Abstract
CCL22, which could induce chondrocyte apoptosis, was identified to be overexpressed in damaged cartilage. This study was conducted with the aim of investigating the effects of CCL22 interference on chondrocyte injury. The osteoarthritis model was established by stimulating chondrocytes with LPS. The expressions of CCL22, CCR4, matrix metallopeptidase (MMP) 3, MMP9, MMP13, (a disintegrin and metalloproteinase with thrombospondin-like motifs) ADAMTS-4, collagen II and inflammatory cytokines were measured using quantitative reverse transcription PCR (RT-qPCR) and western blot. Besides, immunoprecipitation (IP) was employed to verify the binding of CCL22 and CCR4. After CCR4 was overexpressed, cell viability was observed using Cell Counting Kit-8 (CCK-8). Additionally, cell apoptosis as well as its related proteins was detected by TUNEL and western blot, respectively. ng What’s more, glycosaminoglycan (GAG) level was detected using GAG kits. CCL22 and CCR4 expression increased noticeably in LPS-stimulated ATDC5 chondrocytes. CCL22 inhibition could suppress the expression of CCR4 in LPS-induced ATDC5 cells. Likewise, CCL22 inhibition could revive the activation of LPS-induced ATDC5 cells by regulating CCR4. In addition, CCL22 knockdown alleviated inflammatory response and cell apoptosis through CCR4. Furthermore, the cartilage degradation of ADTC5 cells could be relieved by CCL22 silence via regulating CCR4. CCL22/CCR4 expression was increased in osteoarthritic cartilage injury and participated in the inflammation and cartilage degradation of chondrocytes.
Keywords: Osteoarthritis, CCL22, CCR4, Cartilage injury, Cartilage degradation
Introduction
Osteoarthritis (OA) is a joint disease involving degradative cartilage, sclerotic bone and long-term joint inflammation, which is commonly diagnosed in patients over 60 years old or with a history of joint injury (Sacitharan 2019). It is clinically manifested as slow development of joint pain, joint swelling, tenderness, stiffness, functional limitation and joint deformity (Michael et al. 2010). The risk factors, apart from age and injury, include obesity, joint wear and tear, congenital anomalies and so on (Gofton 1971; Hawker 2019). Weight-bearing joints and joints with more activities such as cervical vertebra, lumbar spine, knee joint and hip joint are prone to OA (Goode et al. 2013; Hafer et al. 2019). The global society is now facing a rise in OA incidence and prevalence, possibly due to increased obesity rate (Leyland et al. 2016; Nelson 2018). OA can result in different degrees of disability and seriously bring down the quality of life. Currently, weight loss, assistant crutch, anti-inflammatory drugs and analgesics are clinically adopted to alleviate the symptoms or delay the progression of OA (Bechert 2019; Ghouri and Conaghan 2019). In advanced stage, artificial joint replacement may be an option but is only optimal when the patient can well tolerate it, which, however, can also lead to a series of complications such as periprosthetic infection, deep vein thrombosis and secondary injury (Otto-Lambertz et al. 2017; Shao et al. 2019). Therefore, a more effective, more precise and less harmful therapeutic method is in urgent need for OA treatment.
Chemokines, first discovered around 40 years ago, are a class of small cytokines or signaling proteins secreted by cells (Legler and Thelen 2016). It was reported that chemokines have the ability to induce directed chemotactic migration of nearby reactive cells (Hughes and Nibbs 2018). They have also been identified as mediators of inflammation to respond infection and to control the function of innate immunity (Luster 1998; Sokol and Luster 2015). Macrophage-derived chemokine (MDC/CCL22), a member of chemokine family, has been recently reported to be highly expressed on the site of injured cartilage, inducing the apoptosis of chondrocytes (Ren et al. 2019). C–C chemokine receptor 4 (CCR4), a receptor of CCL22, is regulated by CCL22 and plays a crucial role in inflammatory response and autoimmunity (Scheu et al. 2017). It is suggested that the CCL22/CCR4 axis could be closely associated with the onset of cartilage degradation in OA. Nevertheless, whether CCL22 silence could actually affect the inflammatory injury of chondrocytes and the cartilage degradation in OA remains to be explored. This study was conducted with the aim of investigating the potential of CCL22 as a therapeutic target.
Materials and methods
Cell culture and treatment
ATDC5 mouse chondrocytes were sourced from BioVector National Type Culture Collection (Beijing, China) and cultured in DMEM (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT, USA) in an atmospheric condition with 95% air and 5% carbon dioxide (CO2) at 37 °C. The osteoarthritis model was established by stimulating ATDC cells with lipopolysaccharide (LPS) in a concentration of 1, 5 or 10 μg/ml for 12 h.
Cell transfection
Smalling interfering RNA plasmids targeting CCL22 (siRNA-CCL22-1 and siRNA-CCL22-1), the pcDNA 3.1( +)/CCR4 plasmid (ov-CCR4) and their corresponding negative controls (siRNA-NC and ov-NC) were constructed by GenePharma (Shanghai, China). Cell transfection was performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s protocol.
Cell counting kit-8 (CCK-8) assay
CCK-8 (APExBIO, Houston, TX, USA) was used to detect cell viability in accordance with the product manual. After treatment or transfection in different groups, the cells were seeded into a 96-well plate and incubated overnight at 37 °C. Subsequently, the cells were incubated for 4 h with 10 μl of CCK-8 solution added into each well. The optical density was measured at 450 nm wavelength by a microplate reader.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA extraction was performed by TRIzol LS Reagent (Glpbio Technology, Shanghai, China). Through reverse transcription, the synthesization of extracted RNA with cDNA was conducted with Hifair® III 1st Strand cDNA Synthesis SuperMix for qPCR (Yeasen, Shanghai, China). Next, One-step RT-qPCR (SYBR Green) kit (Lmai-Bio, Shanghai, China) was utilized to perform qPCR on the ProFlex PCR system (Applied Biosystems, Foster City, CA, USA). The amplification procedures followed the manufacturer’s recommendations. 2−ΔΔCt method was applied for the calculation of relative mRNA expression fold change.
Western blot (WB)
Total protein was isolated by lysing the cells on ice with RIPA lysis buffer (Beyotime, Nanjing, China). Protein concentration was determined by a BCA kit (Beyotime, Nanjing, China). Next, the protein samples were bathed in boiling water for 5 min for denaturation, electrophoresed with SDS-PAGE Electrophoresis Buffer (Beyotime, Nanjing, China) for protein separation and transferred onto a PVDF membrane. Subsequently, the membrane was blocked by QuickBlock™ Blocking Buffer for Western Blot (Beyotime, Nanjing, China) and incubated with primary antibodies overnight at 4 °C followed by secondary antibody for 2 h at room temperature. Lastly, protein bands were observed with the adoption of BeyoECL Star kit (Beyotime, Nanjing, China).
Immunoprecipitation (IP)
Cells were washed with phosphate buffered saline (Macklin, Shanghai, China), lysed in non-denaturing lysis buffer (APPLYGEN Technologies, Shanghai, China) and centrifuged at 4 °C. The supernatant was collected to incubate with diluted anti-CCL22 antibody and anti-CCR4 antibody (Santa Cruz Biotechnology) at 4 °C for 6 h, followed which protein G-coupled Sepharose beads (Sigma-Aldrich, St. Louis, MO, USA) were employed to incubate the supernatant for overnight at 4 °C. At last, the immunoprecipitated proteins were eluted by glycine buffer (Solarbio, Beijing, China) and the immunoprecipitation was checked by western blot.
Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL)
1 × 104 cells were inoculated on the sterile coverslip in a 35 mm petri dish and cultured for 24 h. Apoptosis was induced before the cells reached 80% confluency. After that, the cells were washed once with PBS (pH 7.2; Aladdin, Shanghai, China) and fixed with cold methanol (TCI Chemicals, Shanghai, China) for 10 min. The coverslip was then air-dried at room temperature for 5 min and rehydrated by incubation with PBS for 10 min. After incubation with 50 μl TdT labeling buffer (ThermoFisher, Waltham, MA, USA) for 1 h at 37 °C, the coverslip was washed with PBS and fixed with 4% paraformaldehyde (Aladdin, Shanghai, China).
Detection of glycosaminoglycan (GAG) expression
The expression of GAG in ATDC5 cells was detected with GAG Assay Kit (Jiancheng Bioengineering Institute, Nanjing, China) following the product’s instructions.
Statistical analysis
All experiments were performed independently in triplicate. Data were analyzed on SPSS 20.0 and expressed as the mean ± standard deviation (S.D). Statistical difference between two groups was compared with the unpaired two-tailed student’s t test, while one-way ANOVA was utilized for comparisons among multiple groups. A P value of 0.05 or less stands for statistical significance.
Results
CCL22 and CCR4 expressions were increased in LPS-stimulated ATDC5 cells
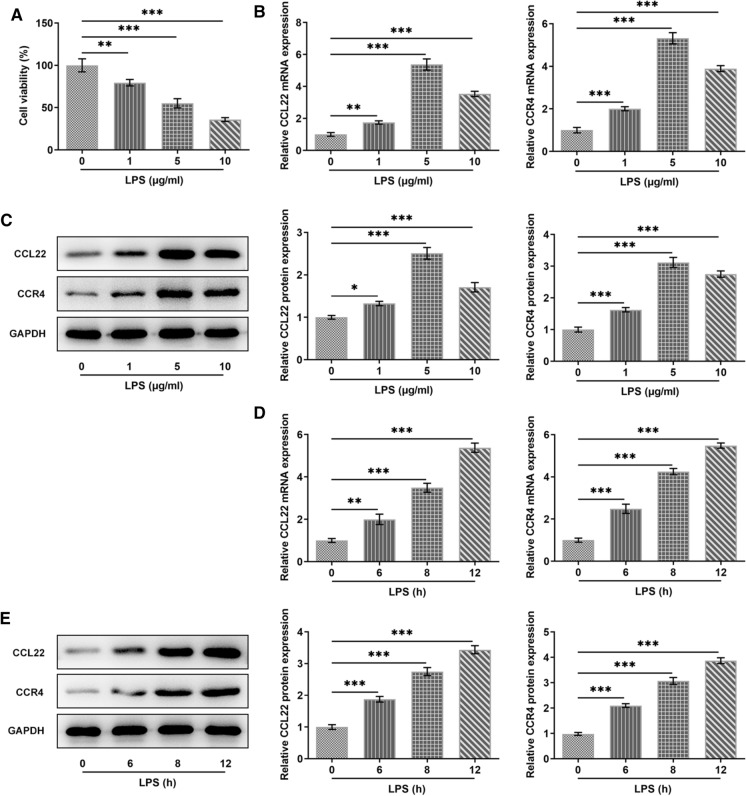
For the establishment of osteoarthritis model, 1, 5 and 10 μg/ml of LPS was respectively chosen to stimulate ATDC5 cells, as previously described (Liu et al. 2019). As Fig. 1a vividly shown, the cell viability was greatly decreased by LPS stimulation. Compared with Control, the relative mRNA expression and protein expression of CCL22 and CCR4 gained a huge growth in LPS-induced ATDC5 cells. Besides, it is noted that LPS with a concentration of 5 μg/ml contributed to the highest expressions of CCL22 and CCR4 in ATDC5 cells (Fig. 1b, c). According to Fig. 1d, e, LPS induction brought about increased CCL22 and CCR4 and its effects on CCL22 and CCR4 were in a time-dependent manner.
Fig. 1.
CCL22 and CCR4 were increased in LPS-stimulated ATDC5 cells. a Cell viability in LPS-stimulated ATDC5 cells was detected by CCK-8. b, c The expressions of CCL22 and CCR4 were measured by RT-qPCR and WB. d–e The expressions of CCL22 and CCR4 were detected by RT-qPCR and WB. *P < 0.05, **P < 0.01, ***P < 0.001
CCL22 knockdown inhibited CCR4 expression in LPS-stimulated ATDC5 cells
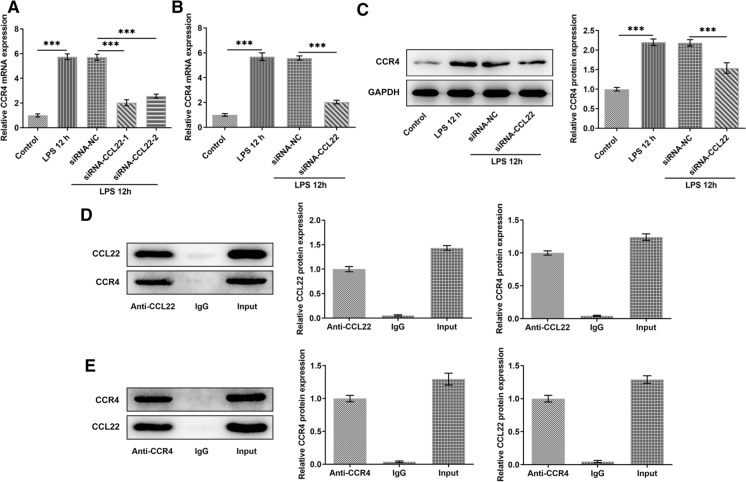
Compared with Control, the expression of CCL22 in ATDC5 cells was greatly upregulated after LPS induction while the increased expression was then suppressed by CCL22 silence (Fig. 2a). SiRNA-CCL22-1 was chosen for subsequently experiments for its better inhibitory effects on CCL22. Furthermore, the increased CCR4 induced by LPS was then decreased by CCL22 silence, revealing that CCL22 silence could reversed the effects of LPS induction on ATDC5 cells (Fig. 2b, c). Results obtained from IP assay indicated that CCL22 existed in Anti-CCR4 and CCR4 existed in anti-CCL22, revealing that CCL22 could be bound to CCR4 (Fig. 2d, e).
Fig. 2.
CCL22 knockdown inhibited CCR4 expression in LPS-stimulated ATDC5 cells. a CCL22 expression in LPS-stimulated ATDC5 cells was detected by RT-qPCR. b–c CCR4 expression in LPS-stimulated ATDC5 cells was detected by RT-qPCR and Western blotting. d The binding of CCL22 and CCR4 was detected by IP assay. ***P < 0.001
CCL22 knockdown revived the viability of LPS-stimulated ATDC5 cells via CCR4
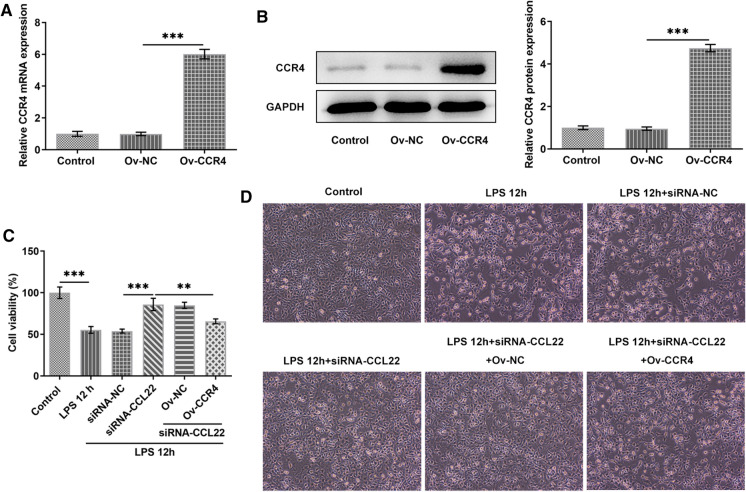
This part explored the potential effect of the interaction between CCL22 and CCR4 on the viability of LPS-stimulated ATDC5 cells. Evidently, CCR4 expression was significantly increased after transfection with CCR4 overexpression plasmids (Fig. 3a, b). CCL2 silence increased the viability of LPS-stimulated ATDC5 cells, which was, however, brought down in the presence of CCR4 overexpression (Fig. 3c). Besides, Fig. 3d displayed the morphology of ATDC5 cells. In a word, the above results indicated that CCL22 knockdown revived the viability of LPS-stimulated ATDC5 cells by regulating CCR4 expression.
Fig. 3.
CCL22 knockdown revived the viability of LPS-stimulated ATDC5 cells via CCR4. a–b Relative mRNA and protein expressions of CCR4 were detected by RT-qPCR and Western blotting, respectively. c Cell viability was detected by CCK-8. d The morphology of chondrocytes was observed. ***P < 0.001
CCL22 knockdown suppressed LPS-induced proinflammatory cytokine secretion in ATDC5 cells via CCR4
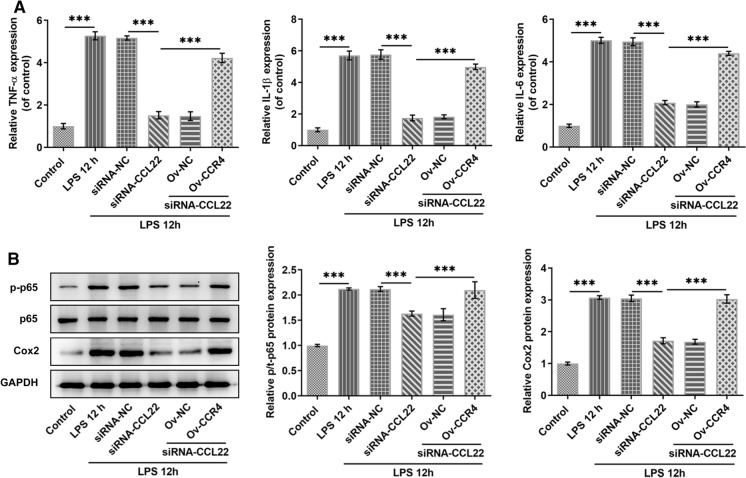
This segment of our study examined the potential effect of the interaction between CCL22 and CCR4 on LPS-induced inflammatory injury of ATDC5 cells. The proinflammatory cytokines including TNF-α, IL-1β, IL-6 and MCP-1 all exhibited higher mRNA expression in LPS-stimulated ATDC5 cells, which was then inhibited by CCL22 knockdown, whereas CCR4 overexpression rescued their expressions (Fig. 4a). Additionally, the promoted protein expressions of proinflammatory p-p65 and COX2 caused by LPS stimulation were then reduced by CCL22 knockdown, while it was elevated again in the presence of CCR4 overexpression (Fig. 4b). These results suggested that CCL22 knockdown suppressed LPS-induced inflammatory cytokine secretion in ATDC5 cells via CCR4.
Fig. 4.
CCL22 knockdown suppressed LPS-induced proinflammatory cytokine secretion in ATDC5 cells via CCR4. a The mRNA expressions of proinflammatory cytokines TNF-α, IL-1β, IL-6 and MCP-1 in LPS-stimulated ATDC5 cells were detected by RT-qPCR. b The protein expressions of proinflammatory p-p65, p65 and COX2 were detected by WB. ***P < 0.001
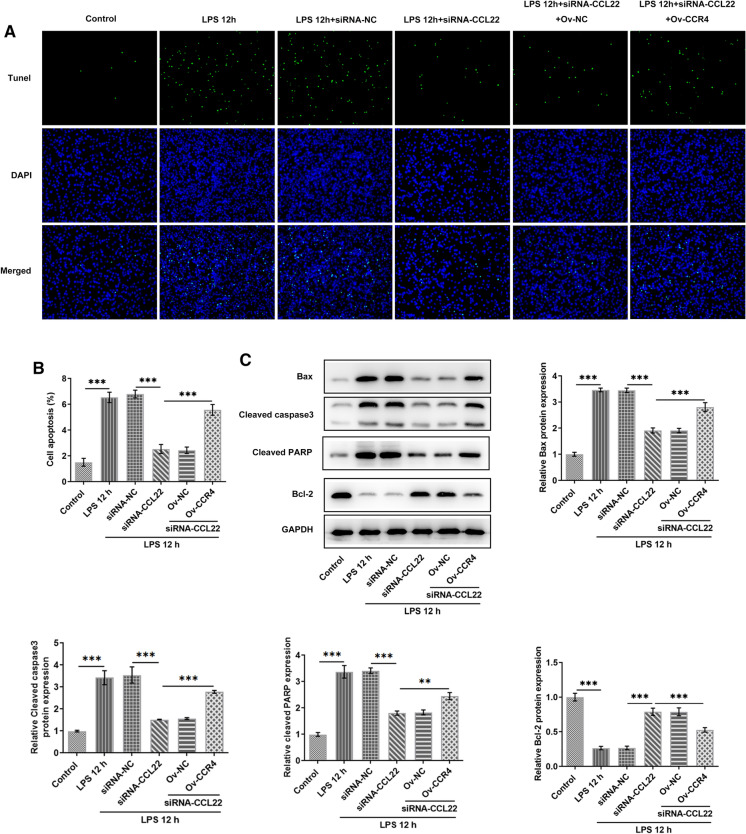
CCL22 knockdown ameliorated LPS-induced apoptosis of ATDC5 cells via CCR4
This part of our study explored the potential effect of the interaction between CCL22 and CCR4 on LPS-induced apoptosis of ATDC5 cells. It was found that CCL22 silence largely decreased the level of LPS-induced cell apoptosis, which was subsequently reversed by CCR4 overexpression (Fig. 5a, b). Meanwhile, decreased expressions of pro-apoptotic Bax, cleaved caspase3 and cleaved PARP as well as increased expression of anti-apoptotic Bcl2 were observed in LPS-stimulated ATDC5 cells after CCL22 knockdown, whereas CCR4 overexpression reversed these changes (Fig. 5c). These results suggested that CCL22 knockdown ameliorated LPS-induced apoptosis of ATDC5 cells through the regulation of CCR4 expression.
Fig. 5.
CCL22 knockdown ameliorated LPS-induced apoptosis of ATDC5 cells via CCR4. a–b Cell apoptosis was detected by TUNEL. c The protein expressions of Bax, cleaved caspase3, cleaved PARP and Bcl2 in LPS-stimulated ATDC5 cells were detected by WB. ***P < 0.001
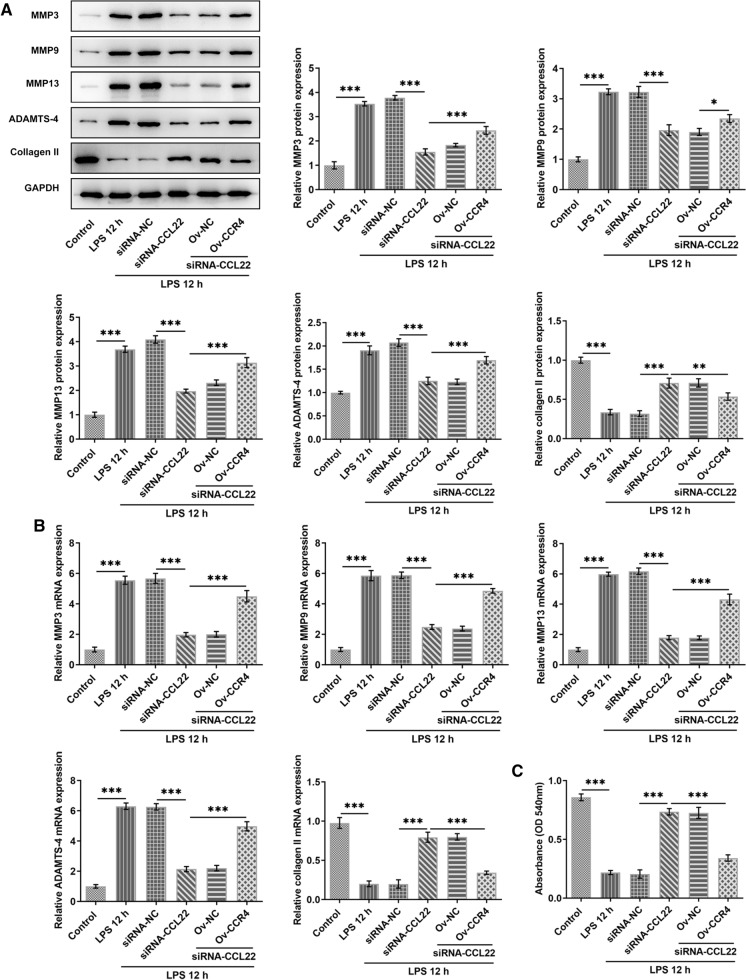
CCL22 knockdown attenuated LPS-induced cartilage degradation of ATDC5 cells via CCR4
This part of our study investigated the potential effect of the interaction between CCL22 and CCR4 on LPS-induced cartilage degradation of ATDC5 cells. Compared with Control, cartilage degradation-associated enzymes MMP3, MMP9, MMP13 and ADAMTS-4 were found to be highly expressed in LPS-stimulated ATDC5 cells, while collagen II expression was decreased (Fig. 6a, b). However, CCL22 silence effectively decreased the expression of MMP3, MMP9, MMP13 and ADAMTS-4 as well as increased that of collagen II, which were then reversed by CCR4 overexpression. Similarly, a significantly decrease in GAG expression was detected in LPS-stimulated ATDC5 cells, which was then elevated by CCL22 knockdown. Nevertheless, CCR4 overexpression downregulated the upregulated GAG expression in comparison with LPS 12 h + siRNA-CCL22 + Ov-NC (Fig. 6c). These results collectively identified that CCL22 knockdown attenuated LPS-induced cartilage degradation of ATDC5 cells via CCR4.
Fig. 6.
CCL22 knockdown attenuated LPS-induced cartilage degradation of ATDC5 cells via CCR4. a–b The protein levels and mRNA expressions of MMP3, MMP9, MMP13, ADAMTS-4 and collagen II in LPS-stimulated ATDC5 cells were detected by Western blotting and RT-qPCR, respectively. c GAG expression in LPS-stimulated ATDC5 cells was detected by a GAG assay kit. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
Osteoarthritis (OA) is the most common joint disease mainly characterized by articular cartilage injury that eventually develops into articular cartilage degeneration, fibrosis, fracture, defect and even damage to the entire articular surface (Buckwalter et al. 2005). Clinical manifestations of OA include joint pain, stiffness, hypertrophy, limited movement and so on. Weight-bearing joints are most likely to be affected by OA, such as knees, hips, cervical vertebrae and lumbar vertebrae, as well as distal interphalangeal joints, proximal interphalangeal joints, first carpometacarpal joints and first metatarsophalangeal joints (Calce et al. 2018). The etiology of OA consists of not a single factor but the combination of many and is generally believed to be related to heredity, age, obesity and joint trauma (Johnson and Hunter 2014). OA patients are mostly middle-aged and senior people, making the disease a main reason for disability for the elderly (Kan et al. 2019). Currently, both pharmacological modalities and surgical therapy in OA treatment are of limited efficacy and inevitably accompanied by serious adverse effects (Taruc-Uy and Lynch 2013). At present, there is no effective approach to full recovery, hence it is of great necessity to identify novel therapeutic targets. Chemokines are cytokines that guide chemotactic migration of immune cells to specific sites. Chemokine (C–C motif) ligand 22 (CCL22), which was mainly expressed on the surface of regulatory T cells to mediate their chemotaxis, is one of the main ligands for chemokine (C–C motif) receptor 4 (CCR4) (Schebesch et al. 1997). CCL22 has been found to be positively related with joint injury pain level and structural change in rat OA model (Ren et al. 2019). Furthermore, CCL22 and its cognate receptor CCR4, which were both highly expressed in the serum of patients with joint replacement, are important participants in osteoclastogenesis (Cadosch et al. 2010). Thus, CCL22 is considered as a possible biomarker and therapeutic target of OA.
The viability of chondrocytes reflects cartilage health as this singular cell type of articular cartilage forms the weight-bearing surface of joints and contributes to cartilage function (Jiang and Tuan 2015). With the development of degenerative joint diseases such as OA, chondrocytes undergo multiple unfavorable alterations in terms of proliferation, viability and so on (Charlier et al. 2019). In our study, LPS stimulation led to conspicuously weakened viability of ATDC5 chondrocytes. Meanwhile, increased expressions of both CCL22 and CCR4 were observed in LPS-stimulated ATDC5 cells, suggesting that they might be involved in chondrocyte progression. After confirmation of the binding relationship between CCL22 and CCR4, the viability of ATDC5 cells was found to be increased by CCL22 knockdown and comparatively decreased in the presence of CCR4 overexpression, implying that CCL22 knockdown may improve chondrocyte viability in OA through CCR4. Furthermore, CCL22/CCR4 axis is activated under inflammatory condition, which can be attributed to its role as a mediator in immune cell trafficking in response to inflammation (Piseddu et al. 2020). For example, increased production of CCL22 has been shown to contribute to pulmonary inflammation, and CCR4 blockade reduced the inflammation level in cellular asthma model (Perros et al. 2009; Beckmann et al. 2020). The results in our study were consistent with these findings in that CCL22 knockdown effectively suppressed the secretion of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, MCP-1, p-p65 and COX2 in LPS-stimulated ATDC5 cells, whereas CCR4 overexpression elevated their expressions. This suggested that CCL22 knockdown may have the potential to inhibit cartilage inflammation in OA through CCR4. Additionally, it is acknowledged that chondrocyte apoptosis performs an essential role in the pathogenesis of OA (Hwang and Kim 2015; Musumeci et al. 2015), therefore, we examined the apoptosis of LPS-stimulated ATDC5 cells and observed significant alleviation after CCL22 knockdown. However, such effect was abated by CCR4 overexpression, indicating that CCL22 knockdown may alleviate chondrocyte apoptosis in OA through CCR4.
Degrading cartilage is a major characteristic of OA, which is presented as extracellular matrix (ECM) breakdown, resulting in the loss of structural support (Gentili and Cancedda 2009). MMP3, MMP9 and MMP13 are matrix metalloproteinases that act as catabolic factors in cartilage extracellular matrix degeneration (Li et al. 2018; Choi et al. 2019). A disintegrin and metalloproteinase with thrombospondin motifs type 4 (ADAMTS-4) is a key enzyme for aggrecan degradation in OA-associated cartilage destruction (Verma and Dalal 2011). Our results revealed that the aberrantly high expression of MMP3, MMP9, MMP13 and ADAMTS-4 in LPS-stimulated ATDC5 cells could be downregulated by CCL22 knockdown, while CCR4 overexpression reversed this effect. Meanwhile, type II collagen (collagen II), the main molecular component of articular cartilage (Luo et al. 2017), showed lower expression in LPS-stimulated ATDC5 cells. The upregulated collagen II caused by CCL22 knockdown was then downregulated by CCR4 overexpression. Glycosaminoglycan (GAG) is a ground substance of ECM that shows significance in the repair of early cartilage injury (Barco et al. 2018). In our study, we observed declined GAG expression in ATDC5 cells after LPS stimulation, whereas it was subsequently increased by CCL22 knockdown and brought down again by CCR4 overexpression. Taken together, these results indicated that CCL22 knockdown may ameliorate cartilage degradation in OA through CCR4.
Conclusion
It can be concluded from the above results that CCL22 participates in chondrocyte inflammation and cartilage degradation in OA via its receptor CCR4. This study illustrates the theoretical possibility of the use of CCL22 and CCR4 as both biomarkers for OA early diagnosis and targets for drug therapy as well as other clinical interventions in OA treatment.
Author contributions
All authors contributed equally to this study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The author declares that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barco A, Ingham E, Fisher J, Fermor H, Davies RPW. On the design and efficacy assessment of self-assembling peptide-based hydrogel-glycosaminoglycan mixtures for potential repair of early stage cartilage degeneration. J Pept Sci. 2018;24(8–9):e3114. doi: 10.1002/psc.3114. [DOI] [PubMed] [Google Scholar]
- Bechert RE. Treatment of posttraumatic osteoarthritis secondary to a chronic plafond fracture: a case report. J Chiropr Med. 2019;18(3):219–224. doi: 10.1016/j.jcm.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Sutton JM, Hoehn RS, Jernigan PL, Friend LA, Johanningman TA, et al. IFNgamma and TNFalpha mediate CCL22/MDC production in alveolar macrophages after hemorrhage and resuscitation. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L864–L872. doi: 10.1152/ajplung.00455.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- Cadosch D, Gautschi OP, Chan E, Simmen HP, Filgueira L. Titanium induced production of chemokines CCL17/TARC and CCL22/MDC in human osteoclasts and osteoblasts. J Biomed Mater Res A. 2010;92(2):475–483. doi: 10.1002/jbm.a.32390. [DOI] [PubMed] [Google Scholar]
- Calce SE, Kurki HK, Weston DA, Gould L. The relationship of age, activity, and body size on osteoarthritis in weight-bearing skeletal regions. Int J Paleopathol. 2018;22:45–53. doi: 10.1016/j.ijpp.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, et al. Chondrocyte dedifferentiation and osteoarthritis (OA) Biochem Pharmacol. 2019;165:49–65. doi: 10.1016/j.bcp.2019.02.036. [DOI] [PubMed] [Google Scholar]
- Choi MC, Jo J, Park J, Kang HK, Park Y (2019) NF-kappaB signaling pathways in osteoarthritic cartilage destruction. Cells 8 [DOI] [PMC free article] [PubMed]
- Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15(12):1334–1348. doi: 10.2174/138161209787846739. [DOI] [PubMed] [Google Scholar]
- Ghouri A, Conaghan PG. Treating osteoarthritis pain: recent approaches using pharmacological therapies. Clin Exp Rheumatol. Suppl. 2019;120(5):124–29. [PubMed] [Google Scholar]
- Gofton JP. Studies in osteoarthritis of the hip. 3. Congenital subluxation and osteoarthritis of the hip. Can Med AssocJ. 1971;104(10):911–915. [PMC free article] [PubMed] [Google Scholar]
- Goode AP, Carey TS, Jordan JM. Low back pain and lumbar spine osteoarthritis: how are they related? Curr Rheumatol Rep. 2013;15(2):305. doi: 10.1007/s11926-012-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafer JF, Kent JA, Boyer KA. Physical activity and age-related biomechanical risk factors for knee osteoarthritis. Gait Posture. 2019;70:24–29. doi: 10.1016/j.gaitpost.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol. Suppl. 2019;120(5):3–6. [PubMed] [Google Scholar]
- Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944–2971. doi: 10.1111/febs.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11(4):206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Kan HS, Chan PK, Chiu KY, Yan CH, Yeung SS, Ng YL, et al. Non-surgical treatment of knee osteoarthritis. Hong Kong Med J. 2019;25(2):127–133. doi: 10.12809/hkmj187600. [DOI] [PubMed] [Google Scholar]
- Legler DF, Thelen M. Chemokines: chemistry biochemistry and biological function. Chimia (aarau) 2016;70(12):856–859. doi: 10.2533/chimia.2016.856. [DOI] [PubMed] [Google Scholar]
- Leyland KM, Judge A, Javaid MK, Diez-Perez A, Carr A, Cooper C, et al. Obesity and the relative risk of knee replacement surgery in patients with knee osteoarthritis: a prospective cohort study. Arthritis Rheumatol. 2016;68(4):817–825. doi: 10.1002/art.39486. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao M, Xiao W. KLF15 regulates the expression of MMP-3 in human chondrocytes. J Interferon Cytokine Res. 2018;38(8):356–362. doi: 10.1089/jir.2017.0135. [DOI] [PubMed] [Google Scholar]
- Liu G, Wang Y, Zhang M, Zhang Q. Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells. Int Immunopharmacol. 2019;66:354–361. doi: 10.1016/j.intimp.2018.11.038. [DOI] [PubMed] [Google Scholar]
- Luo Y, Sinkeviciute D, He Y, Karsdal M, Henrotin Y, Mobasheri A, et al. The minor collagens in articular cartilage. Protein Cell. 2017;8(8):560–572. doi: 10.1007/s13238-017-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338(7):436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Michael JW, Schluter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci G, Castrogiovanni P, Trovato FM, Weinberg AM, Al-Wasiyah MK, Alqahtani MH, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16(9):20560–20575. doi: 10.3390/ijms160920560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthr Cartilage. 2018;26(3):319–325. doi: 10.1016/j.joca.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto-Lambertz C, Yagdiran A, Wallscheid F, Eysel P, Jung N. Periprosthetic Infection in Joint Replacement. Dtsch Arztebl Int. 2017;114(20):347–353. doi: 10.3238/arztebl.2017.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64(7):995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- Piseddu I, Rohrle N, Knott MML, Moder S, Eiber S, Schnell K, et al. Constitutive expression of CCL22 is mediated by T cell-derived GM-CSF. J Immunol. 2020;205(8):2056–2065. doi: 10.4049/jimmunol.2000004. [DOI] [PubMed] [Google Scholar]
- Ren G, Whittaker JL, Leonard C, De Rantere D, Pang DSJ, Salo P, et al. CCL22 is a biomarker of cartilage injury and plays a functional role in chondrocyte apoptosis. Cytokine. 2019;115:32–44. doi: 10.1016/j.cyto.2018.11.030. [DOI] [PubMed] [Google Scholar]
- Sacitharan PK. Ageing and osteoarthritis. Subcell Biochem. 2019;91:123–159. doi: 10.1007/978-981-13-3681-2_6. [DOI] [PubMed] [Google Scholar]
- Schebesch C, Kodelja V, Muller C, Hakij N, Bisson S, Orfanos CE, et al. Alternatively activated macrophages actively inhibit proliferation of peripheral blood lymphocytes and CD4+ T cells in vitro. Immunology. 1997;92(4):478–486. doi: 10.1046/j.1365-2567.1997.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu S, Ali S, Ruland C, Arolt V, Alferink J (2017) The C-C chemokines CCL17 and CCL22 and their receptor CCR4 in CNS autoimmunity. Int J Mol Sci. 18 [DOI] [PMC free article] [PubMed]
- Shao JB, Ni CF, Duan PF, Jin YH. Preventive effects of different drugs on asymptomatic lower extremities deep venous thrombosis after artificial joint replacement: a mixed treatment comparison. Am J Ther. 2019;26(1):e45–e53. doi: 10.1097/MJT.0000000000000438. [DOI] [PubMed] [Google Scholar]
- Sokol CL, Luster AD (2015) The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 7 [DOI] [PMC free article] [PubMed]
- Taruc-Uy RL, Lynch SA. Diagnosis and treatment of osteoarthritis. Prim Care. 2013;40(4):821–36. doi: 10.1016/j.pop.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112(12):3507–3514. doi: 10.1002/jcb.23298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.