Abstract
Growth differentiation 15 (GDF15) is a potential novel biomarker of biological aging. To separate the effects of chronological age and birth cohort from biological age, longitudinal studies investigating the associations of GDF15 levels with adverse health outcomes are needed. We investigated changes in GDF15 levels over 10 years in an age-stratified sample of the general population and their relation to the risk of acute hospitalization and death. Serum levels of GDF15 were measured three times in 5-year intervals in 2176 participants aged 30, 40, 50, or 60 years from the Danish population-based DAN-MONICA cohort. We assessed the association of single and repeated GDF15 measurements with the risk of non-traumatic acute hospitalizations. We tested whether changes in GDF15 levels over 10 years differed according to the frequency of hospitalizations within 2 years or survival within 20 years, after the last GDF15 measurement. The change in GDF15 levels over time was dependent on age and sex. Higher GDF15 levels and a greater increase in GDF15 levels were associated with an increased risk of acute hospitalization in adjusted Cox regression analyses. Participants with more frequent admissions within 2 years, and those who died within 20 years, after the last GDF15 measurement already had elevated GDF15 levels at baseline and experienced greater increases in GDF15 levels during the study. The change in GDF15 levels was associated with changes in C-reactive protein and biomarkers of kidney, liver, and cardiac function. Monitoring of GDF15 starting in middle-aged could be valuable for the prediction of adverse health outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00359-5.
Keywords: Biomarker, GDF15, Aging, Middle-aged-adults, Older adults, Acute hospitalization
Introduction
Acute hospitalizations are associated with a wide range of adverse outcomes such as mobility decline, disability, readmissions, and mortality [1–6] and are often seen as serious and potentially dangerous events for older adults (≥ 65 years). While most studies in this research area have focused on older adults because they represent a large proportion of acutely hospitalized patients, many middle-aged adults (45–65 years) are also hospitalized for infectious, cardiovascular, and respiratory diseases [7–9] and have similar rates of readmissions compared to older patients [10, 11].
Frailty and multimorbidity are commonly observed in hospitalized older patients and indicate a state of vulnerability [12–14]. This vulnerability likely develops well before hospitalization, but few studies have examined or characterized changes in health in the years or months leading up to an acute hospitalization. Frailty is a predictor of hospitalization in older adults [14], but its prevalence is very low in middle-aged populations as frailty instruments are often developed for, and tested on, older adults [15, 16]. Multimorbidity measures do not reflect disease severity and burden [17, 18] and have performed poorly as predictors of hospitalization for older adults [19, 20]. Furthermore, very few studies have investigated how well frailty and multimorbidity measures relate to adverse health outcomes in middle-aged populations.
Blood-based biomarkers could be more responsive measures, compared to frailty and multimorbidity, for the detection of subclinical changes in health status, particularly in middle-aged populations. Biomarkers of chronic inflammation have previously been tested for the prediction of hospitalizations in older populations [17, 21]. One study showed that higher levels of interleukin-6 (IL-6) could identify individuals at risk of repeated hospitalizations but were not associated with time to hospitalization [21]. Another study tested a cumulative score of chronic inflammation based on serum levels of IL-6, C-reactive protein (CRP), albumin, and cholesterol; however, only the highest inflammation score was associated with an increased risk of hospitalization [17].
Growth differentiation factor 15 (GDF15) is a stress-responsive cytokine that has emerged as a potential marker of biological aging in recent years [22, 23]. GDF15 is expressed at low levels in healthy tissues, and circulating levels range between 200 and 1200 pg/mL in healthy adults [24, 25]. In chronic and acute illnesses, GDF15 levels can increase by 1.5- to 27-fold [26–30]. Although elevated levels of GDF15 were initially recognized as a clinically relevant biomarker for cardiovascular disease and cancer [24, 31–36], GDF15 has since been investigated in a large number of diseases [24, 32, 37–40]. In the general population, GDF15 levels are associated with a wide range of biological factors, including chronic inflammation, metabolic profile, and organ function [41, 42] and could be well-suited as a biomarker to track changes in health. However, longitudinal studies are needed to distinguish the effects of chronological and biological aging and to minimize cohort effects. To the best of our knowledge, only few studies have investigated longitudinal changes in GDF15 levels in the years leading up to adverse health outcomes and death [43–47].
We aimed to determine whether GDF15 levels could predict acute hospitalizations in a cohort of the Danish general population. Furthermore, we aimed to describe the longitudinal course of GDF15 levels before acute hospitalization and death. We hypothesized that elevated GDF15 could reflect subtle changes in health over time and that participants with elevated and rapidly increasing GDF15 levels would be more likely to experience the need for an acute hospitalization or death.
Methods
Cohort and participants
We used the data collected as part of the DAN-MONICA I cohort conducted at the Research Center for Prevention and Health in Glostrup, Denmark, and the Biomarker for Cardiovascular Risk Assessment in Europe (BiomarCaRE) project [48]. Results on the predictive value of GDF15 for heart failure and coronary heart disease mortality in this cohort have been previously published [43]. DAN-MONICA I is a longitudinal cohort in which a sample of the population from 11 municipalities in the western part of the Greater Copenhagen Area, Denmark, was included. At baseline in 1982–1984 (round 1), 3785 individuals aged 30, 40, 50, or 60 years were included. Participants were followed up after 5 years in 1987–1988 (round 2, n = 2987) and after an additional 5 years in 1993–1994 (round 3, n = 2656) [49]. The Research Ethics Committee for Copenhagen County approved the study, and all participants gave informed consent.
The present study only included individuals who had GDF15 measurements available at all three rounds (n = 2219). Because GDF15 levels are heavily influenced by pregnancy [50], individuals who reported being pregnant at round 1 or who had pregnancy-related acute hospitalizations (ICD-8 codes, 630-634, Y60-69; and ICD-10 codes, O00-O99) within 9 months of blood sampling were excluded (n = 42).
Serum biomarker measurements
Serum samples were collected at each examination round. GDF15, CRP, cystatin C, creatinine, alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), and high-sensitivity cardiac troponin I (hs-cTnI) levels were measured on the Abbott ARCHITECT platform (Abbot Laboratories, Abbott Park, Illinois, USA) in the MORGAM/BiomarCaRE laboratory (Hamburg, Germany) as previously described [43, 51, 52]. Over half (61.2%) of the ALT measurements were below the lower limit of detection (6 U/L), and therefore, ALT was not included in the analyses. The estimated glomerular filtration rate (eGFR) was calculated from creatinine levels using the CKD-EPI formula [53]. Total cholesterol and high-density lipoprotein (HDL) cholesterol were measured using enzymatic colorimetric methods (Roche, Mannheim, Germany), as previously described [54].
Lifestyle, diagnoses, and anthropometric measurements
The participants’ smoking habits, physical activity levels, and self-reported diagnoses of diabetes, cardiovascular disease (acute myocardial infarction and other heart diseases), and hypertension were obtained via a self-administered questionnaire. Body mass index (BMI, kg/m2) was calculated from weight and height measurements.
Acute hospitalizations and mortality
Information on acute hospitalizations between 01 January 1991 (2 years before blood sampling at round 3) and 31 December 1996 (2 years after blood sampling at round 3) was obtained from the Danish National Patient Register (NPR). To exclude admissions due to traumatic causes, hospitalizations with ICD codes for injury, poisoning, and certain other consequences of external causes (ICD-8 codes, 800-999; and ICD-10 codes, S00-T98) were excluded. Data on death from any causes between the time of blood sampling at round 3 (1993–1994) and 31 December 2014 was obtained from the Danish Register of Causes of Death.
Statistical analyses
For baseline characteristics, continuous variables are expressed as the median and interquartile range (IQR) and categorical variables as the count and percentage.
First, we examined the natural course of GDF15 levels over the 10-year study period. We used mixed models to model the change in GDF15 between each measurement round, within each age category, and according to gender. The model included the measurement round, age category, sex, and the interactions between measurement round and age category, measurement round and sex, and age category and sex as fixed effects. The model also included the measurement round as repeated measure and used an unstructured covariance matrix.
To describe the evolution of GDF15 levels before and after an acute hospitalization, we identified all acute hospitalizations within the 2 years before and after GDF15 measurement at round 3 for each participant. We then calculated the elapsed time between the GDF15 measurement and the acute hospitalization. GDF15 measurements in the 2 years before an acute hospitalization were grouped into five intervals: 0–1 month, 1–6 months, and in three 6-month intervals from 24 to 6 months before hospitalization (24–18, 18–12, 12–6 months). GDF15 measurements made within 2 years after an acute hospitalization were also grouped into five intervals: 0–1 month, 1–6 months, and in three 6-month intervals from 6 to 24 months after hospitalization (6–12, 12–18, 18–24 months). Some participants had multiple hospitalizations during these 4 years, but only the hospitalization with the shortest elapsed time to the GDF15 measurement was used. The median values of GDF15 for participants in each interval and the median GDF15 value for participants not acutely hospitalized during any of the intervals are shown using box plots.
We then examined the association of GDF15 levels with the risk of acute hospitalization. Participants were stratified by age-specific quartiles of GDF15 at each measurement round. The cumulative probability of the first acute hospitalization in the 2 years following round 3 according to the age-specific GDF15 quartile at round 3 is presented in a cumulative incidence plot. We used unadjusted Cox regression analyses to assess the risk of acute hospitalization as a function of age-specific GDF15 quartiles. We also performed the analysis with adjustments for sex, current smoking, self-reported diabetes, cardiovascular disease, or hypertension diagnosis, BMI, and total cholesterol. Death was considered a competing event and loss to follow-up a censored event. Furthermore, we assessed the risk of acute hospitalization as a function of the change in GDF15 levels between rounds 2 and 3 and as a function of the change in GDF15 levels between rounds 1 and 3, for each age-specific quartile of GDF15 at round 2 and round 1, respectively, in unadjusted and adjusted Cox regressions analyses. The change in GDF15 levels was calculated as the absolute difference of log-transformed GDF15 values. Hazard ratios (HR) and 95% confidence intervals (CIs) for each of the age-specific GDF15 quartiles are presented.
Next, we examined whether the change in GDF15 levels over the study period differed according to the frequency of acute hospitalizations within the 2 years following the round 3 measurement and according to the duration of survival up to 20 years after round 3. For the frequency of hospitalizations, we categorized participants as being alive and having (i) no acute hospitalizations, (ii) 1 or 2 acute hospitalizations, (iii) 3 or more acute hospitalizations or (iv) having died within 2 years after round 3. For the duration of survival, we categorized participants as (i) having died within 5 years, (ii) within 5 to 10 years, and (iii) within 10 to 20 years or (iv) participants who lived for at least 20 years after round 3 (end of follow-up). We used mixed linear models to model the change in GDF15 levels between each measurement round for each age category and each hospitalization frequency category or survival category. The model included the age category, measurement round, and hospitalization frequency/survival category, as well as the interactions between hospitalization frequency/survival category and measurement round and hospitalization frequency/survival category and age category as fixed effects. The model also included participant ID as random effect and used an unstructured covariance matrix.
Finally, we tested whether the change in GDF15 levels over the 10-year study period was associated with changes in the levels of CRP and biomarkers of organ function (cystatin C, eGFR, AST, GGT, and hs-cTnI) using multiple linear regressions. The change in each biomarker was tested individually in a model that also included the levels of the biomarker at round 1, age, sex, GDF15 levels at round 1, smoking status, BMI, total cholesterol, and self-reported diabetes, cardiovascular disease, and hypertension diagnosis at round 1. We also tested an extended model that included all the biomarkers simultaneously.
We used SAS Enterprise Guide version 7.1 (SAS Institute, Cary, NC, USA) for statistical analyses and GraphPad Prism v8.0.0 for creating plots. Statistical significance was defined as a p-value < 0.05.
Results
Characteristics of the study participants
A total of 2177 participants had GDF15 measurements available at round 1 (baseline), round 2 (5-year follow-up), and round 3 (10-year follow-up). One participant was excluded due to one GDF15 measurement falling below the detection limit of the assay. Of the remaining 2176 individuals, 25.1% were aged 30 years, 28.6% were 40 years old, 28.1% were 50 years old, and 18.2% were 60 years old at round 1; their baseline characteristics are presented in Table 1. Univariate associations between GDF15 levels and participant characteristics at baseline are presented in Supplementary Table 1. Baseline characteristics of the participants included in the present study (n = 2176), compared to the full cohort of participants included in the DAN-MONICA I study (n = 3785), are presented in Supplementary Table 2. Of the 3785 participants included in the full cohort, 1334 (35.2%) had at least one acute hospitalization, 283 (7.5%) had died, and 10 (0.3%) were lost to follow-up between rounds 1 and 3.
Table 1.
Characteristics of study participants by age at round 1
| 30 years | 40 years | 50 years | 60 years | |
|---|---|---|---|---|
| n = 547 | n = 622 | n = 612 | n = 395 | |
| Demographics and lifestyle | ||||
| Age (years) | 30.8 (30.6–31.0) | 40.8 (40.6–40.9) | 50.8 (50.6–50.9) | 60.8 (60.6–60.9) |
| Male (%) | 260 (47.5) | 304 (48.9) | 313 (51.1) | 171 (43.3) |
| Daily smoker (%) | 298 (54.5) | 292 (47.0) | 319 (52.1) | 145 (36.7) |
| BMI (kg/m2) | 22.3 (20.6–24.5) | 23.7 (21.8–26.3) | 24.8 (22.7–27.4) | 25.0 (22.8–27.2) |
| Physical activity level | ||||
| Almost completely inactive (%) | 145 (26.5) | 161 (25.9) | 144 (23.5) | 82 (20.8) |
| Some physical activity (%) | 273 (49.9) | 299 (48.1) | 336 (54.9) | 234 (59.2) |
| Regular activity (%) | 107 (19.6) | 148 (23.8) | 131 (21.4) | 79 (20.0) |
| Regular hard physical training (%) | 22 (4.0) | 14 (2.3) | 1 (0.2) | 0 (0.0) |
| Cardiovascular risk factors | ||||
| Diabetes (%) | 2 (0.4) | 7 (1.1) | 12 (2.0) | 8 (2.0) |
| Cardiovascular disease (%) | 10 (1.8) | 13 (2.1) | 21 (3.4) | 22 (5.6) |
| Hypertension (%) | 56 (10.2) | 83 (13.3) | 84 (13.7) | 79 (20.0) |
| Total cholesterol (mmol/L) | 5.2 (4.7–5.8) | 5.7 (5.1–6.5) | 6.3 (5.6–7.0) | 6.7 (6.0–7.5) |
| HDL cholesterol (mmol/L) | 1.4 (1.2–1.7) | 1.5 (1.2–1.8) | 1.5 (1.2–1.8) | 1.6 (1.3–1.9) |
| Biomarkers of organ function | ||||
| CRP (mg/L) | 0.8 (0.4–1.7) | 0.9 (0.5–1.9) | 1.3 (0.6–2.8) | 1.7 (0.8–3.2) |
| Cystatin C (mg/L) | 0.7 (0.6–0.7) | 0.7 (0.6–0.7) | 0.7 (0.7–0.8) | 0.8 (0.7–0.9) |
| eGFR (mL/min/1.73m2) | 115.3 (105.3–119.7) | 106.5 (97.2–111.2) | 97.6 (85.3–103.5) | 87.8 (76.7–95.6) |
| AST (U/L) | 25.0 (19.9–31.0) | 25.0 (20.0–31.74) | 26.0 (21.0–32.0) | 29.0 (23.0–35.0) |
| GGT (U/L) | 18.0 (14.0–30.0) | 21.0 (14.0–35.0) | 24.0 (16.49–42.0) | 26.0 (18.0–40.0) |
| hs-cTnI (ng/L) | 2.3 (1.4–3.6) | 2.2 (1.3–3.4) | 2.3 (1.4–3.5) | 3.1 (2.1–4.8) |
Data are presented as median (interquartile range) or n (%).
AST, aspartate transaminase; BMI, body mass index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-cTnI, high-sensitive troponin I; GGT, gamma-glutamyl transferase
Time course of GDF15 levels over the 10-year study period
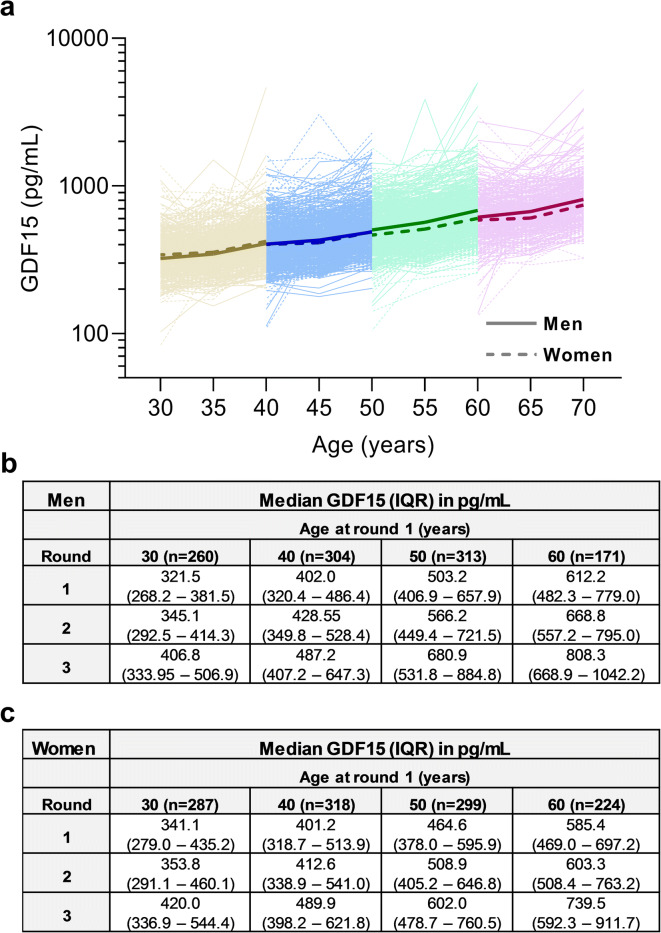
Figure 1 depicts the natural time course of GDF15 levels over the 10-year study period within each age category and for each gender. GDF15 levels increased significantly over the three measurement rounds in all age categories (p < 0.0001 for all comparisons). The increase in GDF15 over time was steeper in the older age categories (aged 50 or 60 years at round 1) compared to the younger age categories (aged 30 or 40 years at round 1; p-value for the interaction between age category and measurement round = 0.006). The interaction between sex and age category (p < 0.0001) and between sex and measurement round (p = 0.0002) were statistically significant, suggesting that differences in baseline GDF15 levels between men and women were dependent on age and that sex also affected the change in GDF15 levels over time. At baseline, GDF15 levels were higher in women than in men for individuals aged 30 years; however, in individuals aged 50 or 60 years, GDF15 levels were higher in men than in women. The increase in GDF15 levels over time was steeper for men compared to women.
Fig. 1.
The natural time course of GDF15 levels over the 10-year study period within each age category and by gender. a Individual GDF15 values for participants aged 30, 40, 50, and 60 years at round 1 are displayed on a logarithmic scale, and median values for men (solid line) and women (dashed line) are shown as bold lines. b Median GDF15 values for men. c Median GDF15 values for women
Time course of GDF15 levels in the 2 years preceding and following acute hospitalization
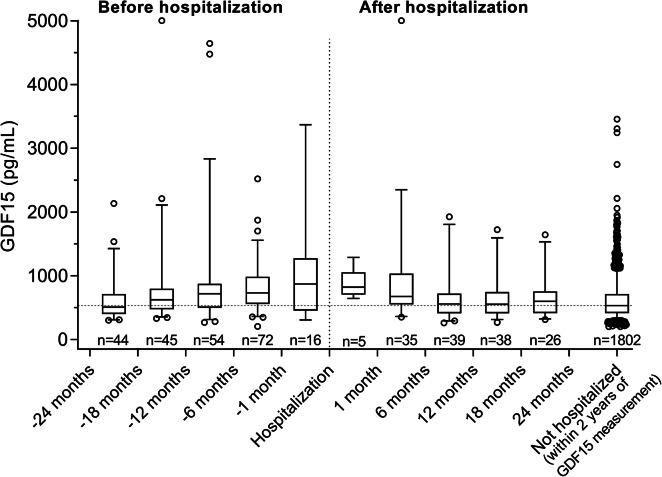
Figure 2 shows GDF15 levels for each participant at round 3 centered around the date of the nearest acute hospitalization within 2 years before or after GDF15 measurement. A total of 374 participants had at least one hospital admission within 2 years of blood sampling at round 3. Median GDF15 levels were gradually higher when the measurement was taken closer to an acute hospitalization. Median GDF15 levels 12 to 18 months before hospitalization were 624.0 pg/mL (IQR, 475.9 to 774.0), whereas they were 719.4 pg/mL (IQR, 505.4 to 874.4) 6 to 12 months before hospitalization and 731.7 pg/mL (IQR, 560.1 to 978.6) 1 to 6 months before hospitalization and reached the highest levels within 1 month before hospitalization: 871.7 pg/mL (IQR, 463.4 to 1254.3). GDF15 levels remained high for participants for whom the measurement occurred during the month following hospitalization (822.2 pg/mL; IQR, 761.0 to 825.8) but were lower by 6 months following hospitalization (677.9 pg/mL; IQR, 549.6 to 1036.3) and were further reduced at 12 months after hospitalization (557.0 pg/mL; IQR, 410.3 to 722.4), after which they remained stable until 2 years after hospitalization (Fig. 2). Median GDF15 levels were lowest for participants with no acute hospitalizations within 2 years before or after GDF15 measurement (535.1 pg/mL; IQR, 415.3 to 714.8).
Fig. 2.
GDF15 levels at round 3 stratified according to the time to the nearest acute hospitalization within 2 years before or after GDF15 measurement for each participant. Box plots depict median and interquartile range; error bars indicate 5–95% percentiles. The dashed line indicates the median GDF15 value for the Not hospitalized participants
GDF15 and risk of acute hospitalization
We identified the first acute hospitalization within 2 years after GDF15 measurement at round 3 for each participant. During this 2-year follow-up after round 3, 244/2176 (11.2%) participants had an acute hospitalization, 3 (0.1%) had died, and one was lost to follow-up. The cumulative incidence of acute hospitalization by age-specific GDF15 quartiles is shown in Fig. 3.
Fig. 3.
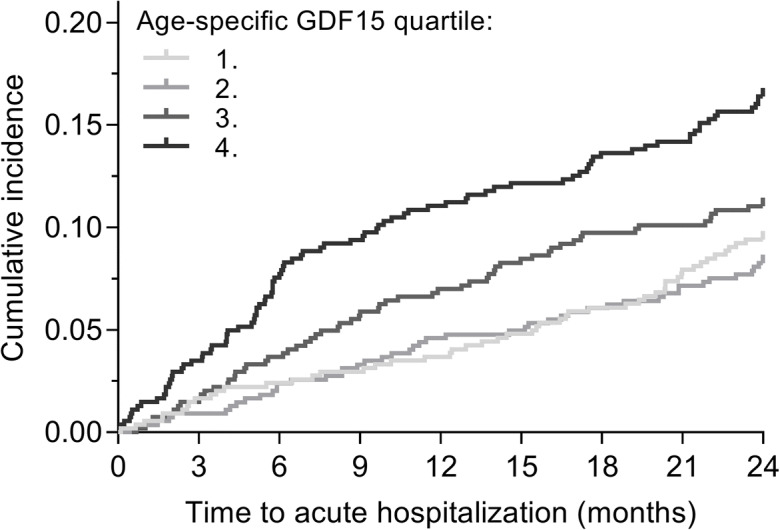
Cumulative incidence plots for first acute hospitalization within 2 years after round 3. GDF15 levels were stratified into age-specific quartiles
In unadjusted and adjusted Cox regression analyses, the HR for acute hospitalization was not different for the second or third age-specific GDF15 quartiles compared to the first quartile (Table 2). However, the HR for the fourth GDF15 quartile was significantly increased compared to the first quartile (Table 2). To explore whether the causes of hospitalization were related to GDF15 levels, we identified the primary diagnoses (ICD codes) for admission for each patient grouped according to the 21 chapters of the ICD-10 classification. Each of the chapters, except for “Diseases of the circulatory system” (n = 44/244, 18.0%),“Diseases of the digestive system” (n = 35/244, 14.3%), and “Diseases of the respiratory system” (n = 34/244, 14.0%), had fewer than 20 participants; regarding cancers, 17 (7.0%) of the 244 participants were diagnosed with malignant neoplasms. Out of the 17 participants hospitalized with cancer, 9 (52.9%) belonged to the fourth age-specific GDF15 quartile. For participants hospitalized with diseases of the cardiovascular, digestive, or respiratory system, these numbers were 16/44 (36.4%), 14/35 (40.0%), and 15/34 (44.1%), respectively, whereas all for all other causes of hospitalization, 36/144 (31.6%) of the participants were in the fourth age-specific GDF15 quartile. We further explored whether participants with higher GDF15 levels were more likely to experience a more severe admission. We determined the number of diagnoses established during the acute hospitalization for each participant. There was no relationship between the number of diagnoses and the proportion of participants with high GDF15 levels: for participants with only one diagnosis, 36.8% belonged to the fourth age-specific GDF15 quartiles; this figure was 35.9% for participants with two diagnoses and 39.1% for participants with more than two established diagnoses during hospitalization.
Table 2.
Hazard ratios for first acute hospitalization within 2 years after round 3 according to age-specific GDF15 quartiles at round 3 and to the change in GDF15 levels between rounds 2 and 3 or between round 1 and 3, for each age-specific GDF15 quartile
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age-specific GDF15 quartiles at round 3 | ||||
| Age-specific GDF15round3 quartile 1 | 1 | — | 1 | — |
| Age-specific GDF15round3 quartile 2 | 0.82 (0.54–1.23) | 0.33 | 0.86 (0.57–1.30) | 0.48 |
| Age-specific GDF15round3 quartile 3 | 1.22 (0.84–1.76) | 0.30 | 1.27 (0.86–1.88) | 0.23 |
| Age-specific GDF15round3 quartile 4 | 1.86 (1.32–2.62) | 0.0004 | 2.09 (1.42–3.08) | 0.0002 |
| Change in GDF15 levels | ||||
| ΔGDF15round3-round2b | ||||
| Age-specific GDF15round2 quartile 1 | 4.42 (1.26–15.58) | 0.02 | 4.77 (1.35–16.79) | 0.01 |
| Age-specific GDF15round2 quartile 2 | 3.56 (0.95–13.34) | 0.06 | 2.69 (0.78–9.24) | 0.12 |
| Age-specific GDF15round2 quartile 3 | 2.30 (0.77–6.83) | 0.13 | 2.59 (0.85–7.84) | 0.09 |
| Age-specific GDF15round2 quartile 4 | 2.74 (1.14–6.58) | 0.02 | 3.00 (1.21–7.40) | 0.02 |
| ΔGDF15round3-round1c | ||||
| Age-specific GDF15round1 quartile 1 | 3.03 (1.51–6.06) | 0.002 | 2.88 (1.41–5.87) | 0.004 |
| Age-specific GDF15round1 quartile 2 | 1.99 (0.59–6.73) | 0.27 | 2.36 (0.66–8.43) | 0.19 |
| Age-specific GDF15round1 quartile 3 | 3.58 (1.76–7.29) | 0.0004 | 3.65 (1.60–8.32) | 0.002 |
| Age-specific GDF15round1 quartile 4 | 2.15 (1.19–3.89) | 0.01 | 2.18 (1.16–4.09) | 0.02 |
aModel adjusted for sex, BMI, smoking, cardiovascular diagnosis, hypertension diagnosis, diabetes diagnosis, and total cholesterol
bModel included GDF15round2, ΔGDF15round3-round2, and the interaction between the two variables as main effects
cModel included GDF15round1, ΔGDF15round3-round1, and the interaction between the two variables as main effects
The change in GDF15 over 5 years (from rounds 2 to 3) was also associated with a significantly higher risk of acute hospitalization for participants who were in the first or fourth age-specific GDF15 quartiles at round 2 (Table 2). The same trend was observed for participants in the second or third age-specific GDF15 quartiles at round 2 but did not reach statistical significance. The 10-year change in GDF15 levels (from rounds 1 to 3) was also associated with an increased risk of acute hospitalization for all but the second age-specific GDF15 quartiles at round 1 (Table 2).
Time course of GDF15 levels according to frequency of acute hospitalization
We examined whether the time course of GDF15 levels over the 10-year study period differed according to the frequency of acute hospitalizations during the 2 years after round 3 (Fig. 4). In the mixed model analysis, the interaction between measurement round and hospitalization frequency was statistically significant (p = 0.001); overall, GDF15 levels tended to increase more steeply for participants who had died within 2 years, compared to participants in the other hospitalization categories, but there were no differences in the change in GDF15 levels over time between the participants who remained alive. The interaction between age category at round 1 and hospitalization frequency was also statistically significant (p = 0.03). In the younger age category (aged 30 years), GDF15 levels at round 1 were higher for participants with 1–2 hospitalizations and those who died compared to those without hospitalizations, whereas in the older age categories (aged 50 and 60 years), GDF15 levels were higher for those with ≥ 3 hospitalizations and those who died compared to those without hospitalizations.
Fig. 4.
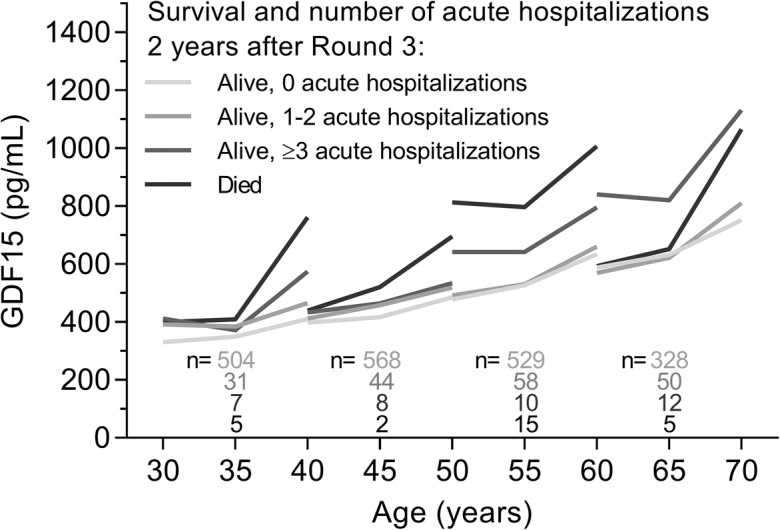
Change in GDF15 levels during the 10-year study period according to the frequency of acute hospitalizations within 2 years after round 3 measurement (alive with 0, 1 to 2, ≥3 acute hospitalizations, or dead) for each age category. Median GDF15 levels are shown
Time course of GDF15 levels according to survival status
We examined whether the time course of GDF15 levels during the 10-year study period differed according to survival status at 20 years after round 3 (Fig. 5). In the mixed model analysis, the interaction between measurement round and survival category was statistically significant (p < 0.0001), indicating that the change in GDF15 levels over the three measurement rounds differed between survival categories. Overall, GDF15 levels increased more steeply for participants who died, compared to participants who survived, and GDF15 levels increased more steeply for participants who died within 5 years, compared to participants who died after 5 years. The interaction between age category at round 1 and survival category was also statistically significant (p < 0.0001). For participants aged 40 years or older at round 1, there were significant differences in baseline GDF15 levels of those who died at any time compared to those who were still alive at 20 years after round 3.
Fig. 5.
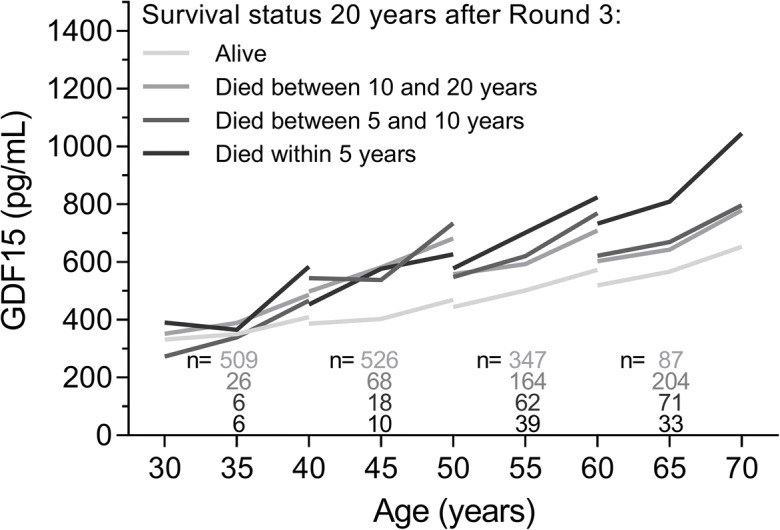
Change in GDF15 levels during the 10-year study period according to survival status 20 years after round 3 measurement (alive, died between 10 and 20 years, 5 and 10 years, or within 5 years) for each age category. Median GDF15 levels are shown
The most frequent causes of death for participants aged 30 years at round 1 were cancer (48.7%), accidental deaths or suicides (18.9%), and diseases of the circulatory system (10.8%). For participants aged 40 and 50 years at round 1, the most frequent causes of death were cancer (46.7 and 38.9%, respectively), diseases of the circulatory system (16.3 and 24.9%), and diseases of the respiratory system (8.7 and 12.5%), while for participants aged 60 years, the most frequent causes of death were diseases of the circulatory system (29.8%), cancer (26.8%), and diseases of the respiratory system (11.4%).
Change in GDF15 concentrations and organ function biomarkers over time
Finally, we tested whether the change in GDF15 concentrations over the 10-year study period was associated with the change in the levels of biomarkers of chronic inflammation (CRP), kidney (cystatin C, eGFR), liver (AST, GGT), and cardiac function (hs-cTnI). In individual linear regressions, for each biomarker, the change in concentration between rounds 1 and 3, and the change in eGFR, was all associated with the change in GDF15 levels (Table 3). In a fully adjusted model that included all biomarkers simultaneously, the changes in CRP, cystatin C, eGFR, and GGT levels between rounds 1 and 3 remained independently associated with the change in GDF15 levels (Table 3).
Table 3.
Associations between the change in GDF15 levels from rounds 1 to 3 and the change in biomarkers of organ function
| Individual analysesa | Fully adjusted modelb | |||||
|---|---|---|---|---|---|---|
| B-estimate | 95% CI | p-value | B-estimate | 95% CI | p-value | |
| ΔCRPround3-round1 (mg/L) | 0.011 | (0.009–0.013) | < 0.0001 | 0.007 | (0.005 – 0.009) | < 0.0001 |
| Δcystatin C round3-round1 (mg/L) | 0.881 | (0.811–0.952) | < 0.0001 | 0.656 | (0.569 – 0.743) | < 0.0001 |
| ΔeGFRround3-round1 (mL/min/1.73m2) | −0.008 | (−0.009–−0.007) | < 0.0001 | −0.004 | (−0.005–−0.003) | < 0.0001 |
| ΔASTround3-round1 (U/L) | 0.003 | (0.003–0.004) | < 0.0001 | 0.000 | (0.000 – 0.001) | 0.279 |
| ΔGGTround3-round1 (U/L) | 0.001 | (0.001–0.001) | < 0.0001 | 0.001 | (0.001–0.001) | < 0.0001 |
| Δhs-cTnIround3-round1 (ng/L) | 0.004 | (0.002–0.006) | < 0.0001 | 0.001 | (−0.000–0.003) | 0.101 |
aEach covariate was tested individually in a model adjusted for baseline levels of the covariate at round 1, GDF15 levels at round 1, age, sex, smoking, BMI, cholesterol, and cardiovascular diagnosis, hypertension diagnosis, diabetes diagnosis
bThe fully adjusted model included the baseline levels and change in level of all the biomarkers listed in the table, GDF15 levels at round 1, age, sex, smoking, and cardiovascular diagnosis, hypertension diagnosis, diabetes diagnosis.
AST, aspartate transaminase; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; hs-cTnI, high-sensitive troponin I; GGT, gamma-glutamyl transferase
Discussion
In this longitudinal observational study of a cohort of the Danish general population, single and repeated measurements of GDF15 levels predicted acute hospitalizations over a 2-year follow-up period. GDF15 levels were already elevated at baseline for participants with more frequent admissions during the 2-year follow-up and for those who died during the 20-year follow-up. Furthermore, GDF15 levels also tended to increase more steeply over the 10-year study period for participants who died within 20 years after the last GDF15 measurement.
GDF15 levels are known to increase with age [22, 23, 55], but to our knowledge, no other study has yet examined the longitudinal course of GDF15 levels in the same individual over 10 years and across such a wide age range. We found that GDF15 levels increased more rapidly for men and after the age of 50. This is in line with the findings from a large proteomic study demonstrating that plasma protein trajectories during aging are not linear [22]. Our results are also in line with other studies demonstrating higher levels of GDF15 in older men compared to women [42, 44]. Interestingly, in participants aged 30 years at baseline, GDF15 levels were higher in women compared to men, and this trend became inverted between the age of 40 and 50 years. This could explain why sex differences in GDF15 levels were not observed in other studies consisting of participants with a wider age range [41, 56]. Despite the strong evidence that GDF15 levels increase with age, the cause and significance of this increase require further studies. Moreover, sex differences in change in GDF15 levels over time require further investigations.
We and others have previously shown that GDF15 levels are increased in older (≥ 60 years) hospitalized patients compared to age-matched controls [57, 58]. In the present study, although the design did not allow for repeated measurements of GDF15 levels before and after hospitalization, we found that, overall, across all participants, GDF15 levels tended to be slightly higher the closer they were measured to an acute hospitalization and tended to resolve to levels similar to non-hospitalized participants within 6 to 12 months after hospitalization. This is in line with our previous observation that GDF15 levels decrease within 30-days after hospital discharge in acutely hospitalized older adults [58]. We also show that the highest age-specific GDF15 quartile was associated with an increased risk of acute hospitalization. This finding indicates that, even in middle-aged adults, high GDF15 levels could reflect subclinical changes in health. There were no differences between the lowest age-specific quartiles of GDF15 and the second and third quartiles. In a study by Salanitro and colleagues, only the highest score of their composite inflammatory score was associated with hospitalization in older adults [17]. Although measures of inflammation, and in our study, GDF15 levels, could reflect subclinical disease progression or severity, the decision to seek care is up to the individual and their perception of the illness burden. Thereby, acute hospitalization itself is a subjective measure of the individual’s perceived health, meaning that acute hospitalization as an outcome could be less directly related to physiological measures, particularly in the less severe cases [17]. Nevertheless, higher GDF15 levels at baseline were also clearly associated with more frequent hospitalizations (from age 50) and early mortality (from age 40). Similarly to our results, another study demonstrated that higher IL-6 levels were associated with more frequent hospitalizations in older adults [21]. We observed that the levels of GDF15 were already strikingly elevated at least 10 years, and even up to 30 years, before death in middle-aged adults (40–60 years old), supporting the strong prognostic potential of GDF15 levels for mortality established in previous studies [36, 43, 44, 47, 59–62]. In the youngest age category (aged 30 years at baseline), GDF15 alone may not be sensitive enough to reflect subtle changes in health. Furthermore, early deaths due to disease in these younger ages were related—to a greater extent—to illnesses with poor prognosis and high short-term mortality such as cancers, rather than chronic diseases such as cardiovascular or respiratory diseases.
Changes in GDF15 levels over time have previously been associated with an increased risk of mortality and heart failure [43, 44]. In the present study, we show that increasing levels of GDF15 over the 5-year and 10-year repeated measurements were associated with an increased risk of hospitalization. Furthermore, for older participants, GDF15 levels tended to increase more steeply for participants who would have more frequent hospitalizations during a 2-year follow-up. In line with previous studies, changes in GDF15 over time were associated with the change in biomarkers of renal (cystatin C, eGFR), cardiac (hs-cTnI), and hepatic (AST, GGT) function, as well as chronic inflammation (CRP) [44, 47]. These results indicate that, rather than reflecting an organ or disease-specific pathological process, GDF15 levels may instead be an indication of general physiological decline. Experimental studies have shown that GDF15 expression is induced in response to a wide variety of cellular stressors and as part of cellular stress responses [63, 64]. The increase in GDF15 levels during aging could reflect the activation of cellular stress responses due to the age-related buildup of cellular damage, and several recent studies also suggest that increased GDF15 levels could reflect the accumulation of senescent cells with age [65, 66]. Elevated GDF15 levels could thereby reflect the underlying cellular processes that drive physiological decline, frailty, and vulnerability and which can eventually lead to an increased risk of acute hospitalization. Thus, even for younger and potentially healthy individuals with low baseline levels of GDF15, a greater increase in GDF15 concentration over time could reflect a faster rate of biological aging predisposing to adverse health outcomes and early mortality.
The limitations of this retrospective cohort study should be acknowledged. We did not evaluate the presence of multimorbidity or frailty, and therefore, we could not determine whether GDF15 reflected these conditions in the studied population. In addition, the number of participants with frequent hospitalizations, as well as the proportion of participants who died in the younger age categories (30–40 years), was small, and we did not have sufficient statistical power to investigate differences in GDF15 levels depending on the causes of hospitalization. Furthermore, the causes of hospitalizations may differ between the age categories and could explain the differences in the change in GDF15 between age categories. To be able to depict the change of GDF15 levels over time, out of all the participants included in the MONICA cohort, only those with all three repeated measurements of GDF15 were included in this study. These participants may have been healthier and have had more resources than those who had dropped out of the original cohort. The study was also limited to individuals aged 60 or younger at baseline, and therefore, other studies are needed to confirm our findings in older populations.
Conclusions
Adding to the previous evidence that GDF15 levels are associated with the incidence of specific diseases and with mortality, our findings demonstrate that both single and repeated measurements of GDF15 have potential in predicting acute hospitalization and mortality in the general population across a wide age range. Monitoring of GDF15, starting in middle-aged, could be valuable for the prediction of adverse health events and as a factor for the estimation of the rate of biological aging.
Supplementary Information
(PDF 75 kb)
Code availability
Not applicable.
Author contribution
JT: Conceptualization, data analysis, interpretation of the results, writing of the original draft, figure preparation, review, and editing; OA: Conceptualization, interpretation of the results, review, and editing. JON: Conceptualization, interpretation of the results, review, and editing. JP: Conceptualization, data analysis, interpretation of the results, figure preparation, review, and editing. All authors read and approved the final manuscript.
Data availability
The datasets are not publicly available due to regulations set out by the Danish Data Protection Agency but are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The study was approved by the Research Ethics Committee for Copenhagen County.
Consent to participate
All participants provided informed consent.
Consent for publication
All the authors have read and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brown CJ, Roth DL, Allman RM, Sawyer P, Ritchie CS, Roseman JM. Trajectories of life-space mobility after hospitalization. Ann Intern Med. 2009;150:372–378. doi: 10.7326/0003-4819-150-6-200903170-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown CJ, Foley KT, Lowman JD, MacLennan PA, Razjouyan J, Najafi B, et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med. 2016;176:921–927. doi: 10.1001/jamainternmed.2016.1870. [DOI] [PubMed] [Google Scholar]
- 3.Volpato S, Onder G, Cavalieri M, Guerra G, Sioulis F, Maraldi C, et al. Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J Gen Intern Med. 2007;22:668–674. doi: 10.1007/s11606-007-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd CM, Ricks M, Fried LP, Guralnik JM, Xue Q-L, Xia J, Bandeen-Roche K. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women’s Health and Aging Study I. J Am Geriatr Soc. 2009;57:1757–1766. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodilsen AC, Klausen HH, Petersen J, Beyer N, Andersen O, Jørgensen LM, Juul-Larsen HG, Bandholm T. Prediction of mobility lmitations after hospitalization in older medical patients by simple measures of physical performance obtained at admission to the emergency department. PLoS One. 2016;11:e0154350. doi: 10.1371/journal.pone.0154350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263–1270. doi: 10.1111/j.1532-5415.2004.52354.x. [DOI] [PubMed] [Google Scholar]
- 7.Søvsø MB, Hermansen SB, Færk E, Lindskou TA, Ludwig M, Møller JM, Jonciauskiene J, Christensen EF. Diagnosis and mortality of emergency department patients in the North Denmark region. BMC Health Serv Res. 2018;18:548. doi: 10.1186/s12913-018-3361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vest-Hansen B, Riis AH, Sørensen HT, Christiansen CF. Acute admissions to medical departments in Denmark: diagnoses and patient characteristics. Eur J Intern Med. 2014;25:639–645. doi: 10.1016/j.ejim.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola V-P, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L, EuroHeart Survey Investigators. Heart Failure Association, European Society of Cardiology EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J Oxford Academic. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 10.Ranasinghe I, Wang Y, Dharmarajan K, Hsieh AF, Bernheim SM, Krumholz HM. Readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia among young and middle-aged adults: a retrospective observational cohort study. PLoS Med [Internet]. 2014 [cited 2020 Sep 20];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4181962/ [DOI] [PMC free article] [PubMed]
- 11.Jain S, Khera R, Mortensen EM, Weissler JC. Readmissions of adults within three age groups following hospitalization for pneumonia: analysis from the Nationwide Readmissions Database. PLoS One. 2018;13:e0203375. doi: 10.1371/journal.pone.0203375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juul-Larsen HG, Petersen J, Sivertsen DM, Andersen O. Prevalence and overlap of disease management program diseases in older hospitalized patients. Eur J Ageing. 2017;14:283–293. doi: 10.1007/s10433-017-0412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juul-Larsen HG, Christensen LD, Bandholm T, Andersen O, Kallemose T, Jørgensen LM, Petersen J. Patterns of multimorbidity and differences in healthcare utilization and complexity among acutely hospitalized medical patients (≥65 years) - a latent class approach. Clin Epidemiol. 2020;12:245–259. doi: 10.2147/CLEP.S226586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 15.Gordon SJ, Baker N, Kidd M, Maeder A, Grimmer KA. Pre-frailty factors in community-dwelling 40–75 year olds: opportunities for successful ageing. BMC Geriatr. 2020;20:96. doi: 10.1186/s12877-020-1490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3:e323–e332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salanitro AH, Ritchie CS, Hovater M, Roth DL, Sawyer P, Locher JL, Bodner E, Brown CJ, Allman RM. Inflammatory biomarkers as predictors of hospitalization and death in community-dwelling older adults. Arch Gerontol Geriatr. 2012;54:e387–e391. doi: 10.1016/j.archger.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salanitro AH, Hovater M, Hearld KR, Roth DL, Sawyer P, Locher JL, Bodner E, Brown CJ, Allman RM, Ritchie CS. Symptom burden predicts hospitalization independent of comorbidity in community-dwelling older adults. J Am Geriatr Soc. 2012;60:1632–1637. doi: 10.1111/j.1532-5415.2012.04121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byles JE, D’Este C, Parkinson L, O’Connell R, Treloar C. Single index of multimorbidity did not predict multiple outcomes. J Clin Epidemiol. 2005;58:997–1005. doi: 10.1016/j.jclinepi.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Boeckxstaens P, Vaes B, Van Pottelbergh G, De Sutter A, Legrand D, Adriaensen W, et al. Multimorbidity measures were poor predictors of adverse events in patients aged ≥80 years: a prospective cohort study. J Clin Epidemiol. 2015;68:220–227. doi: 10.1016/j.jclinepi.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Adriaensen W, Matheï C, Vaes B, van Pottelbergh G, Wallemacq P, Degryse J-M. Interleukin-6 as a first-rated serum inflammatory marker to predict mortality and hospitalization in the oldest old: a regression and CART approach in the BELFRAIL study. Exp Gerontol. 2015;69:53–61. doi: 10.1016/j.exger.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Lehallier B, Gate D, Schaum N, Nanasi T, Lee SE, Yousef H, Moran Losada P, Berdnik D, Keller A, Verghese J, Sathyan S, Franceschi C, Milman S, Barzilai N, Wyss-Coray T. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25:1843–1850. doi: 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conte M, Ostan R, Fabbri C, Santoro A, Guidarelli G, Vitale G, Mari D, Sevini F, Capri M, Sandri M, Monti D, Franceschi C, Salvioli S. Human aging and longevity are characterized by high levels of mitokines. J Gerontol A Biol Sci Med Sci. 2019;74:600–607. doi: 10.1093/gerona/gly153. [DOI] [PubMed] [Google Scholar]
- 24.Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B, Breit SN. MIC-1 Serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res. 2003;9:2642–2650. [PubMed] [Google Scholar]
- 25.Fairlie WD, Moore AG, Bauskin AR, Russell PK, Zhang H-P, Breit SN. MIC-1 is a novel TGF-β superfamily cytokine associated with macrophage activation. J Leukoc Biol. 1999;65:2–5. doi: 10.1002/jlb.65.1.2. [DOI] [PubMed] [Google Scholar]
- 26.Mueller T, Leitner I, Egger M, Haltmayer M, Dieplinger B. Association of the biomarkers soluble ST2, galectin-3 and growth-differentiation factor-15 with heart failure and other non-cardiac diseases. Clin Chim Acta. 2015;445:155–160. doi: 10.1016/j.cca.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Buendgens L, Yagmur E, Bruensing J, Herbers U, Baeck C, Trautwein C, et al. Growth differentiation factor-15 is a predictor of mortality in critically ill patients with sepsis. Dis Markers. 2017;2017:5271203. doi: 10.1155/2017/5271203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, Nicoletti R, Chiu MI, Gyuris J, Garcia JM. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle. 2015;6:317–324. doi: 10.1002/jcsm.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark BJ, Bull TM, Benson AB, Stream AR, Macht M, Gaydos J, Meadows C, Burnham EL, Moss M, the ARDS Network Investigators Growth differentiation factor-15 and prognosis in acute respiratory distress syndrome: a retrospective cohort study. Crit Care. 2013;17:R92. doi: 10.1186/cc12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JS, Kim S, Won CW, Jeong KH. Association between plasma levels of growth differentiation factor-15 and renal function in the elderly: Korean frailty and aging cohort study. Kidney Blood Press Res. 2019;44:405–414. doi: 10.1159/000498959. [DOI] [PubMed] [Google Scholar]
- 31.Kempf T, Björklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, et al. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J. 2007;28:2858–2865. doi: 10.1093/eurheartj/ehm465. [DOI] [PubMed] [Google Scholar]
- 32.Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, et al. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53:284–291. doi: 10.1373/clinchem.2006.076828. [DOI] [PubMed] [Google Scholar]
- 33.Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 34.Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, Ridker PM. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet. 2002;359:2159–2163. doi: 10.1016/S0140-6736(02)09093-1. [DOI] [PubMed] [Google Scholar]
- 35.Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, Peter T, Siegbahn A, Venge P, Drexler H, Wallentin L. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation. 2007;116:1540–1548. doi: 10.1161/CIRCULATIONAHA.107.697714. [DOI] [PubMed] [Google Scholar]
- 36.Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn-Wichmann R̈, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson AC, Ingelsson E, Sundström J, Jesus Carrero J, Gustafsson S, Feldreich T, Stenemo M, Larsson A, Lind L, Ärnlöv J. Use of proteomics to investigate kidney function decline over 5 years. Clin J Am Soc Nephrol. 2017;12:1226–1235. doi: 10.2215/CJN.08780816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho JE, Hwang S-J, Wollert KC, Larson MG, Cheng S, Kempf T, Vasan RS, Januzzi JL, Wang TJ, Fox CS. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin Chem. 2013;59:1613–1620. doi: 10.1373/clinchem.2013.205716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Husebø GR, Grønseth R, Lerner L, Gyuris J, Hardie JA, Bakke PS, Eagan TM. Growth differentiation factor-15 is a predictor of important disease outcomes in patients with COPD. Eur Respir J. 2017;49:1601298. doi: 10.1183/13993003.01298-2016. [DOI] [PubMed] [Google Scholar]
- 40.Lee ES, Kim SH, Kim HJ, Kim KH, Lee BS, Ku BJ. Growth differentiation factor 15 predicts chronic liver disease severity. Gut Liver. 2017;11:276–282. doi: 10.5009/gnl16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krintus M, Braga F, Kozinski M, Borille S, Kubica J, Sypniewska G, Panteghini M. A study of biological and lifestyle factors, including within-subject variation, affecting concentrations of growth differentiation factor 15 in serum. Clin Chem Lab Med. 2019;57:1035–1043. doi: 10.1515/cclm-2018-0908. [DOI] [PubMed] [Google Scholar]
- 42.Bao X, Borné Y, Muhammad IF, Nilsson J, Lind L, Melander O, Niu K, Orho-Melander M, Engström G. Growth differentiation factor 15 is positively associated with incidence of diabetes mellitus: the Malmö Diet and Cancer-Cardiovascular Cohort. Diabetologia. 2019;62:78–86. doi: 10.1007/s00125-018-4751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fluschnik N, Ojeda F, Zeller T, Jørgensen T, Kuulasmaa K, Becher PM, Sinning C, Blankenberg S, Westermann D. Predictive value of long-term changes of growth differentiation factor-15 over a 27-year-period for heart failure and death due to coronary heart disease. PLoS One. 2018;13:e0197497. doi: 10.1371/journal.pone.0197497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eggers KM, Kempf T, Wallentin L, Wollert KC, Lind L. Change in growth differentiation factor 15 concentrations over time independently predicts mortality in community-dwelling elderly individuals. Clin Chem. 2013;59:1091–1098. doi: 10.1373/clinchem.2012.201210. [DOI] [PubMed] [Google Scholar]
- 45.Kempf T, Guba-Quint A, Torgerson J, Magnone MC, Haefliger C, Bobadilla M, Wollert KC. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol. 2012;167:671–678. doi: 10.1530/EJE-12-0466. [DOI] [PubMed] [Google Scholar]
- 46.Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth-differentiation factor-15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 47.Dallmeier D, Brenner H, Mons U, Rottbauer W, Koenig W, Rothenbacher D. Growth differentiation factor 15, its 12-month relative change, and risk of cardiovascular events and total mortality in patients with stable coronary heart disease: 10-year follow-up of the KAROLA study. Clin Chem. 2016;62:982–992. doi: 10.1373/clinchem.2016.254755. [DOI] [PubMed] [Google Scholar]
- 48.Zeller T, Hughes M, Tuovinen T, Schillert A, Conrads-Frank A, den Ruijter H, et al. BiomarCaRE: rationale and design of the European BiomarCaRE project including 300,000 participants from 13 European countries. Eur J Epidemiol. 2014;29:777–790. doi: 10.1007/s10654-014-9952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osler M, Linneberg A, Glümer C, Jørgensen T. The cohorts at the Research Centre for Prevention and Health, formerly ‘The Glostrup Population Studies’. Int J Epidemiol Oxford Academic. 2011;40:602–610. doi: 10.1093/ije/dyq041. [DOI] [PubMed] [Google Scholar]
- 50.Moore AG, Brown DA, Fairlie WD, Bauskin AR, Brown PK, Munier ML, Russell PK, Salamonsen LA, Wallace EM, Breit SN. The transforming growth factor-ss superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J Clin Endocrinol Metab. 2000;85:4781–4788. doi: 10.1210/jcem.85.12.7007. [DOI] [PubMed] [Google Scholar]
- 51.Blankenberg S, Salomaa V, Makarova N, Ojeda F, Wild P, Lackner KJ, Jørgensen T, Thorand B, Peters A, Nauck M, Petersmann A, Vartiainen E, Veronesi G, Brambilla P, Costanzo S, Iacoviello L, Linden G, Yarnell J, Patterson CC, Everett BM, Ridker PM, Kontto J, Schnabel RB, Koenig W, Kee F, Zeller T, Kuulasmaa K, BiomarCaRE Investigators Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J. 2016;37:2428–2437. doi: 10.1093/eurheartj/ehw172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skaaby T, Husemoen LLN, Borglykke A, Jørgensen T, Thuesen BH, Pisinger C, Schmidt LE, Linneberg A. Vitamin D status, liver enzymes, and incident liver disease and mortality: a general population study. Endocrine. 2014;47:213–220. doi: 10.1007/s12020-013-0107-8. [DOI] [PubMed] [Google Scholar]
- 53.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ambye L, Rasmussen S, Fenger M, Jørgensen T, Borch-Johnsen K, Madsbad S, Urhammer SA. Studies of the Gly482Ser polymorphism of the peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) gene in Danish subjects with the metabolic syndrome. Diabetes Res Clin Pract. 2005;67:175–179. doi: 10.1016/j.diabres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka T, Biancotto A, Moaddel R, Moore AZ, Gonzalez-Freire M, Aon MA, Candia J, Zhang P, Cheung F, Fantoni G, CHI consortium. Semba RD, Ferrucci L. Plasma proteomic signature of age in healthy humans. Aging Cell. 2018;17:e12799. doi: 10.1111/acel.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho JE, Mahajan A, Chen M-H, Larson MG, McCabe EL, Ghorbani A, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem. 2012;58:1582–1591. doi: 10.1373/clinchem.2012.190322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herpich C, Franz K, Ost M, Otten L, Coleman V, Klaus S, et al. Associations between serum GDF15 concentrations, muscle mass and strength show sex-specific differences in older hospital patients. Rejuvenation Res. 2020. [DOI] [PubMed]
- 58.Tavenier J, Rasmussen LJH, Andersen AL, Houlind MB, Langkilde A, Andersen O, et al. Association of GDF15 with inflammation and physical function during aging and recovery after acute hospitalization: a longitudinal study of older patients and age-matched controls. The Journals of Gerontology: Series A. 2021;glab010. [DOI] [PubMed]
- 59.Rothenbacher D, Dallmeier D, Christow H, Koenig W, Denkinger M, Klenk J. Association of growth differentiation factor 15 with other key biomarkers, functional parameters and mortality in community-dwelling older adults. Age Ageing. 2019;48:541–546. doi: 10.1093/ageing/afz022. [DOI] [PubMed] [Google Scholar]
- 60.Daniels LB. Clopton Paul, Laughlin Gail A., Maisel Alan S., Barrett-Connor Elizabeth. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults. Circulation. 2011;123:2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiklund FE, Bennet AM, Magnusson PKE, Eriksson UK, Lindmark F, Wu L, Yaghoutyfam N, Marquis CP, Stattin P, Pedersen NL, Adami HO, Grönberg H, Breit SN, Brown DA. Macrophage inhibitory cytokine-1 (MIC-1/GDF15): a new marker of all-cause mortality. Aging Cell. 2010;9:1057–1064. doi: 10.1111/j.1474-9726.2010.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breit SN, Carrero JJ, Tsai VW-W, Yagoutifam N, Luo W, Kuffner T, Bauskin AR, Wu L, Jiang L, Barany P, Heimburger O, Murikami MA, Apple FS, Marquis CP, Macia L, Lin S, Sainsbury A, Herzog H, Law M, Stenvinkel P, Brown DA. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end-stage renal disease. Nephrol Dial Transplant. 2012;27:70–75. doi: 10.1093/ndt/gfr575. [DOI] [PubMed] [Google Scholar]
- 63.Emmerson PJ, Duffin KL, Chintharlapalli S, Wu X. GDF15 and growth control. Front Physiol. 2018;9:1712. doi: 10.3389/fphys.2018.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN. The MIC-1/GDF15-GFRAL pathway in energy homeostasis: implications for obesity, cachexia, and other associated diseases. Cell Metab. 2018;28:353–368. doi: 10.1016/j.cmet.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 65.Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, Campisi J, Schilling B. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599. doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schafer MJ, Zhang X, Kumar A, Atkinson EJ, Zhu Y, Jachim S, et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 75 kb)
Data Availability Statement
The datasets are not publicly available due to regulations set out by the Danish Data Protection Agency but are available from the corresponding author on reasonable request.