Clinical Implications.
Polyethylene glycol monoallergic patients identified through polyethylene glycol and polysorbate 80 skin testing can safely receive polysorbate 80–containing coronavirus disease 2019 vaccines.
During the worldwide coronavirus disease 2019 (COVID-19) vaccination campaign, a limited number of patients have experienced postvaccination anaphylaxis.1 The exact mechanisms remain unknown, yet specific excipients—such as polyethylene glycol (PEG) in the Pfizer/BioNTech and Moderna vaccines and polysorbate 80 (PS80) in the AstraZeneca and Johnson & Johnson vaccines—have been identified as causal allergens in a minority of cases.2, 3, 4, 5 Allergy to PEG and PS80 is considered exceedingly rare, although the exact prevalence remains unknown.6 Current guidelines recommend that patients with an allergy to PEG or PS80 should not be administered a COVID-19 vaccine with this excipient and should seek advice to identify a safe alternative.7 Both excipients are structurally similar, and cross-reactivity has been reported.6 , 8 , 9 As such, there is a theoretical risk of reactions to both PEG- and PS80-containing vaccines in patients with previous reactions to either component. Currently, the tolerability of COVID-19 vaccination in proven PEG- or PS80-allergic patients with an alternative, PS80- or PEG-containing vaccine, respectively, remains unexplored.
Within a prospective study on rare cases of anaphylaxis, tolerability to COVID-19 vaccines was evaluated in a cohort of patients with a skin test–confirmed PEG allergy. Medical record review and enquiry with the general practitioner and/or pharmacist served to identify inadvertent exposure to either PS80 and/or PEG after diagnosis. Those PEG-allergic patients with neither skin tests nor confirmed tolerated exposure to PS80 were recalled for additional skin testing (see Appendix 1 and Table E1; available in this article’s Online Repository at www.jaci-inpractice.org). All patients with negative PS80 skin tests and/or inadvertent exposure to PS80 since their PEG allergy diagnosis received a PS80-containing COVID-19 vaccine (Table I ). Patients with positive skin tests for both excipients were excluded from vaccination. Vaccines were administered either in a monitored hospital setting or in a routine setting in case of prior tolerance to the excipient. Tolerability was evaluated on-site and through telephone interview.
Table I.
Study population characteristics and COVID-19 vaccination outcome
| Patient no. | Sex | Age (y) | Index reaction | Index product | Positive skin tests | Post index PS80 exposure | Negative skin test for PS80 | COVID-19 vaccine∗ |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 53 | ANA | PEG 3350 | PEG: 3350, 4000, 6000 | Yes | Not performed | J&J |
| 2 | M | 67 | ANA | PEG 4000 | PEG: 4000 | Yes | Not performed | J&J |
| 3 | F | 73 | ANA | PEG 4000 | PEG: 4000 | Yes | Not performed | J&J |
| 4 | F | 54 | U, AE | PEG 400 | PEG: 400 | Yes | Not performed | J&J |
| 5 | F | 81 | U | PEG 3350 | PEG: 400, 4000 | Yes | Not performed | AZ |
| 6 | M | 70 | Rash | PEG 3350 | PEG: 3350, 4000 | Yes | Not performed | AZ |
| 7 | F | 21 | U, AE | PEG 3350 | PEG: 3350, 4000, 6000 | Yes | Yes | J&J |
| 8 | M | 39 | ANA | PEG 3350 | PEG: 3350,† 4000† | Yes | Yes | J&J |
| 9 | M | 56 | ANA | PEG 4000 | PEG: 3350, 4000 | No | Yes | J&J |
| 10 | F | 60 | ANA | PEG 3350 | PEG: 3350,† 4000† | No | Yes | J&J |
| 11 | F | 69 | ANA | PEG 3350 | PEG: 3350, 4000, 6000 | No | Yes | J&J |
| 12 | M | 33 | ANA | PEG 3350 | PEG: 1500, 3350, 4000; PS80 | No | No | - |
| 13 | M | 31 | ANA | PEG 6000 | PEG: 6000; PS80 | No | No | - |
| 14 | F | 34 | ANA | PEG 4000 | PS80‡ | No | No | - |
AE, Angioedema; ANA, anaphylaxis (defined as an immediate reaction involving > 1 organ system [World Allergy Organization]; AZ, AstraZeneca (Vaxzevria) COVID-19 vaccine; J&J, Johnson & Johnson (Janssen) COVID-19 vaccine; U, urticaria.
All COVID-19 vaccinations were tolerated.
Systemic reaction during skin testing without positive skin test.
Systemic reaction during skin testing with positive skin test.
Fourteen patients were identified with a skin test–proven primary PEG allergy (Table I). In 10 of 14 patients (71%), the index reaction was anaphylaxis. The culprit drug was PEG as a laxative in 3 of 14 patients (21%) and PEG in an extended-release steroid in 10 of 14 patients (71%). In 4 of 14 patients (29%), multiple events were noted. In 2 of 14 patients (14%), skin test–proven cross-reactivity between PEG and PS80 was observed. One patient (case 14) with a clinical history of multiple reactions to both PEG- and PS80-containing products developed a mild systemic reaction after a positive intradermal test with PS80 at the lowest concentration (Table E1). For safety reasons, no further skin testing with PEG was performed and the diagnosis of PS80 and PEG cross-reactivity was established on a clinical basis. All 11 patients with a monoallergy to PEG, based on negative PS80 skin tests and/or proven tolerance to PS80 after the initial allergy workup, tolerated a subsequent PS80-containing COVID-19 vaccine (Table I).
The COVID-19 pandemic has increased awareness of PEG and PS80 allergy. Nevertheless, PEG and PS80 remain rare causes of drug allergy with only 14 cases identified in our tertiary referral center over a 12-year period. Skin testing protocols for PEG and PS80 have evolved over time and a number of (unexplained) systemic reactions, especially upon intradermal testing, were the basis for the current drug-specific recommendations (outlined in Appendix 1).4 , 8 Although sensitivity of PEG skin testing is estimated to be low (58.8%), specificity is high (99.5%).7 Cross-reactivity with PS80 was initially not systematically evaluated in our patients and was subsequently observed in 21%, in line with the 30% (3 of 10 cases) reported by Bruusgaard-Mouritsen et al,8 but lower than the 100% (2 of 2 cases) in Stone et al.9 Interestingly, 2 out of 3 patients with both PEG and PS80 allergy in our cohort experienced symptoms upon topical exposure to or manipulation of a PEG- containing drug and all had severe index reactions. The uneventful administration of PS80-containing vaccines to all patients with negative PS80 skin tests suggests an excellent negative predictive value of PS80 skin testing.
In addition, we demonstrate the potential of a noninvasive workup encompassing a thorough medical file search and contact with allied health care workers. Using this approach, we confirmed that half of our patients had already tolerated subsequent exposure to a parenteral drug containing PS80, in most cases in another vaccine. Combining both methods, the majority of PEG-allergic patients received a COVID-19 vaccine without incident and only a minority was excluded owing to cross-reactivity with PS80. Currently, there is no procedure for providing a COVID-19 vaccine in this subgroup because all available vaccines contain either PEG or PS80 and, to our knowledge, evidence on the safety and efficacy of (graded) vaccine challenges or desensitization procedures for PEG and PS80 is currently lacking.7 Theoretically, if the risk of not vaccinating significantly outweighs the (uncertain yet potential) risk of an allergic reaction to the vaccine, a challenge could be considered. If dose-splitting and/or dilution of current vaccine formulations for a graded challenge would impact vaccine integrity and/or immunogenicity, a single-dose challenge would be preferred. However, evidently, such an approach would require a process of shared decision making with extensive informed consent as well as the availability of a monitored setting with trained personnel.
In our cohort, we did not identify a previously known PS80 monoallergic patient, although a skin test–proven PS80 monoallergic patient, identified after anaphylaxis to rituximab and Vaxzevria, had negative PEG skin testing and safely received a second dose with a PEG-containing vaccine (Pfizer/BioNTech, data not shown). Although this case indicates that PS80 present in COVID-19 vaccines is sufficient to elicit an allergic reaction in certain patients monosensitized to PS80, it remains unknown whether this holds true for patients with a skin test–proven primary PEG allergy who subsequently also demonstrate skin test positivity to PS80.
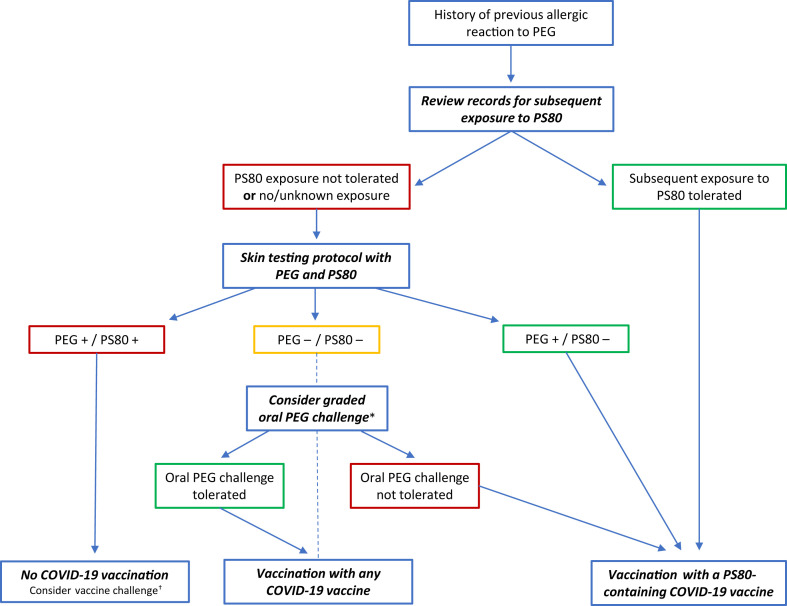
In conclusion, our study emphasizes that an allergist-driven approach (Figure 1 ) can identify safe COVID-19 vaccine alternatives for those initially deemed ineligible for vaccination owing to a PEG or PS80 allergy and assists in further reducing barriers to vaccination.
Figure 1.
Flowchart for the diagnostic evaluation prior to COVID-19 vaccination in patients with a history of PEG allergy. A careful review of the medical records in cooperation with allied health professionals can help identify patients with subsequent tolerated exposure to PS80, eligible for vaccination with a PS80-containing vaccine. If subsequent PS80 tolerability is absent or unclear, the patient should be referred to an allergist for further evaluation (skin testing with PEG and PS80 to identify a safe vaccine alternative can be considered). ∗In patients with double-negative skin tests despite a suggestive clinical history, a graded oral challenge to PEG can be considered on a case-by-case level, in a monitored setting. †In patients with positive skin tests to both PEG and PS80, no safe U.S. Food and Drug Administration– or European Medicines Agency-approved alternative is currently available. In selected cases in which the risk of not vaccinating would outweigh the potential risk of anaphylaxis, a (graded) vaccine challenge could be considered after informed consent or shared decision making and only in a monitored setting with personnel trained in the recognition and management of anaphylaxis.
Acknowledgments
This study was approved by the ethics committee research UZ/KU Leuven (S60734). All patients provided their informed consent.
M. Vandebotermet and R. Schrijvers conceived the initial study. T. Ieven, T. Van Weyenbergh, M. Vandebotermet, and R. Schrijvers wrote the manuscript with input from D. Devolder and C. Breynaert. D. Devolder provided hospital pharmacy expertise. T. Ieven, T. Van Weyenbergh, M. Vandebotermet, C. Breynaert, and R. Schrijvers provided allergy workups and vaccination.
Footnotes
C. Breynaert is supported by a KOOR fellowship (UZ Leuven). R. Schrijvers is a senior clinical investigator fellow of the Fonds Wetenschappelijk Onderzoek—Vlaanderen, National Fund for Scientific research (FWO, 1805518N).
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
Appendix 1
Supplementary methods
All patients were diagnosed at the allergy and clinical immunology unit of the University Hospitals Leuven, Belgium (UZ Leuven). UZ Leuven is a teaching hospital and tertiary referral center with 1,764 beds providing secondary, tertiary, and quaternary care for all specialisms and all age groups. Patients in this study were diagnosed over a 12-year period, between 2009 and 2021. Skin testing procedures evolved over time, in line with the prevailing literature. Skin testing for polysorbate 80 (PS80) was not systematically performed throughout the study period as outlined in Table E1 and complemented prior to coronavirus disease 2019 (COVID-19) vaccination in a subset of patients.
Currently, skin tests for PS80 are performed sequentially using PS80 (Tween, 1 mg/mL) skin prick test (SPT) undiluted and intradermal testing (IDT) up to 0.1 mg/mL. Polyethylene glycol (PEG) is evaluated using sequential SPT undiluted for PEG 400 (undiluted, no concentration provided by the manufacturer), PEG 3350 (Depo-Medrol 40 mg/mL methylprednisolone acetate; and PEG 3350 29 mg/mL), PEG 3350 (Movicol, 100 mg/mL), PEG 4000 (macrogol, 100 mg/mL), PEG 20,000 (Flagyl, metronidazole 500 mg/tablet; and PEG 20,000 1.4 mg/700 mg [(0.2%] tablet). The PEG 6000 (100 mg/mL) and PEG 1500/300 (100 mg/mL), previously used as indicated in Table E1, is no longer available for testing. The SPT dilutions for the different PEG molecules (1/10–1/1000) are used upon a high index of suspicion or severity of the index reaction(s). Skin testing with PEG is currently only performed with SPT and no longer with IDT except for IDT with Depo-Medrol (PEG 3350 up to 2.9 mg/mL, a 1/10 dilution) when probability of PEG allergy is low or in case of confirmed PS80 allergy and necessity to demonstrate tolerance to PEG (as in the context prior to Pfizer/BioNTech, Moderna COVID-19 vaccination). The IDT are only performed at the end of the skin test protocol. All steps (SPT, IDT) are performed with 30-minute intervals and in a monitored setting (with intravenous access in patients with a prior anaphylaxis and/or necessity to receive IDT with Depo-Medrol in this context). The SPT with PEG 20,000 was added to the skin test protocol since May 2021. Positive (histamine [10 mg/mL] and negative [0.9% saline] SPT controls are consistently performed. For IDT, a volume of 0.05 mL is used per injection.
Supplementary patient information
Patient 5 developed urticaria after Depo-Medrol. She experienced a second episode upon topical Bactroban (contains PEG 400, 3350) application after which she developed urticaria distant from the application site. Treatment with loratadine (brand Mylan, containing PEG 400, 6000) caused a paradoxical deterioration.
Patient 8 experienced nausea, vomiting, diffuse urticaria, and pruritus immediately after administration of Depo-Medrol and Marcaïne intra-articular. Skin testing and provocation with Marcaïne (without epinephrine) was negative. After IDT with Depo-Medrol (1/10, 2.9 mg/mL of PEG 3350) and macrogol 4000 (1/10, 10 mg/mL) at 3 separate occasions (twice for macrogol 4000 of which 1 occurred during a single-blinded placebo-controlled skin test), the patient developed systemic urticaria, sneezing, and conjunctival injection (without a local skin test reaction), interpreted as positive, as reported earlier. Placebo IDT (during single-blinded skin testing) was negative. Basophil activation test with macrogol 4000 was negative.
Patient 9 had an initial anaphylaxis after Diprophos with a diagnosis of PEG allergy (based on IDT for PEG 4000 and Diprophos, at that time no SPT for PEG 4000 were performed). He was reevaluated prior to COVID-19 vaccination with negative SPT and IDT (0.1 mg/mL) for PS80. However, SPT for PEG 4000 (100 mg/mL) was also negative but no comparison could be made since initially IDT were performed (IDT was not reperformed because of the valid alternative and practice to avoid IDT in PEG-allergic patients).
Patient 13 experienced anaphylaxis immediately after application of isobetadine gel but had no symptoms upon later exposure to isobetadine solution (not containing PEG).
Supplementary Material
References
- 1.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfson A.R., Robinson L.B., Li L., McMahon A.E., Cogan A.S., Fu X., et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9:3308–3320.e3. doi: 10.1016/j.jaip.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sellaturay P., Nasser S., Islam S., Gurugama P., Ewan P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy. 2021;51:861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol–induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9:670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 5.Habran M., Vandebotermet M., Schrijvers R. Polyethylene glycol allergy and immediate-type hypersensivitity reaction to COVID-19 vaccination: case report. https://doi.org/10.18176/jiaci.0740 J Investig Allergol Clin Immunol. Published online July 26, 2021. [DOI] [PubMed]
- 6.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 7.Greenhawt M., Abrams E.M., Shaker M., Chu D.K., Khan D., Akin C., et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9:3456–3467. doi: 10.1016/j.jaip.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruusgaard-Mouritsen M.A., Jensen B.M., Poulsen L.K., Duus Johansen J., Garvey L.H. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol. 2021;148:89–90. doi: 10.1016/j.jaci.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Stone C.A., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.