Abstract
Despite the identical genomic context, trophocytes and oenocytes in worker bees exhibit aging-related phenotypes, in contrast to the longevity phenotypes in queen bees. To explore this phenomenon at the molecular level, we evaluated the age-associated transcriptomes of trophocytes and oenocytes in worker bees and queen bees using high-throughput RNA-sequencing technology (RNA-seq). The results showed that (i) while gene expression profiles were different between worker and queen bees, they remained similar between young and old counterparts; (ii) worker bees express a high proportion of low-abundance genes, whereas queen bee transcriptomes display a high proportion of moderate-expression genes; (iii) genes were upregulated to a greater extent in queen bees vs. worker bees; and (iv) distinct aging-related and longevity-related candidate genes were found in worker and queen bees. These results provide new insights into the cellular aging and longevity of trophocytes and oenocytes in honey bees. Identification of aging-associated biomarker genes also constitutes a basis for translational research of aging in higher organisms.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00360-y.
Keywords: Transcriptome, Next-generation sequencing, Aging, Longevity, Honey bee
Introduction
Honey bee (Apis mellifera) is a unique model animal for aging and longevity studies because queen bees have a much longer lifespan than worker bees, even though they have the same genome [58–60, 62]. Besides, honey bees live in large colonies, are easily manipulated, and their genome has been sequenced [3, 18, 27, 68].
The difference in lifespan between worker and queen bees is mainly related to food intake [39]. Queen bees feed on royal jelly throughout their lives, whereas worker bees mostly consume glandular secretions, honey, and pollen [69]. Queen bees live in the hive except during mating flights and colony fission and generally lay 1500–2000 eggs per day throughout their lives [7]. By contrast, worker bees spend only the first two weeks of life in the hive and perform tasks such as comb-building, cell-cleaning, and brood-nursing before transitioning to the foraging stage. In the foraging stage, workers fly up to 21 km per day to collect nectar and pollen for the hive [55]. However, while worker and queen bees perform distinct tasks, these differences are rather inconsequential in altering the lifespan of honey bees [32, 39, 59].
The trophocytes and oenocytes of honey bees have been targeted as cells for cellular senescence and longevity studies due to their biological relevance as white adipose tissue or liver, the ease of isolation from the abdomen, and convenient manipulation [11, 26, 56, 64]. Trophocytes, which are large and irregularly shaped, and oenocytes, which are small and spherical, attach to form a single layer of cells around each segment of the honey bee abdomen. Most importantly, these cells do not divide during adulthood, which is a requirement for studying aging and longevity [29, 30]. Previous studies have shown that several metabolic features — mitochondrial energy utilization, energy regulation activity, cellular degradation activity, and cellular metabolism — in the trophocytes and oenocytes of worker bees decrease with age [13, 34, 35]. However, these processes are maintained with age in the trophocytes and oenocytes of queen bees [36–38]. These findings thus indicate that the trophocytes and oenocytes of worker bees exhibit aging phenotypes and that those of queen bees show pro-longevity phenotypes.
To further explore this phenomenon, we analyzed transcriptomes in the trophocytes and oenocytes of worker bees and queen bees using RNA-seq [21, 66]. Comparative analysis of the age-associated transcriptomes in this unique insect model may provide a mechanistic understanding of aging and longevity control in worker and queen bees.
Methods
Honey bees
The honeycomb frames containing pupae from the different colonies were transferred to an incubator (34 °C, 95% relative humidity). Seventy newly emerged worker bees were collected in a cage (15 × 10 × 12 cm), put into a 34 °C thermostat (NK system, Nippon, Japan), and fed honey and fresh pollen grains mixed with honey every day [32]. Young (5-day-old) and old (30-day-old) worker bees were collected for the following studies; thus, the difference between them is only age. According to previous studies [13, 29, 32, 35, 48], worker bees reared in a thermostat at 34 °C throughout their lives can replace worker bees reared in field hives for aging studies. Young (2-month-old) and old (16-month-old) queen bees were collected from different hives for the following experiments. The young and old queen bees were mated with drones and were able to lay eggs. Since queen bees cannot be reared outside hives without altering their normal physiological state, they need to be collected from the field hives. If young queen bees can lay eggs, beekeepers will cut their wings to prevent colony fission. Young queen bees and old queen bees undergo the same behavioral patterns including mating flights, eating royal jelly, the absence of colony fission, and laying eggs. The difference between young and old queen bees is only age [31]. Young and old worker bees and young and old queen bees were collected on the same dates for subsequent experiments.
RNA isolation, library preparation, and sequencing
Trophocytes and oenocytes were isolated from three biological replicates of ten young and ten old worker bees, or five young and five old queen bees. Total RNA was extracted from trophocytes and oenocytes by using TRIzol (15596018; Invitrogen, Carlsbad, CA, USA). The concentration and quality of the RNA were determined by using an ND-1000 spectrophotometer (Nanodrop Technology, Wilmington, DE, USA) and Bioanalyzer 2100 (Agilent Technology, Santa Clara, CA, USA) with the RNA 6000 Labchip Kit (Agilent Technologies, USA). Library preparation and sequencing were performed according to the manufacturer’s instructions from Illumina at Welgene Biotech Co., Ltd. (Taipei, Taiwan). Library construction of all samples was done by TruSeq RNA Sample Prep Kits v2 for 50-bp (Single-End) sequencing on the Solexa platform. The sequence was directly determined using sequencing-by-synthesis technology via the TruSeq SBS Kit. Raw sequences, roughly 10 million reads per sample, were generated from the Illumina GA Pipeline software CASAVA v1.8.
Raw data processing and statistical analysis
The original image data generated by the sequencer were transformed into sequence data by base calling, which was defined as raw reads and stored in fastq format. Initially, the sequences generated were subjected to a filtering process to obtain qualified reads. ConDeTri was implemented to trim or remove the reads according to the quality score. Qualified reads after filtering low-quality data were analyzed using TopHat/Cufflinks for gene expression estimation. The gene expression level was calculated as fragments per kilobase of transcript per million mapped reads (FPKM). For differential expression analysis, CummeRbund (Illumina Inc.) was employed to perform statistical analyses of gene expression profiles. The reference genome and gene annotations were retrieved from the Beebase database. To identify differentially expressed genes (DEGs), the gene dataset of young worker bees was used as the baseline, to which the genes of other samples were compared.
Function and pathway analysis
All of the differential expressed genes were analyzed using Gene Ontology (GO) and then mapped using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
Validation of RNA-sequencing data by quantitative real-time PCR analysis
To verify the RNA-sequencing results, quantitative real-time PCR (qPCR) was used to measure DEGs expression levels. RNA isolation and qPCR were carried out as previously described [35]. Briefly, total RNA was isolated from the trophocytes and oenocytes (isolated from one bee for each experimental group) using TRIzol. Complementary DNA (cDNA) was synthesized by using 1 μg of total RNA and iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). qPCR reaction containing 0.5 μl of 10 μM each primer, 12.5 μl of SYBR Green (170-8882; Bio-Rad, Hercules, CA, USA), 1 μl of diluted cDNA, and 10.5 μl of ddH2O in a final volume of 25 μl was assayed by using iQ5 RT-PCR detection system (Bio-Rad, Hercules). Primers were designed based on GenBank nucleotide sequences (Supplementary Table 1). The actin gene was used as a control housekeeping gene [18, 70]. The relative expression levels of genes were calculated using the 2−ΔΔCt method [46]. All samples were run in three biological replicates.
Statistical analysis
The difference in the mean values among the four groups was determined by one-way ANOVA and by Tukey’s HSD for pairwise comparisons. SPSS software was used for statistical analysis. Statistical significance was set at 0.05.
Results
Raw data processing
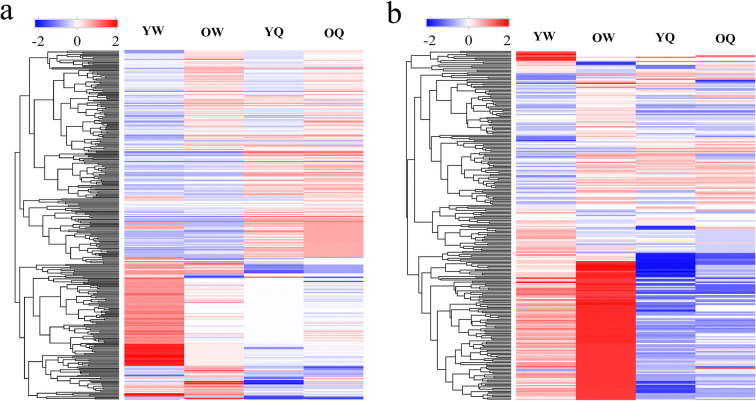
The transcriptome library of young worker bees, old worker bees, young queen bees, and old queen bees on average yielded 30,721,785, 22,072,970, 18,267,899, and 17,796,501 usable reads, respectively. After mapping to the reference genome and the junction database, 15,314 uniquely mapped reads of young worker bees, old worker bees, young queen bees, and old queen bees were acquired (Fig. 1). The overall gene expression profile of young worker bees was similar to that of old worker bees. Likewise, the gene expression profile of young queen bees was similar to that of old queen bees. However, the transcriptomes between the two types of bees were quite different, indicating that developmental fate has a greater impact than age on the transcriptome output. To this end, most upregulated genes identified in worker bees were downregulated in queen bees, and vice versa.
Fig. 1.

Heat map showing clustering of the 15,314 DEGs between worker and queen bees of young and old ages. The hierarchical clustering of samples was arranged as shown above the heat map. Induced genes are represented in red, whereas suppressed genes are shown in blue. YW, young worker bees; OW, old worker bees; YQ, young queen bees; OQ, old queen bees
Expression level analysis
To evaluate the transcriptional status between worker and queen bees at young and old ages, we classified the gene expression levels into eight grades based on their FPKM and defined low-, moderate-, and high-expression genes according to criteria outlined in previous studies [12, 44]. The proportion of low-expression genes (< 1) was higher in worker bees compared with queen bees. In contrast, the proportion of moderate-expression genes (10~100) was higher in queen bees compared with worker bees. The proportion of high-expression genes (500~≥ 1000) was similar between worker and queen bees (Fig. 2).
Fig. 2.
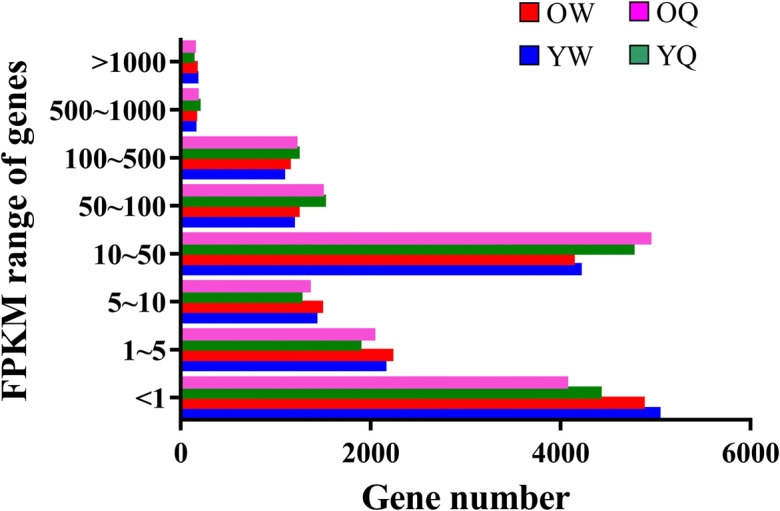
Abundance distribution of unigenes expressed in the indicated samples, as detected by RNA-seq. The expression level ranges are categorized as follows: 0~10, low-expression genes; 10~500, moderate-expression genes; 500~∞, high-expression genes
Screening analysis of DEGs
To screen the genes possibly related to aging and longevity, we analyzed the DEGs between young and old bees. A total of 367 DEGs were found between old vs. young worker bees, of which 224 were upregulated and 143 downregulated. A total of 401 DEGs were found between old vs. young queen bees, of which 356 were upregulated and 45 downregulated. Overall, queen bees were found to express 884–1799 upregulated genes and 405–616 downregulated genes in comparison to worker bees (Fig. 3a). The Venn diagram in Fig. 3b shows the distributions of unique and shared DEGs among different bee groups.
Fig. 3.
Overall transcriptome differences between worker and queen bees of young and old ages. a Distribution of upregulated and downregulated DEGs across different comparisons. b Venn diagram representation of the number of DEGs in the indicated comparison. YW, young worker bees; OW, old worker bees; YQ, young queen bees; OQ, old queen bees
To further associate the DEG candidates with aging and longevity, we identified upregulated and downregulated genes between young and old worker bees as well as between young and old queen bees by using pairwise comparisons. In worker bees, the following genes were highly expressed in old worker bees compared with young worker bees: key lactase metabolism genes encoding L-lactate dehydrogenase (LDH) (GB48134) and l-lactate dehydrogenase-like (GB48315); genes involved in the sensory perception such as odorant receptors (GB52386, GB52385, GB52381, GB52379, GB52387, GB52372, GB52371, GB52388, GB52389); immunity genes such as serine protease 1 (GB45701), serine protease 14 (GB55007), defensin 2 (GB47618), beta-1,3-glucan recognition protein 1 (GB42685) and beta-1,3-glucan recognition protein 2 (GB42981); and dopamine synthesis genes including aromatic-L-amino-acid decarboxylase-like (GB45938) and tyrosine hydroxylase (GB40967). In contrast, the downregulated genes included candidates implicated in critical metabolic pathways: genes involved in carbohydrate metabolic process such as glycogen phosphorylase (GB42835), and chitinase (GB48474); genes involved in lipid metabolic process such as hydroxymethylglutaryl-coenzyme A synthase (GB44420), trans-2,3-enoyl-CoA reductase-like (GB46772), alkyldihydroxyacetonephosphate synthase (GB47736), 1-acyl-sn-glycerol-3-phosphate acyltransferase alpha-like (GB43362), phospholipase A1 member A-like (GB50722), acyl-CoA Delta(11) desaturase-like (GB42218), and elongation of very-long-chain fatty acids protein 4-like (GB54399); cuticular protein (GB50453, GB48981, GB50438, GB40566); genes of mitochondrial respiratory chain complex such as cytochrome b-c1 complex subunit 6, mitochondrial-like (GB50335), ATP synthase, H+ transporting, mitochondrial F1 complex, and alpha subunit 1 (GB41028); and pancreatic triacylglycerol lipase-like (GB43513) (Fig. 4a).
Fig. 4.
Hierarchical clustering analysis of DEGs between young and old ages: a 367 DEGs between young and old worker bees; b 401 DEGs between young and old queen bees. Induced genes are represented in red, while downregulated genes are shown in blue. YW, young worker bees; OW, old worker bees; YQ, young queen bees; OQ, old queen bees
In queen bees, the following genes were highly expressed in the old counterparts: odorant receptor (GB40202, GB52376, GB52363, GB42587, GB40201, GB52362, GB50051); juvenile hormone esterase-like (GB48046); immunity genes such as defensin 1 (GB41428), serine protease 45 (GB43369), serine protease homolog 57 (GB48080), serine protease 47 (GB48081), toll like receptor 8 (GB48399), and apidaecin (GB51306); catalase (GB41427); LOC100576135 glutamate receptor 1-like (GB54881); LOC409777 glutamate receptor; ionotropic kainate 2-like (GB47549); corazonin receptor (GB44824); LOC100578314 monocarboxylate transporter 10-like (GB53682); LOC408646 monocarboxylate transporter 8-like (GB49280); and neurotrimin-like (GB54005, GB44739, GB53527). Conversely, genes downregulated in old vs. young queen bees include Pangolin (GB53166); synaptic vesicle glycoprotein 2B-like (GB50473); adenylate cyclase type 6-like (GB48012); EF-hand domain-containing protein CG10641-like (GB51014); rap guanine nucleotide exchange factor 6-like (GB50393); hemicentin-2-like (GB44248); protein fork head-like (GB53404); and cuticular protein analogous to peritrophins 3-E (GB52854) (Fig. 4b).
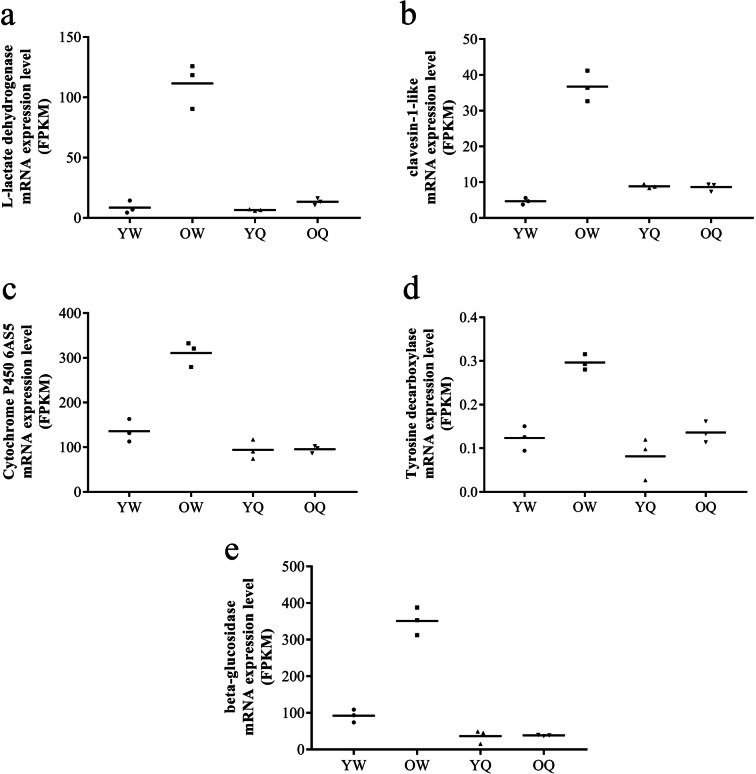
By further integrating these comparisons, we defined aging-related candidate genes as those with higher expression in old worker bees than in the other three bee groups. The examples of these genes included L-lactate dehydrogenase-like (LDH) (GB48135), clavesin-1-like (GB49504), cytochrome P450 6AS5 (CYP6AS5) (GB49890), tyrosine decarboxylase 2 (GB55830), and β-glucosidase (GB54486) (Fig. 5). Likewise, longevity-related candidate genes were defined as those showing higher expression in young worker bees and young and old queen bees (vs. old workers) or unique expression in queen bees but not in worker bees. The examples of these genes included pancreatic triacylglycerol lipase-like (GB43513), epoxide hydrolase (GB42829), Sir2 histone deacetylase (SirT1) (GB53035), major royal jelly protein 1 (MRJP1) (GB55205), epidermal growth factor receptor-like (EGFR) (GB54477), and vitellogenin (Vg) (GB49544) (Fig. 6).
Fig. 5.
Expression profiles of the aging-related candidate genes as revealed by RNA-seq. YW, young worker bees; OW, old worker bees; YQ, young queen bees; OQ, old queen bees
Fig. 6.
Expression profiles of the longevity-related candidate genes as revealed by RNA-seq. YW, young worker bees; OW, old worker bees; YQ, young queen bees; OQ, old queen bees
Validation of RNA-sequencing data by qPCR analysis of cholesterol-hydroxyedysone-Vg pathway genes
To confirm the deep sequencing data, eight randomly selected DEGs were validated by using qPCR in old worker bees, young queen bees, and old queen bees compared with young worker bees. The extents of correlation of old worker bees (0.993179), young queen bees (0.995733), as well as old queen bees (0.996576) in comparison to young worker bees were quite high. The fold changes were also similar among them (Supplementary Table 2). Thus, these qPCR results were consistent with and therefore authenticating the RNA-sequencing findings.
The expression levels of genes involved in the cholesterol-hydroxyecdysone-Vg pathway, such as Cyp314A1, EcR-A, EcR-B1, USP, E74, E75, BR-C, Vg, and VgR, were lower generally in worker bees than in the queen bees [49]. The unique expression patterns of the cholesterol-hydroxyecdysone-Vg pathway observed in this study were consistent with the previous study (Supplementary Fig. 1), demonstrating the reliability of the current RNA-sequencing results and further supporting the link of this pathway to the aging process.
Discussion
Some gene expression studies have previously been conducted to document gene changes associated with queen bee longevity [16], aging [24, 47], reproductive status [22], and flight [53]. However, due to the design of these studies, which are mainly based on gene microarray and real-time PCR analysis of selected genes, they might be limited in the scope of genome-wide changes being detected. In this study, we evaluated the global transcriptomes of trophocytes and oenocytes in young and old worker bees and young and old queen bees using RNA-sequencing and provided a system-wide and comprehensive profile of transcriptome changes associated with caste-specific aging. The findings indicated that the gene expression of trophocytes and oenocytes was different between worker and queen bees, and showed a distinct association with cellular aging in worker bees and cellular longevity in queen bees.
Raw data processing and expression level analysis
RNA-seq has been used to analyze the transcriptomes of the fat body of nurse bees, foragers, and winter worker bees [64], the ovaries of worker and queen bees [57], the hypopharyngeal gland of honeybees [44, 45], queen- and worker-destined larvae of honeybees [12, 19], and the kidneys of rat [66]. As shown by our RNA-seq experiments, gene expression patterns of worker bees were different from those of the queen bees. Importantly, even though they share the same genome, most upregulated genes of worker bees were downregulated in queen bees, and vice versa, indicating that royal jelly reprograms the transcriptome of queen bees [39]. Low-expression genes are mainly expressed in worker bees and moderate-expression genes are mainly expressed in queen bees, suggesting that low-expression genes might be associated with aging and moderate-expression genes might be associated with reproduction and longevity in female honey bees.
Screening analysis of DEGs
The main function of the hypopharyngeal gland is to produce and secrete the protein components of royal jelly. A previous study revealed 614 DEGs in the hypopharyngeal gland between nurse bees and forager, of which 25 were upregulated and 589 downregulated in forager as compared with nurse bees [45]. In trophocytes and oenocytes, DEGs between young and old worker bees were numbered at 314, of which 170 were upregulated and 144 downregulated in old worker bees as compared with young worker bees. The number of DEGs (n = 314) between young and old worker bees is close to the results between nurse bees and foragers [64]. Moreover, identified DEGs in the trophocytes and oenocytes between young and old queen bees are numbered at 401, in which 356 upregulated and 45 downregulated when old queen bees compared with young queen bees.
Functional analysis of DEGs
The genes whose expression altered between young and old worker bees might be associated with aging. The functions of upregulated genes in old worker bees compared to young worker bees corresponded to the detection of chemical stimulus, sensory perception of smell, RNA metabolic process, transcription, and cellular amino acid metabolic process. The genes downregulated in old worker bees might imply the maintenance of youth. This set was enriched in genes associated with lipid metabolic process, structural molecule activity, structural constituent of the cuticle, and calcium ion binding.
In contrast, the upregulated genes detected in old queen bees might be associated with longevity. The putative functions of the detected upregulated genes fell in the categories of olfactory receptor activity, sequence-specific DNA binding, ATP binding, response to stress, oxidation-reduction process, transporter activity, and peptidase activity. The genes downregulated in old queen bees might be associated with youth. In this regard, this set was enriched in genes implicated in the carbohydrate derivative metabolic process and organ nitrogen compound metabolic process. Collectively, these results provided new candidate biomarkers and insights into the molecular mechanisms underlying the aging and longevity of honey bees.
GO annotation of DEGs
All of the DEGs were analyzed using gene ontology (GO). In worker bees, GO terms were generated for 172 items from 314 DEGs. These items were divided into three categories: molecular function, biological process, and cellular component. Binding, metabolic process, and membrane were dominant in the three categories. In terms of molecular functions, GO analysis revealed an over-representation of the genes involved in catalytic activity, ion binding, heterocyclic compound binding, organic cyclic compound binding, and hydrolase activity. In terms of biological processes, GO analysis revealed an over-representation of the genes involved in the single-organism process, cellular process, organic substance metabolic process, single-organism cellular process, and primary metabolic process. In terms of cellular components, GO analysis revealed an over-representation of the genes involved in cell, cell part, membrane part, intracellular, and integral component of membrane.
In queen bees, GO terms were generated for 146 items from 258 DEGs. These items were also divided into three categories: molecular function, biological process, and cellular component. Binding, metabolic process, and membrane were also dominant in the three categories. In terms of molecular functions, GO analysis revealed an over-representation of the genes involved in protein binding, heterocyclic compound binding, organic cyclic compound binding, catalytic activity, and ion binding. In terms of biological processes, GO analysis revealed an over-representation of the genes involved in the cellular process, single-organism process, metabolic process, single-organism cellular process, and biological regulation. In terms of cellular components, GO analysis revealed an over-representation of the genes involved in the intrinsic component of membrane, membrane part, the integral component of membrane, cell, and cell part.
Pathway annotation of DEGs within castes
To further understand the functions of these DEGs, we mapped DEGs by using the Kyoto encyclopedia of genes and genomes (KEGG) database for signaling pathways analysis. In worker bees, 13 pathways were identified and included synthesis and degradation of ketone bodies, oxidative phosphorylation, valine, leucine and isoleucine degradation, tyrosine metabolism; novobiocin biosynthesis, starch and sucrose metabolism, amino sugar and nucleotide sugar metabolism, glycerolipid metabolism, ether lipid metabolism, propanoate metabolism, methane metabolism, folate biosynthesis, and aminoacyl-tRNA biosynthesis. Of those, genes involved in amino sugar and nucleotide sugar metabolism were the most significantly enriched between young worker bees and old worker bees. In queen bees, 4 pathways were identified and included fatty acid elongation, amino sugar, and nucleotide sugar metabolism, glycerolipid metabolism, glyoxylate, and dicarboxylate metabolism.
Aging-related candidate genes
Aging-related candidate genes were defined as higher expression in old worker bees in comparison to young worker bees and young and old queen bees. These genes included LDH-like (GB48135), clavesin-1-like (GB49504), CYP6AS5 (GB49890), tyrosine decarboxylase 2 (GB55830), and β-glucosidase (GB54486).
LDH catalyzes the conversion of lactate to pyruvate and back. LDH gene expression increased in old worker bees, which is consistent with previous studies showing that LDH activity and lactate concentration increase with age in the trophocytes and oenocytes of worker bees (Apis mellifera) [48], in the brain and cerebrospinal fluid of mice [61, 71], and in the cerebrospinal fluid of human [42]. These studies indicate that LDH is associated with aging. Clavesin-1 was essential for the normal morphology of late endosomes and lysosomes [40]. Lysosomal dysfunction is associated with age-related diseases and a decline in lifespan [10]. Clavesin-1 gene expression increased in old worker bees, which is consistent with previous studies showing that lysosomal efficiency decreased with age in the trophocytes and oenocytes of worker bees [35] but remained similar between young and old queen bees [38]. These studies together indicate that clavesin-1 is associated with aging. CYP6AS5 is involved in insecticide metabolism and resistance [1, 4, 14, 52]. Low CYP6AS5 expression may cause low detoxification leading to aging or the accumulation of harmful substances. Tyrosine decarboxylase catalyzes tyrosine to tyramine and CO2. Tyramine can be converted into octopamine, which decreases the number of vitellogenic oocytes and the fecundity in Drosophila [23]. Therefore, tyrosine decarboxylase is associated with aging. β-glucosidase catalyzes the hydrolysis of terminal non-reducing residues in β-glucosides and releases glucose. β-glucosidase gene expression increased in old worker bees, which is consistent with a previous study showing that its activity increased with aging in C. elegans [15].
Longevity-related candidate genes
Longevity-related candidate genes were defined as higher expression in young worker bees and young and old queen bees or young and old queen bees compared with old worker bees. These genes included pancreatic triacylglycerol lipase-like (GB43513), epoxide hydrolase (GB42829), major royal jelly protein 1 (MRJP1) (GB55205), epidermal growth factor receptor-like (EGFR) (GB54477), Sir2 histone deacetylase (GB53035), and Vg (GB49544).
Lipase catalyzes the hydrolysis of triacylglycerol. Lipase gene expression increased in young worker bees and young and old queen bees, which is consistent with previous studies showing that lipase activity decreases with age in the trophocytes and oenocytes of worker bees [48] and rats [5, 9] but remains similar in the trophocytes and oenocytes of young and old queen bees [50]. Epoxide hydrolase cleaves oxirane derivatives to yield the corresponding diols and plays roles in detoxification and metabolism [2, 51]. Epoxide hydrolase gene expression increased in young worker bees and young and old queen bees, which is consistent with previous studies showing that β-oxidation decreases with age in the trophocytes and oenocytes of worker bees [48], human beings [41, 43], rats [65, 67], and mice [28] but remains similar in the trophocytes and oenocytes of young and old queen bees [50]. Besides, epoxide hydrolase participates in lipid catabolism [51].
MRJP1 and EGFR gene expression increased in young and old queen bees, which is consistent with previous studies showing that MRJP1 induces the differentiation of queen and worker bees through EGFR in the larval stage and increases the lifespan of female fly (Drosophila melanogaster) [39] and nematode (C. elegans) through EGFR [17]. SirT1 regulates cellular functions, including cellular stress responses and energy metabolism. SirT1 gene expression increased in young worker bees and young and old queen bees, which is consistent with previous studies showing that SirT1 activity decreases with age in the trophocytes and oenocytes of worker bees [34] and rats [6] but remains similar in the trophocytes and oenocytes of young and old queen bees [36]. In addition, SirT1 activity is increased in calorie restriction [8], ambient temperature reduction [33], and exercise [20], which are all beneficial for longevity. Vg is important storage, transport, and longevity-related protein. Vg gene expression increased in young and old queen bees, which is consistent with previous studies showing that Vg protein and gene maintain at high levels in mature queen bees and long-lived worker bees [47, 49, 63] and decrease in worker bees [25]. In addition, knockdown of Vg decreases the lifespan of worker bees [54].
Supplementary information
Supplementary data are available at GeroScience online.
(JPG 201 kb)
(DOCX 19 kb)
(DOCX 19 kb)
Author contribution
C.Y.H. designed research; C.Y. L. and Y.T.W. performed research; C.Y. L. and Y.T.W. analyzed data; C.Y.H. and B.T. wrote the paper.
Funding
This work was supported by grants (CMRPD1G0582, CMRPD1G0583, and CMRPD1K0481) from the Chang Gung Memorial Hospital, Linkou, Taiwan, and a grant (MOST 108-2320-B-182-037-MY3) from the Ministry of Science and Technology, Taiwan.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alptekin S, Bass C, Nicholls C, Paine MJ, Clark SJ, Field L, et al. Induced thiacloprid insensitivity in honeybees (Apis mellifera L.) is associated with up-regulation of detoxification genes. Insect Mol Biol. 2016;25:171–180. doi: 10.1111/imb.12211. [DOI] [PubMed] [Google Scholar]
- 2.Arand M, Cronin A, Adamska M, Oesch F. Epoxide hydrolases: structure, function, mechanism, and assay. Methods Enzymol. 2005;400:569–588. doi: 10.1016/S0076-6879(05)00032-7. [DOI] [PubMed] [Google Scholar]
- 3.Barchuk AR, Cristino AS, Kucharski R, Costa LF, Simões ZLP, Maleszka R. Molecular determinants of caste differentiation in the highly eusocial honeybee Apis mellifera. BMC Dev Biol. 2007;7:70. doi: 10.1186/1471-213X-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenbaum MR. Postgenomic chemical ecology: from genetic code to ecological interactions. J Chem Ecol. 2002;28:873–896. doi: 10.1023/a:1015260931034. [DOI] [PubMed] [Google Scholar]
- 5.Bey L, Areiqat E, Sano A, Hamilton MT. Reduced lipoprotein lipase activity in postural skeletal muscle during aging. J Appl Physiol. 2001;91:687–692. doi: 10.1152/jappl.2001.91.2.687. [DOI] [PubMed] [Google Scholar]
- 6.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Camazine S. Self-organizing pattern formation on the combs of honey bee colonies. Behav Ecol Sociobiol. 1991;28:61–76. doi: 10.1098/rspb.2008.0793. [DOI] [Google Scholar]
- 8.Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlile SI, Lacko AG. Age-related changes in plasma lipid levels and tissue lipoprotein lipase activities of Fischer-344 rats. Arch Gerontol Geriatr. 1985;4:133–140. doi: 10.1016/0167-4943(85)90027-5. [DOI] [PubMed] [Google Scholar]
- 10.Carmona-Gutierrez D, Hughes AL, Madeo F, Ruckenstuhi C. The crucial impact of lysosomes in aging and longevity. Ageing Res Rev. 2016;32:2–12. doi: 10.1016/j.arr.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan QWT, Mutti NS, Foster LJ, Kocher SD, Amdam GV, Florian W. The worker honeybee fat body proteome is extensively remodeled preceding a major life-history transition. PLoS ONE. 2011;6:e24794. doi: 10.1371/journal.pone.0024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Hu Y, Zheng H, Cao L, Niu D, Yu D, Sun Y, Hu S, Hu F. Transcriptome comparison between honey bee queen-and worker-destined larvae. Insect Biochem Mol Biol. 2012;42:665–673. doi: 10.1016/j.ibmb.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Chuang YL, Hsu CY. Changes in mitochondrial energy utilization in young and old worker honeybees (Apis mellifera) Age. 2013;35:1867–1879. doi: 10.1007/s11357-012-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claudianos C, Ranson H, Johnson RM, Biswas S, Schuler MA, Berenbaum MR, Feyereisen R, Oakeshott JG. A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol. 2006;15:615–636. doi: 10.1111/j.1365-2583.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins JJ, Huang C, Hughes S, Kornfeld K. The measurement and analysis of age-related changes in Caenorhabditis elegans. WormBook. 2008;24:1–21. doi: 10.1895/wormbook.1.137.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mech Ageing Dev. 2005;126:1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Detienne G, De Haes W, Ernst UR, Schoofs L, Temmerman L. Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp Gerontol. 2014;60:129–135. doi: 10.1016/j.exger.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Elsik CG, Worley KC, Bennett AK, Beye M, Camara F, Childers CP, de Graaf DC, Debyser G, Deng J, Devreese B, Elhaik E, Evans JD, Foster LJ, Graur D, Guigo R, HGSC production teams. Hoff K, Holder ME, Hudson ME, Hunt GJ, Jiang H, Joshi V, Khetani RS, Kosarev P, Kovar CL, Ma J, Maleszka R, Moritz RFA, Munoz-Torres MC, Murphy TD, Muzny DM, Newsham IF, Reese JT, Robertson HM, Robinson GE, Rueppell O, Solovyev V, Stanke M, Stolle E, Tsuruda JM, Vaerenbergh M, Waterhouse RM, Weaver DB, Whitfield CW, Wu Y, Zdobnov EM, Zhang L, Zhu D, Gibbs RA, on behalf of Honey Bee Genome Sequencing Consortium Finding the missing honey bee genes: lessons learned from a genome upgrade. BMC Genomics. 2014;15:86. doi: 10.1186/1471-2164-15-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JD, Wheeler DE. Differential gene expression between developing queens and workers in the honey bee, Apis mellifera. Proc Natl Acad Sci USA. 1999;96:5575–5580. doi: 10.1073/pnas.96.10.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 21.Grada A, Weinbrecht K. Next-generation sequencing: methodology and application. J Invest Dermatol. 2013;133:e11. doi: 10.1038/jid.2013.248. [DOI] [PubMed] [Google Scholar]
- 22.Grozinger CM, Fan Y, Hoover SER, Winston ML. Genome-wide analysis reveals differences in brain gene expression patterns associated with caste and reproductive status in honey bees (Apis mellifera) Mol Ecol. 2007;16:4837–4848. doi: 10.1111/j.1365-294X.2007.03545.x. [DOI] [PubMed] [Google Scholar]
- 23.Gruntenko NE, Karpova EK, Alekseev AA, Chentsova NA, Bogomolova EV, Bownes M, Rauschenbach IY. Effects of octopamine on reproduction, juvenile hormone metabolism, dopamine, and 20-hydroxyecdysone contents in Drosophila. Arch Insect Biochem Physiol. 2007;65:85–94. doi: 10.1002/arch.20187. [DOI] [PubMed] [Google Scholar]
- 24.Hagai T, Cohen M, Bloch G. Gene encoding putative takeout/juvenile hormone binding proteins in the honeybee (Apis mellifera) and modulation by age and juvenile hormone of the takeout-like gene GB19811. Insect Biochem Mol Biol. 2007;37:689–701. doi: 10.1016/j.ibmb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Hartfelder K, Engels W. Social insect polymorphism: hormonal regulation of plasticity in development and reproduction in the honeybee. Curr Top Dev Biol. 1998;40:45–77. doi: 10.1016/s0070-2153(08)60364-6. [DOI] [PubMed] [Google Scholar]
- 26.Haunerland NH, Shirk PD. Regional and functional differentiation in the insect fat body. Annu Rev Entomol. 1995;40:121–145. doi: 10.1146/annurev.en.40.010195.001005. [DOI] [Google Scholar]
- 27.Honeybee Genome Sequencing Consortium Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J. The metabolic footprint of aging in mice. Sci Rep. 2011;1:134. doi: 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh YS, Hsu CY. Honeybee trophocytes and fat cells as target cells for cellular senescence studies. Exp Gerontol. 2011;46:233–240. doi: 10.1016/j.exger.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh YS, Hsu CY. The changes of age-related molecules in the trophocytes and fat cells of queen honeybees (Apis mellifera) Apidologie. 2011;42:728–739. doi: 10.1007/s13592-011-0085-x. [DOI] [Google Scholar]
- 31.Hsieh YS, Hsu CY. Oxidative stress and anti-oxidant enzyme activities in the trophocytes and fat cells of queen honeybees (Apis mellifera) Rejuvenation Res. 2013;16:295–303. doi: 10.1089/rej.2013.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu CY, Chan YP. The use of honeybees reared in a thermostatic chamber for aging studies. Age. 2013;35:149–158. doi: 10.1007/s11357-011-9344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu CY, Chiu YC. Ambient temperature influences aging in an annual fish (Nothobranchius rachovii) Aging Cell. 2009;8:726–737. doi: 10.1111/j.1474-9726.2009.00525.x. [DOI] [PubMed] [Google Scholar]
- 34.Hsu CY, Chuang YL. Changes in energy-regulated molecules in the trophocytes and fat cells of young and old worker honeybees (Apis mellifera) J Gerontol A Biol Sci Med Sci. 2014;69:955–964. doi: 10.1093/gerona/glt163. [DOI] [PubMed] [Google Scholar]
- 35.Hsu CY, Chuang Y, Chan YP. Changes in cellular degradation activity in young and old worker honeybees (Apis mellifera) Exp Gerontol. 2014;50:128–136. doi: 10.1016/j.exger.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Hsu CY, Hu TH. Energy-regulated molecules maintain young status in the trophocytes and fat cells of old queen honeybees. Biogerontology. 2014;15:389–400. doi: 10.1007/s10522-014-9509-0. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CY, Lu CY. Mitochondrial energy utilization maintains young status in the trophocytes and oenocytes of old queen honeybees. Apidologie. 2015;46:583–594. doi: 10.1007/s13592-015-0348-z. [DOI] [Google Scholar]
- 38.Hsu CY, Qiu JT, Chan YP. Cellular degradation activity is maintained during aging in long-lived queen bees. Biogerontology. 2016;17:829–840. doi: 10.1007/s10522-016-9652-x. [DOI] [PubMed] [Google Scholar]
- 39.Kamakura M. Royalactin induces queen differentiation in honeybees. Nature. 2011;473:478–483. doi: 10.1038/nature10093. [DOI] [PubMed] [Google Scholar]
- 40.Katoh Y, Ritter B, Gaffry T, Blondeau F, Höning S, McPherson PS. The clavesin family, neuron-specific lipid-and clathrin-binding Sec14 proteins regulating lysosomal morphology. J Biol Chem. 2009;284:27646–27654. doi: 10.1074/jbc.M109.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostyak JC, Kris-Etherton P, Bagshaw D, DeLany JP, Farrell PA. Relative fat oxidation is higher in children than adults. Nutr J. 2007;6:19. doi: 10.1186/1475-2891-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leen WG, Willemsen MA, Wevers RA, Verbeek MM. Cerebrospinal fluid glucose and lactate: age-specific reference values and implications for clinical practice. PLoS ONE. 2012;7:e42745. doi: 10.1371/journal.pone.0042745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levadoux E, Morio B, Montaurier C, Puissant V, Boirie Y, Fellmann N, Picard B, Rousset P, Beaufrere B, Ritz P. Reduced whole-body fat oxidation in women and in the elderly. Int J Obes Relat Metab Disord. 2001;25:39–44. doi: 10.1038/sj.ijo.0801530. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, Ji T, Yin L, Shen J, Shen F, Chen G. Transcriptome sequencing analysis reveals the regulation of the hypopharyngeal glands in the honey bee, Apis mellifera carnica pollmann. PLoS ONE. 2013;8:e81001. doi: 10.1371/journal.pone.0081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Wang ZL, Tian LQ, Qin QH, Wu XB, Yan WY, Zeng ZJ. Transcriptome differences in the hypopharyngeal gland between western honeybees (Apis mellifera) and eastern honeybees (Apis cerana) BMC Genomics. 2014;15:744. doi: 10.1186/1471-2164-15-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Lockett GA, Almond EJ, Huggins TJ, Parker JD, Bourke AF. Gene expression differences in relation to age and social environment in queen and worker bumble bees. Exp Gerontol. 2016;77:52–61. doi: 10.1016/j.exger.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Lu CY, Chuang YL, Hsu CY. Aging results in a decline in cellular energy metabolism in the trophocytes and oenocytes of worker honeybees (Apis mellifera) Apidologie. 2017;48:761–775. doi: 10.1007/s13592-017-0521-7. [DOI] [Google Scholar]
- 49.Lu CY, Huang PJ, Hsu CY. The cholesterol-hydroxyecdysone-vitellogenin pathway is involved in the longevity of trophocytes and oenocytes of queen honey bees (Apis mellifera) Apidologie. 2018;49:721–733. doi: 10.1007/s13592-018-0596-9. [DOI] [Google Scholar]
- 50.Lu CY, Qiu JT, Hsu CY. Cellular energy metabolism is maintained during aging in long-living queen bees. Arch Insect Biochem Physiol. 2018;2018:e21468. doi: 10.1002/arch.21468. [DOI] [PubMed] [Google Scholar]
- 51.Mackert A, Hartfelder K, Bitondi MM, Simões ZL. The juvenile hormone (JH) epoxide hydrolase gene in the honey bee (Apis mellifera) genome encodes a protein which has negligible participation in JH degradation. J Insect Physiol. 2010;56:1139–1146. doi: 10.1016/j.jinsphys.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Mao W, Rupasinghe SG, Johnson RM, Zangerl AR, Schuler MA, Berenbaum MR. Quercetin-metabolizing CYP6AS enzymes of the pollinator Apis mellifera (Hymenoptera: Apidae) Comp Biochem Physiol B Biochem Mol Biol. 2009;154:427–434. doi: 10.1016/j.cbpb.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Margotta JW, Mancinelli GE, Benito AA, Ammons A, Roberts SP, Elekonich MM. Effects of flight on gene expression and aging in the honey bee brain and flight muscle. Insects. 2013;4:9–30. doi: 10.3390/insects4010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007;5:e62. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neukirch A. Dependence of the lifespan of the honeybee (Apis mellifera) upon flight performance and energy consumption. J Comp Physiol. 1982;146:35–40. doi: 10.1016/j.exger.2007.06.002. [DOI] [Google Scholar]
- 56.Nilsen KA, Ihle KE, Frederick K, Fondrk MK, Smedal B, Hartfelder K, Amdam GV. Insulin-like peptide genes in honey bee fat body respond differently to manipulation of social behavioral physiology. J Exp Biol. 2011;214:1488–1497. doi: 10.1242/jeb.050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niu D, Zheng H, Corona M, Lu Y, Chen X, Cao L, Sohr A, Hu F. Transcriptome comparison between inactivated and activated ovaries of the honey bee Apis mellifera L. Insect Mol Biol. 2014;23:668–681. doi: 10.1111/imb.12114. [DOI] [PubMed] [Google Scholar]
- 58.Omholt SW, Amdam GV. Epigenic regulation of aging in honeybee workers. Sci Aging Knowl Environ. 2004;26:pe28. doi: 10.1126/sageke.2004.26.pe28. [DOI] [PubMed] [Google Scholar]
- 59.Page RE, Peng CYS. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp Gerontol. 2001;36:695–711. doi: 10.1016/S0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 60.Remolina SC, Hughes KA. Evolution and mechanisms of long life and high fertility in queen honey bees. Age. 2008;30:177–185. doi: 10.1007/s11357-008-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, et al. High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. Proc Natl Acad Sci U S A. 2010;107:20087–20092. doi: 10.1073/pnas.1008189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rueppell O, Bachelier C, Fondrk MK, Page RE., Jr Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.) Exp Gerontol. 2007;42:1020–1032. doi: 10.1016/j.exger.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc Natl Acad Sci U S A. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seehuus SC, Taylor S, Petersen K, Aamodt RM. Somatic maintenance resources in the honeybee worker fat body are distributed to withstand the most life-threatening challenges at each life stage. PLoS ONE. 2013;8:e69870. doi: 10.1371/journal.pone.0069870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Son N, Hur HJ, Sung MJ, Kim MS, Hwang JT, Park JH, Yang HJ, Kwon DY, Yoon SH, Chung HY, Kim HJ. Liquid chromatography-mass spectrometry-based metabolomic analysis of liver from aged rats. J Proteome Res. 2012;11:2551–2558. doi: 10.1021/pr201263q. [DOI] [PubMed] [Google Scholar]
- 66.Tain YL, Huang LT, Chan JY, Lee CT. Transcriptome analysis in rat kidneys: importance of genes involved in programmed hypertension. Int J Mol Sci. 2015;16:4744–4758. doi: 10.3390/ijms16034744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tucker MZ, Turcotte LP. Impaired fatty acid oxidation in muscle of aging rats perfused under basal conditions. Am J Physiol Endocrinol Metab. 2002;282:E1102–E1109. doi: 10.1152/ajpendo.00175.2001. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Jorda M, Jones PL, Maleszka R, Ling X, Robertson HM, Mizzen CA, Peinado MA, Robinson GE. Functional CpG methylation system in a social insect. Science. 2006;314:645–647. doi: 10.1126/science.1135213. [DOI] [PubMed] [Google Scholar]
- 69.Winston ML. The biology of the honey bee. Cambridge: Harvard University Press; 1987. [Google Scholar]
- 70.Yang X, Cox-Foster DL. Impact of an extoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification. Proc Natl Acad Sci U S A. 2005;102:7470–7475. doi: 10.1073/pnas.0501860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yesavage JA, Holman CA, Sarnquist FH, Berger PA. Elevation of cerebrospinal fluid lactate with aging in subjects with normal blood oxygen saturations. J Gerontol. 1982;37:313–315. doi: 10.1093/geronj/37.3.313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPG 201 kb)
(DOCX 19 kb)
(DOCX 19 kb)