Abstract
Although COVID-19 affects older people more severely, health policies during the first wave of the pandemic often prioritized younger individuals. We investigated whether age had influenced the access to a diagnostic test for SARS-CoV-2 infection and whether clinical complexity and healthcare resources availability could have impacted such differences.
This work included 126,741 Italian participants in the EPICOVID19 web-based survey, who reported having had contacts with known/suspected COVID-19 cases (epidemiological criterion) and/or COVID-19-like signs/symptoms (clinical criterion) from February to June 2020. Data on sociodemographic, medical history and access to SARS-CoV-2 nasopharyngeal swab (NPS) were collected. Logistic regressions estimated the probability of accessing NPS as a function of age and the possible modifying effect of chronic diseases’ number and residential areas in such association.
A total of 6136 (4.8%) participants had undergone an NPS. Older participants had lower NPS frequencies than the younger ones when reporting epidemiological (14.9% vs. 8.8%) or both epidemiological and clinical criteria (17.5% vs. 13.7%). After adjustment for potential confounders, including epidemiological and clinical criteria, the chance of NPS access decreased by 29% (OR=0.71, 95%CI:0.63–0.79) in older vs. younger individuals. Such disparity was accentuated in areas with greater healthcare resources.
In conclusion, in the first wave of the pandemic, age may have affected the access to COVID-19 diagnostic testing, disadvantaging older people.
Keywords: COVID-19 diagnostic testing, Health Resources, Multimorbidity, Ageism
Introduction
Although COronaVIrus Disease 2019 (COVID-19) cases have been reported across all age classes, up to June 2020, the large majority of cases occurred in individuals above the age of 30, with marked increases among those aged 50 years or older [1], [2], [3]. Age-related differences have also been noted in the disease's clinical course, with older patients showing more complicated diseases and worse prognosis than the younger ones, especially in Western countries [2], [3], [4], [5], [6], [7]. Indeed, around eight out of ten deaths due to COVID-19 in the US occurred in individuals aged 65 and over [7]. The greater vulnerability of older people to SARS-CoV-2 infection in terms of incidence and prognosis may be due to several factors. Among these, age-related changes in the immune system [8] and increases in the prevalence of cardiovascular diseases, metabolic disorders, and multimorbidity [4] may play a relevant role. Moreover, a recent study found that frailty, a prevalent condition in old age [9], could predict in-hospital mortality of COVID-19 patients better than age or comorbidities [10].
Despite the greater burden of COVID-19 observed in advanced age, during the first wave of the pandemic, the health policies implemented to face the outbreak have often been prioritizing younger individuals, especially in countries more severely affected by the pandemic [11], [12], [13]. For instance, some national scientific societies hypothesized the need to consider an age cut-off in the decision-making for intensive care allocation in case of an extreme shortage of healthcare resources [14,15]. Regarding the access to diagnostic testing and the implementation of preventive measures in at-risk individuals, such as nursing home residents or frail individuals managed at home, healthcare systems proved to be completely unprepared, often leaving physicians, caregivers and patients without any support [16,17]. However, whether disparities in care delivery between young and older people were driven by ageism or by other factors is still unclear [11], [12], [13].
In this study, we focused on possible differences by age in accessing diagnostic test for SARS-CoV-2 infection. Following national and international guidelines, especially in the first months of the pandemic, the recommendation for a SARS-CoV-2 nasopharyngeal swab (NPS) testing was based on the report of contacts with COVID-19 cases (epidemiological criterion) and signs or symptoms compatible with SARS-CoV-2 infection (clinical criterion) [18]. In this context, evaluating whether age-related disparities in access to diagnostic procedures occurred is not only of social but also clinical relevance. Indeed, early identification and confirmation of COVID-19 could favor the implementation of safe preventive measures (e.g., physical distancing), as well as the effectiveness of the provided treatments.
This study aimed to investigate whether advanced age might have influenced the access to diagnostic testing for SARS-CoV-2 infection. Moreover, we assessed to which extent potential age-related differences were associated with the individual's clinical complexity, i.e., the number of chronic diseases or the availability of healthcare resources in the residence area. We hypothesized that: 1) for a given clinical and/or epidemiological indication, older people might have had less access to diagnostic testing for SARS-CoV-2 compared to younger individuals; and, 2) disparities in access to nasopharyngeal swab (NPS) could have been exacerbated by clinical complexity and lower local availability of healthcare resources.
Methods
Study design and study population
This study used data from EPICOVID19, a national Italian internet-based survey developed by a group of epidemiologists, researchers, and clinicians during the first wave of the COVID-19 pandemic. The survey was launched on April 13th, 2020, and targeted adult volunteers who filled in the online questionnaire until May 31st, 2020. The survey was promoted by social media (WhatsApp, Facebook, Instagram, Twitter), press releases, local radio and TV, and institutional websites. Inclusion criteria were: age >18 years; having the possibility to access a mobile phone, computer, or tablet with internet connectivity; and giving the online informed consent to participate in the study. The EPICOVID19 questionnaire was developed after reviewing scientific literature, WHO protocols, and instruments used for previous pandemics [19,20]. Of the total 38 items composing the questionnaire, some were developed ad-hoc for the purposes of the study, and some others were derived from validated scales in order to guarantee the harmonization and comparability of our data with that of other studies. Details on the questionnaire's content have been previously described [20,21]. Overall, the areas covered were the following: sociodemographic data, clinical status, personal characteristics and health status, housing conditions, lifestyle and behaviors during the lockdown. The EPICOVID19 study protocol was approved by the Ethics Committee of the Istituto Nazionale per le Malattie Infettive I.R.C.C.S. Lazzaro Spallanzani (Protocol No.70, 12/4/2020). At their first access to the online platform, participants filled in the informed consent. The study complies with the principles of the Declaration of Helsinki. Data were handled and stored following the European Union General Data Protection Regulation (EU GDPR) 2016/679 (http://gdpr-info.eu/); data transfer included encrypting/decrypting and password protection.
Data collection
Of the data collected through the EPICOVID19 questionnaire, for the present study we considered the following sociodemographic and lifestyle information: age, sex, education (categorized as low [primary school or less], middle [middle or high school], and high [university or post-graduate degree]), occupational status (categorized as currently employed, student, unemployed, retired, or other), and smoking habit (classified as never, former or current smokers). Whether the responder was a healthcare professional or not was also recorded. Geographical areas of residence were classified as Italian regions, the Republic of San Marino, other countries, and unknown. Italian regions were categorized into four areas based on the following ratio: (total nasopharyngeal swabs [NPS] performed / total individuals tested at least once) / (total COVID-19 cases / total individuals tested at least once), from national data [22]. The numerator of the ratio provides a measure of NPS availability in each region, while the denominator indicates the disease's local prevalence. The ratio between these elements allows the evaluation of the NPS availability weighted by the COVID-19 prevalence in each region, with a higher ratio corresponding to greater regional resources allocated to perform the NPS test. The four areas identified with increasing ratio were: Area 1: Piedmont, Lombardy, Aosta Valley, Emilia Romagna, Liguria, Marche; Area 2: Tuscany, Trentino Alto Adige, Abruzzo, Apulia; Area 3: Veneto, Friuli Venezia Giulia, Lazio, Molise, Campania; Area 4: Sicily, Sardinia, Umbria, Calabria and Basilicata (regional data are reported in Supplementary Table 1).
As far as participants’ health status, self-reported data on chronic conditions and medications regularly taken allowed to detect the presence of the following diseases: lung diseases, cardiovascular diseases (CVD), arterial hypertension, diabetes pharmacologically treated, chronic kidney diseases, immunologic diseases, cancer, metabolic diseases, liver diseases, thyroid diseases pharmacologically treated, and depression and/or anxiety. The sum of the above chronic conditions resulted in the total number of chronic diseases, categorized as none, one, and two or more (the latter indicating multimorbidity status).
Among the COVID-19-specific information, for this study, we considered:
-
a)
signs/symptoms self-reported between February and May 2020, including fever >37.5° for at least three consecutive days, cough, headache, myalgia, smell or taste disorders, shortness of breath, sore throat/rhinorrhea, chest pain, feeling of a fast beat, gastrointestinal disturbances, conjunctivitis, and pneumonia;
-
b)
month at the onset of the symptoms and conditions mentioned above (February, March, April, or May 2020);
-
c)
contact with any confirmed or suspected COVID-19 cases;
-
d)
access to at least one NPS test for diagnosis of SARS-CoV-2 infection.
Having reported at least one sign/symptom among the above mentioned was used to define the “clinical criterion.” Having reported any contact with known or suspected COVID-19 cases was used to determine the “epidemiological criterion.”
Statistical analysis
The sample characteristics are described as mean ± standard deviation (SD) for continuous variables and as frequency and percentage for categorical variables. Comparison of such characteristics between individuals who did vs. did not receive at least one SARS-CoV-2 NPS was performed through Student t-and chi-square tests for continuous and categorical variables, respectively. The chi-square test was also used to compare the frequency of NPS between adult (age <65 years) and older (age ≥65 years) individuals based on the report of epidemiologic and/or clinical criteria to perform the test.
The chance of accessing a SARS-CoV-2 NPS as a function of age (≥ vs. <65 years) was evaluated through binary logistic regression. Models were run first unadjusted and, second, adjusted for factors that may be potential confounders in the association between age and the chance of accessing an NPS, i.e. sex, educational level, epidemiological and/or clinical criteria, respiratory diseases, CVD, arterial hypertension, and month at symptoms’ onset.
The roles of the number of chronic diseases and of the area of residence as effect modifiers on the association between age and the chance of accessing a SARS-CoV-2 NPS were tested through interaction analyses (i.e., by including the multiplicative interaction term “age*number of chronic diseases” or “age*area of residence”, in the multivariable model) and by stratifying the multivariable logistic regressions according to such factors.
As sensitivity analyses, we tested the above associations, first, after excluding participants who worked in healthcare services (n = 11,657); and, second, considering only the subsample of participants who contacted the emergency number and/or the general practitioner to report symptoms of suspected infection by COVID-19 (n = 18,974). Two-tail p-values <0.05 were considered statistically significant. All the analyses were performed using R [23].
Results
From the total 198,828 survey participants, for this study, we excluded 337 nursing home residents and 292 professionals working in care facilities who underwent SARS-CoV-2 NPS test due to screening procedure; and 71,458 individuals who reported neither clinical nor epidemiological criteria to justify the execution of an NPS test. The final study population included 126,741 adult (n = 113,286) and older individuals (n = 13,455). The characteristics of the sample as a whole and categorized based on NPS access are shown in Table 1 . The participants’ mean age was 46.3 ± 14.1 years, 37.9% were men, and 61.3% had a high educational level. Women, individuals with higher education, employed, especially healthcare professionals, were more likely to have done at least one NPS.
Table 1.
Characteristics of the sample as a whole and by access to nasopharyngeal swab.
| Nasopharyngeal swab |
||||
|---|---|---|---|---|
| All n = 126,741) | Not done (n = 120,605) | Done (n = 6136) | p-value | |
| Age (years) | 46.29 (14.10) | 46.28 (14.16) | 46.43 (12.80) | 0.411 |
| Age ≥65 years | 13,455 (10.6) | 13,071 (10.8) | 384 (6.3) | <0.001 |
| Sex (M) | 48,008 (37.9) | 45,858 (38.0) | 2150 (35.0) | <0.001 |
| Educational level | <0.001 | |||
| Low | 6659 (5.3) | 6414 (5.3) | 245 (4.0) | |
| Middle | 42,418 (33.5) | 41,113 (34.1) | 1305 (21.3) | |
| High | 77,664 (61.3) | 73,078 (60.6) | 4586 (74.7) | |
| Occupation | <0.001 | |||
| Employed | 90,952 (71.8) | 85,552 (70.9) | 5400 (88.0) | |
| Student | 9279 (7.3) | 9103 (7.5) | 176 (2.9) | |
| Unemployed | 6049 (4.8) | 5944 (4.9) | 105 (1.7) | |
| Retired | 13,641 (10.8) | 13,355 (11.1) | 286 (4.7) | |
| Other | 6820 (5.4) | 6651 (5.5) | 169 (2.8) | |
| Healthcare professional | 11,657 (9.2) | 8431 (7.0) | 3226 (52.6) | <0.001 |
| Smoking habit | <0.001 | |||
| Never | 72,284 (57.0) | 68,489 (56.8) | 3795 (61.8) | |
| Former | 30,427 (24.0) | 29,059 (24.1) | 1368 (22.3) | |
| Current | 24,030 (19.0) | 23,057 (19.1) | 973 (15.9) | |
| Chronic diseases (number) | 0.325 | |||
| 0 | 71,631 (56.5) | 68,158 (56.5) | 3473 (56.6) | |
| 1 | 31,202 (24.6) | 29,657 (24.6) | 1545 (25.2) | |
| 2+ | 23,908 (18.9) | 22,790 (18.9) | 1118 (18.2) | |
| Respiratory diseases | 8505 (6.7) | 8031 (6.7) | 474 (7.7) | 0.001 |
| Cardiovascular diseases | 8921 (7.0) | 8547 (7.1) | 374 (6.1) | 0.003 |
| Arterial hypertension | 19,084 (15.1) | 18,102 (15.0) | 982 (16.0) | 0.035 |
| Diabetes pharmacologically treated | 2575 (2.0) | 2449 (2.0) | 126 (2.1) | 0.938 |
| Chronic kidney diseases | 1086 (0.9) | 1040 (0.9) | 46 (0.7) | 0.388 |
| Immunologic diseases | 12,310 (9.7) | 11,718 (9.7) | 592 (9.6) | 0.878 |
| Cancer | 3819 (3.0) | 3644 (3.0) | 175 (2.9) | 0.472 |
| Metabolic diseases | 10,903 (8.6) | 10,408 (8.6) | 495 (8.1) | 0.131 |
| Liver diseases | 972 (0.8) | 930 (0.8) | 42 (0.7) | 0.494 |
| Depression-anxiety | 11,478 (9.1) | 11,059 (9.2) | 419 (6.8) | <0.001 |
| Self-reported symptoms and conditions | ||||
| None | 6941 (5.5) | 5947 (4.9) | 994 (16.2) | <0.001 |
| Fever | 16,717 (13.2) | 14,829 (12.3) | 1888 (30.8) | <0.001 |
| Cough | 42,082 (33.2) | 39,670 (32.9) | 2412 (39.3) | <0.001 |
| Headache | 55,096 (43.5) | 52,440 (43.5) | 2656 (43.3) | 0.774 |
| Myalgia | 39,395 (31.1) | 36,912 (30.6) | 2483 (40.5) | <0.001 |
| Olfactory or taste disorders | 11,065 (8.7) | 9572 (7.9) | 1493 (24.3) | <0.001 |
| Shortness of breath | 10,867 (8.6) | 9820 (8.1) | 1047 (17.1) | <0.001 |
| Sore throat/rhinorrhea | 64,871 (51.2) | 62,240 (51.6) | 2631 (42.9) | <0.001 |
| Chest pain | 13,221 (10.4) | 12,213 (10.1) | 1008 (16.4) | <0.001 |
| Feeling of fast beating | 12,545 (9.9) | 11,636 (9.6) | 909 (14.8) | <0.001 |
| Gastrointestinal disturbances | 33,223 (26.2) | 31,211 (25.9) | 2012 (32.8) | <0.001 |
| Conjunctivitis | 18,882 (14.9) | 18,038 (15.0) | 844 (13.8) | 0.010 |
| Pneumonia | 1156 (0.9) | 595 (0.5) | 561 (9.1) | <0.001 |
| Month at symptoms’ onset | <0.001 | |||
| No symptoms | 6941 (5.5) | 5947 (4.9) | 994 (16.2) | |
| February | 64,967 (51.3) | 63,192 (52.4) | 1775 (28.9) | |
| March | 46,842 (37.0) | 43,874 (36.4) | 2968 (48.4) | |
| April | 7953 (6.3) | 7560 (6.3) | 393 (6.4) | |
| May | 38 (0.0) | 32 (0.0) | 6 (0.1) | |
| Criteria | <0.001 | |||
| Epidemiological | 6941 (5.5) | 5947 (4.9) | 994 (16.2) | |
| Clinical | 98,683 (77.9) | 97,196 (80.6) | 1487 (24.2) | |
| Epidemiological and clinical | 21,117 (16.7) | 17,462 (14.5) | 3655 (59.6) | |
| Contact with physicians to report symptoms of suspected COVID-19 | 18,974 (15.0) | 15,910 (13.2) | 3064 (49.9) | <0.001 |
| Area of residence | <0.001 | |||
| Area 1 | 76,692 (61.0) | 73,197 (61.2) | 3495 (57.6) | |
| Area 2 | 14,886 (11.8) | 14,166 (11.8) | 720 (11.9) | |
| Area 3 | 26,458 (21.1) | 24,933 (20.8) | 1525 (25.1) | |
| Area 4 | 7321 (5.8) | 7013 (5.9) | 308 (5.1) | |
| Other | 297 (0.2) | 280 (0.2) | 17 (0.3) | |
Notes: Number are mean (SD) for the continuous variables and frequency (%) for the categorical ones. P-values refer to the Student t-test (for continuous variables) or chi-square test (for categoric variables) to compare the characteristics of individuals who performed vs. did not perform nasopharyngeal swab. Area 1 includes Piedmont, Lombardy, Emilia Romagna, Liguria, Marche, and Aosta Valley. Area 2 includes Tuscany, Trentino Alto Adige, and Apulia. Area 3 includes Veneto, Lazio, Friuli Venezia Giulia, Molise, and Campania. Area 4 includes Sicily, Sardinia, Umbria, Calabria, and Basilicata. Other includes the Republic of San Marino, other countries, and unknown.
Almost half of participants had at least one chronic disease, and around one out of five were multimorbid, without relevant differences by access to NPS. Individuals who received an NPS (n = 6136, 4.8% of the total sample) were more likely to have respiratory diseases and arterial hypertension than those who did not, while they reported a lower prevalence of CVD and depression-anxiety disorders. Considering the criteria to access the NPS, 5.5% met only the epidemiological criterion, 77.9% reported only the clinical criterion (the most frequent symptoms being sore throat/rhinorrhea, headache, cough, and myalgia), and 16.7% met both the epidemiological and clinical criteria.
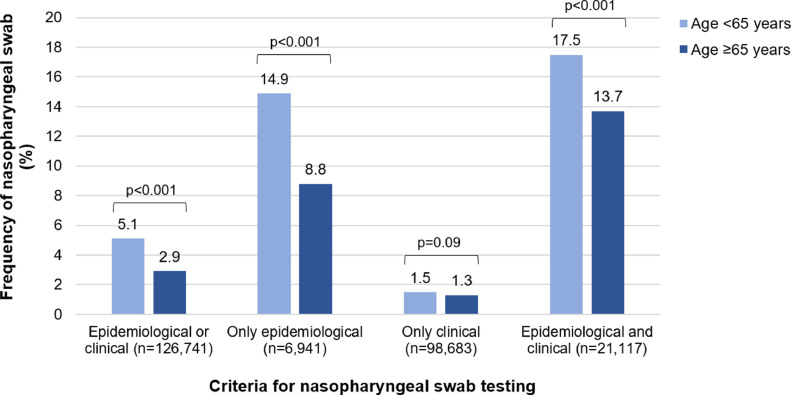
Fig. 1 compares the frequency of having access to at least one NPS between individuals aged <65 vs. ≥65 years, according to the report of epidemiological and/or clinical criteria. As illustrated, older participants showed significantly lower frequencies of NPS access than the younger ones in the presence of only epidemiological (14.9% vs. 8.8%) or both epidemiological and clinical criteria (17.5% vs. 13.7%). Moreover, considering the individuals who performed the NPS and had available test results (5241 individuals aged <65 years, and 361 individuals aged ≥65 years), we found that the ratio between the positive and negative NPS was 32% in the younger and 74% in the older group. In the subsample of 18,974 participants (15.5% of those <65 years, and 10.3% of those ≥65 years) who had contacted the emergency number and/or the general practitioner for suspected symptoms of COVID-19, we found a higher proportion of total performed NPS (16.1% vs. 2.9%) and positive NPS (37.7% vs. 8.7%), compared with individuals who did not seek medical attention. In this subsample, no differences in the frequency of performed NPS between young and older individuals were observed (data not shown).
Fig. 1.
Frequency of swab testing in young and older individuals by epidemiological and clinical criteria
Notes: P-values refer to the chi-square test to compare the frequency of nasopharyngeal swab test between individuals aged < vs. ≥65 years. Epidemiological criterion concerned any reported contact with a suspected or confirmed COVID-19 case. Clinical criterion concerned any reported symptom or condition in the months February to May 2020, among fever, cough, headache, myalgia, olfactory or taste disorders, shortness of breath, sore throat/rhinorrhea, chest pain, feeling of a fast beat, gastrointestinal disorders, conjunctivitis, and pneumonia.
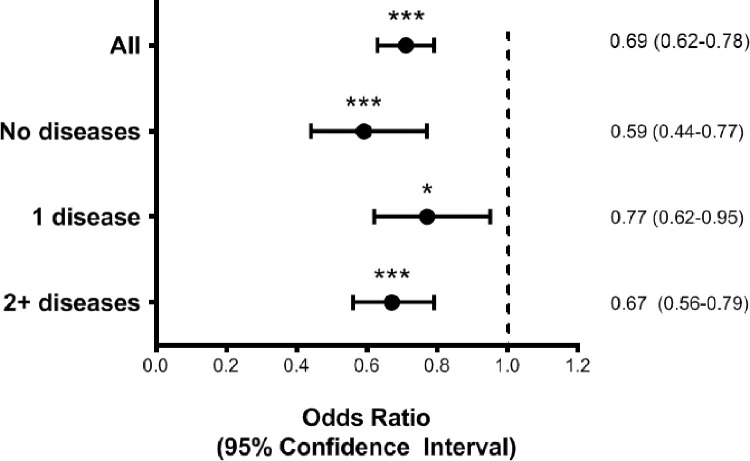
At binary logistic regression (Fig. 2 , Supplementary Table S2), after adjusting for potential confounders, including reported epidemiological and clinical criteria, the chance of accessing an NPS test was reduced by around 30% (OR=0.69, 95%CI:0.62–0.78) in individuals aged ≥65 years compared with those younger. Results did not substantially differ when excluding the participants working in healthcare services (data not shown).
Fig. 2.
Binary logistic regression for the association between age ≥65 years (vs. <65 years) and access to nasopharyngeal swab, in the sample as a whole and stratified by number of chronic diseases
Notes. Odds ratios derive from a binary logistic regression with age≥65 years (reference category <65 years) as main exposure and access to a nasopharyngeal swab (yes vs. no) as the outcome. Model is adjusted for sex, educational level, criteria for a nasopharyngeal swab, respiratory diseases, cardiovascular diseases, arterial hypertension, area of residence, month at symptoms’ onset. *p<0.05, ***p<0.001.
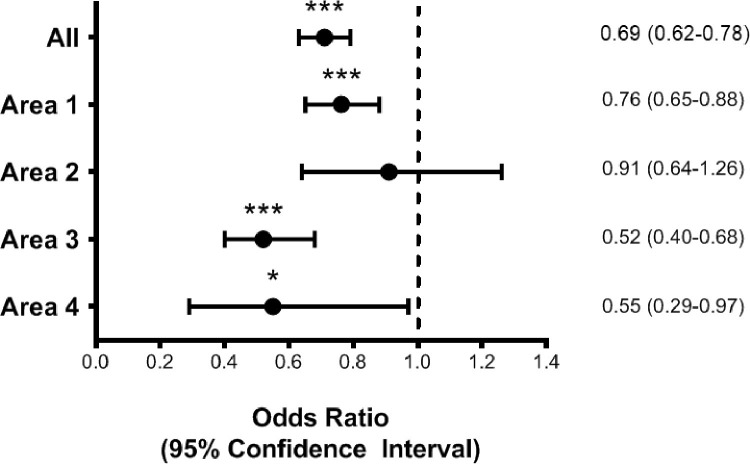
Concerning the possible modifying effect of the number of chronic diseases on the association between age and the chance of NPS access, we did not find any significant interaction between age and the number of chronic diseases (pinteraction = 0.36). However, the reduced probability of performing an NPS in older age seemed to be slightly stronger among individuals with no chronic diseases than those with one or ≥2 conditions (Fig. 2, Supplementary Table S3). The association between age and NPS access seemed to be significantly modified by geographical area (pinteraction = 0.005). When stratifying by area of residence (Fig. 3 , Supplementary Table S4), we found that the reduction in the chance of accessing NPS for older compared with younger individuals ranged from −24% in the regions with lower health resources (Area 1) to −48 and −45% in those with greater resources (Areas 3 and 4).
Fig. 3.
Binary logistic regression for the association between age ≥65 years (vs. <65 years) and access to nasopharyngeal swab, in the sample as a whole and stratified by area of residence
Notes. Odds ratios derive from a binary logistic regression with age ≥65 years (reference category <65 years) as main exposure and access to a nasopharyngeal swab (yes vs. no) as the outcome. Model is adjusted for sex, educational level, criteria for a nasopharyngeal swab, respiratory diseases, cardiovascular diseases, arterial hypertension, month at symptoms’ onset. Area 1 includes Piedmont, Lombardy, Emilia Romagna, Liguria, Marche, and Aosta Valley (proportion of individuals aged ≥65 years: 10.9%). Area 2 includes Tuscany, Trentino Alto Adige, and Apulia (proportion of individuals aged ≥65 years: 10.0%). Area 3 includes Veneto, Lazio, Friuli Venezia Giulia, Molise, and Campania (proportion of individuals aged ≥65 years: 9.9%). Area 4 includes Sicily, Sardinia, Umbria, Calabria, and Basilicata. The 297 individuals living in the Republic of San Marino, other countries, or unknown are excluded from the analysis (proportion of individuals aged ≥65 years: 7.1%). *p<0.05, ***p<0.001.
Discussion
Our results show that, in the first wave of the COVID-19 pandemic, older people, except those with a clear-cut clinical indication, were less likely to be tested for SARS-CoV-2 infection than younger individuals. Such a difference was more evident in the areas with greater healthcare resources availability.
The sample included in this study was composed only of participants who reported either contact with confirmed/suspected COVID-19 cases or signs/symptoms that may be consistent with SARS-CoV-2 infection. However, when considering the access to SARS-CoV-2 NPS, less than 5% of the sample declared to have undergone a diagnostic test. NPS frequency increased for those who reported the epidemiological criterion either alone or combined with the clinical one.
Focusing on the clinical criterion, it is noteworthy that most of the individuals who reported suspected symptoms of SARS-CoV-2 infection did not seek medical attention. This suggests that, in the first months of the outbreak, there was a tendency to under-recognize or underestimate the onset of COVID-19-like symptoms or confusion about the most appropriate actions to be taken in such an event. When considering the epidemiological criterion, it should be kept in mind that recommendations on access to diagnostic tests progressively changed over the first months of the pandemic. Indeed, the indication to perform an NPS test in Italy was initially directed toward individuals who presented both the clinical and epidemiological criteria (including also recent staying or travelling through “COVID-19 areas”). At a later phase, instead, the epidemiological criterion was no longer needed to define suspected cases and plan appropriate diagnostic procedures [24]. Despite these considerations, even within the category reporting both the clinical and the epidemiological criteria, we found that less than one out of five individuals were tested. This figure confirms the lack of resilience of our healthcare system in such an emergency, which was more marked in the first months of the outbreak [17].
As hypothesized, in conditions of scarcity of resources, we found that older individuals were less likely to access a SARS-CoV-2 NPS even when reporting the same criteria to justify the need for a diagnostic test. This difference was more evident in the presence of the epidemiological criterion alone or combined with the clinical criterion. On the contrary, such disparity was not observed among those who contacted the emergency number or their general practitioner for suspected symptoms of SARS-CoV-2 infection. Comparing the individuals who sought medical attention with those who did not, we found that the formers were more likely to have a positive NPS. Therefore, we can argue that the clinical pattern reported by such individuals could have been quite suggestive of COVID-19 to the point of indicating the execution of a diagnostic test, irrespective of individual age. Conversely, individuals who probably contacted their physicians due to general or unclear symptoms or contacts with suspected/confirmed COVID-19 cases had lesser chances to be tested, especially the oldest ones. These findings may underline a tendency of underestimating the exposure to possible COVID-19 cases in older people who, despite having lesser working and social interactions than younger individuals, could equally be at risk of contagion due, for example, to contacts with relatives, caregivers, or healthcare professionals.
Simultaneously, considering the reported symptoms, the lower frequency of NPS in old than in young people may be due to the misperception that participants had of their health status and risk of getting COVID-19 or the under-recognition of symptoms likely attributed to other diseases. However, even when adjusting the analyses by the presence of some chronic conditions that might confound the symptomatologic pattern, as well as by epidemiological/clinical criteria and time, we found that older age was still associated with a 30% lower chance of undergoing a SARS-CoV-2 NPS.
Overall, these results may support the hypothesis that, except for the most suggestive COVID-19 cases, age per se may have been a factor limiting NPS access during the first wave of the pandemic [11], [12], [13]. In line with the possible ageist attitude which considers older people as a homogeneous category of vulnerable individuals [13], in our study, the reduced probability of accessing an NPS in older than in younger individuals did not seem to be influenced by the individual clinical complexity.
Surprisingly, when investigating the modifying effect of the local availability of healthcare resources, we observed the most marked reduction in the probability of accessing an NPS related to older age in the areas with greater healthcare resources. Indeed, older individuals living in regions with the lowest resources had a 24% lower chance of accessing an NPS than their younger counterparts. In comparison, in areas with greater resources, such reduction accentuated up to 48%. Although our findings may have been affected by the low representation of participants in some geographical areas, they could reflect the tendency of health policies during the COVID-19 pandemic to invest more resources in the diagnostic testing for the younger individuals, who were probably primarily responsible for the spread of the disease and, following some guidelines, had priority in accessing intensive care [14,15]. According to our results, such a tendency may have been exacerbated in the areas where greater resources were available, which likely promoted wider screening programs for the young and adult population in respect to the older one. Although it is reasonable addressing diagnostic and screening tests to people with higher social interactions, it should be acknowledged that the disproportional implementation of these policies would be at the expense of the older individuals. Indeed, this issue may have led to the under-identification and undertreatment of COVID-19 in older people, particularly in the frailest categories (e.g., institutionalized individuals [16,17]) and a greater need for more intensive care due to delays in the disease diagnosis. Several studies have demonstrated that the burden of ageism may have substantial detrimental effects worldwide, both at the individual and structural levels [25]. Identifying the areas where age-related inequalities occur and assessing the aspects that influence such phenomena is the basis to assure equal rights to healthcare for everyone [26]. In this regard, the COVID-19 pandemic is just the Pandora's Box that evidenced the need to reinforce primary and secondary care for older people. In particular, high priority should be posed to the implementation of home-based care programs that could facilitate access to health services for people with coexistent conditions of frailty, disability, and multimorbidity [27].
Concerning the work's limitations, first, the urgency of developing the EPICOVID19 survey during the first pandemic wave did not allow us to perform a formal validation of the questionnaire, which is currently ongoing. Second, the collection of self-reported information linked to using survey data may be a possible recall bias source. Third, the web-based media to administrate the questionnaire limited the participation of individuals with low technological skills and, particularly, of the oldest ones, leading to an underrepresentation of this population compared to the general Italian population. This aspect, along with the voluntary basis of the survey, may have determined a selection bias and a possible underestimation of NPS testing in the oldest age categories. On the other hand, the prevalence of NPS testing in our sample was in line with national data for the same period and the geographic representativeness of the survey respondents corresponded to the spread of the pandemic in Italy [28], supporting the generalizability of our findings. Finally, we recognize that the parameter used to estimate healthcare resource availability could be biased by other factors related to the specific health policies implemented at the regional level. However, we think that our results may give important insights at a societal level and in a public health perspective, highlighting the possible emergence of an ageist attitude in the scarcity of resources. On the other hand, the work's strength lies in the large sample of participants who contributed to the present study with a broad set of information on their health condition, COVID-19-related symptoms, and received diagnostic tests.
Conclusions
Our study suggests that older people had a lower probability of access to an NPS than younger individuals in the first wave of the pandemic. This disparity was more marked if the diagnostic test's indication had to be determined based on confounding clinical patterns or contacts with individuals likely affected by COVID-19. Such age-related differences seemed to occur irrespective of healthcare resource availability. Further studies are needed to confirm these findings in other contexts and possibly explore the roots of such behavior to remove any age-related obstacle to healthcare access and delivery.
Funding sources
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
Ethics and consent form
The Ethics Committee of the Istituto Nazionale per le Malattie Infettive IRCCS Lazzaro Spallanzani approved the study protocol of EPICOVID19 (Protocol No. 70, 12/4/2020). Participants filled in the informed consent at their first access to the online platform. The study complies with the principles of the Declaration of Helsinki. Data were handled and stored in accordance with the European Union General Data Protection Regulation (EU GDPR) 2016/679; data transfer included encrypting/decrypting and password protection.
CRediT authorship contribution statement
Caterina Trevisan: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Claudio Pedone: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Stefania Maggi: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft. Marianna Noale: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Mauro Di Bari: Conceptualization, Supervision, Writing – original draft. Aleksandra Sojic: Investigation, Writing – review & editing. Sabrina Molinaro: Conceptualization, Investigation, Supervision, Writing – review & editing. Andrea Giacomelli: Conceptualization, Investigation, Writing – review & editing. Fabrizio Bianchi: Conceptualization, Investigation, Writing – review & editing. Marcello Tavio: Conceptualization, Investigation, Supervision, Writing – review & editing. Stefano Rusconi: Investigation, Supervision, Writing – review & editing. Gabriele Pagani: Investigation, Supervision, Writing – review & editing. Massimo Galli: Conceptualization, Investigation, Supervision, Writing – review & editing. Federica Prinelli: Conceptualization, Data curation, Investigation, Investigation, Methodology, Project administration, Writing – original draft. Fulvio Adorni: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft. Raffaele Antonelli Incalzi: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft.
Declaration of Competing Interest
None.
Acknowledgement
We are thankful to all the study participants for their valuable contribution to this initiative.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.healthpol.2021.10.002.
Appendix. Supplementary materials
References
- 1.Natale F., Ghio D., Tarchi D., Goujon A., Conte A. COVID-19 Cases and Case Fatality Rate by age 2020. https://ec.europa.eu/knowledge4policy/sites/know4pol/files/jrc120420_covid_risk_and_age.pdf.
- 2.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M., et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Guo T., Shen Q., Guo W., He W., Li J., Zhang Y., et al. Clinical characteristics of elderly patients with COVID-19 in Hunan Province, China: a multicenter, retrospective study. Gerontology. 2020:1–9. doi: 10.1159/000508734. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J.F., Korevaar D.A., Matczak S., Brice J., Chalumeau M., Toubiana J. COVID-19-related mortality by age groups in Europe: a meta-analysis. MedRxiv. 2020 doi: 10.1101/2020.04.11.20061721. 2020.04.11.20061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Older Adults and COVID-19 | CDC n.d. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed August 16, 2020).
- 8.Mueller A.L., Mcnamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect the elderly? Preprints. 2020:1–32. doi: 10.20944/preprints202004.0548.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt J., Carter B., Vilches-Moraga A., Quinn T.J., Braude P., Verduri A., et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Heal. 2020:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colenda C.C., Reynolds C.F., Applegate W.B., Sloane P.D., Zimmerman S., Newman A.B., et al. COVID-19 pandemic and ageism: a call for humanitarian care. J Am Geriatr Soc. 2020;68:1627–1628. doi: 10.1111/jgs.16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser S., Lagacé M., Bongué B., Ndeye N., Guyot J., Bechard L., et al. Ageism and COVID-19: what does our society’s response say about us? Age Ageing. 2020 doi: 10.1093/ageing/afaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayalon L. There is nothing new under the sun: ageism and intergenerational tension in the age of the COVID-19 outbreak. Int Psychogeriatr. 2020:1–4. doi: 10.1017/S1041610220000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergano M., et al. Condizioni eccezionali di squilibrio tra necessità e risorse disponibili. 2020. Raccomandazioni di etica clinica per l'ammissione a trattamenti intensivi e per la loro sospensione. [DOI] [PubMed] [Google Scholar]
- 15.Zucker H., Adler K., Berens D. New York State Department of Health Task Force on Life and the Law; Albany: 2015. Ventilator allocation guidelines. [Google Scholar]
- 16.Kemenesi G., Kornya L., Tóth G.E., Kurucz K., Zeghbib S., Somogyi B.A., et al. Nursing homes and the elderly regarding the COVID-19 pandemic: situation report from Hungary. GeroScience. 2020;1 doi: 10.1007/s11357-020-00195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volpato S., Landi F., Incalzi R.A. A frail health care system for an old population: lesson form the COVID-19 outbreak in Italy. J Gerontol A. 2020;XX:1–2. doi: 10.1093/gerona/glaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May 2020. Case definition for coronavirus disease 2019 (COVID-19), as of 29.https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition n.d. (accessed August 17, 2020) [Google Scholar]
- 19.Adorni F., Prinelli F., Bianchi F., Giacomelli A., Pagani G., Bernacchia D., et al. Self-reported symptoms of SARS-CoV-2 infection in a non-hospitalized population: results from the large Italian web-based EPICOVID19 cross-sectional survey. JMIR Public Heal Surveill. 2020 doi: 10.2196/21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastiani L., Fortunato L., Pieroni S., Bianchi F., Adorni F., Prinelli F., et al. EPICOVID19: psychometric assessment and validation of a short diagnostic scale for a rapid Covid-19 screening based on reported symptoms. MedRxiv. 2020 doi: 10.1101/2020.07.22.20159590. 2020.07.22.20159590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prinelli F., Bianchi F., Drago G., Ruggieri S., Sojic A., Jesuthasan N., et al. Association between smoking and SARS-CoV-2 infection: cross-sectional study of the EPICOVID19 internet-based survey. JMIR Public Heal Surveill. 2021;7 doi: 10.2196/27091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COVID-19 scheda Regioni n.d. https://github.com/pcm-dpc/COVID-19/blob/master/schede-riepilogative/regioni/dpc-covid19-ita-scheda-regioni-20200709.pdf (accessed July 9, 2020).
- 23.R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2008. R: a language and environment for statistical computing. ISBN 3-900051-07-0, URL http://www.R-project.org. n.d. [Google Scholar]
- 24.Tartaglione M., Gamberini L., Semeraro F., Lupi C., Coniglio C., Gordini G. COVID-19 suspicion and diagnosis: are we still chasing epidemiological criteria? J Med Virol. 2020 doi: 10.1002/jmv.26042. jmv.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang E.-.S., Kannoth S., Levy S., Wang S.-.Y., Lee J.E., Levy B.R. Global reach of ageism on older persons’ health: a systematic review. PLoS ONE. 2020;15 doi: 10.1371/JOURNAL.PONE.0220857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’cruz Migita, Banerjee Debanjan. An invisible human rights crisis”: the marginalization of older adults during the COVID-19 pandemic - an advocacy review. Psychiatry Res. 2020;292:113369. doi: 10.1016/J.PSYCHRES.2020.113369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebrasseur Audrey, Fortin-Bédart Noémie, Lettre Josiane, Raymond Emilie, Brussières Eveline, Lapierre Nolwenn, et al. Impact of the COVID-19 pandemic on older adults: rapid review. JMIR Aging. 2021;4(2):e26474. doi: 10.2196/26474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GitHub - pcm-dpc/COVID-19: COVID-19 Italia - Monitoraggio situazione n.d. https://github.com/pcm-dpc/COVID-19 (accessed July 16, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.