Abstract
Non-small-cell lung cancer takes up the majority of lung carcinoma-caused deaths. It is reported that targeting PD-1/PD-L1, a well-known immune evasion checkpoint, can eradicate tumor. Checkpoint inhibitors, such as monoclonal antibodies, are actively employed in cancer treatment. Thus, this review aimed to assess the therapeutic and toxic effects of PD-1/PD-L1 inhibitors in treatment of NSCLC. So far, 6 monoclonal antibodies blocking PD-1/PD-L1 interaction are identified and used in clinical trials and randomized controlled trials for NSCLC therapy. These antibody-based therapies for NSCLC were collected by using search engine PubMed, and articles about the assessment of adverse events were collected by using Google search. Route of administration and dosage are critical parameters for efficient immunotherapy. Although antibodies can improve overall survival and are expected to be target-specific, they can cause systemic adverse effects in the host. Targeting certain biomarkers can limit the toxicity of adverse effects of the antibody-mediated therapy. Clinical experts with knowledge of adverse effects (AEs) of checkpoint inhibitors can help manage and reduce mortalities associated with antibody-based therapy of NSCLC.
Keywords: NSCLC, PD-1/PD-L1, checkpoint inhibitors, monoclonal antibodies, toxicity
Introduction
Lung cancer is the foremost reason for deaths related to cancer in the United States. According to statistics of the year 2019, the death toll related to cancers of respiratory system will exceed one-tenth of a million. There are several new treatment regimens endorsed by NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for the management of non-small-cell lung cancer (NSCLC)1. Cancer cells are identified by the immune system, especially T-cells for control or elimination depending on receptors that strengthen the antitumor efficacy2. Such cells take part in multiple mechanisms to prevent this immune attack. PD-1 pathway is one mechanism for immune escape in several mouse tumor models. Manipulating such mechanism can allow the immune system to eliminate tumor3.
Over the past two decades, monoclonal antibodies (mAbs) have emerged as successful therapeutic agents for cancer in a multibillion-dollar market4,5. Recently, Relatlimab, Omburtamab, Etigilimab, Enoblituzumab and Tiragolumab are actively employed mAbs as immune checkpoint inhibitors (ICIs) at clinical level6. Programmed cell death protein (PD-1), a 288 amino acid, is a surface molecule often designated as a membrane protein that is expressed on immune cells including dendritic cells. Programmed death ligand 1 (PD-L1), a major PD-1 ligand and 40-kDa type 1 transmembrane protein, is often expressed in several types of malignant tumors and is related to survival and tumor progression7,8. PD-1 can interact with PD-L1 and result in the release of signals that can regulate T-cell mediated immunity9. This interaction ensures that immune system is activated at the proper timing and thereby the chances of chronic inflammation are reduced10. Cancer cells express proteins including PD-1 on their surface to trick the immune cells and evade their detection, and this action can stop a cytolytic activity11. An increase in PD-1 expression is considered as a hallmark for exhaustion of T-cells12. Notably, according to a meta-analysis based on randomized controlled trials, higher PD-L1 level in NSCLC cells is related to better efficacy of treatment by PD-1/PD-L1 inhibitors13.
Blockade of PD-1/PD-L1 pathway can cause limited toxicity compared to CTLA-4 blockade14. Blockade using monoclonal antibodies is a recently well-studied immune checkpoint inhibition and is used as a therapy for a variety of diseases including cancers of multiple origins15. This blockade of PD-1 using antibodies is now applied to treat patients with recurrence of cancer16. Although therapeutic targeting of PD-1/PD-L1 axis using ICIs can be more efficient than conventional chemotherapy, it can cause immune system related adverse effects (irAEs) predominantly in the digestive system, and these effects can be systemic. Drug resistance emerged with the blockade by ICIs is considered as a consequence of the progress of tumor neoantigens and the increase in immune checkpoint proteins that are not related to PD-1/PD-L1 axis17–19 .
Therefore, present review focuses on therapeutic roles and toxicity of ICIs targeting the PD-1/PD-L1 axis for clinical management of NSCLC. Clinical trials and randomized controlled trials of past decade were searched from PubMed for therapy and toxicity of ICIs in management of NSCLC.
PD-1/PD-L1 Axis As A Target for Therapy of NSCLC
The blockade of PD-1 and PD-L1 ligation can relieve the dysfunction, exhaustion, and tolerance of T-cells, so it has been proved to be a successful way to fight against cancer20. Yet, the antibodies may or may not have similarity in their binding sites on PD-121. Relapse in disease can occur in patients with primary or acquired resistance to the antibody-based monotherapy and new approaches are employed recently to avoid or overcome the resistance to such therapy22. Genetic polymorphisms in PD-1 loci can increase the chances of developing several autoimmune disorders. PD-1/PD-L1 expression may vary with specific types of tumor so PD-1/PD-L1 can act as prognostic or diagnostic markers in several cancers. Therefore, several biomarkers such as elevated lymphocytes and eosinophils, low levels of circulating tumor DNA, and lactate dehydrogenase can help predict the responses to ICIs. Tumor mutational burden related to genes such as Polybromo-1 can also serve the purpose23,24.
Emerging Roles of Monoclonal Antibodies at the Nanoscale
As is known, mAbs are roughly 10 nm in size and possess unique properties. Dynamic light-scattering is used to analyze antibody formulations at the nanoscale. To serve therapeutic purposes, their affinity should be 1 nM or less for a specific antigen. They are absorbed via lymphatic system depending on convection and diffusion. These are plausible mechanisms for the uptake of mAbs25–27 . Glass and silica microparticles can adsorb mAbs via surface layering of 4-nm thickness28. Nanoparticles conjugated with antibodies are effectively delivered to their targeted site because of the size around 50 nm29. Administration of antibody-coated nanoparticles through subcutaneous route at fixed and optimized doses can improve targeted delivery and prevent irAEs30,31. Supportive of the fore-mentioned information, PD-L1 antibodies are conjugated onto copolymer nanoparticular surface and loaded with chemotherapeutic drugs to achieve targeted therapy and inhibition of PD-L1 expression in cancer cells32. Lipids, polymers and metal-based nanoparticles with an ideal size of 200 nm have been used in specific-targeting of lung cancer cells33.
Role of Biomarker Validation
Biomarkers can improve the specific targeting of tumor associated antigens and decrease systemic toxicity34. Tumor mutational burden, tissue polypeptide-specific antigen, and immunohistochemistry assays are valuable tools in biomarker validation for early detection of cancer35–38 . PD-L1 testing based on immunohistochemical platforms such as Dako and Ventana are used with four FDA approved antibodies for binding with precise epitope of PD-L1. Antibodies such as 22C3, 28–8, SP263 and SP142 are used to identify patients who can respond to immunotherapy for PD-L1 positive NSCLC39,40. Elevated expressions of PD-L1 is optimum for treatment using ICIs13. At a safe dose, combinatorial therapy of mAbs with other drugs can increase the OS of patients with NSCLC. Statuses of other oncogenes such as EGFR, ALK, KRAS, MET, ROS1, BRAF, and NTRK are critical in identification of specific ICIs for NSCLC41,42. Host microbiomes are tested and used as alternative biomarkers for NSCLC43. Also, several biomarkers are discovered to predict or influence the toxicity of PD-1/PD-L1 inhibitor-based therapy. For example, cytokines such as IL1Rα, IL-1α/β, IL-2, IL12p70, IL-13, GM-CSF, G-CSF, fractalkine, and IFN-α2 are proved as biomarkers highly expressed in patients having severe irAEs during ICI treatment44. TIM3 is proved to hinder anti-cancer immunity and regulate resistance to PD-1 and PD-L1 inhibitors45. The TLR3-specific adjuvant alleviates resistance to therapy using PD-L1 antibody without toxicity46. Targeting gut microbiota is reported to enhance efficiency and decrease toxicity of current therapy depending on various agents including anti-PD-L147.
Clinical and Randomized Controlled trials for NSCLC Therapy using ICIs Targeting the PD-1/PD-L1 Axis
Atezolizumab, avelumab, durvalumab, cemiplimab, nivolumab, and pembrolizumab are fully humanized IgG1 and IgG4 antibodies that target the PD-1/PD-L1 axis in combinatorial first-line treatment of NSCLC. They can elicit anti-tumor effects through mechanisms involving adaptive and innate immunity48–53 . The search was performed in PubMed using the filter of 2010 to 2020 for clinical trials and randomized controlled trials in treatment of NSCLC targeting the PD-1/PD-L1 axis. A total of 8 trials were identified as a result of the search. We summarized the characters, applications and toxicities of 5 antibodies from the 8 researches in Table 1. The results indicated that the median overall survival (OS) was improved in Phase 2 and 3 trials using atezolizumab by more than 4 months versus docetaxel; increasing OS was coupled with higher PD-L1 level in NSCLC patients, and the adverse event profile for atezolizumab was more favorable than docetaxel 54,55. In an open-label Phase 3 trial, avelumab improved the median OS by one month in comparison with docetaxel, which was insignificant; high PD-L1 expression in NSCLC patients was related to longer OS; and the ratio of treatment-related adverse event in patients receiving avelumab treatment was generally lower than those receiving docetaxel56. There was no report for durvalumab and cemiplimab according to the search. Yet, trials at various phases are ongoing for the treatment of NSCLC using cemiplimab57. Nivolumab had an OS of more than 40% after one year in a Phase 2, single-arm trial in NSCLC patients; the treatment-related immune-mediated adverse events after nivolumab treatment were neither frequent nor severe and the toxicity of nivolumab was weaker than toxic chemotherapy58. In combination with other chemotherapeutic interventions at Phase I level, nivolumab treatment resulted in OS of 12.2 and 17.4 months; combined use of other drugs did not lead to higher risk of nivolumab-related immune-mediated adverse events and these adverse events are generally manageable59,60. Pembrolizumab in combination with ipilimumab improved the OS compared to standard chemotherapy61. The median OS was more than 10 months, but the combined use of pembrolizumab and ipilimumab may lead to a higher risk of adverse events62.
Table 1.
Characters, application and toxicities of antibodies against PD-1/PD-L1 summarized based on 8 clinical trials.
| Antibodies | Descriptions | |
|---|---|---|
| Atezolizumab | Characters | Humanized IgG1 monoclonal anti-PD-L1 Blocks the interaction of PD-L1/PD-1 and PD-L1/B7.1 and leads to anti-tumor T-cell activity restoration and strengthened T-cell priming. |
| Application | Patients: n = 142 (Phase 2 trial); n = 609 (Phase 3 trial) Dose: 1200 mg fixed dose every 3 weeks on day 1 of each 3-week cycle |
|
| Toxicity | Phase 2 trial: Median OS: 12,6 months Patients with AEs: 96% Common AEs: Pneumonia, increased aspartate aminotransferase, increased aspartate aminotransferase, increased alanine aminotransferase, pneumonitis, colitis and hepatitis. Phase 3 trial: Median OS: 18.9 months Patients with AEs: 94% Common AEs: Fatigue, nausea, decreased appetite, and asthenia. |
|
| Avelumab | Characters | Human anti-PD-L1 IgG1 antibody Tolerable safety profile and durable anti-tumor activity. |
| Application | Patients: n = 396 Dose: 10 mg/kg for intravenous injection over 1 h every 2 weeks a time |
|
| Toxicity | Median OS: 11.4 months Patients with treatment-related AEs: 64% Common AEs: Infusion-related reaction, decreased appetite, increased lipase. |
|
| Cemiplimab | Characters | Human monoclonal antibody to PD-1 Exhibits antitumor activity with safety in phase 1 trial of malignancies at advanced stage, including NSCLC. |
| Application | Patients: n = 230 Dose: unclear dose for Q3 W (up to 108 weeks) |
|
| Toxicity | No report yet. | |
| Nivolumab | Characters | Fully human, IgG4 immune checkpoint inhibitor antibody Binds to PD-1 on the activated immune cells and leads to disruption of PD-1/PD-L1/2 interaction, so as to attenuate inhibitory signals and enhance the host anti-tumor response. |
| Application | Patients: n = 117 Dose: 3 mg/kg via intravenous infusion 2 weeks a time (1 cycle until the occurrence of unacceptable toxic effects or disease progression) Patients: n = 292 Dose: 3 mg/kg every 2 weeks Patients: n = 21 Dose: from 3 mg/kg to a flat 240 mg |
|
| Toxicity | Median OS: 8·2 months (n = 117) Patients with grade 3–4 treatment-related AEs: 17% Common AEs: Fatigue, pneumonitis, and diarrhoea. Median OS: 12.2 months (n = 292) Common AEs: Cough, decreased appetite, constipation, pruritis, fatigue, and musculoskeletal pain. Median OS: 17.4 months (n = 21) Common AEs: Injection-site reactions, flu-like symptoms, fever, fatigue, chills, nausea, and pain. |
|
| Pembrolizumab | Characters | Humanized monoclonal antibody against PD-1. Blocks the PD-1/PD-L1/2 interaction and leads to antitumor immune response reactivation. |
| Application | Patients: n = 30 Dose: a fixed dose of 200 mg three weeks a time ((Q3 W), 21 days) for 2 cycles Patients: n = 51 Dose: 10 mg/kg (n = 6) or 2mg/kg (n = 45) three weeks a time (Q3 W) for up to 2 years |
|
| Toxicity | Median OS: 10.9 months (n = 51) Treatment-related AEs: 64% Immune-mediated AEs and infusion reactions: 42% Common AEs: Fatigue, hypothyroidism, decreased appetite, diarrhea, and pruritus. |
|
References: 54-61.
Systemic Toxicity of ICIs
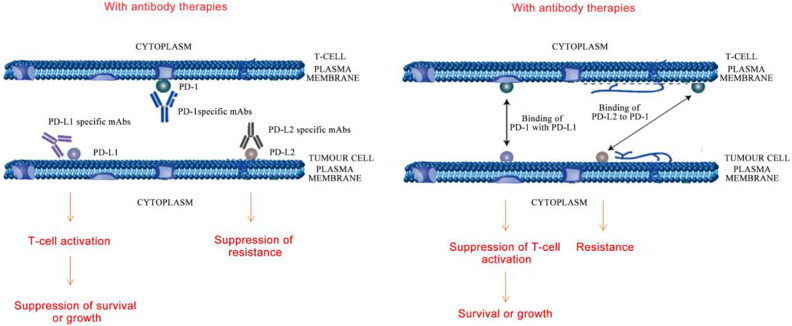
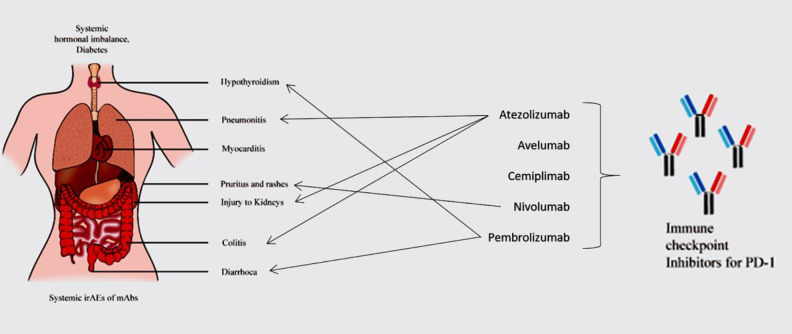
The Immune-Related Adverse Events (irAEs) of ICIs increase at higher doses63. Inhibitors of PD-1/PD-L1 axis can cause systemic toxicities. PD-1 interacts with its alternate ligand PD-L2 when its expression is elevated in certain organs. This can result in resistance, survival or growth of tumor cells by T-cell inactivation, leading to an increase in toxicity (Fig. 1). The irAEs of ICIs include colitis, diarrhoea, hormonal imbalances, hypothyroidism, diabetes, acute injury to kidneys, pneumonitis, and myocarditis. Hepatic, pulmonary, and dermatological reactions such as pruritus and rashes are also evident18,64,65,66–70 .
Figure 1.
Pictorial representation of resistance, survival or growth among tumor cells as a result of T-cell inactivation leading to irAEs. Left: Blocking PD-1/PD-L1 by PD-1 or PD-L1 mAbs caused T-cell activation and suppressed tumor cell growth or survival; blocking PD-1/PD-L2 by PD-L2 mAbs repressed tumor cell drug resistance. Right: PD-1 on T-cell bound to PD-L1 on tumor cell to suppress T-cell activation and facilitate tumor cell growth or survival; PD-1 on T-cell bound to PD-L2 on tumor cell to facilitate tumor drug resistance.
Atezolizumab can induce systemic toxicities comparable to other PD-1 inhibitors such as avelumab and durvalumab by interacting with PD-L1, but is different from the irAEs of conventional chemotherapy. This can avoid interaction of PD-1 with PD-L1. 20 mg/kg is the tolerable dose in clinical trials involving atezolizumab and avelumab intended for NSCLC therapy at Phase I50,71–74 . Nivolumab at 3 mg/kg was the optimum dose in combination with ipilimumab (1 mg/kg) for treatment of NSCLC75. The 2-year OS for this antibody (5∼10 mg/kg) did range between 25∼62% based on the combinatorial agent used76,77. Pembrolizumab can cause thyroid dysfunction and even fatal pneumonitis in NSCLC patients78,79.
Toxicity of ICIs on Different Systems of the Human Body
Fatigue of all grades is one of the common irAEs induced by ICIs80. Macules and papules are skin-related irAEs of grade 1 and 2. Vitiligo-like depigmentation is related to grade 3 or 4 symptoms81. With incidence lower than 1%, AEs of ICIs associated with cardiovascular system are myocarditis, pericarditis, cardiac fibrosis, cardiac arrest, arrhythmias, heart failure, large pericardial effusion, tamponade and Takotsubo syndrome. Electrocardiogram, troponin monitoring and diagnosis of myocarditis are baseline tools to detect abnormalities related to heart82–84 . Disorders of the coagulation-fibrinolysis system can occur85. Grade 1 to 4 diarrhoea and colitis are lower GI-based irAEs. ICIs can induce upper GI tract toxicity, resulting in decline of appetite and nausea86,87. Esophagitis, gastritis, duodenitis, and jejunitis are other upper GI tract-associated irAEs88. Thyroid dysfunction and hypophysitis are the major toxicities associated with the endocrine system, although the effects can be systemic89. Pneumonitis is a late grade irAE in NSCLC patients90. Other pulmonary events such as dyspnoea, hypoxia and lung opacities were also observed91. Polyneuropathy, myasthenia gravis, Bell’s palsy and encephalopathy are neurological irAEs92. Loss of skeletal muscle mass and proteinuria are other irAEs of ICIs for NSCLC (Fig. 2). These irAEs are enlisted in Table 2.
Figure 2.
Systemic toxicity of Immune-Related Adverse Events (irAEs). Toxicity to multiple human organ systems caused by inhibitors for PD-1 block are presented and several of them are reported to be the toxicity of mAbs reviewed in this article.
Table 2.
Systemic Toxicity of ICIs.
| Organ systems | Adverse effects |
|---|---|
| Integumentary system | Grade 1 and 2 macules and papules. Grade 3 or 4 related vitiligo-like depigmentation |
| Cardiovascular system | Disorders of the coagulation-fibrinolysis, myocarditis, pericarditis, cardiac fibrosis, cardiac arrest, arrhythmias, heart failure, large pericardial effusion, tamponade and Takotsubo syndrome |
| Digestive system | Diarrhoea, colitis, decline of appetite and nausea, esophagitis, gastritis, duodenitis, and jejunitis |
| Endocrine system | Thyroid dysfunction and hypophysitis |
| Respiratory system | Pneumonitis, dyspnoea, hypoxia and lung opacities |
| Nervous system | Polyneuropathy, myasthenia gravis, Bell’s palsy and encephalopathy |
| Skeletal system | Loss of skeletal muscle mass |
| Other adverse effects | Fatigue and proteinuria |
References: 76-90
The toxicities associated with immunotherapy may be autoimmune (on target, off-tumor toxicity) or cytokine-associated. The mechanisms related to such toxicities may vary from the reactions similar to immune system-related allergies to the auto-immune reactions, such as reactions observed in chemotherapy or the entry of T-cells into central nervous system. There are several tests that individually identify the irAEs specific for each organ by detecting the specific antigens on toxicity-induced organs using specific antibodies. Steroids and other immunosuppressive agents are used for management of such irAEs93,94.
Challenges and Future Directions for Therapy of NSCLC using Checkpoint Inhibitors
Resistance of NSCLC cells to available drugs can limit the therapeutic potential of a drug and therefore requires proper management to control progression95. Immune escape and evasion in NSCLC patients play a critical role in cancer progression96. Amplification of tumor-infiltrating lymphocytes after collection from the patient, growth at laboratory scale and giving back can boost the immune system97,98. Clinicians are aware that the management of irAEs of ICIs can follow guidelines for cancer care and the challenges imposed99. Early detection is critical to the management of increase in systemic toxicity and mortality100.
Peptide antagonists for PD-1 and its ligand PD-L1 are emerging alternatives for cancer therapy and a few examples have been elucidated101,102. A novel peptide RK-10 identified from RCSB protein data bank has been known to detect PD-L1 in several circulating and tumor cells and tissues of patients suffering from various cancers103. T-cell therapy allowing the same cells to secrete certain peptide fragments may improve both safety and anti-tumor efficacy as such peptides can stay localized to tumor cells104. Non-blocking antibodies for PD-1 possess anti-tumor effect similar to that of anti-PD-1 monotherapy using blocking antibodies105. An interesting study suggests that on-target lifespan of anti-PD-1 antibodies is much shorter than usually expected on the tumor-infiltrating T-cells. According to the authors, second generation antibodies can be a fascinating approach for they have much more specific Fc regions that possess extended binding to such T-cells106.
Analyzing the composition of gut microbiota is a key point in determining the therapeutic efficacy of antibodies against PD-1/PD-L1. Fecal microbiome could improve sensitivity of anti-PD-L1 therapy which differs between melanoma patients who respond to therapy and who do not. Such patients had unique microbiome, and enhanced immunity was observed in mice transplanted with fecus of patients who responded to checkpoint blockade immunotherapy107.
Therefore, novel therapeutic regimens with specific targeting capacity are mandatory for prolonging the survival among NSCLC patients108.
Conclusions
NSCLC can be treated efficiently by targeting the PD-1/PD-L1 axis using checkpoint inhibitors such as monoclonal antibodies. Atezolizumab, avelumab, durvalumab, cemiplimab, nivolumab, and pembrolizumab are fully humanized antibodies used in clinical trials and randomized controlled trials for NSCLC therapy. Treatment modalities are critical parameters in targeted therapy. Identification of biomarkers and targeted therapy can help in limiting the toxicity of adverse effects of antibody-mediated therapy. Further research in identifying checkpoint inhibitors can help eradicate cancers of various origins.
Supplemental Material
Supplemental Material, sj-docx-1-cll-10.1177_09636897211041587 for Therapeutic and Systemic Adverse Events of Immune Checkpoint Inhibitors Targeting the PD-1/PD-L1 axis for Clinical Management of NSCLC by Jing Chen, Yaser Alduais and Baoan Chen in Cell Transplantation
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial statement for research, authorship and/or publication of thiu article: The work was supported by National Natural Science Foundation of China (No.81903091), the Fundamental Research Funds for the Central Universities (No.2242020R20006) and the Jiangsu Planned Projects for Postdoctoral Research Funds.
ORCID iD: Baoan Chen  https://orcid.org/0000-0003-1490-1259
https://orcid.org/0000-0003-1490-1259
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D’Amico TA, et al. NCCN Guidelines insights: non-small cell lung cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464–1472. [DOI] [PubMed] [Google Scholar]
- 2.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94(1):41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dotti G. Blocking PD-1 in cancer immunotherapy. Blood. 2009;114(8):1457–1458. [DOI] [PubMed] [Google Scholar]
- 4.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 5.Rajewsky K. The advent and rise of monoclonal antibodies. Nature. 2019;575(7781):47–49. [DOI] [PubMed] [Google Scholar]
- 6.Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamane H, Isozaki H, Takeyama M, Ochi N, Kudo K, Honda Y, Yamagishi T, Kubo T, Kiura K, Takigawa N. Programmed cell death protein 1 and programmed death-ligand 1 are expressed on the surface of some small-cell lung cancer lines. Am J Cancer Res. 2015;5(4):1553–1557. [PMC free article] [PubMed] [Google Scholar]
- 8.Escors D, Gato-Cañas M, Zuazo M, Arasanz H, García-Granda MJ, Vera R, Kochan G. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct Target Ther. 2018;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. [DOI] [PubMed] [Google Scholar]
- 10.Bose CK. Immune checkpoints, their control by immunotherapy and ovarian cancer. Contemp Oncol (Pozn). 2017;21(3):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carotta S., Targeting NK.Cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol. 2016;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kareva I. Chapter 6 -cancer as a systemic disease that requires a systemic approach. In: Kareva I, ed. Understanding Cancer from a Systems Biology Point of View: Academic Press; 2018. p 79–90. [Google Scholar]
- 13.Xu Y, Wan B, Chen X, Zhan P, Zhao Y, Zhang T, Liu H, Afzal MZ, Dermime S, Hochwald SN, Hofman P, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res. 2019;8(4):413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, He X, Lv Q, Jing J, Shi H.Management of adverse events in cancer patients treated with PD-1/PD-L1 blockade: focus on Asian populations. Front Pharmacol. 2019;10:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblatt J, Avigan D. Targeting the PD-1/PD-L1 axis in multiple myeloma: a dream or a reality™ Blood. 2017;129(3):275–279. [DOI] [PubMed] [Google Scholar]
- 16.Smith K, Chiu A, Parikh R, Yahalom J, Younes A., Chapter 75 -Hodgkin lymphoma: clinical manifestations, staging, and therapy. In: Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, Salama ME, Abutalib SA, editors. Hematology (Seventh Edition: ): Elsevier; 2018. p 1212–1229. [Google Scholar]
- 17.Ahmed M.Checkpoint inhibitors: What gastroenterologists need to know. World J Gastroenterol. 2018;24(48):5433–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajwa R, Cheema A, Khan T, Amirpour A, Paul A, Chaughtai S, Patel S, Patel T, Bramson J, Gupta V, Levitt M, et al. Adverse effects of immune checkpoint inhibitors (programmed death-1 inhibitors and cytotoxic T-lymphocyte-associated protein-4 inhibitors): results of a retrospective study. J Clin Med Res. 2019;11(4):225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyers DE, Bryan PM, Banerji S, Morris DG. Targeting the PD-1/PD-L1 axis for the treatment of non-small-cell lung cancer. Curr Oncol. 2018;25(4):e324–e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response™ Front Immunol. 2017;8:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowicki TS, Hu-Lieskovan S, Ribas A. Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J. 2018;24(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seidel JA, Otsuka A, Kabashima K.Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavacchi D, Pellegrini E, Palmieri VE, Doni L, Mela MM, Di Maida F, Amedei A, Pillozzi S, Carini M, Antonuzzo L. Immune checkpoint inhibitors in the treatment of renal cancer: current state and future perspective. Int J Mol Sci. 2020;21(13):4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas VA, Balthasar JP. Understanding inter-individual variability in monoclonal antibody disposition. Antibodies (Basel). 2019;8(4):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razinkov VI, Treuheit MJ, Becker GW. Accelerated formulation development of monoclonal antibodies (mAbs) and mAb-based modalities: review of methods and tools. J Biomol Screen. 2015;20(4):468–483. [DOI] [PubMed] [Google Scholar]
- 28.Le Basle Y, Chennell P, Tokhadze N, Astier A, Sautou V.Physicochemical stability of monoclonal antibodies: a review. J Pharm Sci. 2020;109(1):169–190. [DOI] [PubMed] [Google Scholar]
- 29.Villanueva-Flores F, Castro-Lugo A, Ramírez OT, Palomares LA. Understanding cellular interactions with nanomaterials: towards a rational design of medical nanodevices. Nanotechnology. 2020;31(13):132002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awwad S, Angkawinitwong U. Overview of antibody drug delivery. Pharmaceutics. 2018;10(3):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oltolina F, Colangelo D, Miletto I, Clemente N, Miola M, Verné E, Prat M, Follenzi A. Tumor targeting by monoclonal antibody functionalized magnetic nanoparticles. Nanomaterials (Basel). 2019;9(11):1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu S, Cui F, Huang D, Zhang D, Zhu A, Sun X, Cao Y, Ding S, Wang Y, Gao E, Zhang F. PD-L1 monoclonal antibody-conjugated nanoparticles enhance drug delivery level and chemotherapy efficacy in gastric cancer cells. Int J Nanomedicine. 2019;14:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babu A, Templeton AK, Munshi A, Ramesh R.Nanoparticle-based drug delivery for therapy of lung cancer: progress and challenges. J Nanomater. 2013;2013:863951. [Google Scholar]
- 34.Liu D. Cancer biomarkers for targeted therapy. Biomark Res. 2019;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim SM, Hong MH, Kim HR. Immunotherapy for non-small cell lung cancer: current landscape and future perspectives. Immune Netw. 2020;20(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howat WJ, Lewis A, Jones P, Kampf C, Pontén F, van der Loos CM, Gray N, Womack C, Warford A. Antibody validation of immunohistochemistry for biomarker discovery: recommendations of a consortium of academic and pharmaceutical based histopathology researchers. Methods. 2014;70(1):34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klempner SJ, Fabrizio D, Bane S, Reinhart M, Peoples T, Ali SM, Sokol ES, Frampton G, Schrock AB, Anhorn R, Reddy P.Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: a review of current evidence. Oncologist. 2020;25(1):e147–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Pascual J, Ayuso-Sacido A, Belda-Iniesta C, Resistance CD. Drug resistance in cancer immunotherapy: new strategies to improve checkpoint inhibitor therapies. Cancer Drug Resist. 2019;2:980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ancevski Hunter K, Socinski MA, Villaruz LC. PD-L1 testing in guiding patient selection for PD-1/PD-L1 inhibitor therapy in lung cancer. Mol Diagn Ther. 2018;22(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ionescu DN, Downes MR, Christofides A, Tsao MS. Harmonization of PD-L1 testing in oncology: a Canadian pathology perspective. Curr Oncol. 2018;25(3):e209–e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elkrief A, Joubert P, Florescu M, Tehfe M, Blais N, Routy B. Therapeutic landscape of metastatic non-small-cell lung cancer in Canada in 2020. Curr Oncol. 2020;27(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui H, Ma N, Wang Y, Li H, Liu X, Su Y, Yang J.Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res. 2018;2018:6984948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teixidó C, Vilariño N, Reyes R, Reguart N. PD-L1 expression testing in non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758835918763493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, Breen EJ, Yang JYH, Ghazanfar S, Kefford RF, Scolyer RA, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res. 2019;25(5):1557–1563. [DOI] [PubMed] [Google Scholar]
- 45.Harding JJ, Moreno V, Bang YJ, Hong MH, Patnaik A, Trigo J, Szpurka AM, Yamamoto N, Doi T, Fu S, Calderon B, et al. Blocking TIM-3 in treatment-refractory advanced solid tumors: a phase Ia/b study of LY3321367 with or without an Anti-PD-L1 antibody. Clin Cancer Res. 2021;27(8):2168–2178. [DOI] [PubMed] [Google Scholar]
- 46.Takeda Y, Kataoka K, Yamagishi J, Ogawa S, Seya T, Matsumoto M. A TLR3-specific adjuvant relieves innate resistance to PD-L1 blockade without cytokine toxicity in tumor vaccine immunotherapy. Cell Rep. 2017;19(9):1874–1887. [DOI] [PubMed] [Google Scholar]
- 47.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14(6):356–365. [DOI] [PubMed] [Google Scholar]
- 48.Yuan M, Huang LL, Chen JH, Wu J, Xu Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther. 2019;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R, Xia W, Wang S, Xu Y, Ma Z, Xu W, Zhang E, Wang J, Fang T, Zhang Q, Dong G, et al. Long noncoding RNA SBF2-AS1 Is critical for tumorigenesis of early-stage lung adenocarcinoma. Mol Ther Nucleic Acids. 2019;16:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mezquita L, Planchard D. Durvalumab for the treatment of non-small cell lung cancer. Expert Rev Respir Med. 2018;12(8):627–639. [DOI] [PubMed] [Google Scholar]
- 52.Chen YM. Immune checkpoint inhibitors for nonsmall cell lung cancer treatment. J Chin Med Assoc. 2017;80(1):7–14. [DOI] [PubMed] [Google Scholar]
- 53.Sezer A, Gogishvili M, Bentsion D, Kilickap S, Lowczak A, Gumus M, Gladkov O, Clingan P, Sriuranpong V, Rizvi N, Lee S, et al. P2.01-01 cemiplimab, a human pd-1 monoclonal antibody, versus chemotherapy in first-line treatment of advanced NSCLC with PD-L1 ≥50%. J Thoracic Oncology. 2019;14(10):S638. [Google Scholar]
- 54.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. [DOI] [PubMed] [Google Scholar]
- 55.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. [DOI] [PubMed] [Google Scholar]
- 57.Rizvi N, Lee S, Curtis P, Caldwell W, Gao B, Rietschel P.P3.04-25 EMPOWER-Lung 3: a phase 3 study of Cemiplimab, Ipilimumab and Chemotherapy in advanced NSCLC with PD-L1 <50%. J Thoracic Oncology. 2018;13(10):S931. [Google Scholar]
- 58.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, Miller JS, Farhad M, Anderton K, Lindsey K, Taffaro-Neskey M, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2018;19(5):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eichhorn F, Klotz LV, Bischoff H, Thomas M, Lasitschka F, Winter H, Hoffmann H, Eichhorn ME. Neoadjuvant anti-programmed death-1 immunotherapy by Pembrolizumab in resectable nodal positive stage II/IIIa non-small-cell lung cancer (NSCLC): the NEOMUN trial. BMC Cancer. 2019;19(1):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gubens MA, Sequist LV, Stevenson JP, Powell SF, Villaruz LC, Gadgeel SM, Langer CJ, Patnaik A, Borghaei H, Jalal SI, Fiore J, et al. Pembrolizumab in combination with ipilimumab as second-line or later therapy for advanced non-small-cell lung cancer: KEYNOTE-021 cohorts D and H. Lung Cancer. 2019;130:59–66. [DOI] [PubMed] [Google Scholar]
- 63.Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abu-Sbeih H, Ali FS, Wang Y. Immune-checkpoint inhibitors induced diarrhea and colitis: a review of incidence, pathogenesis and management. Curr Opin Gastroenterol. 2020;36(1):25–32. [DOI] [PubMed] [Google Scholar]
- 65.Kuo JR, Davis AD, Rodriguez EA, Vela MF, Heigh RI, Salomao MA, Gurudu SR. Severe diarrhea in the setting of immune checkpoint inhibitors. Case Rep Gastroenterol. 2018;12(3):704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyawaki E, Kenmotsu H. [Pneumonitis Induced by Immune Checkpoint Inhibitors]. Gan To Kagaku Ryoho. 2018;45(7):1021–1026. [PubMed] [Google Scholar]
- 67.Patel AB, Pacha O. Skin reactions to immune checkpoint inhibitors. Adv Exp Med Biol. 2018;995:117–129. [DOI] [PubMed] [Google Scholar]
- 68.Sibaud V.Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. [DOI] [PubMed] [Google Scholar]
- 69.Shankar B, Naidoo J. PD-1 and PD-L1 inhibitor toxicities in non-small cell lung cancer. J Thorac Dis. 2018;10(Suppl 33):S4034–S4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin G, Tu X, Li H, Cao P, Chen X, Song J, Han H, Li Y, Guo B, Yang L, Yan P, et al. Long Noncoding RNA p53-stabilizing and activating RNA promotes p53 signaling by inhibiting heterogeneous nuclear ribonucleoprotein K deSUMOylation and suppresses hepatocellular carcinoma. Hepatology. 2020;71(1):112–129. [DOI] [PubMed] [Google Scholar]
- 71.Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akamine T, Toyokawa G, Tagawa T, Seto T. Atezolizumab in non-squamous non-small cell lung cancer. J Thorac Dis. 2018;10(Suppl 26):S3155–S3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spagnuolo A, Gridelli C. ″Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer″: is there a substantial difference or not™ J Thorac Dis. 2018;10(Suppl 33):S4065–S4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gil-Bazo I. Avelumab-a new programmed death-ligand 1 inhibitor against advanced non-small cell lung cancer. Transl Lung Cancer Res. 2017;6(Suppl 1):S35–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, Shepherd FA, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie SA, Goldman JW, Shepherd FA, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction™ J Immunother Cancer. 2016;4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Möller M, Turzer S, Schütte W, Seliger B, Riemann D. Blood immune cell biomarkers in patient with lung cancer undergoing treatment with checkpoint blockade. J Immunother. 2020;43(2):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cortellini A, Vitale MG, De Galitiis F, Di Pietro FR, Berardi R, Torniai M, De Tursi M, Grassadonia A, Di Marino P, Santini D, Zeppola T, et al. Early fatigue in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: an insight from clinical practice. J Transl Med. 2019;17(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamińska-Winciorek G, Cybulska-Stopa B, Lugowska I, Ziobro M, Rutkowski P. Principles of prophylactic and therapeutic management of skin toxicity during treatment with checkpoint inhibitors. Postepy Dermatol Alergol. 2019;36(4):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kennedy LB, Salama AKS. A review of immune-mediated adverse events in melanoma. Oncol Ther. 2019;7(2):101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen DY, Huang WK, Chien-Chia Wu V, Chang WC, Chen JS, Chuang CK, Chu PH. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: a review when cardiology meets immuno-oncology. J Formos Med Assoc. 2020;119(10):1461–1475. [DOI] [PubMed] [Google Scholar]
- 84.Varricchi G, Marone G, Mercurio V, Galdiero MR, Bonaduce D, Tocchetti CG. Immune checkpoint inhibitors and cardiac toxicity: an emerging issue. Curr Med Chem. 2018;25(11):1327–1339. [DOI] [PubMed] [Google Scholar]
- 85.Sato R, Imamura K, Sakata S, Ikeda T, Horio Y, Iyama S, Akaike K, Hamada S, Jodai T, Nakashima K, Ishizuka S, et al. Disorder of coagulation-fibrinolysis system: an emerging toxicity of Anti-PD-1/PD-L1 monoclonal antibodies. J Clin Med. 2019;8(6):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei W, Luo Z.Risk of gastrointestinal toxicities with PD-1 inhibitors in cancer patients: a meta-analysis of randomized clinical trials. Medicine (Baltimore). 2017;96(48): e8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang ZH, Shen L. Management of gastrointestinal adverse events induced by immune-checkpoint inhibitors. Chronic Dis Transl Med. 2018;4(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Onuki T, Morita E, Sakamoto N, Nagai Y, Sata M, Hagiwara K. Severe upper gastrointestinal disorders in pembrolizumab-treated non-small cell lung cancer patient. Respirol Case Rep. 2018;6(6):e00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferrari SM, Fallahi P, Elia G, Ragusa F, Ruffilli I, Patrizio A, Galdiero MR, Baldini E, Ulisse S, Marone G, Antonelli A. Autoimmune endocrine dysfunctions associated with cancer immunotherapies. Int J Mol Sci. 2019;20(10):2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Remon J, Mezquita L, Corral J, Vilariño N, Reguart N. Immune-related adverse events with immune checkpoint inhibitors in thoracic malignancies: focusing on non-small cell lung cancer patients. J Thorac Dis. 2018;10(Suppl 13):S1516–s1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zugazagoitia J, Molina-Pinelo S, Lopez-Rios F, Paz-Ares L. Biological therapies in nonsmall cell lung cancer. Eur Respir J. 2017;49(3): 1601520. [DOI] [PubMed] [Google Scholar]
- 92.Mirabile A, Brioschi E, Ducceschi M, Piva S, Lazzari C, Bulotta A, Viganò MG, Petrella G, Gianni L, Gregorc V. PD-1 Inhibitors-related neurological toxicities in patients with non-small-cell lung cancer: A literature review. Cancers (Basel). 2019;11(3):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer. 2016;54:139–148. [DOI] [PubMed] [Google Scholar]
- 94.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsui DWY, Murtaza M, Wong ASC, Rueda OM, Smith CG, Chandrananda D, Soo RA, Lim HL, Goh BC, Caldas C, Forshew T, et al. Dynamics of multiple resistance mechanisms in plasma DNA during EGFR-targeted therapies in non-small cell lung cancer. EMBO Mol Med. 2018;10(6):e7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sacher AG, Gandhi L. PD-1 and PD-L1 inhibitors in advanced non-small cell lung cancer—promising agents and evolving questions. Oncology & Hematology Review (US). 2015;11(1):36. [Google Scholar]
- 97.Lee HJ, Kim YA, Sim CK, Heo SH, Song IH, Park HS, Park SY, Bang WS, Park IA, Lee M, Lee JH, et al. Expansion of tumor-infiltrating lymphocytes and their potential for application as adoptive cell transfer therapy in human breast cancer. Oncotarget. 2017;8(69):113345–113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.García-Martínez E, Gil GL, Benito AC, González-Billalabeitia E, Conesa MA, García García T, García-Garre E, Vicente V, Ayala de la Peña F. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16(6):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trinh S, Le A, Gowani S, La-Beck NM. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia Pac J Oncol Nurs. 2019;6(2):154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fessas P, Possamai LA, Clark J, Daniels E, Gudd C, Mullish BH, Alexander JL, Pinato DJ. Immunotoxicity from checkpoint inhibitor therapy: clinical features and underlying mechanisms. Immunology. 2020;159(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li C, Zhang N, Zhou J, Ding C, Jin Y, Cui X, Pu K, Zhu Y. Peptide blocking of PD-1/PD-L1 interaction for cancer immunotherapy. Cancer Immunol Res. 2018;6(2):178–188. [DOI] [PubMed] [Google Scholar]
- 102.Chang HN, Liu BY, Qi YK, Zhou Y, Chen YP, Pan KM, Li WW, Zhou XM, Ma WW, Fu CY, Qi YM, et al. Blocking of the PD-1/PD-L1 interaction by a D-Peptide antagonist for cancer immunotherapy. Angew Chem Int Ed Engl. 2015;54(40):11760–11764. [DOI] [PubMed] [Google Scholar]
- 103.Caldwell C, Jr, Johnson CE, Balaji VN, Balaji GA, Hammer RD, Kannan R. Identification and validation of a PD-L1 binding peptide for determination of PDL1 expression in tumors. Sci Rep. 2017;7(1):13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, Yan S, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Craig F, Juan-Luis L-V, Victor J, Celine P, Alex F, Navina R, Thibaut D, Line E-L, Madeleine S, Khalid O, Raphael G, et al. Novel anti-PD-1 antibodies not acting through PD-1/pdl-1 blockade that enhance tumor clearance. J Clin Oncol. 2018;36(15_suppl):e15118–e15118. [Google Scholar]
- 106.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9(389):eaal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang C, Leighl NB, Wu YL, Zhong WZ. Emerging therapies for non-small cell lung cancer. J Hematol Oncol. 2019;12(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-cll-10.1177_09636897211041587 for Therapeutic and Systemic Adverse Events of Immune Checkpoint Inhibitors Targeting the PD-1/PD-L1 axis for Clinical Management of NSCLC by Jing Chen, Yaser Alduais and Baoan Chen in Cell Transplantation