Abstract
Obesity is now recognized as a disease. This study revealed a novel role for pyruvate dehydrogenase kinase (PDK) in diet-induced hypertrophic obesity. Mice with global or adipose tissue-specific PDK2 deficiency were protected against diet-induced obesity. The weight of adipose tissues and the size of adipocytes were reduced. Adipocyte-specific PDK2 deficiency slightly increased insulin sensitivity in HFD-fed mice. In studies with 3T3-L1 preadipocytes, PDK2 and PDK1 expression was strongly increased during adipogenesis. Evidence was found for epigenetic induction of both PDK1 and PDK2. Gain- and loss-of-function studies with 3T3-L1 cells revealed a critical role for PDK1/2 in adipocyte differentiation and lipid accumulation. PDK1/2 induction during differentiation was also accompanied by increased expression of hypoxia-inducible factor-1α (HIF1α) and enhanced lactate production, both of which were absent in the context of PDK1/2 deficiency. Exogenous lactate supplementation increased the stability of HIF1α and promoted adipogenesis. PDK1/2 overexpression-mediated adipogenesis was abolished by HIF1α inhibition, suggesting a role for the PDK-lactate-HIF1α axis during adipogenesis. In human adipose tissue, the expression of PDK1/2 was positively correlated with that of the adipogenic marker PPARγ and inversely correlated with obesity. Similarly, PDK1/2 expression in mouse adipose tissue was decreased by chronic high-fat diet feeding. We conclude that PDK1 and 2 are novel regulators of adipogenesis that play critical roles in obesity.
Subject terms: Mechanisms of disease, Obesity
Obesity: Effects of different forms of key enzyme
The discovery that two forms of a key enzyme appear to play a critical role in fat production triggered by overeating might lead to new approaches to prevent and treat obesity. Hyeon-Ji Kang at Kyungpook National University, Daegu, South Korea, and colleagues in South Korea and the USA examined the role of the enzymes pyruvate dehydrogenase kinase types 1 and 2 (PDK1/2). PDK enzymes regulate the activity of a multi-enzyme complex that catalyzes a key step in the use of glucose to provide energy stores for cells. Mice deficient in PDK2 were protected from diet-induced obesity, and PDK 1 and 2 activity was increased during the generation of fat cells. Studies using mice and human fat tissue confirmed that the enzymes regulate the development and growth of fat cells. Drugs inhibiting PDK enzymes might combat obesity.
Introduction
Obesity is an epidemic in the United States. After decades of study, thousands of publications, and a number of clinical trials, obesity remains—as it has always been—easy to diagnose but impossible to treat for want of an effective pharmacological intervention. A better understanding of the molecular mechanisms controlling the function of adipocytes may provide the insight needed to avoid the negative metabolic consequences and health risks associated with obesity.
The capacity of adipocytes to store triacylglycerol and release free fatty acids, the primary function of adipose tissue, is required for survival during starvation, provides the energy needed for physically demanding work and meets the challenges imposed by the stresses of illnesses and infections. By virtue of their ability to expand, adipocytes also provide a temporary storage depot for energy after overconsumption of food. Pyruvate dehydrogenase kinase 2 (PDK2), one of the four mitochondrial kinases responsible for phosphorylation-mediated regulation of the pyruvate dehydrogenase complex, is required for maintenance of euglycemia during starvation but contributes to hyperglycemia in diabetes1.
Inhibition or genetic deletion of PDK2 lowers blood glucose and improves insulin sensitivity in diabetic models2,3, increases apoptosis and decreases proliferation and tumor growth4, suppresses hypoxia-inducible factor-1α (HIF1α) signaling and angiogenesis in cancer5, suppresses macrophage polarization toward the M1 phenotype6, and is required for induction of apoptosis by p534,7.
This study follows from previous findings that PDK2 deficiency reduces body weight gain, prevents hepatic steatosis, and increases hepatic fatty acid oxidation, ketogenesis, and energy expenditure in mice fed a high-fat diet2. Decreased accumulation of body fat was apparent from the lower body weights of PDK2 knockout mice. In the present study, this finding was confirmed and extended by the demonstration that adipose-specific PDK2 deficiency decreased body fat accumulation and the size of adipocytes and improved insulin sensitivity in high-fat diet (HFD)-fed mice. Upregulation of PDK2 along with PDK1 was also found to occur and to be required for full differentiation of 3T3-L1 adipocytes and primary stromal vascular cells into adipocytes. It is proposed that the metabolic effects of PDK2 deficiency reduce obesity by limiting full differentiation of preadipocytes into adipocytes, which in turn limits adipocyte hypertrophy.
Materials and methods
Animal study
Pdk2lox/+ mice were generated on a C57BL/6 background by Cyagen Bioscience (Guangzhou, China). The targeting construct included LoxP sites flanking exon 2 of Pdk2 as well as a neomycin resistance cassette flanked by FRT sites. Pdk2lox/+ mice were intercrossed to generate Pdk2lox/lox mice. To generate adipocyte-specific PDK2 KO mice, Pdk2lox/lox mice were crossed with adiponectin-Cre mice. Pdk2-/- or Pdk4-/- mice, as well as Pdk2lox/lox and Pdk2ad-/- mice, were maintained on a 12-h light/dark cycle at 22 ± 2 °C. Four-week-old mice were fed either a control diet (low-fat diet; 10 kcal% fat; D12450B, Research Diets, Inc., New Brunswick, NJ, USA) or a high-fat diet (HFD; 60 kcal% fat and 6 kcal% sucrose; D12492, Research Diets). The whole-body lean and fat masses of the mice were measured using a MiniSpec LF 50 body composition analyzer (Bruker Optics, Billerica, MA, USA). All animal experiments were approved by the Institutional Animal Care and Use Committee of Kyungpook National University (2015-0063) and the Deagu-Gyeongbuk Medical Innovation Foundation (DGMIF 19020704-00).
Glucose & insulin tolerance tests (GTT & ITT)
The GTT was performed by injecting mice with D-glucose (1.5 g/kg, i.p.) after 16 h of fasting. Likewise, for the ITT, insulin (0.75 U/kg) was injected i.p. into mice after a 6 h period of fasting. Blood glucose levels were measured with a standard glucometer (Accu-Check Active, Roche Diagnostics GmbH, Mannheim, Germany) using blood collected from cut tail tips at the indicated time points.
Histological and immunofluorescence analyses
Adipose tissues were fixed with 4% formaldehyde in PBS and embedded in paraffin. Sectioned slides were stained with H&E. The adipocyte area was analyzed using ImageJ software (NIH, Bethesda, MD, USA). For immunofluorescence staining of perilipin, sections were permeabilized with 0.1% Triton X-100 for 15 min at room temperature and incubated with an antibody specific for perilipin A (ab3526, Abcam, Cambridge, MA, USA) in zymogen Ab diluent solution overnight at 4 °C prior to incubation with an Alexa Fluor-568-conjugated anti-rabbit secondary antibody (A11011, Thermo Fisher Scientific, Waltham, MA, USA). Samples were visualized with a confocal microscope (FluoView™ FV1000; Olympus, Tokyo, Japan).
Cell culture and differentiation
3T3-L1 preadipocytes were obtained from ATCC (Manassas, VA, USA) and maintained in high glucose-DMEM (HyClone, Logan, UT, USA) supplemented with 10% bovine serum (BS, Gibco 16170, Thermo Fisher Scientific) in a humidified atmosphere of 5% CO2 at 37 °C. For differentiation, confluent 3T3-L1 preadipocytes were treated with 1 µg/ml insulin (Sigma-Aldrich, St. Louis, MO, USA), 1 µM dexamethasone (Sigma-Aldrich), 0.5 mM IBMX (Sigma-Aldrich), and 10% fetal bovine serum (FBS, Gibco 16000, Thermo Fisher Scientific). After 2 days, the medium was replaced with medium containing 10% FBS and 1 µg/ml insulin and was thereafter replaced every 2 days with medium containing 10% FBS. After 6 days, differentiated 3T3-L1 cells were stained with Oil Red O (Sigma-Aldrich). Oil Red O staining was performed as described previously8. Sodium dichloroacetate (DCA, 347795), sodium l-lactate (L7022), and chetomin (C9623) were purchased from Sigma-Aldrich. GSK2837808A (# HY-10031) was purchased from MedChemExpress (Monmouth Junction, NJ, USA).
Western blot analysis
Western blot analysis was performed as described previously8. Proteins were separated by SDS-PAGE, transferred to a PVDF membrane (Millipore, Billerica, MA, USA), and incubated with specific primary antibodies [anti-SREBP-1c (577036, BD Biosciences, Franklin Lakes, NJ, USA), anti-FAS (3180, Cell Signaling, Danvers, MA, USA), anti-C/EBPα (2295, Cell Signaling), anti-PPARγ (2435, Cell Signaling), anti-PDK1 (KAP-PK112, Enzo Life Science, Farmingdale, NY, USA), anti-PDK2 (sc-100534, Santa Cruz, Dallas, TX, USA), anti-PDK3 (sc-365378, Santa Cruz) and anti-PDK4 (ab214938, Abcam), anti-p-PDHE1α Ser232 (AP1063, Calbiochem, San Diego, CA, USA), anti-p-PDHE1α Ser293 (AP1062, Calbiochem), anti-HIF1α (NB100-123, Novus Biologicals, Centennial, CO, USA), anti-HSP90 (4874, Cell Signaling), and anti-β-tubulin (G098, Applied Biological Materials, Richmond, BC, Canada)].
Real-time PCR
Total RNA was extracted using QIAzol reagent (Qiagen, Germantown, MD, USA) as described by the manufacturer. cDNA was synthesized using a RevertAidTM First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Real-time PCR was performed using SYBR Green (SYBR Green Master Mix, Applied Biosystems, Foster City, CA, USA) in a ViiA 7 Real-Time PCR System (Applied Biosystems). The sequences of the primers used for amplification of mouse and human DNA are shown in Supplementary Table 1.
Chromatin immunoprecipitation (ChIP) assay
3T3-L1 cells were crosslinked with formaldehyde for 20 min. The reaction was stopped by the addition of 0.25 M glycine for 10 min. Cells were lysed in lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl (pH 8)). The lysates were sonicated to generate DNA fragments of 500 to 1000 bp and diluted with ChIP buffer (1% SDS, 1% Triton X-100, 16.7 mM Tris-HCl (pH 8.1), 167 mM NaCl, 1.2 mM EDTA). The samples were incubated with antibodies (anti-H3ac (06–599, Millipore), anti-H4ac (06–598, Millipore), anti-H3K4me3 (07–473, Millipore), anti-PCAF (ab12188, Abcam), anti-p300 (ab14984, Abcam), anti-H3K27ac (ab177178, Abcam) and control mouse IgG (I5381, Sigma-Aldrich)) overnight at 4 °C on a rotating wheel. To collect DNA–protein–antibody complexes, protein A/G-agarose beads were added to the mixtures, which were then incubated at 4 °C for 2 h with rotation and pelleted by brief centrifugation. The samples were washed and dissolved in 500 μl of elution buffer, and crosslinking was reversed by treatment with 20 µl of 5 M NaCl at 65 °C for 2 h. After EDTA and proteinase K treatment, DNA was extracted from the supernatants with phenol/chloroform and precipitated with ethanol. Immunoprecipitated DNA was analyzed by quantitative real-time PCR using primers specific for the mouse PDK1 promoter (forward, 5′-ACAGGCTCATTGCCAACGAT-3′ and reverse, 5′-CCGCCCTCCCAGTCTCA-3′) and the mouse PDK2 promoter (forward, 5′-GGGCACATACCAAGTTTTACCAA-3′ and reverse, 5′-CCCTGCCTCTTCCCTGAGA-3′).
Retroviral plasmids and retroviral transduction
The full-length cDNAs of PDK1 and PDK2 were amplified by PCR and inserted into the XhoI and NotI sites in the Vxy-puro retroviral vector. The constructs (Vxy-puro, Vxy-Pdk1-puro, and Vxy-Pdk2-puro) were transfected into Phoenix ecotropic packaging cells using LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA). Viral supernatants were collected after 48 h, clarified by filtration through 0.45-µm pore size syringe filters (Sartorius Stedim Biotech, Bohemia, NY, USA), and used for transduction of 3T3-L1 preadipocytes in the presence of 5 μg/ml polybrene (Sigma-Aldrich) for 24 h. Transduced 3T3-L1 cells were selected with 3 μg/ml puromycin (Sigma-Aldrich) for 7 days.
Establishment of cells with stable knockdown of Pdk1 and Pdk2
Predesigned short hairpin RNA (shRNA) plasmids targeting Pdk1 or Pdk2 were purchased from Qiagen. To generate stable Pdk1/2 knockdown cells, shRNA-Pdk1, shRNA-Pdk2, and control vectors were transiently transfected into 3T3-L1 preadipocytes using Attractene Transfection Reagent (Qiagen). Stably transfected cells were selected with puromycin and hygromycin for 7 days.
Measuring l-Lactate release in cell culture medium
3T3-L1 cell culture medium was collected every 2 days after the differentiation medium was changed. Lactate production was assessed using an EnzyChromTM l-Lactate Assay Kit (BioAssay Systems, Hayward, CA, USA).
Human samples
For human studies, visceral adipose tissue was obtained from 34 healthy subjects who underwent nephrectomy as donors for kidney transplantation between approximately 2015 and 2018 (Seoul National University Bundang Hospital, IRB No. B-1801-445-301). Visceral adipose tissues were collected, and correlations between PDK expression, adipogenic genes, and clinical information such as BMI were examined. The baseline characteristics are summarized in Supplementary Table 2.
Statistical analysis
Data were plotted using GraphPad Prism 8.3 software (GraphPad Inc., San Diego, CA, USA), and statistical analysis was performed using IBM SPSS Statistics (version 21, IBM Corp., Armonk, NY, USA). Statistically significant differences in normally distributed data were identified by 2-tailed Student’s t test. Statistical analysis of group comparisons was performed by one-way or two-way ANOVA followed by the LSD post hoc test. p-values <0.05 were considered statistically significant.
Results
Global Pdk2 knockout mice exhibit decreased adiposity
Pdk2-/- and Pdk4-/- mice were used to test the effect of Pdk2 and Pdk4 on fat mass. Four-week-old male wild-type (WT), Pdk2 -/-, and Pdk4-/- mice were fed a high-fat diet (HFD) for 6 weeks. Body weight and fat mass (both subcutaneous and epididymal fat) gain were significantly attenuated in Pdk2-/- mice but not in Pdk4-/- mice compared with WT mice (Supplementary Fig. 1a–c). To systematically assess weight gain in Pdk2-/- mice, we fed them either a low-fat diet (LFD) or HFD for 4 weeks. Computed tomography (CT) scans showed less lipid accumulation in visceral (apricot-colored) and subcutaneous (blue-colored) depots in Pdk2-/- mice than in WT mice under both LFD and HFD conditions (Fig. 1a). Four weeks after shifting to HFD feeding, Pdk2-/- mice exhibited significantly less weight gain than WT mice. Importantly, Pdk2-/- mice fed a LFD had body weights comparable to those of their WT counterparts throughout the experiment (Fig. 1b). Body composition analysis revealed that Pdk2-/- mice showed a slightly decreased fat mass with LFD feeding (13.5 ± 0.9%, n = 5 to 10.4 ± 0.2%, n = 6), but the reduction was more dramatic when the mice were fed an HFD (20.8 ± 0.9%, n = 6 to 14.7 ± 1.2%, n = 6; Fig. 1c). No significant changes in the masses of other major metabolic organs, such as brown adipose tissue, the heart, and the kidney, were found between WT and Pdk2-/- mice fed either a LFD or a HFD, implying that fat mass is the dominant factor affecting body weight (Supplementary Fig. 1d). The epididymal and subcutaneous fat masses were both smaller in Pdk2-/- mice than in WT mice fed the HFD (Fig. 1d). Histological examination of epididymal fat tissue by hematoxylin and eosin (H&E) staining indicated that the decrease in fat mass was due to a decrease in adipocyte size, which was further confirmed by staining with the lipid droplet-specific marker perilipin, showing a 26% reduction in adipocyte size (from 33.5 ± 1.1 to 24.8 ± 1.9) under HFD conditions (Fig. 1e); however, the reduction in adipocyte size was less notable under LFD conditions (Supplementary Fig. 1e).
Fig. 1. Pdk2 deficiency inhibits high-fat diet-induced fat accumulation.
a Representative images and computed tomography (CT) scans revealed fat accumulation in the epididymal adipose tissue of WT and Pdk2-/- mice fed a LFD or a HFD for 4 weeks. b Body weight was monitored in WT and Pdk2-/- mice fed a LFD or a HFD (n = 5–6 per group). c Body composition (% of body weight) was analyzed in WT and Pdk2-/- mice fed a LFD (n = 5 per group) or a HFD (n = 6 per group) for 3 weeks. d Representative morphology and weight (% of body weight) of subcutaneous and epididymal adipose tissue were evaluated in WT and Pdk2-/- mice fed a LFD or a HFD (n = 5–6 per group). e H&E and perilipin staining of epididymal adipose tissue from WT and Pdk2-/- mice fed a HFD for 4 weeks (n = 6 per group). The adipocyte area was measured with ImageJ. The data are presented as the mean ± SEM values. *P < 0.05, **P < 0.01, ***P < 0.001 by 1-way ANOVA followed by the LSD test.
Pdk2 deficiency in adipocytes leads to decreased fat mass in an organ-intrinsic manner
To directly investigate the role of Pdk2 in adipose tissues, we used a fat tissue-specific animal model established using the Cre-LoxP recombination system involving Adipoq-Cre and Pdk2lox/lox mice. As previous studies verified the adipocyte-specific expression pattern of Adipoq-Cre, we established Adipoq-Cre/Pdk2lox/lox (Pdk2ad-/-) mice, which harbored deletion of exon 2 of the Pdk2 gene in all adipocytes (Supplementary Fig. 2a). Western blot analysis demonstrated the efficiency and specificity of Pdk2 deletion in BAT and WAT but not in nonadipose tissues, such as the liver (Supplementary Fig. 2b).
When fed a HFD, Pdk2ad-/- mice appeared much leaner than WT mice, and after 4 weeks on a HFD, Pdk2ad-/- mice consistently weighed less than WT mice (Fig. 2a). In accordance with the observation in germline Pdk2-/- mice, the fat mass in Pdk2ad-/- mice was lower under either LFD or HFD conditions (Fig. 2b). The necropsy data indicated that the size (Fig. 2c) and weight (Fig. 2d) of both subcutaneous and epididymal fat were decreased in Pdk2ad-/- mice under both HFD and LFD conditions. Histological examination of epididymal fat tissue by H&E staining showed that adipocytes were smaller in Pdk2ad-/-mice fed either a LFD or a HFD (Fig. 2e).
Fig. 2. Pdk2 deficiency in adipose tissue results in decreased fat mass and improved insulin sensitivity.
a Body weight was measured in Pdk2lox/lox and Pdk2ad-/- mice fed a LFD or a HFD (n = 12–16 per group). b Body composition (% of body weight) was analyzed in Pdk2lox/lox and Pdk2ad-/-mice fed a LFD or a HFD for 7 weeks (n = 12–14 per group). c Representative morphology and d weight of subcutaneous and epididymal adipose tissue (% of body weight) from Pdk2lox/lox and Pdk2ad-/- mice fed a LFD or a HFD for 8 weeks (n = 9–11 per group). e Representative images of H&E-stained sections of eWAT from Pdk2lox/lox and Pdk2ad-/- mice fed a LFD or a HFD for 8 weeks. The adipocyte area was measured with ImageJ. f A glucose tolerance test (1.5 g/kg, i.p.) after 16 h of fasting and g an insulin tolerance test (0.75 U/kg, i.p.) after 6 h of fasting were performed in Pdk2lox/lox and Pdk2ad-/- mice fed a LFD or a HFD (n = 7–10 per group). h Serum free fatty acid (FFA), triglyceride (TG) and cholesterol levels were measured in Pdk2lox/lox and Pdk2ad-/- mice fed a HFD (n = 7–10 per group). The data are presented as the mean ± SEM values. *P < 0.05, ***P < 0.001 by 1-way ANOVA followed by the LSD test (a–g) or by 2-tailed Student’s t test (h).
In contrast to HFD-fed global PDK2 knockout mice, which showed dramatic improvements in glucose tolerance and insulin sensitivity2, Pdk2ad-/- mice showed only a slight improvement in insulin sensitivity and no improvement in glucose tolerance (Fig. 2f, g). Serum triglycerides and free fatty acids were also reduced in these mice (Fig. 2h).
To test whether energy expenditure is altered in Pdk2ad-/- mice, we examined the respiratory exchange ratio, energy expenditure, and physical activity. Pdk2ad-/- mice showed no significant differences from their counterparts in food intake (Supplementary Fig. 2c) or in VO2, VCO2, and the corresponding respiratory exchange ratio and energy expenditure (Supplementary Fig. 3), suggesting that the lower fat mass in Pdk2ad-/- mice is not a consequence of an increased metabolic rate.
Expression of PDK1 and PDK2 is increased during adipogenesis
Obesity is closely related to excessive accumulation of WAT resulting from both the hypertrophy of preexisting adipocytes and the differentiation of adipocyte precursors into mature adipocytes9,10. As both Pdk2-/- mice and Pdk2ad-/- mice showed a reduction in adiposity, we reasoned that PDK2 may affect adipocyte differentiation in a cell-intrinsic manner.
In agreement with this hypothesis, microarray analysis of differentiated 3T3-L1 cells11 showed that Pdk2 mRNA was highly expressed during 3T3-L1 cell differentiation, especially 7 days after the initiation of differentiation (Fig. 3a). Pdk1 also showed an expression pattern similar to that of Pdk2 (Fig. 3a).
Fig. 3. The expression levels of PDK1 and PDK2 are increased during adipogenesis.
aPdk isoform expression levels during 3T3-L1 cell differentiation were analyzed in microarray data. Raw expression microarray data were obtained from GSE2075211. The b mRNA (n = 3) and c protein expression levels of PDK isoforms during the differentiation of 3T3-L1 cells were measured by real-time PCR and western blotting, respectively. d ChIP‐Seq profiles of H3K27ac, H3K4me3, and H3K36me3 on gene loci encoding Pdk1 and Pdk2 during the differentiation of 3T3-L1 cells. Raw expression microarray data were obtained from GSE20752. e, f AcH3, AcH4, PCAF, EP300, H3K4me3, and H3K27ac recruitment to e Pdk1 and f Pdk2 loci during 3T3-L1 cell differentiation was analyzed by ChIP-qPCR. IgG was used as the negative control (n = 3). The data are presented as the mean ± SD values. *P < 0.05, **P < 0.01, ***P < 0.001 by 2-tailed Student’s t test.
To further examine the involvement of PDKs in adipogenesis, 3T3-L1 cells were differentiated, and the mRNA and protein expression levels of the Pdk isoforms were measured every 2 days by qPCR and western blotting, respectively. In accordance with the microarray data, the protein and mRNA expression levels of Pdk1 and Pdk2 increased dramatically during differentiation, while those of Pdk3 and Pdk4 were unaltered (Fig. 3b, c).
To confirm the physiological importance of these findings in 3T3-L1 preadipocytes, we cultured and primary stromal vascular cells and differentiated them into adipocytes. qPCR analysis showed that the expression levels of Pdk1 and Pdk2 were increased after differentiation (Supplementary Fig. 4). As observed in 3T3-L1 cells, Pdk3 and Pdk4 were not induced during differentiation. These findings show that Pdk1 and Pdk2 are upregulated during adipocyte differentiation, suggesting that Pdk1 and Pdk2 may play important roles during adipogenesis.
Adipocyte differentiation is an essential process for adipose tissue development, involving complex molecular networks and epigenetic modifications12,13. A series of experiments was performed to investigate whether the transcriptional activities of Pdk1 and Pdk2 are epigenetically regulated. Published chromatin immunoprecipitation sequencing (ChIP-seq) results11 and our own ChIP assay results demonstrated that H3K27 acetylation, which is associated with ‘open’ chromatin (euchromatin) and active cis-regulatory regions, was markedly enriched in the region surrounding the transcriptional start site (TSS) in the Pdk1 and Pdk2 loci (Fig. 3d). H3K4 trimethylation, which is associated with transcriptional initiation, was slightly increased in differentiated 3T3-L1 cells after 7 days (Fig. 3d). Furthermore, H3K36 trimethylation, which is associated with transcriptional elongation, was distributed across active gene bodies and increased markedly in both Pdk1 and Pdk2 as they were upregulated (Fig. 3d). This result suggests that Pdk1 and Pdk2 are upregulated via epigenetic modification during adipocyte differentiation.
Histone acetyltransferases (HATs) are recruited to H3K27 for histone acetylation14–17. Because recruitment of HATs results in transcriptional activation via H3K27 acetylation, we tested whether HATs are recruited to the Pdk1 and Pdk2 promoter regions. The ChIP assay showed that HATs (PCAF and EP300) were markedly recruited to the Pdk1 and Pdk2 promoter regions during adipogenesis and that acetylation of both H3 and H4 was dramatically increased in mature 3T3-L1 adipocytes (Fig. 3e, f). The ChIP assay also showed that both H3K4 trimethylation and H3K27 acetylation were distinctly increased, similar to our findings from ChIP-seq (Fig. 3e, f). These results suggest that Pdk1 and Pdk2 are highly upregulated during adipocyte differentiation via epigenetic modification.
PDK1 and PDK2 are critically involved in adipocyte differentiation in 3T3-L1 cells
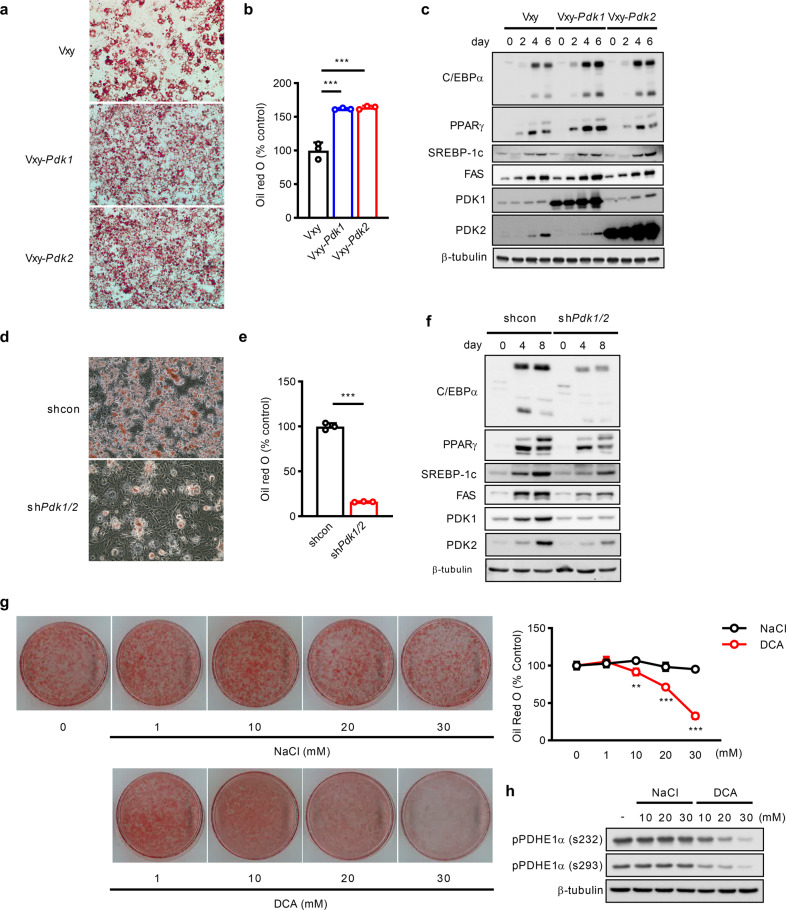
Next, to test whether PDK1 and PDK2 regulate adipocyte differentiation, 3T3-L1 cells stably expressing Pdk1 (Vxy-Pdk1), Pdk2 (Vxy-Pdk2), or a control vector (Vxy-puro) via a retroviral overexpression system were differentiated into adipocytes. Oil Red O staining showed that differentiated 3T3-L1 cells stably expressing either Pdk1 or Pdk2 exhibited an increase in lipid drop formation of approximately 62% compared to that in Vxy-puro cells, suggesting that both Pdk1 and Pdk2 stimulate adipogenesis (Fig. 4a, b). In addition, Pdk1 or Pdk2 overexpression increased both the mRNA and protein expression levels of adipogenic marker genes, including C/EBPα, PPARγ, and SREBP-1c (Fig. 4c, Supplementary Fig. 5c,d). Individual knockdown of Pdk1 or Pdk2 resulted in a modest (10~ 20%) loss of adipogenic capacity, as evidenced by decreased lipid accumulation (Supplementary Fig. 5a, b), suggesting that PDK1 and PDK2 may act synergistically during adipogenesis. Indeed, profound inhibition of adipogenesis was observed when Pdk1 and Pdk2 were simultaneously knocked down in 3T3-L1 cells (Fig. 4d, e). Major adipogenic genes were also dramatically downregulated (Fig. 4f, Supplementary Fig. 5e, f) by Pdk1 and Pdk2 deficiency.
Fig. 4. PDKs are required for adipocyte differentiation in 3T3-L1 cells.
a–c 3T3-L1 cells were retrovirally transduced with empty vector (Vxy-puro) or a retroviral vector encoding Pdk1 or Pdk2. a, b Lipid accumulation in 3T3-L1 adipocytes was measured by Oil Red O staining on day 6 after induction of differentiation (n = 3). c The protein expression levels of adipocyte-specific genes were measured in 3T3-L1 cells stably expressing Vxy-puro, Vxy-Pdk1, or Vxy-Pdk2. d, e Lipid accumulation in differentiated control (shcon) and Pdk1/2-silenced 3T3-L1 cells (shPdk1/2) was measured with Oil Red O staining (n = 3). f The protein expression levels of adipocyte-specific genes were measured in control and Pdk1/2-silenced 3T3-L1 cells. g Two-day postconfluent 3T3-L1 preadipocytes were cultured in differentiation medium with or without dichloroacetate (DCA), a pan-PDK inhibitor, at the indicated concentration for 6 days. On day 6, intracellular lipid accumulation was evaluated by Oil Red O staining (n = 3). h The phosphorylation level of PDHE1a was measured in differentiated 3T3-L1 cells. The data are presented as the mean ± SD values. **P < 0.01, ***P < 0.001 by 2-tailed Student’s t test.
To further confirm that PDK plays important roles during adipogenesis, 3T3-L1 cells were treated with the pan-PDK inhibitor dichloroacetate (DCA) during adipocyte differentiation. DCA inhibited the phosphorylation of PDHE1α and the differentiation of 3T3-L1 cells in a dose-dependent manner (Fig. 4g, h and Supplementary Fig. 5g).
Lactate-mediated stabilization of HIF1α is a key regulator of PDK-induced adipogenesis
Glucose, an essential substrate for triacylglycerol (TAG) storage in WAT, is used for de novo fatty acid synthesis and is converted to lactate during adipogenesis18. Lactate production from glucose is dramatically increased in adipose tissue in obese and diabetic humans19 and is known to be closely related to obesity and insulin resistance20.
To determine whether glycolysis is correlated with adipogenesis, we performed gene set enrichment analysis (GSEA) on the gene expression profile of differentiated 3T3-L1 cells using public databases11. The GSEA results showed a strong positive correlation between the expression of genes related to the glycolysis pathway (Supplementary Fig. 6a), such as Hk2, Pgk1, Gapdh, Pkm, and Ldha (Supplementary Fig. 7), and adipogenesis. Consistent with these findings, the extracellular acidification rate (ECAR) was increased in differentiated 3T3-L1 cells compared with undifferentiated cells (day 0) (Supplementary Fig. 6b). Lactate production was increased in differentiated 3T3-L1 cells (Supplementary Fig. 6c).
Because lactate plays an important role in adipogenesis, we tested whether glucose consumption and lactate production are regulated by PDK1/2 during adipogenesis. Concomitant increases in lactate production and glucose consumption were observed in 3T3-L1 cells approximately 4 days after the initiation of differentiation (Fig. 5a), consistent with previous data indicating that most lactate is formed from glucose21. The rapid glucose consumption and lactate production observed between 2–4 days after initiation of adipocyte differentiation corresponded chronologically to the timepoint at which PDK1 and PDK2 were induced (Fig. 3a–c), suggesting that enhanced glycolysis during adipogenesis may be related to the induction of PDKs (Fig. 5a). As expected, the median lactate levels and glucose levels did not change significantly in shPdk1/2 3T3-L1 cells (Fig. 5a). Similarly, the ECAR was significantly reduced in shPdk1/2 3T3-L1 cells (Fig. 5b). These results suggest that PDK1 and PDK2 are entirely responsible for glucose consumption and lactate production during adipocyte differentiation, prompting us to examine the effect of lactate on adipogenesis.
Fig. 5. PDKs enhance HIF1α stability, leading to aerobic glycolysis during adipogenesis.
a Glucose and lactate concentrations were measured in culture supernatant from control and Pdk1/2-silenced 3T3-L1 cells (n = 3). b Extracellular acidification rates (ECARs) measured in control and Pdk1/2-silenced 3T3-L1 cells (n = 7). c 3T3-L1 cells were exposed to increasing concentrations of lactate over a 24-hr period. The protein expression of HIF1a was measured in lactate-treated 3T3-L1 cells. d, e HIF1a protein expression was measured in 3T3-L1 cells with d stable Pdk1 or Pdk2 overexpression or e Pdk1/2 silencing during adipocyte differentiation. The data are presented as the mean ± SD values. *P < 0.05, **P < 0.01, ***P < 0.001 by 2-tailed Student’s t test.
Lactate supplementation increased lipid droplet formation in a dose-dependent manner (Supplementary Fig. 6e). One of the well-established roles of lactate in metabolic reprogramming is accomplished by HIF1α activation22. Given that HIF1α can also mediate aerobic glycolysis23, we tested whether lactate can stabilize HIF1α in 3T3-L1 cells. Lactate dramatically increased the HIF1α protein level in a dose-dependent manner (Fig. 5c) but did not increase the mRNA level (data not shown), suggesting a role of lactate in HIF1α protein stabilization. The lactate level in the medium was increased by overexpression of either PDK1 or PDK2 (Supplementary Fig. 6d). In addition, the protein level of HIF1α increased continuously during cell differentiation and increased further in the presence of PDK1 or PDK2 (Fig. 5d).
Consistent with these findings, HIF1α stability was not increased during adipogenesis in shPdk1/2 cells (Fig. 5e), indicating that PDK1 and PDK2 are indispensable for HIF1α protein stabilization. Inhibition of HIF1α by chetomin or siRNA decreased adipogenesis mediated by PDK1 or PDK2 (Fig. 6a, Supplementary Fig. 6f), and adipogenic gene expression was suppressed by HIF1α knockdown (Fig. 6b).
Fig. 6. Inhibition of HIF1α expression or lactate production decreases adipogenesis in 3T3-L1 cells with stable Pdk1 or Pdk2 overexpression.
a Oil Red O staining showed the effect of siHif1a on adipogenesis in 3T3-L1 cells stably overexpressing Pdk1 or Pdk2. b The mRNA expression levels of adipogenic genes were measured on day 6 after induction of differentiation (n = 3). c Extracellular lactate level in 3T3-L1 cells stably overexpressing Pdk1 or Pdk2 and treated with the LDH inhibitor GSK2837808A. d, e Lipid accumulation was evaluated by Oil Red O staining in 3T3-L1 cells stably overexpressing Pdk1 or Pdk2 with or without GSK2837808A treatment. The data are presented as the mean ± SD values. *P < 0.05, **P < 0.01, ***P < 0.001 by 2-tailed Student’s t test.
Finally, to confirm that PDK1/2-mediated lactate production is responsible for adipogenesis, the LDH inhibitor GSK2837808A was used. LDH inhibitor treatment successfully decreased the lactate level in the medium (Fig. 6c), leading to a reduction in adipogenesis (Fig. 5d,e); these findings suggest that the PDK-lactate-HIF1α axis plays an important role in adipogenesis.
The expression of PDK1 and PDK2 correlates positively with that of PPARγ in human adipose tissue in a manner dependent on the body mass index
As both global and adipose tissue-specific Pdk2-/- mice are leaner than WT mice and Pdk1/2 expression is increased during adipogenesis due to epigenetic modifications (Fig. 3), we analyzed the expression of PDK1/2 in human adipose tissue.
PPARγ expression leads to the development of an adipogenic phenotype and is required for the differentiation of adipocytes in vivo. However, previous results showed that in mice, HFD feeding increases the levels of adipogenic genes such as PPARγ24 and lipogenic genes such as SREBP-1c25, which are paradoxically decreased in obese humans. To determine whether this pattern is also seen with PDKs, we analyzed the mRNA expression of various genes, including PPARγ, SREBP-1c, and PDK isotypes, in visceral adipose tissue from 34 subjects who had undergone nephrectomy as donors for kidney transplantation. qPCR analysis (bivariate analysis) revealed that the gene expression levels of PDK1 and PDK2 but not PDK3 and PDK4 correlated inversely with body mass index (BMI) in this cohort (Fig. 7a) (clinical and laboratory data are summarized in Supplementary Table 2).
Fig. 7. PDK1 and PDK2 expression levels are decreased in human visceral adipose tissue and are positively correlated with the PPARγ expression level.
a Correlations between the mRNA expression of PDK1, PDK2, PDK3, and PDK4 and body mass index (BMI). b Correlations of PPARγ and SREBP-1c mRNA expression with BMI. c Correlations between the expression of PDK isoforms and that of PPARγ.
In agreement with a previous series of studies24, the PPARγ gene expression level showed an inverse correlation with BMI. Furthermore, the levels of lipogenic genes such as SREBP-1c were inversely correlated with BMI (Fig. 7b). In this cohort, a positive correlation between the PDK1/2 and PPARγ expression levels was noted (Fig. 7c), suggesting that like PPARγ expression, PDK1 and PDK2 expression might be implicated in the pathogenesis of human obesity. In addition, comparison of the protein levels of PDK1 and PDK2 in adipose tissue from normal chow-fed and high-fat diet-fed mice revealed that the levels of these proteins were decreased in mice fed a HFD (Supplementary Fig. 8).
Discussion
In the present study, we observed that body weight gain and adipose tissue mass in Pdk2-/- mice were significantly lower than those in WT mice upon HFD feeding. In addition, adipose tissue-specific Pdk2 deficiency was sufficient to decrease adipose tissue mass independent of changes in whole-body energy expenditure. In 3T3-L1 cells, the mRNA expression levels of Pdk1 and Pdk2 were increased by epigenetic regulation, and the corresponding protein expression levels were increased due to an increase in lactate production during adipocyte differentiation. Stable overexpression of Pdk1 or Pdk2 in 3T3-L1 cells stimulated adipocyte differentiation, whereas knockdown of Pdk1/2 or pharmacological inhibition of PDK activity with DCA attenuated adipocyte differentiation. These results suggest that PDK1 and PDK2 play a critical role in fat accumulation.
Previous studies have shown that HIF1α protein expression is increased in differentiated 3T3-L1 cells as well as in adipose tissue of subjects with obesity26. As shown in Fig. 5, HIF-1α protein expression was elevated in Pdk1- or Pdk2-overexpressing 3T3-L1 cells and decreased in shPdk1/2 cells during adipocyte differentiation. Metabolic conversion of glucose to lactate was increased during adipocyte differentiation in 3T3-L1 cells but was hampered by PDK1/2 deficiency. This effect was dependent on the inhibition of lactate production and subsequent inhibition of HIF1α stabilization. Lactate production is increased during adipocyte differentiation21. In the present study, exogenous lactate supplementation induced adipocyte differentiation. Although the mechanism by which lactate promotes adipocyte differentiation remains largely unknown, our study revealed that HIF1α stabilization by lactate may be one of the key mechanisms linking lactate accumulation and adipocyte differentiation via PDK1/2. This possibility is supported by our finding that genetic and pharmacologic inhibition of HIF1α abrogated adipocyte differentiation in either the presence or absence of PDK1/2 overexpression (Fig. 5e, Supplementary Fig. 6f). Furthermore, the LDH inhibitor phenocopied the effect of the HIF1α inhibitor on adipogenesis (Fig. 6d,e). Accumulating evidence shows that increased lactate production, a result of metabolic reprogramming by PDK induction, can mediate HIF-1a stabilization27,28. Furthermore, in a previous paper29, it was proposed that overexpression of C/EBPδ and HIF-1A upregulates the promoter activity of adipocyte-specific genes such as leptin, CFD, HIG2, LPL, and PGAR.
In conjunction with these findings, it should also be noted that HIF1α is the primary regulator of glycolysis (Supplementary Fig. 7). As both PDK1 and PDK2 are known to be transcriptionally regulated by HIF1α30, not only the PDK1/2-lactate-HIF1α axis but also the inverse axis might play a metabolic signaling role in adipogenesis.
We sought to identify the putative transcription factors that can regulate the transcription of PDK1 and PDK2 during adipocyte differentiation by iRegulon analysis based on microarray and ChIP-seq data (Supplementary Tables 3 and 4)31. Interestingly, we found that JUN and FOS might regulate the transcription of PDK1 and PDK2 during adipocyte differentiation. Previous research has suggested that the expression of JUN and FOS is increased together with that of Myc and C/EBP within 1 h after the addition of methylisobutylxanthine, dexamethasone, and insulin32,33 and that transgenic mice expressing a dominant-negative protein that prevents binding to the basic leucine zipper domain of JUN family proteins under the control of the adipose-enriched aP2 enhancer/promoter lack white adipose tissue34. It has also been reported that PPARγ expression is synergistically upregulated by JUN and FOS35. Considering these observations collectively, we hypothesized that JUN and FOS might activate the transcription of PDK1 and PDK2 in the initial phase of adipocyte differentiation. Increased expression of PDK1 and PDK2 by JUN and FOS enforces HIF1a stability to facilitate adipocyte differentiation and obesity.
One of the unexpected findings in the current study was the involvement of PDK1 in adipogenesis. Unlike the roles of PDK2 and PDK4, the role of PDK1 is underexplored in obesity and diabetes. Unlike in vivo studies that demonstrated that adipose tissue-specific PDK2 deficiency was sufficient to slow adiposity upon HFD feeding, our in vitro studies revealed that deletion of PDK1 or PDK2 alone in 3T3-L1 cells was not sufficient to prevent adipogenesis, suggesting that substantial induction of PDH by dual inhibition of PDK1 and PDK2 might be required for sufficient metabolic reprogramming of pyruvate flux into acetyl-CoA to reduce lactate production. Further evaluation of adipose tissue mass in adipose tissue-specific Pdk1-/- rodents would enhance our understanding of the importance of PDK1 in adipogenesis under pathological conditions. Further inhibition of adipogenesis is anticipated in adipose tissue-specific Pdk1/2-/- mice, which were not available for the current study.
We observed a positive correlation between PPARγ and PDK1/2 expression in visceral adipose tissue obtained from individuals with various degrees of obesity. Similarly, PDK1/2 protein levels were decreased in mice fed a HFD compared with mice fed a normal chow diet. Recent literature has shown that chronic HFD feeding in rodents impairs the expression of adipogenic differentiation-related genes36. Similar findings have also been reproduced in humans37. Notably, whereas PDK1/2 expression was decreased, PDK4 expression was robustly increased in adipose tissue of both HFD-fed mice and obese humans (Fig. 6 and Supplementary Fig. 8). Under fasting or HFD-fed conditions, when glucose uptake by adipose tissue is disrupted by insulin deficiency or insulin resistance, respectively, glyconeogenesis in adipose tissue relies on pyruvate derived from lactate or alanine. This ability requires inhibition of PDC activity by induction of PDK438. In obesity, adipogenic differentiation is typically insufficient to meet metabolic demands, and excess calories are primarily stored in preexisting adipocytes, which become overloaded with lipids. This process could explain the decrease in PDK1/2 expression and increase in PDK4 expression in the adipose tissue of individuals with severe obesity.
In summary, PDK2 deficiency in adipose tissue protects against adiposity induced by HFD feeding without reducing glucose tolerance and simultaneously improves insulin sensitivity. In vitro studies demonstrated that PDK1 and PDK2 regulate adipocyte differentiation by enhancing HIF1α expression through lactate-mediated protein stabilization. As the expression of PDKs has been noted in the adipose tissue of humans, pharmacological inhibition of PDK1/2 might also play a critical role in the prevention and treatment of obesity in humans.
Supplementary information
Acknowledgements
This work was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI16C1501); the Basic Science Research Program through the National Research Foundation (NRF) of Korea (NRF-2017R1A2B3006406); the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A6A3A01012921); and NRF grants funded by the Ministry of Science and ICT (NRF-2020R1C1C1012729, NRF-2018R1A5A2024425 and NRF-2018R1D1A1B07047275).
Author contributions
H.-J.K, B.-K.M., W.-I.C., D.W.K., S.P., Y.G., R.A.H., and I.-K.L. generated the hypothesis and designed the experiment. H.-J.K, B.-K.M., H.-J.K., J.-E.B., Y.G., H.J.H., Y.H.J., M.-J.K., and J.Y.L. performed the experiments. B.-K.M., W.-I.C., D.W.K., S.P., A.R.W., R.A.H., J.-H.J., and I.-K.L. discussed the data. H.-J.K, B.-K.M., W.-I.C., D.W.K., S. P, J.-H.J., and R.A.H. wrote the manuscript. Y.K.L. and S.H.C. collected human specimens and analyzed the data. I.-K.L. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hyeon-Ji Kang, Byong-Keol Min, Won-Il Choi, Jae-Han Jeon.
Contributor Information
Sung Hee Choi, Email: drshchoi@snu.ac.kr.
Robert A. Harris, Email: raharris@iu.edu
In-Kyu Lee, Email: leei@knu.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s12276-021-00672-1.
References
- 1.Jeoung NH, Rahimi Y, Wu P, Lee WN, Harris RA. Fasting induces ketoacidosis and hypothermia in PDHK2/PDHK4-double-knockout mice. Biochem. J. 2012;443:829–839. doi: 10.1042/BJ20112197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go Y, et al. Inhibition of pyruvate dehydrogenase kinase 2 protects against hepatic steatosis through modulation of tricarboxylic acid cycle anaplerosis and ketogenesis. Diabetes. 2016;65:2876–2887. doi: 10.2337/db16-0223. [DOI] [PubMed] [Google Scholar]
- 3.Tao R, Xiong X, Harris RA, White MF, Dong XC. Genetic inactivation of pyruvate dehydrogenase kinases improves hepatic insulin resistance induced diabetes. PLoS ONE. 2013;8:e71997. doi: 10.1371/journal.pone.0071997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang Y, et al. Dichloroacetate restores colorectal cancer chemosensitivity through the p53/miR-149-3p/PDK2-mediated glucose metabolic pathway. Oncogene. 2020;39:469–485. doi: 10.1038/s41388-019-1035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutendra G, et al. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene. 2013;32:1638–1650. doi: 10.1038/onc.2012.198. [DOI] [PubMed] [Google Scholar]
- 6.Min BK, et al. Pyruvate dehydrogenase kinase is a metabolic checkpoint for polarization of macrophages to the M1 phenotype. Front. Immunol. 2019;10:944. doi: 10.3389/fimmu.2019.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contractor T, Harris CR. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer Res. 2012;72:560–567. doi: 10.1158/0008-5472.CAN-11-1215. [DOI] [PubMed] [Google Scholar]
- 8.Kang HJ, et al. Dimethylfumarate suppresses adipogenic differentiation in 3T3-L1 preadipocytes through inhibition of STAT3 activity. PloS ONE. 2013;8:e61411. doi: 10.1371/journal.pone.0061411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dusseault J, et al. Nck2 deficiency in mice results in increased adiposity associated with adipocyte hypertrophy and enhanced adipogenesis. Diabetes. 2016;65:2652–2666. doi: 10.2337/db15-1559. [DOI] [PubMed] [Google Scholar]
- 10.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell. Biol. 2015;17:376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikkelsen TS, et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siersbaek R, et al. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 2011;30:1459–1472. doi: 10.1038/emboj.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014;25:293–302. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Q, et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30:249–262. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzi K, et al. A hybrid mechanism of action for BCL6 in B cells defined by formation of functionally distinct complexes at enhancers and promoters. Cell. Rep. 2013;4:578–588. doi: 10.1016/j.celrep.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creyghton MP, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pradeepa MM, et al. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat. Genet. 2016;48:681–686. doi: 10.1038/ng.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newby FD, Wilson LK, Thacker SV, DiGirolamo M. Adipocyte lactate production remains elevated during refeeding after fasting. Am. J. Physiol. 1990;259:E865–E871. doi: 10.1152/ajpendo.1990.259.6.E865. [DOI] [PubMed] [Google Scholar]
- 20.Nellemann B, Gormsen LC, Sorensen LP, Christiansen JS, Nielsen S. Impaired insulin-mediated antilipolysis and lactate release in adipose tissue of upper-body obese women. Obesity. 2012;20:57–64. doi: 10.1038/oby.2011.290. [DOI] [PubMed] [Google Scholar]
- 21.Sabater D, et al. Cultured 3T3L1 adipocytes dispose of excess medium glucose as lactate under abundant oxygen availability. Sci. Rep. 2014;4:3663. doi: 10.1038/srep03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonveaux P, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE. 2012;7:e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma LJ, et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004;53:336–346. doi: 10.2337/diabetes.53.2.336. [DOI] [PubMed] [Google Scholar]
- 25.Nadler ST, et al. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc. Natl Acad. Sci. USA. 2000;97:11371–11376. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Q, et al. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab. 2011;300:E877–E885. doi: 10.1152/ajpendo.00626.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozlov AM, Lone A, Betts DH, Cumming RC. Lactate preconditioning promotes a HIF-1alpha-mediated metabolic shift from OXPHOS to glycolysis in normal human diploid fibroblasts. Sci. Rep. 2020;10:8388. doi: 10.1038/s41598-020-65193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Saedeleer CJ, et al. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS ONE. 2012;7:e46571. doi: 10.1371/journal.pone.0046571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang C, et al. HIF-1A and C/EBPs transcriptionally regulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia. Stem Cell Res. Ther. 2015;6:21. doi: 10.1186/s13287-015-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prigione A, et al. HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells. 2014;32:364–376. doi: 10.1002/stem.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janky R, et al. iRegulon: from a gene list to a gene regulatory network using large motif and track collections. PLoS Comput. Biol. 2014;10:e1003731. doi: 10.1371/journal.pcbi.1003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephens JM, Butts MD, Pekala PH. Regulation of transcription factor mRNA accumulation during 3T3-L1 preadipocyte differentiation by tumour necrosis factor-alpha. J. Mol. Endocrinol. 1992;9:61–72. doi: 10.1677/jme.0.0090061. [DOI] [PubMed] [Google Scholar]
- 33.Stephens JM, Butts M, Stone R, Pekala PH, Bernlohr DA. Regulation of transcription factor mRNA accumulation during 3T3-L1 preadipocyte differentiation by antagonists of adipogenesis. Mol. Cell. Biochem. 1993;123:63–71. doi: 10.1007/BF01076476. [DOI] [PubMed] [Google Scholar]
- 34.Moitra J, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasenfuss SC, et al. Regulation of steatohepatitis and PPARgamma signaling by distinct AP-1 dimers. Cell Metab. 2014;19:84–95. doi: 10.1016/j.cmet.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee TK, et al. HDAC9 knockout mice are protected from adipose tissue dysfunction and systemic metabolic disease during high-fat feeding. Diabetes. 2014;63:176–187. doi: 10.2337/db13-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giusti V, et al. Expression of peroxisome proliferator-activated receptor-gamma1 and peroxisome proliferator-activated receptor-gamma2 in visceral and subcutaneous adipose tissue of obese women. Diabetes. 2003;52:1673–1676. doi: 10.2337/diabetes.52.7.1673. [DOI] [PubMed] [Google Scholar]
- 38.Jeoung NH, Harris RA. Role of pyruvate dehydrogenase kinase 4 in regulation of blood glucose levels. Korean Diabetes J. 2010;34:274–283. doi: 10.4093/kdj.2010.34.5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.