Abstract
Aging is associated with sleep and circadian alterations, which can negatively affect quality of life and longevity. Importantly, the age-related reduction in light sensitivity, particularly in the short-wavelength range, may underlie sleep and circadian alterations in older people. While evidence suggests that non-image-forming (NIF) light responses may diminish in older individuals, most laboratory studies have low sample sizes, use non-ecological light settings (e.g., monochromatic light), and typically focus on melatonin suppression by light. Here, we investigated whether NIF light effects on endogenous melatonin levels and sleep frontal slow-wave activity (primary outcomes), and subjective sleepiness and sustained attention (secondary outcomes) attenuate with aging. We conducted a stringently controlled within-subject study with 3 laboratory protocols separated by ~ 1 week in 31 young (18–30 years; 15 women) and 16 older individuals (55–80 years; eight women). Each protocol included 2 h of evening exposure to commercially available blue-enriched polychromatic light (6500 K) or non-blue-enriched light (3000 K or 2500 K) at low levels (~ 40 lx, habitual in evening indoor settings). Aging significantly affected the influence of light on endogenous melatonin levels, subjective sleepiness, sustained attention, and frontal slow-wave activity (interaction: P < 0.001, P = 0.004, P = 0.007, P = 0.001, respectively). In young individuals, light exposure at 6500 K significantly attenuated the increase in endogenous melatonin levels, improved subjective sleepiness and sustained attention performance, and decreased frontal slow-wave activity in the beginning of sleep. Conversely, older individuals did not exhibit signficant differential light sensitivity effects. Our findings provide evidence for an association of aging and reduced light sensitivity, with ramifications to sleep, cognition, and circadian health in older people.
Keywords: Aging, Light sensitivity, Circadian photosensitivity, Alertness, Cognition, Sleep
Introduction
Aging is often associated with alterations in sleep and circadian function [1], with adverse consequences for quality of life and longevity [2]. Healthy older individuals often exhibit increased wakefulness during sleep, reduced slow-wave sleep and sleep efficiency, earlier sleep-wake times, attenuated amplitude and/or phase-advanced circadian rhythms, as compared to young individuals [3, 4]. Importantly, these sleep and circadian effects are partly mediated by a reduced light sensitivity in healthy aging, particularly in the short-wavelength light [5, 6]. With advancing age, there is a steady decline in light transmission, because of the clouding and yellowing of the natural crystalline lens [7], especially for the short-wavelength of light [8]. Aging is also associated with reduced pupil size [9] and increased ocular lens absorption [10]. The age-related reduction of photic input to the brain (e.g., suprachiasmatic nucleus [commonly termed the central circadian clock], hypothalamus, thalamic nuclei, among others) can disrupt neuroendocrine and alerting responses to light (see Fig. 1A for a conceptual framework). Light subtends to the dual purpose of visual perception and non-image-forming (NIF) function, the latter being essential for circadian photosensitivity, alertness, cognition, and sleep [11, 12]. As human NIF system is sensitive to ~ 460 to 480 nm [13], NIF light responses predominantly occur when individuals are exposed to short-wavelength light [14–18]. Reduced sensitivity to short-wavelength light with aging affects neuroendocrine responses to light, such as melatonin suppression [6, 19, 20]. Healthy aging is also associated with diminished sensitivity to short-wavelength light in cognitive brain regions required for maintaining alertness, attention and executive function [21]. Evening light exposure can elicit carryover-alerting responses to short-wavelength or blue-enriched polychromatic light in young individuals, including longer sleep latency and altered dynamics of slow-wave activity (SWA) across the sleep episode [22–24]. In contrast, very little is known for healthy aging with mixed results showing either no differential light effects on sleep activity between young and older individuals [25] or a significantly prolonged latency to rapid eye movement (REM) sleep following evening light exposure in older individuals [26]. Of critical relevance, most human laboratory studies have limited sample sizes, and have assessed the influence of (predominatly evening/nighttime) light exposure in healthy aging using monochromtic light settings [20, 21, 24, 27–29], rarely encountered in real-life settings. Furthermore, most of these human studies focus on light effects affecting neuroendocrine responses in healthy aging (e.g., melatonin supression by light), while comparatively less is known about neurophysiological (i.e., alertness, cognition, and sleep) responses to light. Therefore, we investigated whether neuroendocrine (i.e., endogenous melatonin) and neurophysiological (i.e., alertness, cognition, and sleep) responses to evening polychromatic light are attenuated with aging. In our study, 31 healthy young and 16 older participants underwent a balanced within-subject crossover study with three separate laboratory protocols, ~ 1 week apart. In each protocol, participants underwent one of three commercially available polychromatic light (ecological) conditions for 2 h in the evening before habitual sleep. This was either a blue-enriched light condition at 6500 K (melanopic-weighted photon density: 6.96E + 16 photons m−2 s−1), a non-blue-enriched light condition at 2500 K (1.79E + 16 photons m−2 s−1) or at 3000 K (3.32E + 16 photons m−2 s−1) at relatively low light levels (~ 40 lx, typically encountered in indoor settings [30]) (Fig. 1B). Primary outcomes were endogenous melatonin levels and frontal SWA that are, respectively, hallmarks of the circadian system [31] and sleep homeostatic regulation [32]. Secondary outcomes were subjective sleepiness and sustained attention, which are exquisitely sensitive to light exposure [15].
Fig. 1.
Aging is associated with reduced light sensitivity. A Concept of the effects of aging on light sensitivity within the eye and downstream, and study hypothesis, B Our study protocol comprised a within-subject cross-sectional design with three separate laboratory protocols, 1 week apart. Times correspond to an illustrative clock time (e.g., 18:00 h for a participant whose habitual wake-up time derived from ambulatory sleep-wake recordings was 08:00 h). For details, see “Methods”
Methods
Participants
All study participants provided written informed consent. The local Ethics Committee (EKBB/Ethikkommission beider Basel, Switzerland) approved the study protocol, advertisements, screening questionnaires, and the consent form, which were in agreement with the Declaration of Helsinki. Our study sample aimed at enrolling healthy individuals and therefore included multiple inclusion/exclusion criteria, which were as follows: (A) age 20–31 years for the young group and 55–80 years for the older group; (B) body mass index (BMI) > 19 and < 28 kg/m2; (C) no co-existing ocular pathologies, medical comorbidities; (D) compliance with regular sleep-wake schedules (see “Study design”); (E) no shift work experience within at least 1 year; (F) Pittsburgh sleep quality index score ≤ 5; (G) no extreme morning or evening chronotype ratings; (H) no smoking or recreational drug intake.
Regarding medication intake, participants who used antihypertensive, antidiabetic, anti-allergic medications, non-steroidal anti-inflammatory drugs (including aspirin), benzodiazepines, and hormone replacement therapy were excluded. An ophthalmologic examination was performed before the study to exclude volunteers with visual impairments. In the older group, sleep disorders, including narcolepsy, sleep apnea (apnea index > 10/h), periodic limb movements (PLMS index associated with arousal > 15/h), as verified by a polysomnographic adaptation night before the study, were excluded. Our final sample comprised 31 young and 16 older individuals.
Study design
Prior to the laboratory protocols, participants underwent an ambulatory segment of ~ 3 weeks to ensure sleep-wake cycle regularity (from the week before the first laboratory protocol to the third laboratory protocol). On the week prior to each laboratory protocol, participants were requested to abstain from excessive alcohol and caffeine consumption (less than 5 alcoholic beverages per week, and 1 cup of coffee or 1 caffeine-containing beverage per day). On the day of each laboratory protocol, participants were also allowed to consume 1 cup of coffee or 1 caffeine-containing beverage. Caffeine intake was limited to the breakfast, thus at least 8 h before the start of the protocol to minimize the acute effects of caffeine on the study outcome measures. During this study segment, study participants kept a regular sleep-wake schedule of ~ 7 to 8 h per night (bedtimes and wake times within ± 30 min of a self-selected target). We verified compliance by wrist-worn actigraphy (Actiwatch L, Cambridge Neurotechnology, Cambridge, UK) and daily sleep-wake logs. The young and older groups did not significantly differ in their ambulatory sleep and wake-up times or sleep duration (Table 1).
Table 1.
Baseline demographics and study-related characteristics between age groups
| Young group | Older group | P-value* | |
|---|---|---|---|
| n | 31 | 16 | |
| Age (years) | 25.2 (22, 29) | 63.6 (58, 70) | 0.72 |
| Sex | 15 women; 16 men | 8 women; 8 men | 0.97 |
| Body mass index (kg/m2) | 22.6 (21.3 to 23.9) | 23.4 (21.7 to 25.1) | 0.63 |
| Race/ethnicity | All caucasians | All caucasians | 0.99 |
| Pittsburg sleep quality index | 2.2 (1.5 to 2.9) | 2.1 (1.4 to 2.8) | 0.85 |
| Epworth sleepiness scale | 4.3 (1.5 to 7) | 4.9 (2.4 to 6.3) | 0.59 |
| Morning-Eveningness Questionnaire | 58.4 (52.7 to 64.1) | 59.1 (52.2 to 66.0) | 0.78 |
| Sleep time (h:min) | 23:21 + 0:21 | 23:19 + 0:06 | 0.62 |
| Wake-up time (h:min) | 07:03 + 0:23 | 07:11 + 0:10 | 0.59 |
| Sleep duration (h:min) | 07:16 + 0:21 | 06:55 + 0:11 | 0.21 |
Data are mean and 95% Confidence intervals, except for actigraphy sleep and wake-up times, and sleep duration (mean + SEM)
*Chi-square tests for sex and race; T-tests for independent groups for the other outcomes
This study was an observational counterbalanced within-subject crossover design with three laboratory protocols, with a 1 week intervening period between each protocol (Fig. 1B). Participants commenced each laboratory protocol ~ 10 h after their habitual wake-up time, as determined during the ambulatory segment of the study. Thus, the study protocol was timed to occur in the evening hours (e.g., 18:00 h for a participant whose habitual wake-up time was e.g., 08:00 h) and ended the next day (upon usual wake-up time). During each laboratory protocol, participants stayed in an individual laboratory room to ensure a stringent environmental condition that could confound the effects of light exposure (e.g., no windows to avoid external environmental light). During each protocol, participants had 3.5 h of controlled light exposure (1.5 h of dim light < 8 lx and 2 h dark adaptation at < 1 lx; baseline). The baseline ensured controlled and homogenous light exposure prior to the 2 h of evening light exposure thereafter, as it is very-well established that NIF responses depend on specific light properties, including photic history [33]. Thereafter, participants had a 2 h light exposure to a compact fluorescent light source either at 6500 K or 2500 K or to an incandescent light source at 3000 K. Light exposure occurred in the evening hours, as exposure to light at that time of day strongly affects circadian photosensitivity and alertness [22, 34, 35]. Afterwards, participants underwent ~ 30 min of post-light exposure (PL) under dim light (< 8 lx), before an 8-h sleep opportunity that commenced at each participant’s habitual sleep time (based on their ambulatory sleep-wake times). The ~ 30 min of PL allowed assessing light-induced effects on endogenous melatonin beyond the acute 2 h light exposure. Physical activity was controlled for during dark adaptation and light exposure, as participants remained seated. During the scheduled sleep episode, participants remained on bed (in supine position) and in darkness, until their habitual wake-up time. Participants consumed dinner before commencing each protocol. This allowed minimizing the effect of meals on our outcome measures. Upon habitual wake-up time, participants received breakfast. Each laboratory protocol was identical, except for the 2 h evening light exposure that was either 6500 K, 3000 K or 2500 K. For the light exposure settings, we used commercially available compact fluorescent light sources with a highly correlated color temperature (6500 K; melanopic-weighted photon density: 6.96E + 16 photons m−2 s−1) and a lower color temperature (2500 K; melanopic-weighted photon density: 1.79E + 16 photons m−2 s−1). We also used an incandescent light source (3000 K; melanopic-weighted photon density: 3.32E + 16 photons m−2 s−1). For details of the light spectra, see Chellappa et al. [36]. We used two non-blue-enriched light sources given the ecological design of this study, such that light at 2500 K had less irradiance at the short wavelength than light at 6500 K and the incandescent light source was a broadband white light source commonly used in Switzerland (prior to its official banning). We did not perform a control laboratory protocol with 2 h of dim light (< 8 lx) exposure, as this additional fourth protocol could have reduced study feasibility. Illumination levels were set at ~ 40 lx on a white wall located at the central point in the participant’s field of view.
In our counterbalanced crossover study design with 3 laboratory protocols, young and older participants underwent one of three lists with light setting sequences. List 1: 1st exposure, light at 6500 K; 2nd exposure, light at 3000 K; 3rd exposure, light at 2500 K. List 2: 1st exposure, light at 2500 K; 2nd exposure, light at 6500 K; 3rd exposure, light at 3000 K. List 3: 1st exposure, light at 3000 K; 2nd exposure, light at 2500 K; 3rd exposure, light at 6500 K. Therefore, the treatment (6500 K vs. 2500 K vs. 3000 K) was counterbalanced to avoid possible order effects. We used only three potential combinations of light setting sequences to ensure equal distribution of participants per list. Participants were allocated to one of three lists with light setting sequences based on the order in their recruitment/enrolment. Therefore, participants were not assigned in a more random manner, as generated using e.g., minimization-randomization programs. Ten participants in the young group and five participants in the older group underwent list 1 (6500 K–3000 K–2500 K). Eleven participants in the young group and six participants in the older group underwent list 2 (2500 K–6500 K–3000 K). Eleven participants in the young group and five participants in the older group underwent List 3 (3000 K–2500 K–6500 K). The study investigators were not blind to the study outcome measures, to the interventions received by the participants or to the treatment order. The study participants were blind to the study outcome measures and to the treatment order in the laboratory protocols.
Outcome measures
The primary outcome measures were endogenous melatonin levels, a classical marker of the circadian timing system [31], and frontal SWA (all-night and during the first NREM-REM sleep cycle), a hallmark of sleep homeostatic regulation [32]. The secondary outcome measures were subjective sleepiness, indexed as the subjective perception of current sleepiness levels (Karolinska sleepiness scale) and sustained attention performance (psychomotor vigilance task, PVT). These outcomes are exquisitely sensitive to the effects of sleep loss, circadian phase, and light exposure [34, 37, 38].
Salivary melatonin collections were scheduled during wakefulness every 30-40 min throughout each laboratory protocol. A direct double-antibody radioimmunoassay was used for the salivary melatonin assays (validated by gas chromatography–mass spectroscopy with an analytical least detectable dose of 0.65 pm/mL; Bühlmann Laboratory, Schönenbuch, Switzerland) [39]. The minimum detectable dose of melatonin (analytical sensitivity) was determined to be 0.2 pg/ml.
Participants rated their current subjective sleepiness levels every 30-40 min using the Likert-point scale Karolinska Sleepiness Scale (KSS) [40]. This is a 9-point scale (1 = extremely alert, 3 = alert, 5 = neither alert nor sleepy, 7 = sleepy but no difficulty remaining awake, and 9 = extremely sleepy, fighting sleep). Sustained attention performance was assessed using the well-validated PVT [37]. Participants had to press a button as soon as an auditory stimulus occurred (auditory threshold was set at ~ 60% of the maximum volume), presented in randomly varying intervals of 3–7 s. Duration of the PVT was 5 min. We report test sessions during dark adaptation (baseline) and during the light exposure.
Sleep was recorded throughout the scheduled sleep opportunity using the Vitaport Ambulatory system (Vitaport-3 digital recorder TEMEC Instruments BV, Kerkrade, the Netherlands). Sleep stages were visually scored per 20 s epochs (Vitaport Paperless Sleep Scoring Software) [41]. Spectral analysis was conducted using fast Fourier transformation (FFT; 10% cosine 4-s window) with a 0.25 Hz bin resolution. In this study, we assessed EEG power spectra in the frequency range 0.5–25 Hz during sleep. Here, we assessed SWA and focused on the frontal derivation, as this corresponds to the topographical location most sensitive to light effects [23].
Statistical analyses
The sample size was derived from the difference (percentage of change) in the effect of blue-enriched and non-blue-enriched light conditions on endogenous melatonin levels between young and older individuals [42]. To determine an age-dependent difference of ~ 20% in melatonin levels by light exposure (with 95% power), 16 participants per age group were required.
We normalized endogenous melatonin levels (primary outcome measure), subjective sleepiness and sustained attention performance (secondary outcome measures) by using the average of each participant’s levels measured during baseline (the 3.5 h controlled prior light exposure). Therefore, these outcomes correspond to the percentage of change from baseline (average of the 3.5 h dim light-dark exposure levels) to light exposure (average of the 2 h evening light exposure levels). For each outcome measure, we applied mixed-model analyses of variance (PROC MIXED) using within-factor “Light condition” (6500 K, 2500 K, 3000 K), and between-factor “Group” (young vs. older group), and the interaction of “Group” by “Light condition.” The latter was used to assess whether aging significantly affected the influence of light exposure on endogenous melatonin levels, alertness, and cognitive performance.
Sleep EEG activity was log-transformed prior to analyses. To examine all-night frontal EEG power density during NREM sleep, we applied mixed-model analyses (PROC MIXED) using within-factors “Light condition” (6500 K, 2500 K, 3000 K) and between-factor “Group” (young vs. older group), and their interaction for each 0.25 Hz frequency bin in the range of 0.50–25 Hz during NREM sleep. As we observed a significant interaction effect from 2 to 4 Hz (SWA range; F > 1.04; P < .05), we report the all-night NREM EEG activity and the dynamics of SWA for 2–4 Hz (i.e., sum of EEG log-transformed EEG power in the frequency range of 2–4 Hz for NREM-REM sleep cycles 1–4, per participant). For the dynamics of frontal SWA, mixed-models for EEG activity also included “Sleep cycle” (cycles 1–4) as a within-subject effect. We used the interaction of “Group” by “Light condition” by “Sleep cycle” for SWA (2–4 Hz) to assess whether aging significantly affected the influence of light exposure on sleep dynamics.
All mixed-models reported in this study included “Light condition” as a repeated factor, as participants underwent three separate light exposure settings. We report type III tests for fixed effects, as it accounts for the effects of covariates/interactions. Post-hoc comparisons used the Tukey-Kramer test. Missing data were not included in the analyses (4.8% of observations for sleep EEG activity, due to e.g., poor data quality; for the other outcomes, there was virtually no missing data [< 0.5%]). We used the two-sided P-values of .05 for statistical significance. To control overall type I error in null hypothesis testing when conducting multiple comparisons, we adjusted the P-values from the mixed-model analysis for the primary and secondary outcomes using false discovery rates (FDR) (PROC MULTTEST). We used SAS software, version 9.4 (SAS Institute). Unless specified, data correspond to mean and 95% confidence intervals (95% CI), and FDR-adjusted P-values. Because sex differences have been reported for sleep and circadian regulation [43], and BMI influences sleep quality in older individuals [44], we performed additional mixed-model analyses of variance including sex and BMI as covariates of interest. Our power analyses did not originally account for BMI and sex differences between groups. Therefore, we included these variables as covariates but did not test for the interaction of sex and BMI with the main factors “Group” and “Light condition,” as we do not have statistical power to test such effects.
Results
Thirty-one healthy young individuals (mean [standard error of the mean (SEM)] age, 25.2 [3.1] years; 15 women) and 16 healthy older individuals (63.6 [5.6] years; eight women) completed the entire study. We excluded data from one participant in the young group for the sleep activity analyses, due to low quality in the sleep recordings. Participant’s demographics and study-related characteristics did not significantly differ between groups (Table 1). No important harms or unintended adverse effects from the evening light exposure occurred in either group.
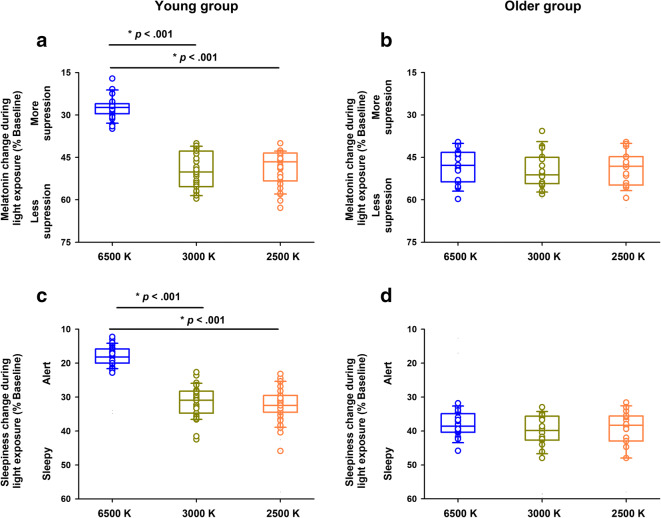
No significant baseline differences were observed for endogenous melatonin levels, subjective sleepiness, and sustained attention performance during baseline (all FDR-adjusted P > .1). Aging significantly affected the differential influence of light exposure on endogenous melatonin levels (interaction “Group” by “Light condition”; FDR-adjusted P < .001). In the young group, we observed an attenuated increase in endogenous melatonin levels during blue-enriched light exposure, with a reduction by 22.8% (difference of means; 95% CI: − 25.7%, − 19.8%), as compared to the non-blue-enriched light conditions (Fig. 2A; see Table 2 for statistical results). Conversely, the older group showed no differences for endogenous melatonin levels across the light conditions (difference of means: − 1.4%; 95% CI: − 6.6%, 3.8%) (Fig. 2B; Table 2).
Fig. 2.
Effects of aging and light on endogenous melatonin levels and subjective sleepiness. The young group showed a significant reduction in the evening rise of endogenous melatonin levels (A) and reduced levels of subjective sleepiness (C) when exposed to blue-enriched light (6500 K, blue circles), as compared to non-blue-enriched light (3000 K, dark yellow circles; 2500 K, orange circles). Conversely, the older group showed no significant differences in endogenous melatonin levels (B) or in subjective sleepiness (D) across the light conditions. Circles correspond to individual data. Boxes correspond to median and interquartile range with 95% confidence intervals as whiskers. Young group: n = 31; older group: n = 16. P-values correspond to post-hocs for the interaction “Group” by “Light condition” (see “Results” and Table 2)
Table 2.
Results of the mixed-model analyses of variance for the primary outcomes (endogenous melatonin and frontal slow-wave activity (SWA) and secondary outcomes (subjective sleepiness and sustained attention performance). P-values adjusted using False discovery rates (FDR). Data presented as mean and 95% confidence intervals. Young group: n = 31 and older group: n = 16
| Young group | Older group | Group | Light condition | Group × light condition | |||||
|---|---|---|---|---|---|---|---|---|---|
| Light condition | 6500 K | 3000 K | 2500 K | 6500 K | 3000 K | 2500 K | P-value* | P-value* | P-value* |
| Melatonin | 26.6 (25, 28.3) | 49.4 (47, 51.7) | 48.3 (46.1, 50.5) | 47.7 (44.5, 50.9) | 49 (45.5, 52.2) | 48.4 (45.2, 51.6) | <0.001 | <0.001 | <0.001 |
| Sleepiness (KSS) | 17.7 (16.7, 19) | 31 (29.3, 32.7) | 31.9 (30.2, 33.7) | 37.7 (35.7, 39.7) | 39.4 (37, 41.8) | 39.1 (36.3, 41.8) | 0.002 | <0.001 | 0.004 |
| PVT median RT | −15.2 (−21, -8.6) | −2 (−4.9, 1) | 0.3 (-2.5, 3.3) | −1.6 (−4.2, 1.1) | 2.2 (−0.1, 4.5) | 2.4 (−9.2, 4.5) | 0.01 | 0.006 | 0.002 |
| PVT fastest RT | −15.3 (−22, -8.5) | −0.03 (−2.9, 2.9) | 0.9 (−1, 2.8) | −1.2 (−3.3, 0.8) | −0.7 (−4, 2.5) | −0.2 (−3.9, 3.5) | 0.01 | 0.002 | 0.007 |
| PVT slowest RT | −4.2 (−7.9, 0.5) | −0.9 (−5.2, 3.4) | 2.9 (-1.5, 7.3) | −6.1 (−14.2, 1.9) | 5.6 (1.6, 9.6) | −3 (−11, 4.9) | 0.8 | 0.02 | 0.1 |
| All-night frontal SWA* | 2 (2, 2.1) | 2.2 (2.1, 2.2) | 2.2 (2.1, 2.2) | 1.9 (1.9, 2) | 1.9 (1.8, 2) | 1.9 (1.8, 2) | <0.001 | 0.006 | 0.07 |
| Dynamics of frontal SWA** | 2.3 (2.2, 2.3) | 2.4 (2.3, 2.4) | 2.3 (2.2, 2.4) | 2 (1.9, 2.1) | 2 (1.9, 2.1) | 2 (1.8, 2.1) | <0.001 | 0.003 | 0.2 |
Melatonin levels, subjective sleepiness levels (Karolinska sleepiness scale, KSS) and psychomotor vigilance task (PVT) reaction time (RT) data expressed as percentage of baseline (see “Statistical analyses” for details on data normalization for each outcome measure)
*Slow-wave activity (SWA) data were log-transformed
**Mean and 95% confidence intervals for 1st non-rapid eye movement (NREM)—rapid eye movement (REM) sleep cycle. Mixed-model analyses of variance also included main effect “Sleep cycle”: p < 0.001, and the interaction of “Group” by “Light condition” by “Sleep cycle”: p = 0.005
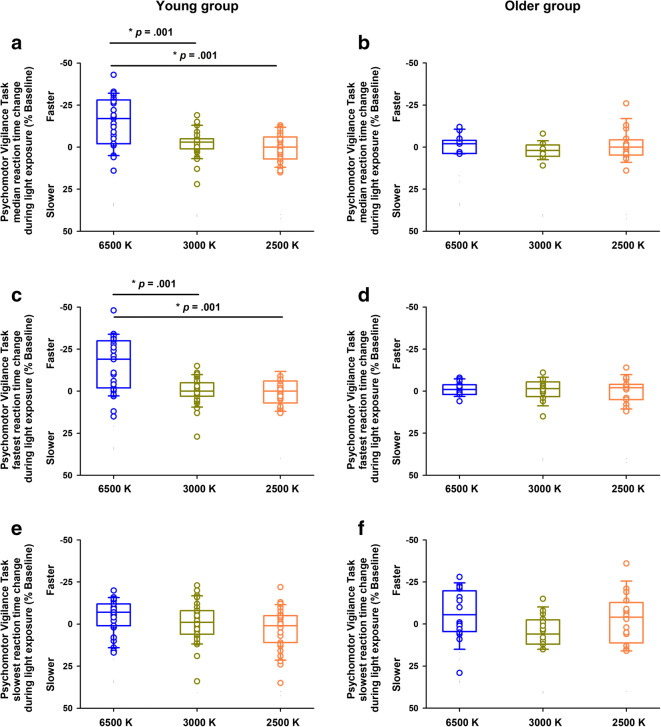
We then tested whether aging was associated with light sensitivity effects on subjective sleepiness and cognitive function, as indexed by tasks mapping allocation of attention resources (PVT; outcomes: median, fastest and slowest reaction times). Aging significantly affected the differential influence of light exposure on alertness (interaction “Group” by “Light condition”; FDR-adjusted P < .001). In the young group, sleepiness levels during the blue-enriched light condition were 13.9% less (difference of means; 95% CI: − 15.2%, − 12.6%), as compared to the non-blue-enriched light conditions. In contrast, the older group had no significant differences for alertness levels across the light conditions (difference of means: − 1.5%; 95% C: − 3.5%, 0.6%) (Fig. 2C–D; Table 2). Furthermore, aging affected the differential influence of light exposure on sustained attention performance (interaction “Group” by “Light condition”; FDR-adjusted P = .002 and P = .007 for PVT median and fastest reaction times, respectively). In the young group, sustained attention performance significantly improved during blue-enriched light exposure, such that their performance was 15.6% faster (difference of means: − 15.6%; 95% CI: − 20.9%, − 10.2%), as compared to both non-blue-enriched light exposure conditions (Fig. 3A, C, E; Table 2). Conversely, the older group did not exhibit significant differences on sustained attention performance across the light conditions (difference of means: − 1.4%; 95% CI: − 4.5%, 1.8%) (Fig. 3B, D, F; Table 2).
Fig. 3.
Effects of aging and light on Psychomotor Vigilance Task (PVT) performance. A and C: The young group exhibited significantly better sustained attention performance (as indexed by the faster reaction times [RT]), when exposed to blue-enriched light (6500 K, blue circles), as compared to non-blue-enriched light (3000 K, dark yellow circles; 2500 K, orange circles), B and D: Conversely, the older group showed no significant differences in sustained attention performance across the light conditions. E and F: There were no significant differential light effects for the PVT slowest RT in either group. Circles correspond to individual data. Boxes correspond to median and interquartile range with 95% confidence intervals as whiskers. Young group: n = 31; older group: n = 16. P-values correspond to post-hocs for the interaction “Group” by “Light condition” (see “Results” and Table 2)
We then assessed whether aging affected the influence of light exposure on sleep phenotypes, here indexed by frontal SWA (all-night and across NREM-REM sleep episodes). Aging showed a trend for a differential influence of light exposure on all-night frontal SWA (2–4 Hz) (interaction “Group” by “Light condition”; FDR-adjusted P = .07). In the young group, we observed a trend for less all-night frontal SWA following blue-enriched light, as compared to one of the non-blue-enriched light conditions (difference of means between 6500 and 3000 K: − 0.08; 95% CI: − 0.12, − 0.04; log-transformed data) (Fig. 4A; Table 2). Conversely, the older group did not exhibit significant differences on all-night frontal SWA across the light conditions (difference of means between 6500 and 3000 K: 0; 95% CI: − 0.04, 0.02; log-transformed data) (Fig. 4B; Table 2). Aging was significantly associated with a differential influence of light exposure on the dynamics of frontal SWA (interaction “Group” by “Light condition” by “Sleep Cycle”; FDR-adjusted P = .005). In the young group, there was significantly less SWA during the first NREM-REM sleep cycle following blue-enriched light exposure, as compared to one of the non-blue-enriched light conditions (difference of means between 6500 and 3000 K: − 0.08; 95% CI: − 0.12, -0.06; log-transformed data) (Fig. 4C, E; Table 2). In contrast, the older group did not exhibit significant differences on the dynamics of SWA across the light conditions (difference of means between 6500 and 3000 K: 0; 95% CI: − 0.05, 0.03; log-transformed data; Tukey-Kramer post-hoc comparisons for the interaction “Group” by “Light condition” by “Sleep Cycle”: P = .035) (Fig. 4D, F; Table 2). Analyses of the influence of light exposure on SWA for NREM-REM sleep cycles 2–4 indicated no significant differences (Tukey-Kramer post hoc comparisons for the interaction “Group” by “Light condition” by “Sleep Cycle”: P > .1). As exploratory analyses, we assessed whether aging affects the influence of light exposure on sleep structure (i.e., NREM sleep stages 1–4 [expressed as percentage of total sleep time], sleep efficiency, REM sleep [expressed as percentage of total sleep time], and wake after sleep onset). Whereas we observed significant effects of “Group,” there were no significant effects of “Light condition” or of the interaction of “Group” by “Light condition” (data not shown).
Fig. 4.
Effects of aging and light on sleep activity. A and B: All-night frontal slow-wave activity (SWA: 2–4 Hz; log-transformed) after exposure to blue-enriched light (6500 K, blue circles) and two non-blue-enriched light (3000 K, dark yellow circles; 2500 K, orange circles). The young group showed a trend for less SWA following exposure to blue-enriched light, as compared to one of the non-blue-enriched light conditions (3000 K). Conversely, the older group showed no significant differences for frontal SWA across the light conditions (see “Results” and Table 2). C and D: Frontal SWA during the first NREM-REM sleep cycle after exposure to blue-enriched light (6500 K, blue circles) and two non-blue-enriched light conditions (3000 K, dark yellow circles; 2500 K, orange circles). The young group showed significantly less SWA following blue-enriched light compared to one of the non-blue-enriched light (3000 K). Conversely, the older group showed no significant differences for frontal SWA across the light conditions (see “Results “and Table 2; P-values correspond to post-hocs derived from the interaction of “Group” by “Light condition” by “Sleep cycle”). Circles correspond to individual data. Boxes correspond to median and interquartile range with 95% confidence intervals as whiskers. E and F: Time–course of frontal SWA per NREM–REM sleep cycles 1–4 after exposure to light at 6500 K (blue lines), 3000 K (dark yellow lines) and 2500 K (orange lines). The young group had significantly less slow-wave activity during the first NREM-REM sleep cycle following blue-enriched light exposure, as compared to one of the non-blue-enriched light conditions (3000 K). In contrast, the older group showed no significant differences for the dynamics of SWA across the light conditions (see “Results” and Table 2). Circles correspond to individual data. Lines represent an exponential decay function [where SWA = SWA~ + (SWA0–SWA~).exp(–rt)] of slow-wave activity fitted to the data separately for each of the three light conditions. Data are plotted by time (in hours) after sleep onset (sleep stage 2 onset) (young group: n = 30; older group: n = 16)
Lastly, we tested potential effects of BMI and sex (covariates of interest) on our primary (endogenous melatonin, frontal SWA) and secondary outcomes (subjective sleepiness and sustained attention performance). BMI did not significantly modify the reported light effects on the primary/secondary outcomes (all FDR-adjusted P > .1; Table 3). Sex significantly affected PVT median and fastest RT, and showed a trend for the dynamics of frontal SWA (FDR-adjusted P = .006, P = .001, P = .07, respectively; Table 3). Accordingly, men had significantly faster PVT performance than the women (P = .001), and showed a trend for more frontal SWA during the first NREM-REM cycle, than the women (P = .08). Importantly, the reported age-related differential influence of light exposure on the primary/secondary outcomes remained significant in our mixed-model analyses with covariates of interest (Table 3).
Table 3.
Results of the mixed-model analyses of variance (P-values) for primary outcomes (endogenous melatonin and frontal slow-wave activity [SWA]) and secondary outcomes (subjective sleepiness and sustained attention performance), including covariates of interest (sex and body mass index, BMI). P-values adjusted using False discovery rates (FDR). Young group: n = 31 and older group: n = 16
| Outcomes | Group | Light condition | Sleep cycle | Sex | Body mass index | Group × light condition | Group × light condition* sleep cycle |
|---|---|---|---|---|---|---|---|
| Melatonin | 0.001 | 0.001 | n/a | 0.7 | 0.7 | 0.001 | n/a |
| Sleepiness | 0.005 | 0.001 | n/a | 0.7 | 0.9 | 0.008 | n/a |
| PVT median RT | 0.02 | 0.008 | n/a | 0.006 | 0.8 | 0.004 | n/a |
| PVT fastest RT | 0.02 | 0.004 | n/a | 0.001 | 0.8 | 0.009 | n/a |
| PVT slowest RT | 0.9 | 0.06 | n/a | 0.7 | 0.8 | 0.3 | n/a |
| All-night frontal SWA | 0.001 | 0.008 | n/a | 0.2 | 0.7 | 0.1 | n/a |
| Dynamics of frontal SWA | 0.001 | 0.004 | 0.001 | 0.07 | 0.7 | 0.3 | 0.01 |
Endogenous melatonin, subjective sleepiness and psychomotor vigilance task (PVT) reaction times (RT) data expressed as percentage of baseline (see “Statistical analyses” for details on data normalization for each outcome measure)
Discussion
Our findings indicate an association of aging with reduced light sensitivity: whereas the young group showed robust blue-enriched light effects at a relatively low illuminance (~ 40 lx) on melatonin levels, alertness, cognition and sleep in the evening, no differential light responses were observed for the older group.
Previous human studies show conflicting results for the association of aging and light sensitivity on neuroendocrine function [19, 29]. Melatonin suppression is attenuated in older individuals exposed to short-wavelength light at ~ 480 nm than to long-wavelength light at ~ 550 nm [19]. Older individuals may show a shift in the peak of NIF light sensitivity (~ 490 nm), as compared to young individuals (~ 480 nm) [29]. Conversely, no age-related difference in melatonin suppression in response to short-wavelength light has also been reported, suggesting preserved circadian photosensitivity with advancing age [29]. In a similar vein, human studies show that circadian phase-shifting responses (e.g., circadian melatonin rhythms) to polychromatic light exposure may remain conserved in healthy aging [20, 27, 45, 46]. Discrepancies across those studies and ours can be ascribed to a myriad of factors. These include differences in statistical power (most of these laboratory studies had limited sample sizes), whether or not there was [sufficient] photic history control), and time-of-day of the light exposure. Concerning the latter, most laboratory protocols, including ours, have investigated light sensitivity effects during the evening/night hours. Studies on the effects of light exposure during the daytime and/or across the daytime and nighttime are currently limited. In a study that used 4 weeks of polychromatic blue-enriched lighting, positive effects (e.g., earlier timing of rest-activity rhythm, increased daytime activity, reduced anxiety) as well as negative effects (e.g., increased nighttime activity, reduced sleep quality) were reported in older individuals [47]. Furthermore, differences in the intensity and photon density of light exposure (some [21, 28] but not all studies [5, 45] including ours, have used light at higher irradiances), and in lens density in the older individuals can account for these mixed findings.
We show that healthy aging is not associated with an increased subjective alerting response to light at 6500 K in the evening. This contrasts previous findings that reported older individuals feeling more alert following exposure to short-wavelength light, as compared to to longer wavelength light [20]. Direct comparisons across studies are difficult given the inherent differences across study protocols and light settings. However, it might be that in naturalistic polychromatic light settings at lower light intensities (typical in most indoor settings [30]) the subjective alerting response to light may be hindered in healthy aging. In contrast, light at higher irradiances may improve this alerting response [26, 42], with comparable subjective sleepiness levels between young and older individuals when they are exposed to evening bright polychromatic light. We then showed that sustained attention performance did not improve across light settings in older individuals. Evidence for the beneficial light effects on cognitive function in healthy aging is very limited. Exposure to polychromatic blue-enriched white light can improve cognitive function (e.g., working memory performance) in older individuals [48]. However, studies confirming such effects are largely absent. Healthy aging can result in a progressive waning in cognitive fitness, although there are large individual differences in healthy older populations [49]. The effect of short-wavelength light on brain responses has been shown to attenuate with aging [21, 28]. In a functional magnetic resonance imaging (fMRI) study, young and older participants performed auditory working memory tasks while under darkness or short-wavelength light exposure during the daytime [21, 28]. Young individuals showed higher impact of light than older individuals did, particularly in brain regions associated with alertness and executive control (e.g., thalamus, tegmentum, hippocampus, and prefrontal cortex) [21, 28]. Ramifications of these brain imaging findings may include a reduced beneficial effect of light on cognition in healthy aging. Moreover, these findings indicate that changes at the brain level (beyond the putative light effects on the central circadian clock) are an important factor underlying the decreased NIF impact of light in older individuals.
An important goal of our study was to identify whether the age-related reduced light sensitvity affects sleep physiology. In young individuals, exposure to short-wavelength light in the evening has been shown to affect homeostatic sleep regulation, with increased sleep latency [24] and attenuated frontal SWA in the beginning of sleep [22, 23]. These findings combined render acute evening exposure to light as potentially hazardous for sleep. Here, we go a step further and show that—despite exposure to light at 6500 K in the evening—older individuals do not exhibit alerting carryover effects on sleep. A recent study in young and older individuals exposed to 40 h of sleep deprivation with different ambient lighting conditions showed that both age groups responded with an equally higher homeostatic sleep response (i.e., SWA during sleep recovery) to light exposure, as compared to a dim light condition [25]. Whereas sleep homeostasis may remain conserved in healthy aging under challenging sleep deprivation conditions, this may not be the case under naturalistic settings with habitual sleep-wake patterns.
NIF responses to light are predominantly mediated via intrinsically photosensitive retinal ganglion cells (ipRGCs) [50], by activation of the photopigment melanopsin. Healthy aging is accompanied by a myriad of changes within the eye and downstream, which may adversely affect NIF responses to light. The natural lens may thicken and attain yellowish discoloration due to increased buildup of chromophores, drastically reducing the amount of light passing through the retina [51]. This is further aggravated in patients with cataract [7, 8], whereas those who undergo intraocular lens replacement show improved circadian photosensitivity, visual comfort, cognitive performance, and sleep [52–55]. Importantly, post-mortem retinas from human donors aged 10–81 years indicate that advanced age is associated with a loss in density and dendritic arborization of the melanopsin-expressing retinal ganglion cells within the retina [56]. Moreover, older individuals may expose themselves to less ambient light resulting in attenuated melatonin secretion at night [57]. In this scenario, a stronger light stimulus (light at a higher irradiance) may ensure adequate neuroendocrine and alerting light responses. Indeed, healthy older individuals exposed to higher irradiance light-emitting diodes (LEDs) (peak wavelength ~ 470 nm) had more melatonin suppression, as compared to low irradiance LEDs [58]. As the ability to use light effectively can decrease with healthy aging, increasing the amount of light exposure may help strengthen and boost the alerting responses to light.
Limitations to our study include the stringent participant inclusion/exclusion criteria and the carefully controlled laboratory protocols (e.g., time-free environment, 3.5 h of prior light history control). While these factors minimized the effect of confounding factors on outcomes (e.g., medical comorbidities, sleep disturbances, medication intake, prior light effects), they differ from the habitual conditions of older individuals in real life. Furthermore, we showed that sex significantly influenced sustained attention performance and had a trend for significance on frontal SWA dynamics. Emerging evidence indicates sex differences in the circadian regulation of sleep and cognitive performance [43] and in light sensitivity [59]. However, the influences of combined individual traits (e.g., sex and age) on light sensitivity remain to be established. Therefore, future studies in real life settings with larger samples will help establish the effects of aging and its interaction with other individual traits on neuroendocrine and alerting responses to light. In summary, our study shows that healthy aging is associated with a reduced sensitivity to typically low (~ 40 lx) evening light exposure on some NIF outputs, including circadian photosensitivity (e.g., endogenous melatonin levels), alertness, sustained attention performance, and sleep physiology. Hence, the typical low light levels occurring in indoor settings may require switching to more efficacious light-delivery systems at e.g., higher irradiances to maximize beneficial light responses in healthy aging.
Acknowledgements
We thank Dr. Julia Krebs, Dr. Thomas Götz, and Dr. Corrado Garbazza for medical screenings, Dr. Christina Schmidt for assistance with the cognitive data preprocessing, Giovanni Balestreri, Claudia Renz, Marie-France Dattler, and all the study helpers for their help in data acquisition. We thank Dr. Roland Steiner, Dr. Dieter Lang, and Prof. Peter Oelhafen for their assistance with the light settings, and the volunteers for participating in this study.
Authors’ contributions
S.L.C. and C.C. conceived and designed the study. S.L.C, V.B., and S.F. performed the experiments and data acquisition. S.L.C. performed the statistical analyses. S.L.C., V.B., S.F., and C.C. drafted the manuscript. All the authors helped interpret the data, provided critical revisions of the manuscript, and approved the final version of the manuscript.
Funding
This study was financially supported by the AXA Foundation (https://www.axa-research.org/en/project/christian-cajochen) and by the Swiss Federal Office for Public Health (Consumer Protection Directorate, 11.007647). The funders had no role in the design and conduct of the study; collection, management, analyses, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability
All deidentified data and related documents (study protocol, statistical analysis plan, informed consent form) will be available by the authors with publication to researchers whose proposed use of the data have been approved. Data will be made available to such researchers for any purpose following approval of a proposal with signed data access agreement.
Declarations
Ethical approval and consent to participate
Our study complied with ethical standards. All study participants provided written informed consent. The local Ethical Committee (EKBB/Ethikkommission beider Basel, Switzerland) approved the study protocol, advertisements, screening questionnaires, and the consent form, which were in agreement with the Declaration of Helsinki.
Consent to participate
All study participants provided written informed consent. The local Ethical Committee (EKBB/Ethikkommission beider Basel, Switzerland) approved the study protocol, advertisements, screening questionnaires, and the consent form, which were in agreement with the Declaration of Helsinki.
Consent for publication
All co-authors have reviewed the content provided in the article and consent for publication.
Conflict of interest
C.C. has had the following commercial interests related to lighting: honoraria, travel, accommodation and/or meals for invited keynote lectures, conference presentations or teaching from Toshiba Materials, Velux, Firalux, Lighting Europe, Electrosuisse, Novartis, Roche, Elite, Servier, and WIR Bank. C.C. is a member of the Daylight Academy. None of these are related to the current study and manuscript publication. S.L.C., V.B., and S.F. declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sarah L. Chellappa, Email: Sarah.Chellappa@outlook.com
Christian Cajochen, Email: Christian.Cajochen@upkbs.ch.
References
- 1.Turner PL, Van Someren EJ, Mainster MA. The role of environmental light in sleep and health: effects of ocular aging and cataract surgery. Sleep Med Rev. 2010;14(4):269–280. doi: 10.1016/j.smrv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Mazzotti DR, Guindalini C, Moraes WA, Andersen ML, Cendoroglo MS, Ramos LR, et al. Human longevity is associated with regular sleep patterns, maintenance of slow wave sleep, and favorable lipid profile. Front Aging Neurosci. 2014;6:134. doi: 10.3389/fnagi.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cajochen C, Munch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23(1-2):461–474. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- 4.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28(5):799–807. doi: 10.1016/j.neurobiolaging.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chellappa SL. Individual differences in light sensitivity affect sleep and circadian rhythms. Sleep. 2021;44(2):zsaa214. 10.1093/sleep/zsaa214. [DOI] [PMC free article] [PubMed]
- 7.Pescosolido N, Barbato A, Giannotti R, Komaiha C, Lenarduzzi F. Age-related changes in the kinetics of human lenses: prevention of the cataract. Int J Ophthalmol. 2016;9(10):1506–1517. doi: 10.18240/ijo.2016.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. 2010;36(2):308–312. doi: 10.1016/j.jcrs.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Bitsios P, Prettyman R, Szabadi E. Changes in autonomic function with age: a study of pupillary kinetics in healthy young and old people. Age Ageing. 1996;25(6):432–438. doi: 10.1093/ageing/25.6.432. [DOI] [PubMed] [Google Scholar]
- 10.Sample PA, Esterson FD, Weinreb RN, Boynton RM. The aging lens: in vivo assessment of light absorption in 84 human eyes. Invest Ophthalmol Vis Sci. 1988;29(8):1306–1311. [PubMed] [Google Scholar]
- 11.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prayag AS, Munch M, Aeschbach D, Chellappa SL, Gronfier C. Light Modulation of human clocks, wake, and sleep. Clocks Sleep. 2019;1(1):193–208. doi: 10.3390/clockssleep1010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mure LS, Cornut PL, Rieux C, Drouyer E, Denis P, Gronfier C, Cooper HM. Melanopsin bistability: a fly’s eye technology in the human retina. PLoS One. 2009;4(6):e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–168. [PubMed] [Google Scholar]
- 15.Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37(2):271–281. doi: 10.5665/sleep.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88(9):4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 17.Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90(3):1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 18.Prayag AS, Najjar RP, Gronfier C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J Pineal Res. 2019;66(4):e12562. doi: 10.1111/jpi.12562. [DOI] [PubMed] [Google Scholar]
- 19.Herljevic M, Middleton B, Thapan K, Skene DJ. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40(3):237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythm. 2009;24(1):73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- 21.Daneault V, Hebert M, Albouy G, Doyon J, Dumont M, Carrier J, et al. Aging reduces the stimulating effect of blue light on cognitive brain functions. Sleep. 2014;37(1):85–96. doi: 10.5665/sleep.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chellappa SL, Steiner R, Oelhafen P, Lang D, Gotz T, Krebs J, et al. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22(5):573–580. doi: 10.1111/jsr.12050. [DOI] [PubMed] [Google Scholar]
- 23.Munch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Phys Regul Integr Comp Phys. 2006;290(5):R1421–R1428. doi: 10.1152/ajpregu.00478.2005. [DOI] [PubMed] [Google Scholar]
- 24.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cajochen C, Reichert CF, Maire M, Schlangen LJM, Schmidt C, Viola AU, Gabel V. Evidence that homeostatic sleep regulation depends on ambient lighting conditions during wakefulness. Clocks Sleep. 2019;1:517–531. doi: 10.3390/clockssleep1040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munch M, Scheuermaier KD, Zhang R, Dunne SP, Guzik AM, Silva EJ, et al. Effects on subjective and objective alertness and sleep in response to evening light exposure in older subjects. Behav Brain Res. 2011;224(2):272–278. doi: 10.1016/j.bbr.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benloucif S, Green K, L'Hermite-Baleriaux M, Weintraub S, Wolfe LF, Zee PC. Responsiveness of the aging circadian clock to light. Neurobiol Aging. 2006;27(12):1870–1879. doi: 10.1016/j.neurobiolaging.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daneault V, Dumont M, Masse E, Forcier P, Bore A, Lina JM, et al. Plasticity in the sensitivity to light in aging: decreased non-visual impact of light on cognitive brain activity in older individuals but no impact of lens replacement. Front Physiol. 2018;9:1557. doi: 10.3389/fphys.2018.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najjar RP, Chiquet C, Teikari P, Cornut PL, Claustrat B, Denis P, Cooper HM, Gronfier C. Aging of non-visual spectral sensitivity to light in humans: compensatory mechanisms? PLoS One. 2014;9(1):e85837. doi: 10.1371/journal.pone.0085837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lok R, Smolders K, Beersma DGM, de Kort YAW. Light, Alertness, and alerting effects of white light: a literature overview. J Biol Rhythm. 2018;33(6):589–601. doi: 10.1177/0748730418796443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skene DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006;43(Pt 5):344–353. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 32.Aeschbach D, Borbely AA. All-night dynamics of the human sleep EEG. J Sleep Res. 1993;2(2):70–81. doi: 10.1111/j.1365-2869.1993.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 33.Chellappa SL, Ly JQ, Meyer C, Balteau E, Degueldre C, Luxen A, et al. Photic memory for executive brain responses. Proc Natl Acad Sci U S A. 2014;111(16):6087–6091. doi: 10.1073/pnas.1320005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cajochen C, Frey S, Anders D, Spati J, Bues M, Pross A, et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol. 2011;110(5):1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- 35.Munch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A, Cajochen C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Phys Regul Integr Comp Phys. 2006;290(5):26. doi: 10.1152/ajpregu.00478.2005. [DOI] [PubMed] [Google Scholar]
- 36.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Gotz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS One. 2011;6(1):e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 38.Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004;80(5):695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Weber JM, Schwander JC, Unger I, Meier D. A direct ultrasensitive RIA for the determination of melatonin in human saliva: comparison with serum levels. J Sleep Res. 1997;26:757. [Google Scholar]
- 40.Gillberg M, Kecklund G, Akerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17(3):236–241. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 41.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda: US Dept of Health, Education and Welfare, Public Health Service; 1968. [Google Scholar]
- 42.Gabel V, Reichert CF, Maire M, Schmidt C, Schlangen LJM, Kolodyazhniy V, Garbazza C, Cajochen C, Viola AU. Differential impact in young and older individuals of blue-enriched white light on circadian physiology and alertness during sustained wakefulness. Sci Rep. 2017;7(1):7620. doi: 10.1038/s41598-017-07060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk DJ. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci U S A. 2016;113(19):E2730–E2739. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Z, Chang-Quan H, Zhen-Chan L, Bi-Rong D. Association between sleep quality and body mass index among Chinese nonagenarians/centenarians. Age (Dordr) 2012;34(3):527–537. doi: 10.1007/s11357-011-9251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheuermaier KD, Lee JH, Duffy JF. Phase shifts to a moderate intensity light exposure in older adults: a preliminary report. J Biol Rhythm. 2019;34(1):98–104. doi: 10.1177/0748730418818655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SJ, Benloucif S, Reid KJ, Weintraub S, Kennedy N, Wolfe LF, Zee PC. Phase-shifting response to light in older adults. J Physiol. 2014;592(1):189–202. doi: 10.1113/jphysiol.2013.262899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hopkins S, Morgan PL, Schlangen LJM, Williams P, Skene DJ, Middleton B. Blue-enriched lighting for older people living in care homes: effect on activity, actigraphic sleep, mood and alertness. Curr Alzheimer Res. 2017;14(10):1053–1062. doi: 10.2174/1567205014666170608091119. [DOI] [PubMed] [Google Scholar]
- 48.Scheuermaier K, Munch M, Ronda JM, Duffy JF. Improved cognitive morning performance in healthy older adults following blue-enriched light exposure on the previous evening. Behav Brain Res. 2018;348:267–275. doi: 10.1016/j.bbr.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eyler LT, Sherzai A, Kaup AR, Jeste DV. A review of functional brain imaging correlates of successful cognitive aging. Biol Psychiatry. 2011;70(2):115–122. doi: 10.1016/j.biopsych.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20(2):600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner PL, Mainster MA. Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92(11):1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chellappa SL, Bromundt V, Frey S, Steinemann A, Schmidt C, Schlote T, Goldblum D, Cajochen C. Association of intraocular cataract lens replacement with circadian rhythms, cognitive function, and sleep in older adults. JAMA Ophthalmol. 2019;137:878. doi: 10.1001/jamaophthalmol.2019.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinemann A, Bromundt V, Chellappa SL, Frey S, Schmidt C, Schlote T, Goldblum D, Cajochen C. Evaluation of visual comfort and mental effort under different light conditions for ultraviolet-absorbing and additional blue-filtering intraocular lenses for cataract surgery. Klin Monatsbl Augenheilkd. 2019;236(4):398–404. doi: 10.1055/a-0810-0302. [DOI] [PubMed] [Google Scholar]
- 54.Chellappa SL, Bromundt V, Frey S, Schlote T, Goldblum D, Cajochen C, et al. Intraocular cataract lens replacement and light exposure potentially impact procedural learning in older adults. J Sleep Res. 2020;1:e13043. doi: 10.1111/jsr.13043. [DOI] [PubMed] [Google Scholar]
- 55.Brondsted AE, Sander B, Haargaard B, Lund-Andersen H, Jennum P, Gammeltoft S, et al. The effect of cataract surgery on circadian photoentrainment: a randomized trial of blue-blocking versus neutral intraocular lenses. Ophthalmology. 2015;122(10):2115–2124. doi: 10.1016/j.ophtha.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 56.Esquiva G, Lax P, Perez-Santonja JJ, Garcia-Fernandez JM, Cuenca N. Loss of melanopsin-expressing ganglion cell subtypes and dendritic degeneration in the aging human retina. Front Aging Neurosci. 2017;9:79. doi: 10.3389/fnagi.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mishima K, Okawa M, Shimizu T, Hishikawa Y. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. J Clin Endocrinol Metab. 2001;86(1):129–134. doi: 10.1210/jcem.86.1.7097. [DOI] [PubMed] [Google Scholar]
- 58.Figueiro MG, Bierman A, Bullough JD, Rea MS. A personal light-treatment device for improving sleep quality in the elderly: dynamics of nocturnal melatonin suppression at two exposure levels. Chronobiol Int. 2009;26(4):726–739. doi: 10.1080/07420520902927809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chellappa SL, Steiner R, Oelhafen P, Cajochen C. Sex differences in light sensitivity impact on brightness perception, vigilant attention and sleep in humans. Sci Rep. 2017;7(1):14215. doi: 10.1038/s41598-017-13973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All deidentified data and related documents (study protocol, statistical analysis plan, informed consent form) will be available by the authors with publication to researchers whose proposed use of the data have been approved. Data will be made available to such researchers for any purpose following approval of a proposal with signed data access agreement.