I vividly recall that spring day four decades ago when I came upon the June 5, 1981, Morbidity and Mortality Reports (MMWR) issue that reported on the first five Pneumocystis carinii pneumonia cases, a signature condition for AIDS, among young gay men in Los Angeles, California. I was sitting at my workstation in the laboratory in Cleveland, Ohio, when my mentor walked in and put that MMWR issue on my desk. Knowing that I would be returning to New York City soon thereafter to pursue a career in clinical infectious diseases and public health, he said, “You might want to take a look at this.” Little did I realize then how this report would be the harbinger of a global epidemic and a catalyst that would transform my career and my life.
Since then, 32.7 million children and adults have died from HIV/AIDS, and currently more than 38 million persons are living with HIV globally.1 At the same time, major advances have been achieved in confronting the HIV epidemic. HIV testing is highly accurate and offers rapid results in minutes, and the scale-up of effective and well-tolerated treatment to millions has been a remarkable achievement. Extensive social and behavioral research has shed light on factors that influence the risk of HIV. HIV prevention, however, has lagged. In 2019, more than 1.7 million new HIV infections were reported, far higher than the target of less than 500 000 annual cases.1 This shortfall raises several questions. Where and among whom are new infections occurring? Why have we lagged in preventing HIV infections? And, most importantly, what can be done to stem the spread of this virus?
In the early years of the HIV epidemic, HIV prevention was focused on influencing sexual and injecting behaviors and promoting the avoidance of higher-risk behaviors, such as multiple sex partners, unprotected sex, and sharing needles and syringes.2 In 1992, a decade after those first five cases were reported, HIV prevention was furthered by the recognition that zidovudine, the first antiretroviral agent, could prevent mother-to-child transmission of HIV.3 This motivated researchers to determine whether antiretroviral drugs could also prevent the sexual transmission of HIV. Two decades later, the landmark HIV Prevention Trials Network (HPTN) 052 study clearly demonstrated that antiretroviral treatment prevented the sexual transmission of HIV among heterosexual serodiscordant couples. Evidence soon followed of similar efficacy among gay men in serodiscordant partnerships.4 The recognition that treatment not only provides individual benefit but also prevents transmission to others further energized efforts to expand global access to antiretroviral therapy.1,5
Prevention research then turned to the next big question, whether antiretroviral drugs would also work for primary prevention (i.e., to prevent HIV acquisition by HIV-negative persons). A pivotal study, the IPrEX study, conducted among men and transgender women who have sex with men, first proved that preexposure prophylaxis was a successful strategy for HIV prevention.6 However, efforts to replicate these findings among heterosexual women and in demonstration projects led to inconclusive findings, largely because of limited adherence with daily oral preexposure prophylaxis.7
The challenges of uptake, adherence, and persistence inspired efforts to identify long-acting antiretroviral drugs for preexposure prophylaxis. Two studies, HPTN 083 and HPTN 084—of injectable long-acting cabotegravir given every two months to men and transgender persons who have sex with men and to heterosexual women in sub-Saharan Africa—recently demonstrated the superiority of this approach when compared with daily oral preexposure prophylaxis. Another long-acting antiretroviral provided via a monthly vaginal dapivirine ring also offered encouraging findings.8 Further innovations continue with exciting efforts in pursuit of long-acting antiretroviral pills, implants, and patches, all in an effort to overcome the challenge of adherence.9
Despite these advances, the successful prevention of HIV transmission requires a fundamental reconceptualization of the overall approach, recognizing that the HIV epidemic is not a monolith but consists of diverse epidemics. The unique characteristics and life experiences of populations at risk need to drive our efforts to protect them from HIV. In the United States, for example, men who have sex with men are disproportionately more severely affected than are other groups, with Black and Latinx men accounting for 25% and 20% of new infections, respectively.10 Transgender persons around the world have a 13-fold higher risk of HIV, and female sex workers are 30 times more likely to acquire HIV than are people in the general population.1 Persons who inject drugs have also borne the brunt of HIV and are at additional high risk for other infectious diseases such as hepatitis and tuberculosis.11 All these groups face profound barriers, particularly stigma and discrimination, two issues that drive them to avoid HIV services (e.g., testing, treatment, prevention).12
At the same time, for young women in sub-Saharan Africa, who continue to account for 59% of new HIV infections in this region, misperceptions regarding personal risk, competing life priorities, and difficulties in negotiating safer sex continue to put them in harm’s way. This, combined with structural impediments (e.g., economic vulnerabilities, lack of supportive services at health facilities and in the community, and the fact that their male partners are often unaware of their HIV-positive status and consequently have an unsuppressed viral load), results in increasing the women’s risk of HIV acquisition.2,13 In addition, although the efficacy of antiretroviral drugs for prevention of mother-to-child transmission of HIV first sparked the truly transformative, game-changing research on the use of antiretroviral drugs for primary and secondary prevention, elimination of mother-to-child transmission remains beyond our reach.14 Tragically, an estimated 150 000 new infections among children were reported in 2019, because of stigma faced by HIV-positive pregnant and breastfeeding women and difficulties in accessing antenatal and HIV services.2
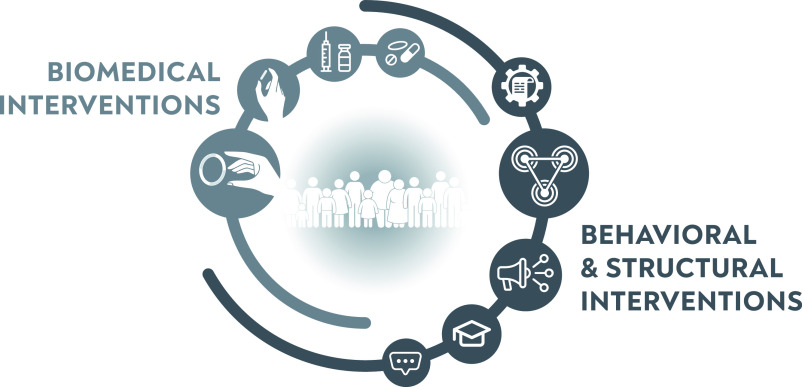
What will it take to get us to the end of the HIV epidemic? Clearly, it will take more than the pursuit of biomedical “magic bullets.” We must celebrate and acknowledge progress in identifying new tools, and we need to harness similar vigor to identify how best to use these tools to maximize their benefits for people at risk. Developing a vibrant, integrated strategy research agenda is critical. This involves combining behavioral and structural interventions with biomedical tools in ways that meet the needs of each population (Figure 1).15 For young women in sub-Saharan Africa, for example, we must seek effective interventions that enhance their accurate perception of risk, empower their agency in negotiating safer sex, and address their economic vulnerabilities. For disenfranchised groups, we need to study ways to change or mitigate the effects of punitive laws and overcome stigmatization by the health system.
FIGURE 1—
Integrated Strategies for HIV Prevention
Forty years on in an epidemic that has changed the lives of millions around the world and the face of global health, we have come to a broader understanding of the factors that must be examined and addressed if we are to successfully realize a world without AIDS. Although biomedical solutions are a critical linchpin, we must also learn how to address wider behavioral and systemic obstacles to adoption and persistence with biomedical prevention methods. However, unfortunately, research into integrated strategies for HIV prevention often does not receive the same priority as research with a purely biomedical focus, perhaps because it is not perceived to be as “scientific” or it is thought that incorporating behavioral or structural interventions is not likely to be of added value. These perceptions must change. Ultimately, to achieve the goal of ending the AIDS epidemic, we must learn how to address the realities and the contexts of the people at its heart.
ACKNOWLEDGMENTS
Partial funding for this work was provided by the HIV Prevention Trials Network, National Institutes of Health (grant UM1AI154468).
Inputs from Jessica Justman, Hugh Siegel, Melissa Reyes, and Joseph Stegemerten are deeply appreciated.
CONFLICTS OF INTEREST
The author has no conflicts of interest from funding or affiliation-related activities to declare.
Footnotes
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS. Global HIV & AIDS statistics—2020 fact sheet. https://www.unaids.org/en/resources/fact-sheet
- 2.CDC AIDS Community Demonstration Projects Research Group. Community-level HIV intervention in 5 cities: final outcome data from the CDC AIDS Community Demonstration Projects. Am J Public Health. 1999;89:336–345. doi: 10.2105/AJPH.89.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACTG 076 and reduction of perinatal transmission. Update Natl Minor AIDS Counc. 1997;4–7:9. [PubMed] [Google Scholar]
- 4.Rodger AJ, Cambiano V. PARTNER Study Group, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428–2438. doi: 10.1016/S0140-6736(19)30418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Sadr WM, Holmes CB, Mugyenyi P, et al. Scale-up of HIV treatment through PEPFAR: a historic public health achievement. J Acquir Immune Defic Syndr. 2012;60(suppl 3):S96–104. doi: 10.1097/QAI.0b013e31825eb27b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR iPrEx Study. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute of Allergy and Infectious Diseases. Pre-exposure prophylaxis (PrEP) to reduce HIV risk. https://www.niaid.nih.gov/diseases-conditions/pre-exposure-prophylaxis-prep
- 8.Baeten JM, Palanee-Phillips T HOPE Study Team. et al. Safety, uptake, and use of a dapivirine vaginal ring for HIV-1 prevention in African women (HOPE): an open-label, extension study. Lancet HIV. 2021;8(2):e87–e95. doi: 10.1016/S2352-3018(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flexner C, Owen A, Siccardi M, Swindells S. Long-acting drugs and formulations for the treatment and prevention of HIV infection. Int J Antimicrob Agents. 2021;57(1):106220. doi: 10.1016/j.ijantimicag.2020.106220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HIV.gov. U.S. statistics. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics
- 11.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wouters E, Sommerland N, Masquillier C. Unpacking the dynamics of double stigma: how the HIV-TB co-epidemic alters TB stigma and its management among healthcare workers. BMC Infect Dis. 2020;20(1):106. doi: 10.1186/s12879-020-4816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camlin CS, Ssemmondo E. SEARCH Collaboration, et al. Men “missing” from population-based HIV testing: insights from qualitative research. AIDS Care. 2016;28(suppl 3):67–73. doi: 10.1080/09540121.2016.1164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton E, Bossiky B, Ditekemena J, et al. Using the PMTCT cascade to accelerate achievement of the global plan goals. J Acquir Immune Defic Syndr. 2017;75(suppl 1):S27–S35. doi: 10.1097/QAI.0000000000001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodenow MM, Gaist P. Importance of HIV/AIDS-related behavioral and social sciences research at the NIH and beyond. J Acquir Immune Defic Syndr. 2019;82(suppl 2):S84–S87. doi: 10.1097/QAI.0000000000002163. [DOI] [PMC free article] [PubMed] [Google Scholar]