental pathology. 2015;8(5):5938–5943. [PMC free article] [PubMed] [Google Scholar]
- 8.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18(55):7908–7916. [DOI] [PubMed] [Google Scholar]
- 9.Jewell KD, Toweill DL, Swanson PE, Upton MP, Yeh MM. Gastric hyperplastic polyps in post transplant patients: a clinicopathologic study. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(9):1108–1112. [DOI] [PubMed] [Google Scholar]
- 10.Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68(4):297–316. [DOI] [PubMed] [Google Scholar]
- 11.Group CsO. Long-Term Follow-Up Guildines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Children’s Oncology Group. 2018;version 5.0:1–232.
- 12.Daly PE, Samiee S, Cino M, et al. High prevalence of adenomatous colorectal polyps in young cancer survivors treated with abdominal radiation therapy: results of a prospective trial. Gut. 2017;66(10):1797–1801. [DOI] [PubMed] [Google Scholar]
- 13.Elhanafi S, Saadi M, Lou W, et al. Gastric polyps: Association with Helicobacter pylori status and the pathology of the surrounding mucosa, a cross sectional study. World journal of gastrointestinal endoscopy. 2015;7(10):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulnigg-Dabsch S Autoimmune gastritis. Wien Med Wochenschr. 2016;166(13–14):424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman HJ. Proton pump inhibitors and an emerging epidemic of gastric fundic gland polyposis. World J Gastroenterol. 2008;14(9):1318–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204–3213. [PubMed] [Google Scholar]
- 17.Yurgelun MB, Hornick JL, Curry VK, et al. Therapy-associated polyposis as a late sequela of cancer treatment. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(6):1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daibo M, Itabashi M, Hirota T. Malignant transformation of gastric hyperplastic polyps. The American journal of gastroenterology. 1987;82(10):1016–1025. [PubMed] [Google Scholar]
- 19.Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118(23):6023–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request within one year of publication.


