Abstract
The interleukin-6 receptor antagonist tocilizumab became widely used early in the coronavirus disease 2019 (COVID-19) pandemic based on small observational studies that suggested clinical benefit in COVID-19 patients with a hyperinflammatory state. To inform our local treatment algorithms in the absence of randomized clinical trial results, we performed a rapid analysis of the first 11 hospitalized COVID-19 patients treated with tocilizumab at our academic medical center. We report their early clinical outcomes and describe the process by which we assembled a team of diverse trainees and stakeholders to extract, analyze, and disseminate data during a time of clinical uncertainty.
Keywords: SARS-CoV-2, Evidence-based decision-making, Tocilizumab, Cytokine release syndrome, COVID-19, Interleukin 6 (IL-6)
The use of unproven therapeutics in a pandemic presents a challenge to evidence-based clinical decision making. In the early months, limited evidence was available to guide treatment of coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Many unproven therapeutics were given to patients worldwide, in an effort to find treatments that would improve patients' clinical course and decrease mortality. Without an evidence base for a novel disease, clinicians have one of three options: liberally treat patients based on small, preliminary case series; withhold unproven therapeutics until randomized clinical trial (RCT) results are available; or pair initial off-label treatment with rapid analysis of patients in one's local practice environment while awaiting RCT results.
Rapid analysis of off-label tocilizumab use
We describe our experience with the latter approach for off-label use of the interleukin-6 (IL-6) receptor inhibitor tocilizumab in severe COVID-19 early in the pandemic. Tocilizumab had been posited to reduce the severity of COVID-19 caused by an IL-6-mediated hyperinflammatory state based on limited observational studies from China and other sites early in the pandemic.1, 2, 3, 4, 5, 6 Given the paucity of data on tocilizumab use in COVID-19 in the United States early on, and with results from randomized controlled trials (RCTs) still months away, we performed a rapid, descriptive analysis of the first 11 patients treated with off-label tocilizumab for COVID-19 and used the results to guide clinical practice at our medical center.
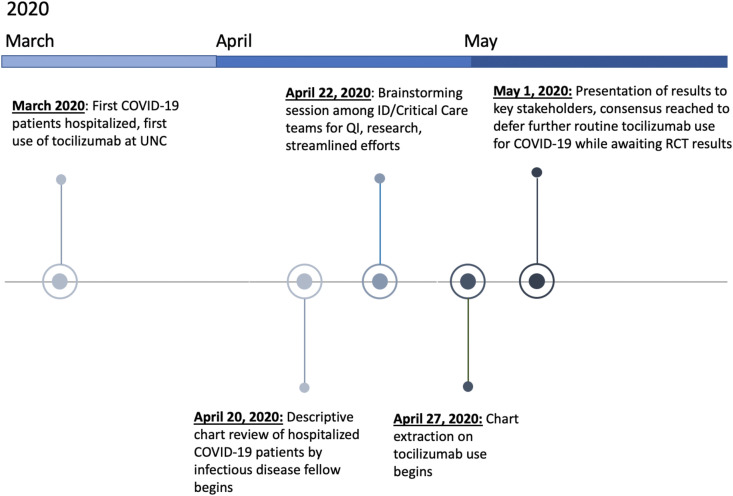
We repurposed a group of trainees who had been sidelined by the COVID-19 pandemic to conduct a rapid-turnaround analysis of tocilizumab use at our medical center. As classes and clinical rotations had been put on hold, a large pool of trainees with different skill sets were available, including graduate students in epidemiology, medical students, medical residents, and fellows in infectious diseases. Covariates of interest were identified by crowdsourcing to faculty in the UNC divisions of infectious diseases, hospital medicine, and pulmonary and critical care and the UNC Eshelman School of Pharmacy via email and web conference; final data fields were determined by consensus. All trainees contributed about a month of time and first took an online refresher course in research, ethics, and compliance available through the Collaborative Institutional Training Initiative (CITI). The study was approved by the University of North Carolina at Chapel Hill (UNC) Institutional Review Board (IRB), with proactive submission to the IRB at the start of the pandemic to enable rapid analysis of local data for practice improvement and dissemination of results. After starting data extraction, a strict 1-week timeline for extraction, analysis, and dissemination was established due to the urgent need for informed clinical decision making (Fig. 1 ).
Fig. 1.
Timeline of events. The COVID-19 Rapid Practice Improvement Deployment (RaPID) study of tocilizumab successfully extracted, analyzed, and disseminated its findings to key stakeholders in less than one week.
We then launched the COVID-19 Rapid Practice Improvement Deployment (RaPID) study of tocilizumab, performing a retrospective cohort study of patients with COVID-19 treated with tocilizumab at UNC Medical Center between March 21st and April 25th, 2020. Clinical data was extracted from the medical record. COVID-19 was diagnosed using nasopharyngeal swabs and reverse-transcriptase polymerase chain reaction. IL-6 testing was conducted at Mayo Clinic Laboratories (Rochester, MN) using an electrochemiluminescence assay. Baseline IL-6 values for two patients were from a multi-cytokine panel (lower-limit of detection [LOD] of 5.0 pg/mL); all others were from a standalone IL-6 assay (LOD 1.8 pg/ml). Disease severity was defined according to the World Health Organization (WHO) COVID-19 clinical status scale with minor modification.7 Descriptive statistics were calculated using JMP 15. GraphPad Prism 8 was used to test for differences between matched measures within a patient 1-day pre- and 5-days post-tolicizumab treatment using Wilcoxon signed-rank test. Data were visualized using the tidyverse collection of packages in R 3.6.0.
Patient characteristics and early outcomes
Detailed patient characteristics and early outcomes of the 11 included patients with confirmed SARS-CoV-2 infection treated with tocilizumab are provided in the Supplementary Results and Table. In brief, most patients had severe or critical COVID-19 by modified WHO criteria at the time of admission. Only three (27%) received corticosteroids. Tocilizumab was administered a median of one day (IQR 1–4) from admission and nine days (IQR 7–14) after symptom onset. Two of six patients with available baseline IL-6 levels had low levels, one below the assay's LOD (≤5 pg/mL) and another of 10.4 pg/mL. Four of five with available IL-6 levels after tocilizumab had IL-6 levels above the upper limit of quantification. IL-6 increased after tocilizumab in all four patients with matched baseline and post-tocilizumab testing. IL-6 levels required several days to return and were not available to guide decisions about tocilizumab use.
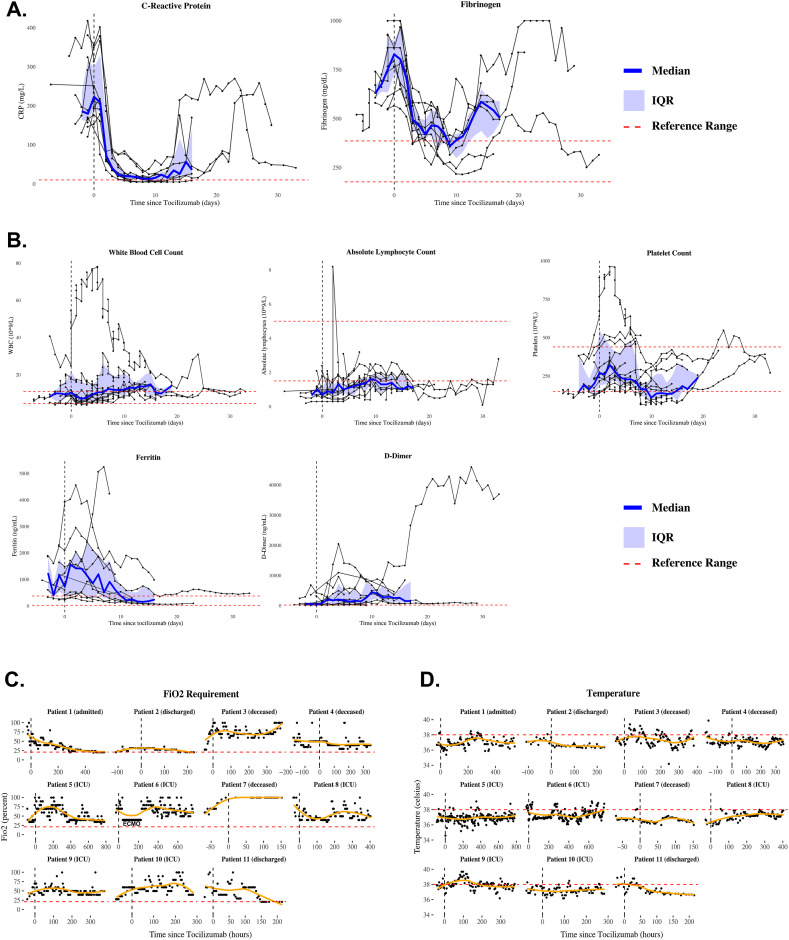
CRP and fibrinogen decreased significantly after tocilizumab (Fig. 2 A). Other laboratory markers did not show clear trends (Fig. 2B). Temperature did not show obvious improvement (Fig. 2C), and only three patients (27%) showed improvement in oxygen requirements (Fig. 2D). At end of follow-up, patients were observed for a median of 17 days (range=7–34) after initial tocilizumab dose. Three died; five remained in the intensive care unit (ICU) on mechanical ventilation, receiving vasopressors or hemodialysis; one was transferred from the ICU to floor status and weaned to room air; and two were discharged home without oxygen. No serious adverse events were observed.
Fig. 2.
Laboratory and clinical outcome data presented to key stakeholders. (A–B) Laboratory trends in eleven COVID-19 patients after tocilizumab treatment. Medians (blue lines), interquartile ranges (IQR, blue shading), and reference ranges (dashed red lines). (C–D) Oxygen requirements and temperature trends, displayed using locally estimated scatterplot smoothing (orange lines). Patient #6 was treated with two doses of tocilizumab; time 0 refers to the time of initial administration.
Applying results to clinical practice
Using an interdisciplinary group of faculty and trainees, we performed a rapid descriptive analysis of patients with COVID-19 treated with tocilizumab at our academic medical center, almost all of whom required ICU care. While tocilizumab was described as a promising therapy for severe COVID-19, our initial descriptive analysis raised questions about the generalizability of early observational reports and prompted removal from our local treatment algorithms. CRP and fibrinogen improved after tocilizumab administration as previously reported,1 , 2 , 8, 9, 10 but improvement in disease severity or mortality was not observed. These findings, in addition to low baseline IL-6 levels observed in some patients, raised questions about tocilizumab use in patients presenting with severe or critical COVID-19. As a result, we elected to defer further tocilizumab use until RCT results became available.
Though our cohort was small and our analysis did not include a control group, our findings were consistent with signs from early RCTs of tocilizumab in COVID-19.11, 12, 13, 14, 15 Our findings contrasted with most previous observational reports16 and did not provide clear evidence of clinical improvement after tocilizumab treatment in patients with severe COVID-19. We considered several explanations as we recommended against routine use of tocilizumab at our medical center. First, our patients were more severely ill than previous cohorts. It was possible tocilizumab was administered too late to offer benefit. Second, our patient cohort had more comorbidities, notably obesity and hypertension, than those in China and Italy, which could influence pathobiology and response to treatment. Our cohort was also more racially and ethnically diverse, which could reflect differences in baseline healthcare access and social determinants of health17 that confound treatment response within a small observational sample. Third, improvements observed in prior, less severely ill cohorts, might have occurred as part of the natural disease process, with or without tocilizumab. While the descriptive nature of our study and its small sample size were insufficient to define tocilizumab's role in the treatment of COVID-19, our findings provided a counterpoint to a growing number of small observational studies and supported our decision to defer further tocilizumab use while waiting for results from rigorous RCTs.
Rapid dissemination of results to local and external stakeholders
Data extraction, analysis, and dissemination was performed over the course of a single week. Two medical students extracted data, with arbitration by an infectious diseases fellow and faculty as needed. Data was visualized by an epidemiology graduate student. Initial results were presented as part of an interdisciplinary meeting including both pulmonary and critical care and infectious diseases faculty. Consensus was reached to remove tocilizumab from routine clinical practice until RCT results became available. Results of the RaPID descriptive analysis of tocilizumab, which contrasted with previously published observational data, were disseminated externally via immediate pre-print publication on medRxiv,18 local news outlets, and via Twitter.
Implications of available evidence and current guidelines
The conclusions of our analysis were consistent with early RCT results, which did not show clear evidence of efficacy in COVID-19 pneumonia.19 However, more recent RCTs demonstrated a mortality benefit when tocilizumab is administered in combination with corticosteroids and using specific clinical criteria.20 , 21 In response, we later resumed tocilizumab use for COVID-19 in our hospital in accordance with revised U.S. Department of Health and Human Services guidelines.22 These guidelines support the use of tocilizumab in combination with dexamethasone for select hospitalized patients exhibiting respiratory decompensation. This evidence-based approach differs from our early ad hoc usage of tocilizumab in critically ill patients with progressive disease. While an argument can be made that broader use of tocilizumab early in the pandemic might have benefited patients, many therapeutic candidates promoted early on ultimately failed to demonstrate efficacy for COVID-19, and some caused harm. This experience emphasizes the importance of building clinical algorithms based on RCT results.
Lessons learned
Our experience highlights the value of rapidly analyzing local data to guide clinical decision making, especially when available evidence is limited. We encourage the use of local hospital data to inform clinical decision-making as a bridge until data from RCTs are available. In this case, reflection on local practice was an important step in our hospital's management of severe COVID-19 and empowered clinicians to prioritize RCT over observational data whenever possible. Established platforms within large academic medical systems are insufficiently nimble for this type of rapid assessment early in the pandemic, though efforts to strengthen data analysis and sharing, such as the U.S. Department of Health and Human Services' National COVID Cohort Collaborative, are now bearing fruit and can be translated to future pandemics. Inclusion of trainees across disciplines served both the project and their professional development, as it offered experience with retrospective data collection and rapid dissemination, as well as mentorship from a broad range of collaborators. In a historical moment of disrupted schedules and systems, efficient collaboration by key stakeholders across research and clinical arenas enabled locally informed treatment decision making. We highlight the value of rapid analysis of local health system data to inform clinical decisions in times of uncertainty.
Declaration of competing interest
JBP reports research support outside the submitted work from Gilead Sciences, Virology Education, and the World Health Organization, and non-financial support from Abbott Laboratories. CLG has received research support outside the submitted work from Viiv Healthcare and Gilead Sciences. HS is now an employee of ViiV Healthcare. SSC reports research support outside of the submitted work from Biomarck Pharmaceuticals.
Acknowledgements
The authors would like to thank the nurses; laboratory staff; and allied health providers involved in the care of these patients. We also thank Carlton Moore and Hannah Little for assistance with initial exploration of data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hjdsi.2021.100581.
Funding
The authors report no designated funding for the project. CAR and CEM receive training support from the University of North Carolina at Chapel Hill Medical Scientist Training Program (T32GM008719). CAR additionally receives training support from the National Institutes of Health MD/PhD Partnership Program.
Author contributions
CAR, CEM extracted data, analyzed data, wrote first draft. GJB analyzed data. MKK extracted data. JBP supervised the study. CAR, CEM, GJB, MKK, TH, AM, BB, HS, SN, JLS, SSC, WAF, JJE, CLG, JBP reviewed and edited the final draft.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. Published online. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. Published online. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrey A.J., Choi G., Hanna R.M., et al. A case of novel coronavirus disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease. Am J Nephrol. 2020;51(5):337–342. doi: 10.1159/000507417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michot J.-M., Albiges L., Chaput N., et al. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. Published online April 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X., Song K., Tong F., et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4(7):1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mihai C., Dobrota R., Schröder M., et al. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis. 2020;79(5):668–669. doi: 10.1136/annrheumdis-2020-217442. [DOI] [PubMed] [Google Scholar]
- 7.WHO . RD Bluepr.; 2020. WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. Published online. [Google Scholar]
- 8.Sciascia S., Aprà F., Baffa A., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020 Published online. [PubMed] [Google Scholar]
- 9.Price C.C., Altice F.L., Shyr Y., et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. CHEST. 2020 doi: 10.1016/j.chest.2020.06.006. 0(0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kewan T., Covut F., Al–Jaghbeer M.J., Rose L., Gopalakrishna K.V., Akbik B. Tocilizumab for treatment of patients with severe COVID–19: a retrospective cohort study. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama C., Han J., Yau L., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosas I.O., Bräu N., Waters M., et al. Tocilizumab in hospitalized patients with severe covid-19 pnemonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvarani C., Dolci G., Massari M., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6615. Published online October 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermine O., Mariette X., Tharaux P.-L., et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6820. Published online October 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone J.H., Frigault M.J., Serling-Boyd N.J., et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2028836. Published online October 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malgie J., Schoones J.W., Pijls B.G. Decreased mortality in coronavirus disease 2019 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin Infect Dis. 2021 doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimland CA, Morgan CE, Bell GJ, et al. Clinical characteristics and early outcomes in patients with COVID-19 treated with tocilizumab at a United States academic center. medRxiv. Published online May 19, 2020:2020.05.13.20100404, version 1. doi:10.1101/2020.05.13.20100404.
- 19.Parr J.B. Time to reassess tocilizumab's role in COVID-19 pneumonia. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6557. Published online October 20. [DOI] [PubMed] [Google Scholar]
- 20.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abani O., Abbas A., Abbas F., et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coronavirus disease 2019 (COVID-19) treatment guidelines. COVID-19 Treatment Guidelines. Accessed July 25, 2021. https://www.covid19treatmentguidelines.nih.gov/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.