Abstract
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic taking the lives of millions. The virus itself not only invades and destroys the angiotensin-converting enzyme 2 (ACE2)-expressing cells of the lungs, kidneys, liver, etc. but also elicits a hyperinflammatory immune response, further damaging the tissue leading to acute respiratory distress syndrome (ARDS) and death. Although vaccines, as a prime example of active immunotherapy, have clearly disrupted the transmission of virus and reduced mortality, hospitalization, and burden of disease, other avenues of immunotherapy are also being explored. One such approach would be to adoptively transfer modified/unmodified immune cells to the critically ill. Here, we compiled and summarized the immunopathogenesis of SARS-CoV-2 and the recent preclinical and clinical data on the potential of cell-based therapies in the fight against COVID-19.
Keywords: SARS-CoV-2, COVID-19, Adoptive immunotherapy, T cell, Vaccine
Abbreviations: MSC, mesenchymal stromal cell; NK cell, natural killer cell; iPSC, inuced pluripotent stem cell; CAR, chimeric antigen receptor; DC, dendritic cell; CTL, cytotoxic T cell; GM-CSF, granulocyte monocyte colony-stimulating factor; VST, virus-specific T cell; ARDS, acute respiratory T cells; HLA, human leukocyte antigen; SARS, sever acute respiratory syndrome; ACE2, angiotensin-converting enzyme 2; COVID-19, Coronavirus disease 2019
1. Introduction
In December 2019, a type of contagious pneumonia was reported in Wuhan, China which was later called the coronavirus disease-19 (COVID-19). One major symptom of the disease was severe acute respiratory syndrome (SARS), and it was caused by a species of the Coronaviridae family [1]. Hence, the virus that rapidly swept across the globe with a pandemic of magnificent proportions was named SARS-CoV-2. Before COVID-19, SARS-CoV and the Middle East respiratory syndrome (MERS)-CoV were the other outbreaks caused by the Coronaviridae family, but none had the global impact that COVID-19 demonstrated [2]. SARS-CoV-2 has more than 80% genomic similarity to that of SARS-CoV, and using the spike (S) glycoproteins of its receptor-binding domains (RBDs) invades the type II alveolar epithelial cells through angiotensin-converting enzyme 2 (ACE2) with high affinity, which might explain why SARS-CoV-2 is highly contagious [3].
Needless to say, the impact of COVID-19 worldwide has prompted the need for developing vaccines and therapies. Immunotherapy seeks to harness the strength of the immune system to fend off pathogens. Most of the current therapies for COVID-19 emphasize the active aspect of immunotherapy, such as the use of vaccines. While many of the vaccines approved through Emergency Use Authorization (EUA) have proved an efficacy of greater than 70%, they have raised some concerns as well, which include the yet-unknown durability of the immune response, no immunity in the upper respiratory tract, and failure in high viral load [4]. In such cases, all therapeutic options must be scrutinized, including adoptive immunotherapy, a form of passive immunotherapy which takes advantage of the cells of the immune system to counter the infection [5]. Some subtypes of adoptive cell therapy, such as dendritic cells (DCs), can also be categorized as vaccine therapy [6]. Here, we focus on the feasibility and efficacy of using the immune cells as treatments for COVID-19.
2. A prelude to COVID-19 immunopathogenesis and immune cell responses against SARS-CoV-2 infection
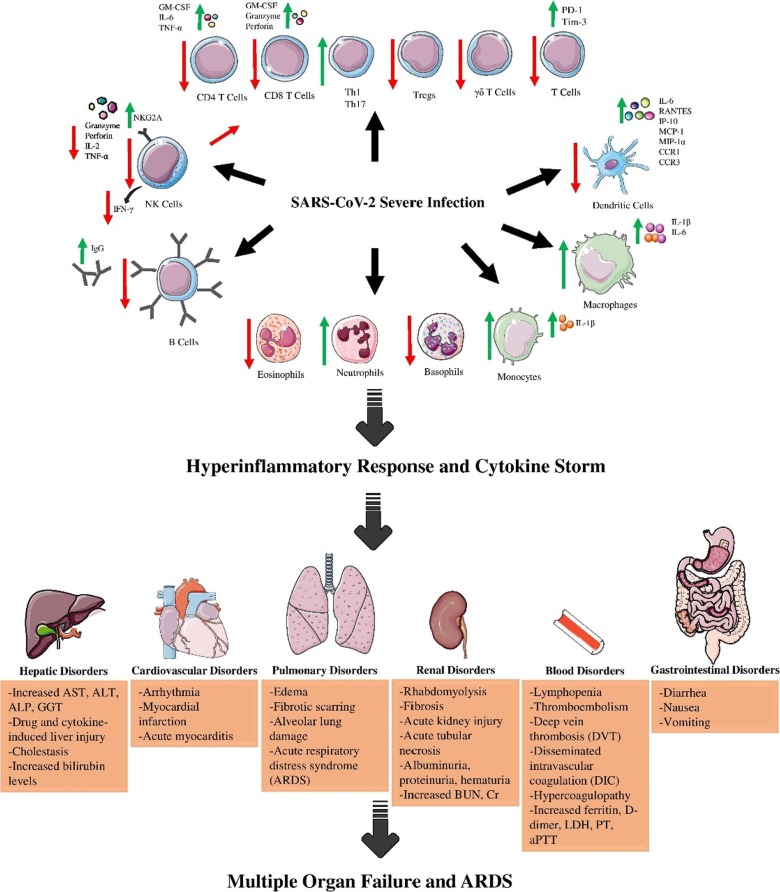
The SARS-CoV-2 virus is distributed through tiny droplets from infected patients. Then, the virus replicates in the lungs and progresses to the lower airway tracts. The immune response is key in defining the outcome of the disease. The primary infection evokes a local immune response which is commenced by innate immunity. The antigen-presenting cells (APCs) such as macrophages and DCs recognize and present the immune cells with pathogen-associated molecular patterns (PAMPs) of the virus to induce a biological response. Natural killer (NK) cells exert their function in the absence of an APC. This immune response culminates in the secretion of chemokines and cytokines such as IL-12, IL-6, IFN-γ, TNF-α, monocyte chemoattractant protein (MCP-1), and IL-1β [7]. Activated APCs also secrete type I IFNs and inflammatory cytokines which galvanize NK cells of the innate immunity and the cells of the adaptive immunity. These cytokines signal the attraction of neutrophils, macrophages/monocytes, and lymphocytes to the infection site to clear the virus (Fig. 1 ).
Fig. 1.
the diagram depicting the process of ARDS creation. Over-activation of the immune cells results in cytokine storm and deleterious effects on organs such as lungs, gastrointestinal system, kidneys, etc. (Figure illustrated with the help of https://smart.servier.com/).
Adaptive immunity initiates when CD4+ helper T-cells (Th) are presented with virus antigens by APCs. Upon stimulation, Th1 cells secrete IL-2, IFN-γ, and TNF-α to activate cytotoxic T-cells (CTLs), a major defense mechanism versus SARS-CoV-2. The humoral aspect of adaptive immunity is governed by Th2 stimulation of B-cells to secrete neutralizing antibodies (short-lasting IgM and long-lasting IgG) after differentiating to plasma cells and memory B cells [8]. The formation of memory T cells is another favorable byproduct of the activation of adaptive immunity, which was previously shown to persist for up to four years after initial exposure to SARS-CoV [9]. In another follow-up study, memory T cells were present six years after recovery, while Ag-specific memory B cells and SARS-CoV IgG were not detected in SARS-CoV recovered patients after six years [10]. This makes looking into T cell-based therapies for the novel SARS-CoV-2 all the more expedient.
Besides the high levels of inflammatory markers, such as ferritin, C-reactive protein (CRP), and D-dimer, lymphopenia, basopenia, and eosinopenia are common features of SARS-CoV-2 infection and are correlated with disease severity [11]. Lymphocytopenia is mostly due to reduced NK cell, B cell, and most importantly T cell numbers. This could be due to direct virus toxicity and infiltration and sequestration of lymphocytes in lung tissue or indirect factors such as diabetes and hypertension [12]. Th17 would be an exception since its inflammatory cytokines exacerbate the pathogenesis of COVID-19. Although T cells secrete activation cytokines, such as IL-2, TNF-α, and IFN-γ and express OX40 and 4-1BB co-stimulatory signals, in severe cases, the expression of these activation markers are reduced and replaced with programmed cell death protein 1 (PD-1) and mucin-domain containing-3 (Tim-3) exhaustion markers [13], [14]. The number of granulocytes and monocytes are also abnormal in COVID-19 patients. Increased neutrophil-to-lymphocyte ratio (NLR) is an indicator of disease severity, which is due to reduced lymphocyte count and increased conversion of marginal neutrophil pools to circulating neutrophil pools [15]. Eosinophil, basophil, and monocyte count are also reduced in severe cases. High levels of serum antibodies are another hallmark of SARS-CoV-2 infection; hence, antibody titer and nucleic acid detection method are the basis for disease detection. Although high antibody titer (especially IgG) is reported to be correlated with the severity of the disease, it is undetectable in most patients within the first seven days [16].
The inflammatory response is usually self-limiting to inhibit collateral normal tissue damage. In case the immune system fails to contain the virus, the unhinged production of inflammatory cytokines leads to further accumulation of immune cells in a feedback loop. In this cytokine storm, the massive infiltration of immune cells and their secretions (e.g. proteases and ROS) triggers lung tissue damage and the disruption of alveolar-capillary barrier, which results in the release of the virus and accumulated cytokines to other organs [17]. The destructive effects of pro-inflammatory cytokines include diffuse lung injury, fibrosis, edema, and hyaline membrane and thrombus formation [18]. Systemic inflammation and multiple-organ failure are other consequences of cytokine storm that disrupt the function of kidneys, heart, liver, etc. [19]. This is mostly due to the presence of neutrophils and monocytes. Considering the role of cytokine storm in determining the pathogenesis and severity of the disease, many strategies are focusing on diminishing inflammatory cytokines and stopping lung and multiple-tissue damage, while simultaneously targeting SARS-CoV-2.
3. Immune cell-based therapies against SARS-CoV-2 infection
Immunotherapy is defined as utilizing and manipulating the host's immune system to eliminate the affliction. Cell therapies using autologous or allogeneic immune cells have shown potential as a therapeutic approach in solid and hematological malignancies [20], chronic viral infections (e.g. HIV) [21], and refractory viral infections in hematopoietic stem cell transplantation (HSCT) patients [22], [23]. There is currently no gold-standard and definitive treatment for COVID-19, and considering its urgency, it has prompted scientists from all fields to investigate this problem, engendering a wide range of potential therapeutic options [24] (Table 1 ). Advances in adoptive cell therapy have engendered an off-branch of cell therapy which, unlike conventional acellular vaccines, uses primed cells as vaccines, such as DC-based vaccines [25].
Table 1.
Active clinical trials using T cells, DC vaccines, NK cells, and MSCs in COVID-19 treatment. Data extracted from https://clinicaltrials.gov/ (last accessed 9/15/2021).
| Treatment | Phase | Drug | Explanation/intervention | Country | NCT number |
|---|---|---|---|---|---|
| NK cell | I/II | CYNK-001 | To assess the safety of NK cells derived from human placental CD34 + stem cells as a treatment option | USA | NCT04365101 |
| I | NK cells/ conventional therapy | To assess the safety of NK cells plus conventional therapy in COVID-19 patients | China | NCT04280224 | |
| I | FT516 | To assess the safety of off-the-shelf iPSC-derived NK cells | USA | NCT04363346 | |
| I | DVX201 | To assess the safety of NK cells derived from CD34 + hematopoietic stem cells | USA | NCT04900454 | |
| I | NK cells | To assess the safety of ex vivo expanded NK cells | Brazil | NCT04634370 | |
| I/II | NK cells/ T cells | To assess the safety of NK cells and memory T cells from convalescent donors | Spain | NCT04578210 | |
| I/II | NKG2D-ACE2 CAR-NK Cells | To assess the safety of IL15 superagonist- and GM-CSF neutralizing scFv-secreting NKG2D-ACE2 CAR-NK derived from cord blood | China | NCT04324996 | |
| I | agenT-797 | To assess the safety and efficacy of an unmodified, allogeneic iNKT therapy in ARDS patients | USA | NCT04582201 | |
| DC | I/II | AV-COVID-19 | To assess the safety of monocyte-derived autologous DCs loaded with spike protein | USA | NCT04386252 |
| I | DC Vaccine | To assess the safety of minigene-containing DC vaccine | China | NCT04299724 | |
| I/II | Minigene Vaccine/ CTLs | Minigene-containing LV-SMENP-DC vaccine plus antigen-specific CTLs | China | NCT04276896 | |
| I | AV-COVID-19 | To assess the safety of autologous DCs loaded with spike protein with or without GM-CSF | Indonesia | NCT04690387 | |
| I | AV-COVID-19 | To assess the safety of autologous DCs loaded with spike protein with or without GM-CSF | Indonesia | NCT04685603 | |
| T cell | I | CSTC-Exo | Exosomes of SARS-CoV-2-specific T cells | Turkey | NCT04389385 |
| – | VSTs | To assess the possibility of VST production from convalescent donors | Singapore | NCT04351659 | |
| I/II | VSTs | To assess the possibility of VST production from convalescent donors and its safety as a treatment | Singapore | NCT04457726 | |
| I | VSTs | To assess the safety of partially HLA-matched VSTs as a treatment option | USA | NCT04401410 | |
| I/II | IMP | To assess the safety of VSTs as a treatment option at various doses | Germany | NCT04762186 | |
| I | VSTs | To assess the safety of HLA-matched CTLs | USA | NCT04742595 | |
| I | VSTs | To assess the safety of CTLs as a treatment | USA | NCT04765449 | |
| I/II | TCB008 | To assess the safety of γδ T cells of unrelated donors as a treatment | UK | NCT04834128 | |
| I | CK0802 | Treatment of ARDS using cord blood derived regulatory T cells | USA | NCT04468971 | |
| I/II | RAPA-501-ALLO | Off-the-shelf allogeneic hybrid Treg/Th2 Cells | USA | NCT04482699 | |
| I/II | AlloStim | Allogenic memory T cells | USA | NCT04441047 | |
| MSC | II | Secretome-MSC | Injection of isolated S-MSCs from culture medium | Indonesia | NCT04753476 |
| I | ExoFlo™ | Injection of bone marrow MSCs' extracellular vesicles to alleviate ARDS | USA | NCT04657458 | |
| II | EXIT-COVID19 | Injection of bone marrow MSCs' extracellular vesicles to alleviate ARDS | USA | NCT04493242 | |
| I | MSC exosome | Inhalation of MSC-derived secretomes to treat COVID-19 pneumonia | China | NCT04276987 | |
| II | MSCs | Injection of MSCs for the treatment of ARDS | Mexico | NCT04416139 | |
| I/II | MSCs | Injection of allogenic pooled olfactory mucosa-derived MSCs | Belarus | NCT04382547 | |
| I/II | MSCs | Injection of human dental pulp-derived MSCs to treat ARDS | China | NCT04336254 | |
| I | MSCs | Injection of umbilical cord-derived MSCs to alleviate COVID-19 pneumonia | USA | NCT04490486 | |
| I/II | Astrostem-V | Injection of allogeneic adipose tissue-derived MSCs to control ARDS | South Korea | NCT04527224 | |
| II | MSCs | Injection of adipose tissue-derived MSCs to control ARDS | NCT04905836 | ||
| II | MSCs | Injection of autologous adipose tissue-derived MSCs to control ARDS | USA | NCT04428801 | |
| I | MSCs | Injection of Wharton's Jelly MSCs for treatment of COVID-19 ARDS | Jordan | NCT04313322 | |
| I | MSCs | Ascertain the positive effects of MSC injection on DNA repair genes | NCT04898088 | ||
| II | NestaCell® | Assess the efficacy of NestaCell® in combination with conventional therapy | Brazil | NCT04315987 | |
| I/II | MSCs | Injection of bone marrow-derived MSCs to treat ARDS | China | NCT04346368 | |
| II | MSCs | Injection of MSCs to treat COVID-19-associated pneumonia | Spain | NCT04361942 | |
| II | MSCs | Injection of bone marrow-derived MSCs to treat COVID-19-associated pneumonia | Pakistan | NCT04444271 | |
| I | MSCs | Investigate the safety of allogenic MSC injection | Brazil | NCT04467047 | |
| I/II | MSCs | Injection of MSCs to treat ARDS | USA | NCT04345601 | |
| I/II | MSCs | Injection of umbilical cord-derived MSCs to alleviate COVID-19 pneumonia | Canada | NCT04400032 | |
| I/II | MSCs | Injection of cord blood-derived MSCs to alleviate COVID-19 pneumonia | USA | NCT04565665 | |
| I | PSC-04 | To assess the safety of allogeneic adipose-derived MSCs | USA | NCT04486001 | |
| II | MSCs | To assess the safety of umbilical cord-derived MSCs | Colombia | NCT04429763 | |
| I | MSCs | Injection of Wharton Jelly-derived MSCs to treat ARDS | Mexico | NCT04456361 | |
| I/II | MSCs | Injection of Wharton Jelly-derived MSCs to treat ARDS | France | NCT04625738 | |
| I/II | MSCs | Injection of human cord tissue MSCs to treat ARDS | USA | NCT04399889 | |
| II | MSCs | Injection of MSCs to treat ARDS | Spain | NCT04615429 | |
| I/II | MSCs | To assess the safety and efficacy of allogeneic, adipose tissue-derived MSCs to alleviate COVID-19 pneumonia | Spain | NCT04366323 | |
| I | MSCs | Injection of Longeveron MSCs to treat COVID-19 and Flu-elicited ARDS | USA | NCT04629105 | |
| I | ADR-001 | To assess the safety and efficacy of adipose tissue-derived MSCs to alleviate COVID-19 pneumonia | Japan | NCT04522986 | |
| I | MSCs | Injection of dental pulp MSCs to treat ARDS | China | NCT04302519 | |
| II | MSCs | Multiple dosing of MSC as a treatment study for COVID-19 ARDS | USA | NCT04466098 | |
| I | MSCs | Injection of human cord tissue MSCs to treat ARDS | Brazil | NCT04525378 | |
| I | MSCs | Injection of autologous adipose tissue-derived MSCs to control ARDS | USA | NCT04352803 | |
| I/II | MSCs | Injection of Wharton's Jelly MSCs for treatment of COVID-19 ARDS | Colombia | NCT04390152 | |
| II | MSCs | To assess the efficacy of MSC injection in COVID-19 patients | Pakistan | NCT04437823 | |
| II | MSCs | Injection of umbilical cord-derived MSCs to alleviate COVID-19 pneumonia | China | NCT04288102 | |
| I | DW-MSCs | To assess the safety and efficacy of MSC injection in COVID-19 patients | Indonesia | NCT04535856 | |
| I | MSCs | Injection of umbilical cord-derived MSCs to alleviate COVID-19 pneumonia | Indonesia | NCT04457609 | |
| I/II | MSCs | Injection of umbilical cord-derived MSCs to alleviate COVID-19 pneumonia | China | NCT04339660 | |
| I | MSCs | To assess the safety of umbilical cord-derived MSCs in COVID-19 patients | China | NCT04273646 | |
| I/II | MSCs | To assess the safety and efficacy of MSC therapy | Belgium | NCT04445454 | |
| I/II | SBI-101 | To assess the safety of MSC therapy in COVID-19 patients with acute kidney injury | USA | NCT04445220 | |
| I | MSCs | Injection of MSCs to alleviate COVID-19 pneumonia | Mexico | NCT04611256 | |
| I/II | MSCs | Injection of umbilical cord-derived MSCs to alleviate COVID-19 ARDS | USA | NCT04355728 | |
| I | MSCs | Injection of MSCs to alleviate COVID-19 pneumonia | China | NCT04252118 | |
| II | HB-adMSCs | Injection of allogeneic adipose-derived MSCs to provide support against COVID-19 | USA | NCT04348435 | |
| II | HB-adMSCs | Injection of allogeneic adipose-derived MSCs to provide support against COVID-19 | USA | NCT04349631 | |
| I | MSCs | To assess the safety of MSC therapy in COVID-19 patients | China | NCT04371601 | |
| I/II | MSCs | Injection of umbilical cord-derived MSCs to alleviate COVID-19 ARDS | France | NCT04333368 | |
| I/II | MSCs | Injection of MSCs to alleviate COVID-19 pneumonia and multiple organ failure | Turkey | NCT04392778 | |
| I/II | XCEL-UMC-BETA | To assess the safety and efficacy of MSC therapy | Spain | NCT04390139 | |
| I | PrimePro | To assess the palliative effects of MSC administration in COVID-19 patients and as prophylaxis in healthcare providers | USA | NCT04573270 | |
| I/II | MSCs | Injection of allogeneic cryopreserved umbilical cord- and placenta-derived MSCs to alleviate COVID-19 ARDS | Ukraine | NCT04461925 | |
| II | PLX-PAD | To assess the efficacy of MSC therapy | USA | NCT04389450 | |
| II | HB-adMSC | To assess the efficacy and safety of allogeneic adipose-derived MSCs | USA | NCT04362189 | |
| I | BM-Allo.MSC | To assess the safety of allogeneic bone marrow-derived MSC therapy | USA | NCT04397796 | |
| I/II | BX-U001 | Injection of umbilical cord-derived MSCs to alleviate COVID-19 ARDS | USA | NCT04452097 | |
| I/II | CYP-001 | To assess the safety of MSC injection in ICU patients with respiratory failure | Australia | NCT04537351 | |
| II | PLX-PAD | To assess the efficacy of MSC therapy | Germany | NCT04614025 | |
| I/II | MSCs | Injection of umbilical cord derived CD362 enriched MSCs to alleviate COVID-19 ARDS | UK | NCT03042143 | |
| II | MSCs | Injection of bone marrow-derived MSCs to treat ARDS | Germany | NCT04377334 | |
| III | Remestemcel-L | To assess the safety and efficacy of MSC plus standard care in ARDS patients | USA | NCT04371393 | |
| II | MSC | Injection of umbilical cord-derived MSCs to alleviate COVID-19 ARDS | China | NCT04269525 | |
| I/II | ACT-20-MSC/ACT-20-CM | To assess the safety and efficacy of umbilical cord-derived MSC in ARDS patients | USA | NCT04398303 |
MSC: mesenchymal stromal cell; NK cell: natural killer cell; iPSC: induced pluripotent stem cell; CAR: chimeric antigen receptor; DC: dendritic cell; CTL: cytotoxic T cell; GM-CSF; granulocyte monocyte colony-stimulating factor; VST: virus-specific T cell; ARDS: acute respiratory distress syndrome; HLA: human leukocyte antigen
3.1. Ag-loaded DC vaccine therapy
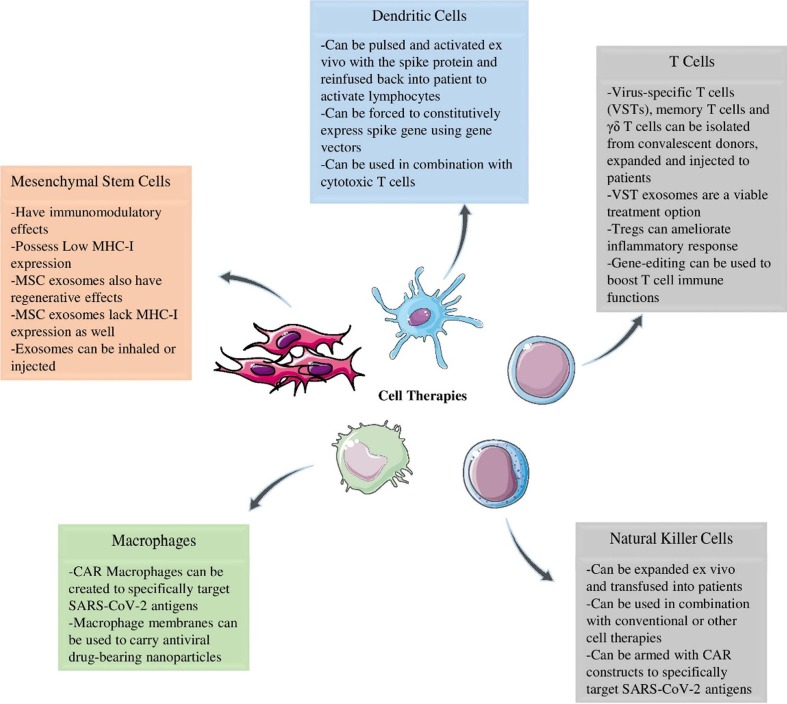
In addition to acting as an APC, type 1 DCs, (DC-I) are involved in antiviral responses by secreting IL-6 inflammatory cytokine, and type 1 IFNs and activating NK cells using NKG2D and NKp46 at an early phase of infection [26]. DC cancer vaccines have been previously proven efficacious in many preclinical and clinical studies involving solid [27] and hematological [28] malignancies, making them a viable strategy against COVID-19. Considering the importance of APCs, especially DCs, to kick-start the adaptive immunity, two viable strategies are being used to create vaccine DCs: 1) manufacturing vaccine DCs reactive to SARS-CoV-2 by loading (pulsing) them with spike protein ex vivo, 2) force surface expression of SARS-CoV-2 peptides on DCs using gene vectors. Monocyte-derived DCs can be pulsed with peptides of the spike protein in order for the DC to present SARS-CoV-2 antigens to T cells (NCT04386252). In the second approach, vaccine DCs are being investigated. Using a lentiviral vector system called NHP/TYF, DC vaccines are transfected and modified with a synthetic minigene to express conserved viral proteins and immunomodulatory genes (NCT04276896).
After subcutaneous injection of the prepared DC vaccine, engineered DCs can proceed to activate CTLs and B cells through CD4+ helper T cells. The germinal centers in the secondary lymphoid organs can house follicular DCs and create memory B cell responses in the long term via B cell stimulation [29]. Since DC-I cytokine overproduction is an underlying cause of ARDS, strategies can thirdly be tailored to inhibit unchecked activation of DC-I. By directly stimulating CD4+ T cells and indirectly stimulating B cells, DC-based therapies hold the promise of sustainable anti-SARS-CoV-2 response; however, the lack of robust immunity of DC vaccines for some challenging viruses, such as HIV, HCV, and CMV should also be noted [30], [31], [32]. Hopefully, the current trials will shed light on the efficacy of DC-based vaccines therapy for COVID-19 (Fig. 2 ).
Fig. 2.
The current applications of the most prominent cell-based therapies in COVID-19. (Figure illustrated with the help of https://smart.servier.com/).
3.2. Virus-specific T cell therapy
T-cells are perhaps the most pivotal cells of adaptive immunity when the body is invaded by viruses, so much so that lymphopenia, especially in the more severe and elderly cases, is a consistent laboratory finding of COVID-19 patients [33]. Low lymphocyte count is, to some extent, due to diminished CD4+ T cells, B cells, and NK cells but largely because of very low CD8+ populations, which are essential in anti-viral responses. The number of CD8+ T cells is correlated with the severity and outcome of the infection. CD8+ T cells count and CD4/CD8 T cell ratio increase and decrease respectively after recuperation [34]. Besides low T cell count, T cell exhaustion is another hallmark of COVID-19, especially in ICU patients. Separate studies have found that PD-1, CTLA-4, and TIGIT [35] and PD-1 and Tim-3 [13] T cell exhaustion markers are greatly elevated in both CD4+ and CD8+ T cells of COVID-19 patients, particularly in the more severe cases.
Based on this evidence, CD8+ T cells are a plausible treatment option for COVID-19. Adoptively transferring Ag-specific T cells is currently being used as an advantageous mode of treatment in cancer and has shown efficacy in rendering HSCT patients free of viral infections [36], [37], [38]. Virus-specific T cells (VSTs) of convalescent donors can be isolated and used to treat the infection since these patients have higher antiviral activity. Recently, Cooper et al. developed a rapid good manufacturing practice (GMP)-compatible protocol for isolation and expansion of SARS-CoV-2 VSTs from convalescent donors [39]. The VSTs were able to respond to virus stimulation by secreting IFN-γ but had to be collected within two months after convalescence since VST numbers drop to less than 0.03% after such time. After two weeks of culture, the cells showed a shift to central memory phenotypes and retained proliferative capacity. A recent study aimed to isolate and expand VSTs of asymptomatic and symptomatic patients as well as healthy individuals. Although VST product were obtained from COVID-19 patients, T cells of healthy donors could not be expanded or primed [40]. Thus, if a VST product is to be made, cell lines must be obtained from positive or convalescent donors. These findings served to cement the results of previous studies which used the same methodology to successfully expand SARS-CoV-2 VSTs [41], [42] and memory T cells [43] and achieve the same results. The presence of memory T cells, which was reported to be up to 11 years in SARS-CoV1 patients, grants a more rapid and robust immunity against the pathogen [44]. Ferreras and colleagues detected virus-specific memory T cells among memory CD45RA– T cells and isolated them by depleting CD45RA T cells. CD45RA– T cells expressed IFN-γ after exposure to three SARS-CoV-2 peptides and could then be activated and proliferated via IL-15, an essential cytokine for memory T cells [43]. This method can potentially be used to create a biobank of SARS-CoV-2-specific memory T cells.
VSTs can indirectly be used as a therapy for COVID-19 as well. Upon activation and expansion in vitro, VSTs release IFN-rich exosomes into the culture media. These exosomes can be harvested and used as a separate treatment which requires no previous human leukocyte antigen (HLA) compatibility. Incompatible HLA of allogenic, unrelated T cells can result in acute or chronic graft-versus-host disease (GVHD), in which the grafted immune cells target the healthy cells of the recipient. CSTC-Exo is one such product. A phase 1 interventional study is investigating the safety and efficacy of these VST-derived exosomes (NCT04389385). CSTC-Exo is administered to early-stage COVID-19 patients via a metered dose inhaler.
The safety and efficacy of different phenotypes of T cells including γδ T cells (NCT04834128) and regulatory T cells (Tregs) (NCT04468971) are being investigated in trials among other unconventional T cell subtypes [45]. γδ T cells constitute a small portion of circulating T cells and exhibit both the innate and adaptive traits of the immune system with a wide antiviral activity independent of the major histocompatibility complex (MHC) molecules [46]. Adoptive transfer of Tregs is beneficial in inflammatory and autoimmune disorders [47] and can be used to dampen the inflammatory response in severe COVID-19 cases. Gladstone et al. reported the treatment of ARDS in two patients using ex vivo expanded, allogenic HLA-matched, cord blood-derived Tregs, which was associated with diminished IL-6 and TNF-α inflammatory cytokines [48].
3.3. NK cell therapy
NK cells, activated via macrophage-derived cytokines and type I IFNs, are members of the innate immune system which target virus-infected cells. Using a slew of inhibitory (e.g. NKG2A and KIR) and activating (e.g. NKG2D) receptors, NK cells rapidly respond to invading pathogens as they require no MHCs to exert their immune response. Adoptive transfer of NK cells to COVID-19 patients is a viable strategy. These cells can be derived from various stem cells such as induced pluripotent stem cells (iPSCs) (NCT04363346). NKG2A is a common exhaustion marker on both NK cells and CTLs reversely correlated with the number of NK cells and CTLs and their cytokine production. NKG2A is downregulated in recovered patients, making NKG2A inhibition a potential therapy [49].
Chimeric antigen receptors (CARs) are genetically engineered constructs on CAR-T cells designed to specifically recognize and bind to an antigen in order for the T-cell to perform its cytotoxic effect. Initially designed to recognize tumor-associated antigens, CARs can be designed to recognize any antigen. CAR-primed cells are similar to NK cells in that their antigen recognition is independent of MHC molecules, and since NK cells are adept at quelling viral infections, CAR-NK cells are proposed as a potential COVID-19 therapy. A phase I/II trial is aiming to use universal CAR-expressing NK cells that recognize S protein and NKG2DL of infected cells via their ACE2 and NKG2D proteins, respectively (NCT04324996). These NKG2D-ACE2 CAR-NK cells are reportedly engineered to secrete IL-15 superagonist to increase NK cells' survival time after adoptive transfer. These cells also secrete GM-CSF-neutralizing scFv to inhibit CAR-T cell-related neurotoxicity and cytokine release syndrome (CRS). An unpublished in vitro study reported the generation of CAR-NK cells armed with scFV of CR3022, a neutralizing antibody for SARS-CoV-2 [50]. Extracellular exosomes of CAR-T cells can also prove to be an effective therapy against SARS-CoV-2. Fu and colleagues have demonstrated that the CAR-T cell-derived exosomes in the CAR-T cell culture media express CAR and contain high concentrations of cytotoxic molecules with no PD-1 expression [51].
3.4. Macrophage therapy
Macrophages can be divided into two subtypes, which are polar opposites of each other. While type 2 (M2) macrophages are designed for an anti-inflammatory response, type 1 (M1) macrophages contribute to inflammation by secreting IL-6 and IL-1β. Any attempt to annul the inflammation caused by M1 or apply M2 macrophage to reduce lung inflammation can be used as a therapeutic approach [52]. One study reported the successful generation of CAR macrophages against SARS-CoV-2 that reduced the viral load while not increasing the expression of inflammatory cytokines [53]. Other proposed macrophage-related therapies would include the use of anti-CSF1-R and anti-GM-CSF antibodies to inhibit the differentiation of monocytes to macrophages or to inhibit recruitment of macrophages to the lungs by targeting chemokines and their receptors [54]. Recently, Tan et al. have developed a drug delivery system that uses macrophage membrane as carriers. PLGA nanoparticles are loaded with lopinavir as antiviral drug and are coated with fragments of macrophage membrane. These mini macrophages release the drug upon contact with SARS-CoV-2 antigen to inactivate the virus. They have also been shown to competitively absorb and neutralize inflammatory cytokines, inhibiting the activation of neutrophils and macrophages [55].
3.5. Mesenchymal stem cell therapy
So far, a large body of studies in the field of cell therapy has been devoted to using stem cells to dampen cytokine storm and ARDS. At the epicenter of these studies, mesenchymal stem/stromal cells (MSCs) have been the main focus [56]. MSCs can be isolated from various sources and are known to have anti-inflammatory and immunomodulatory characteristics [57]; MSCs have been used in the clinic for disorders such as type II diabetes [58] and GVHD [59]. With at least a few dozen clinical trials and scads of preclinical studies, MSCs are suggested to also reduce apoptosis of normal and immune cells, induce an anti-microbial response from innate immunity, prevent damage to alveolar epithelial cells, increase the clearance of alveolar fluids, and reduce multiple organ injuries and ARDS [60]. The immunomodulatory and regenerative effects of MSCs, as well as a lack of ACE2 receptors and their previous success with treating acute lung injury associated with the influenza virus, are the main reasons MSCs are being investigated as a potential treatment for ARDS [61].
A case report of a critically ill patient who was not responding to conventional treatment for 12 days, demonstrated that three times IV administration of allogeneic umbilical cord MSCs (UC-MSCs) improved the overall laboratory indices and symptoms of the patient, and the patient no longer needed mechanical ventilation [62]. In a preliminary study, Leng et al. evaluated the therapeutic potential of intravenous (IV) injection of ACE2/TMPRSS2-negative MSCs in 10 patients whose symptoms ranged from moderate to critically ill [63]. Two days after MSC administration, pulmonary functions and clinical symptoms of the patients improved, and reduced CRP and inflammatory cytokines levels were witnessed with no adverse event. In addition, the number of inflammatory cytokine-secreting T and NK cells diminished and, at the same time, total lymphocyte count and regulatory DC population increased. This was discussed to be the effect of MSC anti-inflammatory cytokines. Interestingly, these cells had the capacity to differentiate to type II alveolar epithelial cells [63]. Using the same MSC source, another study conducted a safety experiment in 12 patients who received a single IV dose of UC-MSCs. Starting day 3, clinical symptoms started to dissipate as oxygen saturation and CT results improved significantly. IL-6 concentration, lymphocyte count, and CRP levels were drastically different from the control group by day 7 [64]. Although there were differences of baseline characteristics between groups, and the sample size was small, the favorable results substantiate the efficacy of MSC treatment in COVID-19 patients.
There are currently at least 60 ongoing or completed trials trying to determine the safety and efficacy of MSC therapy for COVID-19, with the majority of trials being in phase 1 or 2 and a few in phase 3 (Table 1). Trials are using a plethora of sources such as dental pulp (NCT04302519), bone marrow (NCT04346368), adipose tissue (NCT04366323), olfactory mucosa (NCT04382547), embryonic stem cells (NCT04331613), and Wharton's jelly (NCT04313322) to extract MSCs. Umbilical cord, bone marrow (BM), and adipose tissue are the most common sources. Allogeneic transplant is more widely used than autologous donation [65]. Autologous sources minimize disease transmission and immune rejection while allogeneic transplantation is easier and commercially available, yields more cells, and does not expose the patient to biopsy risks. UC-MSCs express the least MHC-1 protein among other sources and do not possess the risk of developing GVHD in allogeneic transplantation [66].
Several trials have focused on evaluating the safety of MSC infusion for ARDS [67], [68], [69]. A phase I controlled non-randomized trial investigated the safety of IV transfusion of 3 × 107 UC-MSCs/infusion in three-day intervals in nine COVID-19 patients. Apart from mild fever and facial flushing in two patients and one case of transient hypoxia, no severe adverse effects or mortalities were reported [70]. It was believed that MSCs exert their function by reducing inflammatory cytokines. The patients with various disease severity but high baseline IL-6 levels experienced a significant drop in serum IL-6. In a recent randomized, phase 1/2a, controlled trial, 12 patients with varying levels of ARDS were injected twice with UC-MSCs in three-day intervals (100 × 106 cell/infusion) plus the standard treatment. The treatment proved efficacious as mortality and inflammatory cytokine levels dropped with no significant adverse events [71].
Although MSCs have exhibited potential as a favorable cell therapy approach, there are still some controversies surrounding their safety and scalability [72]. For instance, MSCs can aggregate in blood vessels that might exacerbate the COVID-19-related coagulation disorder and cause further lung dysfunction [18], [73]. MSCs release intracellular cytokines and growth factors via extracellular vesicles called exosomes. These secretomes also possess anti-inflammatory, regenerative, and immunomodulatory effects of their parent cells [74], making them a potential therapeutic approach for COVID-19 patients. Exosomes are one of the primary reasons for the immunomodulatory effect of MSCs, and such effects have been demonstrated in cancer, lung injury, etc. [75]. They contain a plethora of bioactive molecules and, considering their size, can get past the blood–brain barrier and avert MSC infusion-related pulmonary embolism [76]. Besides the IV injection of secretomes, they can also be administered via inhalation sprays (NCT04276987). Results of clinical studies of exosomes safety and efficacy in COVID-19 patients are scant. In a nonrandomized cohort study, 24 COVID-19 patients received 15 mL of BM-MSC-derived allogeneic exosomes, ExoFloTM, and were monitored for 14 days. No adverse effects were witnessed, and 16% of the patients expired due to reasons other than the therapy [77]. In total, 71% of the patients recovered after one injection, and laboratory values and oxygenation improved, indicating the safety and efficacy of MSC exosomes. Still, the results of clinical trials are needed to elucidate the therapeutic potential of MSC exosomes. Some controversies surround the use of exosomes. Although they are more scalable and sustainable than MSCs, a duality exists regarding their tumorigenesis potential. While some studies suggest exosomes can inhibit tumor growth, others believe they can promote tumor metastasis [78]. Secondly, similar to the heterogeneity in MSC populations, MSC exosomes are also heterogeneous. This difference is observed in both the cytokine content [79] and the adverse events of BM-MSC and adipose MSC exosomes [80]. An immortalized MSC cell line can be created to reduce such variations [79].
4. Conclusion and future perspectives
Immune cell-based therapies have shown therapeutic potential in the treatment of malignancies and viral infections. In the context of COVID-19, they can be used to either moderate the hyperinflammatory response of the immune system or bolster it to stave off the disease. Due to various reasons such as high cost and the labor-intensive and time-consuming process associated with generating customized cells, in many disorders, adoptive cell transfer/therapy is not considered the first line of therapy. The existence of corticosteroids in COVID-19 protocols to manage hyperinflammation nullifies immune cell therapies as well.
A lingering issue with any cell-based therapy is the unmatched allogeneic HLA which could result in GVHD in COVID-19 patients. In the case of T cells, VSTs of HLA-typed donors can be expanded ex vivo and cryopreserved for a partially matched allogeneic transplant [39] or HLA-free exosomes derived from such cells can be utilized. The use of HLA-E-restricted CD8+ T cells can also be investigated to circumvent the alloreactivity issue [81]. The secretion of T cell- and MSC-derived exosomes is particularly interesting in this aspect since it requires no previous HLA matching. Gene-editing, which has gained much attention in the past years with the introduction of clustered regularly interspaced short palindromic repeats (CRISPR) and its associated Cas9 protein, may answer this dilemma. CRISPR/Cas9 has shown great results in preclinical and clinical cancer studies to produce CAR-T cells that are directed against a specific tumor antigen, can constitutively secrete stimulatory cytokines, and are less prone to exhaustion [82]. The expression of endogenous TCR and HLA in these cell can also be knocked out to create off-the-shelf CAR-T cells with no risk of GVHD or host-versus-graft reaction [83]. This platform can potentially be used to create universal CAR-T cells or CAR-NK cells that target SARS-CoV-2 antigens with minimal exhaustion by knocking out PD-1/Tim-3 or NKG2A expression.
Despite the ability of infused MSCs to migrate to inflamed sites, their migration rate is relatively low. MSCs depend on certain surface markers, such as, to be chemoattracted from blood to tissue. MSC coating is a promising method to increase cell migration. Cells can be coated with sialyl Lewis X (SLeX), a carbohydrate crucial in the inflammatory extravasation of immune cells [84]. MSCs can also be coated with antibodies against antigens of a target site. MSC coating with anti-vascular cell adhesion molecule 1 (VCAM-1) increases their migration rate compared to regular MSCs and enhances their immunomodulation in inflammatory bowel disease (IBD) [85]. Genetic modification of MSCs has been used to create cytotoxic MSCs that inhibit the growth of tumor cells in preclinical studies [86], [87]. MSCs can be engineered to deliver TNF-related apoptosis-inducing ligand (TRAIL) to cancer cells to cause apoptosis [88]. Interestingly, ACE2 can alleviate ARDS, and MSCs that overexpress ACE2 alleviate radiation-related lung injury and ARDS in mouse models [89]. ACE2-overexpressing MSCs exert their anti-inflammatory effects by suppressing ERK1/2 and NF-κB signaling pathways [90]. With the emergence of CRISPR/Cas9 and base editors, this mode of therapy appears to be more feasible [83]. Clinical trials and future studies will have to elucidate the efficacy and safety of cell-based therapies for COVID-19 treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors would like to thank the University of Tehran for helping in gathering the required data. No financial support was received for this study.
Author Contributions
SG drafted the manuscript. HK and ASN proposed the concept for the manuscript, helped with extracting the data and revising the manuscript. All authors read and approved the final manuscript.
References
- 1.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S., Baghbanzadeh M., Aghamohammadi N., Zhang W., Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int. J. Epidemiol. 2020;49(3):717–726. doi: 10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan R., Zhang Y., Li Y., Xia L.u., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science (80-) 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashte S., Gulbake A., El-Amin III S.F., Gupta A. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum. Cell. 2021;34(3):711–733. doi: 10.1007/s13577-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masoomikarimi M., Garmabi B., Alizadeh J., Kazemi E., Azari Jafari A., Mirmoeeni S., Dargahi M., Taheri N., Jafari R. Advances in immunotherapy for COVID-19: A comprehensive review. Int. Immunopharmacol. 2021;93:107409. doi: 10.1016/j.intimp.2021.107409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal Yatoo M., Hamid Z., Rather I., Nazir Q.U.A., Bhat R.A., Ul Haq A., Magray S.N., Haq Z., Sah R., Tiwari R., Natesan SenthilKumar, Bilal M., Harapan H., Dhama K. Immunotherapies and immunomodulatory approaches in clinical trials - a mini review. Hum Vaccines Immunother. 2021;17(7):1897–1909. doi: 10.1080/21645515.2020.1871295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L.i. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y.Y., Huang Z.T., Li L., Wu M.H., Yu T., Koup R.A., et al. Characterization of SARS-CoV-specific memory T cells from recovered individuals 4 years after infection. Arch Virol. 2009;154:1093–1099. doi: 10.1007/s00705-009-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang F., Quan Y., Xin Z.-T., Wrammert J., Ma M.-J., Lv H., Wang T.-B., Yang H., Richardus J.H., Liu W., Cao W.-C. Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study. J Immunol. 2011;186(12):7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 11.Sun D.-W., Zhang D., Tian R.-H., Li Y., Wang Y.-S., Cao J., Tang Y., Zhang N., Zan T., Gao L., Huang Y.-Z., Cui C.-L., Wang D.-X., Zheng Y., Lv G.-Y. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: A sentinel? Clin Chim Acta. 2020;508:122–129. doi: 10.1016/j.cca.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toor S.M., Saleh R., Sasidharan Nair V., Taha R.Z., Elkord E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology. 2021;162(1):30–43. doi: 10.1111/imm.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diao B.o., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L.i., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y.i., Wu Y., Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front Immunol [Internet]. 2020;11 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L., Xu L., Lin C. T cell response in patients with COVID-19. Blood Sci. 2020;2:76–78. doi: 10.1097/BS9.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B., Zhou X., Zhu C., Feng F., Qiu Y., Feng J., et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020 doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody Responses to SARS-CoV-2 in Patients with Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Zhou X, Qiu Y, Feng F, Feng J, Jia Y, et al. Clinical characteristics of 82 death cases with COVID-19. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 18.Kohansal Vajari M., Shirin M., Pourbagheri‐Sigaroodi A., Akbari M.E., Abolghasemi H., Bashash D. COVID-19-related coagulopathy: A review of pathophysiology and pharmaceutical management. Cell Biol. Int. 2021;45(9):1832–1850. doi: 10.1002/cbin.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F., Gan R., Zhen Z., Hu X., Li X., Zhou F., Liu Y., Chen C., Xie S., Zhang B., Wu X., Huang Z. Adaptive immune responses to SARS-CoV-2 infection in severe versus mild individuals. Signal Transduct Target Ther. 2020;5(1) doi: 10.1038/s41392-020-00263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monzavi S.M., Naderi M., Ahmadbeigi N., Kajbafzadeh A.-M., Muhammadnejad S. An outlook on antigen-specific adoptive immunotherapy for viral infections with a focus on COVID-19. Cell. Immunol. 2021;367:104398. doi: 10.1016/j.cellimm.2021.104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi J., Ding C., Jiang X., Gao Y. Advances in Developing CAR T-Cell Therapy for HIV Cure. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ottaviano G., Chiesa R., Feuchtinger T., Vickers M., Dickinson A., Gennery A., Veys P., Todryk S. Adoptive T Cell Therapy Strategies for Viral Infections in Patients Receiving Haematopoietic Stem Cell Transplantation. Cells. 2019;8(1):47. doi: 10.3390/cells8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maali A., Atashi A., Ghaffari S., Kouchaki R., Abdolmaleki F., Azad M. A Review on Leukemia and iPSC Technology: Application in Novel Treatment and Future. Curr Stem Cell Res Ther. 2018;13(8):665–675. doi: 10.2174/1574888X13666180731155038. [DOI] [PubMed] [Google Scholar]
- 24.Zaki M.M., Lesha E., Said K., Kiaee K., Robinson-McCarthy L., George H., Hanna A., Appleton E., Liu S., Ng A.H.M., Khoshakhlagh P., Church G.M. Cell therapy strategies for COVID-19: Current approaches and potential applications. Sci. Adv. 2021;7(33):eabg5995. doi: 10.1126/sciadv.abg5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang H.-Y., Wang S.-H., Tang Y.u., Sheng W., Zuo C.-J., Wu D.-W., Fang H., Du Q., Li N. Landscape and progress of global COVID-19 vaccine development. Hum. Vaccines Immunother. 2021;17(10):3276–3280. doi: 10.1080/21645515.2021.1945901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Draghi M., Pashine A., Sanjanwala B., Gendzekhadze K., Cantoni C., Cosman D., Moretta A., Valiante N.M., Parham P. NKp46 and NKG2D Recognition of Infected Dendritic Cells Is Necessary for NK Cell Activation in the Human Response to Influenza Infection. J Immunol. 2007;178(5):2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 27.Song N., Guo H., Ren J., Hao S., Wang X. Synergistic anti-tumor effects of dasatinib and dendritic cell vaccine on metastatic breast cancer in a mouse model. Oncol Lett. 2018;15:6831–6838. doi: 10.3892/ol.2018.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Acker H.H., Versteven M., Lichtenegger F.S., Roex G., Campillo-Davo D., Lion E., Subklewe M., Van Tendeloo V.F., Berneman Z.N., Anguille S. Dendritic Cell-Based Immunotherapy of Acute Myeloid Leukemia. J Clin Med. 2019;8(5):579. doi: 10.3390/jcm8050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabado R.L., Balan S., Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27(1):74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Craenenbroeck A.H., Smits E.L.J., Anguille S., Van de Velde A., Stein B., Braeckman T., Van Camp K., Nijs G., Ieven M., Goossens H., Berneman Z.N., Van Tendeloo V.F.I., Verpooten G.A., Van Damme P., Cools N. Induction of cytomegalovirus-specific T cell responses in healthy volunteers and allogeneic stem cell recipients using vaccination with messenger RNA-transfected dendritic cells. Transplantation. 2015;99(1):120–127. doi: 10.1097/TP.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva L.T., Santillo B.T., de Almeida A., Duarte A.J. da S., Oshiro T.M. Using Dendritic Cell-Based Immunotherapy to Treat HIV: How Can This Strategy be Improved? Front. Immunol. 2018:2993. doi: 10.3389/fimmu.2018.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Yun, Zhang Ying, Yao Zhiqiang, Moorman Jonathan Patrick, Jia Zhansheng. Dendritic cell-based immunity and vaccination against hepatitis C virus infection. Immunology. 2012;136(4):385–396. doi: 10.1111/j.1365-2567.2012.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Zunyou, McGoogan Jennifer M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 34.Liu Zeming, Long Wei, Tu Mengqi, Chen Sichao, Huang Yihui, Wang Shipei, Zhou Wei, Chen Danyang, Zhou Ling, Wang Min, Wu Meng, Huang Qi, Xu Haibo, Zeng Wen, Guo Liang. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J. Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Hong-Yi, Zhang Mi, Yang Cui-Xian, Zhang Nian, Wang Xi-Cheng, Yang Xin-Ping, Dong Xing-Qi, Zheng Yong-Tang. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanley Patrick J., Melenhorst Jan J., Nikiforow Sarah, Scheinberg Phillip, Blaney James W., Demmler-Harrison Gail, Cruz C. Russell, Lam Sharon, Krance Robert A., Leung Kathryn S., Martinez Caridad A., Liu Hao, Douek Daniel C., Heslop Helen E., Rooney Cliona M., Shpall Elizabeth J., Barrett A. John, Rodgers John R., Bollard Catherine M. CMV-specific T cells generated from naïve T cells recognize atypical epitopes and may be protective in vivo. Sci Transl Med. 2015;7(285):285ra63. doi: 10.1126/scitranslmed.aaa2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bollard Catherine M., Heslop Helen E. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood. 2016;127(26):3331–3340. doi: 10.1182/blood-2016-01-628982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moonesi Mohammadreza, Zaka Khosravi Saeed, Molaei Ramshe Samira, Allahbakhshian Farsani Mehdi, Solali Saeed, Mohammadi Mohammad Hossein, Farshdousti Hagh Majid, Mehdizadeh Hanie. IGF family effects on development, stability, and treatment of hematological malignancies. J. Cell. Physiol. 2021;236(6):4097–4105. doi: 10.1002/jcp.30156. [DOI] [PubMed] [Google Scholar]
- 39.Cooper Rachel S., Fraser Alasdair R., Smith Linda, Burgoyne Paul, Imlach Stuart N., Jarvis Lisa M., Turner David M., Zahra Sharon, Turner Marc L., Campbell John D.M. Rapid GMP-Compliant Expansion of SARS-CoV-2–Specific T Cells From Convalescent Donors for Use as an Allogeneic Cell Therapy for COVID-19. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.598402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerreiro Manuel, Aguilar‐Gallardo Cristóbal, Montoro Juan, Francés‐Gómez Clara, Latorre Víctor, Luna Irene, Planelles Dolores, Carrasco María Paz, Gómez María Dolores, González‐Barberá Eva María, Aguado Cristina, Sempere Amparo, Solves Pilar, Gómez‐Seguí Inés, Balaguer‐Rosello Aitana, Louro Alberto, Perla Aurora, Larrea Luis, Sanz Jaime, Arbona Cristina, de la Rubia Javier, Geller Ron, Sanz Miguel Ángel, Sanz Guillermo, Luis Piñana José. Adoptive transfer of ex vivo expanded SARS-CoV-2-specific cytotoxic lymphocytes: A viable strategy for COVID-19 immunosuppressed patients? Transpl Infect Dis. 2021;23(4) doi: 10.1111/tid.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller Michael D., Harris Katherine M., Jensen-Wachspress Mariah A., Kankate Vaishnavi V., Lang Haili, Lazarski Christopher A., Durkee-Shock Jessica, Lee Ping-Hsien, Chaudhry Kajal, Webber Kathleen, Datar Anushree, Terpilowski Madeline, Reynolds Emily K., Stevenson Eva M., Val Stephanie, Shancer Zoe, Zhang Nan, Ulrey Robert, Ekanem Uduak, Stanojevic Maja, Geiger Ashley, Liang Hua, Hoq Fahmida, Abraham Allistair A., Hanley Patrick J., Cruz C. Russell, Ferrer Kathleen, Dropulic Lesia, Gangler Krista, Burbelo Peter D., Jones R. Brad, Cohen Jeffrey I., Bollard Catherine M. SARS-CoV-2–specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood. 2020;136(25):2905–2917. doi: 10.1182/blood.2020008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung Wing, Soh Teck Guan, Linn Yeh Ching, Low Jenny Guek‐Hong, Loh Jiashen, Chan Marieta, Chng Wee Joo, Koh Liang Piu, Poon Michelle Li‐Mei, Ng King Pan, Kuick Chik Hong, Tan Thuan Tong, Tan Lip Kun, Seng Michaela Su‐fern. Rapid production of clinical-grade SARS-CoV-2 specific T cells. Adv CELL GENE Ther. 2020;3(4) doi: 10.1002/acg2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreras C., Pascual-Miguel B., Mestre-Durán C., Navarro-Zapata A., Clares-Villa L., Martín-Cortázar C., De Paz R., Marcos A., Vicario J.L., Balas A., García-Sánchez F., Eguizabal C., Solano C., Mora-Rillo M., Soria B., Pérez-Martínez A. SARS-CoV-2-Specific Memory T Lymphocytes From COVID-19 Convalescent Donors: Identification, Biobanking, and Large-Scale Production for Adoptive Cell Therapy. Front Cell. Dev Biol. 2021;9 doi: 10.3389/fcell.2021.620730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng Oi-Wing, Chia Adeline, Tan Anthony T., Jadi Ramesh S., Leong Hoe Nam, Bertoletti Antonio, Tan Yee-Joo. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34(17):2008–2014. doi: 10.1016/j.vaccine.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.K. Orumaa, M.R. Dunne, The role of unconventional T cells in COVID-19. Ir. J. Med. Sci. 2021. [DOI] [PMC free article] [PubMed]
- 46.Yazdanifar Mahboubeh, Mashkour Narges, Bertaina Alice. Making a case for using γδ T cells against SARS-CoV-2. Crit. Rev. Microbiol. 2020;46(6):689–702. doi: 10.1080/1040841X.2020.1822279. [DOI] [PubMed] [Google Scholar]
- 47.Romano Marco, Fanelli Giorgia, Albany Caraugh Jane, Giganti Giulio, Lombardi Giovanna. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gladstone Douglas E., Kim Bo Soo, Mooney Kathy, Karaba Andrew H., D'Alessio Franco R. Regulatory T Cells for Treating Patients With COVID-19 and Acute Respiratory Distress Syndrome: Two Case Reports. Ann Intern Med. 2020;173(10):852–853. doi: 10.7326/L20-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Meijuan, Gao Yong, Wang Gang, Song Guobin, Liu Siyu, Sun Dandan, Xu Yuanhong, Tian Zhigang. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma M, Badeti S, Geng K, Liu D. Efficacy of Targeting SARS-CoV-2 by CAR-NK Cells. bioRxiv Prepr Serv Biol [Internet]. 2020; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32817942%0A http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC7430572.
- 51.Fu Wenyan, Lei Changhai, Liu Shuowu, Cui Yingshu, Wang Chuqi, Qian Kewen, Li Tian, Shen Yafeng, Fan Xiaoyan, Lin Fangxing, Ding Min, Pan Mingzhu, Ye Xuting, Yang Yongji, Hu Shi. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merad Miriam, Martin Jerome C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu W, Lei C, Qian K, Ma Z, Li T, Lin F, et al. CAR Macrophages for SARS-CoV-2 Immunotherapy 1. bioRxiv [Internet]. 2020;2020.07.26.222208. Available from: https://doi.org/10.1101/2020.07.26.222208.
- 54.Gracia-Hernandez M., Sotomayor E.M., Villagra A. Targeting Macrophages as a Therapeutic Option in Coronavirus Disease 2019. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.577571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan Qingqin, He Lingjie, Meng Xiaojun, Wang Wei, Pan Hudan, Yin Weiguo, Zhu Tianchuan, Huang Xi, Shan Hong. Macrophage biomimetic nanocarriers for anti-inflammation and targeted antiviral treatment in COVID-19. J Nanobiotechnology. 2021;19(1) doi: 10.1186/s12951-021-00926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hossein-khannazer Nikoo, Shokoohian Bahare, Shpichka Anastasia, Aghdaei Hamid Asadzadeh, Timashev Peter, Vosough Massoud. An update to “novel therapeutic approaches for treatment of COVID-19”. J. Mol. Med. 2021;99(2):303–310. doi: 10.1007/s00109-020-02027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Blanc Katarina, Mougiakakos Dimitrios. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 58.Päth Günter, Perakakis Nikolaos, Mantzoros Christos S., Seufert Jochen. Stem cells in the treatment of diabetes mellitus — Focus on mesenchymal stem cells. Metabolism. 2019;90:1–15. doi: 10.1016/j.metabol.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Fisher Sheila A, Cutler Antony, Doree Carolyn, Brunskill Susan J, Stanworth Simon J, Navarrete Cristina, Girdlestone John. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst. Rev. 2019;2019(1) doi: 10.1002/14651858.CD009768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin Hua, Zhao Andong. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein. Cell. 2020;11(10):707–722. doi: 10.1007/s13238-020-00738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Jiajia, Hu Chenxia, Chen Lijun, Tang Lingling, Zhu Yixin, Xu Xiaowei, Chen Lu, Gao Hainv, Lu Xiaoqing, Yu Liang, Dai Xiahong, Xiang Charlie, Li Lanjuan. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering. 2020;6(10):1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang Bing, Chen Junhui, Li Tao, Wu Haiying, Yang Wenjie, Li Yanjiao, Li Jianchun, Yu Congtao, Nie Fangang, Ma Zhaoxia, Yang Mingxi, Xiao Mingying, Nie Panrong, Gao Yanfeng, Qian Chuanyun, Hu Min. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine (Baltimore) 2020;99(31):e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leng Zikuan, Zhu Rongjia, Hou Wei, Feng Yingmei, Yang Yanlei, Han Qin, Shan Guangliang, Meng Fanyan, Du Dongshu, Wang Shihua, Fan Junfen, Wang Wenjing, Deng Luchan, Shi Hongbo, Li Hongjun, Hu Zhongjie, Zhang Fengchun, Gao Jinming, Liu Hongjian, Li Xiaoxia, Zhao Yangyang, Yin Kan, He Xijing, Gao Zhengchao, Wang Yibin, Yang Bo, Jin Ronghua, Stambler Ilia, Lim Lee Wei, Su Huanxing, Moskalev Alexey, Cano Antonio, Chakrabarti Sasanka, Min Kyung-Jin, Ellison-Hughes Georgina, Caruso Calogero, Jin Kunlin, Zhao Robert Chunhua. Transplantation of ACE2- Mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shu Lei, Niu Changming, Li Ruyou, Huang Tingrong, Wang Yan, Huang Mao, Ji Ningfei, Zheng You, Chen Xiaolin, Shi Lei, Wu Mingjing, Deng Kaili, Wei Jing, Wang Xueli, Cao Yang, Yan Jiaxin, Feng Ganzhu. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1) doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abdelgawad Mai, Bakry Nourhan Saied, Farghali Ahmed A., Abdel-Latif Ahmed, Lotfy Ahmed. Mesenchymal stem cell-based therapy and exosomes in COVID-19: current trends and prospects. Stem Cell Res. Ther. 2021;12(1) doi: 10.1186/s13287-021-02542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mason C., Dunnill P. Assessing the value of autologous and allogeneic cells for regenerative medicine. Regen. Med. 2009:835–853. doi: 10.2217/rme.09.64. [DOI] [PubMed] [Google Scholar]
- 67.Yip H.K., Fang W.F., Li Y.C., Lee F.Y., Lee C.H., Pei S.N., et al. Human Umbilical Cord-Derived Mesenchymal Stem Cells for Acute Respiratory Distress Syndrome. Crit Care Med. 2020;E391–9 doi: 10.1097/CCM.0000000000004285. [DOI] [PubMed] [Google Scholar]
- 68.Matthay M.A., Calfee C.S., Zhuo H., Thompson B.T., Wilson J.G., Levitt J.E., et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Z., Chen Y., Luo X., He X., Zhang Y., Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit. Care. 2020 doi: 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5 doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lanzoni G., Linetsky E., Correa D., Messinger Cayetano S., Alvarez R.A., Kouroupis D., et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Metcalfe S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Med Drug Discov. 2020;5 doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.P.W. Askenase, COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies? J. Extracell. Vesicles. 2020. [DOI] [PMC free article] [PubMed]
- 74.Bari E., Ferrarotti I., Saracino L., Perteghella S., Torre M.L., Corsico A.G. Mesenchymal Stromal Cell Secretome for Severe COVID-19 Infections: Premises for the Therapeutic Use. Cells. 2020;9 doi: 10.3390/cells9040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allan D., Tieu A., Lalu M., Burger D. Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: Progress and challenges toward clinical application. Stem Cells Transl Med. 2020;9:39–46. doi: 10.1002/sctm.19-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019 doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao R., Chen X., Song H., Bie Q., Zhang B. Dual Role of MSC-Derived Exosomes in Tumor Development. Stem Cells Int. 2020 doi: 10.1155/2020/8844730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Börger V., Weiss D.J., Anderson J.D., Borràs F.E., Bussolati B., Carter D.R.F., et al. ISEV and ISCT statement on EVs from MSCs and other cells: considerations for potential therapeutic agents to suppress COVID-19. Cytotherapy [Internet]. 2020:1–4. doi: 10.1016/j.jcyt.2020.05.002. Available from: /pmc/articles/PMC7229942/?report=abstract%0Ahttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC7229942/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chance T.C., Rathbone C.R., Kamucheka R.M., Peltier G.C., Cap A.P., Bynum J.A. The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J Trauma Acute Care Surg. 2019;87:S74–S82. doi: 10.1097/TA.0000000000002225. [DOI] [PubMed] [Google Scholar]
- 81.Caccamo N., Sullivan L.C., Brooks A.G., Dieli F. Harnessing HLA-E-restricted CD8 T lymphocytes for adoptive cell therapy of patients with severe COVID-19. Br. J. Haematol. 2020:e185–e187. doi: 10.1111/bjh.16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manriquez-Roman C., Siegler E.L., Kenderian S.S. CRISPR Takes the Front Seat in CART-Cell Development. BioDrugs. 2021;35:113–124. doi: 10.1007/s40259-021-00473-y. [DOI] [PubMed] [Google Scholar]
- 83.Ghaffari S., Nastaran K., Rezaei N. CRISPR/Cas9 revitalizes adoptive T-cell therapy for cancer immunotherapy. J Exp Clin Cancer Res. 2021;40:269. doi: 10.1186/s13046-021-02076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu P.J., Peng H., Li C., Abdel-Latif A., Berron B.J. Adhesive Stem Cell Coatings for Enhanced Retention in the Heart Tissue. ACS Appl Bio Mater. 2020;3:2930–2939. doi: 10.1021/acsabm.9b01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Q., Li Y., Chen Z., Du H., Wan J. Anti-VCAM 1 Antibody-Coated mesenchymal stromal cells attenuate experimental colitis via immunomodulation. Med Sci Monit. 2019;25:4457–4468. doi: 10.12659/MSM.914238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zischek C., Niess H., Ischenko I., Conrad C., Huss R., Jauch K.W., et al. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg. 2009;250:747–752. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- 87.Alieva M., Bagó J.R., Aguilar E., Soler-Botija C., Vila O.F., Molet J., et al. Glioblastoma therapy with cytotoxic mesenchymal stromal cells optimized by bioluminescence imaging of tumor and therapeutic cell response. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0035148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lathrop M.J., Sage E.K., Macura S.L., Brooks E.M., Cruz F., Bonenfant N.R., et al. Antitumor effects of TRAIL-expressing mesenchymal stromal cells in a mouse xenograft model of human mesothelioma. Cancer Gene Ther. 2015;22:44–54. doi: 10.1038/cgt.2014.68. [DOI] [PubMed] [Google Scholar]
- 89.Min F., Gao F., Li Q., Liu Z. Therapeutic effect of human umbilical cord mesenchymal stem cells modifed by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep. 2015;11:2387–2396. doi: 10.3892/mmr.2014.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y., Zeng Z., Cao Y., Liu Y., Ping F., Liang M., et al. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-κB signaling pathways. Sci Rep. 2016;6 doi: 10.1038/srep27911. [DOI] [PMC free article] [PubMed] [Google Scholar]