Abstract
Vero cells have been widely used in the viral vaccine production due to the recommendation of the World Health Organization regarding its safety and non-tumorigenicity. The aim of this study was to describe the development a modified serum-free medium for Vero cell cultures. Two protein hydrolysates (Bacto™ soytone and Bacto™ yeast extract), vitamin C, vitamin B12, SITE liquid media supplement, and recombinant human epidermal growth factor (rEGF) were investigated as serum substitutes. A sequential experiment of fractional factorial and central composite design was applied. A modified serum-free medium obtained (named as SFM01-M) was verified. Contrary to P0, the cell yields obtained at P1, P2, and P3 decreased continuously during the verification experiments indicating that Vero cells could not adapt to SFM01-M as expected according to the empirical mathematical model. To improve cell growth after P0, protein hydrolysates, l-glutamine, and SITE liquid media supplement were further investigated. The results showed that cell yields gradually decreased from P1 to P3 when a fixed concentration of Bacto™ yeast extract (7.0 g/L) combined with various concentrations of Bacto™ soytone (0.1–7.0 g/L) in SFM01-M were used. Similarly, cell yields also gradually decreased from P1 to P3 when a fixed concentration of Bacto™ soytone (7.0 g/L) combined with various concentrations of Bacto™ yeast extract (0.1–7.0 g/L) in SFM01-M were used. However, the combination of Bacto™ soytone at 0.1 g/L and Bacto™ yeast extract at 7.0 g/L or Bacto™ soytone at 7.0 g/L and Bacto™ yeast extract at 0.1 g/L in SFM01-M could give the maximum cell yield at P3 when compared with other combinations. In addition, the addition of SITE liquid media supplement (0.1–2.0% v/v) in SFM01-M in which the concentrations of Bacto™ soytone, Bacto™ yeast extract, and l-glutamine were fixed at 0.1 g/L, 0.1 g/L, and 4.0 mM, respectively, the results showed that the cell yields obtained at P3 were not significantly different. From this study, the optimum concentrations of SFM01-M components were as follows: Bacto™ soytone (0.1 g/L), Bacto™ yeast extract (0.1 g/L), vitamin C (9.719 mg/L), vitamin B12 (0.1725 mg/L), SITE liquid media supplement (0.1–2.0% v/v), rEGF (0.05756 mg/L), l-glutamine (4.0 mM), MEM non-essential amino acids (1.0% v/v), sodium pyruvate (1.0 mM), MEM (9.4 g/L), and sodium hydrogen carbonate (2.2 g/L). However, to evaluate SFM01-M in the long-term subculture of Vero cells, the efficiency of SFM01-M will be further investigated.
Keywords: A modified serum-free medium, Fractional factorial design, Central composite design, Protein hydrolysates, Vero cells
Introduction
Vero is a continuous cell line derived from African green monkey kidney. Vero cells have been widely accepted for viral vaccine productions (WHO 1998). To establish cell cultures in vitro, serum is still necessary for promoting cell growth since it has growth factors, albumin, transferrin, anti-proteases, attachment factors, minerals, hormones, and inhibitors (Hewlett 1991; Jayme 1991; Castle and Robertson 1998; Brunner et al. 2010; Freshney 2010). However, several disadvantages of using serum have been proposed, particularly the risks involved in the contamination with adventitious agents such as mycoplasma, viruses, and prions, agents of transmissible spongiform encephalopathies (TSEs), especially bovine spongiform encephalopathy (BSE). Moreover, serum also has the high concentration of protein which can make downstream processing of recombinant proteins more difficult and costly (Castle and Robertson 1998; Van der Valk et al. 2004; Freshney 2010). Consequently, with the disadvantages of using serum in cell cultures, the attempts to develop serum-free media (SFM) have been widely performed. However, there is no a universal SFM available to fit all the different cellular requirements (Van der Valk et al. 2004; Keenan et al. 2006) since different cell types have different receptors involved in cell survival, growth and differentiation, and also in releasing different factors to their environment (Van der Valk et al. 2010).
Currently, low-cost hydrolysates containing ill-defined mixtures of low-molecular weight constituents such as amino acids, peptides, vitamins, and trace elements, are frequently utilized as SFM additives to provide nutrients in cell cultures, particularly non-animal derived hydrolysates such as plant (soy, wheat gluten, rice) hydrolysates and yeast hydrolysates (Jan et al. 1994; Keen and Rapson 1995; Heidemann et al. 2000; Franĕk et al. 2000; Sung et al. 2004; Chun et al. 2007; Kim and Lee 2009; Rourou et al. 2009; Babcock et al. 2010; Lobo-Alfonso et al. 2010; Michiels et al. 2011a, b; Petiot et al. 2010). Additionally, other components that have been found to be essential for serum-free cultures namely insulin, transferrin, sodium selenite, and ethanolamine are also utilized. Insulin serves as growth and maintenance factors. It stimulates the uptake of uridine and glucose. Additionally, it also stimulates the synthesis of ribonucleic acids (RNA), proteins, and lipids as well as enhancing the synthesis of fatty acids and glycogen. Transferrin is an iron binding glycoprotein that interacts with surface receptors and implicates in iron transport through cell membrane. It also has additional in vitro functions, e.g. the chelation of deleterious trace materials, which are unlikely replaced by other components. Selenium is an essential trace element for normal cell growth and development. It is incorporated into enzymes that protect cells by reducing peroxides, organic hydroperoxides, and peroxynitrites to non-toxic species. Ethanolamine is reported as an essential growth factor for hybridoma in serum-free cultures. Its function is implicated in the synthesis of phosphatidylethanolamine, a major constituent of membrane phospholipid. (Taub et al. 1979; Murakami et al. 1982; Chuman et al. 1982; Jäger et al. 1988; Kovář and Franĕk 1989; Eto et al. 1991; Chen et al. 1993; Okamoto et al. 1996; Lee et al. 1999; Kim et al. 1998; Morris and Schmid 2000; Liu et al. 2001; Liu and Chang 2006; Parampalli et al. 2007; Cervera et al. 2011). Vitamin C or L-ascorbic acid is a micronutrient required for innumerable biological functions. It is an essential cofactor of α-ketoglutarate-dependent dioxygenases, for example, prolyl hydroxylases which play an important role in the collagen and glycosaminoglycan biosynthesis, and in down-regulation of hypoxia-inducible factor (HIF)-1, a transcription factor that regulates many genes responsible for tumor growth, energy metabolism, neutrophil function, and apoptosis. Additionally, it exhibits as antioxidant molecules that provide protection against oxidative stress-induced cell damage by scavenging reactive oxygen species (ROS) whereas other its function is involved in the iron metabolism in mammalian cells (Kao et al. 1990; Arrigoni and Tullio 2000; Traber and Stevens 2011; Arigony et al. 2013; Lane and Richardson 2014). Vitamin B12 or cyanocobalamin is required for methionine synthase which is an important enzyme for sulphur amino acid, folate and polyamine metabolism, S-adenosylmethionine metabolism and also is involved in the methylation pathway of deoxyribonucleic acid (DNA), RNA, proteins, and lipids (Kenyon et al. 1996; Thomas and Thomas 2001; Bjelakovic et al. 2006; Arigony et al. 2013). Epidermal growth factor (EGF) is a small mitogenic polypeptide. It plays an important role in the regulation of cell growth, proliferation, and differentiation (Gospodarowicz and Moran 1976; Bettger et al. 1981; Zhaolie et al. 1996).
The development of a serum-free medium is always time and labor consuming when the traditional one-factor-at-a-time (OFAT) approach is applied. Consequently, the use of systematic design of experiments which is a structured and efficient methodology for planning and analyzing the experimental results, is more effective than the previous OFAT approach with respect to time and labor consuming (Montgomery 2001; Box et al. 2005). The procedure commonly applied for the optimization of culture media can be subdivided into four steps: identification of the most prominent medium components (screening), identification of optimal variable ranges (narrowing), identification of the optimum (optimum search), and experimental verification of the identified optimum (Rourou et al. 2009).
In the present study, the effect of protein hydrolysates (Bacto™ soytone and Bacto™ yeast extract) on Vero cell growth was further investigated. Additionally, some modifications of a modified serum-free medium (SFM01-M) with l-glutamine and SITE liquid media supplement were also investigated. The results shown here indicated that Vero cells could adapt and grow in SFM01-M after some modifications. However, to evaluate SFM01-M in the long-term subculture of Vero cells, the efficiency of SFM01-M will be further investigated.
Materials and methods
Cell line
Vero cells (ATCC® CCL-81™) were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). Vero cells were grown in Eagle’s minimal essential medium (MEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 1.0% (v/v) MEM non-essential amino acids (100 ×), 1.0 mM sodium pyruvate, and 2.0 mM l-glutamine at 37 °C, 5% CO2 in a CO2 incubator (Thermo Electron Corporation, USA) until confluence. Monolayer cells were washed once with phosphate buffered saline (Ca2+, Mg2+-free PBS, pH 7.4), and then detached with the trypsin–EDTA solution (0.25% w/v trypsin plus 0.02% w/v EDTA) for 2–5 min at 37 °C. Prior to use in the experiments, Vero cells were subcultured for at least 3 passages in this medium (serum-containing medium, SCM).
Culture media and chemicals
Eagle’s minimal essential medium (MEM, “Nissui” No. 1) was purchased from Nissui Pharmaceutical Co., Ltd, Japan. l-glutamine, sodium pyruvate, vitamin C, vitamin B12 and SITE liquid media supplement were purchased from Sigma-Aldrich®, St. Louis, MO, USA. Fetal bovine serum (FBS) (EU Approved: South America, Thermo Fisher Scientific, Auckland, New Zealand), MEM non-essential amino acids (100 ×) (Thermo Fisher Scientific, MA, USA), recombinant human epidermal growth factor (rEGF) (Thermo Fisher Scientific, MD, USA), Trypsin (1:250) (Thermo Fisher Scientific, ONT, Canada), and TrypLE™ Select (1 ×) (Thermo Fisher Scientific, MA, USA) were also purchased and used in the experiments. All chemicals were suitable for cell culture.
Protein hydrolysates
Bacto™ soytone and Bacto™ yeast extract were purchased from Becton, Dickinson and Company, New Jersey, USA. Each hydrolysate was prepared in Milli Q water as a stock solution of 100 g/L and sterile filtered using Sartopore2 (Sartorius AG, Goettingen, Germany) with pore size of 0.45 μm + 0.2 μm.
Cell cultures
Screening experiments
In the screening experiment, a 26–2 fractional factorial design was applied and a concentration range of each serum substitute and mean cell yields were shown in Table 1. In Vero cell cultures, a seeding density of 2 × 104 cells/cm2 was used. Starter cells were prepared in SCM before seeding to 6-well plates (Thermo Fisher Scientific, Jiangsu, China) at a final volume of 3 mL. Vero cells in plates were incubated at 37 °C, 5% CO2 for 3 h. After 3 h incubation, spent media were removed and replaced with tested media. Vero cell cultures were carried out in triplicate and cultured at 37 °C, and 5% CO2 for 7 days.
Table 1.
Lay out of the screening experiments using a 26–2 fractional factorial design
| Experiment no | A | B | C | D | E | F | Cell yield (× 105 cells/well) |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 0.1 | 0.05 | 0.01 | 0.1 | 0.005 | 10.24 ± 0.25 |
| 2 | 7.0 | 0.1 | 0.05 | 0.01 | 2.0 | 0.005 | 9.30 ± 0.61 |
| 3 | 0.1 | 7.0 | 0.05 | 0.01 | 2.0 | 0.030 | 13.80 ± 1.48 |
| 4 | 7.0 | 7.0 | 0.05 | 0.01 | 0.1 | 0.030 | 12.04 ± 0.66 |
| 5 | 0.1 | 0.1 | 50.00 | 0.01 | 2.0 | 0.030 | 8.30 ± 0.66 |
| 6 | 7.0 | 0.1 | 50.00 | 0.01 | 0.1 | 0.030 | 10.38 ± 0.43 |
| 7 | 0.1 | 7.0 | 50.00 | 0.01 | 0.1 | 0.005 | 8.10 ± 0.78 |
| 8 | 7.0 | 7.0 | 50.00 | 0.01 | 2.0 | 0.005 | 8.82 ± 0.69 |
| 9 | 0.1 | 0.1 | 0.05 | 0.41 | 0.1 | 0.030 | 10.72 ± 0.43 |
| 10 | 7.0 | 0.1 | 0.05 | 0.41 | 2.0 | 0.030 | 9.98 ± 1.02 |
| 11 | 0.1 | 7.0 | 0.05 | 0.41 | 2.0 | 0.005 | 9.32 ± 0.77 |
| 12 | 7.0 | 7.0 | 0.05 | 0.41 | 0.1 | 0.005 | 8.18 ± 0.45 |
| 13 | 0.1 | 0.1 | 50.00 | 0.41 | 2.0 | 0.005 | 8.65 ± 0.34 |
| 14 | 7.0 | 0.1 | 50.00 | 0.41 | 0.1 | 0.005 | 7.76 ± 0.66 |
| 15 | 0.1 | 7.0 | 50.00 | 0.41 | 0.1 | 0.030 | 9.04 ± 0.84 |
| 16 | 7.0 | 7.0 | 50.00 | 0.41 | 2.0 | 0.030 | 9.54 ± 0.68 |
| 17 | 3.55 | 3.55 | 25.025 | 0.21 | 1.05 | 0.0175 | 9.17 ± 0.30 |
| 18 | 3.55 | 3.55 | 25.025 | 0.21 | 1.05 | 0.0175 | 8.39 ± 0.18 |
| 19 | 3.55 | 3.55 | 25.025 | 0.21 | 1.05 | 0.0175 | 9.04 ± 0.25 |
| Positive control | 13.03 ± 0.70 | ||||||
| Negative control | 1.80 ± 0.06 |
In this study, Vero cells were incubated in SCM in 6-well plates at 37 °C, 5% CO2 for 3 h and then replaced with tested media. Cultures were cultured at 37 °C, 5% CO2 for 4 days. Cell cultures were carried out in triplicate and expressed as mean ± SD. A (Bacto™ soytone, g/L), B (Bacto™ yeast extract, g/L), C (vitamin C, mg/L), D (vitamin B12, mg/L), E (SITE liquid media supplement, % v/v), and F (recombinant human epidermal growth factor (rEGF), mg/L) were the factors (serum substitutes) used in this study. A positive control was MEM + 10% (v/v) FBS + 1.0% (v/v) MEM non-essential amino acids + 1.0 mM sodium pyruvate. A negative control was MEM without any supplements
Optimization experiments
In the optimization experiments, a circumscribed central composite design was applied. Three types of serum substitutes (vitamin C, vitamin B12, and rEGF) which were obtained from the screening experiments were further optimized. The concentration levels of each were designed as shown in Table 2 and carried out according to the instruction of the circumscribed central composite design. Since the experiment no. 3 obtained from the previous screening experiments could give the maximum cell yield at day 4 of culture; therefore, the concentration level of each component in the experiment no. 3 except vitamin C, vitamin B12, and rEGF were fixed as follows: Bacto™ soytone (0.1 g/L), Bacto™ yeast extract (7.0 g/L), SITE liquid media supplement (2.0% v/v), MEM (9.4 g/L), sodium hydrogen carbonate (2.2 g/L), MEM non-essential amino acid (1.0% v/v), and sodium pyruvate (1.0 mM). Starter cells were prepared in SCM before seeding to 6-well plates (Thermo Fisher Scientific, Jiangsu, China) at a final volume of 3 mL. Vero cells in plates were incubated at 37 °C, 5% CO2 for 3 h, and then spent media were removed and replaced with tested media. Vero cell cultures were carried out in triplicate and cultured at 37 °C, and 5% CO2 for 4 days.
Table 2.
Lay out of the optimization experiments using a central composite design
| Experiment no | X1 | X2 | X3 | Cell yield (× 105 cells/well) |
|---|---|---|---|---|
| 1 | 4.097 (− 1) | 0.0692 (− 1) | 0.02864 (− 1) | 8.20 ± 0.10 |
| 2 | 16.097 (+ 1) | 0.0692 (− 1) | 0.02864 (− 1) | 11.20 ± 0.28 |
| 3 | 4.097 (− 1) | 0.2692 (+ 1) | 0.02864 (− 1) | 10.38 ± 0.13 |
| 4 | 16.097 (+ 1) | 0.2692 (+ 1) | 0.02864 (− 1) | 11.80 ± 0.30 |
| 5 | 4.097 (− 1) | 0.0692(− 1) | 0.06864 (+ 1) | 11.80 ± 0.40 |
| 6 | 16.097 (+ 1) | 0.0692(− 1) | 0.06864 (+ 1) | 11.00 ± 0.25 |
| 7 | 4.097 (− 1) | 0.2692 (+ 1) | 0.06864 (+ 1) | 11.60 ± 0.30 |
| 8 | 16.097 (+ 1) | 0.2692 (+ 1) | 0.06864 (+ 1) | 10.03 ± 0.03 |
| 9 | 0.005 (− 1.68) | 0.1692 (0) | 0.04864 (0) | 10.00 ± 0.10 |
| 10 | 20.189 (+ 1.68) | 0.1692 (0) | 0.04864 (0) | 12.00 ± 0.25 |
| 11 | 10.097 (0) | 0.0010 (− 1.68) | 0.04864 (0) | 10.50 ± 1.00 |
| 12 | 10.097 (0) | 0.3374 (+ 1.68) | 0.04864 (0) | 11.00 ± 1.15 |
| 13 | 10.097 (0) | 0.1692 (0) | 0.01500 (− 1.68) | 11.20 ± 1.20 |
| 14 | 10.097 (0) | 0.1692 (0) | 0.08228 (+ 1.68) | 11.60 ± 0.85 |
| 15 | 10.097 (0) | 0.1692 (0) | 0.04864 (0) | 12.10 ± 0.40 |
| 16 | 10.097 (0) | 0.1692 (0) | 0.04864 (0) | 12.07 ± 0.28 |
| 17 | 10.097 (0) | 0.1692 (0) | 0.04864 (0) | 12.05 ± 0.70 |
In this study, Vero cells were incubated in SCM in 6-well plates at 37 °C, 5% CO2 for 3 h and then replaced with tested media. Cells were cultured at 37 °C, 5% CO2 for 4 days. Cell cultures were carried out in triplicate and expressed as mean ± SD. X1, X2, and X3 were respectively vitamin C; vitamin B12 and recombinant human epidermal growth factor (rEGF) and were expressed as mg/L. Code unit that was designed according to a central composite design was shown in parentheses at-1.68, − 1, 0, + 1, and + 1.68 after its actual concentration
Verification experiments
In the verification experiments, a modified serum-free medium (named as SFM01-M) was formulated as follows: MEM (9.4 g/L), MEM non-essential amino acids (1.0% v/v), sodium pyruvate (1.0 mM), l-glutamine (2.0 mM), Bacto™ soytone (0.1 g/L), Bacto™ yeast extract (7.0 g/L), vitamin C (9.719 mg/L), vitamin B12 (0.1725 mg/L), SITE liquid media supplement (2.0% v/v), rEGF (0.05756 mg/L), and sodium hydrogen carbonate (2.2 g/L). Vero cell cultures were carried out in triplicate and cultured at 37 °C, and 5% CO2 for 4 days.
Cell counting
The growth and proliferation of cultured Vero cells was determined daily. Spent media in plates were removed and washed once with PBS (Ca2+, Mg2+-free PBS, pH 7.4). Cultured Vero cells were detached with the trypsin–EDTA solution for 2–5 min at 37 °C. Digested cells were re-suspended in SCM to inactivate the activity of trypsin enzyme. Aliquot of cell suspension was sampled to a 1.5 mL centrifuged tube, and then viable Vero cells were counted after staining with the 0.4% (w/v) trypan blue solution using an improved Neubauer hemocytometer (Boeco, Hamburg, Germany). In the modified serum-free medium condition, the trypsin–EDTA solution was replaced by TrypLE™ Select. Briefly, spent medium was removed and washed once with PBS, and then 0.1 mL of TrypLE™ Select was added to each well (6-well plates). Vero cells were detached at 37 °C for 2–5 min until cells were dislodged. Dislodged cells in each well were added with 0.9 mL of washed medium (SFM01-M + 0.5 g/L soybean trypsin inhibitor (Thermo Fisher Scientific, MA, USA)). Dislodged cells and washed medium were mixed well, and then were placed in a 1.5 mL centrifuge tube. Cells were separated by centrifugation at 1000 rpm for 5–10 min. Washed medium was removed and replaced with SFM01-M. Cell pellets were mixed several times before cell counting.
Experimental design and statistical analysis
In this study, the Minitab® Release 14 Statistical Software was applied for design of experiments and the statistical analysis of data. The serum substitutes which had the effect on the response (cell yield) were screened using a 26–2 fractional factorial design. Estimated effects and coefficients of factors or factor interactions were analyzed by the software. For the optimization experiments, a circumscribed central composite design was applied. The optimum conditions were analyzed using the response optimizer program, and contour plots were generated by the software. Additionally, two-tailed Student’s t test was used to compare mean and p-value < 0.05 was regarded as statistically significant.
Results and discussion
Screening experiments
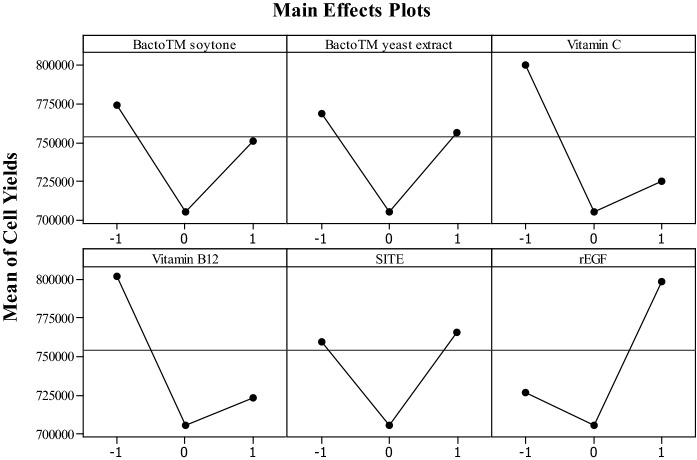
To develop a modified serum-free medium for Vero cell cultures, two protein hydrolysates (Bacto™ soytone and Bacto™ yeast extract) and other four components were used as serum substitutes and were screened using a 26–2 fractional factorial design. Most of cell growth could be observed (Table 1) in the range of 7.76 ± 0.66–13.80 ± 1.48 × 105 cells/well. Cell yield of the positive control was 13.03 ± 0.70 × 105 cells/well, whereas the negative one was 1.80 ± 0.06 × 105 cells/well. The maximum cell yield was the experiment no. 3 that serum substitutes consisted of Bacto™ soytone (0.1 g/L), Bacto™ yeast extract (7.0 g/L), vitamin C (0.05 mg/L), vitamin B12 (0.01 mg/L), SITE liquid media supplement (2.0% v/v) and rEGF (0.030 mg/L). Main effects plots of serum substitutes (Fig. 1) demonstrated that Bacto™ soytone, Bacto™ yeast extract, vitamin C, and vitamin B12 had effect negatively, whereas SITE liquid media supplement and rEGF had effect positively on the cell yields. However, only vitamin C, vitamin B12, and rEGF were statistically significant (p-value < 0.05) as shown in Table 3. As indicated in Table 3, only four factor-interactions (AB, AE, BF, ABF) were statistically significant (p-value < 0.05). However, since the effect hierarchical principle stating that both main effects and lower order effects are more important than the higher order effects and those effects of the same order are equally important; therefore, only two factor-interactions (AB, AE, and BF) were more important than other higher order factor-interactions (ABF).
Fig. 1.
Main effects plots derived from a 26–2 fractional factorial design. − 1, 0 and + 1 denote the “low level”, “middle level” and “high level” of a factor concentration
Table 3.
Estimated effects and coefficients of the response (cell yields)
| Term | Effect | Coefficient | T | p-value |
|---|---|---|---|---|
| Constant | 762,938 | 146.00 | 0.000 | |
| A | − 23,625 | − 11,812 | − 2.27 | 0.151 |
| B | − 12,375 | − 6187 | − 1.19 | 0.356 |
| C | − 75,375 | − 37,687 | − 7.24 | 0.019 |
| D | − 78,625 | − 39,313 | − 7.55 | 0.017 |
| E | 6375 | 3188 | 0.61 | 0.603 |
| F | 72,375 | 36,187 | 6.95 | 0.020 |
| AB | − 53,875 | − 26,938 | − 5.18 | 0.035 |
| AC | 32,125 | 16,062 | 3.09 | 0.091 |
| AD | 8875 | 4437 | 0.85 | 0.484 |
| AE | − 95,125 | − 47,563 | − 9.14 | 0.012 |
| AF | 10,875 | 5437 | 1.04 | 0.406 |
| BD | 25,125 | 12,563 | 2.41 | 0.137 |
| BF | 48,125 | 24,063 | 4.62 | 0.044 |
| ABD | − 15,375 | − 7688 | − 1.48 | 0.278 |
| ABF | − 59,375 | − 29,687 | − 5.70 | 0.029 |
| Center point | – | 57,604 | − 4.40 | 0.048 |
S = 20,816.7, R2 = 99.46%, R2 (adjusted) = 95.13%, Significant at p-value < 0.05. A (Bacto™ soytone), B (Bacto™ yeast extract), C (vitamin C); D (vitamin B12), E (SITE liquid media supplement), and F (recombinant human epidermal growth factor, rEGF) were the factors (serum substitutes) used in a 26–2 fractional factorial design
Optimization experiments
Vitamin C, vitamin B12, and rEGF were designed as shown in Table 2, whereas other components were fixed as follows: MEM (9.4 g/L), sodium hydrogen carbonate (2.2 g/L), MEM non-essential amino acids (1% v/v), sodium pyruvate (1.0 mM), l-glutamine (2.0 mM), Bacto™ soytone (0.1 g/L), Bacto™ yeast extract (7.0 g/L), and SITE liquid media supplement (2.0% v/v). The central composite design which is a 23 factorial design containing six star points (α = ± 1.68) and three center points is applied. In the regression equation, the actual concentration of each factor (vitamin C, vitamin B12, and rEGF) was calculated directly from Eq. (1).
| 1 |
where xi is the code value expressed as − 1.68, − 1, 0, + 1, + 1.68; Xi is the actual concentration of the ith tested factor (vitamin C, vitamin B12, and rEGF); is the actual concentration of Xi at the center point of the investigated area: ΔXi is the step size.
Based on the experimental results derived from the central composite design, all main effects, quadratic and interaction terms were statistically significant (p-value < 0.05) as shown in Table 3. In the statistical analysis, the coefficient of determination (R2 = 95.19%) revealed that 95.19% of the experimental data could be described by the empirical mathematical model except only 4.91% of those experimental data could not be described by this empirical mathematical model. The empirical mathematical model for estimating the cell yield corresponding to the optimized serum substitutes was as shown in Eq. (2).
| 2 |
By using the response optimizer together with contour plots (data not shown), the optimum points of X1, X2 and X3 expressed as code units (actual units) were − 0.063 (9.719 mg/L), 0.033 (0.1725 mg/L), and 0.446 (0.05756 mg/L), respectively. The cell yield estimated according to the empirical mathematical model was 12.1 × 105 cells/well. However, the cell yield in the range of 10.3 × 105–13.9 × 105 cells /well was acceptable.
Verification experiments
Cell yields decreased continuously (Fig. 2) at P1, P2 and P3, whereas P0 was relatively high (11.4 ± 0.8 × 105 cells/well, data not shown in Fig. 2). The better attachment and spreading of cells at P0 (Fig. 3) may resulted from the possible binding of serum components to the cell surface during a brief period of contact with serum (Hewlett 1991). Vitamin C is a micronutrient required for various biological functions. It serves as a cofactor for certain important enzymes and also as an antioxidant. It acts as a promoter for collagen synthesis and secretion including the involvement in the production of glycosaminoglycan by fibroblast cells (Arrigoni and Tullio 2000; Arigony et al. 2013; Traber and Stevens 2011; Kao et al. 1990). Also, it could stimulate the production of laminin and fibronectin by trabecular meshwork cells (Yue et al. 1990). In this study, the optimum concentration of vitamin C was 9.719 mg/L which was relatively low compared to the concentration used as reported by Rourou et al. (2009) at 100 μM (17.612 mg/L) in IPT-AFM medium to avoid medium precipitation. Generally, the concentration of vitamin C in culture medium containing 10% (v/v) FBS is trace, while about 50 μM (8.806 mg/L) is in human serum. Thus, the appropriate concentration of vitamin C in culture medium should be as low as found in human serum. Therefore, the optimal concentration of vitamin C at 9.719 mg/L was close to that found in human serum and also to that suggested for using in cell culture medium (Arigony et al. 2013). Vitamin B12 is required for growth, genetic stability, and the survival of cell in vitro. It acts as unique coenzymes of methionine synthase and methylmalonyl-Co A mutase. The deficiency of vitamin B12 may contribute to acidosis, genome instability, and mitochondria-mediated apoptosis (Arigony et al. 2013). In this study, the optimum concentration of vitamin B12 was 0.1725 mg/L. In serum-free media condition, the requirement of vitamin B12 for a low cell density is necessary as used in this study (2 × 104 cells/cm2). In addition, it might have the synergistic effect associated with hypoxanthine and thymidine, which were found in Bacto™ soytone and Bacto™ yeast extract, which could stimulate cell growth (Bjare 1992). Vitamin B12 in culture medium containing 10% (v/v) FBS is trace and is also relatively low in human serum at 3 × 10–4 μM. However, its concentration in culture medium should be higher than that found in human serum (0.407 μg/L) (Arigony et al. 2013). Although the optimum concentration of vitamin B12 in this study was relatively high compared to that suggested for using in cell culture medium, the excessive concentration of it has still not been identified.
Fig. 2.
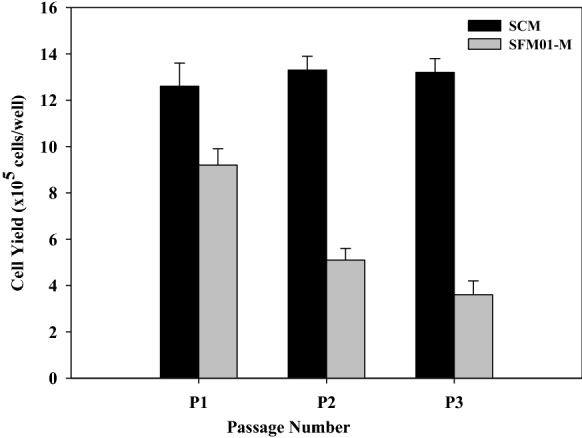
Subcultures of cells in SFM01-M compared with SCM. In SFM01-M cell cultures, a cell density of 2 × 104 cells/cm2 was inoculated in 6-well plates and then cultured at 37 °C, 5% CO2 for 4 days. In SCM cell cultures a cell density of 2 × 104 cells/cm2 was inoculated in 6-well plates and then cultured at 37 °C, 5% CO2 for 4 days. Both cell cultures were carried out in triplicate and viable cell density was expressed as mean ± SD. Comparison of cell yields at P1, P2, and P3 between SFM01-M and SCM was significantly different (p-value < 0.05)
Fig. 3.
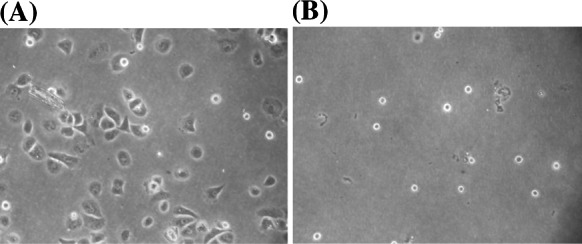
Comparison of inoculated cells, a cells were incubated in SCM at 37 °C, 5% CO2 for 3 h before replaced with tested medium and b cells were washed 3 times with PBS before seeded and incubated at 37 °C, 5% CO2 for 3 h in tested medium
Effect of protein hydrolysates on cell growth
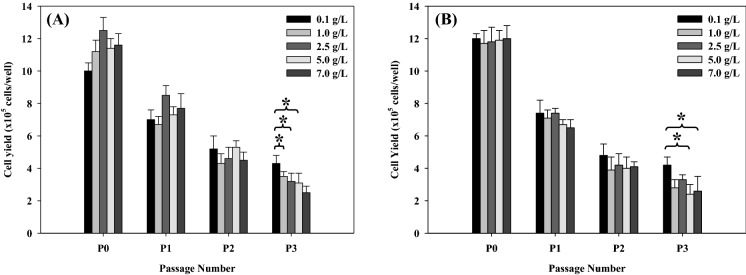
As shown in Fig. 4a and b, cell yields decreased continuously after P0. However, the use of either Bacto™ soytone or Bacto™ yeast extract at a low concentration of 0.1 g/L showed the maximum cell yields at P3 when compared with other combinations although some higher combinations of them showed no significant difference (p-value > 0.05). However, the use of them at a low concentration was probably more suitable than other concentrations during subcultures under the serum-free condition and could also reduce a cost of medium preparation. Bacto™ soytone is rich in high-quality proteins, carbohydrates, calcium, and B vitamins. It is produced using animal-free sources or enzymes of microorganisms that have been grown in animal-free media (BD Bionutrients™ technical manual 2006). The protein hydrolysates provided not only a source of amino acids but also specific peptides that could mimic growth factors or survival factors (Franĕk et al. 2000). For Vero cell cultures under serum-free or animal component-free condition, the optimum concentration of soy peptone was relatively much different as reported by other authors, for example, 0.2 g/L (Lobo-Alfonso et al. 2010) and 5 g/L (Rourou et al. 2009). Generally, soy protein hydrolysates have been used extensively as a medium additive in serum-free or protein-free media to improve cell density and productivity. However, the specific characteristic of quality (impurity) and concentration of each soy protein hydrolysate may lead to the distinct difference of cell responses (Jan et al. 1994; Heidemann et al. 2000; Chun et al. 2005, 2007; Rourou et al. 2009; Michiels et al. 2011a, b; Babcock et al. 2010; Lobo-Alfonso et al. 2010; Davami et al. 2014; Saifuddin et al. 2014; Spearman et al. 2014). Bacto™ yeast extract is the concentrate of the water-soluble portion of Saccharomyces cerevisiae cells that have been autolyzed. Yeast extract provides essential water soluble vitamins, amino acids, peptides and carbohydrates (BD BionutrientsTM technical manual 2006). Yeast extract has been widely used as an additive or serum substitute for mammalian and insect cell lines (Drews et al. 1995; Batista et al. 2008; Vaughn and Fan 1997; Wu and Lee 1998; Ikonomou et al. 2001; Mendonça et al. 2007; Shen et al. 2007; Chou 2013; Sung et al. 2004; Kim and Lee 2009). For Vero cell cultures under serum-free condition, the growth of Vero cells could be maintained when only 0.2 g/L of yeast extract was added to culture medium (MEM) (Lobo-Alfonso et al. 2010). However, the selection of yeast extracts or yeast hydrolysates including their concentrations for each cell type should be much considered because they might cause the undesirable cell production (Chun et al. 2007; Babcock et al. 2010).
Fig. 4.
Effect of Bacto™ soytone (a) and Bacto™ yeast extract (b) on cell growth during subcultures. In a, the concentration of Bacto™ soytone was varied from 0.1 to 7.0 g/L in SFM01-M, whereas the concentration of Bacto™ yeast extract was kept constant at 7.0 g/L. In b, the concentration of Bacto™ yeast extract was varied from 0.1 to 7.0 g/L in SFM01-M, whereas the concentration of Bacto™ soytone was kept constant at 7.0 g/L. Comparison of cell yields at P3 between SFM01-M containing either Bacto™ soytone (0.1 g/L) or Bacto™ yeast extract (0.1 g/L) and other combinations of them in SFM01-M was not significantly different when p-value was higher than 0.05 (*)
Effect of l-glutamine on cell growth
When l-glutamine was increased to 4.0 mM, cell growth was improved (Fig. 5). Glutamine is a major source of energy, carbon, and nitrogen for in vitro culture of mammalian cells. The amount of glutamine present in culture media is 2.0–6.0 mM which is more than other amino acids. It is metabolized via glutamate and α-ketoglutarate, and enters the TCA cycle to provide energy (Huang et al. 2006). The rapid depletion of glutamine and serine during Vero cell cultures in a serum-free medium might relate to cell apoptosis (Quesney et al. 2003). In microcarrier cultures of BHK 21/C13 cells, the optimal glutamine concentration was 4.0 mM (Butler and Spier, 1984). Although the metabolic by-product of l-glutamine is ammonium that can inhibit the growth of animal cells; however, Vero cells were not sensitive to NH4 Cl at 2.0 mM (Hassell et al. 1991), and IC50 at 5.0 mM (Huang et al. 2006). It is possible that the addition of l-glutamine at 4.0 mM in SFM01-M was suitable for Vero cells under serum-free condition.
Fig. 5.
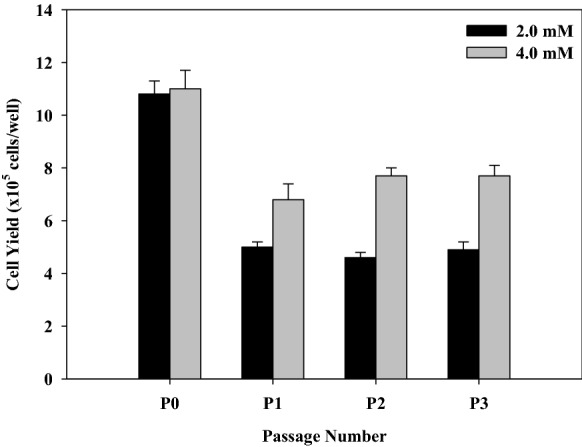
Effect of l-glutamine on cell growth. The concentrations of both Bacto™ soytone and Bacto™ yeast extract in SFM01-M were fixed at 0.1 g/L, whereas other components were fixed as follows: MEM (9.4 g/L), sodium hydrogen carbonate (2.2 g/L), MEM non-essential amino acids (1.0% v/v), sodium pyruvate (1.0 mM), vitamin C (9.719 mg/L), vitamin B12 (0.1725 mg/L), SITE liquid media supplement (2.0% v/v), rEGF (0.5756 mg/L). Comparison of cell yields at P3 between SFM01-M supplemented with 2 mM l-glutamine and SFM01-M supplemented with 4 mM l-glutamine was significantly different (p-value < 0.05)
Effect of SITE liquid media supplement on cell growth
Since no significant difference of cell yields was observed (Fig. 1 and Table 3) when two levels of SITE liquid media supplement (low and high) were studied by using the 26–2 fractional factorial design; therefore, four concentrations of SITE liquid media supplement were further investigated separately. The results showed that the cell yields obtained at P0 for all concentrations of SITE liquid media supplement were relatively high and also higher than those obtained at subsequent passages (P1, P2, and P3). However, the cell yields obtained at P1, P2, and P3 were still relatively constant for all concentrations of SITE liquid media supplement used in this study (Fig. 6). In general, SITE is a mixture of recombinant human insulin, human transferrin (partially iron-saturated), sodium selenite, and ethanolamine which is commonly used to promote cell growth and proliferation in serum-free culture. In this study, the results showed that the concentration of SITE liquid media supplement used (0.1–2.0% v/v) in SFM01-M might be suitable for supporting Vero cell growth and proliferation. However, since no significant difference of cell yields obtained at P1, P2, and P3, hence the preparation of SFM01-M is probably cost-effective when a low concentration of SITE liquid media supplement (0.1–0.5% v/v) is used.
Fig. 6.
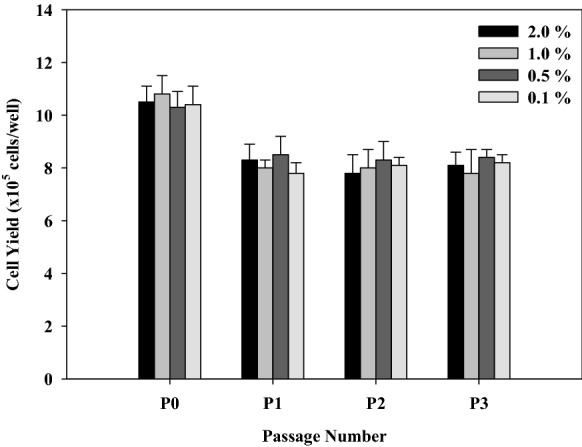
Effect of SITE liquid media supplement on cell growth. The concentrations of both Bacto™ soytone and Bacto™ yeast extract in SFM01-M were fixed at 0.1 g/L and l-glutamine was 4.0 mM, whereas othercomponents were fixed as follows: MEM (9.4 g/L), sodium hydrogen carbonate (2.2 g/L), MEM non-essential amino acids (1.0% v/v), sodium pyruvate (1.0 mM), vitamin C (9.719 mg/L), vitamin B12 (0.1725 mg/L), rEGF (0.5756 mg/L). Comparison of cell yields at P3 among SFM01-M supplemented with different concentration of SITE liquid media supplement was not significantly different (p-value > 0.05)
Conclusion
The aim of this study was to describe the development of a modified serum-free medium by using the systematic design of experiments to screen and optimize the serum substitutes. Two protein hydrolysates (Bacto™ soytone and Bacto™ yeast extract) and other four namely vitamin C, vitamin B12, SITE liquid media supplement and rEGF were used as serum substitutes. To screen the serum substitutes, 26–2 fractional factorial design with resolution III was applied. In the analysis of main effects on cell yields, the results showed that Bacto™ soytone, Bacto™ yeast extract, vitamin C, and vitamin B12 had effect negatively whereas SITE liquid media supplement and rEGF had effect positively. However, the analysis of estimated effects and coefficients of the response (cell yields) acted directly by each factor and factor-interactions showed obviously that vitamin C, vitamin B12, and rEGF had effect significantly (p-value < 0.05). After that, the concentration of three components was further optimized using the central composite design. The optimum concentrations of vitamin C, vitamin B12, and rEGF were respectively 9.719 mg/L, 0.1725 mg/L, and 0.05756 mg/L. A modified serum-free medium (named as SFM01-M) was subsequently verified by the direct adaptation method which Vero cells were cultured in SCM at 37 °C for 3 h before replacing with SFM01-M. However, cell yields decreased continuously during subculture at P1, P2, and P3 whereas only at P0 could give the maximum cell yield. The effect of protein hydrolysates, l-glutamine, and SITE liquid media supplement on cell yields were further investigated. Cell yields gradually decreased from P1 to P3 when a fixed concentration of Bacto™ yeast extract (7.0 g/L) combined with each concentration of Bacto™ soytone (0.1–7.0 g/L) in SFM01-M were used. Similarly, cell yields also gradually decreased from P1 to P3 when a fixed concentration of Bacto™ soytone (7.0 g/L) combined with each concentration of Bacto™ yeast extract (0.1–7.0 g/L) in SFM01-M were used. However, the combination of Bacto™ soytone at 0.1 g/L and Bacto™ yeast extract at 7.0 g/L or Bacto™ soytone at 7.0 g/L and Bacto™ yeast extract at 0.1 g/L in SFM01-M could give the maximum cell yield at P3 when compared with other combinations. In addition, it was found that the addition of SITE liquid media supplement (0.1–2.0% v/v) in SFM01-M in which the concentrations of Bacto™ soytone, Bacto™ yeast extract, and l-glutamine were fixed at 0.1 g/L, 0.1 g/L, and 4.0 mM, respectively, the cell yields obtained at P3 were not significantly different. From this study, the optimum concentrations of SFM01-M components were as follows: Bacto™ soytone (0.1 g/L), Bacto™ yeast extract (0.1 g/L), vitamin C (9.719 mg/L), vitamin B12 (0.1725 mg/L), SITE liquid media supplement (0.1–2.0% v/v), rEGF (0.05756 mg/L), l-glutamine (4.0 mM), MEM non-essential amino acids (1.0% v/v), sodium pyruvate (1.0 mM), MEM (9.4 g/L), and sodium hydrogen carbonate (2.2 g/L). However, to evaluate SFM01-M in the long-term subculture of Vero cells, the efficiency of SFM01-M will be further investigated.
Acknowledgements
The authors would like to acknowledge Asst. Prof. Dr. Kanokwan Poomputsa, Dr. Panit Kitsubun for their critical comments, and Dr. Saengchai Akeprathumchai for his suggestion on experimental design.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manoch Posung, Email: manoch.p@dmsc.mail.go.th.
Anan Tongta, Email: anan.ton@kmutt.ac.th.
References
- Arrigoni O, De Tullio M. The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol. 2000;157:481–488. doi: 10.1016/S0176-1617(00)80102-9. [DOI] [Google Scholar]
- Arigony ALA, De Oliveira IM, Machado M, Bordin DL, Bergter L, Prá D, Henriques JAP. The influence of micronutrients in cell culture: a reflection on viability and genomic stability. BioMed Res Int. 2013;2013:1–22. doi: 10.1155/2013/597282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock J, Wilcox C, Huttinga H. Partial replacement of chemically defined media with plant-derived protein hydrolysates. Biopharm Int. 2010;23:36–41. [Google Scholar]
- Batista FRX, Pereira CA, Mendonça RZ, Moraes ĂM. Formulation of a protein-free medium based on IPL-41 for the sustained growth of Drosophila melanogaster S2 cells. Cytotechnology. 2008;57:11–22. doi: 10.1007/s10616-008-9153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettger WJ, Boyce ST, Walthall BJ, Ham RG. Rapid clonal growth and serial passage of human diploid fibroblasts in a lipid-enriched synthetic medium supplemented with epidermal growth factor, insulin, and dexamethasone. Proc Natl Acad Sci USA. 1981;78:5588–5592. doi: 10.1073/pnas.78.9.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BD BionutrientsTM technical manual . Advanced bioprocessing, 3rd revised edition. New Jersey: BD Biosciences; 2006. [Google Scholar]
- Bjare U. Serum-free cell culture. Pharmacol Ther. 1992;53:355–374. doi: 10.1016/0163-7258(92)90056-6. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Pavlovic D, Jevtovic T, Stojanovic I, Sokolovic D. Vitamin B12 and folic acid effects on polyamine metabolism in rat liver. Pteridines. 2006;17:90–94. doi: 10.1515/pteridines.2006.17.3.90. [DOI] [Google Scholar]
- Box GEP, Hunter JS, Hunter WG. Statistics for experimenters: designs, innovation, and discovery. 2. New York: Wiley-Liss; 2005. [Google Scholar]
- Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. Altex. 2010;27:53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- Butler M, Spier RE. The effects of glutamine utilisation and ammonia production on the growth of BHK cells in microcarrier cultures. J Biotechnol. 1984;1:187–196. doi: 10.1016/0168-1656(84)90004-X. [DOI] [Google Scholar]
- Castle P, Robertson JS. Animal sera, animal sera derivatives and substitutes used in the manufacture of pharmaceuticals. Biologicals. 1998;26:365–368. doi: 10.1006/biol.1998.0165. [DOI] [PubMed] [Google Scholar]
- Cervera L, Gutiérrez S, Gòdia F, Segura MM. Optimization of HEK 293 cell growth by addition of non-animal derived components using design of experiments. BMC Proceedings. 2011;5:126. doi: 10.1186/1753-6561-5-S8-P126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ke Y, Chen Y. A serum-free medium for hybridoma cell culture. Cytotechnology. 1993;11:169–174. doi: 10.1007/BF00749866. [DOI] [PubMed] [Google Scholar]
- Chou CC. Identification of bioactive yeastolate fractions responsible for insect cell growth and baculovirus production. J Biochem Tech. 2013;4:611–615. [Google Scholar]
- Chuman L, Fine LG, Cohen AH, Saier MH., Jr Continuous growth of proximal tubular kidney epithelial cells in hormone-supplemented serum-free medium. J Cell Biol. 1982;94:506–510. doi: 10.1083/jcb.94.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun BH, Lee YK, Bang WG, Chung N. Use of plant protein hydrolysates for varicella virus production in serum-free medium. Biotechnol Lett. 2005;27:243–248. doi: 10.1007/s10529-004-8357-4. [DOI] [PubMed] [Google Scholar]
- Chun BH, Kim JH, Lee HJ, Chung N. Usability of size-excluded fractions of soy protein hydrolysates for growth and viability of Chinese hamster ovary cells in protein-free suspension culture. Bioresour Technol. 2007;98:1000–1005. doi: 10.1016/j.biortech.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Davami F, Baldi L, Rajendra Y, Wurm M. Peptone supplementation of culture medium has variable effects on the productivity of CHO cells. Int J Mol Cell Med. 2014;3:146–156. [PMC free article] [PubMed] [Google Scholar]
- Drews M, Paalme T, Vilu R. The growth and nutrient utilization of the insect cell line Spodoptera frugiperda Sf9 in batch and continuous culture. J Biotechnol. 1995;40:187–198. doi: 10.1016/0168-1656(95)00045-R. [DOI] [Google Scholar]
- Eto N, Yamada K, Shito T, Shirahata S, Murakami H. Development of a protein-free medium with ferric citrate substituting transferrin for the cultivation of mouse-mouse hybridomas. Agri Biol Chem. 1991;55:863–865. [PubMed] [Google Scholar]
- Franĕk F, Hohenwarter O, Katinger H. Plant protein hydrolysates: preparation of defined peptide fractions promoting growth and production in animal cells cultures. Biotechnol Prog. 2000;16:688–692. doi: 10.1021/bp0001011. [DOI] [PubMed] [Google Scholar]
- Freshney RI. Culture of animal cells: a manual of basic technique and specialized applications. 6. New York: Wiley-Blackwell; 2010. [Google Scholar]
- Gospodarowicz D, Moran JS. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- Hassell T, Gleave S, Butler M. Growth inhibition in animal cell culture: the effect of lactate and ammonia. Appl Biochem Biotechnol. 1991;30:29–41. doi: 10.1007/BF02922022. [DOI] [PubMed] [Google Scholar]
- Heidemann R, Zhang C, Qi H, Rule JL, Rozales C, Park S, Chuppa S, Ray M, Michaels J, Konstantin K, Naveh D. The use of peptones as medium additives for the production of a recombinant therapeutic protein in high density perfusion cultures of mammalian cells. Cytotechnology. 2000;32:157–167. doi: 10.1023/A:1008196521213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett G. Strategies for optimising serum-free media. Cytotechnology. 1991;5:3–14. doi: 10.1007/BF00365530. [DOI] [PubMed] [Google Scholar]
- Huang H, Yi X, Zhang Y. Improvement of Vero cell growth in glutamate-based culture by supplementing ammoniagenic compounds. Process Biochem. 2006;41:2386–2392. doi: 10.1016/j.procbio.2006.06.018. [DOI] [Google Scholar]
- Ikonomou I, Bastin G, Schneider YJ, Agathos SN. Design of an efficient medium for insect cell growth and recombinant protein production. Vitro Cell Dev Biol. 2001;37:549–559. doi: 10.1290/1071-2690(2001)037<0549:DOAEMF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Jäger V, Lehmann J, Friedl P. Serum-free growth medium for the cultivation of a wide spectrum mammalian cells in stirred bioreactors. Cytotechnology. 1988;1:319–329. doi: 10.1007/BF00365077. [DOI] [PubMed] [Google Scholar]
- Jan DCH, Jones SJ, Emery AN, Al-Rubeai M. Peptone, a low cost growth-promoting nutrient for intensive animal cell culture. Cytotechnology. 1994;16:17–26. doi: 10.1007/BF00761775. [DOI] [PubMed] [Google Scholar]
- Jayme DW. Nutrient optimization for high density biological production applications. Cytotechnology. 1991;5:15–30. doi: 10.1007/BF00365531. [DOI] [PubMed] [Google Scholar]
- Kao J, Huey G, Kao R, Stern R. Ascorbic Acid stimulates production of glycosaminoglycans in cultured fibroblasts. Exp Mol Pathol. 1990;53:1–10. doi: 10.1016/0014-4800(90)90020-E. [DOI] [PubMed] [Google Scholar]
- Keenan J, Pearson D, Clynes M. The role of recombinant proteins in the development of serum-free media. Cytotechnology. 2006;50:49–56. doi: 10.1007/s10616-006-9002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen MJ, Rapson NT. Development of a serum-free medium for the large scale production of recombinant protein from a Chinese hamster ovary cell line. Cytotechnology. 1995;17:153–163. doi: 10.1007/BF00749653. [DOI] [PubMed] [Google Scholar]
- Kenyon SH, Nicolaou A, Ast T, Gibbons WA. Stimulation in vitro of vitamin B12-dependent methionine synthase by polyamines. Bioch J. 1996;316:661–665. doi: 10.1042/bj3160661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee GM. Development of serum-free medium supplemented with hydrolysates for the production of therapeutic antibodies in CHO cell cultures using design of experiments. Appl Microbiol Biotechnol. 2009;83:639–648. doi: 10.1007/s00253-009-1903-1. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kim NS, Lee GM. Development of a serum-free medium for the production of humanized antibody from Chinese hamster ovary cells using a statistical design. Vitro Cell Dev Biol. 1998;34:757–761. doi: 10.1007/s11626-998-0029-6. [DOI] [PubMed] [Google Scholar]
- Kovář J, Franĕk F. Growth-stimulating effect of transferrin on a hybridoma cell line: relation to transferrin iron-transporting function. Exp Cell Res. 1989;182:358–369. doi: 10.1016/0014-4827(89)90241-3. [DOI] [PubMed] [Google Scholar]
- Lane DJR, Richardson DR. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption. Free Radic Biol Med. 2014;75:69–83. doi: 10.1016/j.freeradbiomed.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Lee GM, Kim EJ, Kim NS, Yoon SK, Ahn YH, Song JY. Development of a serum-free medium for the production of erythropoietin by suspension culture of recombinant Chinese hamster ovary cells using a statistical design. J Biotechnol. 1999;69:85–93. doi: 10.1016/S0168-1656(99)00004-8. [DOI] [PubMed] [Google Scholar]
- Liu CH, Chang TY. Rational development of serum-free medium for Chinese hamster ovary cells. Process Biochem. 2006;41:2314–2319. doi: 10.1016/j.procbio.2006.06.008. [DOI] [Google Scholar]
- Liu CH, Chu IM, Hwang SM. Factorial designs combined with the steepest ascent method to optimize serum-free media for CHO cells. Enzyme Microb Technol. 2001;28:314–321. doi: 10.1016/S0141-0229(00)00346-X. [DOI] [PubMed] [Google Scholar]
- Lobo-Alfonso J, Price P, Jayme D. Benefits and limitation of protein hydrolysates as components of serum-free media for animal cell culture applications. Berlin: Springer Science + Business Media; 2010. [Google Scholar]
- Mendonça RZ, de Oliveira EC, Pereira CA, Lebrun I. Effect of bioactive peptides isolated from yeastolate, lactalbumin and NZCase in the insect cell growth. Bioprocess Biosyst Eng. 2007;30:157–164. doi: 10.1007/s00449-006-0099-3. [DOI] [PubMed] [Google Scholar]
- Michiels JF, Barbau J, De Boel S, Dessy S, Agathos SN, Schneider YJ. Characterisation of beneficial and detrimental effects of a soy peptone, as an additive for CHO cell cultivation. Process Biochem. 2011;46:671–681. doi: 10.1016/j.procbio.2010.11.012. [DOI] [Google Scholar]
- Michiels JF, Sart S, Schneider YJ, Agathos SN. Effects of a soy peptone on γ-IFN production steps in CHO-320 cells. Process Biochem. 2011;46:1759–1766. doi: 10.1016/j.procbio.2011.05.025. [DOI] [Google Scholar]
- Montgomery DC. Design and analysis of experiments. 5. New York: Wiley; 2001. [Google Scholar]
- Morris AE, Schmid J. Effects of insulin and long R3 on serum-free chinese hamster ovary cell cultures expressing two recombinant proteins. Biotechnol Prog. 2000;16:693–697. doi: 10.1021/bp0000914. [DOI] [PubMed] [Google Scholar]
- Murakami H, Masui H, Sato GH, Sueoka N, Chow TP, Kano-Sueoka T. Growth of hybridoma cells in serum-free medium: ethanolamine is an essential component. Proc Natl Acad Sci USA. 1982;79:1158–1162. doi: 10.1073/pnas.79.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Tani R, Yabumoto M, Sakamoto A, Takada K, Sato GH, Sato JD. Effects of insulin and transferrin on the generation of lymphokine-activated killer cells in serum-free medium. J Immunol Methods. 1996;195:7–14. doi: 10.1016/0022-1759(96)00081-6. [DOI] [PubMed] [Google Scholar]
- Parampalli A, Eskridge K, Smith L, Meagher MM, Mowry MC, Subramanian A. Development of serum-free media in CHO-DG44 cells using a central composite statistical design. Cytotechnology. 2007;54:57–68. doi: 10.1007/s10616-007-9074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot E, Fournier F, Gény C, Pinton H, Marc A. Rapid screening of serum-free media for the growth of adherent vero cells by using a small-scale and non-invasive tool. Appl Biochem Biotechnol. 2010;160:1600–1625. doi: 10.1007/s12010-009-8674-0. [DOI] [PubMed] [Google Scholar]
- Quesney S, Marc A, Gerdil C, Gimenez C, Marvel J, Richard Y, Meignier B. Kinetics and metabolic specifications of Vero cells in bioreactor cultures with serum-free medium. Cytotechnology. 2003;42:1–11. doi: 10.1023/A:1026185615650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourou S, Van der Ark A, Van der Velden T, Kallel H. Development of an animal-component free medium for vero cells culture. Biotechnol Prog. 2009;25:1752–1761. doi: 10.1002/btpr.279. [DOI] [PubMed] [Google Scholar]
- Saifuddin N, Johari NA, Anuar N, Samsuddin R. Effect of soy hydrolysates on CHO-K1 cells growth profile in low serum mixed media. Regen Res. 2014;3:56–63. [Google Scholar]
- Shen CF, Kiyota T, Jardin B, Konishi Y, Kamen A. Characterization of yeastolate fractions that promote insect cell growth and recombinant protein production. Cytotechnology. 2007;54:25–34. doi: 10.1007/s10616-007-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman M, Lodewyks C, Richmond M, Butler M. The bioactivity and fractionation of peptide hydrolysates in cultures of CHO cells. Biotechnol Prog. 2014;30:584–593. doi: 10.1002/btpr.1930. [DOI] [PubMed] [Google Scholar]
- Sung YH, Lim SW, Chung JY, Lee GM. Yeast hydrolysate as a low-cost additive to serum-free medium for the production of human thrombopoietin in suspension cultures of Chinese hamster ovary cells. Appl Microbiol Biotechnol. 2004;63:527–536. doi: 10.1007/s00253-003-1389-1. [DOI] [PubMed] [Google Scholar]
- Taub M, Chuman L, Saier MH, Sato G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc Natl Acad Sci USA. 1979;76:3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci. 2001;58:244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, Hellebrekers L, Hyllner J, Jonker FH, Prieto P, Thalen M, Baumans V. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol in Vitro. 2004;18:1–12. doi: 10.1016/j.tiv.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Van der Valk J, Brunner D, Smet KD, Svenningsen ÅF, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, Gstraunthaler G, (2010) Optimization of chemically defined cell culture media-Replacing fetal bovine serum in mammalian in vitro methods. Toxicol in Vitro 24:1053–1063 [DOI] [PubMed]
- Vaughn JL, Fan F. Differential requirements of two insect cell lines for growth in serum-free medium. Vitro Cell Dev Biol. 1997;33:479–482. doi: 10.1007/s11626-997-0067-5. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1998) Requirements for the use of animal cells as in vitro substrates for the production of biological. WHO Technical Report Series 878:20–55 (Annex 1)
- Wu J, Lee KID. Growth promotion by yeastolate and related components on insect cells. Biotechnol Tech. 1998;12:67–70. doi: 10.1023/A:1008811711205. [DOI] [Google Scholar]
- Yue BYJT, Higginbotham E, Chang IL. Ascorbic acid modulates the production of fibronectin and laminin by cells from an eye tissue-trabecular meshwork. Exp Cell Res. 1990;187:65–68. doi: 10.1016/0014-4827(90)90117-S. [DOI] [PubMed] [Google Scholar]
- Zhaolie C, Chengzu X, Hong L, Benchuan W, Xihua J. A novel serum-free medium for the cultivation of Vero cells on microcarriers. Biotechnol Tech. 1996;10:449–452. doi: 10.1007/BF00174231. [DOI] [Google Scholar]