Abstract
Data are mixed on whether patients with semantic variant primary progressive aphasia exhibit a category-selective semantic deficit for animate objects. Moreover, there is little consensus regarding the neural substrates of this category-selective semantic deficit, though prior literature has suggested that the perirhinal cortex and the lateral posterior fusiform gyrus may support semantic memory functions important for processing animate objects. In this study, we investigated whether patients with semantic variant primary progressive aphasia exhibited a category-selective semantic deficit for animate objects in a word-picture matching task, controlling for psycholinguistic features of the stimuli, including frequency, familiarity, typicality and age of acquisition. We investigated the neural bases of this category selectivity by examining its relationship with cortical atrophy in two primary regions of interest: bilateral perirhinal cortex and lateral posterior fusiform gyri. We analysed data from 20 patients with semantic variant primary progressive aphasia (mean age = 64 years, S.D. = 6.94). For each participant, we calculated an animacy index score to denote the magnitude of the category-selective semantic deficit for animate objects. Multivariate regression analysis revealed a main effect of animacy (β = 0.52, t = 4.03, P < 0.001) even after including all psycholinguistic variables in the model, such that animate objects were less likely to be identified correctly relative to inanimate objects. Inspection of each individual patient’s data indicated the presence of a disproportionate impairment in animate objects in most patients. A linear regression analysis revealed a relationship between the right perirhinal cortex thickness and animacy index scores (β = −0.57, t = −2.74, P = 0.015) such that patients who were more disproportionally impaired for animate relative to inanimate objects exhibited thinner right perirhinal cortex. A vertex-wise general linear model analysis restricted to the temporal lobes revealed additional associations between positive animacy index scores (i.e. a disproportionately poorer performance on animate objects) and cortical atrophy in the right perirhinal and entorhinal cortex, superior, middle, and inferior temporal gyri, and the anterior fusiform gyrus, as well as the left anterior fusiform gyrus. Taken together, our results indicate that a category-selective semantic deficit for animate objects is a characteristic feature of semantic variant primary progressive aphasia that is detectable in most individuals. Our imaging findings provide further support for the role of the right perirhinal cortex and other temporal lobe regions in the semantic processing of animate objects.
Keywords: semantic variant primary progressive aphasia, category-selective semantic memory deficit, animacy, semantic memory
Henderson et al. reported that semantic variant primary progressive aphasia patients exhibit a category-selective semantic deficit for animate objects that is uninfluenced by the psycholinguistic features of the stimuli and the magnitude of this deficit is associated with greater atrophy in the bilateral anterior fusiform gyri and right temporal regions.
Graphical Abstract
Graphical Abstract.
Introduction
Semantic memory—memory for words, symbols, concepts, and their relationships, or general knowledge1—can be specifically impaired in patients with a form of anterior temporal lobe-predominant Frontotemporal Lobar Degeneration that may manifest as Semantic Dementia (SD)2–4 or as semantic variant Primary Progressive Aphasia (svPPA).5,6 Neuropsychological studies of patients with impaired semantic memory have identified dissociable patterns of impairment, with some exhibiting a category-selective semantic memory deficit (CSSD) for animate or inanimate categories of information,7,8 whereas others show generalized impairment across categories. Detailed case reports provide well-established evidence that some svPPA cases exhibit a CSSD for animate objects—i.e. a disproportionate semantic memory loss for animate compared to inanimate objects.6,9–19 Although some group studies of patients with SD or svPPA suggest that they have a non-selective general semantic impairment,20,21 other studies of SD/svPPA have found a CSSD for animate objects.22–25
An important factor that likely contributes to these mixed findings pertains to the psycholinguistic characteristics of the stimuli used to assess semantic memory, including frequency, familiarity, typicality and age of acquisition.22,26,27 Performance on tests of semantic memory is better for more familiar, more frequent, more typical and earlier acquired words.21,27–29 These factors are particularly relevant when comparing animate versus inanimate categories21 because many animate objects in commonly used stimulus sets (e.g. rhinoceros) are rated as less familiar and less frequent than inanimate objects30 and are acquired at an older age.31 The few studies accounting for psycholinguistic variables when assessing the presence of a CSSD for animate objects have yielded mixed results in svPPA. While some studies have reported the absence of a CSSD for animate objects after accounting for these factors in naming tasks,22,27 others have demonstrated that a CSSD for animate objects is still present even after controlling for frequency, familiarity and age of acquisition in various naming and recognition tasks.12,25,32
If a CSSD for animate objects is present in some patients with svPPA, this offers a valuable opportunity to advance our understanding of the neural basis of cognitive processes contributing to semantic memory. Such investigations are informed by functional neuroimaging studies of healthy adults which suggest that animate or inanimate stimuli may be represented differentially within unimodal sensory and heteromodal association cortex. Areas preferentially engaged by animate objects in healthy adults include the lateral fusiform gyri, perirhinal and entorhinal cortex, inferior temporal gyrus, and right superior temporal sulcus, whereas areas preferentially engaged by inanimate objects include the left posterior middle temporal gyrus, medial fusiform gyri and inferior parietal lobule.33–38 Functional MRI studies have shown greater activation in the lateral region of the posterior fusiform gyrus using naming, basic-level categorization or semantic decision tasks for animal versus tool stimuli.33,39–46 Neuropsychological studies have also supported the role of the perirhinal cortex in disambiguating perceptually and semantically confusable objects, such as animate objects, which are thought to be more ambiguous than inanimate objects because they share many visual features within their semantic category.47–50
Variability in the absence or presence of a CSSD for animate objects in patients with svPPA may arise from individual variability in regional neurodegeneration. Debate continues about the earliest and most consistent sites of atrophy in the anterior temporal region in patients with svPPA.51–53 Our own work suggests a replicable localization within the tip of the temporal pole,51 but others have found the anterior fusiform and inferior temporal gyrus,54 entorhinal cortex55 and perirhinal cortex56 to be areas of greatest degenerative change. Despite the relatively stereotyped pattern of anterior temporal lobe atrophy in svPPA, heterogeneity is present in the localization and magnitude of atrophy across individual patients.51,53,57 Some of this heterogeneity relates to the severity of semantic memory impairment, some is related to the presence of other impairments, such as visual agnosia58–60 or behavioural symptoms,61 and some is driven by overall dementia severity. This variability within svPPA may offer the opportunity to test hypotheses about relationships between relatively greater regional atrophy and a more prominent CSSD for animate objects.
Here, we sought to address two major gaps in the literature. First, we aimed to determine whether patients with svPPA exhibit a CSSD for animate objects after controlling confounding psycholinguistic features of the stimuli. Based on previous literature12,25,32 and our own clinical experience, we hypothesized that a CSSD for animate objects would be present in svPPA and would not be attributable to differential frequency, familiarity, age of acquisition, or typicality of the stimuli. We predicted that this CSSD for animate objects would be found in the sample as a whole and in the majority of individual patients, since we believe that this is a characteristic feature of svPPA. Second, we investigated the neural basis of the CSSD for animate objects by examining the localization of atrophy that correlates with relatively greater semantic memory impairment for animate than inanimate objects. Based on the literature reviewed above, we hypothesized that a disproportionately poorer performance for animate (relative to inanimate) objects will be associated with greater cortical atrophy in the bilateral perirhinal cortex and the lateral fusiform gyri. To assess the specificity of these regional associations and identify additional regions that may contribute to a CSSD for animate objects in svPPA, we conducted an analysis examining the relationship between the effect of animacy and cortical thickness within the entire temporal lobe. The choice to restrict this analysis to the temporal lobes was motivated by strong evidence supporting the role of temporal lobe structures in semantic memory2–4 and by the typical pattern of atrophy exhibited by patients with svPPA in the temporal lobes.51,53,57 If some patients with svPPA exhibit a CSSD for animate objects that are predictably localizable, this would have important implications for theoretical models of semantic cognition.
Materials and methods
Participants
We analysed data from 20 svPPA patients (mean age = 64 years, S.D. = 6.94; 10 females) who participate in the ongoing Massachusetts General Hospital Frontotemporal Disorders Unit PPA cohort. Participants undergo a comprehensive clinical evaluation as previously described.62,63 We perform a multidisciplinary assessment, including a structured interview by a neurologist or psychiatrist assessing cognition, mood/behaviour, sensorimotor function, and daily activities; a neurologic examination, including office-based cognitive testing (for cases in this report, BCD); a speech-language assessment by a speech-language pathologist (M.Q. or D.H.), including the Progressive Aphasia Severity Scale (PASS) to specifically assess language impairment from a patient’s premorbid baseline62; and an MRI scan with T1- and T2-weighted sequences inspected visually by a neurologist. A clinician also performs a structured interview with a care partner, augmented with standard questionnaires. For most of the participants in this report, the protocol included the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (using version 2.0 previously and currently version 3.0) and supplementary measures.
Cases selected for this study had been diagnosed with svPPA according to consensus guidelines.64,65 All patients presented with impaired confrontation naming and single-word comprehension with intact motor speech. Most patients (85% of our sample) presented with surface dyslexia, as evidenced by regularization errors when asked to spell irregularly spelled words. Multidisciplinary assessment and structured care partner interviews documented the absence of visual agnosia and prominent behavioural disturbance in all patients. Visual inspection of a clinical MRI revealed cortical atrophy that was most prominent in the left anterior temporal lobe and ruled out other causes of focal brain damage. Besides a single left-handed patient, all patients were right-handed and all were native English speakers. All patients and their care partners denied a pre-existing psychiatric disorder, other neurological disorder or developmental cognitive disorder.
All patients and their care partners gave written informed consent in accordance with guidelines established by the Mass General Brigham Healthcare System Institutional Review Boards which govern human subjects research at Massachusetts General Hospital.
Single-word comprehension task
Stimuli and procedures
All participants completed a spoken word-picture matching task from the Cambridge Semantic Battery (CSB)66–68 consisting of 64 black-and-white drawings69 representing animate and inanimate categories. The animate stimuli included a total of 32 items with eight items belonging to the following categories: domestic animals, foreign animals, fruits and birds. The inanimate stimuli included 32 stimuli divided into the categories of small household items, large household items, vehicles and tools.
Black-and-white line drawings were reproduced on vertically oriented white paper with picture arrays, each page consisting of 10 items (i.e. one target and nine foil items) from the same category. For each item, participants heard the examiner say the name of an object and were asked to point to it. The examiner then turned the page and presented a new target item.
Animacy index score
Similar to prior work,70 the magnitude of each participant’s CSSD for animate objects was calculated as the ratio of the difference between inanimate and animate scores and the sum of those scores.
Positive scores indicated poorer performance on animate relative to inanimate objects (i.e. a CSSD for animate objects) and negative scores indicated poorer performance on inanimate relative to animate objects.
Psycholinguistic factors
The original picture matching stimuli from the CSB have been divided into two sets, one matched for familiarity and the other for age of acquisition.66 Each matched set consists of 16 animate and 16 inanimate items. Previous investigations have shown the confounding effects of frequency, familiarity, typicality and age of acquisition on SD/svPPA patients’ picture naming accuracy using the same stimuli from the CSB.27,30 We used ratings from the Morrison et al.31 corpus to account for frequency, familiarity, and age of acquisition, as well as Morrow and Duffy29 ratings to account for typicality (Supplementary Table 1).
MRI data acquisition and analysis
Each participant underwent an MRI scan within six months of the CSB administration. One participant who did not have an MRI scan and a single left-handed participant were excluded from analysis, resulting in an imaging subsample of 18 svPPA patients. Structural MRI data were collected on a 3 T MRI scanner (Siemens TIM Trio 3.0 T, Siemens Medical Systems, Erlingan, Germany) that included the acquisition of a T1-weighted multi-echo magnetization prepared rapid acquisition gradient echo (MPRAGE) structural image using the following parameters: FOV = 256, 192 sagittal slices, thickness = 1.0 mm, matrix 240 × 256, voxel size 1 × 1 × 1 mm, repetition time = 2.53 ms, echo = 3.48 ms, flip angle = 7°.
Cortical reconstructions of the T1-weighted images were performed using the FreeSurfer analysis suite version 6.0 (https://surfer.nmr.mgh.harvard.edu/ Accessed January 2021) using a procedure that has been previously described in detail.71–78 Each structural volume underwent spatial and intensity normalization, skull stripping, and an automated segmentation of cerebral white matter to locate the grey–white boundary. Defects in the surface topology were manually corrected, and the grey–white boundary was deformed outward using an algorithm designed to obtain an optimal representation of the pial surface. Cortical thickness was then derived from the distance between the grey–white boundary and the pial surface across the entire cortical mantle.
Cortical thickness in our first a priori region of interest (ROI), the perirhinal cortex, was extracted using previously published Freesurfer-derived labels.79 The lateral posterior fusiform gyrus ROI was defined using peak Talairach coordinates reported by a previous meta-analysis of functional MRI studies identifying regions more active while naming animate (i.e. animals) relative to inanimate (i.e. tools) objects in cognitively normal adults.40 Talairach coordinates corresponding to the posterior fusiform (Left: −40, −62, −18; Right: 40, −68, −18) were converted to MNI space (Left: −47.17, −66.51, −17.46; Right: 44.28, −67.58, −18.33)80 and a sphere with 10 mm radius was generated to represent our lateral fusiform gyrus a priori ROI. This was then projected to the pial surface, visually inspected for accuracy, and subsequently used to extract cortical thickness values for each participant (Fig. 1). Next, we combined Freesurfer-derived labels to create bilateral temporal lobe masks using the following ROIs from the Desikan–Killiany atlas: temporal pole, entorhinal cortex (which also includes perirhinal cortex in this atlas), parahippocampal gyrus, fusiform gyrus, lingual gyrus, inferior temporal gyrus, middle temporal gyrus, superior temporal gyrus and the superior temporal sulcus (Fig. 1). These masks were used in subsequent general linear model analyses. Finally, the bilateral precentral gyrus was selected as a control region.81
Figure 1.
Regions of interest. The mask used for the vertex-wise general linear model analysis restricted to the temporal lobes is shown along with the two a priori ROIs—the perirhinal cortex and the lateral fusiform gyrus—represented in blue and green, respectively. The medial view is rotated 45 degrees to aid visualization.
Statistical analyses
Behavioural aims
We used a multiple regression model to assess the independent effects of animacy, frequency, familiarity, age of acquisition and typicality on single-word comprehension accuracy across all stimuli (i.e. the percentage of participants who correctly identified each stimuli item). Sixty-one of the 64 stimuli had ratings available for frequency, familiarity and age of acquisition; 50 ratings were available for typicality. Spearman’s correlation analyses were used to determine the relationships between each variable and percent correct per stimulus item (Supplementary Table 2).
Next, we examined the impact of each psycholinguistic variable on single-word comprehension performance in our primary analysis. To investigate the effects of familiarity and age of acquisition, we used the two evenly matched sets of the current CSB word-picture matching stimuli in which half of the animate and inanimate items are matched for familiarity in one subset and half are matched for age of acquisition in the other subset.66 A paired samples t-test was performed to analyse performance on animate and inanimate items in each of the familiarity and age of acquisition matched sets. For frequency and typicality, we divided the total stimuli into higher and lower frequency and typicality animate versus inanimate subsets using the median as the cut point based on the ratings for these items. These 32-item matched sets did not have overlapping items. There were 61 ratings available for frequency, yielding 30 high (mean = 3.31, S.D. = 0.46; range = 2.75–4.70) and 31 low (mean = 2.10, S.D. = 0.29; range = 1.60–2.65) frequency items. There was no difference in average frequency ratings for low frequency animate versus inanimate items (t = −1.43, P = 0.16), or for high frequency animate versus inanimate items (t = −0.73, P = 0.47). For typicality, there were 50 ratings available, yielding 25 high (mean = 6.63, SD = 0.24; range = 6.22–6.94) and 25 low (mean = 5.56, S.D. = 0.55; range = 4.45–6.12) typicality items. There was no difference in average typicality ratings for low typicality animate versus inanimate items (t = −0.07, P = 0.95), or for high typicality animate versus inanimate items (t = 0.59, P = 0.56). We calculated each participant’s performance on each subset. A repeated measures ANOVA was performed with animacy (i.e. animate, inanimate), frequency (i.e. high, low) and typicality (i.e. high, low) as within-subject factors.
Finally, to examine the potential influence of overall language severity on the CSSD for animate objects, we used a repeated measures ANOVA with total performance scores (i.e. percent correct) on animate and inanimate categories as a within-subjects factor and overall language severity (i.e. Clinical Dementia Rating language score of 0.5 or 1) as a between-subjects predictor.
Imaging aims
Linear regression analyses assessed the relationship between animacy index scores and cortical thickness in the bilateral perirhinal cortex and lateral fusiform gyri, as well as the bilateral precentral gyrus, our control region. General linear model analyses were run using FreeSurfer’s mri_glmfit command to determine the relationship between the animacy index and cortical thickness at each vertex point of the cortex82 within the temporal lobes only (with FDR < 0.05 corrections). Statistical significance maps were generated and overlaid onto the average cortical surface template for evaluation.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Additional data are available from the corresponding author, upon reasonable request.
Results
Clinical and demographic characteristics
Baseline speech-language characteristics and demographic information are summarized in Table 1. Mean PASS sum of boxes were 4.6 (S.D. = 1.95), Clinical Dementia Rating Language scores were 0.78 (S.D. = 0.26), and global Clinical Dementia Rating scores were 0.5 (S.D. = 0.16). That is, about half of the sample had very mild aphasia and the other half had mild aphasia, and nearly all patients had very mild global cognitive impairment. The education level of these patients was high (mean = 17.2 years, S.D. = 2.35). All participants were non-Hispanic, and all but two were white (one Caribbean-American and one Asian-American).
Table 1.
Clinical and demographic characteristics of svPPA participants
| Subject | Age | Sex | Education | Handedness | CDR-G | CDR-Lang | PASS domains |
PASS SOB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Art | Flu | Syn | WR | Rep | AC | SWC | Read | Writ | Func | ||||||||
| S1 | 72 | F | 18 | R | 0.5 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.5 | 0.5 | 0 | 3 |
| S2 | 68 | M | 18 | R | 0.5 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0.5 | 0 | 0.5 | 0 | 1.5 |
| S3 | 79 | M | 20 | R | 0.5 | 0.5 | 0 | 0 | 0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 3.5 |
| S4 | 61 | M | 18 | R | 0.5 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0.5 | 0 | 0 | 0 | 1 |
| S5 | 67 | F | 14 | R | 0.5 | 0.5 | 0 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0 | 0.5 | 4.5 |
| S6 | 62 | M | 16 | R | 0.5 | 1 | 0 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 0.5 | 6.5 |
| S7 | 55 | F | 18 | R | 0.5 | 1 | 0 | 1 | 0 | 1 | 0.5 | 1 | 1 | 2 | 1 | 0.5 | 8 |
| S8 | 67 | M | 17 | R | 0.5 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0.5 | 0.5 | 0 | 0.5 | 0.5 | 2.5 |
| S9 | 65 | F | 18 | R | 0.5 | 0.5 | 0 | 0 | 0 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 4.5 |
| S10 | 59 | F | 18 | R | 0.5 | 0.5 | 0 | 0 | 0.5 | 0.5 | 0 | 0 | 0.5 | 0.5 | 0 | 0.5 | 2.5 |
| S11 | 53 | M | 20 | R | 0.5 | 1 | 0 | 0 | 0.5 | 1 | 0.5 | 1 | 1 | 2 | 0.5 | 1 | 7.5 |
| S12 | 63 | F | 16 | R | 0.5 | 1 | 0 | 0.5 | 0.5 | 1 | 0.5 | 0 | 1 | 0.5 | 0.5 | 0.5 | 5 |
| S13 | 62 | M | 22 | L | 0.5 | 0.5 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0.5 | 0.5 | 0.5 | 4.5 |
| S14 | 70 | F | 16 | R | 0.5 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.5 | 0.5 | 0.5 | 3.5 |
| S15 | 62 | F | 13 | R | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0.5 | 1 | 2 | 0.5 | 1 | 6 |
| S16 | 63 | M | 20 | R | 0.5 | 1 | 0 | 0.5 | 0.5 | 1 | 0 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 5 |
| S17 | 79 | M | 18 | R | 0.5 | 1 | 0 | 0 | 0.5 | 1 | 0 | 0.5 | 0.5 | 1 | 1 | 1 | 5.5 |
| S18 | 54 | F | 16 | R | 0.5 | 0.5 | 0 | 0 | 0.5 | 1 | 0.5 | 0 | 0.5 | 1 | 0.5 | 0.5 | 4.5 |
| S19 | 69 | F | 14 | R | 0.5 | 1 | 0 | 0 | 0 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | 6.5 |
| S20 | 56 | M | 14 | R | 1 | 1 | 0 | 0 | 0.5 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 | 7 |
Art, articulation; AC, auditory comprehension; CDR-G, Clinical Dementia Rating (CDR) global score; CDR-Lang, CDR supplemental language score; Flu, fluency; Func, functional communication; PASS SOB, Progressive Aphasia Severity Scale Sum of Boxes; Read, reading; Rep, repetition; SWC, single-word comprehension; Syn, syntax; WR, word retrieval; Writ, writing.
Behavioural aims
Relationships of psycholinguistic variables to the CSSD for animate objects
The multivariate regression analysis revealed a significant main effect of animacy (β = 0.52, t = 4.03, P < 0.001) even after including all psycholinguistic variables in the model [F(5,43) = 8.83, P < 0.001], such that animate items were less likely to be identified correctly relative to inanimate items. A main effect of age of acquisition (β = −0.57, t = −3.50, P = 0.001), but not frequency (β = 0.22, t = 0.91, P = 0.37), familiarity (β = −0.16, t = −0.59, P = 0.56), or typicality (β = 0.04, t = 0.25, P = 0.80), was found. To account for the potential problem of multicollinearity in the regression analysis (Supplementary Table 2), we computed the variance inflation factor (VIF) from the standardized regression coefficient. Given the relatively higher VIF (>5) for familiarity and frequency compared to the other variables (where recommendations for acceptable levels of VIF include a maximum of 483,84), multivariate regression analysis was re-run without these two variables. Results were consistent with the initial analysis (Supplementary Text 1).
Impact of psycholinguistic variables on single-word comprehension
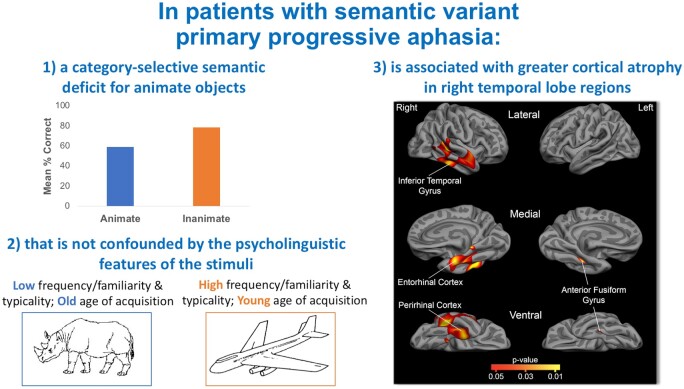
We explored the role of each individual psycholinguistic variable on CSSD for animate objects. Participants performed worse for animate than inanimate items in the familiarity-matched (t = −3.77, P = 0.001) and age of acquisition-matched (t = −4.35, P < 0.001) sets (Fig. 2B). A repeated measures ANOVA with frequency and animacy (i.e. animate, inanimate) revealed main effects of frequency [F(1,19) = 22.99, P < 0.001] and animacy [F(1,19) = 16.90, P = 0.001], with participants correctly identifying fewer animate and lower frequency items (Fig. 2A). No frequency by animacy interaction was found [F(1,19) = 1.77, P = 0.20], suggesting that the effect of animacy is independent of item frequency. A repeated measures ANOVA with typicality and animacy revealed main effects of typicality [F(1,19) = 31.78, P < 0.001] and animacy [F(1,19) = 8.19, P = 0.01], with participants correctly identifying fewer animate and lower typicality items (Fig. 2C). A typicality by animacy interaction was not found [F(1,19) = 1.29, P = 0.27], suggesting that the effect of animacy is independent of item typicality. Table 2 shows the consistent presence of the CSSD for animate objects in the vast majority of participants on nearly all stimulus classes. Across all 64 stimuli, a CSSD for animate objects was found in 75% of individuals (15 patients) in our sample. Of the five individuals who did not present with a CSSD for animate objects, two were at ceiling (S2 and S3), one showed equivalent performance on both categories (S4), and two showed worse performance on inanimate relative to animate objects (S1 and S7).
Figure 2.
Effects of frequency, familiarity, age of acquisition, and typicality on individual performance. Individual patient data illustrate CSSD for animate objects in most patients across matched sets of stimuli. When performance is not at ceiling, the vast majority of individual svPPA patients show a CSSD for animate objects across stimuli with various psycholinguistic characteristics. (A) Individual accuracy scores on low (top right) versus high (top left) frequency stimuli. A repeated measures ANOVA with frequency and animacy (i.e. animate, inanimate) revealed main effects of frequency [F(1,19) = 22.99, P < 0.001] and animacy [F(1,19) = 16.90, P = 0.001]. No frequency by animacy interaction was found [F(1,19) = 1.77, P = 0.20]. (B) Individual accuracy scores on the familiarity (middle left) and age of acquisition-matched (middle right) stimuli sets. Participants performed worse for animate than inanimate items in the familiarity-matched (t = −3.77, P = 0.001) and age of acquisition-matched (t = −4.35, P < 0.001) sets. (C) Individual accuracy scores on low (bottom right) versus high (bottom left) typicality stimuli. A repeated measures ANOVA with typicality and animacy revealed main effects of typicality [F(1,19) = 31.78, P < 0.001] and animacy [F(1,19) = 8.19, P = 0.01]. A typicality by animacy interaction was not found [F(1,19) = 1.29, P = 0.27]. + indicates worse performance on animate relative to inanimate objects (i.e. equivalent to A in Table 2).
Table 2.
Summary of presence (i.e. a positive animacy index score) or absence of a CSSD for animate objects in individual patients across frequency, familiarity, typicality and age of acquisition matched stimuli
| Subjects | High frequency stimuli | Low frequency stimuli | AoA matched stimuli | Familiarity matched stimuli | High typicality stimuli | Low typicality stimuli |
|---|---|---|---|---|---|---|
| S1 | N | I | A | I | I | I |
| S2 | C | C | C | C | C | C |
| S3 | C | C | C | C | C | C |
| S4 | N | I | N | N | I | I |
| S5 | N | A | A | N | N | I |
| S6 | A | I | A | N | I | A |
| S7 | A | I | I | A | I | I |
| S8 | A | I | N | A | A | I |
| S9 | A | A | A | N | I | A |
| S10 | A | A | A | A | A | A |
| S11 | A | A | A | A | I | A |
| S12 | A | A | A | A | A | A |
| S13 | A | A | A | A | A | A |
| S14 | A | A | A | A | I | A |
| S15 | A | A | A | A | A | A |
| S16 | A | A | A | A | A | I |
| S17 | A | A | A | A | A | A |
| S18 | A | A | A | A | A | A |
| S19 | A | A | A | A | A | I |
| S20 | A | A | A | A | A | A |
The designated subject numbers above are used consistently throughout the paper and denote the same individuals. A, worse performance on animate relative to inanimate objects; AoA, age of acquisition; C, ceiling on both animate and inanimate categories; I, worse performance on inanimate relative to animate objects; N, equivalent performance on animate and inanimate objects.
Impact of overall language severity on single-word comprehension
A repeated measures ANOVA with total performance scores (i.e. total percent correct) and overall language severity (i.e. Clinical Dementia Rating language score of 0.5 versus 1) revealed a main effect of animacy [F(1,18) = 21.12, P < 0.001] and overall language severity [F(1,18) = 14.33, P = 0.001], such that worse single-word comprehension performance was associated with worse aphasia severity and with animate objects (Fig. 3). No disease severity by animacy interaction was found [F(1,18) = 1.70, P = 0.21], suggesting that the animacy effect did not differ across levels of language severity, at least in these very mild to mildly impaired patients (Fig. 3). Despite the absence of an interaction, the difference between inanimate and animate scores showed a larger effect size in the Clinical Dementia Rating Language 1 group (Cohen’s d = 0.96) than the 0.5 group (Cohen’s d = 0.82).
Figure 3.
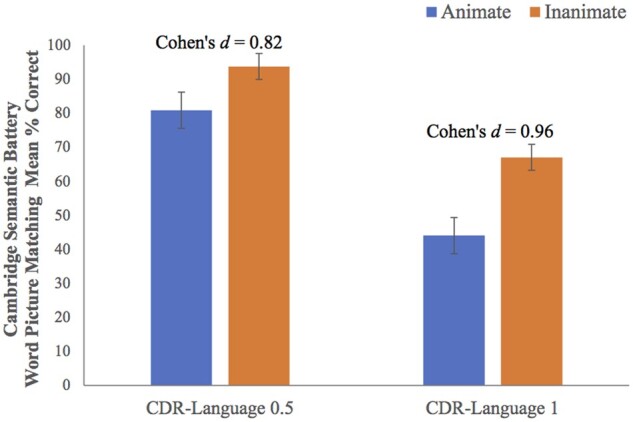
Effect of overall language severity on group performance. While the group means are lower for animate than inanimate objects for both groups based on overall severity of language impairment, a larger effect size is found in the CDR-Language 1 group (Cohen’s d = 0.96) than in the CDR-Language 0.5 group (Cohen’s d = 0.82). A repeated measures ANOVA with total performance scores (i.e. total percent correct) and overall language severity (i.e. Clinical Dementia Rating language box score of 0.5 versus score of 1) revealed a main effect of animacy [F(1,18) = 21.12, P < 0.001] and overall language severity [F(1,18) = 14.33, P = 0.001]. No disease severity by animacy interaction was found [F(1,18) = 1.70, P = 0.21].
Brain–behavior relationships
A priori ROIs
A linear regression analysis revealed a relationship between right perirhinal cortex thickness and animacy index scores (β = −0.57, t = −2.74, P = 0.015) such that worse performance on animate relative to inanimate items was associated with a thinner right perirhinal cortex (Fig. 4). The relationships between animacy index scores and cortical thickness did not reach statistical significance in the left perirhinal cortex (β = −0.13, t = −0.54, P = 0.60), left lateral posterior fusiform gyrus (β = −0.30, t = −1.25, P = 0.23), or the right lateral posterior fusiform gyrus (β = 0.08, t = 0.34, P = 0.74). A linear regression analysis did not reveal a significant relationship between animacy index scores and the control ROIs: the left precentral gyrus (β = −0.17, t = −0.70, P = 0.49) or the right precentral gyrus (β = −0.12, t = −0.49, P = 0.63).
Figure 4.
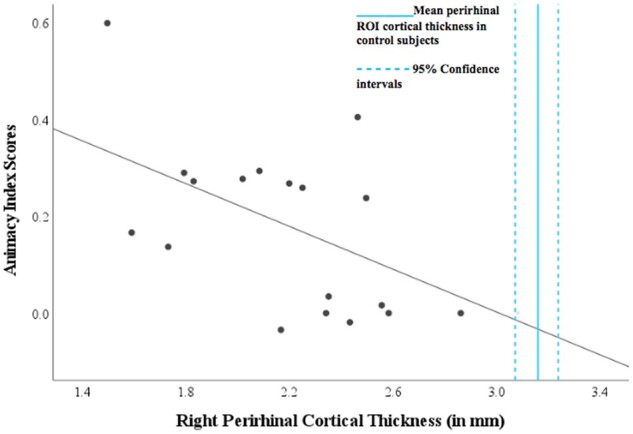
Association between perirhinal cortical atrophy and the magnitude of a CSSD for animate objects in svPPA. Patients with svPPA all exhibit atrophy in the perirhinal cortex, and in this sample more atrophy in the right perirhinal cortex is associated with a more prominent CSSD for animate objects (β = −0.57, t = −2.74, P = 0.01). The scatterplot shows each svPPA patient’s right perirhinal cortical thickness plotted against animacy index score, with a positive value indicating the presence of a CSSD for animate objects. A reference is provided for the average right perirhinal ROI cortical thickness in the healthy control group (3.16 mm, S.D. = 0.35), indicating the presence of atrophy in this ROI in all patients.
Figure 4 shows the mean cortical thickness of the right perirhinal cortex with 95% confidence intervals from a sample of MRI scans from control subjects, age and gender matched cognitively unimpaired participants (n = 79; mean age = 66 years, S.D. = 5.74), from the Massachusetts Alzheimer’s Disease Research Center Longitudinal Cohort. These participants had undergone comprehensive clinical assessments at the time of their MRI scan, including with the NACC UDS battery, and were determined to be cognitively normal (Clinical Dementia Rating = 0; no neurologic or psychiatric history).
Cortical surface-based analysis within the temporal lobe
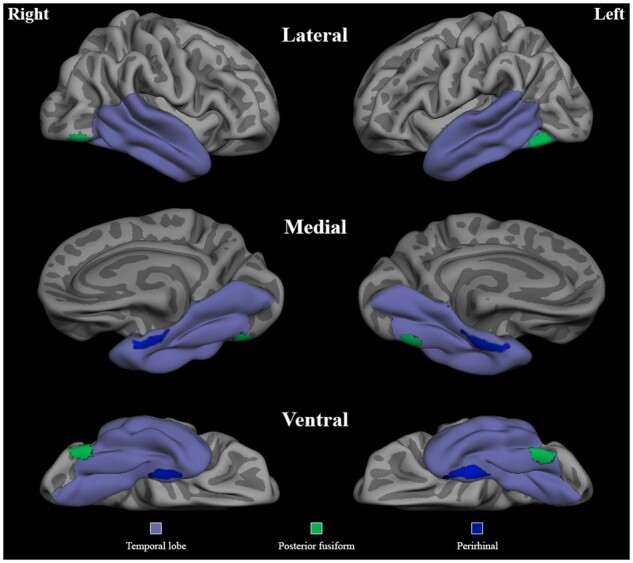
A vertex-wise general linear model analysis restricted to the temporal lobes revealed a stronger association between positive animacy index scores and cortical atrophy in the right hemisphere including regions of the perirhinal cortex, superior, middle, and inferior temporal gyri, and the anterior fusiform gyrus, which falls within Brodmann Area 20 (Fig. 5). Peak MNI coordinates were found in the right inferior temporal gyrus (51.78, −45.06, −18.27), superior temporal gyrus (55.40, −6.27, −6.50), perirhinal cortex (30.83, −14.96, −36.13), anterior fusiform gyrus (41.61, −34.81, −17.20), and the middle temporal gyrus (61.98, −39.07, −5.25). Within the left hemisphere, a CSSD for animate objects was associated with atrophy within the anterior fusiform gyrus only (within Brodmann Area 20; −36.45, −34.56, −20.49).
Figure 5.
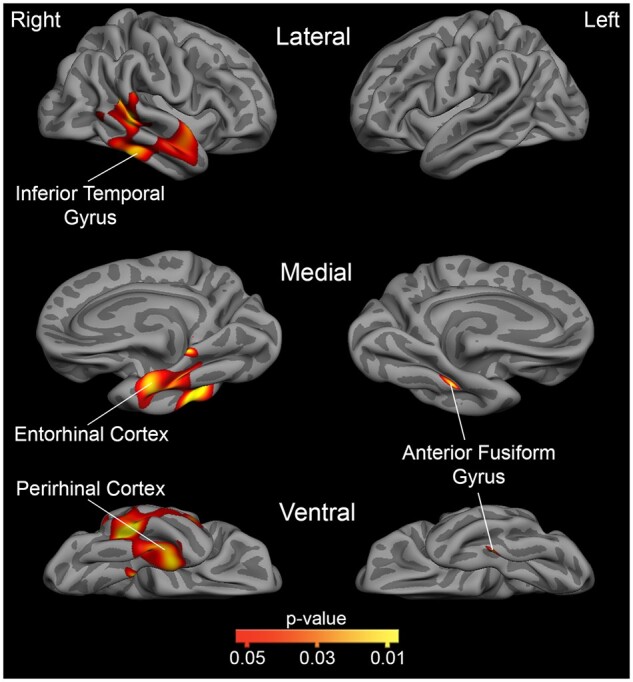
Regions within the temporal lobe mask that are associated with a greater CSSD for animate objects. This statistical map shows where cortical atrophy is associated with a greater CSSD for animate objects, as measured by the animacy index, within a priori hypothesized temporal lobe regions (FDR < 0.05 corrected; depicted as red-to-yellow heat gradient). The medial view is rotated 45 degrees to aid visualization. Within these svPPA patients, a larger CSSD for animate objects is associated with greater cortical atrophy in the perirhinal cortex, anterior fusiform gyrus (Brodmann Area 20), inferior temporal gyrus, and portions of the middle and superior temporal gyrus in the right hemisphere, and with only the anterior fusiform gyrus (Brodmann Area 20) in the left hemisphere.
Discussion
Data have been mixed as to whether svPPA patients exhibit a CSSD for animate objects when controlling for the potentially confounding psycholinguistic characteristics of the stimuli. In the present sample of 20 mildly impaired svPPA patients, we found a CSSD for animate objects in nearly all participants that was not attributable to differential frequency, familiarity, age of acquisition or typicality of words. In cases where a CSSD for animate objects was not observed, it was usually due to a ceiling effect. Consistent with the hypothesized role of the perirhinal cortex in subserving processes important for understanding animate objects, greater atrophy in the right perirhinal cortex in these patients was associated with a more prominent CSSD for animate objects. Other temporal lobe regions where greater atrophy was associated with a more prominent CSSD for animate objects included the inferior temporal, middle and superior temporal, and anterior fusiform gyri (Brodmann Area 20). Interestingly, the only left hemisphere region to show this effect was the anterior fusiform gyrus (Brodmann Area 20). These findings not only extend our understanding of the characteristics of impaired semantic memory in svPPA, but also provide further evidence for the role of the anterior ventral temporal cortex in the representation and semantic processing of animate objects.
svPPA patients exhibit a characteristic CSSD for animate objects
Consistent with previous case reports,6,9–19 a disproportionately poorer performance with animate compared to inanimate objects was found in our sample as whole and in nearly all individual patients. Our results provide strong evidence that a CSSD for animate objects may be an early and consistent clinical characteristic of this illness.
Several previous investigations of svPPA/SD patients have argued that psycholinguistic confounds are responsible for the CSSD for animate objects, such that the effect of animacy was not present after controlling for the psycholinguistic features of the stimuli.22,27,85,86 Moreover, prior studies have shown an effect of typicality, but not animacy, on picture naming and word-picture matching accuracy.27,87 In contrast, a typicality effect was not found in the present study. Besides a main effect of animacy, a main effect of age of acquisition was found; that is, age of acquisition was a predictor of performance when accounting for other confounding variables. However, when the task stimuli were matched for age of acquisition across animate and inanimate categories in the present study, 80% of svPPA patients still exhibited worse performance on animate than on inanimate items (Fig. 2B).
Discrepant findings may be related to the stimulus materials employed to assess semantic memory and the type of response elicited by the task. Other word-picture matching and associative tasks (e.g. category membership of pictures/words) have reported a CSSD for animate objects.25,88,89 In a semantic sorting task aimed to assess fine-grained dissociations among animate entities, greater categorical impairment for animals than tools and kitchenware but a lesser impairment of fruits and vegetables was reported in SD that was not modulated by confounds like frequency.24 In these studies, variability exists in the focus of category selectivity (e.g. within animate category versus animate/inanimate dissociation), types of stimuli employed (e.g. pictures versus words), psycholinguistic variables that were controlled for (e.g. word length versus representativeness), and the response required (e.g. verbal versus non-verbal). Our motivation to employ a word-picture matching task was influenced by (i) a single world comprehension deficit characteristic of svPPA5,6; (ii) unbiased loading of verbal and non-verbal processing to account for the differential contributions of the left and right anterior temporal lobes (ATLs) in semantic processing23,90,91; and (iii) the comparative advantage of using a standardized test with associated psycholinguistic confounds.
Previous discrepant findings may also reflect differences related to the characteristics of the patient sample. Most prior group studies have smaller sample sizes and examined a CSSD for animate objects among samples of SD patients18,20,24,88; only a handful of studies have restricted their analysis to a sample of svPPA patients meeting strict diagnostic criteria when investigating a CSSD for animate objects.92 While often used synonymously with svPPA, individuals with SD can have a heterogenous clinical presentation including impairments outside of language, such as visual agnosia, prosopagnosia, loss of empathy, behavioural rigidity, a variety of preoccupations, or executive dysfunction.3,4,93–97 Many studies have neither reported the details of the patient characteristics nor presented individual patient data to examine within-group between-subject variability of clinical and neuroanatomical features, which might contribute to discrepant findings of the CSSD for animate objects.
A CSSD for animate objects is associated with atrophy in the right temporal regions and left anterior fusiform gyrus
We found a strong association between cortical thickness in the right perirhinal cortex and animacy index scores in our sample. To our knowledge, this has not been previously reported within a group of svPPA/SD cases, but this finding is consistent with results in other patient populations. In patients with a broad range of neurodegenerative diseases including SD, Brambati et al.22 reported a correlation between gray matter volume in the right anteromedial temporal pole at the level of the entorhinal cortex and naming performance for animate objects. In mild Alzheimer’s disease dementia patients, Kivisaari et al.70 found perirhinal cortex atrophy to be associated with a disproportionate difficulty in naming animate compared to inanimate objects. Similarly, patients with herpes simplex virus encephalitis with lesions in the anteromedial temporal structures including the perirhinal cortex have also been found to present with a CSSD for animate objects,7,22,98–102 but these patients often have lesions that cover a relatively large territory of the anterior temporal lobe.
Debate continues about the functional role of the perirhinal cortex in semantic processing and why damage to the perirhinal cortex may lead to a CSSD for animate objects. An important putative function associated with the perirhinal cortex is high-level visual object processing. According to the hierarchical account of object processing, the complexity of neural representations increases from posterior occipital to the anterior and the anteromedial temporal regions, and the bilateral perirhinal cortex is thought to be crucial in the processing of the distinct characteristics of animate objects that require more complex feature computations (which are not thought to be as important for inanimate objects).103–106
In this study, an association between left perirhinal cortical thickness and the magnitude of the CSSD for animate objects was not found. One potential explanation for the null finding may be a floor effect, such that the left ATL regions including the perirhinal cortex are so atrophic that it is no longer possible to find a relationship with this behavioural measure, although there still appears to be a reasonable amount of variance in this anatomical measure (Supplementary Fig. 1). Since the perirhinal cortex is involved early in the course of svPPA/SD, typically starting in the left hemisphere before spreading bilaterally, this account would predict the preferential degradation of semantic representations for animate objects early in the disease course. Patients with the mildest impairment in this study did not consistently show a CSSD for animate objects, but further investigation with more sensitive tasks is warranted. It is possible that the degeneration of the left perirhinal cortex relates to loss of conceptual and lexical knowledge of animate objects and the degeneration of the right perirhinal cortex relates to the loss of visual object knowledge of animate objects,107–109 but this too deserves further study.
In addition to the right perirhinal cortex, our results revealed the right inferior, middle, and superior temporal gyri, as well as the bilateral anterior fusiform gyrus, as regions where atrophy was greater in individuals with a more prominent impairment for animate than inanimate objects. Damasio et al.110 first reported that a CSSD for animate objects was associated with damage to the left temporal lobe in Brodmann Area 20/36 in subjects with lesions caused by cerebrovascular disease, herpes simplex virus encephalitis or temporal lobectomy.7,8 Since then, the anterior fusiform gyrus has been widely cited as a critical region for semantic processing.111–113 Our results complement previous findings by further supporting a critical role of the anterior fusiform gyrus (Brodmann Area 20/36) in semantic processing114 and support the preferential involvement of the right and left anterior fusiform gyri in linking visual feature information for animate objects to the words that represent them.88,115 These results are convergent with those of Libon et al.,25 who reported that a CSSD for animate objects in svPPA (using a category membership judgement task) was associated with atrophy in ventromedial temporal lobe regions, including a left inferior temporal/fusiform cluster (MNI: −34, −22, 28) in close proximity to that reported here (MNI: −36.45, −34.56, −20.49).
Evidence is converging from studies of patients with diverse lesions that right temporal lobe lesions may be associated with a CSSD for animate objects. In patients with unilateral lesions due to stroke, herpes simplex encephalitis or temporal lobectomy, Tranel et al116 found dissociable patterns of categorical impairment specific to persons, animals, and tools, and laterality of lesion.117 They found a strong association between impaired recognition of animals with lesions in the right ventral temporal and mesial occipital region. Similarly, a greater naming deficit for animate than inanimate objects has been reported in patients with focal lesions in the right occipitotemporal lobe, including the anterior fusiform gyrus (cases 1 and 2118), and in temporal lobe epilepsy patients who underwent right (but not left) temporal lobe resection, including the superior, middle, inferior temporal and fusiform gyri.119 In patients with bilateral, right or left temporal lobe necrosis due to cranial irradiation therapy, Chan et al. found greater impairment in naming and attribute judgement of animate relative to inanimate objects in patients with right temporal lobe lesions (than that seen in those with left temporal lobe lesions). Lesion sites specifically related to animate object knowledge loss included the right fusiform gyrus, inferior and superior temporal gyri—regions that directly overlap with our findings.114,120
While individuals diagnosed with svPPA/SD typically show an asymmetric, left-lateralized pattern of atrophy, for a proportion of patients (roughly estimated around 30%) atrophy may be more prominent in the right temporal lobe.93,121 The relationship between laterality of atrophy and the emergence of a CSSD for animate object is largely debated, with some reports suggesting the prevalence of the right ATL atrophy/lesions in individuals with a CSSD for animate objects22,88,91, and others stressing the role of the left ATL in the semantic representation and recognition of animate items.101,122,123 Woollams et al.23 investigated whether asymmetry of atrophy underpins the likelihood of a category effect across patients with SD and found a larger CSSD for animate objects in SD patients with R > L ATL atrophy than those with L > R ATL atrophy. To our knowledge, our study is the first to show a CSSD for animate objects in a cohort of svPPA patients with L > R ATL atrophy and strong associations between the magnitude of a CSSD for animate objects and the magnitude of atrophy in right ATL regions. Our findings contribute to this controversial issue of laterality and suggest that right temporal lobe regions may play a critical role in the representation and semantic processing of animate objects, even in svPPA patients with the prototypical left-lateralized ATL atrophy.
The lateral posterior fusiform gyrus was another a priori ROI in this study. Our hypothesis was developed based on the differential activation patterns often found for animate versus inanimate objects.33–38 Previous functional MRI studies of healthy subjects have shown enhanced activity in the lateral posterior fusiform gyrus using naming, basic level categorization, or semantic decision tasks with animate pictures and/or their written names.33,39–46 Contrary to our hypothesis, our results did not reveal any relationship between atrophy in the lateral posterior fusiform gyri and animacy index scores. This may be related to differences in the task we used compared to those functional activation studies or because our sample of patients did not include individuals with atrophy localized so posteriorly.
Surprisingly, atrophy in the temporal pole, the presumed localization of the most prominent neurodegeneration in svPPA,51 did not correlate with animacy index scores bilaterally. While one explanation could be that atrophy is already maximal in this region in our sample, previous investigations have also found the anterior fusiform gyrus, but not the temporal pole, to be associated with semantic impairment.114,120
The presence of a CSSD for animate objects in svPPA has important implications for our understanding of the theoretical models of semantic cognition. The cognitive mechanism underlying the dissociation between animate and inanimate objects has been previously interpreted in the context of theoretical frameworks such as the domain-specific theory, where distinct neural systems for categorical domains such as animals and plant life formed due to evolutionary pressures.122 In addition, while proponents of the sensory and functional theory propose that animate objects require the processing of more perceptual features (e.g. shape, colour, sound) than inanimate objects which require greater processing of functional properties (i.e. relevant to action and motor scheme),7,123,124 other distributed semantics and correlated structure theories argue in favour of a model where animate and inanimate objects are distinguished by the correlations between their perceptual and functional features.125,126 Moreover, the animate–inanimate dichotomy has also influenced theories pertaining to the neuroanatomical localization of semantic memory, such as embodied cognition127,128 and the hub-and-spoke model.129,130 The presence of an animate–inanimate difference in this study strengthens the proposal that specific regions within the temporal lobes (i.e. right perirhinal cortex, bilateral anterior fusiform gyrus, and portions of the right superior and middle temporal gyri) may be more important in the semantic processing and representation of animate relative to inanimate entities.
Interpreting these data in the context of some of our and others’ recent work, we suggest that in svPPA (i) neurodegeneration begins in the temporal pole51; (ii) semantic memory impairment develops as neurodegeneration expands into the networks that converge on this hub131 with heterogeneity between patients132–134; and (iii) the nature of the early semantic memory impairment is determined by the specific node or region that is affected most prominently, with anterior fusiform and perirhinal cortical involvement leading to a selective loss of semantic memory for animate relative to inanimate objects.
Limitations and future directions
Although we posit that a CSSD for animate objects is a clinical characteristic found in many svPPA individuals, our study has limitations. While we observed a CSSD for animate objects after accounting for several psycholinguistic confounds, we were unable to exhaustively examine all possible confounds in our primary analysis. Additional analyses to assess the effects of visual complexity, concreteness, imageability, and semantic neighbourhood density were carried out post-hoc and the results are summarized in the Supplementary materials. The main effect of animacy remained significant even after including each of these additional variables in the model. This type of work also points towards the value of developing stimulus sets that better control for a wider variety of picture and word features. To our knowledge, the discriminability between targets and foils was not controlled for between categories in the CSB. The investigation of this question using stimulus materials that more rigorously control for these factors is warranted.
Variability in cortical atrophy exists in patients with svPPA, with some patients having more involvement of the ventral visual semantic or lateral language circuits while others exhibit more involvement of the medial affective/paralimbic networks.131 Like many lesion neuropsychology studies, this presents challenges when attempting to compare to other reports. And while larger than most other similar types of reports, our sample size may not have been large enough to detect small effects. In a post hoc power analysis with G*Power 3135 using the observed effect size (f2 = 1.02) and sample size (n = 20) from our multiple regression model to assess the effect of animacy, we achieved a power of 0.78. This was clearly adequate to detect the effects reported here, but larger single- or multi-centre studies may enable the investigation of larger samples to replicate effects.
Future studies should include longitudinal investigations to improve our understanding of how the localization, magnitude, and spread of atrophy in svPPA relates to the presence and magnitude of a CSSD for animate objects or other categories of information, including emotionally-charged information.136,137 These next steps will improve our understanding of brain systems that subserve dissociable categories of semantic memory and how their degeneration impacts patients’ understanding of the world around them.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We greatly appreciate the dedication of the patients and families who participated in this research, without whom we would not have been able to perform this study. We appreciate the efforts of the MGH FTD Unit staff whose contributions to data collection and quality control are invaluable.
Funding
This research was conducted with funding from National Institutes of Health grants R01 DC014296 (BCD) and P30 AG062421 (BCD, Imaging Core), and was carried out at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital (MGH), using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and the Neuroimaging Analysis Center, P41EB015902, a P41 supported by NIBIB. This work also involved the use of instrumentation supported by the National Institutes of Health Shared Instrumentation Grant Program; specifically, S10RR017208-01A1, S10RR026666, S10RR022976, S10RR019933, S10RR023043 and S10RR023401. We also appreciate the philanthropic support of the Friends of the MGH Frontotemporal Disorders Unit and the Tommy Rickles Endowed Chair in Primary Progressive Aphasia Research.
Competing interests
B.C.D. Disclosures 6 January 2021—research support from NIH, Alzheimer’s Drug Discovery Foundation, consulting for Acadia, Alector, Arkuda, Axovant, Biogen, Denali, Eisai, Life Molecular Sciences, Lilly, Merck, Novartis, Takeda and Wave LifeSciences. Editorial duties with payment for Elsevier (Neuroimage: Clinical and Cortex). Royalties from Oxford University Press and Cambridge University Press.
Glossary
- ATL =
anterior temporal lobe
- CSB =
Cambridge Semantic Battery
- CSSD =
category-selective semantic memory deficit
- ROI =
region of interest
- svPPA =
Semantic variant Primary Progressive Aphasia
- SD =
Semantic Dementia
References
- 1.Tulving E.Episodic and semantic memory. In: Organization of memory. Oxford, England: Academic Press; 1972:xiii–423. [Google Scholar]
- 2.Warrington EK.The selective impairment of semantic memory. Q J Exp Psychol. 1975;27(4):635–657. [DOI] [PubMed] [Google Scholar]
- 3.Snowden JS, Goulding PJ, Neary D.. Semantic dementia: A form of circumscribed cerebral atrophy. Behav Neurol. 1989;2(3):167–182. [Google Scholar]
- 4.Hodges JR, Patterson K, Oxbury S, Funnell E.. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115 (Pt 6):1783–1806. [DOI] [PubMed] [Google Scholar]
- 5.Mesulam MM, Grossman M, Hillis A, Kertesz A, Weintraub S.. The core and halo of primary progressive aphasia and semantic dementia. Ann Neurol. 2003;54 (Suppl 5):S11–14. [DOI] [PubMed] [Google Scholar]
- 6.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warrington EK, Shallice T.. Category specific semantic impairments. Brain. 1984;107 (Pt 3):829–854. [DOI] [PubMed] [Google Scholar]
- 8.Hillis AE, Caramazza A.. Category-specific naming and comprehension impairment: A double dissociation. Brain. 1991;114 (Pt 5):2081–2094. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy RA, Warrington EK.. Evidence for modality-specific meaning systems in the brain. Nature. 1988;334(6181):428–430. [DOI] [PubMed] [Google Scholar]
- 10.Basso A, Capitani E, Laiacona M.. Progressive language impairment without dementia: A case with isolated category specific semantic defect. J Neurol Neurosurg Psychiatry. 1988;51(9):1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breedin SD, Saffran EM, Coslett HB.. Reversal of the concreteness effect in a patient with semantic dementia. Cogn Neuropsychol. 1994;11(6):617–660. [Google Scholar]
- 12.Barbarotto R, Capitani E, Spinnler H, Trivelli C.. Slowly progressive semantic impairment with category specificity. Neurocase. 1995;1(2):107–119. [Google Scholar]
- 13.Cardebat D, Demonet JF, Celsis P, Puel M.. Living/non-living dissociation in a case of semantic dementia: A SPECT activation study. Neuropsychologia. 1996;34(12):1175–1179. [DOI] [PubMed] [Google Scholar]
- 14.Gentileschi V, Sperber S, Spinnler H.. C rossmodal agnosia for familiar people as a consequence of right infero polar temporal atrophy. Cogn Neuropsychol. 2001;18(5):439–463. [DOI] [PubMed] [Google Scholar]
- 15.Joubert S, Felician O, Barbeau E, et al. Impaired configurational processing in a case of progressive prosopagnosia associated with predominant right temporal lobe atrophy. Brain. 2003;126 (Pt 11):2537–2550. [DOI] [PubMed] [Google Scholar]
- 16.Carroll E, Garrard P.. Knowledge of living, nonliving and “sensory quality” categories in semantic dementia. Neurocase. 2005;11(5):338–350. [DOI] [PubMed] [Google Scholar]
- 17.Zannino GD, Perri R, Pasqualetti P, Di Paola M, Caltagirone C, Carlesimo GA.. The role of semantic distance in category-specific impairments for living things: Evidence from a case of semantic dementia. Neuropsychologia. 2006;44(7):1017–1028. [DOI] [PubMed] [Google Scholar]
- 18.Noppeney U, Patterson K, Tyler LK, et al. Temporal lobe lesions and semantic impairment: A comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007;130 (Pt 4):1138–1147. [DOI] [PubMed] [Google Scholar]
- 19.Papagno C, Capasso R, Miceli G.. Reversed concreteness effect for nouns in a subject with semantic dementia. Neuropsychologia. 2009;47(4):1138–1148. [DOI] [PubMed] [Google Scholar]
- 20.Lambon Ralph MA, Lowe C, Rogers TT.. Neural basis of category-specific semantic deficits for living things: Evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130 (Pt 4):1127–1137. [DOI] [PubMed] [Google Scholar]
- 21.Rogers TT, Patterson K, Jefferies E, Lambon Ralph MA.. Disorders of representation and control in semantic cognition: Effects of familiarity, typicality, and specificity. Neuropsychologia. 2015;76:220–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brambati SM, Myers D, Wilson A, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18(10):1644–1653. [DOI] [PubMed] [Google Scholar]
- 23.Woollams AM, Patterson K.. Cognitive consequences of the left-right asymmetry of atrophy in semantic dementia. Cortex. 2018;107:64–77. [DOI] [PubMed] [Google Scholar]
- 24.Merck C, Jonin P-Y, Vichard H, Boursiquot SLM, Leblay V, Belliard S.. Relative category-specific preservation in semantic dementia? Evidence from 35 cases. Brain Lang. 2013;124(3):257–267. [DOI] [PubMed] [Google Scholar]
- 25.Libon DJ, Rascovsky K, Powers J, et al. Comparative semantic profiles in semantic dementia and Alzheimer's disease. Brain. 2013;136(Pt 8):2497–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambon Ralph MA, Graham KS, Patterson K, Hodges JR.. Is a picture worth a thousand words? Evidence from concept definitions by patients with semantic dementia. Brain Lang. 1999;70(3):309–335. [DOI] [PubMed] [Google Scholar]
- 27.Woollams AM, Cooper-Pye E, Hodges JR, Patterson K.. Anomia: A doubly typical signature of semantic dementia. Neuropsychologia. 2008;46(10):2503–2514. [DOI] [PubMed] [Google Scholar]
- 28.Patterson K, Lambon Ralph MA, Jefferies E, et al. “Presemantic” cognition in semantic dementia: Six deficits in search of an explanation. J Cogn Neurosci. 2006;18(2):169–183. [DOI] [PubMed] [Google Scholar]
- 29.Morrow LI, Duffy MF.. The representation of ontological category concepts as affected by healthy aging: Normative data and theoretical implications. Behav Res Methods. 2005;37(4):608–625. [DOI] [PubMed] [Google Scholar]
- 30.Lambon Ralph MA, Howard D, Nightingale G, Ellis AW.. Are living and non-living category-specific deficits causally linked to impaired perceptual or associative knowledge? evidence from a category-specific double dissociation. Neurocase. 1998;4(4-5):311–338. [Google Scholar]
- 31.Morrison CM, Chappell TD, Ellis AW.. Age of acquisition norms for a large set of object names and their relation to adult estimates and other variables. Q J Exp Psychol Sect A. 1997;50(3):528–559. [Google Scholar]
- 32.Lambon Ralph MA, Patterson K, Garrard P, Hodges JR.. Semantic dementia with category specificity: A comparative case-series study. Cogn Neuropsychol. 2003;20(3):307–326. [DOI] [PubMed] [Google Scholar]
- 33.Anzellotti S, Mahon BZ, Schwarzbach J, Caramazza A.. Differential activity for animals and manipulable objects in the anterior temporal lobes. J Cogn Neurosci. 2011;23(8):2059–2067. [DOI] [PubMed] [Google Scholar]
- 34.Martin A, Chao LL.. Semantic memory and the brain: Structure and processes. Curr Opin Neurobiol. 2001;11(2):194–201. [DOI] [PubMed] [Google Scholar]
- 35.Devlin JT, Moore CJ, Mummery CJ, et al. Anatomic constraints on cognitive theories of category specificity. Neuroimage. 2002;15(3):675–685. [DOI] [PubMed] [Google Scholar]
- 36.Mahon BZ, Anzellotti S, Schwarzbach J, Zampini M, Caramazza A.. Category-specific organization in the human brain does not require visual experience. Neuron. 2009;63(3):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bright P, Moss HE, Stamatakis EA, Tyler LK.. The anatomy of object processing: The role of anteromedial temporal cortex. Q J Exp Psychol B. 2005;58(3-4):361–377. [DOI] [PubMed] [Google Scholar]
- 38.Mahon BZ, Milleville SC, Negri GA, Rumiati RI, Caramazza A, Martin A.. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55(3):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao LL, Weisberg J, Martin A.. Experience-dependent modulation of category-related cortical activity. Cereb Cortex. 2002;12(5):545–551. [DOI] [PubMed] [Google Scholar]
- 40.Chouinard PA, Goodale MA.. Category-specific neural processing for naming pictures of animals and naming pictures of tools: An ALE meta-analysis. Neuropsychologia. 2010;48(2):409–418. [DOI] [PubMed] [Google Scholar]
- 41.Devlin JT, Rushworth MF, Matthews PM.. Category-related activation for written words in the posterior fusiform is task specific. Neuropsychologia. 2005;43(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mechelli A, Sartori G, Orlandi P, Price CJ.. Semantic relevance explains category effects in medial fusiform gyri. Neuroimage. 2006;30(3):992–1002. [DOI] [PubMed] [Google Scholar]
- 43.Okada T, Tanaka S, Nakai T, et al. Naming of animals and tools: A functional magnetic resonance imaging study of categorical differences in the human brain areas commonly used for naming visually presented objects. Neurosci Lett. 2000;296(1):33–36. [DOI] [PubMed] [Google Scholar]
- 44.Price CJ, Noppeney U, Phillips J, Devlin JT.. How is the fusiform gyrus related to category-specificity? Cogn Neuropsychol. 2003;20(3):561–574. [DOI] [PubMed] [Google Scholar]
- 45.Rogers TT, Hocking J, Mechelli A, Patterson K, Price C.. Fusiform activation to animals is driven by the process, not the stimulus. J Cogn Neurosci. 2005;17(3):434–445. [DOI] [PubMed] [Google Scholar]
- 46.Wheatley T, Weisberg J, Beauchamp MS, Martin A.. Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cogn Neurosci. 2005;17(12):1871–1885. [DOI] [PubMed] [Google Scholar]
- 47.Humphreys GW, Forde EM.. Hierarchies, similarity, and interactivity in object recognition: “category-specific” neuropsychological deficits. Behav Brain Sci. 2001;24(3):453–476. discussion 476–509. [PubMed] [Google Scholar]
- 48.Taylor KI, Moss HE, Tyler LK.. The conceptual structure account: A cognitive model of semantic memory and its neural instantiation. In: Neural basis of semantic memory. New York, NY, US: Cambridge University Press; 2007:265–301. [Google Scholar]
- 49.Randall B, Moss HE, Rodd JM, Greer M, Tyler LK.. Distinctiveness and correlation in conceptual structure: Behavioral and computational studies. J Exp Psychol Learn Mem Cogn. 2004;30(2):393–406. [DOI] [PubMed] [Google Scholar]
- 50.Tyler LK, Moss HE.. Towards a distributed account of conceptual knowledge. Trends Cogn Sci. 2001;5(6):244–252. [DOI] [PubMed] [Google Scholar]
- 51.Collins JA, Montal V, Hochberg D, et al. Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain. 2017;140(2):457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR.. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47(1):36–45. [PubMed] [Google Scholar]
- 53.Rohrer JD, Warren JD, Modat M, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72(18):1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD.. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan D, Fox NC, Scahill RI, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol. 2001;49(4):433–442. [PubMed] [Google Scholar]
- 56.La Joie R, Landeau B, Perrotin A, et al. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer's and semantic dementia-targeted networks. Neuron. 2014;81(6):1417–1428. [DOI] [PubMed] [Google Scholar]
- 57.Brambati SM, Rankin KP, Narvid J, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: A tensor-based morphometry study. Neurobiol Aging. 2009;30(1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robert PH, Lafont V, Snowden JS, Lebert F.. [Diagnostic criteria for fronto-temporal lobe degeneration]. Encephale. 1999;25(6):612–621. [PubMed] [Google Scholar]
- 59.Miller JB, Banks SJ, Leger GC, Cummings JL.. Randomized controlled trials in frontotemporal dementia: Cognitive and behavioral outcomes. Transl Neurodegener. 2014;3:12- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hurley RS, Mesulam MM, Sridhar J, Rogalski EJ, Thompson CK.. A nonverbal route to conceptual knowledge involving the right anterior temporal lobe. Neuropsychologia. 2018;117:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosen HJ, Allison SC, Ogar JM, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67(10):1752–1756. [DOI] [PubMed] [Google Scholar]
- 62.Sapolsky D, Domoto-Reilly K, Dickerson BC.. Use of the Progressive Aphasia Severity Scale (PASS) in monitoring speech and language status in PPA. Aphasiology. 2014;28(8-9):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sapolsky D, Domoto-Reilly K, Negreira A, Brickhouse M, McGinnis SM, Dickerson BC.. Monitoring progression of primary progressive aphasia: Current approaches and future directions. Neurodegen Dis Manag. 2011;1(1):43–55. [Google Scholar]
- 64.Mesulam MM.Primary progressive aphasia. Ann Neurol. 2001;49(4):425–432. [PubMed] [Google Scholar]
- 65.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adlam AL, Patterson K, Bozeat S, Hodges JR.. The Cambridge Semantic Memory Test Battery: Detection of semantic deficits in semantic dementia and Alzheimer's disease. Neurocase. 2010;16(3):193–207. [DOI] [PubMed] [Google Scholar]
- 67.Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR.. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38(9):1207–1215. [DOI] [PubMed] [Google Scholar]
- 68.Hodges JR, Salmon DP, Butters N.. Semantic memory impairment in Alzheimer's disease: Failure of access or degraded knowledge? Neuropsychologia. 1992;30(4):301–314. [DOI] [PubMed] [Google Scholar]
- 69.Snodgrass JG, Vanderwart M.. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6(2):174–215. [DOI] [PubMed] [Google Scholar]
- 70.Kivisaari SL, Tyler LK, Monsch AU, Taylor KI.. Medial perirhinal cortex disambiguates confusable objects. Brain. 2012;135 (Pt 12):3757–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins DL, Neelin P, Peters TM, Evans AC.. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 72.Dale AM, Fischl B, Sereno MI.. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 73.Fischl B, Sereno MI, Dale AM.. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 74.Fischl B, Sereno MI, Tootell RB, Dale AM.. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischl B, Dale AM.. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fischl B, Liu A, Dale AM.. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. [DOI] [PubMed] [Google Scholar]
- 77.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. [DOI] [PubMed] [Google Scholar]
- 78.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. [DOI] [PubMed] [Google Scholar]
- 79.Augustinack JC, Huber KE, Stevens AA, et al. ; Alzheimer's Disease Neuroimaging Initiative. Predicting the location of human perirhinal cortex, Brodmann's area 35, from MRI. Neuroimage. 2013;64:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28(11):1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 82.Dickerson BC, Fenstermacher E, Salat DH, et al. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rogerson P. Statistical methods for geography: A student's guide, Los Angeles: SAGE Publications Inc; 2015.
- 84.Pan Y, Jackson RT.. Ethnic difference in the relationship between acute inflammation and serum ferritin in US adult males. Epidemiol Infect. 2008;136(3):421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambon Ralph MA, Patterson K, Garrard P, Hodges JR.. Semantic dementia with category specificity: A comparative case-series study. Cogn Neuropsychol. 2003;20(3):307–326. [DOI] [PubMed] [Google Scholar]
- 86.Funnell E, Sheridan J.. Categories of knowledge? unfamiliar aspects of living and nonliving things. Cogn Neuropsychol. 1992;9(2):135–153. [Google Scholar]
- 87.Mayberry EJ, Sage K, Ralph MA.. At the edge of semantic space: The breakdown of coherent concepts in semantic dementia is constrained by typicality and severity but not modality. J Cogn Neurosci. 2011;23(9):2240–2251. [DOI] [PubMed] [Google Scholar]
- 88.Silveri MC, Brita AC, Liperoti R, Piludu F, Colosimo C.. What is semantic in semantic dementia? The decay of knowledge of physical entities but not of verbs, numbers and body parts. Aphasiology. 2018;32(9):989–1009. [Google Scholar]
- 89.Laiacona M, Barbarotto R, Capitani E.. Perceptual and associative knowledge in category specific impairment of semantic memory: A study of two cases. Cortex. 1993;29(4):727–740. [DOI] [PubMed] [Google Scholar]
- 90.Mesulam M, Rogalski E, Wieneke C, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009;132(Pt 9):2553–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Drane DL, Ojemann GA, Ojemann JG, et al. Category-specific recognition and naming deficits following resection of a right anterior temporal lobe tumor in a patient with atypical language lateralization. Cortex. 2009;45(5):630–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mesulam MM, Wieneke C, Hurley R, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136(Pt 2):601–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan D, Anderson V, Pijnenburg Y, et al. The clinical profile of right temporal lobe atrophy. Brain. 2009;132(Pt 5):1287–1298. [DOI] [PubMed] [Google Scholar]
- 94.Josephs KA, Whitwell JL, Knopman DS, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology. 2009;73(18):1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mummery CJ, Patterson K, Wise RJ, et al. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122 (Pt 1):61–73. [DOI] [PubMed] [Google Scholar]
- 96.Snowden JS.Semantic dysfunction in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 1999;10 (Suppl 1):33–36. [DOI] [PubMed] [Google Scholar]
- 97.Neary D, Snowden J.. Fronto-temporal dementia: Nosology, neuropsychology, and neuropathology. Brain Cogn. 1996;31(2):176–187. [DOI] [PubMed] [Google Scholar]
- 98.Pietrini V, Nertempi P, Vaglia A, Revello MG, Pinna V, Ferro-Milone F.. Recovery from herpes simplex encephalitis: Selective impairment of specific semantic categories with neuroradiological correlation. J Neurol Neurosurg Psychiatry. 1988;51(10):1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moss HE, Tyler LK, Durrant-Peatfield M, Bunn EM.. ‘Two eyes of a see-through’: Impaired and intact semantic knowledge in a case of selective deficit for living things. Neurocase. 1998;4(4):291–310. [Google Scholar]
- 100.Moss HE, Rodd JM, Stamatakis EA, Bright P, Tyler LK.. Anteromedial temporal cortex supports fine-grained differentiation among objects. Cereb Cortex. 2005;15(5):616–627. [DOI] [PubMed] [Google Scholar]
- 101.Gainotti G.What the locus of brain lesion tells us about the nature of the cognitive defect underlying category-specific disorders: A review. Cortex. 2000;36(4):539–559. [DOI] [PubMed] [Google Scholar]
- 102.Taylor KI, Stamatakis EA, Tyler LK.. Crossmodal integration of object features: Voxel-based correlations in brain-damaged patients. Brain. 2009;132(Pt 3):671–683. [DOI] [PubMed] [Google Scholar]
- 103.Bussey TJ, Saksida LM.. The organization of visual object representations: A connectionist model of effects of lesions in perirhinal cortex. Eur J Neurosci. 2002;15(2):355–364. [DOI] [PubMed] [Google Scholar]
- 104.Buckley MJ, Gaffan D.. Perirhinal cortical contributions to object perception. Trends Cogn Sci. 2006;10(3):100–107. [DOI] [PubMed] [Google Scholar]
- 105.Saksida LM, Bussey TJ.. The representational-hierarchical view of amnesia: Translation from animal to human. Neuropsychologia. 2010;48(8):2370–2384. [DOI] [PubMed] [Google Scholar]
- 106.Mishkin M, Ungerleider LG, Macko KA.. Object vision and spatial vision: Two cortical pathways. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- 107.Bruffaerts R, Dupont P, Peeters R, De Deyne S, Storms G, Vandenberghe R.. Similarity of fMRI activity patterns in left perirhinal cortex reflects semantic similarity between words. J Neurosci. 2013;33(47):18597–18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clarke A, Tyler LK.. Object-specific semantic coding in human perirhinal cortex. J Neurosci. 2014;34(14):4766–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liuzzi AG, Dupont P, Peeters R, et al. Left perirhinal cortex codes for semantic similarity between written words defined from cued word association. Neuroimage. 2019;191:127–139. [DOI] [PubMed] [Google Scholar]
- 110.Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR.. A neural basis for lexical retrieval. Nature. 1996;380(6574):499–505. [DOI] [PubMed] [Google Scholar]
- 111.Binney RJ, Embleton KV, Jefferies E, Parker GJ, Ralph MA.. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cereb Cortex. 2010;20(11):2728–2738. [DOI] [PubMed] [Google Scholar]
- 112.Visser M, Embleton KV, Jefferies E, Parker GJ, Ralph MA.. The inferior, anterior temporal lobes and semantic memory clarified: Novel evidence from distortion-corrected fMRI. Neuropsychologia. 2010;48(6):1689–1696. [DOI] [PubMed] [Google Scholar]
- 113.Binney RJ, Hoffman P, Lambon Ralph MA.. Mapping the multiple graded contributions of the anterior temporal lobe representational hub to abstract and social concepts: Evidence from distortion-corrected fMRI. Cereb Cortex. 2016;26(11):4227–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mion M, Patterson K, Acosta-Cabronero J, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133(11):3256–3268. [DOI] [PubMed] [Google Scholar]
- 115.Gainotti G.Cognitive and anatomical locus of lesion in a patient with a category-specific semantic impairment for living beings. Cogn Neuropsychol. 1996;13(3):357–390. [Google Scholar]
- 116.Tranel D, Damasio H, Damasio AR.. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35(10):1319–1327. 10.1016/S0028-3932(97)00085-7 [DOI] [PubMed] [Google Scholar]
- 117.Bruffaerts R, De Weer AS, De Grauwe S, et al. Noun and knowledge retrieval for biological and non-biological entities following right occipitotemporal lesions. Neuropsychologia. 2014;62:163–174. [DOI] [PubMed] [Google Scholar]
- 118.Luckhurst L, Lloyd-Jones TJ.. A selective deficit for living things after temporal lobectomy for relief of epileptic seizures. Brain Lang. 2001;79(2):266–296. [DOI] [PubMed] [Google Scholar]
- 119.Chan AS, Sze SL, Cheung MC.. Neuroanatomical basis in the temporal lobes for processing living things. Neuropsychology. 2004;18(4):700–709. [DOI] [PubMed] [Google Scholar]
- 120.Ding J, Chen K, Chen Y, et al. The left fusiform gyrus is a critical region contributing to the core behavioral profile of semantic dementia. Front Hum Neurosci. 2016;10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kumfor F, Landin-Romero R, Devenney E, et al. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain. 2016;139(Pt 3):986–998. [DOI] [PubMed] [Google Scholar]
- 122.Caramazza A, Shelton JR.. Domain-specific knowledge systems in the brain the animate-inanimate distinction. J Cogn Neurosci. 1998;10(1):1–34. [DOI] [PubMed] [Google Scholar]
- 123.Warrington EK, McCarthy R.. Category specific access dysphasia. Brain. 1983;106 (Pt 4):859–878. [DOI] [PubMed] [Google Scholar]
- 124.Warrington EK, McCarthy RA.. Categories of knowledge. Further fractionations and an attempted integration. Brain. 1987;110 (Pt 5):1273–1296. [DOI] [PubMed] [Google Scholar]
- 125.Garrard P, Patterson K, Watson PC, Hodges JR.. Category specific semantic loss in dementia of Alzheimer's type. Functional-anatomical correlations from cross-sectional analyses. Brain. 1998;121 (Pt 4):633–646. [DOI] [PubMed] [Google Scholar]
- 126.Moss HE, Tyler LK, Devlin J.. The emergence of category specific deficits in a distributed semantic system. In: Category-specificity in brain and mind. Hove, UK: Psychology Press; 2002. 115–148. [Google Scholar]
- 127.Mahon BZ, Caramazza A.. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J Physiol Paris. 2008;102(1-3):59–70. [DOI] [PubMed] [Google Scholar]
- 128.Aziz-Zadeh L, Wilson SM, Rizzolatti G, Iacoboni M.. Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Curr Biol. 2006;16(18):1818–1823. [DOI] [PubMed] [Google Scholar]
- 129.Rogers TT, McClelland JL.. Semantic cognition: A parallel distributed processing approach. Cambridge, MA, US: MIT Press; 2004. [DOI] [PubMed] [Google Scholar]
- 130.Visser M, Lambon Ralph MA.. Differential contributions of bilateral ventral anterior temporal lobe and left anterior superior temporal gyrus to semantic processes. J Cogn Neurosci. 2011;23(10):3121–3131. [DOI] [PubMed] [Google Scholar]
- 131.Pascual B, Masdeu JC, Hollenbeck M, et al. Large-scale brain networks of the human left temporal pole: A functional connectivity MRI study. Cereb Cortex. 2015;25(3):680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Montembeault M, Brambati SM, Gorno-Tempini ML, Migliaccio R.. Clinical, anatomical, and pathological features in the three variants of primary progressive aphasia: A review. Front Neurol. 2018;9:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borghesani V, Battistella G, Mandelli ML, et al. Regional and hemispheric susceptibility of the temporal lobe to FTLD-TDP type C pathology. Neuroimage Clin. 2020;28:102369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murley AG, Coyle-Gilchrist I, Rouse MA, et al. Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. Brain. 2020;143(5):1555–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Faul F, Erdfelder E, Lang AG, Buchner A.. GPower 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 136.Lindquist KA, Gendron M, Barrett LF, Dickerson BC.. Emotion perception, but not affect perception, is impaired with semantic memory loss. Emotion. 2014;14(2):375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Veronelli L, Makaretz SJ, Quimby M, Dickerson BC, Collins JA.. Geschwind syndrome in frontotemporal lobar degeneration: Neuroanatomical and neuropsychological features over 9 years. Cortex. 2017;94:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Additional data are available from the corresponding author, upon reasonable request.