Abstract
Background
The value of preoperative breast MRI as an adjunct technique regarding its effect on re-excision rates has been a subject of discussion. No survival data regarding preoperative breast MRI are available from randomized studies.
Methods
Ten-year follow-up of the POMB randomized multicentre study was analysed, evaluating MRI and its effect on disease-free survival (DFS) and overall survival (OS). Patients with newly diagnosed breast cancer were randomized to either preoperative MRI or conventional imaging. Kaplan–Meier plots were used to analyse DFS and OS, and Cox regression to estimate hazard ratios (HRs).
Results
A total of 440 patients, aged 56 years or less, with newly diagnosed breast cancer were randomized to either preoperative MRI (220) or conventional imaging (220; control). Median follow-up for each group was 10 years. DFS rates were 85.5 and 80.0 per cent for the MRI and control groups respectively (P = 0.099). The risk of relapse or death was 46 per cent higher in the control group (HR 1.46, 95 per cent c.i. 0.93 to 2.29). OS rates after 10 years were 90.9 and 88.6 per cent in the MRI and control groups respectively (P = 0.427). The risk of death was 27 per cent higher in the control group (HR 1.27, 0.71 to 2.29). Locoregional, distant, and contralateral recurrence outcomes combined were increased in the control group (P = 0.048). A subgroup analysis of patients with breast cancer stages I–III showed that preoperative MRI improved DFS compared with conventional imaging, but this did not reach statistical significance (P = 0.057).
Conclusion
After 10 years of follow-up, preoperative breast MRI as an adjunct to conventional imaging resulted in slightly, but non-significantly, improved DFS and OS. Registration number: NCT01859936 (http://www.clinicaltrials.gov).
This 10-year follow-up of the POMB randomized multicentre study evaluated preoperative breast MRI in terms of disease-free survival (DFS) and overall survival (OS). DFS and OS were slightly but non-significantly improved.
Introduction
Surgical treatment for breast cancer has become less invasive after several randomized studies showed that breast-conserving surgery (BCS) followed by radiotherapy for local control provides survival equivalent to that observed after mastectomy for invasive breast cancer1–3. Even poorer survival has been reported for patients who receive mastectomy than those who receive BCS and radiotherapy4–8. Preoperative evaluation using adequate imaging techniques to determine tumour size and extent within the affected breast facilitates surgical planning to avoid positive tumour margins and additional surgery. Re-excision is associated with a greater risk of complication, increased patient anxiety levels, more challenging surgical procedures, delayed initiation of adjuvant therapies, and increased medical costs9,10.
Presently, MRI is deemed the most sensitive method for detecting occult findings in the ipsilateral and contralateral breast in the preoperative setting, especially for women with dense breasts. However, preoperative breast MRI results in an increased proportion of false-positive findings requiring further investigation. This imaging modality is resource- and time-consuming, may increase patient anxiety, and can lead to unnecessary mastectomies11,12. Whether all MRI-detected additional findings that may have caused changes to previous treatment plans are biologically relevant remains unclear13.
Only a few randomized studies14–16 have evaluated the effect of preoperative MRI, albeit with inconclusive results. The POMB (preoperative MRI of the breast) study17 evaluated the effect of MRI findings on surgical decision-making. In contrast to other studies, it showed that preoperative breast MRI did result in altered treatment in 20 per cent of patients, and a reduction in re-excision rate by 30 per cent without an increase in the total number of mastectomies compared with conventional imaging. In a follow-up study, it was also found that the MRI findings were highly accurate compared with histopathological analysis of the surgical specimens18.
A reasonable hypothesis, corroborated by the findings of these earlier studies, is that the detection of confirmed additional disease on preoperative breast MRI to achieve radical surgery would decrease recurrence and mortality. Fisher and colleagues19 in 1986 had suggested that occult residual carcinoma is a relevant cause of tumour recurrence and, because local recurrence often leads to distant metastases, it is likely that overall survival (OS) will eventually decrease20–25. Nevertheless, the importance of occult lesions is questionable. Anatomical studies have shown that foci in the breast in quadrants away from the primary tumour have no impact on prognosis26. The Dutch breast cancer guidelines27 recommend treatment of focally positive margins after BCS in invasive tumours using only complementary whole-breast irradiation including boost, thereby omitting re-excision.
No randomized studies have examined the long-term relationship between preoperative MRI, breast cancer recurrence, and survival. The purpose of this study was to report the 10-year follow-up of the POMB study with a focus on the long-term outcomes disease-free survival (DFS) and OS.
Methods
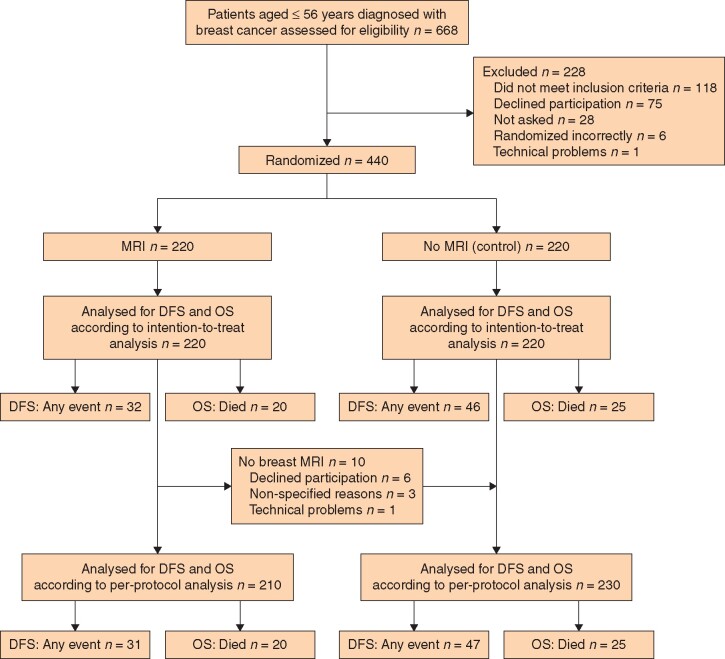
This randomized multicentre study was approved by the Ethical Board Committee in Stockholm and Uppsala (2007/1057–31/4 and 2020–00351). The design of the POMB study (NCT01859936) has been described in detail previously17. It included 440 patients aged 56 years or less with newly diagnosed invasive and/or non-invasive clinical and screen-detected breast cancer, regardless of the stage of disease and prognosis. Patients were randomized at three different breast cancer units to undergo preoperative breast MRI in addition to standard preoperative assessment (MRI group) or conventional imaging only (control group). Two hundred and twenty patients were randomly assigned to each group. Patients excluded from the study were those with previous malignant disease in the ipsilateral breast, pregnancy/lactation, kidney disease, metal implants, overweight and reduced mobility, claustrophobia, mental illness, or difficulties in understanding the study (Fig. 1).
Fig. 1.
CONSORT diagram for trial
DFS, disease-free survival; OS, overall survival.
Information regarding patient demographics, clinical data, tumour biology, histopathological tumour characteristics, surgical treatment, and neoadjuvant/adjuvant therapy was collected at the time of the initial POMB study, and was supplemented by review of all available recorded electronic charts between March and May 2020.
Adjuvant therapy was provided according to the national guidelines based on stage and prognostic markers. Patients who underwent BCS or mastectomy with chemotherapy were followed annually using bilateral mammography, sonography, and clinical examination at the breast clinic or department of oncology for the following 10 years to detect any locoregional and contralateral breast cancer recurrence or distant metastatic disease. Patients who had undergone mastectomy alone were examined annually for 5 years. All patients were thereafter followed via the national mammography screening programme, which includes women aged between 40 and 74 years. The presence, location, and extent of distant metastases were assessed using conventional chest radiography, CT, or PET–CT in patients with locally advanced breast cancer.
Local recurrence was defined as any new invasive or in situ breast cancer limited to the ipsilateral breast or chest wall/mastectomy site previously affected by cancer after radiotherapy or at least 3 months after primary surgical treatment. Regional recurrence was defined as any ipsilateral malignancy detected in the axilla and/or in the supraclavicular, infraclavicular or internal mammary lymph nodes after adjuvant radiotherapy or 3 months after surgery. Distant metastases were recorded when metastatic cells or findings were detected outside the regional lymph nodes, either by cytological/histological confirmation or radiological assessment. Distant metastases detected before the date of surgery were regarded as synchronous with the primary tumour and were not listed as an event. Contralateral breast cancer was defined as any breast cancer diagnosed in the untreated breast during follow-up.
The follow-up interval was calculated as the number of months from the date of randomization to the date of death or emigration, or the date of last known follow-up. For six patients in the MRI group, the date of randomization was missing. For these patients, the date of diagnosis was used instead, because it most probably differed from the date of randomization by only a few days.
DFS was defined as the interval between the date of randomization and the date of any breast cancer recurrence or death, even if the patients were not at risk of recurrence until the final surgery. OS was defined as the interval from randomization to death from any cause. Breast cancer-specific survival was calculated similarly, but included only deaths due to breast cancer.
Statistical analysis
The sample size in this study was based on a power calculation supported by data from a study by Bedrosian and colleagues28. Patients accepting participation entered the original POMB trial by means of a telephone call to the randomization centre after disclosure of the cancer diagnosis. A computer-generated algorithm was used for randomization, and patients were assigned randomly on a 1 : 1 basis to the preoperative breast MRI group or the control group.
Kaplan–Meier plots were used to estimate and analyse the primary endpoint, DFS, as well as the secondary endpoint, OS, for each group. Log rank tests were used for comparison between groups. Cox regression analysis was used to estimate hazard ratios (HRs). All primary analyses were performed according to the intention-to-treat principle. A per-protocol analysis was undertaken excluding 10 patients from the MRI group who had not undergone MRI; these patients were added to the control group. A subgroup analysis of patients with tumour stages I–III, excluding those with more advanced disease, was also performed.
All reported P values were based on two-sided tests. P < 0.050 was considered statistically significant. All statistical analyses were performed using SPSS® version 26.0 (IBM, Armonk, NY, USA).
Results
Demographic data for the 440 included patients are shown in Table 1 according to the randomization group. The mean age of the patients was 46 years in each group, and the median follow-up time for OS from randomization until the end of the study was 10 years. Regarding survival, no patients were lost to follow-up, but two patients moved outside the study region, and the date of last screening was noted as the end of follow-up for DFS. There were only minor differences between groups in terms of patient demographics, clinical data, tumour biology, histopathological tumour characteristics, and neoadjuvant/adjuvant therapy.
Table 1.
Patient demographics, clinical data, tumour characteristics, and treatment of 440 patients included in the POMB study randomized to preoperative MRI or conventional imaging
| MRI | Control | |
|---|---|---|
| (n = 220) | (n = 220) | |
| Age at randomization (years)* | 46 (27–55) | 46 (21–56) |
| Menopausal status | ||
| Premenopausal | 157 (74.4) | 163 (74.1) |
| Perimenopausal | 28 (13.3) | 26 (11.8) |
| Postmenopausal | 10 (4.7) | 17 (7.7) |
| Unknown | 25 (7.6) | 14 (6.4) |
| Screen-detected breast cancer | ||
| Yes | 83 (37.7) | 83 (37.7) |
| No | 137 (62.3) | 137 (62.3) |
| Breast density, right† | ||
| 1 | 106 (48.2) | 103 (46.8) |
| 2 | 85 (38.6) | 83 (37.7) |
| 3 | 24 (10.9) | 28 (12.7) |
| 4 | 5 (2.3) | 5 (2.2) |
| Unknown | 0 (0) | 1 (0.6) |
| Breast density, left† | ||
| 1 | 104 (47.3) | 102 (46.4) |
| 2 | 85 (38.6) | 85(38.6) |
| 3 | 26 (11.8) | 29 (13.2) |
| 4 | 5 (2.3) | 4 (1.8) |
| Tumour size (cm) | ||
| Tis | 19 (8.6) | 25 (11.4) |
| < 2 | 120 (54.5) | 129 (58.6) |
| 2–5 | 62 (28.2) | 45 (20.5) |
| > 5 | 19 (8.6) | 20 (9.1) |
| Unknown | 0 (0) | 1 (0.5) |
| Lymph node metastasis | ||
| 0 | 120 (54.5) | 136 (61.8) |
| 1–3 | 69 (31.4) | 65 (29.5) |
| 4–9 | 15 (6.8) | 8 (3.6) |
| >10 | 4 (1.8) | 4 (1.8) |
| Unknown | 12(5.5)‡ | 7 (3.2)§ |
| Type of invasive carcinoma | ||
| Ductal | 146 (66.4) | 166 (75.5) |
| Ductal and lobular | 6 (2.7) | 5 (2.3) |
| Lobular | 15 (6.8) | 11 (5.0) |
| Other | 16 (7.3) | 10 (4.5) |
| Type of in situ carcinoma | ||
| DCIS | 108 (49.1) | 129 (58.6) |
| DCIS and LCIS | 1 (0.5) | 5 (2.3) |
| LCIS | 11 (5.0) | 6 (2.7) |
| Other | 1 (0.5) | 0 (0) |
| ER status | ||
| Positive | 162 (73.6) | 158 (71.8) |
| Negative | 37 (16.8) | 48 (21.8) |
| Unknown | 21 (9.6) | 14 (6.4) |
| PR status | ||
| Positive | 149 (67.7) | 146 (66.4) |
| Negative | 50 (22.7) | 59 (26.8) |
| Unknown | 21 (9.1) | 15 (6.9) |
| HER2 status | ||
| Positive | 30 (13.6) | 32 (14.5) |
| Negative | 168 (76.4) | 172 (78.2) |
| Unknown | 22 (10.0) | 16 (7.3) |
| Herceptin | ||
| Yes | 32 (14.5) | 30 (13.6) |
| No | 186 (84.5) | 189 (85.9) |
| Unknown | 2 (0.9) | 1 (0.5) |
| Molecular subtype by proxy¶ | ||
| Luminal A | 62 (28.2) | 67 (30.5) |
| Luminal B HER2– | 84 (38.2) | 71 (32.3) |
| Luminal B HER2+ | 15 (6.8) | 15 (6.8) |
| HER2+ | 11 (5.0) | 17 (7.7) |
| Triple-negative | 24 (10.9) | 30 (13.6) |
| Unknown | 24 (10.9) | 20 (9.1) |
| Breast-conserving surgery | ||
| Yes | 123 (55.9) | 129 (58.6) |
| No | 97 (44.1) | 91 (41.4) |
| Radiotherapy | ||
| Breast | 73 (33.2) | 78 (35.3) |
| Locoregional | 65 (29.5) | 60 (27.3) |
| Breast + boost | 33 (15.0) | 38 (17.3) |
| Locoregional + boost | 5 (2.3) | 8 (3.6) |
| No | 31 (14.1) | 35 (15.9) |
| Unknown | 13 (5.9) | 1 (0.5) |
| Chemotherapy | ||
| Yes | 140 (63.6) | 137 (62.2) |
| No | 79 (35.9) | 82 (37.3) |
| Unknown | 1 (0.5) | 1 (0.5) |
| Endocrine therapy | ||
| Yes | 160 (72.7) | 153 (69.5) |
| No | 59 (26.8) | 66 (30.0) |
| Unknown | 1 (0.5) | 1 (0.5) |
| Chemotherapy and endocrine therapy | ||
| Yes | 104 (47.3) | 93 (42.2) |
| No | 115 (52.3) | 126 (57.3) |
| Unknown | 1 (0.5) | 1 (0.5) |
Values in parentheses are percentages unless indicated otherwise;
values are median (range).
Breast density according to American College of Radiology Breast Imaging Reporting and Data System: 1, 0–24 per cent breast parenchyma; 2, 25–50 per cent breast parenchyma; 3, 51–75 per cent breast parenchyma; 4, 76–100 per cent breast parenchyma.
Eight and
four patients had no axillary surgery.
Luminal A: oestrogen receptor (ER)-positive and/or progesterone receptor (PR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, Ki-67 below 20 per cent; luminal B HER2–: ER-positive and/or PR-positive, HER2-negative, Ki-67 20 per cent or more;luminal B HER2+: ER-positive and/or PR-positive any Ki 67; HER2+: ER-negative and PR-negative, HER2-poistive, any Ki 67; Tripple-negative: ER-negtaive, PR-negative, HER2-negative, any Ki67; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
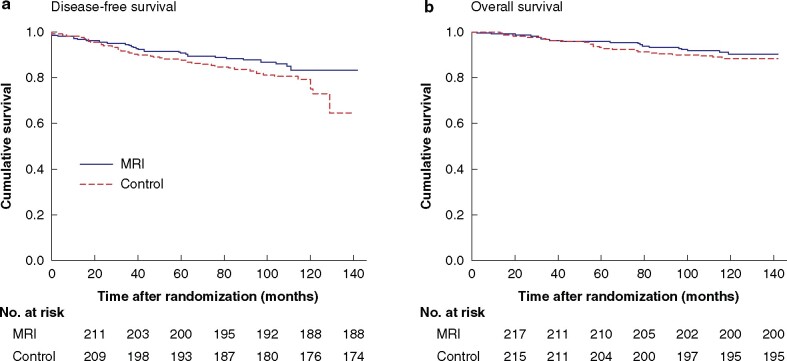
Ten patients in the MRI group did not undergo MRI, but all patients assigned to the MRI group were included in accordance with the intention-to-treat study plan17,18. Of the 440 women in the analysis, 85.5 and 80.0 per cent in the MRI and control groups respectively were alive and free from cancer after 10 years (Fig. 2a). The difference was not statistically significant (P = 0.099). Cox regression analysis revealed that the risk of relapse or death was 46 per cent higher in the control group than in the MRI group (HR 1.46, 95 per cent c.i. 0.93 to 2.29).
Fig. 2.
Kaplan–Meier survival curves over 10 years of follow-up for 440 patients included in the POMB study with newly diagnosed breast cancer who did or did not undergo preoperative MRI
a Breast cancer disease-free survival and b overall survival. The results of intention-to-treat analysis are shown. a P = 0.099, b P = 0.427 (log rank test).
Moreover, 90.9 and 88.6 per cent of women in the MRI and control groups respectively were alive after 10 years (P = 0.427) (Fig. 2b). The risk of death was 27 per cent higher in the control group than in the MRI group (HR 1.27, 0.71 to 2.29). Breast cancer-specific survival data were similar to OS data, because only five patients died from other causes. Data related to disease relapse are presented in Table 2. The control group was associated with a statistically significantly increased risk of any type of recurrence compared with the MRI group (HR 1.64, 1.00 to 2.67).
Table 2.
Disease recurrence and survival data after 10 years of follow-up for 440 patients included in the POMB study randomized to preoperative MRI or conventional imaging
| MRI | Control | P † | Hazard ratio* | |
|---|---|---|---|---|
| (n = 220) | (n = 220) | |||
| Locoregional recurrence | 13 (5.9) | 19 (8.6) | 0.275 | 1.48 (0.73, 3.00) |
| Distant recurrence | 16 (7.3) | 26 (11.8) | 0.116 | 1.65 (0.88, 3.07) |
| Locoregional and distant recurrence | 24 (10.9) | 37 (16.8) | 0.087 | 1.57 (0.94, 2.62) |
| Contralateral recurrence | 2 (0.9) | 5 (2.3) | ||
| Any recurrence | 26 (11.8) | 42 (19.1) | 0.048 | 1.64 (1.00, 2.67) |
| Any event (DFS) | (14.5) | (20.9) | 0.101 | 1.46 (0.93, 2.29) |
| Death (OS) | 20 (9.1) | (11.4) | 0.427 | 1.27 (0.71, 2.29) |
| Breast cancer death (breast cancer-specific survival) | 17 (7.7) | 23 (10.5) | 0.321 | 1.38 (0.73, 2.57) |
Values in parentheses are percentages unless indicated otherwise;
*values in parentheses are 95 per cent confidence intervals. Hazard ratios indicate the risk of recurrence and death in the control group compared with the MRI group. DFS, disease-free survival; OS, overall survival.
†Cox regression.
Per-protocol analysis for DFS and OS was undertaken after limiting the sample to all eligible patients, excluding 10 who did not undergo MRI and adding them to the control group (DFS: HR 1.40, 0.89 to 2.20; OS: HR 1.16, 0.65 to 2.09).
Because patients with more advanced disease were considered not likely to benefit from MRI, a subgroup analysis was undertaken including patients without extensive spread of disease at diagnosis. It showed that preoperative MRI resulted in a borderline statistically significant improvement in DFS when seven patients with stage IV disease were excluded (P = 0.057).
A separate sensitivity analysis for DFS excluded two patients with previous breast cancer and three patients without confirmed breast cancer findings on histopathological analysis (HR 1.46, 0.93 to 2.29). Results of the main analyses were qualitatively similar to the findings reported above.
In addition, analyses of DFS and OS were stratified according to breast cancer unit, and the results were almost identical.
Discussion
The use of MRI in the assessment of newly diagnosed breast cancer has been incorporated into clinical practice in resource-rich environments. It is being discussed whether detecting additional cancers using breast MRI and reducing re-excision rates yields any benefit in terms of survival. The POMB study, with its long and complete follow-up using a randomized population, addressed a previously unexplored concern related to the impact of preoperative breast MRI on DFS and OS. After 10 years of follow-up, there was a slight, although non-significant, improvement in DFS and OS among women aged 56 years or younger with breast cancer randomized to undergo breast MRI as an adjunct technique to standard preoperative assessment, especially in those without extensive disease.
The relative 10-year survival rate of women diagnosed with breast cancer was 86 per cent in 2016 according to the National Board of Health and Welfare29. However, prognosis tends to be poorer in younger patients with breast cancer. In this study, the OS rates were 90.9 and 88.6 per cent in the MRI and control groups respectively, which reflected adequate and efficient treatment.
In the present follow-up study, reasons for the low number of contralateral breast cancer occurrences in the two groups might have been the relatively small study population and an effect of adjuvant systemic therapy or hormone therapy. These outcomes may also be reflected in the previous POMB study results17, wherein MRI was found to be associated with a considerable number of contralateral and multifocal findings that would have remained undetected in the absence of preoperative MRI. Because of such findings, the surgical procedure and adjuvant therapy were adjusted for 20 per cent of patients in the MRI group. Thus, correct primary treatment could potentially be translated into improved long-term outcomes.
There are no other comparable randomized studies of preoperative breast MRI reporting survival data. Retrospective studies reported conflicting findings regarding breast cancer recurrence. In 2004, Fischer and co-workers30 compared 121 patients who underwent preoperative MRI with 225 patients who did not. The study demonstrated that preoperative MRI resulted in a statistically significant reduction in ipsilateral breast cancer recurrence rate (from 6.5 to 1.2 per cent) and a reduction in contralateral breast cancer rate (from 4.0 to 1.7 per cent) during 3 years of follow-up. The groups, however, showed a tendency to be unbalanced regarding patient age, risk of recurrence, and tumour size. Additionally, the recurrence rates were rather high during the short follow-up. Another retrospective study by Solin et al.31 compared an MRI group comprising 215 patients with a group of 541 women who did not undergo breast MRI. There was no difference in the 8-year rate of any local recurrence or OS between the groups. Local recurrence rates (3 per cent among patients who underwent preoperative breast MRI and 4 per cent for those who did not) were rather low and demonstrating an improvement in such rates would be extremely difficult in a retrospective cohort study.
Sung and colleagues32 published a retrospective study that included 348 patients, of whom 50 per cent underwent preoperative MRI. In this group, there was a significantly increased proportion of patients with extremely dense breasts and mammographically occult tumours. More tumours were synchronous and contralateral compared with those in the control group. The re-excision rate was lower in the MRI group, but no significant difference in locoregional recurrence or DFS was observed.
Houssami et al.33 investigated the potential association between preoperative MRI and breast cancer recurrence in a meta-analysis of individual-patient data including 3169 women. They found that preoperative MRI was not associated with reduced risk of local and distant recurrences during 8 years of follow-up. However, some of the included studies did not have an optimal design and the results are difficult to interpret because the groups of patients are not directly comparable. In addition, as outlined by the authors33, longer follow-up could possibly show a trend towards a more obvious MRI-related benefit.
The present study has several strengths. It is a randomized study with long follow-up. Because of the national security numbers in Sweden, no patients were lost to follow-up regarding survival. The MRI findings were evaluated by a few experienced specialists in radiology, all of whom were part of the diagnostic and therapeutic team. Women aged 56 years or less were selected, as they were more likely to have dense breasts, with the aim of including patients who would benefit most from preoperative breast MRI.
While interpreting the results, it should be considered that only a few events had occurred even after long-term follow-up, owing to the excellent prognosis of early-stage breast cancers. It is known that a small non-significant difference in survival between breast-conserving treatment with and without radiotherapy translates into a significant difference after 15 years of follow-up34. Whether this will also occur in the POMB cohort remains unknown, but it would indicate the need for longer follow-up.
To date, the most sensitive imaging modality for breast cancer detection has been MRI. However, in terms of cancer specificity, mammography is still superior. The continued development of imaging modalities has enabled the rise of contrast-enhanced digital mammography (CESM) as an alternative with greater breast cancer specificity than MRI. CESM could be of use for patients with contraindications to MRI as well as in regions with limited MRI availability35. However, whether improvement in diagnostic accuracy, leading to more accurate primary surgery, generally extrapolates into a better prognosis remains an unresolved issue.
Funding
This research work was supported by stipends and grants from the Capio St Goran’s Hospital Research Foundation; Centre for Clinical Research, Vastmanland Hospital, Vasteras; the Percy Falk Foundation; and the Vastmanland Research Foundation against Cancer. The sponsors of the POMB trial financed all breast MRI examinations and supported the implementation of the study, but had no role in the study design, data collection, data analyses, or manuscript writing.
Disclosure. The authors declare no conflict of interest.
Acknowledgements
K.S. and S.E. shared authorship. The authors are grateful to medical staff and surgeons at the breast cancer units for the inclusion of patients, and assistance in retrieving patients’ medical records and data. They thank A. Karlsson, radiologist, for his contribution and P. Wagner for statistical support. This research was preregistered with an analysis plan in the independent institutional registry http://clinicaltrials.gov.
Contributor Information
V Gonzalez, Region Vastmanland—Uppsala University, Centre for Clinical Research, Hospital of Vastmanland Vasteras, Vasteras, Sweden.
B Arver, Department of Oncology and Pathology, Karolinska Institutet, Stockholm, Sweden.
L Löfgren, Department of Surgery, St Goran Hospital, Stockholm, Sweden.
L Bergkvist, Region Vastmanland—Uppsala University, Centre for Clinical Research, Hospital of Vastmanland Vasteras, Vasteras, Sweden.
K Sandelin, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
S Eriksson, Region Vastmanland—Uppsala University, Centre for Clinical Research, Hospital of Vastmanland Vasteras, Vasteras, Sweden; Department of Surgery, Hospital of Vastmanland Vasteras, Vasteras, Sweden.
References
- 1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–1241. [DOI] [PubMed] [Google Scholar]
- 2. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery plus radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 3. Litiere S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Limbergen EV et al. Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412–419. [DOI] [PubMed] [Google Scholar]
- 4. Fisher S, Gao H, Yasui Y, Dabbs K, Winget M. Survival in stage I–III breast cancer patients by surgical treatment in a publicly funded health care system. Ann Oncol 2015;26:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen K, Liu J, Zhu L, Su F, Song E, Jacobs LK. Comparative effectiveness study of breast-conserving surgery and mastectomy in the general population: a NCDB analysis. Oncotarget 2015;6:40 127–40 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 2013;119:1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurian AW, Lichtensztajn DY, Keegan THM, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA 2014;312:902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 2014;149:267–274. [DOI] [PubMed] [Google Scholar]
- 9. Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of breast asymmetry after breast-conserving operation for breast cancer. J Am Coll Surg 2008;206:274–280. [DOI] [PubMed] [Google Scholar]
- 10. Olsen M, Nickel KB, Margenthaler J, Wallace AE, Mines D, Miller JP et al. Increased risk of surgical site infection among breast-conserving surgery re-excisions. Ann Surg Oncol 2015;22:2003–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 2008;246:116–124. [DOI] [PubMed] [Google Scholar]
- 12. Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008;26:3248–3258. [DOI] [PubMed] [Google Scholar]
- 13. Morrow M, Freedman G. A clinical oncology perspective on the use of breast MR. Magn Reson Imaging Clin N Am 2006;14:363–378. [DOI] [PubMed] [Google Scholar]
- 14. Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P et al. ; COMICE Trial Group. Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE). Health Technol Assess 2010;14:1–182. [DOI] [PubMed] [Google Scholar]
- 15. Peters NHGM, van Esser S, van den Bosch MAAJ, Plaisier PW, van Dalen T, Diepstraten SC et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET—randomised controlled trial. Eur J Cancer 2011;47:879–886. [DOI] [PubMed] [Google Scholar]
- 16. Brück N, Koskivuo I, Boström P, Saunavaara J, Aaltonen R, Parkkola R. Preoperative magnetic resonance imaging in patients with stage I invasive ductal breast cancer: a prospective randomized study. Scand J Surg 2018;107:14–22. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez V, Sandelin K, Karlsson A, Åberg W, Löfgren L, Iliescu G et al. Preoperative MRI of the breast (POMB) influences primary treatment in breast cancer: a prospective, randomized, multicenter study. World J Surg 2014;38:1685–1693. [DOI] [PubMed] [Google Scholar]
- 18. Karlsson A, Gonzalez V, Jonmarker JS, Bottai M, Sandelin K, Arver B et al. The accuracy of incremental pre-operative breast MRI findings—concordance with histopathology in the Swedish randomized multicenter POMB trial. Eur J Radiol 2019;114:185–191. [DOI] [PubMed] [Google Scholar]
- 19. Fisher ER, Sass R, Fisher B, Gregorio R, Brown R, Wickerham L; Collaborating NSABP Investigators. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). II. Relation of local breast recurrence to multicentricity. Cancer 1986;57:1717–1724. [DOI] [PubMed] [Google Scholar]
- 20. Hellman S. Stopping metastases at their source. N Engl J Med 1997;337:996–997. [DOI] [PubMed] [Google Scholar]
- 21. van Tienhoven G, Voogd AC, Peterse JL, Nielsen M, Andersen KW, Mignolet F et al. Prognosis after treatment for locoregional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). Eur J Cancer 1999;35:32–38. [DOI] [PubMed] [Google Scholar]
- 22. Brooks JP, Danforth DN, Albert P, Sciuto LC, Smith SL, Camphausen KA et al. Early ipsilateral breast tumor recurrences after breast conservation affect survival: an analysis of the National Cancer Institute randomized trial. Int J Radiat Oncol Biol Phys 2005;62:785–789. [DOI] [PubMed] [Google Scholar]
- 23. Doyle T, Schultz DJ, Peters C, Harris E, Solin LJ. Long-term results of local recurrence after breast conservation treatment for invasive breast cancer. Int J Radiat Oncol Biol Phys 2001;51:74–80. [DOI] [PubMed] [Google Scholar]
- 24. Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute randomized trial. Cancer 2003;98:697–702. [DOI] [PubMed] [Google Scholar]
- 25. van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143–1150. [DOI] [PubMed] [Google Scholar]
- 26. Vaidya JS, Vyas JJ, Chinoy RF, Merchant N, Sharma OP, Mittra I. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer 1996;74:820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breast Cancer Guideline, NABON 2012. http://www.richtlijnendatabase.nl/ (accessed 1 September 2020).
- 28. Bedrosian I, Mick R, Orel SG, Schnall M, Reynolds C, Spitz FR et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer 2003;98:468–473. [DOI] [PubMed] [Google Scholar]
- 29. Socialstyrelsen. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2018-6-10.pdf (accessed 1 September 2020).
- 30. Fischer U, Zachariae O, Baum F, von Heyden D, Funke M, Liersch T. The influence of preoperative MRI of the breasts on recurrence rate in patients with breast cancer. Eur Radiol 2004;14:1725–1731. [DOI] [PubMed] [Google Scholar]
- 31. Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol 2008;26:386–391. [DOI] [PubMed] [Google Scholar]
- 32. Sung JS, Li J, Da Costa G, Patil S, Van Zee KJ, Dershaw DD et al. Preoperative breast MRI for early-stage breast cancer: effect on surgical and long-term outcomes. AJR Am J Roentgenol 2014;202:1376–1382. [DOI] [PubMed] [Google Scholar]
- 33. Houssami N, Turner R, Macaskill P, Turnbull LW, McCready DR, Tuttle TM et al. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. J Clin Oncol 2014;32:392–401. [DOI] [PubMed] [Google Scholar]
- 34. Clark M, Collins R, Darby S, Davies C, Elphinstone P, Evans V et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087–2106. [DOI] [PubMed] [Google Scholar]
- 35. Richter V, Hatterman V, Preibsch H, Bahrs DS, Hahn M, Nikolaou K et al. Contrast-enhanced spectral mammography in patients with MRI contraindications. Acta Radiol 2018;59:798–805. [DOI] [PubMed] [Google Scholar]