Abstract
Small breed dogs have longer lifespans than their large breed counterparts. Previous work demonstrated that primary fibroblast cells isolated from large breed young and old dogs have a persistent glycolytic metabolic profile compared with cells from small breed dogs. Here, we cultured primary fibroblast cells from small and large, young and old dogs and treated these cells with three commercially available drugs that show lifespan and health span benefits, and have been shown to reduce glycolytic rates: rapamycin (rapa), resveratrol (res) and metformin (met). We then measured aerobic and anaerobic cellular respiration in these cells. We found that rapa and res increased rates of non-glycolytic acidification in small and large breed puppies and basal oxygen consumption rates (OCR) in small and large breed puppies. Rapa increased proton leak and non-mitochondrial respiration in small and large breed puppies. Maximal respiration was significantly altered with rapa treatment but in opposing ways: large breed puppies showed a significant increase in maximal respiration when treated with rapa, and small old dogs demonstrated a significant decrease in maximal respiration when treated with rapa. In opposition to rapa treatments, met significantly decreased basal OCR levels in cells from small and large breed puppies. Our data suggest that rapa treatments may be metabolically beneficial to dogs when started early in life and more beneficial in larger breeds.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-021-00349-7.
Keywords: Domestic dog, Lifespan, Rapamycin, Resveratrol, Metformin, Cellular metabolism
Introduction
Dogs present an interesting physiological challenge: smaller dogs tend to live significantly longer than larger dogs across all breeds [18, 28, 38, 46, 55], opposing patterns generally found across mammalian species. Underlying physiological mechanisms for this pattern have only begun to be unraveled. Previously, we have compared cellular oxygen consumption, glycolysis, and oxidative stress in primary dermal fibroblasts cells isolated from puppies and senior dogs of small and large breeds to describe a potential physiological mechanism of the disparity of aging rates in dogs [29]. To our surprise, we found differences between small (usually longer-lived) and large breed (usually shorter-lived) size classes with respect to glycolytic parameters, where larger breed puppies have significantly higher glycolytic capacity compared with smaller breeds. We also found that older dogs, shorter-lived breeds have significantly higher glycolytic phenotypes. Thus, from puppies to seniors, large breed/shorter-lived dogs seem to have a persistent glycolytic phenotype, though we have not yet determined whether an increase in glycolytic rates implies increases in lactate production or glucose oxidation. However, the activity of lactate dehydrogenase, the enzyme that catalyzes the conversion of anaerobically produced lactate to pyruvic acid, increased with age in blood from aging Beagles [36], and urine lactate also increased with age in Labradors [57]. This suggests that increases in glycolysis from our previous study may be leading to an increase in anaerobic metabolism and the formation of lactate. This seemingly chronic glycolytic phenotype may suggest that cells from the larger breeds can be metabolically pre-disposed to a tumor-like phenotype [29, 58].
There are three known commercially available drug interventions that seem to decrease glycolysis and seem to have lifespan and health span benefits in humans and rodents. Those drugs are rapamycin (rapa), resveratrol (res) and metformin (met) [43]. Rapa, an inhibitor of the mTOR pathway (mTORC1), at the whole-organism level, has shown promise in delaying aging effects on rodent model systems [60]. Rapa has also been demonstrated to reduce cancer incidence, along with other positive health span benefits [60]. Res is a polyphenol most notoriously found in grape skin and wine [52], which claims to be a molecule with anti-aging, anti-inflammation and anti-tumor properties [40]. Met is a biguanide used to commonly treat type 2 diabetes in humans [37] and is said to cause minimal lactic acidosis long term [32]. Met exerts its effects by activating AMP-activated protein kinase (AMPK), which is a cellular sensor for homeostasis, glucose and fat metabolism [37]. During cellular stress, ATP/AMP levels increase, which activates AMPK, thus inhibiting anabolic processes and activating catabolic processes in cells to produce energy while inhibiting complex I in the mitochondria [16, 19, 37]. Treatments with met also lead to decreased insulin levels and decreased IGF-1 signaling, a reduction in DNA damage, inflammation, autophagy, and cellular senescence [8]. It should be noted that of these three compounds, rapa has demonstrated the most promising extensions in lifespan in mammals. While res has also been shown to increase lifespan, the treatment effect is dependent on dietary supplementation [21, 39]. Additionally, res has only demonstrated lifespan extension in invertebrate model systems ([43]; Table 1). That these three drugs have reported increases in longevity through alteration of metabolic pathways is of great interest in dog aging, where small and large dog breeds demonstrate not just lifespan differences, but also cellular metabolic differences as discussed above.
Table 1.
Summary of whole-animal results using rapa, res, and met as those related to lifespan extensions and aging delays
| Species | Treatment | Outcome | Reference |
|---|---|---|---|
| Mice (UM-HET3) | rapa | Delay in development of age-related pathologies | [60] |
| Mice (UM-HET3) | rapa | Lifespan extension | [39] |
| Mice (C57BL/6J) | rapa | Lifespan extension | [45] |
| Caenorhabditis elegans | res | Lifespan extension | [61] |
| Saccharomyces cerevisae | res | Lifespan extension | [25] |
| Dosophila melagonaster | res | Lifespan extension | [9] |
| Mice (C57BL/6NIA) | res | Delay in development of age-related pathologies | [47] |
| Mice (outbred SHR) | met | Lifespan extension | [2] |
| Mice (inbred 129/Sv) | met | No lifespan extension in male, slight lifespan extension in females | [3] |
| Mice (female outbred SHR) | met | Lifespan extension when started early in life | [4] |
| Mice (male C57BL/6) | met | Lifespan extension when started in middle age | [37] |
Cellular resistance and aging rates have been tested using primary fibroblasts in eight different species of mammals ranging in body masses from 0.1 to 450 kg [33]. Cellular resistance is a corollary of the free radical theory of aging, where longer-lived, larger mammals should have higher resistance to chemical insults compared with shorter-lived, smaller mammals. Kapahi et al. [33] found that primary fibroblasts cells isolated from large, longer-lived mammals resisted chemical stress better than primary fibroblasts isolated from smaller, shorter-lived mammals suggesting that primary fibroblasts are an excellent model for the whole-animal phenotype. Others have used primary fibroblast from bird models and found that birds with longer lives demonstrated higher cellular resistance as well [23].
Here, we used primary fibroblast cells from small (longer-lived) and large (shorter-lived) dog breeds, of young and old age classes, and exposed them to treatments of rapa, res, and met before measuring aerobic and anaerobic cellular metabolism. We predicted that each of these drugs may decrease glycolysis compared with control values more drastically in large breed dogs compared with small. We also predicted that these drugs may increase levels of cellular aerobic metabolism to compensate for the reduction in glycolytic pathways.
Materials and methods
Isolation of dog primary fibroblasts
We isolated primary fibroblast cells from puppies (N = 121) and senior dogs (N = 27) of two size classes, a total of N = 148 subjects. The small breed size class was composed of breeds with an adult body mass of 15 kg or less (N = 57), and the large breed size class included breeds or mixes with an adult body mass of 20 kg or more (N = 91). These size classes are based on American Kennel club (AKC) standards of each breed, and described in Jimenez [28]. Table 2 provides a thorough summary of the data collected about the dogs in the study, i.e., information about breed, sex, body mass, and reason for euthanasia.
Table 2.
Information about breeds, sample sizes, sex, body mass and euthanasia for dogs included in this study. Mean breed lifespan was obtained from Jimenez [28]. All individuals included in this table were included in control experiments
| Breed | Control sample size | Sex/fixed? | Age (years/days) | Body mass (kg) | Size class | Age class | Reason for euthanasia | Treatment sample size |
|---|---|---|---|---|---|---|---|---|
| Autralian Shepherd | 3 | 2M, 1NM | 8.5, 13, 15.5 | 17, 25, 29 | L | Old | Renal failure/neoplasia/pericardial effusion | 1 met, 3 rapa, 3 res |
| Boxer | 1 | N/A | 13.5 | 29.5 | L | Old | degenerative myopathy | 1 met, 1 rapa, 1 res |
| Boxer/Shepherd | 1 | M | 13 | 27.7 | L | Old | seizures, arthritis, cancer in chest | 1 rapa, 1 res |
| Golden retriever | 1 | M | 11 | 41.7 | L | Old | chronic severe bacterial otitis | 1 met, 1 rapa, 1 res |
| Labrador mix | 2 | M, F | 12, 14 | 32, 20 | L | Old | Vomit, weight loss/severe arthritis | 2 met, 2 rapa, 1 res |
| Labrador retriever | 1 | M | 13 | 34 | L | Old | Presumptive brain tumor | 2 rapa, 1 res |
| Pitbull | 1 | NM | 15 | 35.8 | L | Old | Cancerous mass in abdomen | 1 rapa, 1 res |
| Pitbull mix | 1 | SF | 11 | 27.2 | L | Old | Bad injuries due to dog fight | 1 met, 1 rapa, 1 res |
| Rottweiler | 1 | M | 9 | 46.3 | L | Old | Mast cell tumors | 1 rapa, 1 res |
| Shepherd mix | 1 | N/A | 13 | 27.7 | L | Old | Kidney failure | 1 rapa, 1 res |
| Siberian Husky | 2 | N/A, NM | 11, 6 | 20, 38 | L | Old | Inflammatory breast cancer/Cancer | 1 rapa, 1 res |
| Airdale terrier | 7 | 6M, 1F | 5 days old | 0.34 | L | Puppy | 5 met, 7 rapa, 7 res | |
| Australian cattle dog | 5 | 3F, 2M | 3 days old | 0.47 | L | Puppy | 5 rapa, 5 res | |
| Boxer | 6 | 2F, 4M | 3-4 days old | 0.35 | L | Puppy | 5 met, 6 rapa, 6 res | |
| Bracco Italiano | 1 | M | 5 days old | 0.59 | L | Puppy | 1 met, 1 rapa, 1 res | |
| Cane corso mastiff | 1 | M | 10 weeks | 10.9 | L | Puppy | 1 rapa, 1 res | |
| Doberman | 5 | 4M, 1F | 8-10 weeks old | 6.8 | L | Puppy | 5 met, 5 rapa, 5 res | |
| Doberman mix | 1 | M | 9 weeks | 5.9 | L | Puppy | 1 met, 1 rapa, 1 res | |
| German short-haired pointer | 11 | 7M, 4F | 2 days old | 0.5 | L | Puppy | 5 met, 11 rapa, 11 res | |
| German wire hair pointer | 5 | 1M, 4F | 2 days old | 0.5 | L | Puppy | 3 met, 5 rapa, 5 res | |
| Great dane | 6 | 4M, 2F | 5 days old | 0.8 | L | Puppy | 6 met, 6 rapa, 6 res | |
| Labradoodle | 5 | 4M, 2F | 3 days old | 0.52 | L | Puppy | 4 met, 5 rapa, 5 res | |
| Old english sheepdog | 14 | 8M, 6F | 2-3 days old | 0.35 | L | Puppy | 12 met, 14 rapa, 13 res | |
| Pit bull mix | 1 | M | 8 weeks old | 4.5 | L | Puppy | 1 met, 1 rapa, 1 res | |
| Rottweiler | 5 | 2M, 3F | 2-3 days old | L | Puppy | 3 met, 5 rapa, 5 res | ||
| Standard poodle | 3 | N/A | 4 days old | 0.45 | L | Puppy | 1 met, 3 rapa, 3 res | |
| Basenji | 1 | F | 12.5 | 9.6 | S | Old | Unregulated diabetes, poor thrift | 1 res |
| Bichon Frise | 1 | F | 12 | 7.4 | S | Old | Abdominal cancer | 1 met, 1 rapa, 1 res |
| Border terrier | 1 | F | 15 | 7.4 | S | Old | Suspected lymphoma | 1 res |
| Cocker Spaniel mix | 1 | F | 14 | 7.4 | S | Old | Bladder neoplasia/cell carsinoma | 1 rapa, 1 res |
| Jack Russell Terrier | 1 | F | 14.5 | 4.5 | S | Old | Cognitive loss likely brain lesion | 1 rapa, 1 res |
| Miniature schnauzer | 1 | F | 11 | 8.6 | S | Old | Immune-mediated thromboytopenia | 1 rapa, 1 res |
| Shetland sheepdog | 3 | 3M | 14, 12, 14 | 6.5, 19, 8 | S | Old | Cogestive heart failure/Cushing’s disease/Matastatic anal gland adenocarsinoma | 1 met, 2 rapa, 3 res |
| Shih Tzu | 1 | F | 14 | 10.4 | S | Old | Tumor in SI | 1 rapa, 1 res |
| Terrier mix | 1 | F | 14 | 12.7 | S | Old | Mobility issues | 1 res |
| Yorkshire terrier | 1 | F | 17 | 2.3 | S | Old | Chronic kidney disease | 1 rapa, 1 res |
| Cavalier king charles spaniel | 11 | 6M, 5F | 4 days old | 0.3 | S | Puppy | 6 met, 10 rapa, 11 res | |
| Chihuahua | 4 | 1M, 3F | mins old | 0.1 | S | Puppy | 2 rapa, 4 res | |
| Corgi | 8 | 5N/A, 2M, 1F | mins-6 days old | 0.5 | S | Puppy | 5 met, 8 rapa, 8 res | |
| Havanese | 1 | M | 4 days old | 0.2 | S | Puppy | 1 rapa, 1 res | |
| Pomeranian | 2 | 1M, 1F | 1 hrs old | 0.2 | S | Puppy | 2 res | |
| Soft coated wheaten terrier | 7 | 3M, 4F | 4 days old | 0.4 | S | Puppy | 4 met, 7 rapa, 7 res | |
| Yorkshire terrier | 6 | 6N/A | 3-5 days old | 0.07 | S | Puppy | 3 met, 6 rapa, 6 res | |
| Toy poodle | 6 | 3M, 3F | 3-4 days old | 0.2 | S | Puppy | 6 rapa, 6 res |
Puppy samples were obtained from routine tail docks, ear clips and dewclaw removals performed at veterinarian offices in Central New York and Michigan. Senior dog samples were collected from ear clips immediately after euthanasia. The samples were placed in cold transfer media (Dulbecco’s modified Eagle medium [DMEM], with 4.5 g/L glucose, sodium pyruvate, and 4 mM L-glutamine supplemented with 10% heat-inactivated fetal bovine serum and antibiotics [100 U/mL pen/strep], containing 10 mM HEPES) and transferred to Colgate University on ice.
To isolate primary fibroblast cells, skin samples were sterilized in 70% ethanol and 10% bleach. Once any fat and bone were removed, skin was minced and incubated in sterile 0.5% Collagenase Type 2 (Worthington Chemicals, Cat. No. LS004176) overnight in an atmosphere of 37 °C, 5% CO2, and 5% O2. After incubation, the collagenase mixture was filtered through a 20-μm sterile mesh and centrifuged at 1000 rpm for 5 min. The resulting supernatant was removed, and the pellet was resuspended with 7 mL of mammal media (Dulbecco’s modified Eagle medium [DMEM], with 4.5 g/L glucose, sodium pyruvate, and 4-mM L-glutamine supplemented with 10% heat-inactivated fetal bovine serum and antibiotics [100 U/mL pen/strep]). Cells were grown in Corning T-25 culture flasks at 37 °C in an atmosphere of 5% O2 and 5% CO2. When cells reached 90% confluence, they were trypsinized (0.25%) and cryopreserved at 106 cells/mL in DMEM supplemented with 40% fetal bovine serum and dimethylsulfoxide (DMSO) at a final concentration of 10%. We stored cells in liquid N2 prior to any experiments, and cells were thawed by continuously swirling the frozen aliquot in a 37 °C water bath until only a small amount of ice remained. We resuspended the pellet by pipetting 6 mL of chilled mammal media and plated the resuspension in a T-25 culture flask at 37 °C in an atmosphere of 5% O2 and 5% CO2. All experiments highlighted below were run at passage 2 (P2). Once confluent, cells from each individual dog were manually counted before being plated.
All the procedures within this study were approved by Colgate University’s Institutional Care and Use committeee’s under protocol number 1819-13.
Treatments
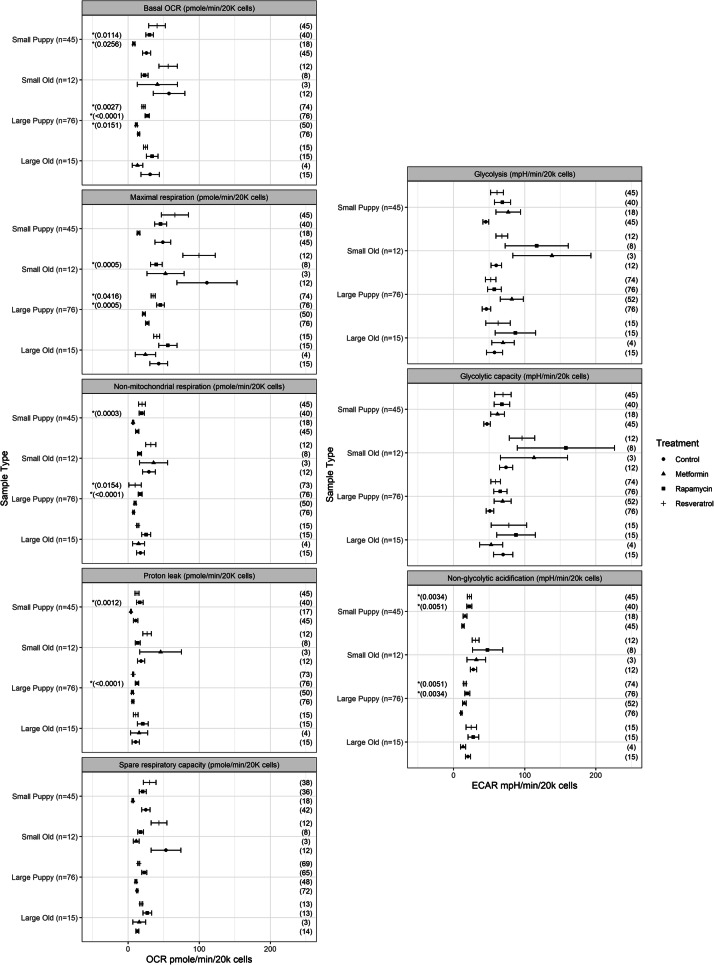
We plated control cells without any drugs. Control wells were also incubated with 5 μl of methanol, as it was the vehicle used. Within the same plate as control cells, we plated cells from the same dogs for each of the drugs used. Each set of duplicate wells per individual dog were treated individually with each compound. We plated a total of 9 individual dogs per plate per day. We plated cells in a Seahorse XFe96 plate at a concentration of 10,000 cells/well in duplicate per dog per treatment and allowed them to attach overnight, and then treatment groups received either 1 μM rapa for 24 h [50], 10 μM res for 24 h [52] or 0.5 mM met for 24 h [54, 59]. Sample sizes per treatment group are highlighted in Fig. 1. Following treatments, we ran one plate for oxygen consumption rates (OCR) and another plate for extracellular acidification rates (ECAR). At the concentrations we used for each drug, we saw no discernable differences in cell loss or cell proliferation rates.
Fig. 1.
Using primary fibroblast cells isolated from young and old, small breed and large breed dogs, we measured aerobic respiration (left: basal OCR, maximal respiration, non-mitochondrial respiration, proton leak, and spare respiratory capacity) and glycolysis (right: non-glycolytic acidification, total glycolysis, and glycolytic capacity) in cells treated with 1 μM rapa for 24 h, 10 μM res for 24 h or 0.5 mM met for 24 h. Results from the full transformed model show that (a) there were no significant differences in glycolysis measurements across treatments. (b) However, there were significant differences in aerobic respiration measurements across met and rapa treatments. Resulting p values from post hoc tests describing the treatment effect across sample types are summarized in Table 3. Small puppies, Small old, Large puppies, and large old values for control, metformin, rapamycin, and resveratrol treatments are plotted as averages ± SEMs. Numbers to the right of each bar represents the sample size of individual dogs used per sample type per treatment, and an asterisk denotes that the post hoc tests describing the treatment effect from control reached statistically significant levels after p value adjustment. Note that there are differing sample sizes due to growth of cells after resuspension
Measuring cellular metabolism
OCRs were determined using XFe96 FluxPaks from Agilent Technologies. We measured OCRs after cells were equilibrated to running media, which contains 10-mM glucose, 1-mM sodium pyruvate, and 2-mM glutamine, pH = 7.4, for 1 h. Baseline measurements of OCRs were made three times prior to injecting a final well concentration of 2 μM oligomycin, which inhibits ATP synthesis by blocking the proton channel of the Fo portion of the ATP synthase. This baseline is used to distinguish the percentage of O2 consumption devoted to ATP synthesis and O2 consumption required to overcome the natural proton leak across the inner mitochondrial membrane plus any non-mitochondrial O2 consumption. We then injected a final well concentration of 0.125-μM carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP), an uncoupling agent that disrupts ATP synthesis by collapsing the proton gradient across the mitochondrial membrane leading to uncoupled consumption of energy and O2 without generating ATP, providing a theoretical maximal respiratory rate. Finally, we injected a final well concentration of 0.5 μM Antimycin A, a Complex III inhibitor and rotenone, a Complex I inhibitor. This combination stops mitochondrial respiration and enables non-mitochondrial respiration to be evaluated [12, 22, 24]. After measurements were completed, we used a 1:200 concentration of CyQUANT dye to quantify final counts of cells in each well [31] and normalized all rates to 20,000 cells.
To normalize accurately, we plated cells from an individual of each breed at three different concentrations (10,000, 20,000, and 30,000 cells per well in duplicate) determined by manual counts and exposed them to two concentrations of CyQuant (1:200 and 1:400). We compared fluorescence of each of these to manual counts of each breed at each cell concentration. We then determined a linear detection range that extended from about 50 cells per well to about 250k cells per well, which corresponded with the higher (1:200) concentration of CyQUANT.
ECAR was measured in units of mpH, which is the pH change in the media surrounding the cells due to proton flux in glycolysis. Measurements of ECAR were performed after the cells are equilibrated to running media for 1 h. Running media contained no glucose and 2 mM L-glutamine in all experiments, pH = 7.4. Baseline rates were measured three times prior to any injections. We, first, injected a final well concentration of 10 mM glucose into media surrounding cells, which provides a measure of glycolytic rate, and then injected a final well concentration of 2-μM Oligomycin, giving an estimate of glycolytic capacity in cells. Finally, we injected a final well concentration of 50-mM 2-DG, a glucose analog that inhibits glycolysis, providing an estimate of non-glycolytic acidification [24]. After measurements were completed, we used a 1:200 concentration of CyQUANT dye to quantify final counts of cells in each well [31] and normalized all rates to 20,000 cells.
Statistics
Data from every assay were first tested for normality using a Shapiro-Wilkes test. If necessary, the data were transformed in a manner informed by a Box Cox power transformation [11]. This meant a log transform for all variables. First, we analyzed all data in a single MANOVA to test for differences in the collection of dependent variables across body size (small or large, as stated above), age (puppies or old), and treatment (control or rap/res/met), their two-way interactions, and a three-way interaction. Follow-up analyses were conducted using three-way ANOVAs on each dependent variable. The models are fit in R [48] and the mixed effects models are fit using the lmerTest package [34] for R. Best-subset model selection according to the Bayesian information criterion (BIC) [53] was completed via complete enumeration [42] for each model. Best-subset model selection compares all possible model specifications given a set of predictors and interactions and supplies the best fitting model according to BIC, which balances model fit with model parsimony. We adjusted the p values in the MANOVA analyses, within each model, and all post hoc comparisons reported using the approach in Benjamini and Hochberg [10] rather than using the overly conservative Bonferonni adjustment [20]. This approach allows for the control of the expected proportion of false discoveries instead of the probability of making at least one false discovery, thus preserving power. Post hoc testing involved estimating marginal means for specified values of independent variables and comparing these means via statistical contrasts. We performed these post hoc tests via the emmeans package for R [35]. Results were considered significant if the adjusted p value was less than 0.05.
Limitations
Due to the nature of working with pet dogs, our sample collection was limited in several ways. Firstly, our sample collections are limited to the age and life stage corresponding to when isolating skin tissue is appropriate. Particularly, our samples do not include a “middle aged” population. Secondly, the number of puppies per breed or group was limited to those breeds that are altered as a breed requirement (tail docks and dewclaw removal). The number of older euthanized dogs was limited due to the owners’ willingness to provide us ear clips at the time of euthanasia. Thirdly, we were not given complete medical charts for any dogs included in this study; thus, we have no records of diet, or exercise, which may be variants in OCR and ECAR. At times, veterinarians did not provide sex or weight for puppies, either. We do know that none of the dogs included in this study were taking any metabolic, neurological, or endocrine medications and none were obese. We also note that due to the sample size of large dogs, we may not have adequate power to detect true treatment effects that may exist, particularly in post hoc testing to break down results by age and size classes.
Results
Averages for each variable’s rate per treatment and group are plotted in Fig. 1 and can be found on Table S0. In the subsections below, we explore statistical results from full and best-subsets model specifications. These results are summarized in Table 3.
Table 3.
A table of statistically significant results, after p value adjustment; “*” denotes that the results met traditional levels of statistical significance after adjustment. Rows labeled “Overall” indicate whether the treatment effect was significant in the best-subsets model. Subsequent rows break down results from post hoc testing the full models to show significant treatment differences for specific age and size classes, averaged across control variables
| Non-glycolytic | Basal | Proton | Maximal | Non-mito | ||
|---|---|---|---|---|---|---|
| Acidification | OCR | Leak | Respiration | Respiration | ||
| Met | Overall | 0.3106 | 0.0001* | 0.1340 | 0.0214* | 0.4011 |
| Small Puppy | 0.4672 | 0.0256* | 0.5006 | 0.3653 | 0.7482 | |
| Large Puppy | 0.3671 | 0.0151* | 0.2616 | 0.3653 | 0.2975 | |
| Small Old | 0.7254 | 0.4908 | 0.2406 | 0.4199 | 0.7934 | |
| Large Old | 0.3049 | 0.1788 | 0.8383 | 0.4581 | 0.8581 | |
| Rapa | Overall | < 0.0001* | < 0.0001* | < 0.0001* | 0.0093* | < 0.0001* |
| Small Puppy | 0.0051* | 0.0114* | 0.0012* | 0.1663 | 0.0003* | |
| Large Puppy | 0.0034* | < 0.0001* | < 0.0001* | 0.0005* | < 0.0001* | |
| Small Old | 0.7000 | 0.0813 | 0.7235 | 0.0005* | 0.4160 | |
| Large Old | 0.5834 | 0.5296 | 0.2406 | 0.8946 | 0.2975 | |
| Res | Overall | < 0.0001* | 0.0003* | 0.0250* | 0.0214* | 0.0014* |
| Small Puppy | 0.0034* | 0.4465 | 0.5323 | 0.7179 | 0.2030 | |
| Large Puppy | 0.0051* | 0.0027* | 0.2406 | 0.0416* | 0.0154* | |
| Small Old | 0.7204 | 0.2960 | 0.2406 | 0.7179 | 0.5551 | |
| Large Old | 0.5834 | 0.3701 | 0.6458 | 0.7179 | 0.8581 | |
MANOVA and ANOVAs
Individual ANOVA results can be found in Tables S2-S9. Any significant results, without the presence of treatment, with respect to with age class and size class, have been previously described and discussed by Jimenez et al. [29]. Details on these differences can be found, with descriptions of the general trends, within the supplementary tables. Due to the focus of the current study, we are limiting the scope of the results and discussion to the differences found between control and cells treated with met, res, or rap.
Post hoc analysis of full transformed model
We saw a significantly higher non-glycolytic acidification in cells treated with rapa in small breed (p = 0.0051; Table 3) and large breed (p = 0.0034; Table 3) puppies, but not on older dogs of either size class (p = 0.7000 for small, p = 0.5834 for large; Table 3) when compared with control values. We also saw a significant increase in non-glycolytic acidification in cells treated with res in small breed (p = 0.0034; Table 3) and large breed (p = 0.0051; Table 3) puppies, but not in older dogs of either size (p = 0.7204 for small, p = 0.5834 for large; Table 3) when compared with control values. We saw a significantly lower basal OCR in cells treated with met in small breed (p = 0.0256; Table 3) and large breed (p = 0.0151; Table 3) puppies, but no differences in older small breed (p = 0.4908; Table 3) or large breed (p = 0.1788; Table 3) dogs. There was a significant effect of res on large breed puppies such that it increased basal OCR (p = 0.0027; Table 3) and maximal respiration (p = 0.0416; Table 3). Additionally, we found a significantly higher basal OCR in cells treated with rapa in small breed (p = 0.0114; Table 3) and large breed (p < 0.0001; Table 3) puppies, but not in older small breed (p = 0.0813; Table 3) or large breed (p = 0.5296; Table 3) dogs when compared with control values. We saw a significantly higher proton leak in cells treated with rapa in small breed (p = 0.0012; Table 3) and large breed (p < 0.0001; Table 3) puppies, but not in older small breed (p = 0.7235; Table 3) or large breed (p = 0.2406; Table 3) dogs when compared with control values. We saw a significant decrease in maximal respiration in cells treated with rapa in old small breed (p = 0.0005; Table 3) and a significant increase in maximal respiration in cells treated with rapa in large breed puppies (p = 0.0005; Table 3), when compared with control values. However, there were no differences in rapa-treated cell values for maximal respiration in small breed puppies (p = 0.1663; Table 3) and large breed old (p = 0.8946; Table 3) dogs. There was a significant increase in non-mitochondrial respiration in cells treated with rapa in small breed (p = 0.0003; Table 3) and large breed (p < 0.0001; Table 3) puppies, but not in older small breed (p = 0.4160; Table 3) or large breed (p = 0.2975; Table 3) dogs.
Best subsets model
We expect a 10.46, 40.93, and 38.57% increase in non-glycolytic acidification compared with control measurements on average when dogs are treated with met (p = 0.3106; Table 3), rapa (p < 0.0001; Table 3), and res (p < 0.0001; Table 3), respectively.
We expect a 35.99% decrease in basal OCR compared with control measurements on average when dogs are treated with met (p = 0.0001; Table 3). We expect a 56.86 and 36.19% increase in basal OCR compared with control measurements on average when dogs are treated with rapa (p < 0.0001; Table 3) and res (p = 0.0003; Table 3), respectively.
We expect a 11.48% decrease in proton leak compared with control measurements on average when dogs are treated with met (p = 0.1340; Table 3). We expect a 55.91% and 16.44% increase in proton leak compared with control measurements on average when dogs are treated with rapa (p < 0.0001; Table 3) and res (p = 0.0250; Table 3), respectively.
We expect a 27.01% decrease in maximal respiration compared with control measurements on average when dogs are treated with met (p = 0.0214; Table 3). We expect a 34.02 and 27.15% increase in maximal respiration compared with control measurements on average when dogs are treated with rapa (p = 0.0093; Table 3), and res (p = 0.0214; Table 3), respectively. We expect a 7.39, 68.22, and 25.72% increase in non-mitochondrial respiration compared with control measurements on average when dogs are treated with met (p = 0.4011; Table 3), rapa (p < 0.0001; Table 3), and res (p = 0.0014; Table 3), respectively.
Discussion
Many studies have focused on the aerobic metabolic consequences of met, rapa, and res; however, our study is the first to measure anaerobic metabolic consequences as well. Similarly, our study is the first to use these three commercially available drugs in a dog model. We predicted that each of these drugs may decrease rates of glycolysis in large breed dog, and potentially increase rates of aerobic respiration to compensate.
However, our data showed that, overall, rapamycin treatment had the most profound effects. Rapa increased rates of non-glycolytic acidification, basal OCR, proton leak, and non-mitochondrial respiration in small and large breed puppies. Maximal respiration was significantly altered with rapa treatment but in opposing ways: large breed puppies showed a significant increase, and small breed old dogs demonstrated a significant decrease. Most of this information points to the fact that rapa treatments may be metabolically more beneficial in younger dogs compared with older dogs.
Res and met treatments did not have metabolic effects as pronounced as rapa. We found that res also increased rates of non-glycolytic acidification in small and large breed puppies and increased basal OCR and maximal respiration in large breed puppies. Unlike rapa treatments, met decreased basal OCR levels in cells from small and large breed puppies. We will discuss the potential physiological mechanisms for our observed responses to rapa, res, and met below.
In cardiac fibroblast cells, a prolonged treatment of rapa on young and senescent cells brought the metabolic phenotype of senescent cells back to early passage, or young, cells [44]. While initiating a rapa treatment early to mid-life seems to increase longevity, this intervention has shown to have side effects [60]. Because of this, rapa was administered to dogs in a short-term trial at two concentrations, which revealed no overt effects [56]. Our data on primary fibroblast cells isolated from dogs support that rapa treatments seem to demonstrate more pronounced effects on cells from puppies of both size classes, supporting whole-animal studies that have assessed treatment with rapa [60]. The increase in non-glycolytic acidification may be related to the previously reported reprogramming in pyruvate oxidation due to rapa treatments [44]. Previous work suggests that proton leak is significantly lower in puppies of both size classes compared with older dogs. We attributed this pattern to greater ATP coupling during the growing phase in puppies [29, 30]. High efficiency in ATP coupling, however, comes at the cost of increased mitochondrial membrane potential, which in turn can increase reactive oxygen species (ROS) production. Thus, increases in proton leak have been associated with decreased ATP coupling and decreased ROS production [51]. In our current study, we saw a significant increase in proton leak in small and large breed puppies treated with rapa compared with control values. This pattern may be associated with the activity of uncoupling proteins (UCP) found in the mitochondrial membrane, which have been previously demonstrated to be upregulated during rapa treatments [44]. We have observed higher basal oxygen consumption rates and a higher degree of ATP coupling efficiency in large breed dogs compared with small breed dogs [30], but no differences in mitochondrial content with respect to any body size variable [29]. This indicates that mitochondria across breed sizes classes may be working differently. To this end, an increase in basal OCR in puppies of both size classes may be due to a transient increase in mitochondrial biogenesis [15]; this increase in mitochondrial content could also explain the increase in proton leak in puppies of both size classes. It should be noted, however, that C2C12 mouse cells treated with a 250–nM rapa treatment for 24 h did not show any differences between control and rapa-treated cells [62]. Thus, the metabolic adjustments we see in primary fibroblasts may not be homogenous across all tissues. Large breed puppies showed a significant increase in maximal respiration when treated with rapa, which may be associated with an increase in mitochondrial content. However, if it is associated with improving mitochondrial function instead, then this may be a beneficial phenotype for large breed dogs because aerobic scope is increased, which could shift cells away from relying on aerobic glycolysis. In opposition, a decrease in maximal respiration with rapa treatment in small breed old dogs is a physiologically challenging phenotype, as cells have less ability to deal with any increases in ATP demand when overall aerobic scope is decreased.
Res has been shown to modify tumor initiation, promotion and progression [27], as well as extend lifespan [7]. These cellular alterations also seem to mimic those found during caloric restriction (CR) phenotype, a demonstrated mechanism for increased lifespan in mammals. Some suggest that the similarities in these pathways stem primarily from a reduction in glucose uptake and a decrease in lactate production, though this is not the sole mechanism of action previously found in this drug [52]. A 10-μM treatment with res has been found to reverse the Warburg effect and cancerous phenotype in colon cancer cells by increasing the ability of these cells to oxidize glucose into pyruvate while decreasing the production of lactate [52]. Res treatment of canine hemangiosarcoma, as low as 20–50 μM, significantly reduced cell viability via apoptosis [14]. Treating primary dermal fibroblasts with 0.01 μM of res for 24 h provided evidence that res is therapeutically effective [40]. Thus, it seems that res treatment can be effective over a wide range of concentrations in different cell types [17]. In opposition, some suggest that a higher dose of res (6.25–12.5 μM) blocked the cell cycle in non-cancerous vascular smooth muscle cells [41]. A treatment of 10 μM res has been found to increase colon cancer’s cell ability to oxidize glucose. Furthermore, the treatment also significantly increased OCRs of both normal and colon cancer cells after 48 h of treatment [52], which is similar to our results, Specifically, large breed puppies demonstrated higher basal OCR and maximal respiration with res treatment. ECAR rates of colon cancer cells were significantly reduced after 48 h of treatment, but normal cells’ ECAR remained unchanged after the same treatment [52], suggesting that res’ effect on glycolysis is more pronounced in cancerous cells. In our study, we found that a 10-μM treatment of res for 24 h showed an increase in rates of non-glycolytic acidification in cells from puppies of both size classes. The two main sources of non-glycolytic acidification are the tricarboxylic acid (TCA) cycle and the breakdown of intracellular glycogen [26]. Res treatments have also been found to increase the activity of pyruvate dehydrogenase (PDH), which is a key mitochondrial enzyme that links glycolysis and the TCA cycle [52]. If PDH activity is increased due to res, it may be that there is an increase in processing through the TCA, leading to a potential increase on non-glycolytic acidification.
Met has been demonstrated to increase health span and lifespan in mice concomitant with reduction in oxidative stress, increased antioxidants, and a lack of accumulation of chronic inflammation [4, 37]. Met treatments have also shown anti-cancer effects, reducing the incidence of various cancers such as hepatocellular, colorectal, pulmonary, and pancreatic [1, 49]. Primary fibroblast cells isolated from mice fed met at 11, 16, 19, and 23 months of age prevented age-related senescent markers to accumulate with cells, thus slowing the aging process [6]. In hepatocytes, met enhanced the activity of pyruvate kinase and decreased glucose production from lactate [5]. Met treatment for 24 h reduced dog mammary cancer cell line viability and suppressed their growth [49]. Our results may indicate that longer treatments may be necessary for tissue culture experiments with met. Others have found the uptake of met to be slow in tissue culture experiments and have seen similar non-significant glycolysis increases as those we report here [13]. In this study, we found that a 0.5-mM met treatment for 24 h significantly decreased basal OCR levels in cells from puppies of both size classes. It is likely that this decrease in basal OCR is stemming from the previously reported inhibition of complex I decrease ATP synthesis by oxidative phosphorylation and decreased in AMP/ATP ratio linked to met treatments [16, 59].
Here, we tested cellular metabolic changes in primary fibroblast cells isolated from small and large breeds and young and old dogs when treated with three compounds that have reported previous increases health span and lifespan in other model organisms (such as mice and yeast). Overall, we found that rapa treatments demonstrated the most drastic and potentially beneficial outcomes compared with met and res. These data are the first empirical evidence of the cellular metabolic effects of these three drugs on canine primary cell lines and may serve to inform ongoing experiments at the whole-animal level with rapa. We should point out, as highlighted in the introduction, that different doses and length of exposure with these drugs, as well as differing cell types, may demonstrate variability in response. Future research may consider how dose amount and length of exposure can be optimized for treatment effect.
Supplementary Information
(PDF 443 kb)
Acknowledgments
We are grateful to the following veterinarians and veterinary practices for providing us with samples: Dr. Kerri Hudson, Dr. James Gilchrist, Dr. Heather Culbertson and Morgan Peppenelli at Waterville Veterinary Clinic (New York); Dr. Frank Capella from Village Vet in Wampsville, NY. Pet Street Station Animal Hospital (New York); Dr. Jim Bader at Mapleview Animal Hospital (Michigan). We are also grateful to the following breeders for participating in our study: Rhonda Poe, Bob Stauffer, Allison Mitchell, Nancy Secrist, Valeria Rickard, Joanne Manning, Lita Long, Betsy Geertson, Susan Banovic, Lisa Uhrich, Sheryl Beitch, Al Farrier, Barbara Hoopes, and Rachel Sann.
Authors’ contributions
AGJ designed the experiments, collected tissue, grew cells, collected data, and wrote the first draft of the manuscript. SL and WC cleaned the raw data, performed the data analyses, wrote the supplement, and edited the manuscript.
Funding
The Seahorse XFe96 oxygen flux analyzer was purchased via a National Science Foundation Major Research Instrument grant (NSF MRI 1725841 to AGJ). A Research Council grant from Colgate University to AGJ partly funded this work.
Data Availability
Data is available in its raw form by contacting the corresponding author. After publication, this dataset will be deposited onto figshare.
Code availability
Code will be available on Dr. William Cipolli’s website or by contacting him directly via email.
Declarations
Conflicts of Interest
The authors declare no conflict of interest
Ethics approval
All the procedures within this study were approved by Colgate University's Institutional Care and Use Committee’s under protocol number 1819-13.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anisimov VN. Metformin for cancer and aging prevention: is it a time to make the long story short? Oncotarget. 2015;6(37):39398–39407. doi: 10.18632/oncotarget.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7(17):2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 3.Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2010;2(12):945–958. doi: 10.18632/aging.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, Poroshina TE. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3(2):148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argaud D, Roth H, Wiernsperger N, LEVERVE XM. Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes. Eur J Biochem. 1993;213(3):1341–1348. doi: 10.1111/j.1432-1033.1993.tb17886.x. [DOI] [PubMed] [Google Scholar]
- 6.Arkad'eva AV, Mamonov AA, Popovich IG, Anisimov VN, Mikhel'son VM, Spivak IM. Metformin slows down ageing processes at the cellular level in SHR mice. Tsitologiia. 2011;53(2):166–174. [PubMed] [Google Scholar]
- 7.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3(6):e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23(6):1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci. 2004;101(35):12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 11.Box GE, Cox DR. An analysis of transformations. J R Stat Soc Ser B Methodol. 1964;26(2):211–243. [Google Scholar]
- 12.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462(3):475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson A, Alderete KS, Grant MKO, Seelig DM, Sharkey LC, Zordoky BNM. Anticancer effects of resveratrol in canine hemangiosarcoma cell lines. Vet Comp Oncol. 2018;16(2):253–261. doi: 10.1111/vco.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, et al. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 2016;8(2):314–327. doi: 10.18632/aging.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, et al. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci. 2014;111(24):E2501–E2509. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM. Resveratrol and the mitochondria: from triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta (BBA) Gen Subj. 2016;1860(4):727–745. doi: 10.1016/j.bbagen.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Deeb BJ, Wolf NS. Studying longevity and morbidity in giant and small breeds of dogs. Vet Med. 1994;89(suppl):702–713. [Google Scholar]
- 19.Del Barco S, Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, et al. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2(12):896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56(293):52–64. doi: 10.1080/01621459.1961.10482090. [DOI] [Google Scholar]
- 21.Fernández AF, Fraga MF. The effects of the dietary polyphenol resveratrol on human healthy aging and lifespan. Epigenetics. 2011;6(7):870–874. doi: 10.4161/epi.6.7.16499. [DOI] [PubMed] [Google Scholar]
- 22.Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81(16):6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007;6(1):1–13. doi: 10.1111/j.1474-9726.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill BG, Benavides GA, Lancaster JR, Ballinger S, Dell’Italia L, Zhang J, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem. 2012;393(12):1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 26.Ipata PL, Balestri F. Glycogen as a fuel: metabolic interaction between glycogen and ATP catabolism in oxygen-independent muscle contraction. Metabolomics. 2012;8(4):736–741. doi: 10.1007/s11306-011-0372-6. [DOI] [Google Scholar]
- 27.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez AG. Physiological underpinnings in life-history trade-offs in man’s most popular selection experiment: the dog. J Comp Physiol B. 2016;186(7):813–827. doi: 10.1007/s00360-016-1002-4. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez AG, Winward J, Beattie U, Cipolli W. Cellular metabolism and oxidative stress as a possible determinant for longevity in small breed and large breed dogs. PLoS One. 2018;13(4):e0195832. doi: 10.1371/journal.pone.0195832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimenez AG, Winward JD, Walsh KE, Champagne AM. Effects of membrane fatty acid composition on cellular metabolism and oxidative stress in dermal fibroblasts from small and large breed dogs. J Exp Biol. 2020;223(12):jeb221804. doi: 10.1242/jeb.221804. [DOI] [PubMed] [Google Scholar]
- 31.Jones LJ, Gray M, Yue ST, Haugland RP, Singer VL. Sensitive determination of cell number using the CyQUANT® cell proliferation assay. J Immunol Methods. 2001;254(1-2):85–98. doi: 10.1016/S0022-1759(01)00404-5. [DOI] [PubMed] [Google Scholar]
- 32.Kalyanaraman B, Cheng G, Hardy M, Ouari O, Sikora A, Zielonka J, Dwinell M. Mitochondria-targeted metformins: anti-tumour and redox signaling mechanisms. Interface Focus. 2017;7(2):20160109. doi: 10.1098/rsfs.2016.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999;26(5):495–500. doi: 10.1016/S0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- 34.Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 35.Russell Lenth (2020) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.8. https://CRAN.R-project.org/package=emmeans
- 36.Lowseth LA, Gillett NA, Gerlach RF, Muggenburg BA. The effects of aging on hematology and serum chemistry values in the beagle dog. Vet Clin Pathol. 1990;19(1):13–19. doi: 10.1111/j.1939-165X.1990.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 37.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michell AR. Longevit of British breeds of dog and its relationships with-sex, size, cardiovascular variables and disease. Vet Rec. 1999;145(22):625–629. doi: 10.1136/vr.145.22.625. [DOI] [PubMed] [Google Scholar]
- 39.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, De Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol Ser A. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizuguchi Y, Hatakeyama H, Sueoka K, Tanaka M, Goto YI. Low dose resveratrol ameliorates mitochondrial respiratory dysfunction and enhances cellular reprogramming. Mitochondrion. 2017;34:43–48. doi: 10.1016/j.mito.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Mnjoyan ZH, Fujise K. Profound negative regulatory effects by resveratrol on vascular smooth muscle cells: a role of p53–p21WAF1/CIP1 pathway. Biochem Biophys Res Commun. 2003;311(2):546–552. doi: 10.1016/j.bbrc.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 42.Morgan JA, Tatar JF. Calculation of the residual sum of squares for all possible regressions. Technometrics. 1972;14(2):317–325. doi: 10.1080/00401706.1972.10488918. [DOI] [Google Scholar]
- 43.Mouchiroud L, Molin L, Dallière N, Solari F. Life span extension by resveratrol, rapamycin, and metformin: The promise of dietary restriction mimetics for an healthy aging. Biofactors. 2010;36(5):377–382. doi: 10.1002/biof.127. [DOI] [PubMed] [Google Scholar]
- 44.Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. GeroScience. 2018;40(3):243–256. doi: 10.1007/s11357-018-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123(8):3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol Ser A Biol Med Sci. 1997;52(3):B171–B178. doi: 10.1093/gerona/52A.3.B171. [DOI] [PubMed] [Google Scholar]
- 47.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
- 49.Saeki K, Watanabe M, Tsuboi M, Sugano S, Yoshitake R, Tanaka Y, et al. Anti-tumour effect of metformin in canine mammary gland tumour cells. Vet J. 2015;205(2):297–304. doi: 10.1016/j.tvjl.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 50.Saha B, Cypro A, Martin GM, Oshima J. Rapamycin decreases DNA damage accumulation and enhances cell growth of WRN-deficient human fibroblasts. Aging Cell. 2014;13(3):573–5. [DOI] [PMC free article] [PubMed]
- 51.Salin K, Villasevil EM, Anderson GJ, Auer SK, Selman C, Hartley RC, Mullen W, Chinopoulos C, Metcalfe NB. Decreased mitochondrial metabolic requirements in fasting animals carry an oxidative cost. Funct Ecol. 2018;32(9):2149–2157. doi: 10.1111/1365-2435.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saunier E, Antonio S, Regazzetti A, Auzeil N, Laprévote O, Shay JW, Coumoul X, Barouki R, Benelli C, Huc L, Bortoli S. Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci Rep. 2017;7(1):6945. doi: 10.1038/s41598-017-07006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- 54.Seo-Mayer PW, Thulin G, Zhang L, Alves DS, Ardito T, Kashgarian M, Caplan MJ. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol-Renal Physiol. 2011;301(6):F1346–F1357. doi: 10.1152/ajprenal.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urfer SR, Wang M, Yang M, Lund EM, Lefebvre SL. Risk Factors Associated with Lifespan in Pet Dogs Evaluated in Primary Care Veterinary Hospitals. J Am Anim Hosp Assoc. 2017a. [DOI] [PubMed]
- 56.Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DE, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39(2):117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Lawler D, Larson B, Ramadan Z, Kochhar S, Holmes E, Nicholson JK. Metabonomic investigations of aging and caloric restriction in a life-long dog study. J Proteome Res. 2007;6(5):1846–1854. doi: 10.1021/pr060685n. [DOI] [PubMed] [Google Scholar]
- 58.Warburg O. On respiratory impairment in cancer cells. Science (New York, NY) 1956;124(3215):269–270. [PubMed] [Google Scholar]
- 59.Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 62.Yu Z, Wang R, Fok WC, Coles A, Salmon AB, Pérez VI. Rapamycin and dietary restriction induce metabolically distinctive changes in mouse liver. J Gerontol Ser Biomed Sci Med Sci. 2014;70(4):410–420. doi: 10.1093/gerona/glu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 443 kb)
Data Availability Statement
Data is available in its raw form by contacting the corresponding author. After publication, this dataset will be deposited onto figshare.
Code will be available on Dr. William Cipolli’s website or by contacting him directly via email.