Abstract
Objective
The aim was to describe direct health-care costs for adults with SLE in the UK over time and by disease severity and encounter type.
Methods
Patients aged ≥18 years with SLE were identified using the linked Clinical Practice Research Datalink–Hospital Episode Statistics database from January 2005 to December 2017. Patients were classified as having mild, moderate or severe disease using an adapted claims-based algorithm based on prescriptions and co-morbid conditions. We estimated all-cause health-care costs and incremental costs associated with each year of follow-up compared with a baseline year, adjusting for age, sex, disease severity and co-morbid conditions (2017 UK pounds).
Results
We identified 802 patients; 369 (46.0%) with mild, 345 (43.0%) moderate and 88 (11.0%) severe disease. The mean all-cause cost increased in the 3 years before diagnosis, peaked in the first year after diagnosis and remained high. The adjusted total mean annual increase in costs per patient was £4476 (95% CI: £3809, £5143) greater in the year of diagnosis compared with the baseline year (P < 0.0001). The increase in costs per year was 4.7- and 1.6-fold higher among patients with severe SLE compared with those with mild and moderate SLE, respectively. Primary care utilization was the leading component of costs during the first year after diagnosis.
Conclusion
The health-care costs for patients with SLE in the UK are substantial, remain high after diagnosis and increase with increasing severity. Future research should assess whether earlier diagnosis and treatment might reduce disease severity and associated high health-care costs.
Keywords: SLE and autoimmunity, health economics, primary care rheumatology, DMARDs, immunosuppressants
Key messages
The direct costs of health care for patients with SLE in the UK are substantial.
The cost to manage patients with moderate and severe SLE doubled 10 years after diagnosis.
Patients with SLE have increasingly high health-care costs driven by primary care and prescription drugs.
Introduction
SLE is a chronic inflammatory autoimmune disease characterized by alternating periods of increased disease activity, SLE flares, disease inactivity and remission. SLE affects multiple organs, including the skin, musculoskeletal, renal, pulmonary and nervous systems, leading to a wide range of clinical manifestations [1]. Common co-morbidities include cardiovascular disease [2, 3], stroke [4], osteoporosis [5] and infection [6]. Involvement of the renal system, referred to as LN [7], occurs in ∼60% of patients [8]. Organ damage in lupus can occur as a direct consequence of the disease or can be associated with long-term CS treatment [9–12], which offers rapid symptom relief and effective short-term disease control [13] but is associated with significant adverse effects. The high prevalence of SLE-related co-morbidities and the adverse effects associated with treatment can result in significant health-care resource utilization and costs. SLE has been associated with high health-care utilization and costs in several countries [12, 14–16]; however, evidence is currently limited in the UK. Furthermore, long-term longitudinal trends in health-care utilization and costs among patients with SLE are lacking.
We assessed the health-care utilization (primary care, hospitalizations, outpatient visits and selected prescription drugs) and costs among patients with SLE over a 13-year period (2005–2017), using population-based data for the UK from the linked Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES) database.
Methods
Study design and data
This study adopted an observational, retrospective cohort design using the UK CPRD- and HES-linked health-care administrative database and the Office for National Statistics mortality files between 1 January 2005 and 31 December 2017. The CPRD has been used previously to describe the epidemiology of SLE in the UK [3, 17–27]. It contains routinely collected primary care medical records data for ∼5.5 million registered patients from ∼590 general practices covering 8% of the UK population and has been shown broadly to be representative of the demographic distribution of the UK population. Linkage to HES is possible for approximately half of patients in the CPRD primary care database. Hospital data on the length, type, reasons and current diagnoses for all UK National Health Service (NHS) inpatient hospital admissions and outpatient clinic attendances were captured regardless of payer (private or government) or geographical residency of the patient [28]. Approval for this study was granted by the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency on 8 March 2018 (CPRD00023132 PROTOCOL 17_281R).
Study population
Adult patients aged ≥18 years and older who had a verified SLE diagnosis in the linked CPRD–HES database during the inclusion period were included in the study. Patients were required to have ≥12 months of prior history in the CPRD GOLD database without a diagnosis of SLE to confirm an incident diagnosis. Patients with a first diagnosis (index date) between 1 January 2005 and 31 December 2017 with ≥12 months of follow-up were selected.
Identification of SLE was based on the presence of one or more definitive diagnostic read codes in CPRD GOLD, confirmed by using International Classification of Diseases, 10th Revision (ICD-10) diagnosis codes in the HES data, by evidence of referral to a rheumatologist, or by treatment with one or more SLE-targeted prescription medications (including oral prednisolone, immunosuppressive therapy and antimalarials) using an algorithm modified from Nightingale et al. [23] (Supplementary Table S1 and S2, available at Rheumatology Advances in Practice online). An index date was assigned corresponding to the earliest SLE diagnosis anywhere in the linked CPRD–HES dataset. Prescriptions for SLE treatment alone were not considered enough to identify SLE incidence; however, if an SLE-specific prescription was identified before the first SLE diagnosis, the time of that prescription was taken to be the index diagnosis date.
Patients were excluded if they had read codes indicating cutaneous, drug-induced or discoid lupus rather than systemic lupus; if they did not have a definite code anywhere in their CPRD record or in HES to confirm diagnosis; or if they transferred out of the practice before the index event date.
Study time line
Patients were followed for 3 years before diagnosis (i.e. before their index diagnosis date) until the earliest of the following events: end of study period; leaving the database/date of patient’s last observed visit; or death. Person-time denominators were used to handle the varying lengths of follow-up of patients.
Assessment of disease severity
Disease severity (mild, moderate or severe) was defined using an algorithm adapted from a US retrospective, observational study [16], which combined SLE medications with SLE-related conditions (Supplementary Table S3, available at Rheumatology Advances in Practice online). The assigned disease severity was the highest severity experienced by a patient during a 1-year baseline period (12 months before index). SLE disease severity was defined as mild, moderate or severe. SLE was categorized as severe if treatment included CYC or an oral CS (prednisone-equivalent) prescription of ≥60 mg/day, or diagnosis of a severe co-morbid condition (e.g. end-stage renal disease, arterial/venous thrombosis). A moderate SLE category was assigned if treatment did not include CYC or oral CSs ≥60 mg/day, if there was a presence of a diagnosis of a moderate co-morbid condition (e.g. nephritis, haemolytic anaemia), or if treatment included an oral CS prescription of ≥7.5 to <60 mg/day or use of an immunosuppressive agent (excluding CYC). When patients did not meet criteria for moderate or severe disease, they were assigned mild SLE.
Assessment of health-care utilization and costs
Mean all-cause health-care costs were estimated using standard unit costing methods [29, 30]. We focused on all-cause health-care costs in order to capture the cost associated with treatment of SLE, related co-morbidities and adverse effects from treatment. Primary care costs were calculated by multiplying the duration of each consultation by the average cost per minute based on a comprehensive estimate of general practice expenses [31]. Outpatient attendances with and without procedures were assigned the appropriate unit costs from the NHS Reference Costs publication [32] by treatment specialty. Inpatient care was costed using the Health Resource Group 2017–2018 Reference Costs Grouper software [33] before applying reference costs for each category of stay, taken from the UK National Cost Schedule [32]. Medications were costed by mapping CPRD to British National Formulary codes [34] data and multiplying the quantity prescribed by the unit costs from the British National Formulary. Manual checks were carried out to examine the validity of quantity, strength and dosage of prescriptions.
Mean all-cause health-care costs per patient per year were estimated for the pre- and post-diagnosis periods for identified patients with SLE. Costs were examined for ≤10 years after the index date as the sum of costs by type of care (primary care, hospital inpatient, outpatient and prescription drugs) in the respective year. For patients with >12 months of pre-index disease-free data, we considered ≤3 years before diagnosis as reference for post-diagnosis cost comparisons.
To account for inflation and variations in pricing over time, 2017 unit costs were applied to all years from the UK NHS perspective. Additional detail on the methods used to estimate costs for each type of care is provided in Supplementary Table S4, available at Rheumatology Advances in Practice online.
Data analysis
We used means, standard deviations and frequencies to describe the characteristics of patients with SLE overall and by disease severity. We estimated unadjusted means and the 25th, 50th (median) and 75th quartiles to summarize annual counts by type of utilization, including inpatient and outpatient hospital and primary care visits, in addition to prescriptions. We estimated the unadjusted mean all-cause health-care costs per patient per year in the 3 years before the index date to 10 years after by type of care (primary care, inpatient, outpatient hospital and prescription drugs) and by disease severity (mild, moderate and severe SLE).
We used generalized estimating equations (GEEs) to compare mean all-cause health-care costs in each year with the reference year (3 years before the index date), adjusting for age and disease severity. We used the third year before diagnosis as a reference, to avoid distortion by the higher expected costs in the 24 months preceeding formal SLE diagnosis. Specifically, previous research has demonstrated that patients present with symptoms that might not be recognized immediately; for example, Al Sawah et al. [35] reported an average of 2.1 years between first lupus symptoms and seeking of medical care. We then used a random-effects (random intercepts) model to estimate patient-specific annual trends in mean all-cause health-care costs, adjusting for age and disease severity. Only the main effect from these models (trend in costs) is shown, because the effects of covariates were very similar to the ones from the GEE models. All available years of data were included for patients with variable amounts of pre-diagnosis and follow-up information. All analyses were conducted using SAS software v.9.4 (SAS Institute, Cary, NC, USA).
Results
Descriptive characteristics
A total of 802 individuals with 12 months of pre- and post-index data were identified, of whom 369 (46.0%) had mild SLE, 345 (43.0%) moderate SLE and 88 (10.9%) severe SLE. Table 1 presents descriptive information on patient characteristics, overall and by disease severity (mild, moderate and severe). About 88% were female, and the average age was 48.4 years. Patients had an average of 5.2 years of follow-up data after diagnosis. Of these 802 patients, 682 (85%) had ≥3 years of health records prior to the index diagnosis of SLE, and 569 (71%) had ≥3 years of follow-up data after diagnosis.
Table 1.
Characteristics of patients with SLE (Clinical Practice Research Datalink–Hospital Episodes Statistics database, 2005–2017)
| Characteristic | All patients (n = 802) | Mild disease (n = 369) | Moderate disease (n = 345) | Severe disease (n = 88) |
|---|---|---|---|---|
| Female, n (%) | 709 (88.4) | 326 (88.4) | 311 (90.1) | 72 (81.8) |
| Age at index, mean (S.d.), years | 48.4 (15.3) | 47.1 (14.4) | 48.2 (15.7) | 53.9 (16.0) |
| Age, n (%) | ||||
| 18–44 years | 348 (43.4) | 169 (45.8) | 152 (44.1) | 27 (30.7) |
| 45–64 years | 321 (40.0) | 149 (40.4) | 134 (38.8) | 38 (43.2) |
| ≥65 years | 133 (16.6) | 51 (13.8) | 59 (17.1) | 23 (26.1) |
| Follow-up, years | ||||
| Mean (S.d.) | 5.2 (3.0) | 5.0 (3.0) | 5.6 (3.0) | 4.7 (2.8) |
Direct health-care utilization
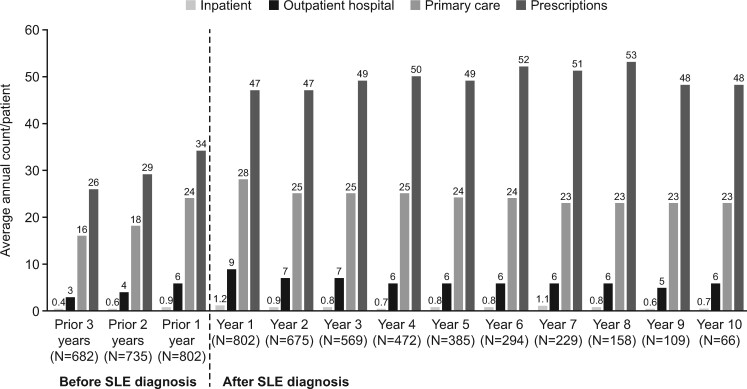
The average number of primary care visits, inpatient stays, outpatient visits and prescriptions in the year of diagnosis was 28.4, 1.2, 8.8 and 46.9, respectively (Fig. 1). For all types of health-care use, there was a pattern of increasing utilization in the 3 years before diagnosis, with a peak during the year of diagnosis, after which health-care utilization remained fairly constant over 10 years of follow-up (Fig. 1; Supplementary Table S5, available at Rheumatology Advances in Practice online).
Fig. 1.
Health-care utilization by category from 3 years before to 10 years after the index date (Clinical Practice Research Datalink–Hospital Episodes Statistics database, 2005–2017)
Mean all-cause direct health-care costs
The mean, unadjusted, all-cause health-care cost for patients with SLE increased progressively in the 3 years before diagnosis, and during the first year after diagnosis rose to £7532 (Table 2). The mean all-cause health-care cost held relatively steady throughout 7 years of follow-up. The highest health-care costs were observed in years 8–10 (from fewer patient numbers) reaching £12 195 in year 10 (Table 2). The adjusted total mean annual increase in all-cause health-care costs from 3 years before the index date compared with each available follow-up year, adjusted for age, sex, disease severity and co-morbid conditions, followed a similar pattern. In the year after diagnosis, adjusted costs reached £4476 (95% CI: £3809, £5092; P < 0.0001) and remained higher in the years after diagnosis compared with the pre-diagnosis period (Table 2; Supplementary Table S6, available at Rheumatology Advances in Practice online).
Table 2.
Mean health-care costs from 3 years before to 10 years after the index date (Clinical Practice Research Datalink–Hospital Episodes Statistics database, 2005–2017)
| Year (number of observations) | Unadjusted mean (S.d.) all-cause health-care costs per person per year, £a |
Adjusted mean all-cause health-care costs vs 3 years before index
b
|
|||
|---|---|---|---|---|---|
| Estimate, £ a , c | 95% CIs, £ | P value | |||
| Before diagnosis | |||||
| Year −3 (n = 682) | 3250 (4475) | – | – | – | – |
| Year −2 (n = 735) | 4171 (6760) | 1020 | 654 | 1386 | <0.0001 |
| Year −1 (n = 802) | 6114 (8183) | 3058 | 2545 | 3571 | <0.0001 |
| After diagnosis | |||||
| Year 1 (n = 802) | 7532 (9634) | 4476 | 3861 | 5092 | <0.0001 |
| Year 2 (n = 675) | 6769 (11 502) | 3898 | 3124 | 4672 | <0.0001 |
| Year 3 (n = 569) | 6809 (12 325) | 4172 | 3276 | 5069 | <0.0001 |
| Year 4 (n = 472) | 6367 (10 028) | 4043 | 3233 | 4854 | <0.0001 |
| Year 5 (n = 385) | 6950 (11 442) | 4950 | 3944 | 5957 | <0.0001 |
| Year 6 (n = 294) | 6593 (8759) | 4947 | 4014 | 5881 | <0.0001 |
| Year 7 (n = 229) | 7614 (10 870) | 6172 | 4889 | 7455 | <0.0001 |
| Year 8 (n = 158) | 10 023 (14 807) | 8506 | 6506 | 10 507 | <0.0001 |
| Year 9 (n = 109) | 10 398 (17 777) | 9239 | 6350 | 12 128 | <0.0001 |
| Year 10 (n = 66) | 12 195 (20 286) | 10 550 | 6592 | 14 508 | <0.0001 |
Costs are expressed in 2017 UK pounds. bPerson-time denominators were used to account for varying lengths of follow-up for individual patients.cCalculated using generalized estimating equations to compare mean all-cause health-care costs in each year with the reference year (3 years before the index date), adjusting for age and disease severity.
Direct health-care costs by type of encounter
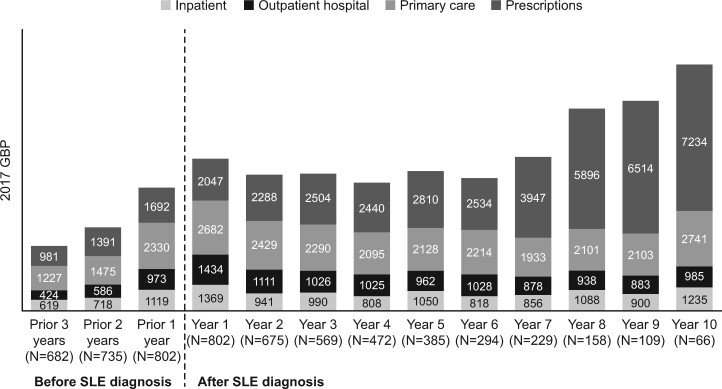
Primary care utilization was the leading component of health-care costs during the first year of diagnosis, representing an unadjusted mean cost of £2682 (Fig. 2). The proportion of health-care cost utilization attributable to primary care and the other utilization categories remained steady until year 6, after which the largest increase in health-care costs was observed (Fig. 2).
Fig. 2.
Mean annual health-care costs by category before and after the index date (Clinical Practice Research Datalink–Hospital Episodes Statistics database, 2005–2017)
Unadjusted costs are expressed in 2017 UK pounds. Costs were estimated using a health system perspective and included direct medical resource use only. Other costs, such as out-of-pocket expenditure by patients and costs of informal and formal caregiving, were not captured. GBP: UK pounds.
All-cause direct health-care costs by disease severity
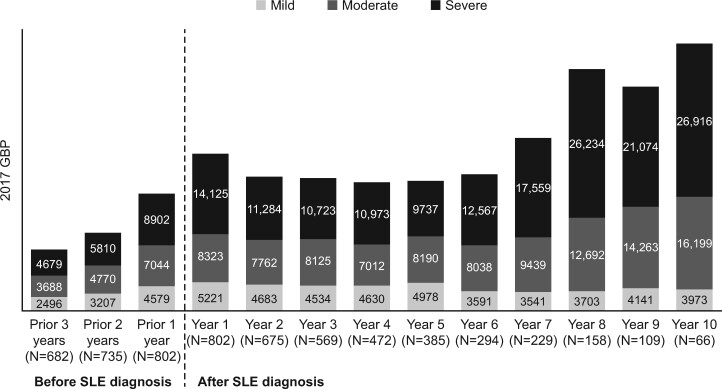
All-cause health-care costs increased over time for patients with severe and moderate SLE but remained relatively flat over the study period for patients with mild SLE. These unadjusted mean costs in the year of diagnosis were £14 125, £8323 and £5221, for severe, moderate and mild SLE, respectively (Fig. 3). Adjusted mean all-cause health-care costs were greater for patients with moderate vs mild SLE (£2786; 95% CI: £1737, £3835; P < 0.0001) and with severe vs mild SLE (£5207; 95% CI: £3277, £7138; P < 0.0001). Models of individual trajectories of mean all-cause health-care costs (Table 3) showed an increase of £616 (95% CI, £560, £672) per year, controlling for age, sex and disease severity. The increased costs were most pronounced among patients with severe SLE (£1228; 95% CI, £981, £1476), followed by moderate (£788; 95% CI, £699, £878) and mild SLE (£262; 95% CI, £194, £330).
Fig. 3.
Mean annual total health-care costs by disease severity before and after the index date (Clinical Practice Research Datalink–Hospital Episodes Statistics database, 2005–2017)
Unadjusted costs are expressed in 2017 UK pounds. SLE disease severity was classified as mild, moderate or severe using an adapted claims-based algorithm that uses SLE-related conditions and medications. GBP: UK pounds.
Table 3.
Change in total health-care costs per year by disease severity (Clinical Practice Research Datalink–Hospital Episodes Statistics database, 2005–2017)
| Group | Change in all-cause health-care costs/year, £ a , b | 95% CIs, £ | P-value | |
|---|---|---|---|---|
| All patients (n = 802) | 616 | 560 | 672 | <0.0001 |
| Mild SLE (n = 369)c | 262 | 194 | 330 | <0.0001 |
| Moderate SLE (n = 345)c | 788 | 699 | 878 | <0.0001 |
| Severe SLE (n = 88)c | 1228 | 981 | 1476 | <0.0001 |
aCosts are expressed in 2017 UK pounds. bCalculated using random intercept patient-specific models. cSLE disease severity was classified as mild, moderate or severe using an adapted claims-based algorithm that uses SLE-related conditions and medications.
Discussion
Direct health-care costs increased gradually in the 3 years before diagnosis, with high costs in the year after diagnosis, which remained relatively stable for several years, possibly reflecting the establishment of treatment regimens. Among patients who remained in the database, mean all-cause health-care costs rose sharply in follow-up years 8–10. This might represent costs associated with long-term SLE care and co-morbid disease; however, this rise should be viewed with caution given the smaller sample size in later years and might be explained by outlier cases (e.g. those with organ damage as a result of SLE). Health-care costs increased with increasing severity. Patients with moderate or severe SLE consistently incurred greater all-cause health-care costs over time compared with patients with mild SLE during a 3-year pre-diagnosis period and after diagnosis, until 10 years of follow-up; costs doubled for patients with moderate and severe SLE, whereas the cost for mild SLE did not increase. The increase in adjusted mean all-cause health-care costs per year were 4.7- and 1.6-fold higher among patients with severe SLE compared with those with mild and moderate SLE, respectively.
There are limited data evaluating health-care costs associated with SLE over time. The substantial health-care costs found in the present study are consistent with studies from other countries that have reported a significant economic burden associated with SLE. A review of articles published between 2007 and 2013 reported high medical costs and high levels of unemployment and absenteeism associated with the disease [36]. An earlier review of 11 articles reported that average direct costs per patient-year ranged from $3735 to $14 410 (2008 US dollars), mostly driven by inpatient care [12]; in another review of 14 studies, the direct annual costs were between $2214 and $16 685 (2010 US dollars) [15]. A recent study also reported that the direct health-care costs associated with SLE were $13 038 (2013 Canadian dollars) [14]. Comparisons between the costs estimated in our study and those estimated in previous studies would need to account for the relevant currency exchange and inflation rates and should be made cautiously, owing to differences in the methodology used to estimate costs, in addition to structural differences in the underlying health-care delivery landscape across countries.
Our study also complements the limited literature on costs of SLE in the UK, which has been based on small samples. One cross-national comparison of patients in the US, Canada and UK, the Tri-Nation study [37], relied on self-reported data for 215 UK-based patients. Another study consisted of 86 patients recruited from four specialist rheumatology centres in England (the LUCIE study) [38, 39]. Detailed data on inpatient and specialist care were obtained from medical chart review for a cohort of patients with prevalent SLE over a follow-up period of 2 years. The LUCIE study was not designed to track either the impact on cost of a nascent diagnosis or the effect on costs over the medium to long term. Furthermore, primary care costs were not captured. We add to this literature by estimating costs across all settings covered by the NHS in the UK for an extended period before incident diagnosis of SLE and following patients for as long as data were available, ≤10 years after diagnosis.
In contrast to studies from other countries [12, 14–16], which found that the largest component of medical costs associated with SLE was inpatient hospitalization, we found that primary care utilization represented a larger share of the mean all-cause health-care cost compared with inpatient stays. This might reflect differences in care delivery, the generally lower costs of inpatient care in the UK compared with the USA, or differences among the costing methodologies used. Prescription medications made up a substantial and growing share of the mean all-cause cost over time in our study. This might be attributable to an increasing need to manage co-morbid conditions and/or the sequelae of SLE as the disease progresses.
Our study demonstrated an increase in adjusted mean all-cause health-care costs over time, with the increase in costs being most pronounced among patients with severe SLE, followed by those with moderate SLE. This is likely to reflect the extent of organ damage in these patients. Greater organ damage has been associated with increased health-care resource use [40]. Potentially, cost savings could be achieved by earlier diagnosis and treatment, including careful monitoring, to reduce the onset of irreversible organ damage and the occurrence of co-morbidities. This aligns with clinical guidelines that stress the goal of treatment aimed at improving long-term patient outcomes and quality of life, in addition to preventing damage accrual [41].
Observational studies using routinely collected electronic health record data are subject to several limitations, including the possibility of missing or misclassified data. To reduce potential misclassification of SLE diagnosis, we required that an SLE diagnosis be confirmed using an algorithm, modified from Nightingale et al. [23], to identify additional criteria in the patient record. It is possible to have underestimated the number of cases, because patients without active disease might have been excluded owing to lack of supporting data on treatment in the medical record. Electronic health records do not routinely include the information needed to generate established scores for disease severity and activity [23, 42]. However, we used a validated algorithm to assign patients to mild, moderate and severe disease categories in the year after index diagnosis [16, 43]. It is possible, given the criteria used in the algorithm, that we have underestimated the proportion of patients with severe SLE and overestimated those with moderate and mild disease. For example, one of the criteria for severe disease is a prescription of prednisolone of >60 mg/day. Other disease severity scores might use lower milligram per day thresholds for severe disease [44]. However, our analysis clearly shows an association between costs and severity.
CPRD GOLD linkage data are available for only 50% of contributing CPRD GOLD practices in the UK; therefore, health-care resource use is not available for all SLE patients captured in the CPRD database. Our study included health-care costs to the NHS only and did not include other societal costs, such as informal care (e.g. by family members and friends), out-of-pocket costs for non-prescription medications and other services not covered by the NHS, and a range of non-health-care costs, such as lost productivity. Other studies have found these indirect costs in individuals with SLE to be much higher than the direct medical costs [45]. In addition, costs of biologics and drugs prescribed at specialty centres are not captured in the CPRD database. Together, these factors suggest that our results might be an underestimation of the true SLE costs in the UK.
Finally, our estimates are based on the missing-at-random assumption, implying that loss to follow-up was not related to health-care costs. As in most longitudinal studies, this assumption could not be tested directly, because cost data on patients after they left the dataset were not available. However, there is no reason to expect that leaving the dataset is associated with the severity of SLE and, therefore, with health-care utilization and cost of care.
Conclusions
Our findings suggest that the direct costs of health care for patients with SLE in the UK are substantial and increase in the years before and after diagnosis. Patients with moderate or severe SLE consistently incur greater all-cause health-care costs over time compared with patients with mild SLE during the 3 years before and after diagnosis, up to 10 years. For all patients, health-care costs gradually increase during the 3 years before diagnosis, suggesting that patients might initiate encounters with the health-care system in quest of a diagnosis. This study sheds light on the importance of disease management for the moderate to severe SLE patient. Earlier diagnosis and treatment might reduce disease severity and occurrence of co-morbidities and the high health-care costs associated with SLE.
Supplementary Material
Acknowledgements
Editing assistance was provided by Rebecca S. Jones, PhD of JK Associates Inc., a part of Fishawack Health. This support was funded by AstraZeneca.
Funding: This work was supported by funding from AstraZeneca.
Disclosure statement: M.S., S.W., J.L. and S.L. are consultants and have worked on behalf of AstraZeneca. V.B., X.W. and BD. are employees of AstraZeneca. B.D. is a shareholder of AstraZeneca. E.H. was an employee of AstraZeneca at the time this study was conducted.
Data availability statement
This study is based in part on data from CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The Office for National Statistics provided the mortality data. The interpretation and conclusions contained in this study are those of the authors alone. The authors do not own these data and hence are not permitted to share the data in the original form.
Supplementary data
Supplementary data are available at Rheumatology Advances in Practice online.
References
- 1.Maidhof W, Hilas O.. Lupus: an overview of the disease and management options. P T 2012;37:240–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Ward MM.Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum 1999;42:338–46. [DOI] [PubMed] [Google Scholar]
- 3.Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR.. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol 2004;93:198–200. [DOI] [PubMed] [Google Scholar]
- 4.Mok CC, Ho LY, To CH.. Annual incidence and standardized incidence ratio of cerebrovascular accidents in patients with systemic lupus erythematosus. Scand J Rheumatol 2009;38:362–8. [DOI] [PubMed] [Google Scholar]
- 5.Almehed K, Forsblad d'Elia H, Kvist G, Ohlsson C, Carlsten H.. Prevalence and risk factors of osteoporosis in female SLE patients—extended report. Rheumatology (Oxford) 2007;46:1185–90. [DOI] [PubMed] [Google Scholar]
- 6.Mosca M, Tani C, Aringer M. et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis 2010;69:1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz N, Goilav B, Putterman C.. The pathogenesis, diagnosis and treatment of lupus nephritis. Curr Opin Rheumatol 2014;26:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxena R, Mahajan T, Mohan C.. Lupus nephritis: current update. Arthritis Res Ther 2011;13:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zonana-Nacach A, Barr SG, Magder LS, Petri M.. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum 2000;43:1801–8. [DOI] [PubMed] [Google Scholar]
- 10.Thamer M, Hernán MA, Zhang Y, Cotter D, Petri M.. Prednisone, lupus activity, and permanent organ damage. J Rheumatol 2009;36:560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petri M, Purvey S, Fang H, Magder LS.. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum 2012;64:4021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon C, Amissah-Arthur M-B, Gayed M. et al. ; British Society for Rheumatology Standards, Audit and Guidelines Working Group. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford) 2018;57:e1–e45. [DOI] [PubMed] [Google Scholar]
- 13.Zhu TY, Tam LS, Li EK.. Cost-of-illness studies in systemic lupus erythematosus: a systematic review. Arthritis Care Res 2011;63:751–60. [DOI] [PubMed] [Google Scholar]
- 14.McCormick N, Marra CA, Sadatsafavi M, Aviña-Zubieta JA.. Socioeconomic status at diagnosis influences the incremental direct medical costs of systemic lupus erythematosus: a longitudinal population-based study. Semin Arthritis Rheum 2020;50:77–83. [DOI] [PubMed] [Google Scholar]
- 15.Meacock R, Dale N, Harrison MJ.. The humanistic and economic burden of systemic lupus erythematosus: a systematic review. Pharmacoeconomics 2013;31:49–61. [DOI] [PubMed] [Google Scholar]
- 16.Garris C, Jhingran P, Bass D. et al. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ 2013;16:667–77. [DOI] [PubMed] [Google Scholar]
- 17.Bernier M-O, Mikaeloff Y, Hudson M, Suissa S.. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Rheum 2009;61:476–81. [DOI] [PubMed] [Google Scholar]
- 18.Rees F, Doherty M, Grainge MJ. et al. Mortality in systemic lupus erythematosus in the United Kingdom 1999–2012. Rheumatology (Oxford) 2016;55:854–60. [DOI] [PubMed] [Google Scholar]
- 19.Rees F, Doherty M, Lanyon P. et al. Early clinical features in systemic lupus erythematosus: can they be used to achieve earlier diagnosis? A risk prediction model. Arthritis Care Res 2017;69:833–41. [DOI] [PubMed] [Google Scholar]
- 20.Bultink IE, Harvey NC, Lalmohamed A. et al. Elevated risk of clinical fractures and associated risk factors in patients with systemic lupus erythematosus versus matched controls: a population-based study in the United Kingdom. Osteoporosis Int 2014;25:1275–83. [DOI] [PubMed] [Google Scholar]
- 21.De Jong HJI, van Staa TP, Lalmohamed A. et al. Pattern of risks of systemic lupus erythematosus among statin users: a population-based cohort study. Ann Rheum Dis 2017;76:1723–30. [DOI] [PubMed] [Google Scholar]
- 22.Meier CR, Sturkenboom MC, Cohen AS, Jick H.. Postmenopausal estrogen replacement therapy and the risk of developing systemic lupus erythematosus or discoid lupus. J Rheumatol 1998;25:1515–9. [PubMed] [Google Scholar]
- 23.Nightingale AL, Davidson JE, Molta CT, Kan HJ, McHugh NJ.. Presentation of SLE in UK primary care using the Clinical Practice Research Datalink. Lupus Sci Med 2017;4:e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nightingale AL, Farmer RDT, de Vries CS.. Incidence of clinically diagnosed systemic lupus erythematosus 1992–1998 using the UK General Practice Research Database. Pharmacoepidemiol Drug Saf 2006;15:656–61. [DOI] [PubMed] [Google Scholar]
- 25.Nightingale AL, Farmer RDT, de Vries CS.. Systemic lupus erythematosus prevalence in the UK: methodological issues when using the General Practice Research Database to estimate frequency of chronic relapsing-remitting disease. Pharmacoepidemiol Drug Saf 2007;16:144–51. [DOI] [PubMed] [Google Scholar]
- 26.Somers EC, Thomas SL, Smeeth L, Schoonen WM, Hall AJ.. Incidence of systemic lupus erythematosus in the United Kingdom, 1990–1999. Arthritis Rheum 2007;57:612–8. [DOI] [PubMed] [Google Scholar]
- 27.Rees F, Doherty M, Grainge M. et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann Rheum Dis 2016;75:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hospital Episode Statistics. Hospital Episode Statistics: hospital outpatient activity 2011–12 summary report, 2012. http://content.digital.nhs.uk/catalogue/PUB09379/hosp-outp-acti-11-12-summ-repo-rep.pdf (11 December 2016, date last accessed).
- 29.Onukwugha E, McRae J, Kravetz A. et al. Cost-of-illness studies: an updated review of current methods. Pharmacoeconomics 2016;34:43–58. [DOI] [PubMed] [Google Scholar]
- 30.Drummond MF, Jefferson TO.. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ (Clinical research ed.) 1996;313:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis L, Burns A. Unit costs of health and social care 2017, 2017. 10.22024/UniKent/01.02/65559 (11 May 2019, date last accessed). [DOI]
- 32.Department of Health. NHS reference costs 2017/18, 2018. https://improvement.nhs.uk/resources/reference-costs/#rc1718 (11 May 2019, date last accessed).
- 33.NHS Digital. HRG4+ 2017/18 reference costs grouper and documentation, 2019. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/costing-hrg4-2017-18-reference-costs-grouper (3 April 2019, date last accessed).
- 34.British National Formulary. British National Formulary, 2019. http://www.bnf.org/bnf/org_450080.htm (9 September 2019, date last accessed).
- 35.Al Sawah S, Daly RP, Foster S. et al. SAT0423 Understanding delay in diagnosis, access to care and satisfaction with care in lupus: findings from a cross-sectional online survey in the United States. Ann Rheum Dis 2015;74:812. [Google Scholar]
- 36.Holloway L, Humphrey L, Heron L. et al. Patient-reported outcome measures for systemic lupus erythematosus clinical trials: a review of content validity, face validity and psychometric performance. Health Qual Life Outcomes 2014;12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke AE, Petri M, Manzi S. et al. ; Tri-Nation Study Group. The systemic lupus erythematosus Tri-nation Study: absence of a link between health resource use and health outcome. Rheumatology (Oxford) 2004;43:1016–24. [DOI] [PubMed] [Google Scholar]
- 38.Khamashta MA, Bruce IN, Gordon C. et al. The cost of care of systemic lupus erythematosus (SLE) in the UK: annual direct costs for adult SLE patients with active autoantibody-positive disease. Lupus 2014;23:273–83. [DOI] [PubMed] [Google Scholar]
- 39.Doria A, Amoura Z, Cervera R. et al. Annual direct medical cost of active systemic lupus erythematosus in five European countries. Ann Rheum Dis 2014;73:154–60. [DOI] [PubMed] [Google Scholar]
- 40.Barber MRW, Hanly JG, Su L. et al. Economic evaluation of damage accrual in an international systemic lupus erythematosus inception cohort using a multistate model approach. Arthritis Care Res (Hoboken) 2020;72:1800–8. [DOI] [PubMed] [Google Scholar]
- 41.Fanouriakis A, Kostopoulou M, Alunno A. et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 42.Mikdashi J, Nived O.. Measuring disease activity in adults with systemic lupus erythematosus: the challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res Ther 2015;17:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speyer CB, Li D, Guan H. et al. Comparison of an administrative algorithm for SLE disease severity to clinical SLE Disease Activity Index scores. Rheumatol Int 2020;40:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isenberg DA, Rahman A, Allen E. et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:902–6. [DOI] [PubMed] [Google Scholar]
- 45.Bexelius C, Wachtmeister K, Skare P, Jönsson L, van Vollenhoven R.. Drivers of cost and health-related quality of life in patients with systemic lupus erythematosus (SLE): a Swedish nationwide study based on patient reports. Lupus 2013;22:793–801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study is based in part on data from CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The Office for National Statistics provided the mortality data. The interpretation and conclusions contained in this study are those of the authors alone. The authors do not own these data and hence are not permitted to share the data in the original form.