Abstract
Background: This presented study was aimed to evaluate the diagnostic and prognostic value of PD-L1+Neutrophils (PD-L1+NEUT) and neutrophil to lymphocyte ratio (NLR) based on our previous experience of Foxp3+Treg in transplantation. Methods: the NLR cutoff value of 1.79 was used to include 136 cases from the 204 patients with hepatocellular carcinoma (HCC) confirmed by clinical pathology, which were divided into highly-moderately and poorly differentiated HCC groups. The expressions of PD-L1+NEUT and Foxp3+Treg in peripheral blood and cancer tissue were detected with flow cytometry, meanwhile, PD-L1 and Foxp3 expressed in carcinoma and para-carcinoma tissues were marked by immunohistochemistry. Survival rates, including overall survival and disease-free survival, were calculated by the Kaplan–Meier curve and evaluated with the log-rank test. Finally, Cox risk regression model was used to analyze the independent risk factors for prognostic survival. Results: The level of PD-L1+NEUT, Foxp3+Treg, and NLR in peripheral blood of patients with poorly differentiated HCC were significantly increased (all P < .001). Both PD-L1+NEUT and NLR were positively correlated with Foxp3+Treg (r = 0.479, P = .0017; r = 0.58, P < .0001). The level of PD-L1+NEUT and Foxp3+Treg as well as PD-L1 and Foxp3 in cancer tissue and patients with poorly differentiated HCC were obviously increased (all P < .01), respectively. Cox regression analysis indicated that PD-L1+NEUT, NLR, and Foxp3+Treg were independent risk factors for the prognosis (P = .000, .000, .006) with a RR and 95%CI of 2.704-(2.155-3.393), 3.139-(2.361-4.173), 1.409-(1.105-1.798), respectively. Conclusion: PD-L1+NEUT, NLR, and Foxp3+Treg are independent risk factors for prognosis which maybe new marker of lower survival benefits.
Keywords: PD-L1+NEUT, Foxp3+Treg, NLR, hepatocellular carcinoma, Cox regression model
Introduction
Hepatocellular carcinoma (HCC) remains one of the most common malignant tumor in humans1 with the second common cause of death for men and third for women.1,2 Over the past decade, the incidence of HCC has continually increased and onset age getting younger world wide.2,3 At present, liver resection (LR) and liver transplantation (LT) are considered as the most effective radical therapies for HCC, which can completely resect the cancer lesions,4-6 but the tumor postoperative recurrence is still the major factor which affects long-term survival.7 Until now, there is no recognized suitable method for prevention and treatment of tumor recurrence after surgery.8,9 Tandem transplantation, re-resection, radiofrequency ablation, and interventional therapy such as transcatheter hepatic arterial chemoembolization are possible alternative approaches.8-10 After the ASCO in 2019, Lenvatinib is considered to be the first-line recommended treatment for HCC compared with sorafenib.11 Although the results still unsatisfactory, further observation is needed to determine its long-term effectiveness. Thus, the earlier the diagnosis, the sooner the operation, the better the prognosis. The earlier diagnosis is the first important step in improving the survivals. To explore more sensitive marker which has advantage both on diagnosis and prognosis will help to improve the operative resection rate and survival benefits.
Despite alpha fetoprotein (AFP) is a highly specific and sensitive tumor marker in HCC diagnosis, more attention should be paid to the negativity of about 30% in HCC patients and false positive results.12 As one of the most abundant types of immune cells, neutrophils in the tumor microenvironment participate in nearly every step of tumor progression and CD4+CD25+FoxP3+regulatory T cells (FoxP3+Tregs) have been demonstrated to perform significant functions in tumor immunity and graft immune tolerance, except that, it has been reported that elevated Foxp3+Treg and neutrophil to lymphocyte ratio (NLR) preoperative in HCC may associate with tumor relapse and low survival rate.13,14 We have also proved that a higher expression of Foxp3+Treg in HCC and HCC recurrence after LT.15 There also has a positive correlation between Foxp3+Treg and AFP.15,16 This may indicated that a well diagnostic and prognostic value for HCC applied with AFP. Furthermore, tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression via CCL17 pathway and negatively regulate adaptive immunity via PD-L1/PD-1 signaling pathway.13,17 In the present study, we mainly investigate the diagnostic and prognostic value of PD-L1+NEUT and NLR in HCC under our previous study on Foxp3+Treg,16 which aims to improve the positive rates and observe the new bio-markers.
Methods
Patient Selection and Operative Techniques
Patient Selection
At the present study, there have nearly 200 cases of HCC patients from January 2016 to June 2017. Finally, according to the sample estimation, we enrolled 136 patients who were diagnosed with HCC and performed LR with strict criteria as follows. This study was approved by the Ethical Committee of PLA General Hospital. All patients provided full written informed consent about the use with biological samples and study participation, written informed consent was obtained in accordance with the Declaration of Helsinki of the World Medical Association (Ethics approval and consent to participate: S2108-013-01). The authors are accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sample size: According to the global cancer statistics released by CA 2008 and 2020, the incidence of liver cancer in China was estimated to be 50% to 55%. Application with the sampling calculation formula of rate: with calculation assumptions: α = 0.05, π = 0.5-0.55, δ = 0.1. The minimum sample size calculated is 96 in this study.
Included criteria: (1) Preoperative image indicated liver malignant; (2) AFP abnormal > 40 ng/ml; (3) pathology results indicated HCC; (4) child A-B classification; (5) age from 18 to 70 years old; (6) no lung, bone, and other further parts metastasis; (7) all with completely case data and given informed consent.
Excluded criteria: (1) Preoperative image indicated liver malignancy, but the pathology results proved not HCC or benign; (2) normal AFP; (3) child C classification, severe cardiopulmonary disease not tolerate surgery; (4) age beyond 70 or below 18 years old; (5) hepatitis C virus or alcoholic liver disease patients with HCC; (6) with lung, bone, and other further parts metastasis; (7) secondary surgery or acceptation of radiotherapy and chemotherapy.
Operative detection
Preoperative tumor evaluation was done by diagnostic imaging methods, including abdominal ultrasonography, computed tomography (CT) including lung and abdominal or abdominal magnetic resonance imaging. The laboratory measurement included liver function, tumor marker, hepatitis index, blood routine examination, and thromboxane function.
Group and Operation
The patient compliance with the study criteria was admitted into the group, after fully operative assessment and then accepted LR. About 136 patients of 115 males and 21 females were grouped according to pathological results named highly-moderately differentiated HCC group and poorly differentiated HCC group. The follow up began at the time of diagnosis and was carried out at the hospital, with all data recorded. The overall survival (OS) and disease-free survival (DFS) were the main indicators of survival benefit.
Reagents and Drugs
Monoclonal antibodies are used for flow cytometry including anti-human CD4/FITC/550628, CD8/PE/562292, CD3/APC/555576, CD25/PE/555432, anti-human Foxp3/APC/4289565 (BD Pharm.), Buffer are used for flow cytometry containing AKC Lysing buffer/340503, phosphate buffered solution (007-16005, Corning), Foxp3 Staining Buffer Set (00-5523-00, BD Pharmingen). Immunohistochemistry (IHC) antibodies contains rat Foxp3 (22228-1-AP, Proteinteck), PD-L1 (SP142, SPRING) and reaction enhancer kits (PV-9000, OriGene Technologies, Inc). IL-10/E0056h, TGF-β/E0124h, IL-2/E0073h, and INF-γ/E0049h enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) were used to cytokine detection.
Sample Detection
Sample Preparation
Peripheral blood samples were collected immediately. Then, centrifuged at 3000 rpm for 10 min, and extracted serum storing at −80 °C until use. The liver tissue specimens were obtained immediately after tumor resection, and fixed with 10% formaldehyde solution.
Preparation of Lymphoid Cell Suspensions of Liver
Lymphocyte suspension of liver tissue were prepared by mincing the tissue on a wire mesh and then filtered with mesh in order to remove cell clumps. After that, the suspension was washed with RPMI-1640 (11835030, Gibco) medium in 15 mL centrifuge tube repeatedly. Resuspended cells, centrifuged at 1200 rpm for 10 min. The supernatant was discarded and resuspended with FBS for a single cell suspension. The last, obtained mononuclear cells by density gradient centrifugation (Sigma, USA) according to the manufacturer's protocol.
Measurement Approaches
The expression of CD3+CD4+T and CD3+CD8+T cell populations was detected by flow cytometric for control, peripheral blood, and lymphocyte suspensions of liver tissue. The Foxp3+Treg and PD-L1+NEUT also were detected by flow cytometric for the same samples.
Hematoxylin and Eosin Stain
Liver cancer tissue samples were fixed by 10% formalin and embedded in paraffin. Then, cutting into 5 μm thin slice by micro-tome, heated at 60 °C on slides warmer for 30 min, undergo the steps of dewaxing, benzene removal, hematoxylin and eosin staining, then dehydration and fixation. Finally observed and photographed under a microscope.
Immunohistochemistry
Paraffin-embedded tissues were sliced into thin tissue sections of 5 μm, heated at 60 °C on slides warmer for 30 min. First, underwent the step of dewaxing, 3% H2O2 inactivation, antigen retrieval in a microwave at 100 °C for 20 min, and closed antibody incubation for 30 min. Next, incubated with primary antibodies of Foxp3 and PD-1/PD-L1 with a 1:100 antibody titer for night. Then, incubated with second antibody PV-9000 enhance kits for 60 min, and tissue color staining with DAB kit (NC9276270, Vector) under an Nikon microscope system (TS1000-F).
ELISA With Cytokines
The cryopreserved serum was used to detect cytokine levels, cytokines concentrations in serum such as IL-10, TGF-β, IL-2, and INF-γ were determined using the ELISA kits, according to the manufacturer's protocol.
Statistical Analysis
Immunohistochemical and pathological results images were collected under optical microscopy for 40×, 100×, and 200× visual fields. Image J and Image Pro Plus were used for analysis of mean fluorescence density (MFI) or average optical (AOD) of the IHC images. All data analysis was carried out by SPSS 13.0 software, each index was expressed by Means ± Sd. Survival rates, including OS and DFS, were calculated using the Kaplan–Meier method and evaluated with the log-rank test. Cox proportional model was used to analyze the multivariate survival, and the independent risk factors affecting the survival time. Qualitative variables were compared using χ2 tests, and quantitative variables were compared using Wilcoxon tests (multigroup) or t-test (2 groups). Statistical significance was defined as p < .05.
Results
NLR and Inclusion Criterion
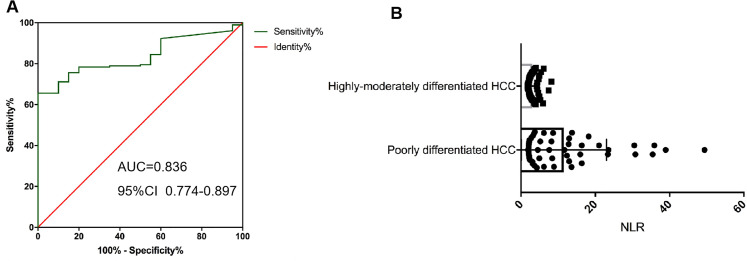
The receiver operating characteristic (ROC) curve is used to determine the cutoff value. According to the ROC curve, the NLR cutoff selected as 1.79 which was based on the NLR corresponding to the maximum YOUDEN index was used as Cutoff = 1.79 (YOUDEN index = Sensitivity + Specificity −1) (Figure 1A).
Figure 1.
Neutrophil–lymphocyte ratio (NLR) in different groups. A, ROC curve analysis of NLR; B, differences in peripheral blood NLR of tumor patients with different degrees of differentiation with t test.
The NLR of 136 enrolled patients ranged from 1.79 to 49.42 with a median value of 2.15. In addition, NLR in peripheral blood of patients with highly-moderately differentiated HCC was significantly lower than that of patients with poorly differentiated HCC, with a significant difference (P < .005) (Figure 1B).
Common Data and Pathology
The 136 enrolled patients who underwent LR for HCC at PLA General Hospital between January 2016 and June 2017 consisted of 115 males and 21 females. Their mean age was 54.62 ± 10.97 years with male 54.68 ± 10.99 years and female 54.37 ± 9.97 years. Pathology confirmed that 89 patients were complicated with cirrhosis, while the other patients had no obvious cirrhosis. Five and 2 patients were with multiple tumors in highly-moderately and poorly differentiated HCC group, respectively. The operative index of age, AFP, ALT was not significant (Table S1).
Pathological analysis of highly-moderately differentiation group and poorly differentiation group showed that 66.75% and 65.08% of hepatitis B cirrhosis complicated with HCC, respectively. The average size of tumors was 4.66 ± 0.41 and 6.62 ± 0.61 cm, respectively, and 20.55% and 19.05% with vascular invaders. Compared with the 2 groups, there was only the tumor size with statistical difference (Table S1).
The date of operation was the starting point of follow-up and ended in Jan 2020. The longest follow-up time was 49 months, the shortest was 24 months, and the median follow-up time was 41 months in the highly-moderately differentiated group. The longest follow-up time in the poorly differentiated group was 48 months, the shortest was 19 months, and the median follow-up time was 38 months. No patients were lost or withdraw during the study preformed.
Tumor Correlation Analysis Between Foxp3+Treg, NEUT and NLR in Peripheral Blood With Different HCC
Foxp3+Treg, PDL1+NEUT, and NLR Expression in Peripheral Blood
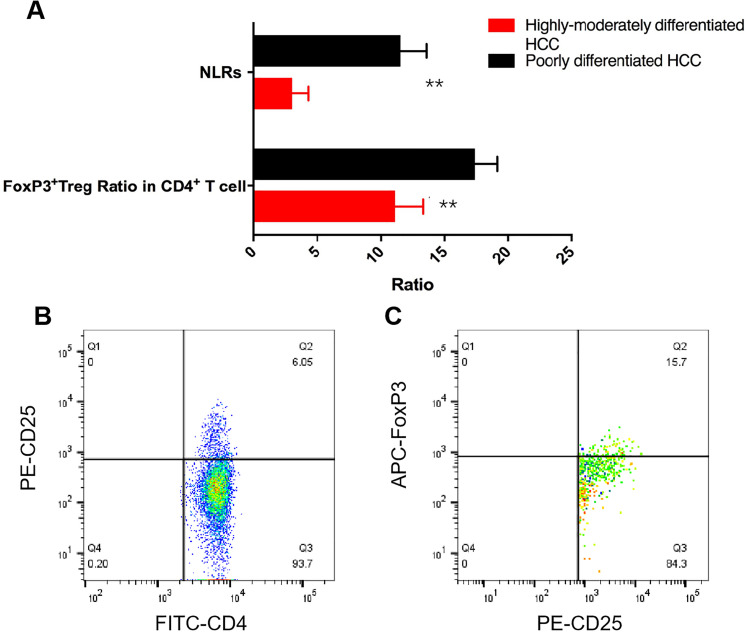
Flow cytometry detection with the marker of CD4, CD25, and Foxp3 indicated that the expression of Foxp3+Treg in peripheral blood of patients with poorly differentiated HCC was significantly increased compared with that of highly-moderately differentiated HCC, and the difference was statistically significant (P < .001) (Figure 2). Also, NLR in peripheral blood of patients with poorly differentiation HCC was significantly increased than that of highly-moderately differentiated HCC (Figure 2A) (P = .027).
Figure 2.
NLR and Foxp3+Treg expression levels in different HCC group, A, statistical analysis of NLR and Foxp3+Treg in CD4+T cell of different differentiation HCC with t test (n = 136), B, C, were the flow cytometry scatter plot of Foxp3+Treg in different HCC groups.
Abbreviations: NLR, neutrophil–lymphocyte ratio; HCC, hepatocellular carcinoma.
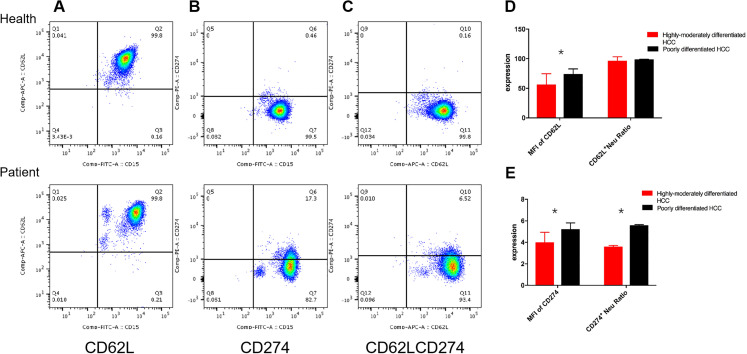
Neutrophils in peripheral blood were first labeled with CD15, and then with the marker of CD62L and PD-L1, flow cytometry detection showed that neutrophils normally expressed CD62L, but unexpressed/almost unexpressed PD-L1 in healthy people (Figure 3A to C, Health). Oppositely, the PD-L1 expression was significantly increased, and the proportion of CD62L and PD-L1 double positive cells increased significantly in peripheral blood of patients with HCC, that is, CD62L+PDL1+NEUT, which we called PDL1+NEUT (Figure 3A to C, Patient) in this presented study.
Figure 3.
Expression difference of neutrophils in hepatocellular carcinoma (HCC) patients with different degree of differentiation. A, Flow cytometry scatter plot of CD15+CD62L+NEUT, B, flow cytometry scatter plot of CD15+PDL1+NEUT, C, flow cytometry scatter plot of CD15+CD62L+PDL1+NEUT. D, The MFI of CD62L and CD62L+NEUT in different HCC groups by t test, E, the MFI of PDL1 and PDL1+NEUT in different HCC groups by t test (n = 64).
And then, we analyzed the difference of CD62L+NEUT and PD-L1+NEUT in different group. It was found that there was no significant difference of CD62L+NEUT in percentage between the highly-moderately and poorly differentiated HCC (P = .984), but a significant difference in the MFI intensity between them (P = .027) (Figure 3D); while double positive expression rates of PD-L1 and CD62L were upregulated in poorly differentiated HCC (Figure 3E), which indicated that the PDL1+NEUT in HCC with different degrees of differentiation was statistically significant (P < .001), and the AOD intensity was also significantly different (P = .021).
Correlation Analysis of Foxp3+Treg, PDL1+NEUT, and NLR Expression
The correlation analysis showed that a positive correlation between Foxp3+Treg and NLR (r = 0.58, P < .0001); and a positive correlation between Foxp3+Treg and PD-L1+NEUT(r = 0.479, P = .0017), and a positive correlation between NLR and PD-L1+NEUT (r = 0.672, P = .0001).
Cd8+T Cell Expression in Peripheral Blood
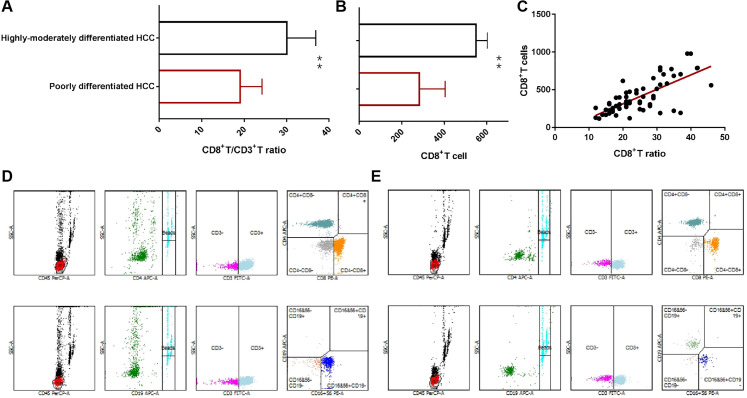
Flow cytometry is used to detect the absolute number and percentage of lymphocyte subsets for precisely analysis the cytotoxic T lymphocyte changes (Figure 4). It is found that whether the proportion of CD8+T cells to lymphocytes or not the level of absolute number of CD8+T cells in peripheral blood are both significantly lower in poorly differentiated HCC patients than that of highly-moderately differentiated HCC patients (Figure 4A and B). Meanwhile, there was a positive correlation between CD8+T ratio and absolute count (r = 0.524, P < .0001) (Figure 4C), and a negative correlation between absolute count of CD8+T cell and Foxp3+Treg (r = −0.625, P < .0001) and PD-L1+NEUT, (r = −0.687, P < .0001), respectively.
Figure 4.
Expression difference of CD8+T cell in hepatocellular carcinoma (HCC) patients with different degree of differentiation. A, The CD8+T/CD3+T cell ratio in different HCC groups analysis by Chi square test, B, the absolute CD8+T cells in different HCC groups analysis by t test, C, the correlation analysis of CD8+T/CD3+T cell ratio and CD8+T cells (n = 64). D, E, Flow cytometry scatter plot of lymphocyte subsets.
Levels of Cytokines in Peripheral Blood
The levels of IL-10 and TGF-β in peripheral blood of patients with poorly differentiated HCC were (176.3 ± 0.67) and (299.8 ± 7.17) pg/ml, respectively. Those of patients with high-moderately differentiated HCC were (162.3 ± 0.330), (256 ± 3.55) pg/ml, respectively. There were significant differences between the 2 groups (all P < .0001). The auxiliary responsive cytokines level of IL-2 was significantly higher in patients with poorly differentiated HCC (P = .0019), but there was no significant difference in the level of INF-γ, suggesting that high levels of Foxp3+Treg and neutrophils secreted suppressive cytokines such as IL-10 and TGF-β to decrease the activation of effector T cells in immune response.
Tumor Correlation Analysis Between Foxp3+Treg, NEUT and NLR in Liver Cancer Tissue With Different HCC
Expression of Foxp3+Treg in Liver Cancer and its Pathological Classification
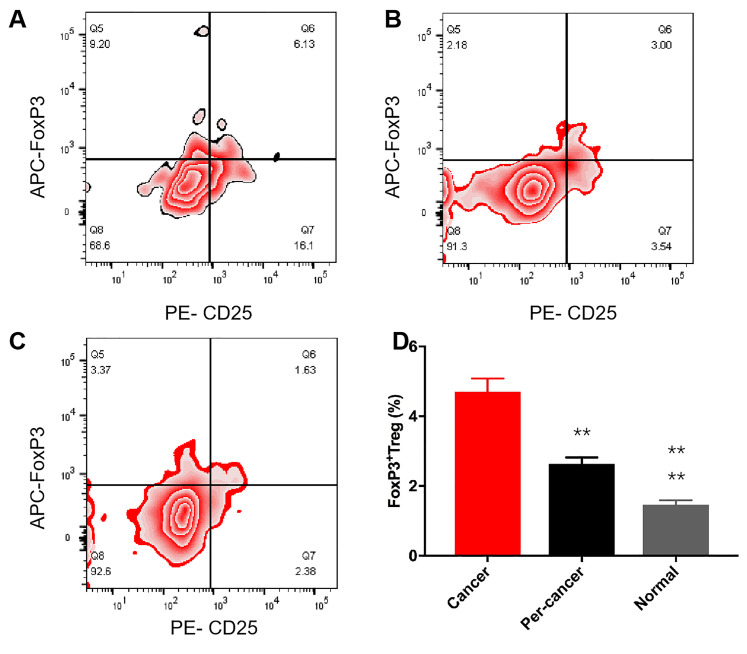
Flow cytometry analysis showed that the expression of Foxp3+Treg in liver cancer tissue, para-carcinoma tissue, and normal tissue was (4.70 ± 0.38)%, (2.63 ± 0.19)%, and (1.46 ± 0.13)%, respectively (Figure 5A and C), and there was a significant difference between the analysis of variance groups (P < .0001) (Figure 5). In the analysis within the group, compared with the normal tissue, the infiltration expression level of the cancer tissue was the highest, followed by the peritumoral tissues, and the difference was statistically significant (Figure 5D) (P all< .01).
Figure 5.
Foxp3+Treg in different liver cancer tissues. (A-C)The flow cytometry scatter plot of Foxp3+Treg A, cancer tissue; B, peritumoral tissue; C, normal liver tissue), D, statistical analysis of Foxp3+Treg in different liver tissue by analysis of variance (ANOVA) (n = 64).
The expression level of Foxp3+Treg was significantly different in different types of HCC (P < .05), which was negatively correlated with the degree of tumor differentiation (r = −0.98; P = .001).
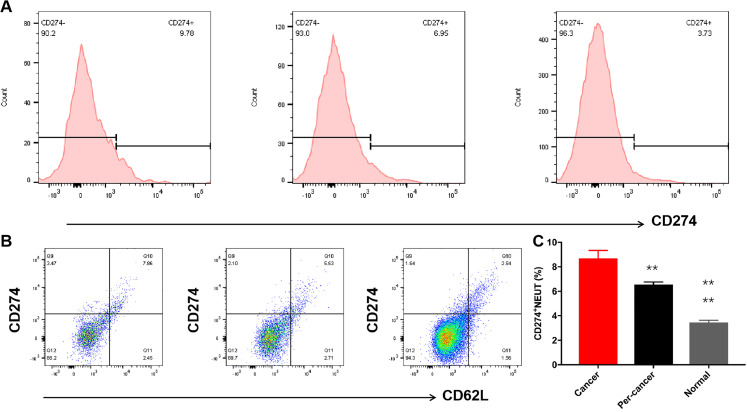
Expression of NEUT in Liver Cancer and Pathological Classification
Flow cytometry analysis suggests that the PD-L1+ NEUT expression in liver cancer tissue, peritumoral tissue, and normal tissue was (8.69 ± 0.65) %, (6.55 ± 0.22) %, (3.45 ± 0.18) % (Figure6A and B), respectively, and analysis of variance significant difference between groups (P < .0001) (Figure 6C). In the analysis within groups, compared with normal tissue, the infiltration expression level of cancer tissue was the highest, and the next was peritumoral tissue, the difference was statistically significant (P < .01) (Figure 6A and B). The expression level of PD-L1+NEUT was significantly different in different types of HCC, which was negatively correlated with the degree of tumor differentiation (r = −0.87; P < .001).
Figure 6.
PDL1+NEUT in different liver cancer tissues. A, The flow cytometry peak value chart of PDL1+NEUT (from left to right is cancer tissue, peritumoral tissue, and normal liver tissue, respectively), B, the flow cytometry scatter plot of PDL1+NEUT (from left to right is cancer tissue, peritumoral tissue, and normal liver tissue, respectively), C, statistical analysis of PDL1+NEUT in different liver tissue by analysis of variance (ANOVA) (n = 64).
Correlation Analysis of Foxp3+Treg, PD-L1+NEUT, and NLR Expression
The correlation analysis showed that the expression of Foxp3+ Treg and PD-L1+NEUT were positively correlated (r = 0.468, P = .0012) and the level of NLR was positively correlated with the expression of PD-L1+NEUT(r = 0.521, P = .0073).
Immunohistochemical Staining Indexes With Differentiation and Prognosis of Liver Cancer
Pathology in Various Differentiated HCC Group
HE stains were used to differentiate the differentiated HCC tissues in each case for further immunohistochemical staining. In comparison with highly-moderately differentiated HCC group, it displayed high heteromorphism, disordered organization arrangement, loss of normal organization structure of the low differentiated HCC (Figure S1).
Foxp3 and PD-L1 expression
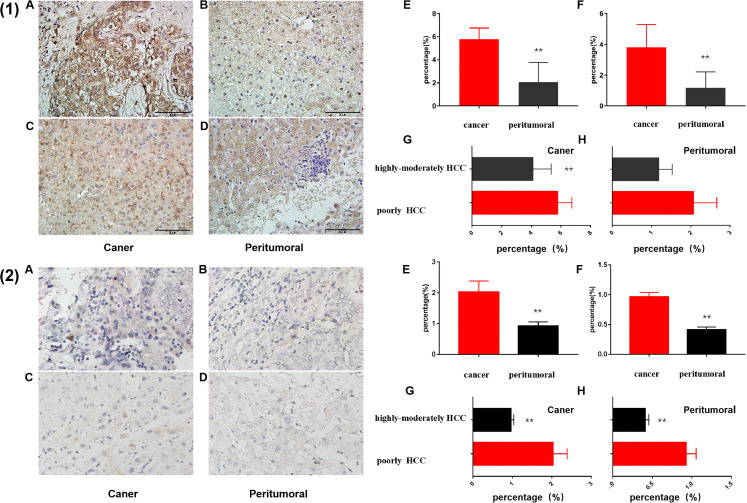
Foxp3 immunohistochemical staining mainly focused on the nucleus (Figure 7), the nuclear staining of liver cancer tissue was yellow brown. The staining intensity of poorly differentiated HCC was higher (Figure 7(1)A) than that of highly-moderately differentiated HCC (Figure 7(1)C) and the staining intensity of carcinoma tissue was higher than that of peritumoral tissue (Figure 7[1]B and D). Mean integrated optical density (MIOD) analysis suggests that the differential degree of differentiation HCC, the expression of FoxP3 in cancerous tissue were significantly higher than that in peritumoral tissue (Figure 7(1)E, P = .0001 vs .0001). For the same tissue site, the expression of FoxP3 in the cancer tissues with poorly differentiation of HCC was significantly increased than that of highly-moderately differentiated HCC (Figure 7(1)F) (P = .0022), while there was no significant difference between the 2 levels of differentiated liver cancer in the peritumoral tissues (Figure 7(1)F) (P = .202).
Figure 7.
Foxp3 (1) and PDL-1(2) expression levels of different degrees of differentiation HCC by IHC staining (200 × ). A, B, The staining results of cancer and peritumoral tissue of poorly differentiation HCC, respectively, C, D, staining results of cancer and peritumoral tissue of highly-moderately differentiation HCC, respectively. E, The statistical analysis of expression levels in different tissues of the same differentiation degree HCC by t test. F, In same tumor tissues with different degrees of differentiation by t test (n = 136).
Abbreviations: HCC, hepatocellular carcinoma; IHC, immunohistochemistry.
Immunohistochemical staining of PD-L1 mainly focused on cytoplasm (Figure 7), and the cytoplasm was stained with light yellow granules. The staining intensity of poorly differentiated tissues (Figure 7(2)A and B) was higher than that of highly-moderately differentiated tissues (Figure 7(2)C and D). MIOD analysis suggests that the differential degree of differentiation HCC, the expression of PD-L1 in cancerous tissue were significantly higher than that in peritumoral tissue (Figure 7(2)E, P = .016 vs < .0001). For the same tissue site, PD-L1 expression was significantly higher in the cancer tissues and peritumoral tissues with poorly differentiation of liver cancer (Figure 7(2)F) than that of highly-moderately differentiated liver cancer (P = .0008 vs .0001).
Survival Benefits Analysis
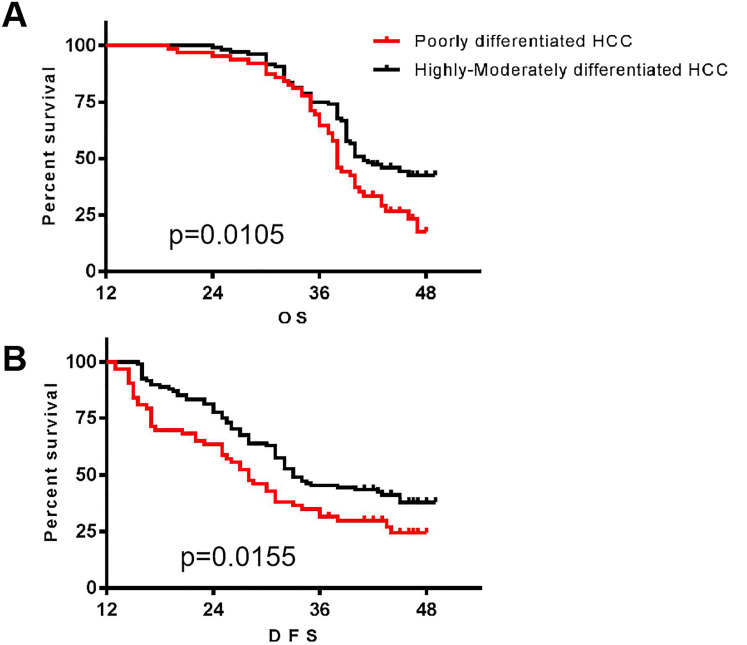
A follow up was performed until the end of January 2020 of the highly-moderately and poorly differentiation patients. OS survival curve analysis indicated that it had no significant difference between 2 groups when follow-up less than 1 year; survival time gradually extended of the highly-moderately differentiation group when followed up beyond 12 months, the difference between the 2 groups was significantly difference (P = .0105) (Figure 8A). DFS survival curve analysis also demonstrated a significant difference between the 2 groups (P = .0155) (Figure 8B).
Figure 8.
Survival benefits of different degrees of differentiation hepatocellular carcinoma (HCC) by Kaplan–Meier analysis. A, The overall survival time of the various differentiation patients; B, the disease free survival time of the various differentiation patients (n = 136).
Cox Proportional Hazard Regression Model Analysis
Significant difference in 2 groups of patients with OS circumstances, we performed multivariable Cox regression model analysis between the NLR, FoxP3+Treg, PD-L1+NEUT, tumor size, whether the metastasis, whether hepatitis and cirrhosis, AFP, and so on. At the same time, the basic confounding factors such as age and sex were analyzed to look for the independent risk factors associated with the prognosis of HCC.
The results of multivariate Cox risk model indicated that NLR was an independent risk factor after surgery, P = .000, RR = 3.319, 95%CI (2.316-4.173). Then, Cox analysis with step forward method (conditional LR) indicated that NLR and PDL1+NEUT were independent risk factors affecting the prognosis of HCC, with the relative risk to RR and 95%CI were 1.362-(1.085-1.709), 2.704-(2.155-3.393), respectively. Under the different differentiation degree (Group), Cox analysis with step forward method (conditional LR) indicated that except NLR and PDL1+NEUT, Foxp3+Treg was the independent risk factor affecting the prognosis of HCC, with the relative risk to RR and 95%CI of Foxp3+Treg was 1.409 (1.105-1.798). Furthermore, AFP (RR = 1) is not an independent risk factor for HCC prognosis with unclearly correlation which maybe not a large enough sample size compared with NLR, PD-L1+NEUT, and Foxp3+Treg both in different NLR and differentiation degrees. This all suggested that preoperative NLR is an independent prognostic factor for HCC, the combination of PD-L1+NEUT and Foxp3+Treg preoperative meanwhile were more conducive to predicting the prognosis and survival of patients (Table S2).
Discussion
Since majority of patients with HCC are already in the middle and advanced stage when diagnosed18 which lose the opportunity of radical surgical resection,11 and have limited long-term survival benefit. Even for patients undergoing surgical resection,19 recurrence and metastasis after surgery are the crucial problems affecting the long-term survival.7,20 In recent years, parallel with targeted therapy and biological immunotherapy, the mechanism study of tumor immune microenvironment of invasive neutrophils,21-24 Foxp3+ regulatory T cells,16,25-29 and myeloid suppressor cells have become the research focus in the field of tumor immunity and tumor microenvironment.
Literature research has confirmed that14,27,29 infiltrated Foxp3+Treg of preoperative peripheral blood and tissue were significantly negative correlation with the prognosis of patients with HCC and other solid tumor. In addition, the function of effector T cells can be reduced or the dysfunction of effector T cells can be caused by directly contact inhibition and indirectly secretion of inhibitory cytokines such as TGF-β and IL-10 of Foxp3+Treg,16,28,30 which can help tumor escape to promote proliferation and invasion.
Previous studies on Foxp3+Treg in the field of organ transplantation have found that,29,30-32 which can upregulate under the action of some immunosuppressive drugs, has the potential to induce immune tolerance, suggesting that the high expression of Foxp3+Treg in transplantation is benefit to the graft survival.31-33 For transplant patients of HCC, our previous studies have found that Foxp3+Treg in the peripheral blood was significantly upregulated in patients with tumor recurrence after transplantation,15,32 which significantly higher than that of patients with non HCC transplantation in stable status, and the level of inhibitory cytokines also significantly increased, so it was concluded that the over expression of Foxp3+Treg in the recipients after LT of HCC may be related to tumor recurrence.
We further confirmed that Foxp3+Tregs play inhibitory effect by secreting inhibitory cytokines of IL-10 and TGF-β which ultimately leads to the damage viability and decrease number of CD8+T cells to promote tumor immune escape with a growth effect of HCC in a simulative rat model of HCC relapse after LT.16 SRL-based therapy exerted an anti-tumor effect by reducingFoxP3+Tregs and their secreted inhibitory cytokines, which may through the AKT-mTOR signaling pathway, and the application PS-T and Zadaxin enhanced the synergistic effect of SRL.15,16 For the further study of the correlation between regulatory T cells and the prognosis and occurrence of HCC, we extended the study scope to HCC patients with no LT.
In this presented study, we found that the level of Foxp3+Treg in peripheral blood and cancer tissue were highly related to the degree of differentiation of HCC and the expression level was notably upregulated in poorly differentiated HCC, which further confirmed the correlation between Foxp3+Treg and tumor. Meanwhile, Foxp3 immunohistochemical staining carried out on the histopathology was found that the staining intensity in the cancer tissue was significantly higher than that in the adjacent tissue, which was consistent with the change trend of Foxp3+Treg in the liver cancer tissue detected by flow cytometry, which was significantly higher than that in the normal tissue and the adjacent tissue. Further detection and analysis of lymphocyte subsets suggested that the absolute count and ratio of CD8+T cells in peripheral blood decreased obviously, so we believed that Foxp3+Treg play inhibitory role in T cells may be one of the mechanisms for promoting the growth of HCC.
Inhibition effect of Foxp3+Treg may be one reason of infiltrating lymphocytes’ activity and function decline in tumor microenvironment,26,33 because inflammation may be the basic and underlying causes for tumor. Professor Shalapour and Kairn,34 confirmed that the liver chronic inflammation promote cancerous by inhibiting immune surveillance function which subvert the traditional ideas about inflammatory activation to stimulate cancer cell division.35 Based on nonalcoholic fatty hepatitis (NASH) in mice tumor model who further confirmed that NASH leading to local accumulation of “immune inhibitory cells” can combine the receptors of PD-1 on the surface of effector lymphocytes such as cytotoxic T cells derived from NASH mutations excitation by secretion or expression of PD-L1. They observed a higher occurrence rate of cancer in experimental mice with low level of cytotoxic T cells and concluded that this process may promote tumor formation and development in inflammatory conditions by causing the hypofunction even incapability of T cells. 34,35 When the expression of PDL-1 was inhibited by blocking or genetic engineering methods, the activity of effector T cells was found to be enhanced which had an anti-tumor effect.36
Like Foxp3+Treg, neutrophils as the largest number of inflammatory cells infiltrating in tumor microenvironment37-39 are involved in almost in every step in the process of tumorigenesis. According to the theory of Shalaour,34 inflammation occurred earlier than cancerogenesis in the early stage or prophase of cancer development, during which the chemotaxis of neutrophils (neutrophils chemotaxis) towards the malignant and mutated sites plays the role of cytotoxicity and immune activation to inhibit the occurrence of tumor.37 In the local microenvironment after tumor development, neutrophils concentrate on the tumor matrix through polarization, promote angiogenesis and lymph angiogenesis by inhibiting the body's natural immunity,39 and reshape the extracellular matrix components, inhibit tumor specific antigen inducing antitumor response of T cells to promote the cancer proliferation, invasion, and metastasis.27,39 Therefore, studies have repeatedly suggested that the high level of local neutrophil infiltration and NLR indicates the worseness of the differentiation degree and low prognosis,12,40-44. In this study, we detected that neutrophils are highly expression in peripheral blood and tissues, and significantly higher than tissue adjacent to carcinoma. On the basis of CD15 and CD62L positive marked by flow cytometry, further labeled the PD-L1 positive subgroup (called PD-L1+NEUT), which was higher in peripheral blood and cancerous tissue synchronization, and this may be the subset of neutrophils that play an inhibitory role.
Mishalian,17 found that tumor associated neutrophils recruitment Treg in tumor microenvironment by secreting CCL17, that plays indirectly suppress on the antitumor immune responses, meanwhile, both in the tumor immune escape reaction has coordinate correlation, plays an promoting role mutually in tumor proliferation and metastasis, therefore, correlation analysis between PD-L1+NEUT and Foxp3+Treg found that the changes were positively correlated. In addition, we carried out immunohistochemical staining of PD-L1 on the cancer and found that PD-L1 expression increased significantly in the cancer tissue, at the same time the invasive cell staining and poorly differentiated HCC expression was higher than in the highly-moderately differentiation of HCC was found, so we think PD-L1+NEUT and Foxp3+Treg as suppression inhibitory of immune cells in HCC proliferation and invasion may have synergy, the change of the causal relationship and the interaction mechanism needs further study.
At the same time, we found that the NLR with poorly-differentiated HCC was significantly higher than that of patients with highly-moderately differentiated HCC in peripheral blood, and was positively correlated with the changes of PD-L1+NEUT and Foxp3+Treg, which further confirmed its inhibitory function and promoted the occurrence of HCC.
In conclusion, we believe that PD-L1+NEUT and Foxp3+Treg play an important role in the occurrence of HCC. The survival curves of the 2 groups showed that the survival time of patients in the poorly-differentiation group was significantly lower than that in the highly-moderately differentiation group, suggesting that the higher level of NLR was closely related to the worse prognosis and differentiation. High expression of PD-L1+NEUT and Foxp3+Treg significantly promoted tumor growth. Cox multifactor risk ratio model analysis suggested that preoperative NLR, PD-L1+NEUT, Foxp3+Treg were significantly correlated with the differentiation degree of patients, and NLR, PD-L1+NEUT, and Foxp3+Treg were independent risk factors for the prognosis of patients with HCC, further confirming the promoting effect of PD-L1+NEUT, Foxp3+Treg on HCC. This was a single-center retrospective study with some limitations. It needs large sample estimated with morbidity and multicenter researches on different type of cancers to provide more reliable evidence for its use as a biomarker.
The core content of tumor cell immune escape and tolerance research, the change of immune microenvironment can promote the growth of tumor, and the cell metabolism of tumor can maintain the stability of local immune microenvironment. We are conducting a prospective clinical study on this project and animal experiments which may help to clear the mechanism of PDL1+NEUT regulating Foxp3+Treg in promoting tumor growth.
Conclusions
PD-L1+NEUT, NLR, and Foxp3+Treg are independent risk factors for prognosis which can serviced as new prognostic or diagnosis marker in HCC.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338211045820 for PD-L1+NEUT, Foxp3+Treg, and NLR as New Prognostic Marker with Low Survival Benefits Value in Hepatocellular Carcinoma by Lin Zhou, Jing Wang, Shao-cheng Lyu, Li-chao Pan, Xian-jie Shi, Guo-sheng Du and Qiang He in Technology in Cancer Research & Treatment
Abbreviations
- PD-L1+NEUT
programmed death-ligand 1+Neutrophils
- NLR
neutrophil to lymphocyte ratio
- HCC
hepatocellular carcinoma
- LR
liver resection
- LT
liver transplantation
- ASCO
American Society of Clinical Oncology
- AFP
alpha fetoprotein
- FoxP3+Tregs
FoxP3+ regulatory T cells
- HCV
hepatitis C virus
- ALD
alcoholic liver disease
- CT
computed tomography
- MRI
magnetic resonance imaging
- OS
overall survival
- DFS
disease-free survival
- MFI
mean fluorescence density
- AOD
average optical
- IHC
immunohistochemistry
- CTL
cytotoxic T lymphocyte.
Footnotes
Authors’ Contributions: Lin Zhou, Xian-jie Shi, and Qiang He designed the study; Lin Zhou performed most of the experiments; Jing Wang and Shao-cheng Lyu assisted with all experiments; Li-chao Pan and Jing Wang collected the data; Lin Zhou analyzed the data; Lin Zhou, Jing Wang, and Shao-cheng Lyu drafted figures; Lin Zhou and Guo-sheng Du drafted the manuscript; Guo-sheng Du, Qiang He, and Lin Zhou made critical revisions and approved the manuscript; all authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Research & Cultivation Fund of Capital Medical University, China (Grant No. PYZ20014) and Beijing Natural Science Foundation, China (Grant No. 7212042).
Ethics Approval: This study was conducted with the approval and guidance of the Ethical Committee of PLA General Hospital and Chaoyang Hospital. All patients provided full written informed consent (Ethics approval and consent to participate: S2108-013-01).
Availability of Data and Materials: The data used and analyzed in this study are included in the article or are available from the corresponding and first authors on reasonable request.
ORCID iD: Qiang He https://orcid.org/0000-0003-2434-1275
Supplemental material: Supplemental material for this article is available online.
References
- 1.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun (Lond). 2019;39(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 3.Thomas MB, Jaffe D, Choti MM, et al. Hepatocellular carcinoma: consensus recommendations of the national cancer institute clinical trials planning meeting. J Clin Oncol. 2010;28(25):3994-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587-2596. [DOI] [PubMed] [Google Scholar]
- 5.Dutkowski P, Linecker M, Deoliveira ML, et al. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148(2):307-323. [DOI] [PubMed] [Google Scholar]
- 6.Tranchart H, Ceribelli C, Ferretti S, et al. Traditional versus robot-assisted full laparoscopic liver resection: a matched-pair comparative study. World J Surg. 2014;38(11):2904-2909. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Ruan DY, Jia CC, et al. Surgical resection versus liver transplantation for hepatocellular carcinoma within the Hangzhou criteria: a preoperative nomogram-guided treatment strategy. Hepatobiliary Pancreat Dis Int. 2017;16(5):480-486. [DOI] [PubMed] [Google Scholar]
- 8.Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947-955. [DOI] [PubMed] [Google Scholar]
- 9.Au KP, Chok K. Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: a proposed management algorithm. World J Gastroenterol. 2018;24(45):5081-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan F, Bai YH, Cui L, et al. Simultaneous transarterial chemoembolization and radiofrequency ablation for large hepatocellular carcinoma. World J Gastrointest Oncol. 2020;12(1):92-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanduzzi-Zamparelli M, Diaz-Gonzalez A, Reig M. New systemic treatments in advanced hepatocellular carcinoma. Liver Transpl. 2019;25(2):311-322. [DOI] [PubMed] [Google Scholar]
- 12.Luo P, Wu S, Yu Y, et al. Current status and perspective biomarkers in AFP negative HCC: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathol Oncol Res. 2020;26(2):599-603. [DOI] [PubMed] [Google Scholar]
- 13.Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150(7):1646-1658. e17. [DOI] [PubMed] [Google Scholar]
- 14.Zahran AM, Nafady-Hego H, Mansor SG, et al. Increased frequency and FOXP3 expression of human CD8( + )CD25(High + ) T lymphocytes and its relation to CD4 regulatory T cells in patients with hepatocellular carcinoma. Hum Immunol. 2019;80(7):510-516. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Pan LC, Zheng YG, et al. Novel strategy of sirolimus plus thymalfasin and huaier granule on tumor recurrence of hepatocellular carcinoma beyond the UCSF criteria following liver transplantation: a single center experience. Oncol Lett. 2018;16(4):4407-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L, Pan LC, Zheng YG, et al. Reduction of FoxP3( + ) Tregs by an immunosuppressive protocol of rapamycin plus Thymalfasin and Huaier extract predicts positive survival benefits in a rat model of hepatocellular carcinoma. Ann Transl Med. 2020;8(7):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishalian I, Bayuh R, Eruslanov E, et al. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17--a new mechanism of impaired antitumor immunity. Int J Cancer. 2014;135(5):1178-1186. [DOI] [PubMed] [Google Scholar]
- 18.Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kow Awc. Transplantation versus liver resection in patients with hepatocellular carcinoma. Transl Gastroenterol Hepatol. 2019;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao GS, Liu Y, Zhang Q, et al. Transarterial chemoembolization combined with huaier granule for the treatment of primary hepatic carcinoma: safety and efficacy. Medicine (Baltimore). 2017;96(29):e7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Y, Li H, Deng Y, et al. Cancer-associated fibroblasts induce PDL1 + neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9(4):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He G, Zhang H, Zhou J, et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Yao R, Zhang D, et al. Circulating neutrophils predict poor survival for HCC and promote HCC progression through p53 and STAT3 signaling pathway. J Cancer. 2020;11(13):3736-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang LY, Luo Q, Lu L, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y, Chen X, Huang ZM, et al. Increased frequency of Foxp3 + regulatory T cells in mice with hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13(8):3815-3819. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki A, Tanaka F, Mimori K, et al. Prognostic value of tumor-infiltrating FOXP3 + regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2008;34(2):173-179. [DOI] [PubMed] [Google Scholar]
- 27.Shang B, Liu Y, Jiang S, Liu Y. Prognostic value of tumor-infiltrating FoxP3 + regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5(1):15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteside TL. FOXP3 + Treg As a therapeutic target for promoting anti-tumor immunity. Expert Opin Ther Targets. 2018;22(4):353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karczewski M, Karczewski J, Kostrzewa A, et al. The role of Foxp3 + regulatory T cells in kidney transplantation. Transplant Proc. 2009;41(5):1527-1529. [DOI] [PubMed] [Google Scholar]
- 30.Singh AK, Chan JL, Seavey CN, et al. CD4 + CD25(Hi) Foxp3 + regulatory T cells in long-term cardiac xenotransplantation. Xenotransplantation. 2018;25(2):e12379. [DOI] [PubMed] [Google Scholar]
- 31.Geissler EK, Schnitzbauer AA, Zulke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter. Open-Label Phase 3 Trial. Transplantation. 2016;100(1):116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L, Du GS, Pan LC, et al. Sirolimus treatment for cirrhosis or hepatocellular carcinoma patients accompanied by psoriasis after liver transplantation: a single center experience. Oncol Lett. 2017;14(6):7817-7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathai AM, Kapadia MJ, Alexander J, et al. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol. 2012;36(7):980-986. [DOI] [PubMed] [Google Scholar]
- 34.Shalapour S, Lin XJ, Bastian IN, et al. Inflammation-induced IgA + cells dismantle anti-liver cancer immunity. Nature. 2017;551(7680):340-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inflammation-Induced IgA( + ) cells promote hepatocellular tumorigenesis. Cancer Discov 2018; 8(1):OF10. [Google Scholar]
- 36.Febbraio MA, Reibe S, Shalapour S, et al. Preclinical models for studying NASH-driven HCC: how useful are they? Cell Metab. 2019;29(1):18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huo X, Li H, Li Z, et al. Transcriptomic profiles of tumor-associated neutrophils reveal prominent roles in enhancing angiogenesis in liver tumorigenesis in zebrafish. Sci Rep. 2019;9(1):1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016, 6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. 2019;9:1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong Z, Liu L, Zheng Y, et al. Predictive value of preoperative peripheral blood neutrophil/lymphocyte ratio for lymph node metastasis in patients of resectable pancreatic neuroendocrine tumors: a nomogram-based study. World J Surg Oncol. 2017;15(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y, Liao H, Zhang Y, et al. Prognostic value of tumor-infiltrating FoxP3 + T cells in gastrointestinal cancers: a meta analysis. PLoS ONE. 2014;9(5):e94376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H, Luo G, Lu Y, et al. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology. 2016;16(6):1080-1084. [DOI] [PubMed] [Google Scholar]
- 43.Gao Y, Wang WJ, Zhi Q, et al. Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget. 2017;8(51):88835-88844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadokura M, Ishida Y, Tatsumi A, et al. Performance status and neutrophil-lymphocyte ratio are important prognostic factors in elderly patients with unresectable pancreatic cancer. J Gastrointest Oncol. 2016;7(6):982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338211045820 for PD-L1+NEUT, Foxp3+Treg, and NLR as New Prognostic Marker with Low Survival Benefits Value in Hepatocellular Carcinoma by Lin Zhou, Jing Wang, Shao-cheng Lyu, Li-chao Pan, Xian-jie Shi, Guo-sheng Du and Qiang He in Technology in Cancer Research & Treatment