Abstract
Immunoglobulin G4 (IgG4)-related disease (IgG4-RD) is a systemic fibroinflammatory disorder that can involve multiple organs. It is often challenging to distinguish IgG4-related sclerosing cholangitis (IgG4-SC) from cholangiocarcinoma because of overlap in their clinical findings. A 75-year-old man presented to a hospital for a detailed examination of the elevation of some biliary enzymes. Radiographic examination revealed segmental bile duct with wall thickening of the common hepatic bile duct, and dilation of the peripheral branches. Transampullary biopsy showed a non-specific inflammatory reaction with several IgG4-positive cell infiltrations. There were no signs of malignancy. The liver biopsy showed bile duct injury accompanied by IgG4-positive cell infiltration. We then performed bile duct biopsy and finally diagnosed the patient with cholangiocarcinoma. We should remember that the IgG4 reaction is neither completely sensitive nor specific for IgG4-RD and avoid resting solely on the IgG4 reaction to precisely distinguish IgG4-SC from cholangiocarcinoma.
Keywords: Cholangiocarcinoma, immunoglobulin G4 (IgG4) reaction, immunoglobulin G4 (IgG4) IgG4-related disease, IgG4-related sclerosing cholangitis
Introduction
Immunoglobulin G4 (IgG4)-related disease (IgG4-RD) is a systemic multiorgan fibroinflammatory disorder that is characterized by prominent infiltrates of lymphoplasmacytic cells with abundant IgG4-positive cells, storiform fibrosis, and elevated serum IgG4 concentrations [1-3]. IgG4-related sclerosing cholangitis (IgG4-SC) is a manifestation of bile duct lesions in systemic IgG4-related diseases, including autoimmune pancreatitis (AIP). IgG4-SC often presents clinical manifestations similar to those of cholangiocarcinoma, and differentiating IgG4-SC from cholangiocarcinoma is still often challenging, particularly when it manifests as an isolated biliary tract lesion [4,5]. Furthermore, some cases of IgG4-SC have been reported to be associated with cholangiocarcinoma or its precursor lesion [6-8]. However, for clinicians, precise differentiation between IgG4-SC and cholangiocarcinoma is strictly required at the point of subsequent treatment and prognosis by a combination of clinical, imaging, and biochemical characteristics.
Increased serum IgG4 values (usually ≥135 mg/dL) and IgG4-positive plasma cell infiltration in affected organs are characteristics of IgG4-related disease. This marked infiltration of IgG4-positive cells in a given organ is known as the IgG4 reaction and could support IgG4-SC. However, an IgG4 reaction is frequently observed not only in patients with IgG4-SC, but also in cholangiocarcinoma [9]. This leads to further confusion in distinguishing IgG4-SC from cholangiocarcinoma.
Here, we present a diagnostically challenging case of cholangiocarcinoma with extensive IgG4 reaction that was finally diagnosed by repeat biopsies.
Material and methods
Case presentation
A 75-year-old man presented to a hospital for a detailed examination of the elevation of some biliary enzymes that was detected 4 months prior to admission. The initial laboratory data were as follows: total bilirubin (1.6 mg/dL), aspartate aminotransferase (133 IU/L), alanine aminotransferase (90 IU/L), gamma-glutamyltransferase (496 IU/L), alkaline phosphatase (529 IU/L), IgG (1350 mg/dL; normal range 861-1747 mg/dL), IgG4 (113 mg/dL; normal range 5-117 mg/dL), ANA (160; normal level < 40, nucleolar), carcinoembryronic antigen level (2.2 ng/mL; normal range 0-5.0 ng/mL) and cancer antigen 19-9 (9.2 U/mL; normal range 0-37 µg/mL). Magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) revealed obstruction of the hilar bile duct, originating from the common hepatic duct to the intrahepatic bile duct in the left and right lobes, and dilation of the intrahepatic bile duct (Figure 1). T2-weighted MRI showed segmental and ill-shaped wall thickening from the common hepatic duct to the intrahepatic bile duct in the left and right lobes, with high signal intensity under diffusion-weighted imaging (Figure 2). Contrast-enhanced computed tomography (CE-CT) showed segmental wall thickening with enhancement of the common hepatic duct, and a stricture from the common hepatic duct to the bilateral intrahepatic bile ducts. (Figures 2 and 3). Endoscopic retrograde cholangiopancreatography (ERCP) also showed hilar bile duct stricture from the common hepatic bile duct to approximately 25 mm in the left lobe intrahepatic bile duct, and approximately 10 mm in the right lobe intrahepatic bile duct. In particular, the intrahepatic bile duct in the left hepatic lobe had severe stenosis with peripheral bile duct dilation. The accumulation of fluorodeoxyglucose (FDG) was not detected by FDG-PET position emission tomography in any organ, including the common bile duct.
Figure 1.
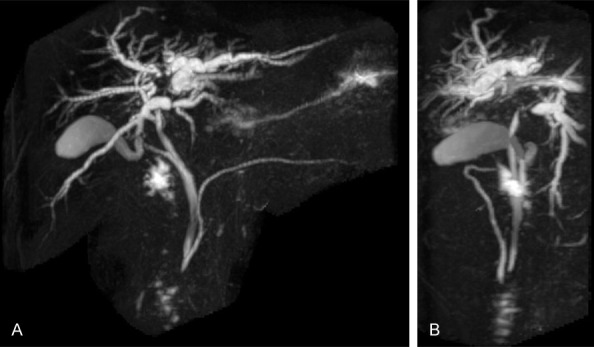
A, B. MRCP revealed common bile duct and intrahepatic stenosis, with upstream intrahepatic bile duct dilation, in the left and right hepatic lobes.
Figure 2.
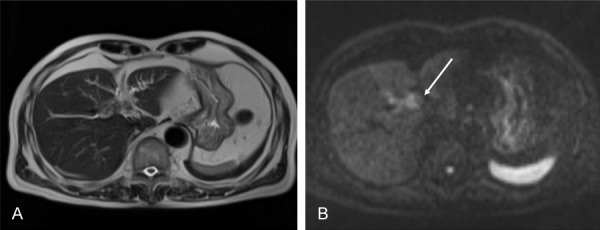
A, B. T2-weighted MRI showed segmental and poorly-formed wall thickening with high-signal intensity under diffusion-weighted imaging as indicated by the arrow.
Figure 3.
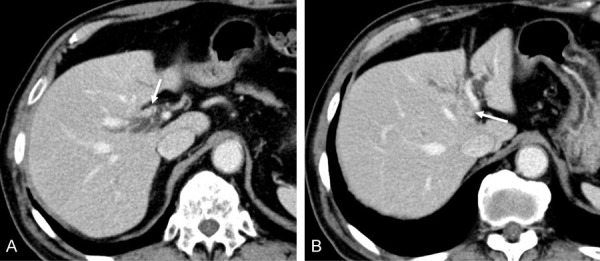
A, B. Contrast-enhanced computed tomography (CE-CT) showed segmental wall thickening with enhancement of the common hepatic duct, and the stricture from the common hepatic duct to the bilateral intrahepatic bile duct (indicated by the arrows).
Results
Diagnosis
Transampullary biopsy showed moderate inflammation with several IgG4-positive cell infiltrations, whereas brush cytology showed reactive changes (Figure 4). These diagnostic investigations were not conclusive. Two months later, he was admitted to the hospital for a detailed examination. On admission, laboratory data showed further elevation of the biliary enzymes as follows: gamma-glutamyl transferase (1,147 IU/L) and alkaline phosphatase (771 IU/L). Pathologic examination of the liver revealed mild portal inflammation, bile duct injury, and infiltration of IgG4-positive cells (> 10 cells per high-power field, IgG4/IgG > 40%) (Figure 5). No cancer cells were detected in these samples, and the typical findings of primary sclerosing cholangitis (PSC) were not detected. These histopathologic findings were primarily indicative of IgG4-SC, but histologic results met the criteria for IgG4-SC. We also considered the possibility of hilar cholangiocarcinoma with IgG4 reaction and included this as a differential diagnosis. Considering that sufficient histopathologic samples had not been obtained by prior biopsies, we performed intraductal ultrasonography (IDUS) and bile duct biopsy to confirm the diagnosis. Finally, bile duct biopsy revealed cholangiocarcinoma (Figure 6). IgG4-staining revealed only a few IgG4-positive cells. Taken together, the biliary lesion was diagnosed as a cholangiocarcinoma accompanied by significant IgG4 positive cell infiltration.
Figure 4.
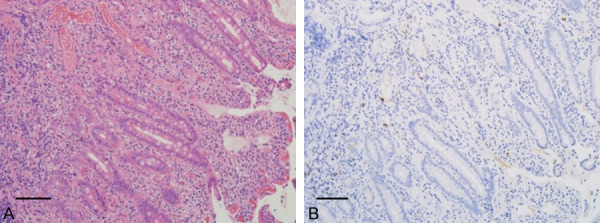
Histopathologic examination of the transampullary biopsy. A. Hematoxylin & eosin (H & E) stain of the transampullary biopsy showed a non-specific inflammation (magnification × 200). B. Reaction with several IgG4-positive cell infiltration (magnification × 200). Bars, each 100 μm.
Figure 5.
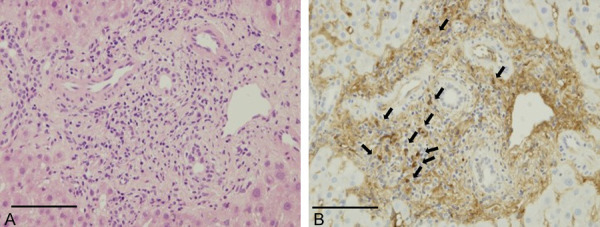
Histopathologic examination of the liver revealing mild portal inflammation, bile duct injury, and infiltration of IgG4-positive cells (> 10 per high power field, IgG4/IgG > 40%; indicated by the arrow). A. Hematoxylin and eosin staining (magnification × 400). B. IgG4 immunostaining (magnification × 400). Bars, each 100 μm.
Figure 6.
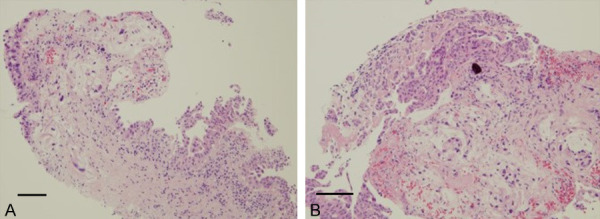
Histopathologic examination of the bile duct biopsy revealed cholangiocarcinoma by hematoxylin and eosin staining ((A) magnification × 100, (B) magnification × 400). Bars, each 100 μm.
Management and outcome
A metallic biliary stent was inserted across the obstruction sites and he underwent chemoradiation therapy for 12 months and radiation therapy (20 Gy × 25 times) after the final diagnosis. However, the cholangiocarcinoma had progressed and the patient elected comfort care about 1 year after the initiation of therapy, and he died two months later.
Discussion
Significant infiltration of IgG4-positive cells in a given organ is known as the IgG4 reaction. Induction of the IgG4 reaction is essential to the pathogenesis of IgG4-related diseases, and IgG4 reaction is one of the histopathologic hallmarks of IgG4-SC. However, the reaction is frequently observed in patients not only with IgG4-SC but also with cholangiocarcinoma or PSC [10,11]. It is extremely challenging to differentiate between the IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy. Here, we present a challenging case of cholangiocarcinoma with extensive IgG4 positive cell infiltration that spread from the duodenal mucosa to the portal vein area, finally resulting in a diagnosis of cholangiocarcinoma by repeat biopsy.
We must consider cholangiocarcinoma, IgG4-SC, and PSC in patients with stenosis and dilation of the hilar or intrahepatic biliary ducts. In our case, pathologic examination of the ampullary and liver biopsy showed IgG4 positive cell infiltration with no obvious malignant findings, especially in the liver biopsy, with over 10 IgG4-positive cells per high-power field, and the IgG4/IgG positive cell ratio was more than 40%. IgG4-positive cell infiltration is one of the clues to the diagnosis of IgG4-SC; other findings that are in the consensus statement on the pathology of IgG4-SC, such as storiform pattern fibrosis and obliterative phlebitis, were not detected [2]. Umehara et al. reported in their study on the histologic features of liver biopsies, that portal inflammation patterns with significant IgG4-positive cell infiltration into portal areas of the liver tended to be seen in autoimmune pancreatitis and were not seen in patients with primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), or chronic viral hepatitis [12]. We could not exclude the possibility of cholangiocarcinoma that produced IgG4, and decided to perform a biopsy of the bile duct for further examination to reach an accurate diagnosis. We finally reached the final diagnosis of cholangiocarcinoma. The complicated relationship between cholangiocarcinoma and IgG4-SC remains to be clarified, and there are few reports that describe cases suspected to be IgG4-SC and were later finally diagnosed as cholangiocarcinoma.
In cholangiocarcinoma, IgG4 reactions are considered localized cancer-associated immune reactions designed to evade immune surveillance. A previous report noted that the existence of an IL-10-based regulatory cytokine network associated with cholangiocarcinoma tissue and speculated that the IgG4 reaction in cholangiocarcinoma is a localized cancer-associated immune reaction to evade immune surveillance in an IL-10-related regulatory cytokine milieu [9]. The IgG4 reaction could be speculated to be non-specific in several pathologic conditions, including cholangiocarcinoma, and we should remember that we should not exclude cholangiocarcinoma even though significant infiltration of IgG4-positive cells is seen, and avoid missing cholangiocarcinoma [13,14].
Furthermore, we should also consider the possibility of cholangiocarcinoma with IgG4 reaction, or the coexistence of IgG4-RD and cholangiocarcinoma in this patient. Although the precise mechanism remains to be investigated, there have been reports of cholangiocarcinoma development in several IgG4-SC cases [6,8,15]. This often makes it more difficult to differentiate between cholangiocarcinoma and IgG4-SC. In our case, high concentrations of IgG4-positive cells were seen in the liver, but other characteristic histopathological features of IgG4-SC, such as a storiform pattern of fibrosis and obliterative phlebitis were not detected in repeated biopsies. In addition, bile duct biopsy revealed few IgG4-positive cells. Based on the clinical and histopathologic results, we finally made a diagnosis of cholangiocarcinoma alone.
In differentiating IgG4-SC from cholangiocarcinoma, diagnostic confirmation is extremely difficult if the histological material is insufficient. In our case, transampullary biopsy and liver biopsy were favorable for IgG4-SC, but not crucial and could not completely exclude the possibility of cholangiocarcinoma. This case confirms that adequate biopsy is needed to distinguish precisely cholangiocarcinoma from IgG4-SC.
Involvement of other organs could suggest IgG4-SC [4,5], but our case did not involve other organs including AIP. It is further difficult to differentiate isolated IgG4-SC without AIP from cholangiocarcinoma. It remains challenging to distinguish between cholangiocarcinoma and IgG4-SC, especially when no organ involvement is seen. Again, we herein demonstrated a case of cholangiocarcinoma where IgG4 reaction spread widely from duodenal mucosa to the portal vein area. In practice, it is extremely difficult to exclude the possibility of the cooccurrence of cholangiocarcinoma and IgG4-RD, especially when an extensive IgG4-reaction is seen like our case [16,17]. We reached the accurate diagnosis as cholangiocarcinoma by repeating biopsies with careful consideration of the possibility of IgG4 reaction accompanied by cholangiocarcinoma. Finally, we considered that the extensive IgG4-reaction of our case may be a localized cancer-associated immune reaction designed to evade immune surveillance. This case indicates that caution should be exercised when distinguishing between IgG4-SC and cholangiocarcinoma; that is, care must be taken not to be overly influenced by the diagnostic criteria for IgG4-SC that overemphasize IgG4-positive cell infiltration in the affected sites. It is particularly important to rule out malignant tumors through biopsies of the bile duct and the collection of multiple specimens from the same site.
Disclosure of conflict of interest
None.
Abbreviations
- IgG4-RD
Immunoglobulin G4 (IgG4)-related disease
- IgG4-SC
IgG4-related sclerosing cholangitis
References
- 1.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 3.Zen Y, Kawakami H, Kim JH. IgG4-related sclerosing cholangitis: all we need to know. J Gastroenterol. 2016;51:295–312. doi: 10.1007/s00535-016-1163-7. [DOI] [PubMed] [Google Scholar]
- 4.Nakazawa T, Ohara H, Sano H, Ando T, Imai H, Takada H, Hayashi K, Kitajima Y, Joh T. Difficulty in diagnosing autoimmune pancreatitis by imaging findings. Gastrointest Endosc. 2007;65:99–108. doi: 10.1016/j.gie.2006.03.929. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa T, Naitoh I, Hayashi K, Miyabe K, Simizu S, Joh T. Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol. 2013;19:7661–7670. doi: 10.3748/wjg.v19.i43.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straub BK, Esposito I, Gotthardt D, Radeleff B, Antolovic D, Flechtenmacher C, Schirmacher P. IgG4-associated cholangitis with cholangiocarcinoma. Virchows Arch. 2011;458:761–765. doi: 10.1007/s00428-011-1073-2. [DOI] [PubMed] [Google Scholar]
- 7.Ohtani H, Ishida H, Ito Y, Yamaguchi T, Koizumi M. Autoimmune pancreatitis and biliary intraepithelial neoplasia of the common bile duct: a case with diagnostically challenging but pathogenetically significant association. Pathol Int. 2011;61:481–485. doi: 10.1111/j.1440-1827.2011.02684.x. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A, Mori M, Kubota K, Naitoh I, Nakazawa T, Takikawa H, Unno M, Kamisawa T, Kawa S, Okazaki K. Epidemiological features of immunoglobulin G4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci. 2020;27:598–603. doi: 10.1002/jhbp.793. [DOI] [PubMed] [Google Scholar]
- 9.Harada K, Shimoda S, Kimura Y, Sato Y, Ikeda H, Igarashi S, Ren XS, Sato H, Nakanuma Y. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology. 2012;56:157–164. doi: 10.1002/hep.25627. [DOI] [PubMed] [Google Scholar]
- 10.Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Takahashi S, Joh T. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009;44:1147–1155. doi: 10.1007/s00535-009-0108-9. [DOI] [PubMed] [Google Scholar]
- 11.Zen Y, Quaglia A, Portmann B. Immunoglobulin G4-positive plasma cell infiltration in explanted livers for primary sclerosing cholangitis. Histopathology. 2011;58:414–422. doi: 10.1111/j.1365-2559.2011.03763.x. [DOI] [PubMed] [Google Scholar]
- 12.Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007;46:463–471. doi: 10.1002/hep.21700. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T, Nakazawa T, Tazuma S, Zen Y, Tanaka A, Ohara H, Muraki T, Inui K, Inoue D, Nishino T, Naitoh I, Itoi T, Notohara K, Kanno A, Kubota K, Hirano K, Isayama H, Shimizu K, Tsuyuguchi T, Shimosegawa T, Kawa S, Chiba T, Okazaki K, Takikawa H, Kimura W, Unno M, Yoshida M. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2019;26:9–42. doi: 10.1002/jhbp.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resheq YJ, Quaas A, von Renteln D, Schramm C, Lohse AW, Lüth S. Infiltration of peritumoural but tumour-free parenchyma with IgG4-positive plasma cells in hilar cholangiocarcinoma and pancreatic adenocarcinoma. Dig Liver Dis. 2013;45:859–865. doi: 10.1016/j.dld.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Koopman KE, Bloemena E, Kazemier G, Klemt-Kropp M. Immunoglobulin G4-mediated sclerosing cholangitis as a risk factor for cholangiocarcinoma: a case report. Mol Clin Oncol. 2016;5:786–788. doi: 10.3892/mco.2016.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azeem N, Ajmera V, Hameed B, Mehta N. Hilar cholangiocarcinoma associated with immunoglobulin G4-positive plasma cells and elevated serum immunoglobulin G4 levels. Hepatol Commun. 2018;2:349–353. doi: 10.1002/hep4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerdsirichairat T, Manivel JC. An unusual lesion presenting as cholangiopathy. Gastroenterology. 2016;150:e3–5. doi: 10.1053/j.gastro.2015.10.015. [DOI] [PubMed] [Google Scholar]


