Abstract
Background
Hepatocellular carcinoma occurs mostly in people with chronic liver disease and ranks sixth in terms of global incidence of cancer, and fourth in terms of cancer deaths. In clinical practice, computed tomography (CT) is used as a second‐line diagnostic imaging modality to confirm the presence of focal liver lesions suspected as hepatocellular carcinoma on prior diagnostic test such as abdominal ultrasound or alpha‐foetoprotein, or both, either in surveillance programmes or in clinical settings. According to current guidelines, a single contrast‐enhanced imaging study CT or magnetic resonance imaging (MRI) showing typical hallmarks of hepatocellular carcinoma in people with cirrhosis is valid to diagnose hepatocellular carcinoma. However, a significant number of hepatocellular carcinomas do not show typical hallmarks on imaging modalities, and hepatocellular carcinoma is, therefore, missed. There is no clear evidence of the benefit of surveillance programmes in terms of overall survival: the conflicting results can be a consequence of inaccurate detection, ineffective treatment, or both. Assessing the diagnostic accuracy of CT may clarify whether the absence of benefit could be related to underdiagnosis. Furthermore, an assessment of the accuracy of CT in people with chronic liver disease, who are not included in surveillance programmes is needed for either ruling out or diagnosing hepatocellular carcinoma.
Objectives
Primary: to assess the diagnostic accuracy of multidetector, multiphasic contrast‐enhanced CT for the diagnosis of hepatocellular carcinoma of any size and at any stage in adults with chronic liver disease, either in a surveillance programme or in a clinical setting.
Secondary: to assess the diagnostic accuracy of CT for the diagnosis of resectable hepatocellular carcinoma in adults with chronic liver disease.
Search methods
We searched the Cochrane Hepato‐Biliary Trials Register, Cochrane Hepato‐Biliary Diagnostic‐Test‐Accuracy Studies Register, the Cochrane Library, MEDLINE, Embase, LILACS, Science Citation Index Expanded, and Conference Proceedings Citation Index – Science until 4 May 2021. We applied no language or document‐type restrictions.
Selection criteria
Studies assessing the diagnostic accuracy of CT for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease, with cross‐sectional designs, using one of the acceptable reference standards, such as pathology of the explanted liver and histology of resected or biopsied focal liver lesion with at least a six‐month follow‐up.
Data collection and analysis
At least two review authors independently screened studies, extracted data, and assessed the risk of bias and applicability concerns, using the QUADAS‐2 checklist. We presented the results of sensitivity and specificity, using paired forest plots, and tabulated the results. We used a hierarchical meta‐analysis model where appropriate. We presented uncertainty of the accuracy estimates using 95% confidence intervals (CIs). We double‐checked all data extractions and analyses.
Main results
We included 21 studies, with a total of 3101 participants. We judged all studies to be at high risk of bias in at least one domain because most studies used different reference standards, often inappropriate to exclude the presence of the target condition, and the time‐interval between the index test and the reference standard was rarely defined. Regarding applicability in the patient selection domain, we judged 14% (3/21) of studies to be at low concern and 86% (18/21) of studies to be at high concern owing to characteristics of the participants who were on waiting lists for orthotopic liver transplantation.
CT for hepatocellular carcinoma of any size and stage: sensitivity 77.5% (95% CI 70.9% to 82.9%) and specificity 91.3% (95% CI 86.5% to 94.5%) (21 studies, 3101 participants; low‐certainty evidence).
CT for resectable hepatocellular carcinoma: sensitivity 71.4% (95% CI 60.3% to 80.4%) and specificity 92.0% (95% CI 86.3% to 95.5%) (10 studies, 1854 participants; low‐certainty evidence).
In the three studies at low concern for applicability (861 participants), we found sensitivity 76.9% (95% CI 50.8% to 91.5%) and specificity 89.2% (95% CI 57.0% to 98.1%).
The observed heterogeneity in the results remains mostly unexplained. The sensitivity analyses, which included only studies with clearly prespecified positivity criteria and only studies in which the reference standard results were interpreted without knowledge of the results of the index test, showed no variation in the results.
Authors' conclusions
In the clinical pathway for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease, CT has roles as a confirmatory test for hepatocellular carcinoma lesions, and for staging assessment. We found that using CT in detecting hepatocellular carcinoma of any size and stage, 22.5% of people with hepatocellular carcinoma would be missed, and 8.7% of people without hepatocellular carcinoma would be unnecessarily treated. For resectable hepatocellular carcinoma, we found that 28.6% of people with resectable hepatocellular carcinoma would improperly not be resected, while 8% of people without hepatocellular carcinoma would undergo inappropriate surgery. The uncertainty resulting from the high risk of bias in the included studies and concerns regarding their applicability limit our ability to confidently draw conclusions based on our results.
Keywords: Adult; Humans; Carcinoma, Hepatocellular; Carcinoma, Hepatocellular/diagnostic imaging; Cross-Sectional Studies; Liver Neoplasms; Liver Neoplasms/diagnostic imaging; Sensitivity and Specificity; Tomography, X-Ray Computed; Ultrasonography
Plain language summary
How accurate are computerised tomography (CT) scans for detecting liver cancer?
Key messages
In people with chronic liver disease,
· computerised tomography (CT: cross‐sectional scans inside the body) probably misses liver cancer in 22.5% of people who would not receive timely or appropriate treatment, and also, CT incorrectly finds liver cancer in 8.7% of people who would receive unnecessary treatment.
· CT probably misses liver cancer in 28.6% of people with liver cancer who could have surgery to remove part of their liver, and CT incorrectly finds liver cancer in 7.7% of people who undergo inappropriate surgery.
· The studies were too different from each other to allow us to draw firm conclusions based on the evidence.
Why is it important to diagnose liver cancer accurately?
Liver cancer, or ‘hepatocellular carcinoma’ occurs mostly in people with chronic liver disease, regardless of the cause. It is the sixth most common cancer in the world and the fourth most common cause of death due to cancer. It is difficult to diagnose because early symptoms are similar to those of liver disease. People with blood test or ultrasound results that suggest liver cancer may go on to have further tests, such as scans that produce images of the liver, or biopsy where a small piece of the liver is removed and examined. If liver cancer is detected early, people may be treated with surgery to remove part of the liver (a liver resection) or with a liver transplant. If the liver cancer is more advanced, people may need chemotherapy. If liver cancer is missed, people will not receive appropriate treatment. However, incorrectly diagnosing liver cancer when it is not present means that people may undergo unnecessary testing or treatment.
What is computed tomography and how might it diagnose liver cancer?
Computed tomography (CT) produces images that show a cross‐section or ‘slice’ of the bones, blood vessels and tissues inside the body. The images consist of a series of X‐rays that are directed and combined by a computer. CT scans can detect the presence of abnormalities in the liver that might be cancer. Current guidelines recommend using either CT or another type of imaging, magnetic resonance imaging (MRI), to confirm the presence of liver cancer in people who might have liver cancer, and to judge the size and spread (stage) of the cancer.
What did we want to find out?
We wanted to find out if CT is accurate enough to diagnose liver cancer in adults with chronic liver disease. We were interested firstly, in liver cancers of any size and stage and secondly, in liver cancers that were suitable for resection.
What did we do?
We searched for studies that assessed the accuracy of CT scans compared to the best available tests to confirm liver cancer in adults with chronic liver disease. The best available tests are examination of the liver, or part of the liver under a microscope.
What did we find?
We found a total of 21 studies with 3101 people.
Based on the studies, around 520 (52%) out of 1000 adults with chronic liver disease have confirmed liver cancer. Of these 1000 people, CT may:
· correctly detect liver cancer in 403 people
· miss liver cancer in 117 people
· incorrectly detect liver cancer in 42 cancer‐free people
· correctly detect no liver cancer in 438 people.
Based on the studies, around 350 (35%) out of 1000 adults with chronic liver disease have confirmed resectable liver cancer. Of these 1000 people, CT may:
· correctly detect resectable liver cancer in 250 people
· miss resectable liver cancer in 100 people
· incorrectly detect resectable liver cancer in 50 people; and
· correctly detect no resectable liver cancer in 600 people.
What are the limitations of the evidence?
Our confidence in the evidence is limited because the studies used different methods to select study participants and used different definitions for the presence of liver disease. This means CT scans could be more or less accurate than suggested by the evidence.
How up to date is this evidence?
The evidence is up to date to 4 May 2021.
Summary of findings
Summary of findings 1. Diagnostic accuracy of computed tomography for the diagnosis of hepatocellular carcinoma.
| Review question: what is the diagnostic accuracy of CT for the diagnosis of HCC in people with chronic liver disease? | |||||||||
| Population: adults with chronic liver disease | |||||||||
| Setting: clinical setting (secondary or tertiary care setting) or surveillance programmes | |||||||||
| Study design: prospective and retrospective cross‐sectional studies | |||||||||
| Index test: CT | |||||||||
| Target condition: HCC of any size, any stage | |||||||||
Reference standards
| |||||||||
Limitations in the evidence: risk of bias and applicability concerns
| |||||||||
| Findings | |||||||||
| Implications in a hypothetical cohort of 1000 people | |||||||||
| Index test | Number of studies (participants) |
Sensitivity (95% CI) |
Specificity (95% CI) |
Prevalencea% | True positives will receive appropriate treatment (surgery or local ablative therapy or systemic chemotherapy) | False negatives will be misdiagnosed and not receive appropriate treatment | True negatives will not undergo inappropriate treatment or unnecessary further testing | False positives will undergo inappropriate treatment | Certainty of the evidence |
| CT | 21 (3101) |
77.5% (70.9% to 82.9%) | 91.3% (86.5% to 94.5%) | 20 | 155 | 45 | 730 | 70 | Lowb |
| 52 | 403 | 117 | 438 | 42 | |||||
| 60 | 465 | 135 | 365 | 35 | |||||
| CI: confidence interval; CT: computed tomography; HCC: hepatocellular carcinoma | |||||||||
aWe chose for exemplification three values of hepatocellular carcinoma prevalence: 20% for a population with low clinical suspicion, 52% as a median derived from our study analysis, and 60% for population with high clinical suspicion (assessment of nodules detected by ultrasound). bDowngraded by two levels: for risk of bias, and indirectness.
Summary of findings 2. Diagnostic accuracy of computed tomography for the diagnosis of resectable hepatocellular carcinoma.
| Review question: what is the diagnostic accuracy of CT for the diagnosis of resectable HCC in people with chronic liver disease? | |||||||||
| Population: adults with chronic liver disease | |||||||||
| Setting: clinical setting (secondary or tertiary care setting) or surveillance programmes | |||||||||
| Study design: cross‐sectional studies | |||||||||
| Index test: computed tomography | |||||||||
| Target condition: resectable HCC | |||||||||
Reference standards
| |||||||||
Limitations in the evidence: risk of bias and applicability concerns (total 12 studies which had > 90% of participants with resectable HCC)
| |||||||||
| Findings | |||||||||
| Implications in a hypothetical cohort of 1000 people | |||||||||
| Index test | Number of studies (participants) | Sensitivity (95% CI) | Specificity (95% CI) | Prevalencea% | True positives will receive appropriate treatment (surgical resection) | False negatives will be misdiagnosed and not undergo surgical resection | True negatives will not undergo inappropriate further testing or surgical resection | False positives will undergo inappropriate further testing or surgical resection | Certainty of the evidence |
| CT | 10 (1854) |
71.4% (60.3% to 80.4%) | 92.0% (86.3% to 95.5%) | 20 | 143 | 57 | 740 | 60 | Lowb |
| 35 | 250 | 100 | 600 | 50 | |||||
| 60 | 434 | 166 | 370 | 30 | |||||
| CI: confidence interval; CT: computed tomography; HCC: hepatocellular carcinoma | |||||||||
aWe chose for exemplification three values of hepatocellular carcinoma prevalence: 20% for a population with low clinical suspicion, 35% as a median derived from our study analysis, and 60% for population with high clinical suspicion (assessment of nodules detected by ultrasound). bDowngraded by two levels: for risk of bias, and indirectness.
Background
Hepatocellular carcinoma is the most common primary liver neoplasm, usually developing in the setting of a chronic liver disease. It is the sixth most commonly diagnosed cancer type and the fourth leading cause of death from cancer worldwide; 782,000 deaths due to hepatocellular carcinoma were reported in 2018 (Bray 2018). Exceedingly high rates are present in East and Southeast Asia, several areas of Africa and Southern Europe (Bertuccio 2017). In the last decade, hepatocellular carcinoma was one of the few cancers that showed increasing incidence and mortality trends in several areas of the world including Europe, and North and Latin America (Bosetti 2013; Hashim 2016; Ryerson 2016). Mortality rates, even with a recently downward reported trend, are reported to be still two to five times higher in Japan, Hong Kong, and Korea than in most European countries and the Americas (Bertuccio 2017). Most common risk factors include liver cirrhosis, severe liver fibrosis, chronic infections with hepatitis B and C, heavy alcohol intake, tobacco use, diabetes, metabolic syndrome, aflatoxins (poisonous carcinogens produced by Aspergillus flavus and Aspergillus parasiticus, which grow in soil, decaying vegetation, hay, and grains), nonalcoholic fatty liver disease, and being overweight (Yang 2011; Bosetti 2014; Stanaway 2016; Bertuccio 2017). However, people who have developed hepatocellular carcinoma without known risk factors have been reported (Bralet 2000; Young 2012). Hepatocellular carcinoma is rare among adolescents with an incidence of 0.3 to 0.45 people per million per year and accounts for less than 1% of all malignant neoplasms among children younger than 20 years (Mann 1990). The reported hepatocellular carcinomas were associated with hepatitis B virus infection or with inherited metabolic disorders, specifically hereditary tyrosinaemia, a‐1‐antitrypsin deficiency, and glycogen storage disease type 1. Only approximately 30% of paediatric hepatocellular carcinomas are associated with cirrhosis, and the carcinogenesis and the clinical course are considered peculiar (Ni 2004; Omata 2017; Mogul 2018).
Clinically, hepatocellular carcinoma is frequently diagnosed in the late stages of liver disease because of the absence of specific symptoms, other than those related to chronic liver disease. Less than 20% of people are eligible for curative treatment ‐ such as liver resection, transplantation or ablation ‐ due to advanced tumour stage, liver dysfunction or shortage of liver donors (Davila 2012). Furthermore, curative treatment options are unfeasible in most instances due to severe clinical deterioration at the moment of diagnosis, or due to the inaccuracy of the preoperative clinical evaluation and staging procedure.
Despite the poor initial prognosis (the mortality‐to‐incidence overall ratio has been reported as 0.95; Ferlay 2019), a five‐year survival rate of more than 50% can be achieved if the hepatocellular carcinoma is detected at an early stage (Forner 2012). According to the Barcelona Clinic Liver Cancer (BCLC) staging system, only people with early‐stage hepatocellular carcinoma are eligible for curative treatment (Llovet 1999). Therefore, accurate and early diagnosis of hepatocellular carcinoma is of high importance.
Prior to advancements in medical imaging, biopsy and cytologic examination of the liver specimen were used to make a definitive diagnosis of hepatocellular carcinoma (Tao 1984). With the development of advanced imaging techniques, hepatocellular carcinoma has become unique among tumours in that its characteristics can be accurately detected using imaging, thus reducing the need for invasive
biopsy (Forner 2008; Sangiovanni 2010; Manini 2014). Currently, biopsy is not preferred for the diagnosis of hepatocellular carcinoma due to concerns regarding tumour seeding, bleeding, and rate of false‐negative results (Silva 2008; Pomfret 2010). However, it is reserved for lesions with atypical appearance and when imaging results are equivocal (Bruix 2011).
Computer tomography (CT) and contrast‐enhanced magnetic resonance imaging (MRI) have been established as the non‐invasive imaging modalities for detection and evaluation of liver lesions (Lee 2012a; O'Neill 2015). In comparison with single‐detector CT, multidetector computed tomography (MDCT) is superior due to greater speed, thinner slices, and multiphasic scanning; these factors improve spatial and temporal resolution and provide more precise evaluation of liver tumour haemodynamics, and consequently, diagnostic accuracy (O'Neill 2015). The ability of CT to detect hepatocellular carcinoma rests on characterising the enhancement patterns in arterial, portal venous, and subsequent phases relative to the surrounding liver tissue. The differences in blood flow and extracellular volume between hepatocellular carcinoma and normal liver tissue lead to the main radiological hallmarks of hepatocellular carcinoma (Hennedige 2012; Choi 2014; Shah 2014; LI‐RADS 2018).
According to the American Association for the Study of Liver Disease (AASLD) and European Association for the Study of the Liver (EASL) guidelines, a single contrast‐enhanced imaging study (CT or MRI), performed in high‐volume centres with up‐to‐date radiological equipment showing typical radiological hallmarks in people with cirrhosis, is valid to diagnose hepatocellular carcinoma (Bruix 2011; EASL‐EORTC 2012; EASL 2018). However, if a detected lesion presents with some (but not all) of the hallmarks of hepatocellular carcinoma, another imaging study or biopsy is warranted.
According to current relevant guidelines, there are some differences in recommendations for management with regards to the size of a suspected focal liver lesion. In the AASLD guideline, lesions with a diameter less than 1 cm and those with a diameter more than 1 cm without hepatocellular carcinoma hallmarks are labelled as indeterminate lesions and require follow‐up (Heimbach 2018). The EASL guideline proposes a diagnostic algorithm for management of suspected focal liver lesions and group lesions in two categories (with a diameter less than 1 cm, and more than 1 cm; EASL 2018). The Asian Pacific Association for the Study of the Liver (APASL) diagnostic pathways focus more on lesion characteristics than on size (Omata 2017).
Previous systematic reviews have assessed the performance of CT in detecting hepatocellular carcinoma, and they have included different studies and yielded different results (Colli 2006; Xie 2011; Chen 2013; Floriani 2013; Chou 2015; Lee 2015; Ye 2015; Guo 2016; Hanna 2016; Roberts 2018; Li 2019). These reviews are comparative reviews that compare two or more tests (ultrasound, CT, MRI) and include studies conducted before 2016, when CT diagnostic criteria were not clearly defined (LI‐RADS). Evaluation of risk of bias and definition of inclusion criteria, type of studies, and reference standards are often inconsistent and questionable. Furthermore, these reviews did not put the index tests into context and did not define clearly their role, instead comparing all the available tests as they were used simultaneously. The aim of the present systematic review and meta‐analysis is to determine the accuracy of CT for the diagnosis of hepatocellular carcinoma of any size, as well as the diagnosis of resectable hepatocellular carcinoma in people with chronic liver disease.
Target condition being diagnosed
Hepatocellular carcinoma is the most common primary liver cancer that occurs in people with chronic liver disease. The incidence of hepatocellular carcinoma increases in individuals with chronic hepatitis B and C, alcohol use, and nonalcoholic fatty liver disease, and in those with liver cirrhosis of various aetiologies (Bruix 2011). There is no definite threshold in the definition of lesion size, although literature tends to classify lesions with a diameter equal to or less than 2 cm as 'small' (Hussain 2002; Choi 2014; Park 2017). The histological diagnosis of hepatocellular carcinoma poses many challenges, particularly when dealing with liver biopsy specimens, due to the heterogeneity of hepatocellular carcinoma and occasional difficulties confirming hepatocellular differentiation. Primary liver tumours should be considered as a continuum with typical hepatocellular and cholangiocarcinoma as the two ends of the spectrum. In between, a whole range of tumours showing both hepatocellular and cholangiocellular differentiation with or without an associated progenitor/stem cell component should be differentiated. Characterisation of combined (or mixed) hepatocellular‐cholangiocarcinoma can be very challenging. In advanced‐stage chronic liver disease, the main challenge for the histopathologist is still to differentiate between hepatocellular carcinoma and its precursors, large regenerative nodule, and a dysplastic nodule, with the potential to progress to hepatocellular carcinoma. The transition from dysplastic nodule to hepatocellular carcinoma is thought to be associated with a change in the lesional vascular supply, from a dual portal‐arterial to a predominantly arterial, due to neoangiogenesis (Quaglia 2018). The radiological counterpart of these changes is contrast uptake in the arterial phase and rapid washout in the venous phase, which is considered to be sufficient for a diagnosis of hepatocellular carcinoma (Omata 2017; EASL 2018; Heimbach 2018). An international consensus defined the diagnostic criteria and highlighted the difficulties in histological differentiation between the different stages of hepatocellular carcinoma progression (International Consensus Group for HCN 2009).
In clinical practice, and according to pertinent guidelines, multiphasic CT or MRI with intravascular contrast allow for a highly accurate diagnosis of hepatocellular carcinoma without an invasive liver biopsy. The diagnosis of hepatocellular carcinoma is usually obtained on the basis of cross‐sectional CT or MRI features, and liver histology is required only for undefined lesions (Omata 2017; EASL 2018; Heimbach 2018; LI‐RADS 2018).
A number of staging systems for hepatocellular carcinoma have been proposed and developed, however, there is no globally applicable staging system (Kinoshita 2015). Among different staging protocols, the BCLC staging system has a notable feature of treatment recommendations for each stage, based on the best treatment options currently available (Llovet 1999; Llovet 2003; Llovet 2008). It is comprised of four elements: tumour extension, liver functional reserve, physical status, and cancer‐related symptoms. According to the BCLC, only people with early‐stage hepatocellular carcinoma are eligible for curative treatment such as surgical resection or percutaneous locoregional treatment. Orthotopic liver transplantation is reserved for people with decompensated cirrhosis, and it is considered a definite curative treatment for hepatocellular carcinoma. When orthotopic liver transplantation for hepatocellular carcinoma was initially introduced in the 1980s, it was associated with poor five‐year survival and high recurrences, which led to the treatment being contraindicated for hepatocellular carcinoma (Yokoyama 1990). In 1996, specific criteria, known as the Milan criteria (Mazzaferro 1996), were developed for the selection of people for liver transplantation. These criteria have been repeatedly validated and their value is considerable (EASL 2018). With their implementation, overall five‐year survival of people with post‐orthotopic liver transplantation exceeded 70% (Mazzaferro 2011). The criteria for people eligible for orthotopic liver transplantation include a single hepatocellular carcinoma lesion with a diameter equal to or less than 5 cm, or up to three hepatocellular carcinoma lesions, each with a diameter equal to or less than 3 cm; no vascular invasion; and no extrahepatic involvement (no metastasis; Mazzaferro 1996).
Index test(s)
Contrast‐enhanced multidetector and multiphasic CT is an advanced imaging modality that includes rapid intravenous injection of contrast agent with fast data acquisition using ionising radiation. Minimal CT requirements for the detection of hepatocellular carcinoma include performance on multidetector CT with 8 or more detector rows, acquisition of images in arterial, portal venous, and delayed phase with multiplanar reformations. If people have undergone prior locoregional hepatocellular carcinoma treatment, acquisition of precontrast images is required (LI‐RADS 2018).
Although uncommon, physicians should be aware of the acute adverse reactions to iodine contrast which are categorised into mild (nausea, mild vomiting, urticaria, and itching), moderate (severe vomiting, marked urticaria, bronchospasm, facial/laryngeal oedema, and vasovagal attack), and severe (hypotensive shock, respiratory arrest, cardiac arrest, and convulsion). Also, the administration of iodinated contrast agent may lead to contrast‐induced nephropathy. However, this entity is more uncommon than the aforementioned adverse reactions (Thomsen 2014). Ionising radiation produced by CT scanners is, by definition, harmful to the molecular structure of human tissue. However, many technological improvements, dose reduction strategies, and radiation effect campaigns have been made for the benefit of reducing radiation risks in people undergoing a CT exam (Kaira 2015; Parakh 2016).
The American College of Radiology established the Liver Reporting and Data System (LI‐RADS), with the aim of standardising the terminology, interpretation, and reporting of imaging findings in people with suspected hepatocellular carcinoma. Several versions have been published since the initial release in 2008, most recently in 2018 (LI‐RADS 2018). The LI‐RADS assign a diagnostic category to each focal liver lesion/observation based on major, ancillary, and other imaging features. Major features include non‐rim‐like hyperenhancement in arterial phase, non‐peripheral washout in portal venous and subsequent phases, enhancing capsule, lesion diameter, and threshold growth (LI‐RADS 2018). Based on the presence of major features and morphological suspicion of hepatocellular carcinoma, each lesion is assigned with a category ranging from LR‐1 (definitely benign) to LR‐5 (definitely hepatocellular carcinoma). Other categories include suspicion for malignancy, but not necessarily hepatocellular carcinoma (LRM) and tumour in vein (LR‐TIV). If assigning a category is doubtful, many ancillary features have been defined to favour the presence of hepatocellular carcinoma, malignancy other than hepatocellular carcinoma, or benign lesion. Features favouring hepatocellular carcinoma include non‐enhancing capsule, nodule in nodule appearance, mosaic architecture, blood products, and fat in the lesion. The main aim of this categorisation is to clearly define the probability that a certain lesion is indeed a hepatocellular carcinoma, and to help guide multidisciplinary clinical management (LI‐RADS 2018; Van der Pol 2019).
Clinical pathway
Surveillance of hepatocellular carcinoma (screening performed at regular intervals) in the at‐risk population, that is, people with chronic liver disease, regardless of aetiology, is carried out by abdominal ultrasound for detection of nodules. Once a suspected nodule has been detected, other imaging methods are considered according to the size of the nodule and appropriate guidelines. For a flow diagram of the clinical pathway and placement of tests, see Figure 1.
1.

Flow diagram of the diagnostic pathway for the diagnosis of hepatocellular carcinoma
American Association for the Study of Liver Disease (AASLD) diagnostic guidelines
According to the AASLD guidelines, adults with cirrhosis and suspected hepatocellular carcinoma should undergo diagnostic evaluation with either multiphasic CT or multiphasic MRI. Lesions that do not meet the positivity criteria (i.e. arterial phase hyperenhancement in combination with washout appearance and/or capsule appearance), or whose size is less than 1 cm, are considered indeterminate. For indeterminate lesions, several options are suggested including follow‐up imaging, imaging with an alternative imaging modality or alternative contrast agent, or biopsy. No option is preferred and recommended over another. Biopsy may be required in selected instances, but its routine use is not advocated (Bruix 2011; Heimbach 2018).
European Association for the Study of the Liver (EASL) diagnostic guidelines
In cirrhotic liver disease, the diagnostic algorithm proposed by the EASL divides suspected focal liver lesions into two categories: lesions smaller than 1 cm, and those larger than 1 cm. Lesions larger than 1 cm need to be evaluated by CT or MRI straight away. If at least one of these imaging modalities is positive, i.e. proves the existence of hepatocellular carcinoma hallmarks, diagnosis of hepatocellular carcinoma is considered certain. If the results are equivocal, the use of other multiphasic imaging modality is required: multiphasic contrast‐enhanced CT or multiphasic contrast‐enhanced MRI, gadoxetic‐enhanced MRI, or contrast‐enhanced ultrasound. If these studies prove the hallmarks of hepatocellular carcinoma, the diagnosis is certain; otherwise, biopsy is warranted. If biopsy appears to be unclear, re‐biopsy is to be considered or a repeat ultrasound follow‐up every four months is needed. Lesions smaller than 1 cm are to be followed up by ultrasound every four months: if the size of the lesion does not increase, then further ultrasound follow‐up is recommended; otherwise, multiphasic contrast‐enhanced CT, multiphasic contrast‐enhanced MRI, or gadoxetic‐enhanced MRI is required (EASL 2018).
Asian Pacific Association for the Study of the Liver (APASL) diagnostic guidelines
Under the APASL guidelines, a single dynamic contrast‐enhanced MRI or CT is warranted regardless of the size of suspected liver nodule. If typical hallmarks of hepatocellular carcinoma are shown (presence of arterial hyperenhancement, followed by washout in the portal venous or delayed phases, or both), diagnosis is confirmed. If the lesion is hypervascular but shows no washout, another contrast‐enhanced MRI study is needed. If the lesion proves to be hypointense, hepatocellular carcinoma diagnosis is confirmed. However, if the lesion is isointense or hyperintense, biopsy is warranted. If the lesion on the first dynamic MRI or CT study is non‐hypervascular, a dynamic MRI study in hepatobiliary phase is needed. If the lesion is isointense or hyperintense, surveillance by ultrasound is recommended every six months, and if the lesion is hypointense, contrast‐enhanced ultrasound of the liver nodule is warranted. Depending on lesion features on contrast‐enhanced ultrasound, biopsy or another dynamic CT or MRI study is recommended every three to six months (Omata 2017).
The expected downstream consequences of the CT results are: people with true‐positive results, that is, those with hepatocellular carcinoma and positive test results, will receive the appropriate treatment (surgery, local ablative therapy, or systemic chemotherapy); people with true‐negative results, that is, those without hepatocellular carcinoma and negative test results, will not undergo inappropriate treatment or unnecessary further testing; people with false‐negative results, that is, those with hepatocellular carcinoma and negative test results, will be misdiagnosed, not receive the appropriate treatment and might be detected later as a more severe case; people with false‐positive results, that is, those without hepatocellular carcinoma and positive test results, will undergo further testing and possibly inappropriate treatment. In people on a waiting list for orthotopic liver transplantation for an indication not related to an hepatocellular carcinoma, the consequences of false‐negative results of preoperative CT are not completely known and might be less severe: indeed studies report no significant difference in terms of overall survival and tumour recurrence between people with and without previously diagnosed hepatocellular carcinomas (Castillo 2009; Senkerikova 2014; Madaleno 2015; El Moghazy 2016).
Prior test(s)
For surveillance purposes, abdominal ultrasound is recommended as a first‐line imaging modality in people with chronic liver disease, regardless of aetiology, who are at risk of developing a hepatocellular carcinoma (Omata 2017; EASL 2018; Heimbach 2018). It is also used as a diagnostic tool in people with clinical suspicion of hepatocellular carcinoma for detecting liver lesions. Alpha‐foetoprotein has been used as a diagnostic biomarker even before technological advancements (Kew 1975). However, its role as a screening tool is still a matter of debate. The diagnosis of chronic advanced liver disease is based on clinical judgement derived from history, laboratory testing, physical examination, imaging, liver stiffness measurement, liver histology, or a combination of the aforementioned. Due to the accuracy of non‐invasive tests, liver histology is reserved for only a minority of people with unclear diagnosis, and a non‐invasive diagnosis of chronic advanced liver disease is considered equivalent to a histological diagnosis of cirrhosis (de Franchis 2015).
Role of index test(s)
Computer tomography is used as an add‐on test after ultrasound detection of liver lesions suspected for hepatocellular carcinoma in surveillance programmes or hospital settings in people with clinical suspicion. Based on CT findings, biopsy and other imaging modalities could be avoided, therefore further testing could be reserved for a minority of patients.
Alternative test(s)
An alternative imaging modality in detecting hepatocellular carcinoma is contrast‐enhanced dynamic MRI with extracellular and cell‐specific gadolinium‐based contrast agents. A recent meta‐analysis aimed to determine the diagnostic benefit between multiphasic contrast‐enhanced CT, extracellular contrast‐enhanced MRI, and cell‐specific gadoxetate‐enhanced MRI for detection of hepatocellular carcinoma in people with cirrhosis (Roberts 2018). No definitive recommendation could be made for the systematic use of gadolinium‐enhanced MRI over CT, although other previous meta‐analyses reported a preference for MRI (Lee 2015; Ye 2015; Guo 2016).
Contrast‐enhanced ultrasound is an advanced form of ultrasound examination in which images are acquired using intravenously injected microbubble contrast agent. Dynamic contrast‐enhanced ultrasound images are obtained similarly to contrast‐enhanced CT and MRI studies: depending on the time of image acquisition after intravenous contrast injection, the study differentiates arterial and portal venous phases in which sonographic hallmarks for hepatocellular carcinoma, such as arterial hyperenhancement and subsequent washout appearance, are investigated (Chung 2015; LI‐RADS). Unlike CT and MRI contrasts, ultrasound contrast agent is a purely intravascular agent; therefore, it is highly accurate in detecting tumour angiogenesis (Schirner 2004).
Lipiodol computerised tomography (Lipiodol‐CT) was used in the past as a diagnostic modality for the detection of hepatocellular carcinoma. The method included intra‐arterial injection of iodised oil (Lipiodol) through the hepatic arterial supply, following which Lipiodol was deposited within the hepatocellular carcinoma nodule. The hepatocellular carcinoma was visualised as a hyperattenuating nodule on the subsequent CT, and it showed high sensitivity in detecting small hepatocellular carcinoma (Takayasu 1990). In the context of transarterial chemoembolisation, Lipiodol may be used as an intraprocedural diagnostic modality (C‐arm Lipiodol CT) for additional detection of small‐size hepatocellular carcinoma (Li 2015).
Rationale
Hepatocellular carcinoma is currently detected by liver ultrasound in people with normal or high alpha‐foetoprotein during surveillance programmes of people with chronic liver disease. Following ultrasound, the diagnosis is usually confirmed with high levels of alpha‐foetoprotein and contrast‐enhanced ultrasound, CT, or MRI. The latter two imaging modalities are also appropriate for staging and allow the choice of the most appropriate treatment. There is no clear evidence of the benefits of surveillance programmes in terms of overall survival: the conflicting results can be a consequence of inaccurate detection, ineffective treatment, or both. Assessing the diagnostic accuracy of CT, the most used confirmatory test after first‐line tests, may clarify whether the absence of benefit in surveillance programmes might be related to underdiagnosis or understaging. Furthermore, an assessment of the accuracy of CT for the diagnosis of hepatocellular carcinoma is needed for either ruling out, diagnosing, or supporting further testing in people with chronic liver disease who are not included in surveillance programmes.
This review represents a part of a series of reviews about the diagnostic accuracy of the most commonly used modalities for diagnosing hepatocellular carcinoma in people with chronic liver disease. The first part includes assessment of the diagnostic accuracy of ultrasound and alpha‐foetoprotein levels, which are used as triage tests in surveillance (Colli 2021). The second part focuses on the diagnostic accuracy of contrast‐enhanced ultrasound in characterising suspected lesions as hepatocellular carcinoma as a second‐line diagnostic modality (Fraquelli 2019). The present review focuses on the assessment of CT as a second‐line imaging modality in assessing focal liver lesions detected on ultrasound suspected for hepatocellular carcinoma. A comparable review assessing the accuracy of MRI for diagnosing hepatocellular carcinoma is in progress (Nadarevic 2021). We are planning to produce an overview of the reviews that will assess abdominal ultrasound and alpha‐foetoprotein, contrast‐enhanced ultrasound, CT, and MRI for the diagnosis of hepatocellular carcinoma.
Objectives
To assess the diagnostic accuracy of multidetector, multiphasic contrast‐enhanced CT for the diagnosis of hepatocellular carcinoma (hepatocellular carcinoma) of any size, and at any stage, in adults with chronic liver disease, either in a surveillance programme or in a clinical setting.
Secondary objectives
To assess the diagnostic accuracy of multidetector, multiphasic contrast‐enhanced CT for the diagnosis of resectable hepatocellular carcinoma in adults with chronic liver disease. The definition of resectable hepatocellular carcinoma is a neoplasm amenable to surgical radical resection according to the current guidelines (the Milan criteria): a single lesion with a maximum diameter of less than 5 cm, or fewer than three lesions with a maximum diameter of 3 cm (Mazzaferro 1996).
-
To investigate the following sources of heterogeneity:
study date (studies published before the year 2005 compared to studies published after the year 2005, due to advancements in technology);
study date (studies published before 2016 compared to studies published after 2016, due to changes in diagnostic criteria);
inclusion of participants without cirrhosis (studies including more than 10% participants without cirrhosis compared to studies including less than 10% participants without cirrhosis);
study location (population differences): studies conducted in North and South America compared to Europe compared to Asia;
patient selection (patients recruited from planned surveillance programmes compared to clinical cohorts);
different hepatocellular carcinoma stage (studies in which 20% or more of participants have resectable hepatocellular carcinoma compared to studies in which less than 20% of participants have resectable hepatocellular carcinoma);
different reference standard (histology of the explanted liver compared to liver biopsy compared to another reference standard);
different liver cirrhosis aetiology (hepatitis C or hepatitis B virus‐associated cirrhosis compared to all other aetiologies);
number of CT detector rows (exams conducted on 64‐slice or fewer compared with more than 64‐slice, due to advancements in technology);
hepatocellular carcinoma mean diameter;
prevalence of the target condition (above median compared to below median);
prior detection of nodules, studies including study participants with prior tests to detect nodules compared to studies including study participants without prior tests.
We chose the variables listed above for the following reasons. Due to advancements in technology and change in diagnostic criteria, we considered the date of study publication. The proportion of participants without cirrhosis is relevant because hepatocellular carcinoma in absence of cirrhosis has different CT characteristics, prognosis, and treatment. There are differences in epidemiology, and clinical and radiological characteristics of hepatocellular carcinoma in Asia and in Western countries. Selection of patients can induce variability of results: participants recruited from screening or surveillance programmes may be different mainly in severity of the underlying liver disease and consequently in radiological characteristics of the liver. The hepatocellular carcinoma prevalence in included studies can change according to selection and epidemiology. The proportion of resectable hepatocellular carcinoma found in the studies reflects different epidemiology and patient selection. The clinical and radiological characteristics of hepatocellular carcinoma varies according to the aetiology of the underlying liver disease, mainly in the case of chronic infection with hepatitis C or hepatitis B, compared to other aetiologies. The accuracy of CT may vary according to the diameter of the neoplastic lesion and the number of detector rows in the CT equipment. Prior testing and the inclusion of participants with nodules might produce differences in CT accuracy estimates secondary to this different selection. The investigation of this last possible source of heterogeneity was not planned in the protocol and was added subsequently.
Methods
Criteria for considering studies for this review
Types of studies
We included studies that, irrespective of publication status and language, have evaluated the diagnostic accuracy of multidetector, multiphasic contrast‐enhanced CT for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. These studies should have used one of the acceptable reference standards (see Reference standards).
We considered studies of cross‐sectional design that included participants with clinical suspicion of hepatocellular carcinoma. We excluded studies of case‐control design that compared people with known hepatocellular carcinoma to matched control as these are considered to have high risk of bias due to inflated accuracy estimates (Colli 2014). We excluded studies that analysed data only per lesion, rather than per participant, unless study authors made participant data available.
Participants
We included participants aged 18 years and older, of any sex, at risk of developing hepatocellular carcinoma, and with chronic liver disease, irrespective of aetiology, severity of disease, and duration of illness, with or without prior tests, ultrasound, and alpha‐foetoprotein. The review focused on diagnostic questions related to adults with a first diagnosis of hepatocellular carcinoma. People with previous diagnosis and treatment of hepatocellular carcinoma make up a distinct group for which the diagnosis or natural history of hepatocellular carcinoma has been modified. These people were not the focus of this review; therefore, we excluded studies that included such participants unless they represented less than 5% of all the included participants, or if study authors had presented data in such a way as to allow this group of participants to be isolated from the remaining included participants.
Index tests
We included multiphasic contrast‐enhanced CT for the detection of hepatocellular carcinoma in adults with chronic liver disease. Regarding positivity criteria, we accepted any definition of positive/negative test results. This judgment usually, even if implicitly, considers the presence of suspected liver lesion, which shows non‐rim‐like arterial hyperenhancement and subsequent non‐peripheral washout appearance in later phases.
Target conditions
Hepatocellular carcinoma of any size and at any stage
Resectable hepatocellular carcinoma (see Secondary objectives)
Reference standards
We accepted one of the following as a reference standard for the diagnosis of hepatocellular carcinoma.
The pathology of the explanted liver in case of transplantation
The histology of resected focal liver lesion(s), or the histology of biopsied focal liver lesion(s) with a follow‐up period of at least three months to exclude the presence of focal lesions not detected by the index test.
These reference standards, even if commonly used in clinical practice, are not perfect. The pathology of the explanted liver is possible only when all the included participants have undergone liver transplantation; therefore, the setting does not represent the whole spectrum of liver disease severity as only people with advanced and decompensated liver disease are candidates for orthotopic liver transplantation. In the case of histology of resected focal lesion and histology of biopsied liver lesions, the negative result can be confirmed only with an adequate follow‐up period. This would introduce an unavoidable differential verification bias.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group (CHBG) Controlled Trials Register and the CHBG Diagnostic Test of Accuracy Studies Register (both registers are maintained and searched internally by the CHBG Information Specialist via the Cochrane Register of Studies Web), The Cochrane Library, MEDLINE Ovid, Embase Ovid, LILACS (Bireme), Science Citation Index ‐ Expanded (Web of Science), and Conference Proceedings Citation Index – Science (Web of Science) until 04 May 2021. Appendix 1 gives the search strategies with the time spans of the searches.
We did not apply any restrictions on language or document type.
Searching other resources
We tried to identify additional references by manually searching articles retrieved from digital databases and relevant review articles. We sought information on unpublished studies by contacting experts in the field. In addition, we handsearched abstract books from meetings of the AASLD, the EASL, and APASL held during the past 10 years. We also searched for other kinds of grey literature in the System for Information on Grey Literature in Europe 'OpenGrey' (www.opengrey.eu/).
Data collection and analysis
We followed available guidelines as provided in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (DTA Handbook 2013).
Selection of studies
Two review authors (VG and TN) independently scrutinised titles and abstracts identified by electronic literature searching to identify potentially eligible studies. We selected any citation, identified by either of the two review authors, as potentially eligible for full‐text review. The same review authors independently assessed full‐text papers for study eligibility, using predefined inclusion and exclusion criteria. We resolved any discrepancies by discussion. After full‐text assessment, we recorded all studies and their reasons for exclusion, in the 'Characteristics of excluded studies' table and illustrated the study selection process using a PRISMA diagram (Salameh 2020; Page 2021).
Data extraction and management
We developed a standardised data extraction form and piloted the form on five of the included studies. Based on the pilot, we finalised the form. Then, two review authors (VG and TN) independently completed the data extraction form for each included study. Each review author independently retrieved study data. In cases of disagreement, we reached consensus through discussion with a third review author (AC).
We extracted the following data.
General information: title, journal, year, publication type, study design and data collection (prospective versus retrospective), surveillance programme or clinical cohorts
Sample size: number of participants meeting the criteria and total number of participants included and tested
Baseline characteristics: baseline diagnosis, age, sex, race, and presence of cirrhosis and mean diameter of hepatocellular carcinoma
Index test with predefined positivity criteria
Target condition
Time interval between the index test and the reference standard
Reference standard tests
Numbers of true‐positive, true‐negative, false‐positive, and false‐negative findings. We extracted these data for the two target conditions (hepatocellular carcinoma of any size and stage and resectable hepatocellular carcinoma)
Number of uninterpretable results
The unit of analysis was the study participant, and we extracted data per participant. We summarised the data from each study in 2x2 tables (true positive, false positive, false negative, true negative), and we entered the data into Review Manager 5 software (Review Manager 2020).
Missing data
We contacted primary authors of nine primary studies by email to ask for additional information regarding per‐patient analyses and data needed to design the 2x2 tables. Two study authors responded but did not provide any additional data. We did not receive a reply from any other study authors. After two weeks we sent a second email but still did not receive a reply. We eventually excluded all the studies in question.
Assessment of methodological quality
Two review authors (VG and TN) independently assessed the risk of bias of included studies and applicability of their results using QUADAS‐2 (revised tool for quality assessment of diagnostic accuracy studies; Whiting 2011). In cases of disagreement, we reached a consensus through discussion. We addressed aspects of study quality involving the participant spectrum, index tests, target conditions, reference standards, and flow and timing. Regarding the index test positivity criteria definition, we assessed whether studies reported a clear definition. We recognise that, even if positivity criteria do not present explicit thresholds, they are nevertheless vulnerable to implicit thresholds. We defined a time interval between the index test and the reference standard of three months as appropriate. According to a recent systematic review, the approximate hepatocellular carcinoma volume doubling time is 4 months to 5 months with significant range of 2.2 moths to 11.3 months (Nathani 2021). In accordance with suggestions from a previous systematic review, which noted the acceptable time interval being from 1 month to 3 months (Kim 2008), we assumed 90 days to be the most acceptable threshold. The visualisation of the liver can sometimes be suboptimal due to patient characteristics; therefore, lack of reporting or exclusion of uninterpretable results from analyses could overestimate the accuracy of CT. We considered the study to be at high risk of bias if uninterpretable results were excluded from the analysis.
We classified a study at a high risk of bias if we judged at least one of the QUADAS‐2 study domains as high risk (Appendix 2).
Statistical analysis and data synthesis
We provided a description of the included studies by calculating median values and interquartile ranges (IQR) across studies for some characteristics of our interest, defined at study level. In particular, we considered hepatocellular carcinoma mean diameter and the prevalence of participants with the following characteristics: hepatocellular carcinoma, resectable hepatocellular carcinoma, liver cirrhosis, and viral aetiology of cirrhosis.
We designed 2x2 tables for each primary study for the index test (see Data extraction and management). We planned the following strategy of analyses.
Firstly, we performed a graphical descriptive analysis of the included studies and presented forest plots (sensitivity and specificity separately, with their 95% confidence intervals (CIs)). Secondly, we performed a meta‐analysis using the bivariate model and provided estimates of summary sensitivity and specificity (Macaskill 2010). We used the pooled estimates obtained from the fitted models to calculate summary estimates of positive and negative likelihood ratios (LR+ and LR−, respectively).
In case of uninterpretable results, we planned to analyse data according to the intention‐to‐diagnose principle (Schuetz 2012), also described as worst‐case scenario in Cohen 2015. Participants with uninterpretable index test results were classified as false positive if they had a negative reference standard or a false negative for participants with a positive reference standard. If data for the intention‐to‐diagnose analyses were not retrievable from the text, we contacted publication authors with provided email addresses. If we received no reply, we included the study in the analyses with data retrievable from the published manuscript and we considered it as having a high risk of bias. However, no study reported uninterpretable index test results.
We performed all statistical analyses using SAS statistical software (SAS), and macro METADAS (DTA Handbook 2013).
Investigations of heterogeneity
We investigated the effects of the following sources of heterogeneity.
Study date (studies published before the year 2005 compared to studies published after the year 2005, due to advancements in technology (categorical)
Study date (studies published before 2016 compared to studies published after 2016, due to changes in diagnostic criteria (categorical)
Inclusion of participants without cirrhosis, studies including more than 10% participants without cirrhosis compared to studies including less than 10% participants without cirrhosis (categorical)
Study location (population differences): studies conducted in North and South America compared to Europe compared to Asia (categorical)
Participant selection, participants recruited from planned surveillance programmes compared to clinical cohorts (categorical)
Different hepatocellular carcinoma stage, studies in which 20% or more of participants have resectable hepatocellular carcinoma compared to studies in which less than 20% of participants have resectable hepatocellular carcinoma (categorical)
Different reference standard, histology of the explanted liver compared to liver biopsy compared to another reference standard)
Different liver cirrhosis aetiology (hepatitis C or hepatitis B virus‐associated cirrhosis compared to all other aetiologies (categorical)
Number of CT detector rows, exams conducted on 64‐slice or fewer compared with more than 64‐slice, due to advancements in technology (categorical)
Hepatocellular carcinoma mean diameter (continuous)
Prevalence of the target condition, above median compared to below median (categorical)
Prior detection of nodules, studies including participants with prior tests to detect nodules compared to studies including participants without prior tests (categorical)
We estimated the effects of the predefined sources of heterogeneity by adding covariates to the bivariate model. We assessed the statistical significance of the covariate effect by using the log‐likelihood ratio test for comparison of models with and without the covariate term. We considered two‐sided P values of less than 0.05 as statistically significant. For interpretation of the results of heterogeneity analysis, we considered the uncertainty of accuracy estimates in the different subgroups, quantified by 95% CIs of the estimated sensitivity and specificity, as an assessment of the degree to which these subgroups could influence diagnostic accuracy.
Sensitivity analyses
We assessed the effects of risk of bias of included studies on diagnostic accuracy by performing a sensitivity analysis in which we excluded studies classified as having high or unclear risk of bias in at least one of the QUADAS‐ 2 domains (Appendix 2). In addition, we defined the following signalling questions as most relevant, and conducted sensitivity analyses in which we excluded studies with answers of 'no' or 'unclear'.
Were the positivity criteria defined?
Were the reference standard results interpreted without the knowledge of the results of the index test?
We also conducted sensitivity analyses in which we excluded studies published only in abstract or letter form, and by limiting the analysis to studies we considered at low concern for applicability.
Assessment of reporting bias
In order to reduce reporting bias, we did not plan to use a filter search strategy nor to implement any language or sample. We did not plan to test for publication bias due to the lack of validated methods for diagnostic test accuracy reviews.
Summary of findings table and assessment of the certainty of evidence
We prepared summary of findings tables to present the main results and key information regarding the certainty of evidence. We assessed the certainty of evidence as recommended using the GRADE approach (Balshem 2011; Schünemann 2008; Schünemann 2016; GRADEpro GDT). We rated the certainty of evidence as either high (when not downgraded), moderate (when downgraded by one level), low (when downgraded by two levels), or very low (when downgraded by more than two levels) based on five domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. For each outcome, the certainty of evidence started as high when there were high‐quality observational studies (cross‐sectional or cohort studies) that enrolled participants with diagnostic uncertainty. If we found a reason for downgrading, we used our judgement to classify the reason as either serious (downgraded by one level) or very serious (downgraded by two levels) (Schünemann 2020a; Schünemann 2020b).
Five authors (TN, VG, MF, AC, GC) discussed judgements and applied GRADE in the following way.
Risk of bias: we used QUADAS‐2 to assess risk of bias.
Indirectness: we assessed indirectness in relation to the population (including disease spectrum), setting, interventions, and outcomes (accuracy measures). We also used prevalence as a guide to whether there was indirectness in the population.
Inconsistency: we carried out prespecified analyses to investigate potential sources of heterogeneity and downgraded when we could not explain inconsistency in the accuracy estimates based on whether the individual point estimates were similar and if the confidence intervals overlapped sufficiently in the forest plots.
Imprecision: we looked at the confidence intervals of sensitivity and specificity estimates and at the unexplained heterogeneity of the results.
Publication bias: we did not evaluate publication bias due to the lack of validated methods for diagnostic test accuracy reviews.
Results
Results of the search
We ran the search on 4 May 2021. We identified 33,282 references by searching the following databases: the Cochrane Hepato‐Biliary Group Controlled Trials Register (n = 350), the Cochrane Hepato Biliary Group Diagnostic Test of Accuracy Studies Register (n = 8), The Cochrane Library (n = 1255), MEDLINE Ovid (n = 5785), Embase Ovid (n = 19,833), LILACS (n = 102), and Science Citation Index – Expanded with Conference Proceedings Citation Index ‐ Science (n = 5949). After exclusion of 8055 duplicates, 25,230 references remained for possible eligibility. We retrieved three additional references through handsearching. After reading the title and the abstract of these references, we excluded 25,065 of them, as they did not meet the inclusion criteria. We retrieved full texts of the remaining 165 records, and after reading the full texts, we excluded 144 studies for various reasons (see Characteristics of excluded studies). Finally, we included in our review 21 references reporting data on 21 studies (Salameh 2020; Page 2021; Figure 2), including a total of 3101 participants (Pozzato 1997; Chalasani 1999; Gambarin‐Gelwan 2000; Mortele 2001; de Ledinghen 2002; Libbrecht 2002; Lim 2002; Freeny 2003; Teefey 2003; Van Thiel 2004; Golfieri 2009; Sangiovanni 2010; Haberman 2011; Kim 2011; Yu 2011; Serste 2012; Maiwald 2014; Lin 2016; Villacastin Ruiz 2016; Hsiao 2019; Langenbach 2019). Three additional studies, which were retrieved through handsearching, were all included in the analysis (Chalasani 1999; Van Thiel 2004; Maiwald 2014). We applied no language restrictions in the inclusion criteria, which resulted in retrieving full‐text articles of 24 studies published in non‐English languages of which we included two in the final analysis (Pozzato 1997; Haberman 2011). We requested further information by email for two studies, but did not receive a reply. The studies were conducted from 1997 to 2019.
2.
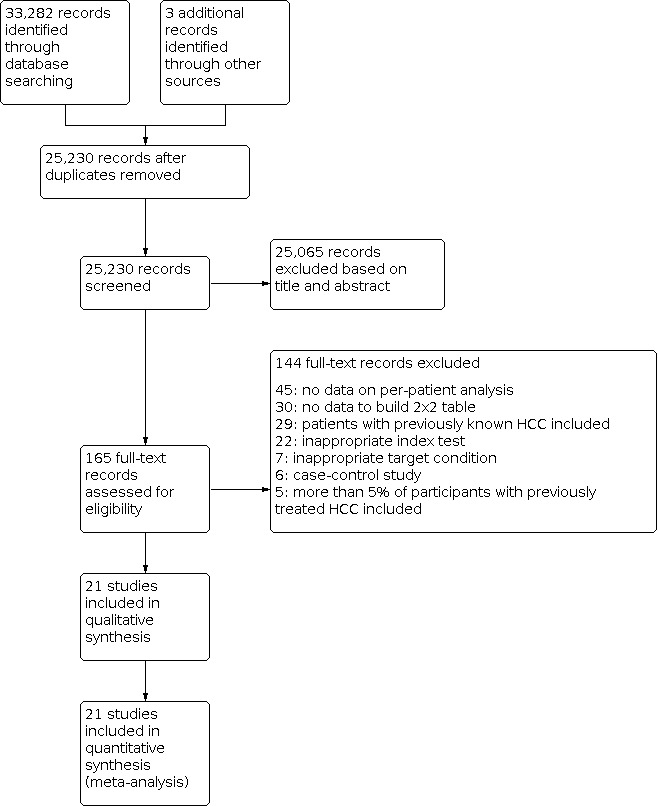
Study flow diagram. Date of search 4 May 2021
We reported in the Characteristics of included studies tables the main characteristics of the 21 studies. All studies are reported as full‐text publications.
Methodological quality of included studies
We have reported in detail results of the quality assessment of included studies in the Characteristics of included studies tables, and we have summarised this information in Figure 3 and Figure 4.
3.
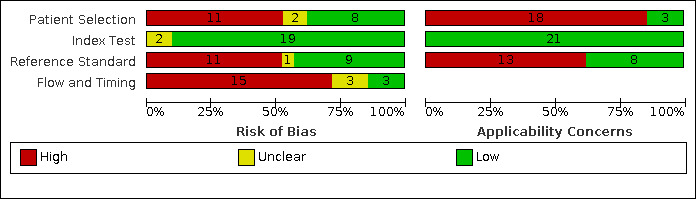
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
4.
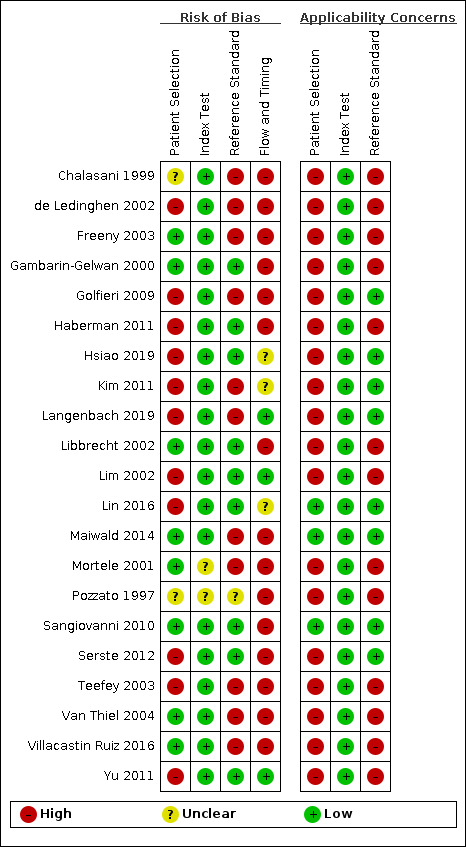
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Patient selection
We included only studies with a cross‐sectional design.
Risk of bias
Eight studies were at low risk of bias in this domain (Gambarin‐Gelwan 2000; Mortele 2001; Libbrecht 2002; Freeny 2003; Van Thiel 2004; Sangiovanni 2010; Maiwald 2014; Villacastin Ruiz 2016). We judged two studies unclear for this domain, as they did not provide any data on the presence of exclusion criteria (Pozzato 1997; Chalasani 1999). Eleven studies were at high risk, due to exclusion criteria that we considered inappropriate: missing results of the index test, hepatocellular carcinoma diameter, time interval between index test and reference standards, inconclusive diagnosis, CT performed in institutions outside the study centre, no pathology fibrosis score analysis, absence of liver tumour at the pathology of the explanted liver, or participants removed from the transplant waiting list (de Ledinghen 2002; Lim 2002; Teefey 2003; Golfieri 2009; Haberman 2011; Kim 2011; Yu 2011; Serste 2012; Lin 2016; Hsiao 2019; Langenbach 2019).
Applicability
We judged three studies at low concern (Sangiovanni 2010; Maiwald 2014; Lin 2016). The other 18 studies we judged at high concern because they included only participants with end‐stage liver disease on the waiting list for orthotopic liver transplantation (Pozzato 1997; Chalasani 1999; Gambarin‐Gelwan 2000; Mortele 2001; de Ledinghen 2002; Libbrecht 2002; Lim 2002; Freeny 2003; Teefey 2003; Van Thiel 2004; Haberman 2011; Yu 2011; Villacastin Ruiz 2016), participants with a defined hepatocellular carcinoma diameter (Golfieri 2009; Kim 2011; Serste 2012; Hsiao 2019), or participants with indeterminate nodules on MRI (Langenbach 2019).
Index test
Risk of bias
We judged 19 studies at low risk, because they clearly predefined the CT positivity criteria (Chalasani 1999; Gambarin‐Gelwan 2000; de Ledinghen 2002; Libbrecht 2002; Lim 2002; Freeny 2003; Teefey 2003; Van Thiel 2004; Golfieri 2009; Sangiovanni 2010; Haberman 2011; Kim 2011; Yu 2011; Serste 2012; Maiwald 2014; Lin 2016; Villacastin Ruiz 2016; Hsiao 2019; Langenbach 2019). We judged two studies as unclear for this domain, due to lack of information on CT positivity criteria (Pozzato 1997; Mortele 2001).
Applicability
We judged all studies at low concern.
Reference standard
In 11 studies the reference standard was the pathology of the explanted liver (Pozzato 1997; Gambarin‐Gelwan 2000; Mortele 2001; de Ledinghen 2002; Libbrecht 2002; Lim 2002; Freeny 2003; Van Thiel 2004; Haberman 2011; Yu 2011; Villacastin Ruiz 2016), in five studies it was the histology of biopsied focal lesions in all participants (Sangiovanni 2010; Serste 2012; Lin 2016; Hsiao 2019; Langenbach 2019), and in three studies it was the histology of biopsied focal lesions in some participants and follow‐up in the others (Chalasani 1999; Kim 2011; Maiwald 2014). Two studies (Golfieri 2009; Teefey 2003), had a mix of pathology of the explanted liver, resection, biopsy, and follow‐up.
Risk of bias
We judged nine studies at low risk (Gambarin‐Gelwan 2000; Libbrecht 2002; Lim 2002; Sangiovanni 2010; Haberman 2011; Yu 2011; Serste 2012; Lin 2016; Hsiao 2019), 11 at high risk (Chalasani 1999; Mortele 2001; de Ledinghen 2002; Freeny 2003; Teefey 2003; Van Thiel 2004; Golfieri 2009; Kim 2011; Maiwald 2014; Villacastin Ruiz 2016; Langenbach 2019), and one at uncertain risk (Pozzato 1997). The main reasons for judging studies at high risk of bias included statements explaining that reference standard results were interpreted with the knowledge of the results of the index test, and in cases of biopsy, the interventionist had to have knowledge of the presence and location of the lesion in order to perform the procedure. We judged uncertain risk of bias due to lack of detailed information regarding the reference standard.
Applicability
We judged eight studies at low concern (Golfieri 2009; Sangiovanni 2010; Kim 2011; Serste 2012; Maiwald 2014; Lin 2016; Hsiao 2019; Langenbach 2019), and 13 studies at high concern due to orthotopic liver transplantation being the only reference standard (Pozzato 1997; Chalasani 1999; Gambarin‐Gelwan 2000; Mortele 2001; de Ledinghen 2002; Libbrecht 2002; Lim 2002; Freeny 2003; Teefey 2003; Haberman 2011; Yu 2011; Van Thiel 2004; Villacastin Ruiz 2016).
Flow and timing
Risk of bias
We judged three studies at low risk of bias (Lim 2002; Yu 2011; Langenbach 2019), 15 studies at high risk (Pozzato 1997; Chalasani 1999; Gambarin‐Gelwan 2000; Mortele 2001; de Ledinghen 2002; Libbrecht 2002; Freeny 2003; Teefey 2003; Van Thiel 2004; Golfieri 2009; Sangiovanni 2010; Haberman 2011; Serste 2012; Maiwald 2014; Villacastin Ruiz 2016), and three at unclear risk (Kim 2011; Lin 2016; Hsiao 2019). Reasons for assessing studies at high risk of bias included inappropriate time between index test and reference standard (> 90 days; Pozzato 1997; Gambarin‐Gelwan 2000; Mortele 2001; de Ledinghen 2002; Libbrecht 2002; Freeny 2003; Teefey 2003; Van Thiel 2004; Haberman 2011; Villacastin Ruiz 2016)), not all participants underwent the same reference standard (Chalasani 1999; Teefey 2003; Golfieri 2009; Maiwald 2014), and participants missing in the final analysis with no explanations (Freeny 2003; Sangiovanni 2010; Serste 2012; Villacastin Ruiz 2016). We the risk to be unclear due to lack of information on time interval between index test and reference standard. No study reported non‐evaluable results.
Overall assessment
We assessed all included studies at high risk of bias. We judged three studies at low concern for applicability for all three QUADAS‐2 domains (Sangiovanni 2010; Maiwald 2014; Lin 2016).
Findings
Twenty‐one studies with 3101 participants provided data assessing CT for the diagnosis of hepatocellular carcinoma. The median prevalence of the target disease was 52% (IQR 25% to 62%).
Twenty‐one studies reported the prevalence of participants with hepatic cirrhosis, and in 16 of them the reported prevalence was 100%. Five studies reported the Child Pugh classification with a median of 54% (IQR 19% to 73%) classified as Child‐Pugh class A. Eighteen studies reported information on liver disease aetiology with a median of 51% (IQR 44% to 73%) having viral aetiology. Sixteen studies reported the proportion of participants with resectable hepatocellular carcinoma, among which 12 reported having more than 90% of participants with resectable hepatocellular carcinoma. Thirteen studies reported the mean diameter of the lesions, with a median of 21 mm (IQR 16 mm to 24 mm).
The studies were conducted from 1997 to 2019. Regarding study location, 10 studies were conducted in Europe, seven in North and South America, and four in Asia. Nineteen studies were conducted in people with clinical suspicion of having a hepatocellular carcinoma, and two studies were conducted in the context of a surveillance programme (Chalasani 1999; Sangiovanni 2010). No study reported uninterpretable index test results.
Among the 11 studies with the pathology of explanted liver as the reference standard, five reported no alternative diagnosis in participants without hepatocellular carcinoma (Pozzato 1997; Gambarin‐Gelwan 2000; Lim 2002, Van Thiel 2004; Haberman 2011), Mortele 2001 reported seven macro regenerative nodules in 36 participants without hepatocellular carcinoma, de Ledinghen 2002 reported 16 dysplastic or regenerative nodules in 34 participants without hepatocellular carcinoma, Libbrecht 2002 reported one haemangioma and one focal nodular hyperplasia in 14 participants without hepatocellular carcinoma, Freeny 2003 reported 296 regenerative nodules in 331 participants without hepatocellular carcinoma, Yu 2011 reported six dysplastic or regenerative macronodules two haemangiomas and one focal infarct in 247 participants without hepatocellular carcinoma, and Villacastin Ruiz 2016 reported six cholangiocarcinomas, two haemangiomas, and six dysplastic nodules in 273 participants without hepatocellular carcinoma.
In the five studies with histology of biopsied focal lesions in all participants, one reported no diagnosis other than hepatocellular carcinoma (Hsiao 2019), one reported 24 out of 60 participants with regenerative nodules (Langenbach 2019), one reported "other liver tumours" without any other specification (Lin 2016), whereas Sangiovanni 2010 reported two out of 69 participants with cholangiocarcinoma, and 21 out of 69 macro regenerative nodules or low‐grade dysplastic nodules, and Serste 2012 reported one out of 74 cholangiocarcinoma, one out of 74 epithelioid haemangioendothelioma, nine out of 74 regenerative macro nodule, and nine out of 74 participants with biopsy showing features of chronic liver disease without any features of dysplastic nodule or hepatocellular carcinoma.
Figure 5 shows a forest plot of sensitivity and specificity with 95% CIs. For the 21 studies, the reported sensitivity ranged from 20% to 97% and the specificity ranged from 56% to 100%.
5.
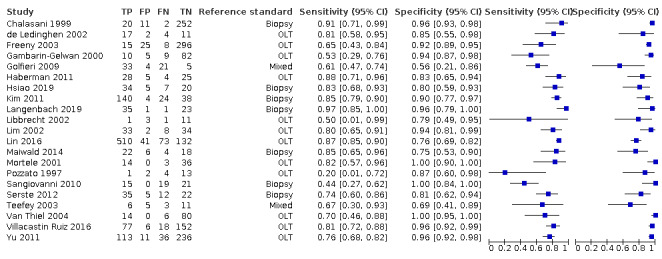
Forest plots of sensitivity and specificity of computed tomography for detection of hepatocellular carcinoma of any size and stage against different reference standards in 21 studies in alphabetical order. Reference standards were: the pathology of the explanted liver in case of transplantation, the histology of resected focal liver lesions, or the histology of biopsied focal liver lesions with a follow‐up period of at least six months.
Values between square brackets are the 95% confidence intervals (CIs) of sensitivity and specificity. The figure shows the estimated sensitivity and specificity of the study (blue square) and its 95% CI (black horizontal line).
CI: confidence interval; FN: false negative; FP: false positive; OLT: orthotopic liver transplantation; TN: true negative; TP: true positive
We performed a meta‐analysis of all 21 included studies using the bivariate model, and we obtained the following pooled estimates: sensitivity 77.5% (95% CI 70.9% to 82.9%), specificity 91.3% (95% CI 86.5% to 94.5%), likelihood ratio: LR+ 8.87 (95% CI 5.67 to 13.86), LR− 0.25 (95% CI 0.19 to 0.32).
Table 3 shows post‐test probabilities, calculated using pooled likelihood ratios, according to three different pre‐test probabilities.
1. Post‐test probabilities.
| Pre‐testprobability | Likelihood ratio | Post‐test probability | |
| 20% | if CT positive | 8.87a | 69% |
| 20% | if CT negative | 0.25b | 6% |
| 52% | if CT positive | 8.87a | 91% |
| 52% | if CT negative | 0.25b | 21% |
| 60% | if CT positive | 8.87a | 93% |
| 60% | if CT negative | 0.25b | 27% |
| CT: computed tomography | |||
aPositive likelihood ratio. bNegative likelihood ratio.
We assessed the diagnostic accuracy for resectable hepatocellular carcinoma as a secondary objective. We found 10 studies that included participants who all had resectable hepatocellular carcinoma (Pozzato 1997; Gambarin‐Gelwan 2000; Mortele 2001; de Ledinghen 2002; Libbrecht 2002; Freeny 2003; Sangiovanni 2010; Yu 2011; Serste 2012; Lin 2016). We performed a meta‐analysis and obtained the following estimates: sensitivity 71.4% (95% CI 60.3% to 80.4%) and specificity 92.0% (95% CI 86.3% to 95.5%). Figure 6 shows the forest plot of sensitivity and specificity with their 95% CIs.
6.
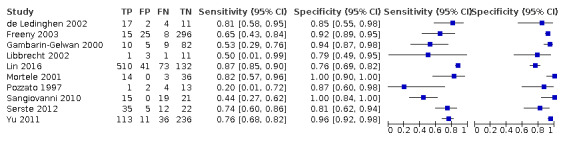
Forest plots of sensitivity and specificity of computed tomography for detection of resectable hepatocellular carcinoma against different reference standards in 12 studies in alphabetical order. Reference standards were: the pathology of the explanted liver in case of transplantation, the histology of resected focal liver lesions, or the histology of biopsied focal liver lesions with a follow‐up period of at least six months.
Values between brackets are the 95% confidence intervals (CIs) of sensitivity and specificity. The figure shows the estimated sensitivity and specificity of the study (blue square) and its 95% CI (black horizontal line).
CI: confidence interval; FN: false negative; FP: false positive; TN: true negative; TP: true positive
Heterogeneity analysis
We investigated heterogeneity for all the predefined potential sources (Secondary objectives). Table 4 shows the comparisons of different predefined subgroups. The prevalence of the target disease, reflecting the selection of participants, may in part explain the inconsistency of the overall results. In fact, studies with a prevalence higher than 52% (the median prevalence in the included studies) show a higher sensitivity (81.0%, 95% CI 72.5% to 87.4% compared to 71.1%, 95% CI 60.7% to 79.7%) and a lower specificity (85.5%, 95% CI 78.4% to 90.5% compared to 94.0%, 95% CI 89.8% to 96.5%) than studies with a prevalence lower than 52%. Another possible source of heterogeneity was the inclusion of more than 90% of study participants with resectable hepatocellular carcinoma, that is, the selection of participants with earlier hepatocellular carcinoma. The sensitivity is marginally lower (72.5%, 95% CI 63.2% to 80.1% compared to 76.2%, 95% CI 63.2% to 85.6%) and specificity higher (93.9%, 95% CI 88.7% to 96.9% compared to 82.4%, 95% CI 61.9% to 93.1%) than in studies including less than 90% of resectable hepatocellular carcinoma. The comparison of the other subgroups, assessing the possible role of study date and location, inclusion of participants without cirrhosis, from surveillance programme or clinical cohorts, previous testing with the detection of nodules, and the use of different reference standard did not show differences. Hepatocellular carcinoma mean diameter had no effect on diagnostic accuracy (P = 0.930).
2. Heterogeneity and sensitivity analyses for computed tomography.
| Subgroup | No of studies | Sensitivity (95% CI) | Specificity (95% CI) | P value |
| All | 21 | 77.5% (70.9% to 82.9%) | 91.3% (86.5% to 94.5%) | ‐ |
| Positivity criteria clearly defined | 19 | 78.3% (72.0% to 83.6%) | 90.7% (85.7% to 94.1%) | ‐ |
| Reference standard blinded | 9 | 77.5% (68.8% to 84.3%) | 91.0% (83.4% to 95.4%) | ‐ |
| Low concern for applicability | 3 | 76.9% (50.8% to 91.5%) | 89.2% (57.0% to 98.1%) | ‐ |
| Before 2005 | 10 | 71.4% (60.5% to 80.3%) | 93.6% (87.7% to 96.7%) | 0.340 |
| After 2005 | 11 | 80.5% (72.3% to 86.7%) | 88.7% (81.1% to 93.5%) | |
| Cirrhosis > 90% | 16 | 75.5% (66.2% to 82.8%) | 93.5% (89.0% to 96.2%) | 0.225 |
| Cirrhosis < 90% | 4 | 85.2% (80.8% to 88.7%) | 81.5% (73.3% to 87.5%) | |
| Europe | 10 | 74.3% (59.7% to 85.0%) | 90.5% (80.8% to 95.6%) | 0.622 |
| North and South America | 7 | 75.0% (65.7% to 82.4%) | 93.7% (87.4% to 96.9%) | |
| Asia | 4 | 85.5% (81.7% to 88.7%) | 85.7% (75.1% to 92.3%) | |
| HCC prevalence ≥ 52% | 11 | 81.0% (72.5% to 87.4%) | 85.5% (78.4% to 90.5%) | 0.051 |
| HCC prevalence < 52% | 10 | 71.1% (60.7% to 79.7%) | 94.0% (89.8% to 96.5%) | |
| Clinically suspect | 19 | 78.5% (72.7% to 83.3%) | 90.2% (84.9% to 93.8%) | 0.333 |
| Surveillance | 2 | 72.3% (29.4% to 94.2%) | 97.3% (88.7% to 99.4%) | |
| HCC resectable 100% | 10 | 71.4% (60.3% to 80.4%) | 92.0% (86.3% to 95.5%) | ‐ |
| HCC resectable < 20% | 2 | 75.6% (55.0% to 88.8%) | 78.3% (42.6% to 94.6%) | 0.116 |
| HCC resectable ≥ 20% | 14 | 73.3% (65.0% to 80.2%) | 93.2% (87.8% to 96.3%) | |
| HCC resectable < 90% | 4 | 76.2% (63.2% to 85.6%) | 82.4% (61.9% to 93.1%) | 0.081 |
| HCC resectable ≥ 90% | 12 | 72.5% (63.2% to 80.1%) | 93.9% (88.7% to 96.9%) | |
| Biopsy | 8 | 82.9% (69.7% ‐ 91.2%) | 90.8% (82.8% ‐ 95.3%) | 0.119 |
| OLT | 11 | 78.8% (73.6% ‐ 83.2%) | 93.2% (88.7% ‐ 96.0%) | |
| Mixed | 2 | 61.9% (49.4% ‐ 73.0%) | 64.0% (44.0% ‐ 80.1%) | |
| Viral < 80%a | 15 | 81.1% (75.5% to 85.7%) | 92.4% (87.5% to 95.4%) | 0.332 |
| Viral ≥ 80%a | 3 | 62.8% (44.6% to 78.0%) | 93.9% (48.2% to 99.6%) | |
| Prior detection of nodules: no | 13 | 79.3% (74.4% to 83.6%) | 92.5% (87.6% to 95.6%) | 0.797 |
| Prior detection of nodules: yes | 8 | 80.9% (67.5% to 89.6%) | 88.6% (78.9% to 94.2%) | |
| CI: confidence interval; HCC: hepatocellular carcinoma; OLT: orthotopic liver transplantation; | ||||
aData not reported in three studies.
Sensitivity analysis
When considering only the 19 studies that clearly prespecified the positivity criteria, we obtained a pooled sensitivity of 78.3% (95% CI 72.0% to 83.6%) and a specificity of 90.7% (95% CI 85.7 to 94.1%; Table 4).
When considering only the nine studies in which the reference standard results were interpreted without knowledge of the results of the index test, we obtained a pooled sensitivity of 77.5% (95% CI 68.8% to 84.3%) and a specificity of 91.0% (95% CI 83.4% to 95.4%; Table 4).
When considering only the three studies at low concern for applicability (Sangiovanni 2010; Maiwald 2014; Lin 2016), we obtained a pooled sensitivity of 76.9% (95% CI 50.8% to 91.5%) and a specificity of 89.2% (95% CI 57.0% to 98.1%).
We did not perform the planned sensitivity analysis in which studies published only in abstract or letter form were excluded because all included studies were published as full texts.
We did not perform the planned sensitivity analysis in which studies at high risk of bias were excluded as we judged all the included studies to be at high risk of bias.
Summary of findings tables
Discussion
Summary of main results
The aim of this review was to assess the diagnostic accuracy of CT for the diagnosis of hepatocellular carcinoma of any size and at any stage in people with chronic liver disease, either in a surveillance programme or in a clinical setting. We included 21 studies that assessed a total of 3101 participants, 19 were conducted in a clinical setting, and two in a surveillance programme. The main results are presented in Table 1 and Table 2.
For the 21 included studies we performed a meta‐analysis using the bivariate model, and we obtained the following pooled estimates: sensitivity 77.5% (95% CI 70.9% to 82.9%) and specificity 91.3% (95% CI 86.5% to 94.5%) for the diagnosis of hepatocellular carcinoma at any size and stage (primary outcome). In Table 3 we show the post‐test probability of having hepatocellular carcinoma in the case of positive or negative result of the index test, assuming different values of pretest probability.
Ten studies included only participants with hepatocellular carcinoma amenable for surgical resection, and the pooled estimate of sensitivity was 71.4% (95% CI 60.3% to 80.4%) and specificity 92.0% (95% CI 86.3% to 95.5%) for the diagnosis of resectable hepatocellular carcinoma (secondary outcome).
We judged all included studies to be at high risk of bias in at least one domain, and we assessed the results of 18 out of 21 studies to be at high concern for applicability in the patient selection domain.
Considering only the three studies at low concern for applicability for patient selection (Sangiovanni 2010; Maiwald 2014; Lin 2016), we obtained a pooled sensitivity of 76.9% (95% CI 50.8% to 91.5%) and a specificity of 89.2% (95% CI 57.0% to 98.1%).
We summarised these main results of analyses in Table 1 and Table 2, assuming three different prevalence values (20%, 52% and 60%). The prevalence of hepatocellular carcinoma varied widely in all included studies, from 7% to 86%, according to the study design and different settings. For exemplification, we considered three values of hepatocellular carcinoma prevalence: 20% for a population with low clinical suspicion, 52% as a median derived from our study analysis, and 60% for a population with high clinical suspicion (assessment of nodules detected by ultrasound).
For participants with hepatocellular carcinoma at any size and stage, we assumed the following consequences of test results: people with true‐positive results, that is, those with hepatocellular carcinoma and positive test results, will receive the appropriate treatment (surgery, local ablative therapy or systemic chemotherapy); people with true‐negative results, that is, those without hepatocellular carcinoma and negative test results, will not undergo inappropriate treatment or unnecessary further testing; people with false‐negative results, that is, those with hepatocellular carcinoma and negative test results, will be misdiagnosed, not receive the appropriate treatment, and might be detected later as more severe hepatocellular carcinoma patient; people with false‐positive results, that is, those without hepatocellular carcinoma and positive test results, will undergo further testing and possibly an inappropriate treatment. Considering a hypothetical cohort of 1000 people with hepatocellular carcinoma prevalence of 52% (the median value in the included studies), we can expect 117 false‐negative and 42 false‐positive results; with a lower prevalence of 20%, we can expect 45 false‐negative and 70 false‐positive results, and with a higher prevalence of 60%, we can expect 135 false‐negative and 35 false‐positive results. We judged the certainty of evidence to be low, downgrading by two levels due to high risk of bias and indirectness.
For participants with resectable hepatocellular carcinoma, considering a hypothetical cohort of 1000 people with hepatocellular carcinoma prevalence of 35%, we can expect 100 false‐negative and 50 false‐positive results; with a prevalence of 20%, we can expect 57 false‐negative and 60 false‐positive results; with a prevalence of 60%, we can expect 166 false‐negative and 30 false‐positive results. We judged the certainty of evidence to be low, downgrading by two levels due to high risk of bias and indirectness.
Strengths and weaknesses of the review
Strengths and weaknesses of included studies
This review included a total of 21 studies, covering a time span of 22 years, from 1997 to 2019 and wide geographical areas, including areas with high and low prevalence of chronic liver disease and hepatocellular carcinoma. Ten studies were conducted in Europe, seven in North and South America, and four in Asia. In terms of number of participants, studies performed in the Americas included 1318 participants, in Asia 1105, and in Europe 678. We found no study from Africa, where hepatocellular carcinoma is highly prevalent (Ferlay 2019).
An overall quality assessment of the studies showed their methodological weaknesses. We assessed all studies at high risk of bias mainly due to inappropriate exclusion criteria, reference standard results interpreted with knowledge of the index test (unavoidable in cases of biopsy), and time interval between index test and reference standard of more than 90 days. The choice of reference standard represents a major concern for all studies, and we recognise none is perfect. The most common reference standard was pathology of the explanted liver (11 studies), the most accurate, allowing the histological evaluation of the whole liver in all participants. However, this almost perfect reference standard is possible only in studies conducted on participants with advanced and decompensated liver diseases on a waiting list for transplantation, that do not represent the intended spectrum of liver disease severity. In fact, we aimed to assess CT accuracy in participants with the whole spectrum of liver disease severity without any exclusion for severity of liver disease or hepatocellular carcinoma volume. Accordingly, correct estimates of CT accuracy can be obtained only at the expense of their applicability. The other reference standards were histology of biopsied focal lesions with adequate follow‐up (8 studies) and mix of pathology of the explanted liver, resection, biopsy, and follow‐up (2 studies). We judged 10 studies in which the time interval between the index test and reference standard was longer than 90 days to be at high risk of bias. In fact, in diagnostic test accuracy assessment, it is necessary to have the time interval between index test and reference standard as short as possible (Colli 2014). Longer time intervals impair accurate assessment due to possible changes in lesion size and morphologic features during certain periods of time. According to the latest systematic review, the approximate hepatocellular carcinoma volume doubling time is four to five months, with significant range of 2.2 months to 11.3 months (Nathani 2021). In accordance with suggestions from a previous systematic review, which noted the acceptable time interval being from one to three months (Kim 2008), we assumed 90 days to be the most acceptable threshold.
We found no studies that reported on uninterpretable results of the index test. Such a failure of reporting or excluding them from the analysis could have produced an overestimation of the obtained accuracy estimates. In fact, in the process of visual interpretation of CT examinations, sometimes it is impossible for the radiologist to make a definite diagnosis of hepatocellular carcinoma. This is primarily due to unclear visual representation and absence of morphological criteria needed for a definite diagnosis (non‐rim like hyperenhancement, non‐peripheral washout in portal‐venous and subsequent phases, enhancing capsule, etc.; LI‐RADS 2018). Technical aspects of a CT examination such as participant movement and breath‐hold, scanning protocol, application of adequate type and amount of contrast, and acquisition of correct phases (arterial, portal‐venous, late phase) can impair liver imaging and its correct interpretation.
Using QUADAS 2, we judged 18 out of 21 studies at high concern for applicability mainly due to the selective inclusion of participants with decompensated advanced liver disease or a definite hepatocellular carcinoma diameter, and the use of pathological examination of the whole liver as the reference standard.
Not all studies reported on all covariates that we planned to assess as a possible source of heterogeneity, and this might have impaired the analyses. Most information on MELD (Model for End‐Stage Liver Disease), Child A and CT detector number was missing.
Strengths and weaknesses of the review process
Search strategy
Our search strategy provided a significant number of studies that were performed in various geographical areas with high and low prevalence of chronic liver disease and hepatocellular carcinoma. Manually searching the references of the included studies and previous narrative and systematic reviews identified three additional studies, which were ultimately included in the final analysis. We applied no language restrictions in the inclusion criteria, which resulted in retrieving full‐text articles of 24 studies published in non‐English languages, two of which we included in the final analysis. We requested further information from study authors regarding two studies, but they did not provide any information. We are confident that the search strategy resulted in the detection of most eligible studies, with a low probability of undetected relevant studies.
Quality assessment and data extraction
We consider our attempts to reduce subjectivity in our judgements to minimise errors and miscalculations in data extraction to be the strength of this review. Two review authors independently assessed the risk of bias of the included studies and applicability of their results using the QUADAS‐2 tool. We extracted data using a proper form. In case of disagreement, we reached consensus through discussion. Disagreements were most frequent for the two QUADAS‐2 domains patient selection (six studies), and reference standard (five studies). All agreements were reached through discussion between two review authors, and the conclusions were discussed and approved by a third review author. For data extraction, most of the discordances were due to miscalculations and typos, which were easily resolved. The same review authors assessed the certainty of evidence using the GRADE approach and the level of agreement was high.
Review analysis
We performed a meta‐analysis using the bivariate model, as the results of the index test were reported as dichotomous (positive or negative) with no explicit threshold. Anyway, we recognise that, nevertheless, implicit thresholds cannot be excluded. The pooled estimates of sensitivity ranged from 20% to 97% and those of specificity from 56% to 100%. Two studies included fewer than 20 participants and their results were quite imprecise with a very wide confidence interval (Pozzato 1997; Libbrecht 2002; Figure 5). Studies with a prevalence of the target disease higher than the median shows higher sensitivity and lower specificity, suggesting that the selection of participants may in part explain the inconsistency of the overall results. The difference in the results between studies that included more than 90% of participants with resectable hepatocellular carcinoma compared to studies that included less than 90% may confirm the possible role of participant selection. Whereas the case mix of the participants in the included studies, at least when adequately reported, seems homogeneous, showing similar numbers and types of alternative diagnosis, encompassing regenerative and dysplastic nodules, and more rarely haemangioma cholangiocarcinoma and focal nodular hyperplasia. Moreover, the study setting (clinical or surveillance programme), different geographic areas, advancements in technology (before and after the year 2005), aetiology of the underlying liver disease, or its severity (prevalence of cirrhosis), hepatocellular carcinoma diameter, and difference in the choice of the reference standards and even prior testing with detection of nodules seem unable to explain the observed inconsistencies.
However, some of our planned investigations were not possible due to lack of data (number of CT detector rows, Child‐Pugh classification of severity of cirrhosis), and lack of published studies (before and after 2016 to assess the changes in diagnostic criteria). Furthermore, we were able to investigate only characteristics that could be assessed at study level whereas participants' factors or hepatocellular carcinoma characteristics can only be assessed by aggregate statistics with the inherent risk of ecological bias. Therefore, some important relationships, such as the one with hepatocellular carcinoma volume, could have been missed. In addition, many of the included studies did not report data on the covariates of interest. Finally, other potential sources of heterogeneity were not planned, and might be assessed in future studies, such as CT slice thickness, contrast injection rate, contrast type, number of exam phases, and collimation.
We excluded studies that reported only per‐lesion analyses and included only studies with per‐patient analyses. Per‐patient and per‐lesion analyses represent two different approaches to diagnostic accuracy assessment and their choice depends on the type of clinical or scientific question, and requires different and appropriate statistical methodology. In the present review, we aimed to assess the accuracy of CT for the diagnosis hepatocellular carcinoma. Consequently, we chose to include studies that evaluated how CT is able to detect patients with hepatocellular carcinoma at any size and any stage, therefore we applied a per‐patient approach. Otherwise, per‐lesion analysis is properly used to assess accuracy in detecting multiple lesions on a single image, providing information that is relevant for hepatocellular carcinoma staging. Studies planning per‐lesion analysis require a different methodological approach and cannot be pooled together with studies using a per‐patient approach (Chang 2006; Zwinderman 2008). Furthermore, the inclusion criteria of studies planning a per‐lesion analysis are quite different and do not match our review question. In fact, they usually do not include participants with chronic liver disease and suspected hepatocellular carcinoma, but participants with known focal liver lesions, encompassing hepatocellular carcinomas, cholangiocarcinomas, benign liver tumours and even metastases from abdominal or extra‐abdominal primary cancers.
We assessed only the impact of the presence of diagnostic criteria on diagnostic accuracy, and we did not assess the differences in various criteria. In our review, most primary studies used perfusion positivity criteria to assess the lesion as hepatocellular carcinoma: non‐rim like hyperenhancement and washout in subsequent phases. A significant number of around 40% of the liver biopsies is reported to be morphologically atypical hepatocellular carcinomas, which surely may impact the accuracy (Kim 2019). The use of perfusion criteria without any additional criteria for non‐hyperenhancing hepatocellular carcinoma in arterial phase could explain the high proportion (more than 25%) of false‐negative results.
We were unable to assess the effect of uninterpretable results on diagnostic accuracy as no study reported such data. Indeed, it is possible that failures in obtaining adequate images were not reported, with consequent overestimation of CT accuracy.
The sensitivity analysis shows that the obtained results are arguably robust with no variation, after including only studies that clearly prespecified the positivity criteria, and including only those in which the reference standard results were interpreted without the knowledge of the results of the index test.
Comparison with previous research
We found 11 non‐Cochrane systematic reviews that assessed the accuracy of CT for detection of hepatocellular carcinomas (Colli 2006;Xie 2011; Chen 2013;Floriani 2013;Chou 2015;Lee 2015;Ye 2015;Guo 2016; Hanna 2016;Roberts 2018; Li 2019). All reviews assessed the accuracy of CT and MRI, and some also assessed ultrasound (Colli 2006; Floriani 2013; Chou 2015; Hanna 2016), contrast‐enhanced ultrasound (Xie 2011), and alpha‐foetoprotein (Colli 2006). Due to differences in methodological approach, inclusion and exclusion criteria, and in statistical analysis, these results are not comparable to each other nor to our present results. The pooled sensitivity of CT for the diagnosis of hepatocellular carcinoma in these reviews ranged from 61% to 86% and the specificity from 64% to 94% (Table 5). Three systematic reviews performed per‐patient analysis (Colli 2006; Floriani 2013; Chou 2015), and the pooled sensitivity and specificity of CT for detection of hepatocellular carcinoma ranged from 67.5% to 83% and 72% to 92.5% (Table 5). These results are in accordance with our present results, despite methodological differences and the number of included studies. We additionally evaluated all the primary studies included in these systematic reviews and assessed them for inclusion in our analysis.
3. Other systematic reviews on diagnostic accuracy of computed tomography for hepatocellular carcinoma.
| Systematicreview | Analysistype | No of included studies | No of patients analysed | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Statistical model |
| Colli 2006 | Per‐patient | 10 | 979 | 67.5 (55 to 80) | 92.5 (89 to 96) | Random‐effects model |
| Floriani 2013 | Per‐patient | 2 | Not reported | 61 (29 to 93) | 72 (50 to 94) | Bivariate random‐effects model |
| Chou 2015 | Per‐patient | 17 | Not reported | 83 (76 to 88) | 91 (84 to 95) | Bivariate logistic mixed random‐effects model |
| Xie 2011 | Per‐lesion | 8 | 1134 | 86 (84 to 88) | 82 (77 to 86) | |
| Chen 2013 | Per‐lesion | 15 | Not reported | 81 (74 to 86) | 93 (88 to 96) | |
| Floriani 2013 | Per‐lesion | 10 | Not reported | 68 (56 to 79) | 64 (21 to 100) | |
| Chou 2015 | Per‐lesion | 80 | Not reported | 76 (72 to 80) | 89 (84 to 93) | |
| Ye 2015 | Per‐lesion | 9 | 469 | 74 (70 to 77) | 93 (91 to 94) | |
| Lee 2015 | Per‐lesion | 17 | 1135 | 72 (75 to 84) | Not estimated | |
| Hanna 2016 | Per‐lesion | 105 | Not reported | 73.6 (70 to 76) | Not estimated | |
| Guo 2016 | Per‐lesion | 12 | 627 | 70 (58 to 80) | 94 (92 to 96) | |
| Roberts 2018 | Per‐lesion | 33 | 2250 | 66 (60 to 72) | 92 (84 to 96) | |
| Li 2019 | Per‐lesion | 8 | 498 | 68 (51 to 81) | 92 (84 to 96) | |
| CI: confidence interval | ||||||
Applicability of findings to the review question
Using the QUADAS‐2 tool, we assessed the applicability of the results of the included studies. We judged most studies to be at high concern in the domains of patient selection and reference standard. In the patient selection domain, the main concerns were the inclusion of only patients on the waiting list for orthotopic liver transplantation with decompensated chronic advanced liver disease or the inclusion of participants according to the hepatocellular carcinoma diameter. The choice of the pathology of the explanted liver as the reference standard also impairs the applicability of the results as this reference standard is applied exclusively to transplanted patients.
Authors' conclusions
Implications for practice.
Hepatocellular carcinoma is a frequent complication of chronic liver disease. The detection of a tumour amenable to surgical resection, thermal ablation, or liver transplantation could improve the prognosis, which in the absence of indications to radical treatment is severe. Being the fourth leading cause of death from cancer worldwide, accurate tests are needed to diagnose hepatocellular carcinoma. In the clinical pathway for the diagnosis of hepatocellular carcinoma in people with chronic liver disease, computed tomography (CT) is currently the second step after ultrasound and alpha‐foetoprotein or the combination of the two, and its main role is to confirm the presence of the disease.
As an ideal diagnostic test, CT should ensure a low proportion of false‐negative results because people with undetected hepatocellular carcinoma cannot receive proper treatment. People with false‐positive results are exposed to unnecessary further diagnostic workup and possible invasive treatment. The estimated pooled sensitivity and specificity derived from our analysis suggest that 22.5% of people with hepatocellular carcinoma would be missed, and 8.7% of people would be unnecessarily treated.
An important piece of clinical information, which is meaningful for further patient workup, is the possibility of surgical resection. Ideally, CT should ensure a low proportion of false‐negative results because people with false‐negative results will not undergo surgical resection, and people with false‐positive results will undergo inappropriate surgical resection. Based on our results 28.6% of people with hepatocellular carcinoma would be incorrectly classified as without any hepatocellular carcinoma and would improperly not be resected, while 7.7% of people with non‐resectable hepatocellular carcinoma will undergo inappropriate surgery. For people on a waiting list for orthotopic liver transplantation for an indication not related to a hepatocellular carcinoma, the consequences of false‐negative results of preoperative CT are not completely known and might be less severe: indeed studies report no significant difference in terms of overall survival and tumour recurrence compared to people with previously diagnosed hepatocellular carcinomas (Castillo 2009; Senkerikova 2014; Madaleno 2015; El Moghazy 2016).
The main hallmarks of hepatocellular carcinoma on a CT study are non‐rim‐like hyperenhancement in arterial phase, and washout in portal‐venous and delayed phases. However, around 40% of hepatocellular carcinomas present with atypical morphological features, which pose a significant diagnostic challenge for radiologists. This significant number of atypical hepatocellular carcinomas may influence the sensitivity, and the radiologist should be acquainted to these atypical appearances to correctly interpret CT findings. Another issue is the presence of hepatocellular carcinoma mimickers, such as intrahepatic cholangiocarcinoma, combined hepatocellular carcinoma‐cholangiocarcinoma, arterioportal shunt, and haemangioma in cirrhotic liver (Lee 2012b; Shirki 2015; Kim 2019).
Apart from correctly classifying people of having hepatocellular carcinoma and assessing them amenable for resection, another important role of CT is to correctly stage the disease as local, regional, or disseminated disease. However, this issue was not the aim of this review.
Overall, caution is needed in interpreting our review results as we judged all the studies at high risk of bias, and most of them with high concern regarding their applicability, mainly due to patient selection and reference standard domain.
Implications for research.
Currently available evidence on the diagnostic accuracy of CT for diagnosis of hepatocellular carcinoma is not conclusive. Therefore, more high‐quality primary studies are needed. With introduction of LI‐RADS criteria, the results of CT studies are no longer needed to be dichotomised allowing inconclusive and probable results to be assessed also. Apart from typical hepatocellular carcinoma appearances, atypical features of hepatocellular carcinoma need to be taken into consideration, so we hypothesise that further studies using LI‐RADS positivity criteria may improve sensitivity. Also, it may be possible that including additional major features such as threshold growth, along with arterial hyperenhancement and subsequent washout may improve sensitivity. Therefore, we welcome future cross‐sectional studies using score systems of positivity criteria. In further research, other sources of heterogeneity may be assessed such as CT slice thickness, contrast injection rate, contrast type, number of exam phases, and collimation.
History
Protocol first published: Issue 6, 2019
Acknowledgements
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of Cochrane Hepato‐Biliary through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, The Capital Region, Rigshospitalet, Copenhagen, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or Copenhagen Trial Unit.
Peer reviewers (CHBG): Rita Golfieri, Italy; Jamie Franklin, UK Peer reviewers through the Cochrane Diagnostic Accuracy Reviews Editorial Team: Joost Daams, The Netherlands; Matthew DF McInnes, Canada; Matthew Grainge, UK Contact Editor from the Cochrane Diagnostic Accuracy Reviews Editorial Team: Karen Steingart, UK Sign‐off editor (CHBG): Christian Gluud, Denmark
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | May 2021 | (computed tomograph* or CT or CECT or MDCT or MSCT or magnetic resonance imaging or MRI or emission tomography or PET) AND (((liver or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) AND (advanc* and chronic and (liver* or hepat*)) |
| The Cochrane Hepato‐Biliary Group Diagnostic Test of Accuracy Studies Register | May 2021 | (computed tomograph* or CT or CECT or MDCT or MSCT or magnetic resonance imaging or MRI or emission tomography or PET) AND (((liver or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) AND (advanc* and chronic and (liver* or hepat*)) |
| The Cochrane Library | 2021, Issue 5 | #1 MeSH descriptor: [Tomography, Emission‐Computed] explode all trees #2 MeSH descriptor: [Tomography, X‐Ray Computed] explode all trees #3 MeSH descriptor: [Magnetic Resonance Imaging] explode all trees #4 (computed tomograph* or CT or CECT or MDCT or MSCT or magnetic resonance imaging or MRI or emission tomography or PET) #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Carcinoma, Hepatocellular] explode all trees #7 MeSH descriptor: [Liver Neoplasms] explode all trees #8 (((liver or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) #9 #6 or #7 or #8 #10 (advanc* and chronic and (liver* or hepat*)) #11 #5 and #9 and #10 |
| MEDLINE Ovid | 1946 to May 2021 | 1. exp Tomography, Emission‐Computed/ 2. exp Tomography, X‐Ray Computed/ 3. exp Magnetic Resonance Imaging/ 4. (computed tomograph* or CT or CECT or MDCT or MSCT or magnetic resonance imaging or MRI or emission tomography or PET).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 5. 1 or 2 or 3 or 4 6. exp Carcinoma, Hepatocellular/ 7. exp Liver Neoplasms/ 8. (((liver or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 9. 6 or 7 or 8 10. (advanc* and chronic and (liver* or hepat*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 11. 5 and 9 and 10 |
| Embase Ovid | 1974 to May 2021 | 1. exp computer assisted tomography/ 2. exp positron emission tomography/ 3. exp nuclear magnetic resonance imaging/ 4. (computed tomograph* or CT or CECT or MDCT or MSCT or magnetic resonance imaging or MRI or emission tomography or PET).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 5. 1 or 2 or 3 or 4 6. exp liver cell carcinoma/ 7. exp liver tumor/ 8. (((liver or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 9. 6 or 7 or 8 10. (advanc* and chronic and (liver* or hepat*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] 11. 5 and 9 and 10 |
| LILACS (Bireme) | 1982 to May 2021 | (computed tomograph$ or CT or CECT or MDCT or MSCT or magnetic resonance imaging or MRI or emission tomography or PET) [Words] and (((liver or hepato$) and (carcinom$ or cancer$ or neoplasm$ or malign$ or tumo$)) or HCC) [Words] and (advanc$ and chronic and (liver$ or hepat$)) [Words] |
| Science Citation Index Expanded (Web of Science) | 1900 to May 2021 | #4 #3 AND #2 AND #1 #3 TS=(advanc* and chronic and (liver* or hepat*)) #2 TS=(((liver or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) #1 TS=(computed tomograph* or CT or CECT or MDCT or MSCT or magnetic resonance imaging or MRI or emission tomography or PET) |
| Conference Proceedings Citation Index – Science (Web of Science) | 1990 to May 2021 | #4 #3 AND #2 AND #1 #3 TS=(advanc* and chronic and (liver* or hepat*)) #2 TS=(((liver or hepato*) and (carcinom* or cancer* or neoplasm* or malign* or tumo*)) or HCC) #1 TS=(computed tomograph* or CT or CECT or MDCT or MSCT or magnetic resonance imaging or MRI or emission tomography or PET) |
Appendix 2. QUADAS 2
| Domain | 1. Patient selection | 2. Index test | 3. Reference standard | 4. Flow and timing |
| Signalling questions and criteria | Q1: "Was a consecutive or random sample of participants enrolled?" Yes ‐ If the study reports on a consecutive or a random selection of patients. No ‐ if the study reports on another form of selection of patients. Unclear ‐ if the study does not report on how the patients were enrolled. Q.2: "Did the study avoid inappropriate exclusions?" Yes ‐ if definitions of exclusion criteria are appropriate (i.e. previous surgery or treatment for hepatocellular carcinoma; patients with cholangiocarcinoma) and all exclusions are reported. No ‐ if exclusion criteria are inappropriate and exclusions are not reported. Unclear ‐ if the study does not report causes of exclusions. |
Q1: "Were the index test results interpreted without knowledge of the results of the reference standard?" Yes ‐ if the study reports that the results of the index test were interpreted without the knowledge of the results of the reference standard. No ‐ if the study reports that results of the index test were interpreted with the results of the reference standard. Unclear ‐ if the study does not report information about blinding of the results of the index test and reference standard. Q2: "Were positivity criteria clearly defined?" Yes ‐ if the study clearly reports positivity criteria (i.e. the minimum diameter of a detectable lesion, exclusion of benign criteria). No ‐ if the study does not report the positivity criteria. Unclear ‐ if the study does not report information about the definition of positivity criteria |
Q1: "Is the reference standard likely to correctly classify the target condition?" Yes ‐ if the reference standard correctly defines the presence/absence of HCC (pathology of explanted liver in a transplant cohort). No ‐ if other reference tests than pathology of explanted liver were used. Unclear ‐ if the study does not report on the reference standard used. Q2: "Were the reference standard results interpreted without the knowledge of the results of the index test?" Yes ‐ if the study reports that the results of the reference standard were interpreted without the knowledge of the results of the index test. No ‐ if the study reports that the results of the reference standard were interpreted with the knowledge of the results of the index test. Unclear ‐ if the study does not report information about blinding of the results of the reference standard and the index test. |
Q1: "Was there an appropriate interval between the index test and the reference standard?" Yes ‐ if the interval between the index test and the reference standard was less than 3 months. No ‐ if the interval was longer than 3 months. Unclear ‐ if the study does not report the interval between the index test and the reference standard. Q2: "Did all participants receive the same reference standard?" Yes ‐ if the study has only one reference standard for all the participants No ‐ if the study has more than one reference standard.(histology of resected focal liver lesion(s), or the histology of focal liver lesion(s) with a follow‐up period of at least six months in the participants with a negative result of the index test) Unclear ‐ if the study information regarding the use of reference standard are unclear Q3: "Were all participants included in the analysis and analysed according to intention to diagnose principle (uninterpretable results considered as false)?" Yes ‐ if all enrolled participants were included in the analysis and uninterpretable index test results were analysed according to the intention to diagnose principle). No ‐ if any participant was excluded from the analysis for any reason or uninterpretable index test results were not analysed according to intention to diagnose principle. Unclear ‐ if the exclusion of participants from the analysis is unclear. |
| Risk of bias |
Could the selection of patients have introduced bias? Low risk: "Yes" for all signalling questions. High risk: "No" for at least one signalling question. |
Could the conduct or interpretation of the index test have introduced bias? Low risk: "Yes" for all signalling questions. High risk: "No" for at least one signalling question or "Unclear" for the Q2. |
Could the reference standard, its conduct, or its interpretation have introduced bias? Low risk: "Yes" for all signalling questions. High risk: "No" for at least one signalling question or "Unclear" for the Q 2. |
Could the participant flow have introduced bias? Low risk: "Yes" for all signalling questions. High risk: "No" for at least one signalling question. |
| Concerns about applicability |
Are there concerns that included participants and setting do not match the review question? Low concern: the participants included in the review represent the participants in whom the tests is used in clinical practice (i.e. surveillance programme in patients with chronic advanced liver disease; clinical cohort of patients with chronic advanced liver disease). High concern: the participants included in the review differ from the participants in whom the tests is used in clinical practice (cohort of patients with advanced and decompensated liver disease, candidates for orthotopic liver transplantation). |
Are there concerns that the index test, its conduct, or interpretation differ from the review question? Low concern: the index test, its conduct or its interpretation does not differ from the way it is used in clinical practice. High concern: the index test, its conduct or its interpretation differs from the way it is used in clinical practice. |
Are there concerns that the target condition as defined by the reference standard does not match the question? High concern: the definition of the target condition as defined by the reference standard does not match the question (i.e. pathology of the explanted liver is feasible only in the case of liver transplant; the natural history and prognosis of HCC detected in explanted liver might be different). Low concern: the definition of the target condition as defined by the reference standard does match the question for all included patients. |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 CT | 21 | 3101 |
| 2 CT for resectable HCC | 10 | 1854 |
1. Test.
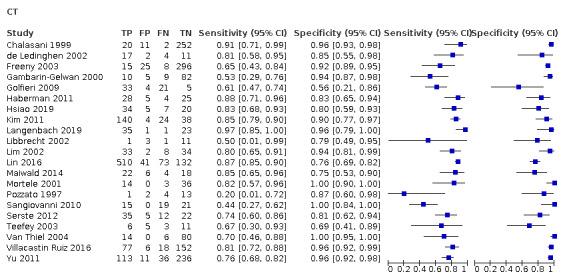
CT
2. Test.
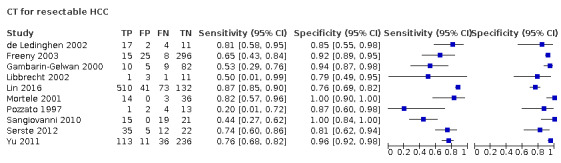
CT for resectable HCC
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chalasani 1999.
| Study characteristics | |||
| Patient Sampling | This was a retrospective analysis of all patients with cirrhosis who were evaluated for liver transplantation in a tertiary referral center from January 1994–December 1997. 166 patients were considered eligible for transplantation, and 27 patients were found to have HCC. |
||
| Patient characteristics and setting | The study included only participants with cirrhosis who underwent liver transplantation. | ||
| Index tests | All abdominal CT scans were performed on a spiral CT scanning machine (CT‐Twin; Elscint, Inc; Rockleigh, NJ). The scanning was dual‐phased in nature and performed after IV injection of 150 mL of nonionic contrast at 4 mL/s. The scanning was performed with a collimated slice width of 5 mm and a reconstruction increment of 4 mm. A suspicious lesion on CT was defined as a solid mass that showed enhancement on arterial phase and was hypo‐, iso‐, or hypervascular on venous‐phase images. In general, ultrasonography technicians performed all the US examinations. Any lesions suspicious for HCC on US or abdominal CT were biopsied under US guidance. Only 1 lesion with the easiest accessibility was biopsied in those who had multiple lesions. | ||
| Target condition and reference standard(s) | The diagnosis of HCC was based on histology in 26 participants. In 1 participant, the diagnosis of HCC was based on characteristic CT and hepatic angiographic findings. The absence of HCC was based on follow‐up with US and CT until liver transplantation or death. | ||
| Flow and timing | No data on interval between index test and reference standard | ||
| Comparative | |||
| Notes | No data on conflict of interest | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
de Ledinghen 2002.
| Study characteristics | |||
| Patient Sampling | This prospective study included 34 participants from February 1997‐July 1999, who had both MRI and spiral CT performed before OLT. In the participant selection process 20 patients were excluded because they did not have both MRI and spiral CT for medical or economic reasons. | ||
| Patient characteristics and setting | The study included patients with cirrhosis who underwent liver transplantation. | ||
| Index tests | On spiral CT, all enhanced nodules during arterial phase were interpreted as HCC. | ||
| Target condition and reference standard(s) | Liver histology of the whole explanted liver was considered as the gold standard for HCC. In all cases, the pathologists were aware of the presence or absence of a HCC diagnosed at radiology and, most of the time, the gross location (right or left lobe) of the tumour was known. | ||
| Flow and timing | Range of the interval between index test and reference standard was 1‐161 days. | ||
| Comparative | |||
| Notes | No data on conflict of interest | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Freeny 2003.
| Study characteristics | |||
| Patient Sampling | This study evaluated 354 consecutive patients who had hepatic transplantation between December 1992‐March 1999. All participants underwent a dual‐phase CT as part of their routine pretransplantation evaluation. All participants underwent OLT only. 61 hyperattenuating nodules were identified on arterial phase CT in 43 participants. | ||
| Patient characteristics and setting | Only study participants who underwent OLT were analysed. | ||
| Index tests | CT ‐ all arterial phase hyperattenuating nodules were considered as potential tumours. | ||
| Target condition and reference standard(s) | After transplantation, all explanted livers were sectioned at 10 mm intervals and each section was evaluated by gross inspection. Nodules suspicious for HCC, were evaluated histologically by hematoxylin and eosin and reticulin‐stained sections. Participants with suspicious liver nodules on pretransplant CT scans were identified at the time of liver sectioning and the scans correlated with the 10 mm sections of the explanted liver. All included participants underwent OLT only. | ||
| Flow and timing | Time interval between index test and ref. standard is 11‐704 days. In the final count of results, 10 participants were missing. | ||
| Comparative | |||
| Notes | No information on conflict of interest | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Gambarin‐Gelwan 2000.
| Study characteristics | |||
| Patient Sampling | This study analysed retrospectively the charts of 106 consecutive adult patients who underwent OLT for treatment of cirrhosis over a 1‐year period at Mount Sinai Hospital. All participants had US, CT, and serum AFP measurements within 6 months of OLT. The results were compared to explant histology. Pathological analysis of 106 explants revealed HCC in 19 participants. | ||
| Patient characteristics and setting | Only patients who underwent OLT were analysed. | ||
| Index tests | CT: All participants underwent conventional CT scans, performed on a GE 9800 CT scanner (General Electric, Milwaukee, WI). Serial transaxial scans were obtained from the diaphragm to the iliac crests by 10 mm collimation sections. Scans were obtained during suspended respiration after administration of oral contrast (E‐Z‐CAT, E‐Z‐M, Inc., Westbury, NY), as well as 150 mL of IV contrast (Omnipaque 240, Sterling Pharmaceuticals, Inc., Barceloneta, Puerto Rico). Radiological examinations were performed by radiologists specialising in the hepatobiliary system. A physician who was blinded to pathology results reviewed US and CT reports. Reports were given a semiquantitative score of 1–4, based upon level of suspicion for HCC |
||
| Target condition and reference standard(s) | HCC. A pathologist specialising in the hepatobiliary system reviewed all liver explants. Each liver explant was sectioned every 1 cm. The presence of tumour nodules, their size, and their location were recorded. The underlying liver pathology was evaluated. | ||
| Flow and timing | The time interval is < 180 days, therefore some participants had interval > 90 days. | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Golfieri 2009.
| Study characteristics | |||
| Patient Sampling | This prospective study was performed at a tertiary liver care centre. 283 consecutive cirrhotic patients were recruited between July 2003‐October 2004. The final study group included 63 participants, out of whom 54 had HCC. 220 participants were excluded for the following reasons: having no nodules or benign regenerative nodules RN (n = 122), large (> 3 cm) HCC (n = 4), previously treated HCC (n = 94). |
||
| Patient characteristics and setting | Patients with HCC > 3 cm were excluded. | ||
| Index tests | CT: quadruple‐phase MDCT (i.e. unenhanced, hepatic arterial, portal‐venous and delayed phases) was performed using a
multidetector‐row CT scanner (Emotion 6, Siemens Medical Systems, Erhlangen, Germany). Positivity criteria: at MDCT and dynamic MRI, nodules showing arterial enhancement plus washout or a coronal enhancement in the portal and/or delayed phase were considered to be typical HCCs, according to the EASL criteria. |
||
| Target condition and reference standard(s) | The final diagnosis was established at pathology on the explanted liver (n = 10), resection (n = 6) and biopsy (n = 38) specimens or at 2 years’ follow‐up (n = 9). | ||
| Flow and timing | The mean imaging‐resection interval was 3 months (range 12‐88 days). | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Haberman 2011.
| Study characteristics | |||
| Patient Sampling | This was a retrospective analysis of patients who were evaluated and had liver transplantation at a tertiary institution from January 2007‐March 2010. All participants included were transplanted and histological analysis of the explanted liver was considered as the final reference of the diagnosis of HCC. Pathological reports were correlated with tomographic reports performed at the time of the comprehensive evaluation of the participants. Patients with time interval between index test and reference standard of > 6 months were excluded. |
||
| Patient characteristics and setting | All participants underwent OLT. | ||
| Index tests | CT. The anatomopathological reports were correlated with the tomographic reports made at the time of the comprehensive evaluation of the participants. The definition of HCC by MDCT ‐ a solid, hypodense lesion without contrast, with moderate to intense and inhomogeneous enhancement in the arterial phase, with isodensity or decreased enhancement (washout) in the portal phase and with confirmation of the enhancement washout in the late phase. |
||
| Target condition and reference standard(s) | Pathology of explanted liver. The anatomopathological reports were correlated with the tomographic reports made at the time of the comprehensive evaluation of the participants. | ||
| Flow and timing | Interval between index test and reference standard is < 180 days | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Hsiao 2019.
| Study characteristics | |||
| Patient Sampling | This is a prospective study, which began on 1 December 2017 and finished on 1 December 2018. The first participant was recruited on 14 December 2017 and the last was recruited on 26 November 2018. Participants were recruited from outpatient clinics. Patients with lesions> 3 cm were excluded. | ||
| Patient characteristics and setting | The study included solitary liver tumour < 3 cm only, and patients without CLD. | ||
| Index tests | CT. Dynamic CT represented quadruple‐phase imaging series (pre‐contrast phase, arterial phase, portal‐venous phase, and equilibrium phase), in which non‐ionic iodine‐based contrast medium was used. Physicians involved in this study were blinded from the results of other examinations while formulating their interpretations. Positivity criteria: 0—not detected, 1—HCC, 2—metastasis, 3—benign tumour, 4—uncertainty |
||
| Target condition and reference standard(s) | The pathology results were treated as a reference diagnosis, which was originally reported by textual description and later classified using the following schema: 0—no tumour, 1—HCC, 2—metastasis, 3—benign tumour. Physicians involved in pathology analysis were blinded from the results of other examinations while formulating their interpretations. | ||
| Flow and timing | No information on interval between index test and reference standard. | ||
| Comparative | |||
| Notes | The authors declare no conflict of interest. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Kim 2011.
| Study characteristics | |||
| Patient Sampling | This study prospectively investigated 206 consecutive patients with hepatic masses > 2 cm. Patients who had undergone CT as a staging work‐up for a known primary extrahepatic malignancy were excluded. Patients who were in the terminal stages of disease and/or who had severe coagulopathy were also excluded because confirmation of the diagnosis would not be helpful for clinical decision and treatment. Patients with intraperitoneal bleeding from spontaneously ruptured tumours were also excluded to perform emergent transarterial chemoembolisation. Patients with inconclusive FNB results were excluded from the analysis and 68 had hepatic nodules between 1‐2 cm in diameter and were therefore excluded from this study. |
||
| Patient characteristics and setting | Patients with liver mass > 2 cm included only | ||
| Index tests | CT examinations were performed using a helical CT (GE Light Speed VCTXT, General Electric Medical Systems, Milwaukee, WI, USA) with a 4‐phase (precontrast, arterial, portal and delayed phases) technique. Index test results were interpreted before reference standard (prospective study). Positivity criteria: CT enhancement patterns of lesions hypervascular in the arterial phase and washed out in the portal/delayed phase were classified as typically vascular. Tumour(s) composed of mixed areas of arterial hyper‐ and hypovascularity, but > 70% hypervascular area, were arbitrarily considered as showing a typical enhancement pattern to exclude the possibility of hepatocholangiocarcinoma. |
||
| Target condition and reference standard(s) | HCC. Biopsy results were considered the gold standard with follow‐up 12 months for benign lesions | ||
| Flow and timing | No information on interval between index test and reference standard | ||
| Comparative | |||
| Notes | The authors declare no conflict of interest. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Langenbach 2019.
| Study characteristics | |||
| Patient Sampling | This is a retrospective study performed at a single centre. All patients participating in this study underwent a Lipiodol‐based angiography, which was followed by percutaneous CT‐guided liver biopsy. From March 2016‐February 2017, 92 consecutive eligible patients were screened, and 36 had HCC. Exclusion criteria: the study excluded all patients whose biopsies showed a histopathologic entity other than HCC or benign regenerative nodules. Additionally, patients without a contrast‐enhanced MRI prior to the angiography were excluded. |
||
| Patient characteristics and setting | Quote: "All patients participating in this study underwent a Lipiodol‐based angiography which was followed by percutaneous CT‐guided liver biopsy. This is according to our local standard procedure for unclear hepatic lesions". The study excluded all patients whose biopsies showed a histopathologic entity other than HCC or benign regenerative nodules. |
||
| Index tests | CT. The evaluation of the lesions in angiography and CT was performed blind by 2 senior radiologists independently, each with > 5 years of experience in diagnostic and interventional radiology. Diagnostic criteria for HCC: combination of 2 independent HCC suspicious criteria | ||
| Target condition and reference standard(s) | Quote: "Biopsy results were considered the gold standard". | ||
| Flow and timing | CT evaluation was performed using the plain CT scan dataset of the upper abdomen used as biopsy planning scan. | ||
| Comparative | |||
| Notes | The authors declare no conflict of interest. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Libbrecht 2002.
| Study characteristics | |||
| Patient Sampling | Between January 2000‐July 2001, a total of 52 patients with liver cirrhosis underwent liver transplantation. Within the 6 months before transplantation, contrast‐enhanced CT was performed in 16 patients (33%). Out of 16 patients, 2 had HCC. 3 patients without chronic HCV infection for whom it was clear that their tumours exceeded the mentioned number and size limits received a donor liver from a patient with positive serological markers for HCV. These 3 patients were excluded from the study. |
||
| Patient characteristics and setting | All participants underwent OLT only. | ||
| Index tests | CT examinations were performed in the setting of pretransplantation evaluation and collected after pathological examination of the explant liver. These reports were made by a fellow and 1 of three different abdominal radiologists who interpreted results of imaging examinations in consensus according to internationally accepted criteria. All cirrhotic explant livers were examined without
knowledge of clinical or imaging data. On contrast‐enhanced CT, nodular lesions that were hypodense during the arterial phase were interpreted as DNs, and enhanced nodules during the arterial phase were interpreted as HCCs. |
||
| Target condition and reference standard(s) | Reference standard: pathology of the whole explanted liver. All cirrhotic explant livers were examined without knowledge of clinical or imaging data. | ||
| Flow and timing | For participants who underwent CT, time interval range was 22‐179 | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Lim 2002.
| Study characteristics | |||
| Patient Sampling | 3‐phase helical dynamic CT in 77 patients with advanced liver cirrhosis was evaluated prospectively before OLT. From February 1998‐April 2002, a total of 108 consecutive adult patients underwent whole liver transplantation. Among them, 77 patients had 3‐phase helical dynamic CT for the evaluation of the liver in terms of hepatic anatomy and liver volume as well as detection of tumour before transplantation, and this group formed the basis of this study. A total of 72 HCCs were confirmed histopathologically in 41 patients. 31 patients who had conventional CT at outside hospitals using third‐generation scanners were excluded from the analysis. |
||
| Patient characteristics and setting | All participants underwent OLT. | ||
| Index tests | 3‐phase helical CT scanning was performed in all of the 77 participants using HiSpeed Advantage helical scanners (General Electric Medical Systems, Milwaukee, WI). The CT criteria for the diagnosis of hepatocellular carcinoma were a nodule showing the enhancement pattern of the hepatic arterial supply and lack of the portal venous supply (e.g. hyperattenuation on the hepatic arterial or portal‐venous phases, low attenuation or isoattenuation on the portal‐venous and delayed phases compared to the adjacent parenchyma). Low attenuating nodules ≥ 2 cm in diameter showing a distinct margin during all 3 phases or on delayed phase were considered HCC. |
||
| Target condition and reference standard(s) | Sectioning of the liver specimens was independently performed without information of CT findings. | ||
| Flow and timing | The time interval between CT examination and surgery was 0∼76 days (mean, 27.3 days), < 90 days | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lin 2016.
| Study characteristics | |||
| Patient Sampling | In this retrospective study between January 2006‐October 2010, 1016 patients underwent liver tumour resections or liver transplantation in the Chang Gang Memorial Hospital, Kaohsiung, Taiwan. Of these, 841 patients underwent liver CT or MRI examinations or had a pathological fibrosis score analysis, and were therefore enrolled in this study. The exclusion criteria were patients who did not undergo liver CT or MRI examination before surgery, did not have a pathological fibrosis score analysis, or did not have liver tumours in the explanted liver. |
||
| Patient characteristics and setting | Participant characteristics and setting match the scope of this review, patients were included regardless of the stage of CLD. No restrictions in HCC lesion size were applied. | ||
| Index tests | CT examinations were performed using a helical CT (Toshiba, 120KVP) with a 4‐phase (non‐contrast, arterial, portal and delayed phases) technique. The study defined typical HCC imaging characteristics as early enhancement in the artery phase and early washout in the venous phase. 4‐phase liver CT or dynamic liver MRI images were read by radiologists with extensive experience in liver and HCC imaging. |
||
| Target condition and reference standard(s) | Reference standard: histological and surgical reports were reviewed to confirm HCC; resection or transplantation. Pathological results were read by pathologists with sufficient experience in the field and who were blinded to the clinical and radiological results. |
||
| Flow and timing | No data on time interval between index test and reference standard | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Maiwald 2014.
| Study characteristics | |||
| Patient Sampling | 50 patients with suspected or proven HCC were included in this prospective single‐centre study to evaluate the diagnostic performance of contrast‐enhanced CT and Gd‐EOB‐DTPA‐enhanced MRI in terms of lesion detection. 26 patients had HCC. Exclusion criteria: renal failure, allergy to contrast agents, hyperthyreoidism, pregnancy and, especially for the MRI‐examination, pacemaker or other non‐compatible implants and claustrophobia |
||
| Patient characteristics and setting | Participant characteristics and setting match the scope of this review, patients were included regardless of the stage of CLD. No restrictions in HCC lesion size were applied. | ||
| Index tests | Multiphase‐CT was performed using 2 different scanners (Brilliance 64/iCT; Philips Healthcare, Eindhoven, Netherlands) with identical parameters. Positivity criteria: diagnosis of HCC was based on hypervascularisation in the arterial phase and washout in the portal‐venous phase or delayed phase. |
||
| Target condition and reference standard(s) | Reference standard: the histopathological report after resection or biopsy of a lesion served as the gold standard for diagnosis, whereas a surrogate of follow‐up (after 6 months) or complementary imaging technique (US, digital subtraction angiography) in combination with clinical (loss of weight, general state) and paraclinical parameters (especially AFP) was used in unresected lesions. Reference standard for positive test were biopsy and liver resection. |
||
| Flow and timing | No information on interval between index test and reference standard. | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Mortele 2001.
| Study characteristics | |||
| Patient Sampling | In this retrospective study from May 1991‐January 1999, 235 patients received hepatic transplants at a tertiary centre, and 53 of them who underwent spiral CT were included in the study. 17 of them had HCC. Exclusion criteria: 49 children (up to age 16 years) because of low incidence of malignancies in childhood, 104 patients operated on before December 1995 (no resected specimens were stored), 32 patients because no or inadequate CT scanning |
||
| Patient characteristics and setting | Patients underwent OLT only | ||
| Index tests | CT scans were performed on a CT unit with available helical CT scanning mode (Somatom Plus 4, Siemens, Erlangen, Germany). | ||
| Target condition and reference standard(s) | Reference standard: final diagnosis was established at pathology on the explanted liver. Specimens were re‐examined in direct correlation with CT results. | ||
| Flow and timing | Mean interval between index test and reference standard was 103 days (range 2‐367 days). | ||
| Comparative | |||
| Notes | No data on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Pozzato 1997.
| Study characteristics | |||
| Patient Sampling | 51 patients with liver cirrhosis were examined in a general hospital and 20 of them had OLT from August 1992‐November 1995. 20 patients were examined by CT, and 5 had HCC. | ||
| Patient characteristics and setting | All participants underwent OLT only as a reference standard. | ||
| Index tests | CT: for each participant the evaluation of each examination was carried out by a radiologist deliberately unaware of the results of the other imaging and laboratory investigations. | ||
| Target condition and reference standard(s) | The final diagnosis was established at pathology on the explanted liver. | ||
| Flow and timing | Mean time interval between index test and reference standard was 165 days (range 60‐420 days) | ||
| Comparative | |||
| Notes | No data on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Sangiovanni 2010.
| Study characteristics | |||
| Patient Sampling | In this prospective study, 64 patients with 67 de novo liver nodules (55 with a size of 1‐2 cm) were consecutively examined by contrast enhanced‐US, CT, MRI, and FNB as diagnostic standard. Excluded were patients with a pre‐existing nodule, poor liver function (ChildePugh C) indicating liver transplantation independently of HCC, or an echo‐coarse US pattern of the liver without a well‐defined nodule. |
||
| Patient characteristics and setting | Participant characteristics and setting match the scope of this review, patients were included regardless of the stage of CLD. No restrictions in HCC lesion size were applied. | ||
| Index tests | CT: CT scan was performed with a 64‐multidetector row CT (MDCT; Definition Siemens, Erlangen, Germany. The typical radiological pattern of HCC was arterial hypervascularisation followed by portal/venous contrast washout of the nodule. |
||
| Target condition and reference standard(s) | Reference standard: the diagnostic gold standard was histology through an FNB performed within the nodule and the surrounding liver parenchyma. All participants underwent biopsy of US‐detected nodule even if the nodule was not detected by CT. | ||
| Flow and timing | No information on interval between index test and reference standard Out of 64 included patients, only 55 were analysed. |
||
| Comparative | |||
| Notes | The authors declare no CoI. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Serste 2012.
| Study characteristics | |||
| Patient Sampling | 75 consecutive patients with CLD with US‐detected 1‐2 cm nodules underwent, within 1 month, multiphasic CT, MRI, and biopsy of the nodule. Exclusion criteria: 1 patient was excluded due to inconclusive diagnosis, and underwent RFA. |
||
| Patient characteristics and setting | Patients with solitary liver tumour 1‐2 cm included only. | ||
| Index tests | CT and MRI results were read by two radiologists in consensus (V.B. and M‐P.V.) who were blind to biopsy results. Vascular pattern was qualified as ‘‘conclusive’’ for HCC if contrast washout occurred, defined as the presence of hypervascularity during the arterial phase followed up by a hypodense/hypointense appearance in later phases defining washout. Nodules in which an enhancement was found during the arterial phase without washout were qualified as ‘‘suspicious.’’ |
||
| Target condition and reference standard(s) | The diagnostic ‘‘gold standard,’’ or the reference method for diagnosis, was the results of biopsy. All biopsies were routinely read by 1 pathologist (P.B.), then independently reviewed in a blinded manner by a second pathologist (V.P.) who was unaware of the previous pathological diagnosis and imaging results. |
||
| Flow and timing | Interval between index test and ref. standard was < 1 month. 1 patient was withdrawn from the study because the studied nodule with washout on both examinations, without conclusive diagnosis on biopsy, underwent RFA. |
||
| Comparative | |||
| Notes | No data on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Teefey 2003.
| Study characteristics | |||
| Patient Sampling | Between August 1996‐December 1998, this prospective study examined 37 patients with end‐stage liver disease who had been listed for hepatic transplantation. 10 of the patients either died prior to liver transplantation or their names were removed from the transplant list. 2 patients whose names had been on the transplant list for > 2 years were not included in the study because of an inability to obtain follow‐up images. The remaining 25 patients formed the study population. 9 patients were confirmed to have HCC. | ||
| Patient characteristics and setting | Only participants with end‐stage liver disease on waiting list for OLT were included | ||
| Index tests | CT studies were performed on Somatom Plus 4; Siemens Medical Systems, Iselin, NJ. The following criteria were used to evaluate HCC.
|
||
| Target condition and reference standard(s) | The criterion standard for diagnosis of HCC was histologic examination of the resected liver and liver biopsy. 21 patients underwent liver transplantation, and 4 underwent biopsy of the pertinent liver lesion observed at ≥ 1 of the imaging tests, with unclear follow‐up). The presence or absence of all lesions identified with ≥ 1 of the imaging tests (CT, MRI, US, or PET) was determined histologically on a lesion‐by‐lesion basis. |
||
| Flow and timing | The interval between the last imaging study and the liver transplantation in the 21 participants who had a liver transplant ranged from 1‐15 months (mean, 5.3 months). | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Van Thiel 2004.
| Study characteristics | |||
| Patient Sampling | Individuals with end‐stage liver disease due to any cause, who were evaluated and found to be free of an identifiable HCC and who met United Network for Organ Sharing (UNOS) criteria for listing for liver transplantation, were included in the study.
From October 1998 through July 2003, a total of 300 individuals were evaluated and presented to the liver transplant review board at Loyola University Medical Center. Of these, 282 were listed for transplantation. 15 of these cases were identified as having an HCC at the time of listing and 5 were listed because of fulminant hepatic failure. These cases were eliminated from the subsequent analysis leaving a total of 262 listed liver transplant candidates. Of these, 105 (41%) were transplanted with 4 individuals receiving two and 1 individual receiving three transplants. These later cases receiving multiple transplants were eliminated leaving 100 cases for analysis.Of these, 5 cases (5%) were thought to have
developed an HCC while on the waiting list utilising the US findings and were treated with ethanol injections but were found to have no evidence of HCC when the explanted liver was examined pathologically and included in no HCC group. Exclusion criteria: exclusion criteria: participants having an HCC at the time of listing, listed because of fulminant hepatic failure and receiving multiple transplants. |
||
| Patient characteristics and setting | All participants underwent OLT. | ||
| Index tests | CT positivity criteria: the criteria used to identify a hepatic tumour by CT consisted of the identification of a focal lesion 6 mm in diameter with early arterial enhancement. | ||
| Target condition and reference standard(s) | Reference standard: pathologist’s identification of a tumour on the explanted liver The most recent CT findings were utilised by the pathologist to identify lesions that were identified prior the transplantation for the presence of tumour. |
||
| Flow and timing | The time from recognition of the hepatic tumour to the date of liver transplant was 247.2 +/‐ 53.8 days | ||
| Comparative | |||
| Notes | No information on CoI | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Villacastin Ruiz 2016.
| Study characteristics | |||
| Patient Sampling | From November 2001‐December 2011, 323 OLTs were performed on 313 patients at our centre. Our study is based on the retrospective analysis of data from 273 patients (213 men and 60 women), of an average age of 55 years (31–79), who underwent scheduled transplants because of cirrhosis. A total of 273 consecutive patients with 218 hepatocellular carcinoma (HCC) nodules, who underwent imaging and subsequent transplantation, were examined. Exclusion criteria were as follows: having undergone urgent nonelective transplants; having undergone retransplantation; and absence of cirrhosis. |
||
| Patient characteristics and setting | All participants underwent OLT. | ||
| Index tests | CT: abdominal examinations were performed using a 2‐slice C Siemens (Siemens Medical Solutions, Forchheim, Germany; November 2001‐May 2009) and a 64‐slice TC Siemens Somaton Sensation 64 (Siemens Medical Solutions; June 2009‐December 2011). Positivity criteria: lesions suggesting HCC were typically characterised by hypervascularity, especially when accompanied by venous‐phase washout. |
||
| Target condition and reference standard(s) | The pathological analysis of the explant livers provided the reference standard. Correlation of nodules between the image and pathological results was based primarily on location and secondarily on size. | ||
| Flow and timing | Time interval between index test and reference standard was 105 days. Out of 273 patients, 253 patients were analyzed. |
||
| Comparative | |||
| Notes | The authors declare no CoI. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Yu 2011.
| Study characteristics | |||
| Patient Sampling | The study analysed data from 638 consecutive adult patients with cirrhosis who received liver transplants within 6 months of imaging at a tertiary care institution. Exclusion criteria: patients with studies at outside imaging centres were not included in the study. Previously detected lesions diagnosed as HCC that had undergone locoregional treatment, including thermal or chemical ablation and transarterial chemoembolisation (TACE), before imaging were excluded from analysis. |
||
| Patient characteristics and setting | All participants underwent OLT. | ||
| Index tests | CT examinations were performed on single‐slice (HighSpeed CT/i, GE Medical Systems; Picker PQ 6000, Picker International,
Cleveland, OH), 4‐slice multidetector (LightSpeed QX/I; GE Medical Systems), or 16 to 64‐slice multidetector (Sensation 16, Sensation 64, or Definition 64; Siemens Medical Solutions, Erlangen, Germany) helical scanners with a multiphasic protocol. Positivity criteria: lesions suspicious for HCC were typically characterised by ≥ 1 of the following features:
|
||
| Target condition and reference standard(s) | Imaging reports and serum AFP levels were compared with results from pathology analysis of explants as the reference standard. Pathologists were not specifically provided with the imaging reports regarding number and locations of any suspected lesions. |
||
| Flow and timing | Mean imaging‐transplantation interval was 2.1 months. | ||
| Comparative | |||
| Notes | The authors declare no CoI. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| Were positivity criteria clearly defined? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis and analysed according to intention to diagnose principle (non‐evaluable results considered as false)? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
AFP: alpha‐foetoprotein; CLD: chronic liver disease; CoI: conflict of interest; CT: computed tomography; EASL: European Association for the Study of the Liver; FNB: fine‐needle biopsy; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; IV: intravenous; MDCT: multidetector computed tomography; MRI: magnetic resonance imaging; OLT: orthotopic liver transplantation; PET: positron emission tomography; RFA: radiofrequency ablation; US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelfattah 2013 | No data on per‐patient analysis are available as well as no data for CT. |
| Addley 2011 | This is a case‐control study. |
| Alaboudy 2011 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. The study included a group with previously known HCC, and no data exist on per‐patient analysis. |
| Alhasan 2019 | The purpose of this study was to evaluate the diagnostic performance of LI‐RADS version 2017 major features, ancillary features, and categories on CT for the diagnosis of HCC. There is no data on per‐patient analysis. |
| Amadei 2008 | A group of patients with previously known HCC was included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| An 2019 | This is a case‐control study. |
| Aqel 2005 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Ascha 2009 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Aubé 2017 | No data on per‐patient analysis were reported. |
| Babar 2019 | Data were provided only for the accuracy of CT in detecting metastatic liver lesions, and primary liver tumours were grouped as a single entity. |
| Baron 1996 | The study included participants with previously known HCC, and no data on per‐patient analysis was provided. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Basha 2018 | No data on per‐patient analysis were reported. |
| Beal 2014 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Bhattacharjya 2004 | The study patient group included patients with known HCC. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Born 1998 | No data on per‐patient analysis were reported. The target condition was not HCC, rather focal liver lesions in general. |
| Brancatelli 2003 | No data on per‐patient analysis were reported. |
| Brehmer 2018 | No data on per‐patient analysis were reported. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Burrel 2003 | This is a case‐control study. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Camera 1999 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. No data on per‐patient analysis were reported. |
| Chen 1982 | The study included patients with previously known HCC. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Chen 2006 | The index test was contrast‐enhanced US, not CT. Patients with previously known HCC were included. |
| Choi 2018 | Patients with previously known HCC were included. No data on per‐patient analysis were reported. |
| Clevert 2009 | The target condition was malignant or benign hepatic tumours in general, no data specific for HCC were present. |
| Dai 2008 | No data on per‐patient analysis were reported. |
| Denies 2002 | No data on per‐patient analysis were reported. |
| De Santis 1992 | No data on per‐patient analysis were reported. |
| Di Martino 2010 | No data on per‐patient analysis were reported. |
| Di Martino 2013 | No data on per‐patient analysis were reported. |
| Fasani 1999 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Fausto 2011 | No data on per‐patient analysis were reported. |
| Frey 2015 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Fukunaga 2007 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Furlan 2012 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Gaiani 2004 | The index test was US not CT. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Garetti 1988 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Gattoni 1993 | Patients with previously known HCC were included. |
| Giangregorio 2009 | Patients with previously treated HCC were included. |
| Giorgio 2004 | Patients with previously known were HCC included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Granito 2013 | The index test was MRI, not CT. No data on per‐patient analysis were reported. |
| Grat 2018 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Gul 2018 | The index test was MRI not CT. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Haberman 2013 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Habermann 2002 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Hafeez 2011 | The aim of the study was to assess the diagnostic accuracy of triphasic spiral CT in differentiating benign from malignant focal tumoral liver lesions, not HCC specifically. No data on per‐patient analysis were reported. |
| Hafeez 2020 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Hasinuzzaman 2018 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Hidaka 2013 | No data on per‐patient analysis were reported. |
| Hirakawa 2011 | Patients with treated HCC were included. No data on per‐patient analysis were reported. |
| Hori 2002 | No data on per‐patient analysis were reported. |
| Iavarone 2019 | No data on per‐patient analysis were reported. |
| Ichikawa 2002 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. No data on per‐patient analysis were reported. |
| Ichikawa 2006 | Patients with previously known HCC were included. |
| Ichikawa 2010 | No data on per‐patient analysis were reported. |
| Ichikawa 2021 | No data on per‐patient analysis were reported. |
| Imbriaco 2017 | No data on per‐patient analysis were reported. |
| Inoue 1994 | No data on per‐patient analysis were reported. |
| Ismail 1990 | No data on per‐patient analysis were reported. |
| Itai 1981 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Iwamura 1982 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Jia 2017 | This is a case‐control study. |
| Jin 2013 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Jin 2016 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Jonczyk 2017 | Patients with previously treated HCC were included. |
| Jung 2005 | The target condition were focal liver lesions in general, not HCC specifically. |
| Jung 2007 | The index test was contrast‐enhanced US with quantitative evaluation, not CT. |
| Kader 2017 | The target condition were focal liver lesions in general, not HCC specifically. |
| Kakihara 2014 | No data on per‐patient analysis were reported. |
| Kan 2010 | The index test was contrast‐enhanced US, not CT. No data on per‐patient analysis were reported. |
| Kanematsu 1997a | The index test was combined CT hepatic arteriography with CT arterial portography, not the type of CT imaging of interest for this review. |
| Kanematsu 1997b | No data on per‐patient analysis were reported. |
| Kato 2004 | The index test is MRI, not CT. |
| Kawamori 1991 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Kawata 2002 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Khalili 2011 | No data on per‐patient analysis were reported. |
| Kim 2005 | The aim of this study was to assess the value of contrast‐enhanced sonography for the characterisation of focal hepatic lesions, not HCC specifically. |
| Kim 2006 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Kim 2007a | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Kim 2007b | One of the index tests was the combination of CT hepatic arteriography with CT arterial portography, not the type of CT imaging of interest for this review. Patients with previously known HCC were included. |
| Kim 2009 | This is a case‐control study. |
| Kim 2017 | This is a case‐control study. |
| Kim 2018 | Patients with previously known HCC included. |
| Kim 2019 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Kurucay 2017 | Index test was CT perfusion imaging, not the type of CT imaging of interest for this review. |
| Laroia 2013 | The index test was contrast‐enhanced US. |
| Laroia 2016 | The index test was dual‐energy CT, not the type of CT imaging of interest for this review. |
| Lee 2003 | Patients with previously known HCC were included. No data on per‐patient analysis were reported. |
| Lee 2009 | > 5% of patients with previously treated HCC were included. |
| Lee 2012c | Patients with previously known HCC were included. |
| Li 2018 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Lin 2011 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Liu 2012 | No data on per‐patient analysis were reported. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Liu 2016 | No data on per‐patient analysis were reported. |
| Luca 2010 | No data on per‐patient analysis were reported. |
| Lucatelli 2020 | No data on per‐patient analysis were reported. |
| Maciel 2006 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Maetani 2008 | Patients with previously known HCC were included. No data on per‐patient analysis were reported. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Malagari 1999 | The index test was CT portography and post‐Lipiodol CT, not the type of CT imaging of interest for this review. |
| Manini 2013 | No data on per‐patient analysis were reported. |
| Marcato 1999 | The index test was Lipiodol CT, not the type of CT imaging of interest for this review. |
| Marin 2009a | No data on per‐patient analysis were reported. |
| Marin 2009b | No data on per‐patient analysis were reported. |
| Masuda 2017 | No data on per‐patient analysis were reported. |
| Mehana 2019 | No data on per‐patient analysis were reported. |
| Miller 1991 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Miller 1994 | No data on per‐patient analysis were reported. |
| Min 2020 | No data on per‐patient analysis were reported. |
| Mok 2004 | The index test was Lipiodol CT, not the type of CT imaging of interest for this review. |
| Moudgil 2017 | No data on per‐patient analysis were reported. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Nakamura 2000 | Patients with previously known HCC were included. No data on per‐patient analysis were reported. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Nusbaum 2015 | Patients with previously known HCC were included. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Oberstein 1996 | Target conditions were different liver diseases, not HCC specifically. |
| Paul 2007 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Peterson 2000 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Pocha 2013 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Putz 2019 | The index test was contrast‐enhanced US for detection of liver lesions, not HCC specifically. |
| Ren 2015 | No data on per‐patient analysis were reported. |
| Ronzoni 2007 | > 5% of patients with previously treated HCC were included. |
| Ryu 2014 | Target condition was focal liver masses, not HCC specifically. No data on per‐patient analysis were reported. |
| Saada 1994 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Sano 2011 | Patients with previously known HCC were included. |
| Sekoguchi 1994 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Semaan 2020 | Patients previously treated for HCC were included. |
| Semelka 1992 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. No data on per‐patient analysis were reported. |
| Seo 2019 | Patients with previously known HCC were included. |
| Shapiro 1996 | Patients with previously known HCC were included. |
| Silberhumer 2004 | Patients with previously known HCC were included. No data on per‐patient analysis were reported. |
| Singh 2007 | Target conditions were primary liver tumours, not HCC specifically. |
| Sofue 2011 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Soyer 1994 | The index test was CT arterial portography for detection of primary malignant liver neoplasms, not HCC specifically. |
| Suarez‐Munoz 2006 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Sugimoto 2015 | Patients with previously known HCC were included, No data on per‐patient analysis were reported. |
| Takayasu 1990 | Patients with previously known HCC were included. |
| Tang 2018 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Valls 2004 | No data on per‐patient analysis were reported. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Wang 1997 | No data on per‐patient analysis were reported. |
| Wang 2007 | The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Wang 2018 | Patients with previously known HCC were included. No data on per‐patient analysis were reported. |
| Wang 2019 | Patients with previously known HCC were included. |
| Watanabe 1986 | Patients with previously known HCC were included. |
| Yamashita 1996 | No data on per‐patient analysis were reported. The study did not report the 2x2 table and we could not calculate/extract it based on reported data. |
| Yim 2016 | No data on per‐patient analysis was reported, index test was CT hepatic arteriography and portography, not the type of CT of interest for this review. |
| Yukisawa 2007 | No data on per‐patient analysis were reported. |
| Zacherl 2002 | Patients with previously known HCC included and only analysis per lesion is presented. |
| Zhao 2007 | No data on per‐patient analysis. |
CT: computed tomography; HCC: hepatocellular carcinoma; MRI: magnetic resonance imaging; US: ultrasound
Differences between protocol and review
We did not use Covidence to manage the selection of studies (Covidence).
We added an additional potential source of heterogeneity: prior testing with detection of liver nodules. We recognised that the retesting was different in the included studies, and some studies included participants with previously detected liver nodules.
We performed a sensitivity analysis considering only the studies at low concern for applicability.
Contributions of authors
TN wrote the protocol and performed searches for references, evaluated references for obtaining the full reports, evaluated studies for inclusion, extracted data from studies, assessed the risk of bias, and wrote the final review. VG commented on the protocol and performed searches for references, evaluated references for obtaining the full reports, evaluated studies for inclusion, extracted data from studies, assessed the risk of bias, and wrote the final review. AC co‐ordinated the protocol design and designed and wrote the final review. MF performed searches for references and critically commented on the review. GC wrote the protocol, provided statistical expert opinion and critically commented on the final review. DM commented on the protocol and will critically comment on the final review. DŠ critically commented on the protocol, will act as arbiter if review authors cannot reach a consensus, and critically commented on the final review.
All authors approved the publication of the review.
Sources of support
Internal sources
None, Other
External sources
None, Other
Declarations of interest
TN: none known VG: none known AC: none known MF: none known GC: none known DM: none known DŠ: none known
New
References
References to studies included in this review
Chalasani 1999 {published data only}
- Chalasani N, Horlander JC Sr, Said A, Hoen H, Kopecky KK, Stockberger SM Jr, et al. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. American Journal of Gastroenterology 1999;94(10):2988-93. [DOI] [PubMed] [Google Scholar]
de Ledinghen 2002 {published data only}
- Lédinghen V, Laharie D, Lecesne R, Le Bail B, Winnock M, Bernard PH, et al. Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? A prospective study of 88 nodules in 34 patients. European Journal of Gastroenterology & Hepatology 2002;14(2):159-65. [DOI] [PubMed] [Google Scholar]
Freeny 2003 {published data only}
- Freeny PC, Grossholz M, Kaakaji K, Schmiedl UP. Significance of hyperattenuating and contrast-enhancing hepatic nodules detected in the cirrhotic liver during arterial phase helical CT in pre-liver transplant patients: radiologic-histopathologic correlation of explanted livers. Abdominal Imaging 2003;28(3):333-46. [DOI] [PubMed] [Google Scholar]
Gambarin‐Gelwan 2000 {published data only}
- Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. American Journal of Gastroenterology 2000;95(6):1535-8. [DOI] [PubMed] [Google Scholar]
Golfieri 2009 {published data only}
- Golfieri R, Marini E, Bazzocchi A, Fusco F, Trevisani F, Sama C, et al. Small (≤3 cm) hepatocellular carcinoma in cirrhosis: the role of double contrast agents in MR imaging vs. multidetector-row CT. Radiologia Medica 2009;114:1239. [DOI] [PubMed] [Google Scholar]
Haberman 2011 {published data only}
- Haberman D, Mela M, Martínez A, Mancinelli A, Laguens R, Gruz F, et al. Accuracy of multislice computed tomography in the diagnosis of hepatocellular carcinoma in patients with cirrhosis evaluated for liver transplantation [Precisión de la tomografía multidetector en el diagnóstico de hepatocarcinoma en pacientes con cirrosis evaluados para trasplante hepático]. Acta Gastroenterologica Latinoamericana 2011;41(3):190-8. [PubMed] [Google Scholar]
Hsiao 2019 {published data only}
- Hsiao CY, Chen PD, Huang KW. A prospective assessment of the diagnostic value of contrast-enhanced ultrasound, dynamic computed tomography and magnetic resonance imaging for patients with small liver tumors. Journal of Clinical Medicine 2019;8(9):1353-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim SE, Lee HC, Shim JH, Park HJ, Kim KM, Kim PN, et al. Noninvasive diagnostic criteria for hepatocellular carcinoma in hepatic masses > 2 cm in a hepatitis B virus-endemic area. Liver International 2011;31(10):1468-76. [DOI] [PubMed] [Google Scholar]
Langenbach 2019 {published data only}
- Langenbach MC, Vogl TJ, den Driesch I, Kaltenbach B, Scholtz JE, Hammerstingl RM, et al. Analysis of Lipiodol uptake in angiography and computed tomography for the diagnosis of malignant versus benign hepatocellular nodules in cirrhotic liver. European Radiology 2019;29(12):6539-49. [DOI] [PubMed] [Google Scholar]
Libbrecht 2002 {published data only}
- Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, et al. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transplantation 2002;8(9):749-61. [DOI] [PubMed] [Google Scholar]
Lim 2002 {published data only}
- Lim JH, Kim MJ, Chiang LW, Lim HK, Park CK, Paik SW, et al. CT detection of hepatocellular carcinoma in advanced liver cirrhosis: correlation of helical CT and explanted liver. Taehan Kan Hakhoe Chi 2002;8(2):201-8. [PubMed] [Google Scholar]
Lin 2016 {published data only}
- Lin MT, Wang CC, Cheng YF, Eng HL, Yen YH, Tsai MC, et al. Comprehensive comparison of multiple-detector computed tomography and dynamic magnetic resonance imaging in the diagnosis of hepatocellular carcinoma with varying degrees of fibrosis. PLOS One 2016;11(11):e0166157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maiwald 2014 {published data only}
- Maiwald B, Lobsien D, Kahn T, Stumpp P. Is 3-Tesla Gd-EOB-DTPA-enhanced MRI with diffusion-weighted imaging superior to 64-slice contrast-enhanced CT for the diagnosis of hepatocellular carcinoma? PLOS One 2014;9(11):e111935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mortele 2001 {published data only}
- Mortelé KJ, De Keukeleire K, Praet M, Van Vlierberghe H, Hemptinne B, Ros PR. Malignant focal hepatic lesions complicating underlying liver disease: dual-phase contrast-enhanced spiral CT sensitivity and specificity in orthotopic liver transplant patients. European Radiology 2001;11(9):1631-8. [DOI] [PubMed] [Google Scholar]
Pozzato 1997 {published data only}
- Pozzato C, Baldini U, Gattoni F, Raiteri R, Lazzerini F, Mevoli A, et al. Diagnostic imaging to select the candidates to orthotopic liver transplantation: experience in a general hospital [Diagnostica per immagini nella selezione dei candidati al trapianto ortotopico del fegato: esperienza in un ospedale non dotatto di centro per trapianti]. Radiologia Medica 1997;93:715-9. [PubMed] [Google Scholar]
Sangiovanni 2010 {published data only}
- Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59(5):638-44. [DOI] [PubMed] [Google Scholar]
Serste 2012 {published data only}
- Sersté T, Barrau V, Ozenne V, Vullierme MP, Bedossa P, Farges O, et al. Accuracy and disagreement of computed tomography and magnetic resonance imaging for the diagnosis of small hepatocellular carcinoma and dysplastic nodules: role of biopsy. Hepatology (Baltimore, Md.) 2012;55(3):800-6. [DOI] [PubMed] [Google Scholar]
Teefey 2003 {published data only}
- Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology 2003;226(2):533-42. [DOI] [PubMed] [Google Scholar]
Van Thiel 2004 {published data only}
- Van Thiel DH, Yong S, Li SD, Kennedy M, Brems J. The development of de novo hepatocellular carcinoma in patients on a liver transplant list: frequency, size, and assessment of current screening methods. Liver Transplantation 2004;10(5):631-7. [DOI] [PubMed] [Google Scholar]
Villacastin Ruiz 2016 {published data only}
- Villacastín Ruiz E, Caro-Patón Gómez A, Calero Aguilar H, Pérez Saborido B, García Pajares F, Sánchez Antolín G, et al. Review of imaging techniques in the diagnosis of hepatocellular carcinoma in patients who require a liver transplant. European Journal of Gastroenterology & Hepatology 2016;28(4):412-20. [DOI] [PubMed] [Google Scholar]
Yu 2011 {published data only}
- Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clinical Gastroenterology and Hepatology 2011;9(2):161-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelfattah 2013 {published data only}
- Abdelfattah MR, Al-Mana H, Neimatallah M, Elsiesy H, Al-Sebayel M, Broering DC. Radiological and pathological correlation for HCC treated with liver transplantation. Hepatology (Baltimore, Md.) 2013;58(4 Suppl):955A. [Google Scholar]
Addley 2011 {published data only}
- Addley HC, Griffin N, Shaw AS, Mannelli L, Parker RA, Aitken S, et al. Accuracy of hepatocellular carcinoma detection on multidetector CT in a transplant liver population with explant liver correlation. Clinical Radiology 2011;66(4):349-56. [DOI] [PubMed] [Google Scholar]
Alaboudy 2011 {published data only}
- Alaboudy A, Inoue T, Hatanaka K, Chung H, Hyodo T, Kumano S, et al. Usefulness of combination of imaging modalities in the diagnosis of hepatocellular carcinoma using Sonazoid-enhanced ultrasound, gadolinium diethylene-triamine-pentaacetic acid-enhanced magnetic resonance imaging, and contrast-enhanced computed tomography. Oncology 2011;81(Suppl 1):66-72. [DOI] [PubMed] [Google Scholar]
Alhasan 2019 {published data only}
- Alhasan A, Cerny M, Olivié D, Billiard JS, Bergeron C, Brown K, et al. LI-RADS for CT diagnosis of hepatocellular carcinoma: performance of major and ancillary features. Abdominal Radiology (NY) 2019;44(2):517-28. [DOI] [PubMed] [Google Scholar]
Amadei 2008 {published data only}
- Amadei E, Orefice R, Koch M. Diagnosis of hepatocellular carcinoma in patients with cirrhosis: contrast-enhanced ultrasound versus contrast-enhanced helical CT. Digestive and Liver disease 2008;40(Suppl):S103. [Google Scholar]
An 2019 {published data only}
- An C, Lee CH, Byun JH, Lee MH, Jeong WK, Choi SH, et al. Intraindividual comparison between gadoxetate-enhanced magnetic resonance imaging and dynamic computed tomography for characterizing focal hepatic lesions: a multicenter, multireader study. Korean Journal of Radiology 2019;20(12):1616-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aqel 2005 {published data only}
- Aqel B, Sachs W, Tetrick LL, Cummings M, Ho SB. Comparison of ultrasound (US) and triphasic computed tomography (CT) for screening for hepatocellular carcinoma (HCC) in patients with advanced liver disease. Gastroenterology 2005;128(4):A758. [Google Scholar]
Ascha 2009 {published data only}
- Ascha, MS, Hanouneh I, Lopez R, Tarek I, Ramimi AR, Albeldawi M, et al. Cumulative incidence and risk factors of hepatocellular carcinoma (HCC) in patients with end-stage liver disease secondary to nonalcoholic steatohepatitis (NASH). Gastroenterology 2009;136(5 Suppl 1):A797. [Google Scholar]
Aubé 2017 {published data only}
- Aubé C, Oberti F, Lonjon J, Pageaux G, Seror O, N'Kontchou G, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver International 2017;37(10):1515-25. [DOI] [PubMed] [Google Scholar]
Babar 2019 {published data only}
- Babar K, Salah-Ud-Din H, Tanvir I, Shahbaz B, Bakkar MA, Ali MS, et al. Diagnostic correlation of histopathological and radiological findings in hepatic lesions keeping histopathology as gold standard. Pakistan Journal of Medical and Health Sciences 2019;13(2):279-81. [Google Scholar]
Baron 1996 {published data only}
- Baron RL, Oliver JH, Dodd GD, Nalesnik M, Holbert BL, Carr B. Hepatocellular carcinoma: evaluation with biphasic, contrast-enhanced, helical CT. Radiology 1996;199:505-11. [DOI] [PubMed] [Google Scholar]
Basha 2018 {published data only}
- Basha MA, Al Azzazy MZ, Ahmed AF, Yousef HY, Shehata SM, El Sammak DA, et al. Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. European Radiology 2018;28(6):2592-603. [DOI] [PubMed] [Google Scholar]
Beal 2014 {published data only}
- Beal EW, Albert S, McNally M, Shirley LA, Hanje J, Michaels AJ, et al. An indeterminate nodule in the cirrhotic liver discovered by surveillance imaging is a prelude to malignancy. Journal of Surgical Oncology 2014;110(8):967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhattacharjya 2004 {published data only}
- Bhattacharjya S, Bhattacharjya T, Quaglia A, Dhillon AP, Burroughs AK, Patch DW, et al. Liver transplantation in cirrhotic patients with small hepatocellular carcinoma: an analysis of pre-operative imaging, explant histology and prognostic histologic indicators. Digesive Surgery 2004;21(2):152-60. [DOI] [PubMed] [Google Scholar]
Born 1998 {published data only}
- Born M, Layer G, Kreft B, Schwarz N, Schild H. MRI, CT and CT arterial portography in the diagnosis of malignant liver tumors in liver cirrhosis. RöFo: Fortschritte auf dem Gebiete der Röntgenstrahlen und der Nuklearmedizin 1998;168(6):567-72. [DOI] [PubMed] [Google Scholar]
Brancatelli 2003 {published data only}
- Brancatelli G, Baron RL, Peterson MS, Marsh W. Helical CT screening for hepatocellular carcinoma in patients with cirrhosis: frequency and causes of false-positive interpretation. American Journal of Roentgenology 2003;180(4):1007-14. [DOI] [PubMed] [Google Scholar]
Brehmer 2018 {published data only}
- Brehmer K, Brismar TB, Morsbach F, Svensson A, Stal P, Tzortzakakis A, et al. Triple arterial phase CT of the liver with radiation dose equivalent to that of single arterial phase CT: initial experience. Radiology 2018;289(1):111-8. [DOI] [PubMed] [Google Scholar]
Burrel 2003 {published data only}
- Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology (Baltimore, Md.) 2003;38(4):1034-42. [DOI] [PubMed] [Google Scholar]
Camera 1999 {published data only}
- Camera L, Imbriaco M, Marano I, Brunetti A, Selva G, Salvatore M. Dual-phase vs triphasic helical CT in the detection of hepato-cellular carcinoma in patients with liver cirrhosis. Radiology 1999;213P:469-70. [Google Scholar]
Chen 1982 {published data only}
- Chen DS, Sheu JC, Sung JL, Lai MY, Lee CS, Su CT, et al. Small hepatocellular carcinoma: a clinicopathological study in thirteen patients. Gastroenterology 1982;83(5):1109-19. [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen MH, Dai Y, Yan K, Fan ZH, Yin SS, Yang W, et al. The role of contrast-enhanced ultrasound on the diagnosis of small hepatocellular carcinoma (</=3 cm) in patients with cirrhosis. Hepatology Research 2006;35(4):281-8. [DOI] [PubMed] [Google Scholar]
Choi 2018 {published data only}
- Choi YR, Chung JW, Yu MH, Lee M, Kim JH. Diagnostic accuracy of contrast-enhanced dynamic CT for small hypervascular hepatocellular carcinoma and assessment of dynamic enhancement patterns: results of two-year follow-up using cone-beam CT hepatic arteriography. PLOS One 2018;13(9):e0203940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clevert 2009 {published data only}
- Clevert DA, Jung EM, Stock KF, Weckbach S, Feuerbach S, Reiser M, et al. Evaluation of malignant liver tumors: biphasic MS-CT versus quantitative contrast harmonic imaging ultrasound. Zeitschrift für Gastroenterologie 2009;47(12):1195-202. [DOI] [PubMed] [Google Scholar]
Dai 2008 {published data only}
- Dai Y, Chen MH, Fan ZH, Yan K, Yin SS, Zhang XP. Diagnosis of small hepatic nodules detected by surveillance ultrasound in patients with cirrhosis: comparison between contrast-enhanced ultrasound and contrast-enhanced helical computed tomography. Hepatology Research 2008;38(3):281-90. [DOI] [PubMed] [Google Scholar]
Denies 2002 {published data only}
- Denies L, Ernst O, Sergent-Baudson G, Duvet S, L'Hermine C. Contribution of spiral CT for the early diagnosis of hepato-cellular carcinoma in cirrhotic patients. Journal de Radiologie 2002;83(5):635-40. [PubMed] [Google Scholar]
De Santis 1992 {published data only}
- De Santis M, Romagnoli R, Cristani S, Cioni G, Casolo A, Vici FF, et al. MRI of small hepatocellular carcinoma: comparison with US, CT, DSA, and Lipiodol-CT. Journal of Computer Assisted Tomography 1992;16(2):189-97. [DOI] [PubMed] [Google Scholar]
Di Martino 2010 {published data only}
- Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 2010;256(3):806-16. [DOI] [PubMed] [Google Scholar]
Di Martino 2013 {published data only}
- Di Martino M, De Filippis G, De Santis A, Geiger D, Del Monte M, Lombardo CV, et al. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. European Radiology 2013;23(4):887-96. [DOI] [PubMed] [Google Scholar]
Fasani 1999 {published data only}
- Fasani P, Sangiovanni A, De Fazio C, Borzio M, Bruno S, Ronchi G, et al. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology (Baltimore, Md.) 1999;29(6):1704-7. [DOI] [PubMed] [Google Scholar]
Fausto 2011 {published data only}
- Fausto CS. Sensitivity and specificity of imaging diagnostic methods (ultrasonography, magnetic resonance and computed tomography) in the detection of liver nodules in cirrhotic patients, with histological correlation of the explanted liver [PhD Dissertation]. Sao Paulo: University of Sao Paulo, Faculty of medicine, 2011. [Google Scholar]
Frey 2015 {published data only}
- Frey RS, Boldanova T, Heim M. Ultrasound surveillance for hepatocellular carcinoma: real-life performance in a hepatology outpatient clinic. Swiss Medical Weekly 2015;145:w14200. [DOI] [PubMed] [Google Scholar]
Fukunaga 2007 {published data only}
- Fukunaga T, Kudo M, Tochio H, Okabe Y, Orino A. Natural course of small nodular lesions with intranodular preserved portal supply in cirrhotic liver. Oncology 2007;72(Suppl 1):24-9. [DOI] [PubMed] [Google Scholar]
Furlan 2012 {published data only}
- Furlan A, Marin D, Cabassa P, Taibbi A, Brunelli E, Agnello F, et al. Enhancement pattern of small hepatocellular carcinoma (HCC) at contrast-enhanced US (CEUS), MDCT, and MRI: intermodality agreement and comparison of diagnostic sensitivity between 2005 and 2010 American Association for the Study of Liver Diseases (AASLD) guidelines. European Journal of Radiology 2012;81(9):2099-105. [DOI] [PubMed] [Google Scholar]
Gaiani 2004 {published data only}
- Gaiani S, Celli N, Piscaglia F, Cecilioni L, Losinno F, Giangregorio F, et al. Usefulness of contrast-enhanced perfusional sonography in the assessment of hepatocellular carcinoma hypervascular at spiral computed tomography. Journal of Hepatology 2004;41(3):421-6. [DOI] [PubMed] [Google Scholar]
Garetti 1988 {published data only}
- Garretti L, Fauciglietti P, Regge D, Bonino F, Brunetto M, Gandini G. Evaluation of the usefulness of ultrasonics in the diagnosis of cancerous cirrhosis. Comparison with CT. Radiologica Medica 1988;76(3):187-92. [PubMed] [Google Scholar]
Gattoni 1993 {published data only}
- Gattoni F, Baldini U, Raiteri R, Pozzato C, De Cobelli F, Uslenghi C. Arterial CT in the diagnosis of hepatocellular carcinoma: initial experience with 12 patients. Radiologia Medica 1993;86(4):484-8. [PubMed] [Google Scholar]
Giangregorio 2009 {published data only}
- Giangregorio F, Comparato G, Marinone MG, Di Stasi M, Sbolli G, Aragona G, et al. Imaging detection of new HCCs in cirrhotic patients treated with different techniques: comparison of conventional US, spiral CT, and 3-dimensional contrast-enhanced US with the Navigator technique (Nav 3D CEUS). Journal of Ultrasound 2009;12(1):12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giorgio 2004 {published data only}
- Giorgio A, Ferraioli G, Tarantino L, Stefano G, Scala V, Scarano F, et al. Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced helical CT appearance. American Journal of Radiology 2004;183(5):1319-26. [DOI] [PubMed] [Google Scholar]
Granito 2013 {published data only}
- Granito A, Galassi M, Piscaglia F, Romanini L, Lucidi V, Renzulli M, et al. Impact of gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance on the non-invasive diagnosis of small hepatocellular carcinoma: a prospective study. Alimentary Pharmacology & Therapeutics 2013;37(3):355-63. [DOI] [PubMed] [Google Scholar]
Grat 2018 {published data only}
- Grat K, Grat M, Rowinski O, Patkowski W, Zieniewicz K, Pacho R. Accuracy of computed tomography in the assessment of Milan criteria in liver transplantation for hepatocellular carcinoma. Transplantation Proceedings 2018;50(7):2002-5. [DOI] [PubMed] [Google Scholar]
Gul 2018 {published data only}
- Gul H, Ahmed A. Sensitivity of diffusion weighted images in detection of small hepatocellular carcinoma in patients with cirrhosis liver. Journal of Medical Sciences (Peshawar) 2018;26(2):120-2. [Google Scholar]
Haberman 2013 {published data only}
- Haberman D, Castignola M, Mela M, Paladini H, Santilli JP, Gruz F, et al. Findings in multidetector computed tomography in the diagnosis of hepatocellular carcinoma in patients with cirrhosis and correlation with pathology of liver explants [Hallazgos en tomografía computada multidetector en el diagnóstico del carcinoma hepatocelular en pacientes con cirrosis y su correlación con la anatomía patológica del explante hepático]. Revista Argentina de Radiología 2013;77(3):209-17. [Google Scholar]
Habermann 2002 {published data only}
- Habermann CR, Weiss F, Hillner M, Staedtler C, Schoder V, Welger J, et al. Value of triple phase helical CT for the detection of hepatocellular carcinoma in cirrhotic liver. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2002;174(1):96-100. [DOI] [PubMed] [Google Scholar]
Hafeez 2011 {published data only}
- Hafeez S, Alam MS, Sajjad Z, Khan ZA, Akhter W, Mubarak F. Triphasic computed tomography (CT) scan in focal tumoral liver lesions. Journal of the Pakistan Medical Association 2011;61(6):571-5. [PubMed] [Google Scholar]
Hafeez 2020 {published data only}
- Hafeez M, Nadeem M, Ahmed M, Faheem-Ur-Rehman. Hepatocellular carcinoma (HCC), where do we stand? Current situation. Pakistan Journal of Medical Sciences 2020;36(3):344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasinuzzaman 2018 {published data only}
- Hasinuzzaman RT, Roy S, Hossain MS, Paul F, Akther M, Barua, S. Triple-phase multidetector computed tomography: an evaluation of hepatic space occupying lesion in cirrhotic patients. Bangladesh Medical Research Council Bulletin 2018;44(1):23-9. [Google Scholar]
Hidaka 2013 {published data only}
- Hidaka M, Takatsuki M, Okudaira S, Soyama A, Muraoka I, Tanaka T, et al. The expression of transporter OATP2/OATP8 decreases in undetectable hepatocellular carcinoma by Gd-EOB-MRI in the explanted cirrhotic liver. Hepatology International 2013;7(2):655-61. [DOI] [PubMed] [Google Scholar]
Hirakawa 2011 {published data only}
- Hirakawa M, Yoshimitsu K, Irie H, Tajima T, Nishie A, Asayama Y, et al. Performance of radiological methods in diagnosing hepatocellular carcinoma preoperatively in a recipient of living related liver transplantation: comparison with step section histopathology. Japanese Journal of Radiology 2011;29(2):129-37. [DOI] [PubMed] [Google Scholar]
Hori 2002 {published data only}
- Hori M, Murakami T, Kim T, Tsuda K, Takahashi S, Okada A, et al. Detection of hypervascular hepatocellular carcinoma: comparison of SPIO-enhanced MRI with dynamic helical CT. Journal of Computer Assisted Tomography 2002;26(5):701-10. [DOI] [PubMed] [Google Scholar]
Iavarone 2019 {published data only}
- Iavarone M, Vigano M, Piazza N, Occhipinti V, Sangiovanni A, Maggioni M, et al. Contrast imaging techniques to diagnose hepatocellular carcinoma in cirrhotics outside regular surveillance. Annales of Hepatology 2019;18(2):318-24. [DOI] [PubMed] [Google Scholar]
Ichikawa 2002 {published data only}
- Ichikawa T, Kitamura T, Nakajima H, Sou H, Tsukamoto T, Ikenaga S, et al. Hypervascular hepatocellular carcinoma: can double arterial phase imaging with multidetector CT improve tumor depiction in the cirrhotic liver? American Journal of Roentgenology 2002;179(3):751-8. [DOI] [PubMed] [Google Scholar]
Ichikawa 2006 {published data only}
- Ichikawa T, Nakajima H, Nanbu A, Hori M, Araki T. Effect of injection rate of contrast material on CT of hepatocellular carcinoma. American Journal of Roentgenology 2006;186(5):1413-8. [DOI] [PubMed] [Google Scholar]
Ichikawa 2010 {published data only}
- Ichikawa T, Saito K, Yoshioka N, Tanimoto A, Gokan T, Takehara Y, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Investigative Radiology 2010;45(3):133-40. [DOI] [PubMed] [Google Scholar]
Ichikawa 2021 {published data only}
- Ichikawa S, Motosugi U, Morisaka H, Kozaka K, Goshima S, Ichikawa T. Optimal combination of features on gadoxetate disodium-enhanced MR imaging for non-invasive differential diagnosis of hepatocellular carcinoma: the JAMP-HCC Study. Magnetic Resonance in Medical Sciences 2021;20(1):47-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imbriaco 2017 {published data only}
- Imbriaco M, De Luca S, Coppola M, Fusari M, Klain M, Puglia M, et al. Diagnostic accuracy of Gd-EOB-DTPA for detection hepatocellular carcinoma (HCC): a comparative study with dynamic contrast enhanced magnetic resonance imaging (MRI) and dynamic contrast enhanced computed tomography (CT). Polish Journal of Radiology 2017;82:50-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue 1994 {published data only}
- Inoue E, Kuroda C, Fujita M, Hosomi N, Kadota T, Narumi Y, et al. Evaluation of multislice dynamic MR imaging of the whole liver. Nippon Acta Radiologica 1994;54(5):363-70. [PubMed] [Google Scholar]
Ismail 1990 {published data only}
- Ismail T, Angrisani L, Gunson BK, Hubscher SG, Buckels JA, Neuberger JM. Primary hepatic malignancy: the role of liver transplantation. British Journal of Surgery 1990;77(9):983-7. [DOI] [PubMed] [Google Scholar]
Itai 1981 {published data only}
- Itai Y, Araki T, Furui S, Tasaka A. Differential diagnosis of hepatic masses on computed tomography, with particular reference to hepatocellular carcinoma. Journal of Computer Assisted Tomography 1981;5(6):834-42. [DOI] [PubMed] [Google Scholar]
Iwamura 1982 {published data only}
- Iwamura KM. A contribution to early diagnosis of primary hepatic cell carcinoma occurring in patients with liver cirrhosis. Tokai Journal of Experimental and Clinical Medicine 1982;7(3):397-411. [PubMed] [Google Scholar]
Jia 2017 {published data only}
- Jia GS, Feng GL, Li JP, Xu HL, Wang H, Cheng YP, et al. Using receiver operating characteristic curves to evaluate the diagnostic value of the combination of multislice spiral CT and alpha-fetoprotein levels for small hepatocellular carcinoma in cirrhotic patients. Hepatobiliary & Pancreatic Diseases International 2017;16(3):303-9. [DOI] [PubMed] [Google Scholar]
Jin 2013 {published data only}
- Jin YJ, Nah SY, Lee JW, Lee JI, Jeong S, Lee DH, et al. Utility of adding Primovist magnetic resonance imaging to analysis of hepatocellular carcinoma by liver dynamic computed tomography. Clinical Gastroenterology and Hepatology 2013;11(2):187-92. [DOI] [PubMed] [Google Scholar]
Jin 2016 {published data only}
- Jin YJ, Lee JW, Kwon OS, Jung YK, Kwon JH, Jang JW, et al. Clinical effect of add-on Primovist-enhanced magnetic resonance imaging on preoperative tumor staging in hepatocellular carcinoma patients. Journal of Surgical Oncology 2016;114(1):106-11. [DOI] [PubMed] [Google Scholar]
Jonczyk 2017 {published data only}
- Jonczyk M, Chapiro J, Collettini F, Geisel D, Schnapauff D, Streitparth F, et al. Diagnostic accuracy of split-bolus single-phase contrast-enhanced cone-beam CT for the detection of liver tumors before transarterial chemoembolization. Journal of Vascular and Interventional Radiology 2017;28(10):1378-85. [DOI] [PubMed] [Google Scholar]
Jung 2005 {published data only}
- Jung G, Poll L, Cohnen M, Saleh A, Vogler H, Wettstein M, et al. Differential diagnosis of focal liver lesions using contrast-enhanced MRI with SHU 555 A in comparison with unenhanced MRI and multidetector spiral-CT [Dignitätsbeurteilung fokaler Leberläsionen mit der kontrastverstärkten MRT mit SHU 555 A im Vergleich zur nativen MRT und zur Mehrzeilen-Detektor-Spiral-CT]. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2005;177(11):1571-7. [DOI] [PubMed] [Google Scholar]
Jung 2007 {published data only}
- Jung EM, Clevert DA, Schreyer AG, Schmitt S, Rennert J, Kubale R, et al. Evaluation of quantitative contrast harmonic imaging to assess malignancy of liver tumors: a prospective controlled two-center study. World Journal of Gastroenterology 2007;13(47):6356-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kader 2017 {published data only}
- Kader MA, Ghani HS, Saad ZM, Abdalla NH, Razek EA. To what extent the DW-MRI and ADC value can be used in assessment of hepatic focal lesions in cirrhotic patients. Egyptian Journal of Radiology and Nuclear Medicine 2017;48:825-37. [Google Scholar]
Kakihara 2014 {published data only}
- Kakihara D, Nishie A, Harada N, Shirabe K, Tajima T, Asayama Y, et al. Performance of gadoxetic acid-enhanced MRI for detecting hepatocellular carcinoma in recipients of living-related-liver-transplantation: comparison with dynamic multidetector row computed tomography and angiography-assisted computed tomography. Journal of Magnetic Resonance Imaging 2014;40(5):1112-20. [DOI] [PubMed] [Google Scholar]
Kan 2010 {published data only}
- Kan M, Hiraoka A, Uehara T, Hidaka S, Ichiryu M, Nakahara H, et al. Evaluation of contrast-enhanced ultrasonography using perfluorobutane (Sonazoid(®)) in patients with small hepatocellular carcinoma: comparison with dynamic computed tomography. Oncology Letters 2010;1(3):485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanematsu 1997a {published data only}
- Kanematsu M, Hoshi H, Imaeda T, Murakami T, Inaba Y, Yokoyama R, et al. Detection and characterization of hepatic tumors: value of combined helical CT hepatic arteriography and CT during arterial portography. American Journal of Roentgenology 1997;168(5):1193-8. [DOI] [PubMed] [Google Scholar]
Kanematsu 1997b {published data only}
- Kanematsu M, Hoshi H, Murakami T, Inaba Y, Kim T, Yamada T, et al. Detection of hepatocellular carcinoma in patients with cirrhosis: MR imaging versus angiographically assisted helical CT. American Journal of Roentgenology 1997;169(6):1507-15. [DOI] [PubMed] [Google Scholar]
Kato 2004 {published data only}
- Kato H, Kanematsu M, Kondo H, Goshima S, Matsuo M, Hoshi H, et al. Ferumoxide-enhanced MR imaging of hepatocellular carcinoma: correlation with histologic tumor grade and tumor vascularity. Journal of Magnetic Resonance Imaging 2004;19(1):76-81. [DOI] [PubMed] [Google Scholar]
Kawamori 1991 {published data only}
- Kawamori Y, Matsui O, Kadoya M, Gabata T, Takashima T, Ida M, et al. Diagnosis of hepatocellular carcinomas using CT arterial portography. Japanese Journal of Clinical Medicine 1991;49(8):1783-88. [PubMed] [Google Scholar]
Kawata 2002 {published data only}
- Kawata S, Murakami T, Kim T, Hori M, Federle MP, Kumano S, et al. Multidetector CT: diagnostic impact of slice thickness on detection of hypervascular hepatocellular carcinoma. American Journal of Roentgenology 2002;179(1):61-6. [DOI] [PubMed] [Google Scholar]
Khalili 2011 {published data only}
- Khalili K, Kim TK, Jang HJ, Haider MA, Khan L, Guindi M, et al. Optimization of imaging diagnosis of 1-2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. Journal of Hepatology 2011;54(4):723-8. [DOI] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim SH, Lee JM, Lee JY, Han JK, An SK, Han CJ, et al. Value of contrast-enhanced sonography for the characterization of focal hepatic lesions in patients with diffuse liver disease: receiver operating characteristic analysis. American Journal of Roentgenology 2005;184(4):1077-84. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim YK, Kwak HS, Kim CS, Chung GH, Han YM, Lee JM. Hepatocellular carcinoma in patients with chronic liver disease: comparison of SPIO-enhanced MR imaging and 16-detector row CT. Radiology 2006;238(2):531-41. [DOI] [PubMed] [Google Scholar]
Kim 2007a {published data only}
- Kim SR, Ando K, Mita K, Fuki S, Ikawa H, Kanbara Y, et al. Superiority of CT arterioportal angiography to contrast-enhanced CT and MRI in the diagnosis of hepatocellular carcinoma in nodules smaller than 2 cm. Oncology 2007;72(Suppl 1):58-66. [DOI] [PubMed] [Google Scholar]
Kim 2007b {published data only}
- Kim YK, Kwak HS, Han YM, Kim CS. Usefulness of combining sequentially acquired gadobenate dimeglumine-enhanced magnetic resonance imaging and resovist-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: comparison with computed tomography hepatic arteriography and computed tomography arterioportography using 16-slice multidetector computed tomography. Journal of Computed Assisted Tomography 2007;31(5):702-11. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim KW, Lee JM, Klotz E, Park HS, Lee DH, Kim JY, et al. Quantitative CT color mapping of the arterial enhancement fraction of the liver to detect hepatocellular carcinoma. Radiology 2009;250(2):425-34. [DOI] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim BR, Lee JM, Lee DH, Yoon JH, Hur BY, Suh KS, et al. Diagnostic performance of gadoxetic acid-enhanced liver MR imaging versus multidetector CT in the detection of dysplastic nodules and early hepatocellular carcinoma. Radiology 2017;285(1):134-46. [DOI] [PubMed] [Google Scholar]
Kim 2018 {published data only}
- Kim B, Lee JH, Kim JK, Kim HJ, Kim YB, Lee D. The capsule appearance of hepatocellular carcinoma in gadoxetic acid-enhanced MR imaging: correlation with pathology and dynamic CT. Medicine (Baltimore) 2018;97(25):e11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2019 {published data only}
- Kim YY, An C, Kim DY, Aljoqiman KS, Choi JY, Kim MJ. Failure of hepatocellular carcinoma surveillance: inadequate echogenic window and macronodular parenchyma as potential culprits. Ultrasonography 2019;38(4):311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurucay 2017 {published data only}
- Kurucay M, Kloth C, Kaufmann S, Nikolaou K, Bösmüller H, Horger M, et al. Multiparametric imaging for detection and characterization of hepatocellular carcinoma using gadoxetic acid-enhanced MRI and perfusion-CT: which parameters work best? Cancer Imaging 2017;17(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laroia 2013 {published data only}
- Laroia ST, Bawa SS, Jain D, Mukund A, Sarin S. Contrast ultrasound in hepatocellular carcinoma at a tertiary liver center: first Indian experience. World Journal of Radiology 2013;5(6):229-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laroia 2016 {published data only}
- Laroia ST, Bhadoria AS, Venigalla Y, Chibber GK, Bihari C, Rastogi A, et al. Role of dual energy spectral computed tomography in characterization of hepatocellular carcinoma: initial experience from a tertiary liver care institute. European Journal of Radiology Open 2016;3:162-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2003 {published data only}
- Lee JM, Kim IH, Kwak HS, Youk JH, Han YM, Kim CS. Detection of small hypervascular hepatocellular carcinomas in cirrhotic patients: comparison of superparamagnetic iron oxide-enhanced MR imaging with dual-phase spiral CT. Korean Journal of Radiology 2003;4(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee DH, Kim SH, Lee JM, Park HS, Lee JY, Yi NJ, et al. Diagnostic performance of multidetector row computed tomography, superparamagnetic iron oxide-enhanced magnetic resonance imaging, and dual-contrast magnetic resonance imaging in predicting the appropriateness of a transplant recipient based on Milan criteria: correlation with histopathological findings. Investigative Radiology 2009;44(6):311-21. [DOI] [PubMed] [Google Scholar]
Lee 2012c {published data only}
- Lee CH, Kim KA, Lee J, Park YS, Choi JW, Park CM. Using low tube voltage (80kVp) quadruple phase liver CT for the detection of hepatocellular carcinoma: two-year experience and comparison with Gd-EOB-DTPA enhanced liver MRI. European Journal of Radiology 2012;81(4):e605-11. [DOI] [PubMed] [Google Scholar]
Li 2018 {published data only}
- Li J, Li X, Weng J, Lei L, Gong J, Wang J, et al. Gd-EOB-DTPA dynamic contrast-enhanced magnetic resonance imaging is more effective than enhanced 64-slice CT for the detection of small lesions in patients with hepatocellular carcinoma. Medicine (Baltimore) 2018;97(52):e13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin MT, Chen CL, Wang CC, Cheng YF, Eng HL, Wang JH, et al. Diagnostic sensitivity of hepatocellular carcinoma imaging and its application to non-cirrhotic patients. Journal of Gastroenterology and Hepatology 2011;26(4):745-50. [DOI] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu YI, Kamaya A, Jeffrey RB, Shin LK. Multidetector computed tomography triphasic evaluation of the liver before transplantation: importance of equilibrium phase washout and morphology for characterizing hypervascular lesions. Journal of Computer Assisted Tomography 2012;36(2):213-9. [DOI] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu JJ, Wang D, Li HX, Zhou Z, Zhao SF, Ding ZL, et al. Comparison of contrast-enhanced ultrasound and contrast-enhanced helical CT in the diagnosis of high echo-level small focal liver lesions. International Journal of Clinical and Experimental Medicine 2016;9(5):8176-82. [Google Scholar]
Luca 2010 {published data only}
- Luca A, Caruso S, Milazzo M, Mamone G, Marrone G, Miraglia R, et al. Multidetector-row computed tomography (MDCT) for the diagnosis of hepatocellular carcinoma in cirrhotic candidates for liver transplantation: prevalence of radiological vascular patterns and histological correlation with liver explants. European Journal of Radiology 2010;20(4):898-907. [DOI] [PubMed] [Google Scholar]
Lucatelli 2020 {published data only}
- Lucatelli P, De Rubeis G, Ginnani Corradini L, Basilico F, Di Martino M, Lai Q, et al. Intra-procedural dual phase cone beam computed tomography has a better diagnostic accuracy over pre-procedural MRI and MDCT in detection and characterization of HCC in cirrhotic patients undergoing TACE procedure. European Journal of Radiology 2020;124:108806. [DOI] [PubMed] [Google Scholar]
Maciel 2006 {published data only}
- Maciel AC, Cerski CT, Moreira RK, Resende VL, Zanotelli ML, Matiotti SB. Hepatocellular carcinoma in patients undergoing orthotopic liver transplantation: radiological findings with anatomopathological correlation in Brazil. Arquivos de Gastroenterologia 2006;43(1):24-9. [DOI] [PubMed] [Google Scholar]
Maetani 2008 {published data only}
- Maetani YS, Ueda M, Haga H, Isoda H, Takada Y, Arizono S, et al. Hepatocellular carcinoma in patients undergoing living-donor liver transplantation. Accuracy of multidetector computed tomography by viewing images on digital monitors. Intervirology 2008;51(Suppl 1):46-51. [DOI] [PubMed] [Google Scholar]
Malagari 1999 {published data only}
- Malagari K, Koskinas J, Brountzos E, Thanos L, Papathanasiou M, Dailiana T, et al. CT portography and post-Lipiodol CT in the preinterventional work-up of primary and secondary liver tumors. A single center experience. Hepato-gastroenterology 1999;46(26):2901-8. [PubMed] [Google Scholar]
Manini 2013 {published data only}
- Manini MA, Sangiovanni A, Fornari F, Piscaglia F, Biolato M, Fanigliulo L, et al. Clinical and economical impact of 2010 AASLD guidelines for the diagnosis of hepatocellular carcinoma. Journal of Hepatology 2014;60(5):995-1001. [DOI] [PubMed] [Google Scholar]
Marcato 1999 {published data only}
- Marcato N, Abergel A, Alexandre M, Boire JY, Darcha C, Duchene B, et al. Hepatocellular carcinoma in cirrhosis: semeiology and performance of magnetic resonance imaging and Lipiodol computed tomography. Gastroenterologie Clinique et Biologique 1999;23(1):114-21. [PubMed] [Google Scholar]
Marin 2009a {published data only}
- Marin D, Catalano C, De Filippis G, Di Martino M, Guerrisi A, Rossi M, et al. Detection of hepatocellular carcinoma in patients with cirrhosis: added value of coronal reformations from isotropic voxels with 64-MDCT. American Journal of Roentgenology 2009;192(1):180-7. [DOI] [PubMed] [Google Scholar]
Marin 2009b {published data only}
- Marin D, Di Martino M, Guerrisi A, De Filippis G, Rossi M, Ginanni Corradini S, et al. Hepatocellular carcinoma in patients with cirrhosis: qualitative comparison of gadobenate dimeglumine-enhanced MR imaging and multiphasic 64-section CT. Radiology 2009;251(1):85-95. [DOI] [PubMed] [Google Scholar]
Masuda 2017 {published data only}
- Masuda K, Kaneko J, Kawaguchi Y, Togashi J, Arita J, Akamatsu N, et al. Diagnostic accuracy of indocyanine green fluorescence imaging and multidetector row computed tomography for identifying hepatocellular carcinoma with liver explant correlation. Hepatology Research 2017;12:1299-307. [DOI] [PubMed] [Google Scholar]
Mehana 2019 {published data only}
- Mehana SM. Assessment of the follow-up interval changes of the less than 2 cm arterial phase enhancing hepatic nodules in correlation with Liver Imaging Reporting and Data System (LI-RADS) classification version 18 using contrast-enhanced multidetector computed tomography. Egyptian Journal of Radiology and Nuclear Medicine 2019;50(1):107. [Google Scholar]
Miller 1991 {published data only}
- Miller WJ, Federle MP, Campbell WL. Diagnosis and staging of hepatocellular carcinoma: comparison of CT and sonography in 36 liver transplantation patients. American Journal of Roentgenology 1991;157(2):303-6. [DOI] [PubMed] [Google Scholar]
Miller 1994 {published data only}
- Miller WJ, Baron RL, Dodd GD 3rd, Federle MP. Malignancies in patients with cirrhosis: CT sensitivity and specificity in 200 consecutive transplant patients. Radiology 1994;193(3):645-50. [DOI] [PubMed] [Google Scholar]
Min 2020 {published data only}
- Min JH, Kim JM, Kim YK, Cha DI, Kang TW, Kim H, et al. Magnetic resonance imaging with extracellular contrast detects hepatocellular carcinoma with greater accuracy than with gadoxetic acid or computed tomography. Clinical Gastroenterology and Hepatology 2020;18(9):2091-100. [DOI] [PubMed] [Google Scholar]
Mok 2004 {published data only}
- Mok TS, Yu SC, Lee C, Sung J, Leung N, Lai P, et al. False-negative rate of abdominal sonography for detecting hepatocellular carcinoma in patients with hepatitis B and elevated serum alpha-fetoprotein levels. American Journal of Roentgenology 2004;183(2):453-8. [DOI] [PubMed] [Google Scholar]
Moudgil 2017 {published data only}
- Moudgil S, Kalra N, Prabhakar N, Dhiman RK, Behera A, Chawla YK, et al. Comparison of contrast enhanced ultrasound with contrast enhanced computed tomography for the diagnosis of hepatocellular carcinoma. Journal of Clinical and Experimental Hepatology 2017;7(3):222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakamura 2000 {published data only}
- Nakamura H, Ito N, Kotake F, Mizokami Y, Matsuoka T. Tumor-detecting capacity and clinical usefulness of SPIO-MRI in patients with hepatocellular carcinoma. Journal of Gastroenterology 2000;35(11):849-55. [DOI] [PubMed] [Google Scholar]
Nusbaum 2015 {published data only}
- Nusbaum JD, Smirniotopoulos J, Wright HC, Dash C, Parpia T, Shechtel J, et al. The effect of hepatocellular carcinoma surveillance in an urban population with liver cirrhosis. Journal of Clinical Gastroenterology 2015;49(10):e91-5. [DOI] [PubMed] [Google Scholar]
Oberstein 1996 {published data only}
- Oberstein A, Kauczor HU, Mildenberger P, Ibe M, Teifke A, Rieker O, et al. Triphasic spiral CT in the diagnosis of liver diseases: comparison with CT arteriography and CT arterio-portography. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1996;164(6):449-56. [DOI] [PubMed] [Google Scholar]
Paul 2007 {published data only}
- Paul SB, Gulati MS, Sreenivas V, Madan K, Gupta AK, Mukhopadhyay S, et al. Evaluating patients with cirrhosis for hepatocellular carcinoma: value of clinical symptomatology, imaging and alpha-fetoprotein. Oncology 2007;72(Suppl 1):117-23. [DOI] [PubMed] [Google Scholar]
Peterson 2000 {published data only}
- Peterson MS, Baron RL, Marsh JW Jr, Oliver JH 3rd, Confer SR, Hunt LE. Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: epidemiology and CT-based tumor detection rate in 430 cases with surgical pathologic correlation. Radiology 2000;217(3):743-9. [DOI] [PubMed] [Google Scholar]
Pocha 2013 {published data only}
- Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Alimentary Pharmacology & Therapeutics 2013;38(3):303-12. [DOI] [PubMed] [Google Scholar]
Putz 2019 {published data only}
- Putz FJ, Verloh N, Erlmeier A, Schelker RC, Schreyer AG, Hautmann MG, et al. Influence of limited examination conditions on contrast-enhanced sonography for characterising liver lesions. Clinical Hemorheology and Microcirculation 2019;71(2):267-76. [DOI] [PubMed] [Google Scholar]
Ren 2015 {published data only}
- Ren WP, Yu MH, Xu P. Contrast-enhanced ultrasound vs contrast-enhanced computed tomography for diagnosis of small nodules (<3 cm) in the liver of patients with liver cirrhosis. World Chinese Journal of Digestology 2015;23(7):1149-53. [Google Scholar]
Ronzoni 2007 {published data only}
- Ronzoni A, Artioli D, Scardina R, Battistig L, Minola E, Sironi S, et al. Role of MDCT in the diagnosis of hepatocellular carcinoma in patients with cirrhosis undergoing orthotopic liver transplantation. American Journal of Roentgenology 2007;189(4):792-8. [DOI] [PubMed] [Google Scholar]
Ryu 2014 {published data only}
- Ryu SW, Bok GH, Jang JY, Jeong SW, Ham NS, Kim JH, et al. Clinically useful diagnostic tool of contrast enhanced ultrasonography for focal liver masses: comparison to computed tomography and magnetic resonance imaging. Gut and Liver 2014;8(3):292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saada 1994 {published data only}
- Saada J, Bhattacharya S, Dhillon AP, Dick R, Burroughs AK, Rolles K, et al. Detection of small hepatocellular carcinomas in cirrhotic livers using iodised oil computed tomography. Gut 1997;41(3):404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sano 2011 {published data only}
- Sano K, Ichikawa T, Motosugi U, Sou H, Muhi AM, Matsuda M, et al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology 2011;261(3):834-44. [DOI] [PubMed] [Google Scholar]
Sekoguchi 1994 {published data only}
- Sekoguchi B, Horiguchi Y, Imai H, Suzuki T, Miyoshi A, Itoh H, et al. Integrated diagnosis of small hepatocellular carcinoma with imaging diagnosis. Rinsho Byori - Japanese Journal of Clinical Pathology 1994;42(10):1036-42. [PubMed] [Google Scholar]
Semaan 2020 {published data only}
- Semaan S, Vietti Violi N, Lewis S, Chatterji M, Song C, Besa C, et al. Hepatocellular carcinoma detection in liver cirrhosis: diagnostic performance of contrast-enhanced CT vs. MRI with extracellular contrast vs. gadoxetic acid. European Radiology 2020;30(2):1020-30. [DOI] [PubMed] [Google Scholar]
Semelka 1992 {published data only}
- Semelka RC, Shoenut JP, Kroeker MA, Greenberg HM, Simm FC, Minuk GY, et al. Focal liver disease: comparison of dynamic contrast-enhanced CT and T2- weighted fat-suppressed, FLASH, and dynamic gadolinium-enhanced MR imaging at 1.5T. Radiology 1992;184(3):687-94. [DOI] [PubMed] [Google Scholar]
Seo 2019 {published data only}
- Seo N, Kim MS, Park MS, Choi JY, An C, Han K, et al. Optimal criteria for hepatocellular carcinoma diagnosis using CT in patients undergoing liver transplantation. European Radiology 2019;29(2):1022-31. [DOI] [PubMed] [Google Scholar]
Shapiro 1996 {published data only}
- Shapiro RS, Katz R, Mendelson DS, Halton KP, Schwartz ME, Miller CM, et al. Detection of hepatocellular carcinoma in cirrhotic patients: sensitivity of CT and ultrasonography. Journal of Ultrasound in Medicine 1996;15(7):497-502. [DOI] [PubMed] [Google Scholar]
Silberhumer 2004 {published data only}
- Silberhumer GR, Steininger R, Laengle F, Muehlbacher F, Zacherl J, Pokieser P, et al. Intraoperative ultrasonography in patients who undergo liver resection or transplantation for hepatocellular carcinoma. Surgical Technology International 2004;12:145-51. [PubMed] [Google Scholar]
Singh 2007 {published data only}
- Singh P, Erickson RA, Mukhopadhyay P, Gopal S, Kiss A, Khan A, et al. EUS for detection of the hepatocellular carcinoma: results of a prospective study. Gastrointestinal Endoscopy 2007;66(2):265-73. [DOI] [PubMed] [Google Scholar]
Sofue 2011 {published data only}
- Sofue K, Tsurusaki M, Kawasaki R, Fujii M, Sugimura K. Evaluation of hypervascular hepatocellular carcinoma in cirrhotic liver: comparison of different concentrations of contrast material with multi-detector row helical CT - a prospective randomized study. European Journal of Radiology 2011;80(3):e237-42. [DOI] [PubMed] [Google Scholar]
Soyer 1994 {published data only}
- Soyer P, Bluemke DA, Hruban RH, Sitzmann JV, Fishman EK. Primary malignant neoplasms of the liver: detection with helical CT during arterial portography. Radiology 1994;192(2):389-92. [DOI] [PubMed] [Google Scholar]
Suarez‐Munoz 2006 {published data only}
- Suárez-Muñoz MA, Leiva-Vera MC, Santoyo-Santoyo J, Fernández-Aguilar JL, Pérez-Daga JA, Sánchez-Pérez B, et al. Detection of neoplastic lesions in cirrhotic patients waiting for liver transplantation [Detección de lesiones neoplásicas en pacientes cirróticos candidatos a trasplante hepático]. Cirugía Española 2006;80(3):157-61. [DOI] [PubMed] [Google Scholar]
Sugimoto 2015 {published data only}
- Sugimoto K, Kim SR, Imoto S, Tohyama M, Kim SK, Matsuoka T, et al. Characteristics of hypovascular versus hypervascular well-differentiated hepatocellular carcinoma smaller than 2 cm - focus on tumor size, markers and imaging detectability. Digestive Diseases 2015;33(6):721-7. [DOI] [PubMed] [Google Scholar]
Takayasu 1990 {published data only}
- Takayasu K, Moriyama N, Muramatsu Y, Makuuchi M, Hasegawa H, Okazaki N, et al. The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients. American Journal of Roentgenology 1990;155(1):49-54. [DOI] [PubMed] [Google Scholar]
Tang 2018 {published data only}
- Tang W, Qin J, Hu B, Zhang L, Guo R, Wang J. Comparison of gadoxetic acid disodium-enhanced MRI and biphasic spiral CT in detection of hepatocellular carcinoma in patients meeting the Milan criteria. International Journal of Clinical and Experimental Medicine 2018;11(3):2551-8. [Google Scholar]
Valls 2004 {published data only}
- Valls C, Cos M, Figueras J, Andía E, Ramos E, Sánchez A, et al. Pretransplantation diagnosis and staging of hepatocellular carcinoma in patients with cirrhosis: value of dual-phase helical CT. American Journal of Roentgenology 2004;182(4):1011-7. [DOI] [PubMed] [Google Scholar]
Wang 1997 {published data only}
- Wang J, Tsang YM, Lee PH, Wei TC, Lai MY, Hsu HC, et al. Detection of hepatic neoplasms by computed tomographic arterial portography in cirrhotic patients. Journal of the Formosan Medical Association 1997;96(12):955-61. [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang H, Mu XT, Zhong X. Comparative study of dynamic MRI and MSCT in hepatocellular carcinoma with cirrhosis. Chinese Journal of Medical Imaging Technology 2007;23(7):1046-8. [Google Scholar]
Wang 2018 {published data only}
- Wang M, Wei C, Shi Z, Zhu J. Study on the diagnosis of small hepatocellular carcinoma caused by hepatitis B cirrhosis via multi-slice spiral CT and MRI. Oncology Letters 2018;15(1):503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2019 {published data only}
- Wang G, Zhu S, Li X. Comparison of values of CT and MRI imaging in the diagnosis of hepatocellular carcinoma and analysis of prognostic factors. Oncology Letters 2019;17(1):1184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 1986 {published data only}
- Watanabe A, Yamamoto H, Ito T, Nagashima H. Diagnosis, treatment and prognosis of small hepatocellular carcinoma. Hepato-gastroenterology 1986;33(2):52-5. [PubMed] [Google Scholar]
Yamashita 1996 {published data only}
- Yamashita Y, Mitsuzaki K, Yi T, Ogata I, Nishiharu T, Urata, J, et al. Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology 1996;200(1):79-84. [DOI] [PubMed] [Google Scholar]
Yim 2016 {published data only}
- Yim SY, Park BJ, Um SH, Han NY, Sung DJ, Cho SB, et al. Diagnostic performance of gadoxetic acid (Primovist)-enhanced MR imaging versus CT during hepatic arteriography and portography for small hypervascular hepatocellular carcinoma. Medicine (Baltimore) 2016;95(39):e4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yukisawa 2007 {published data only}
- Yukisawa S, Okugawa H, Masuya Y, Okabe S, Fukuda H, Yoshikawa M, et al. Multidetector helical CT plus superparamagnetic iron oxide-enhanced MR imaging for focal hepatic lesions in cirrhotic liver: a comparison with multi-phase CT during hepatic arteriography. European Journal of Radiology 2007;61(2):279-89. [DOI] [PubMed] [Google Scholar]
Zacherl 2002 {published data only}
- Zacherl J, Pokieser P, Wrba F, Scheuba C, Prokesch R, Zacherl M, et al. Accuracy of multiphasic helical computed tomography and intraoperative sonography in patients undergoing orthotopic liver transplantation for hepatoma: what is the truth? Annals of Surgery 2002;235(4):528-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2007 {published data only}
- Zhao H, Yao JL, Wang Y, Zhou KR. Detection of small hepatocellular carcinoma: comparison of dynamic enhancement magnetic resonance imaging and multiphase multirow-detector helical CT scanning. World Journal of Gastroenterology 2007;13(8):1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Bertuccio 2017
- Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. Journal of Hepatology 2017;67(2):302-9. [DOI] [PubMed] [Google Scholar]
Bosetti 2013
- Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, et al. Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Annals of Oncology 2013;24:2657-71. [DOI] [PubMed] [Google Scholar]
Bosetti 2014
- Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Practice & Research. Clinical Gastroenterology 2014;28:753-70. [DOI] [PubMed] [Google Scholar]
Bralet 2000
- Bralet MP, Regimbeau JM, Pineau P, Dubois S, Loas G, Degos F, et al. Hepatocellular carcinoma occurring in nonfibrotic liver: epidemiologic and histopathologic analysis of 80 French cases. Hepatology (Baltimore, Md.) 2000;32(2):200-4. [DOI] [PubMed] [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
Bruix 2011
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md.) 2011;53(3):1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castillo 2009
- Castillo E, Pelletier S, Kumer S, Abouljoud M, Divine G, Moonka D. Incidental hepatocellular carcinoma after liver transplantation: population characteristics and outcomes. Transplant Proceedings 2009;41(1):219-21. [DOI] [PubMed] [Google Scholar]
Chang 2006
- Chang KC, Leung CC, Tam CM. Per lesion analysis is misleading. Thorax 2006;61(4):364. [PMC free article] [PubMed] [Google Scholar]
Chen 2013
- Chen L, Zhang L, Bao J, Zhang J, Li C, Xia Y, et al. Comparison of MRI with liver-specific contrast agents and multidetector row CT for the detection of hepatocellular carcinoma: a meta-analysis of 15 direct comparative studies. Gut 2013;62:1520-1. [DOI] [PubMed] [Google Scholar]
Choi 2014
- Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology 2014;272:635-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chou 2015
- Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginburg A, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Annals of Internal Medicine 2015;162:697-711. [DOI] [PubMed] [Google Scholar]
Chung 2015
- Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography 2015;34(1):3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2015
- Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016;6:e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colli 2006
- Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and apha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. American Journal of Gastroenterology 2006;101:513-23. [DOI] [PubMed] [Google Scholar]
Colli 2014
- Colli A, Fraquelli M, Casazza G, Conte D, Nikolova D, Duca P, et al. The architecture of diagnostic research: from bench to bedside - research guidelines using liver stiffness as an example. Hepatology (Baltimore, Md.) 2014;60(1):408-18. [DOI] [PubMed] [Google Scholar]
Colli 2021
- Colli A, Nadarevic T, Miletic D, Giljaca V, Fraquelli M, Štimac D, et al. Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database of Systematic Reviews 2021, Issue 4. Art. No: CD013346. [DOI: 10.1002/14651858.CD013346.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 19 June 2019. Available at covidence.org.
Davila 2012
- Davila JA, Duan Z, McGlynn KA, El-Serag HB. Utilization and outcomes of palliative therapy for hepatocellular carcinoma: a population-based study in the United States. Journal of Clinical Gastroenterology 2012;46:71-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Franchis 2015
- Franchis R. Expanding consensus in portal hypertension. Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63:743-52. [DOI] [PubMed] [Google Scholar]
DTA Handbook 2013
- Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
EASL 2018
- Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. Journal of Hepatology 2018;69(1):182-236. [DOI] [PubMed] [Google Scholar]
EASL‐EORTC 2012
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of Hepatology 2012;56(4):908-43. [DOI] [PubMed] [Google Scholar]
El Moghazy 2016
- El Moghazy W, Kashkoush S, Meeberg G, Kneteman N. Incidence, characteristics, and prognosis of incidentally discovered hepatocellular carcinoma after liver transplantation. Journal of Transplantation 2016;2016:1916387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2019
Floriani 2013
- Floriani I, D'Onofrio M, Rulli E, Chen MH, Li R, Musicco L. Performance of imaging modalities in the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ultraschall in der Medizin 2013;34:454-62. [DOI] [PubMed] [Google Scholar]
Forner 2008
- Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology (Baltimore, Md.) 2008;47:97-104. [DOI] [PubMed] [Google Scholar]
Forner 2012
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379(9822):1245-55. [DOI] [PubMed] [Google Scholar]
Fraquelli 2019
- Fraquelli M, Nadarevic T, Giljaca V, Colli A, Miletic D, Štimac D, et al. Contrast-enhanced ultrasound for the diagnosis of hepatocellular carcinoma in advanced chronic liver disease. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD013483. [DOI: 10.1002/14651858.CD013483] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 5 November 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guo 2016
- Guo J, Seo Y, Ren S, Hong S, Lee D, Kim S, et al. Diagnostic performance of contrast-enhanced multidetector computed tomography and gadoxetic acid disodium-enhanced magnetic resonance imaging in detecting hepatocellular carcinoma: direct comparison and a meta-analysis. Abdominal Radiology 2016;41:1960-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanna 2016
- Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, et al. Comparative 13-year meta-analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdominal Radiology 2016;41:71-90. [DOI] [PubMed] [Google Scholar]
Hashim 2016
- Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. The global decrease in cancer mortality: trends and disparities. Annals of Oncology 2016;27:926-33. [DOI] [PubMed] [Google Scholar]
Heimbach 2018
- Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md.) 2018;67:358-80. [DOI] [PubMed] [Google Scholar]
Hennedige 2012
- Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging 2012;12(3):530-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hussain 2002
- Hussain SM, Zondervan PE, IJzermans JN, Schalm SW, Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics 2002;22:1023-39. [DOI] [PubMed] [Google Scholar]
International Consensus Group for HCN 2009
- The International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology (Baltimore, Md.) 2009;49(2):658-64. [DOI] [PubMed] [Google Scholar]
Kaira 2015
- Kalra MK, Sodickson AD, Mayo-Smith WW. CT radiation: key concepts for gentle and wise use. Radiographics 2015;35(6):1706-21. [DOI] [PubMed] [Google Scholar]
Kew 1975
- Kew MC. Alpha-fetoprotein. In: Read AE, editors(s). Modern Trends in Gastroenterology. Vol. 5. London: Butterworths, 1975:91. [Google Scholar]
Kim 2008
- Kim SH, Choi BI, Lee JY, Kim SJ, So YH, Eun HW, et al. Diagnostic accuracy of multi-/single-detector row CT and contrast-enhanced MRI in the detection of hepatocellular carcinomas meeting the Milan criteria before liver transplantation. Intervirology 2008;51(Suppl 1):52-60. [DOI] [PubMed] [Google Scholar]
Kinoshita 2015
- Kinoshita A, Onoda H, Fushiya N, Koike K, Nishino H, Tajiri H. Staging systems for hepatocellular carcinoma: current status and future perspectives. World Journal of Hepatology 2015;7(3):406-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012a
- Lee JM, Yoon JH, Kim KW. Diagnosis of hepatocellular carcinoma: newer radiological tools. Seminars in Oncology 2012;39:399-409. [DOI] [PubMed] [Google Scholar]
Lee 2012b
- Lee JH, Lee JM, Kim SJ, Baek JH, Yun SH, Kim KW, et al. Enhancement patterns of hepatocellular carcinomas on multiphasic multidetector row CT: comparison with pathological differentiation. British Journal of Radiology 2012;85(101):e573-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2015
- Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging - a systematic review and meta-analysis. Radiology 2015;275:97-109. [DOI] [PubMed] [Google Scholar]
Li 2015
- Li JJ, Zheng JS, Cui SC, Cui XW, Hu CX, Fang D, et al. C-arm Lipiodol CT in transcatheter arterial chemoembolisation for small hepatocellular carcinoma. World Journal of Gastroenterology 2015;21(10):3035-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2019
- Li J, Wang J, Lei L, Yuan G, He S. The diagnostic performance of gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced multi-detector computed tomography in detecting hepatocellular carcinoma: a meta-analysis of eight prospective studies. European Radiology 2019;29(12):6519-28. [DOI] [PubMed] [Google Scholar]
LI‐RADS
- American College of Radiology. Liver imaging reporting and data system. www.acr.org/quality-safety/resources/LIRADS (accessed 4 May 2021).
LI‐RADS 2018
- American College of Radiology. Liver imaging reporting and data system. www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf?la=en 2018 (accessed 14 September 2021).
Llovet 1999
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in Liver Disease 1999;19:329-38. [DOI] [PubMed] [Google Scholar]
Llovet 2003
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [DOI] [PubMed] [Google Scholar]
Llovet 2008
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. Journal of the National Cancer Institute 2008;100:698-711. [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0 [updated September 2010]. The Cochrane Collaboration, 2010. Available from: srdta.cochrane.org.
Madaleno 2015
- Madaleno J, Alves R, Silva N, Calretas S, Tome L, Ferrao J, et al. Incidentally discovered hepatocellular carcinoma in explanted liver: clinical, histopathologic features and outcome. Transplantation Proceedings 2015;47(4):1051-4. [DOI] [PubMed] [Google Scholar]
Manini 2014
- Manini MA, Sangiovanni A, Fornari F, Piscaglia F, Biolato M, Fanigliulo L, et al. Clinical and economical impact of 20120 AASLD guidelines for the diagnosis of hepatocellular carcinoma. Journal of Hepatology 2014;60:995-1001. [DOI] [PubMed] [Google Scholar]
Mann 1990
- Mann JR, Kasthuri N, Raafat F, Pincott JR, Parkes SE, Muir KR, et al. Malignant hepatic tumours in children: incidence, clinical features and aetiology. Paediatric and Perinatal Epidemiology 1990;4(3):276-89. [DOI] [PubMed] [Google Scholar]
Mazzaferro 1996
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New England Journal of Medicine 1996;334:693-9. [DOI] [PubMed] [Google Scholar]
Mazzaferro 2011
- Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence based analysis of 15 years of experience. Liver Transplantation 2011;17:S44-S57. [DOI] [PubMed] [Google Scholar]
Mogul 2018
- Mogul DB, Ling SC, Murray KF, Schwarzenberg SJ, Rudzinski ER, Schwarz KB. Characteristics of hepatitis B virus-associated hepatocellular carcinoma in children: a multi-center study. Journal of Pediatric Gastroenterology and Nutrition 2018;67(4):437-40. [DOI] [PubMed] [Google Scholar]
Nadarevic 2021
- Nadarevic T, Colli A, Giljaca V, Fraquelli M, Casazza G, Manzotti C, et al. Magnetic resonance imaging for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD014798. [DOI: 10.1002/14651858.CD014798] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathani 2021
- Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut 2021;70(2):401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ni 2004
- Ni YH, Chang MH, Wang KJ, Hsu HY, Chen HL, Kao JH, et al. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology 2004;127(6):1733-8. [DOI] [PubMed] [Google Scholar]
O'Neill 2015
- O'Neill EK, Cogley JR, Miller FH. The ins and outs of liver imaging. Clinics in Liver Disease 2015;19:99-121. [DOI] [PubMed] [Google Scholar]
Omata 2017
- Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatology International 2017;11:317-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed.) 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parakh 2016
- Parakh A, Kortesniemi M, Schindera ST. CT radiation dose management: a comprehensive optimization process for improving patient safety. Radiology 2016;280(3):663-73. [DOI] [PubMed] [Google Scholar]
Park 2017
- Park HJ, Choi BI, Lee ES, Park SB, Lee JB. How to differentiate borderline hepatic nodules in hepatocarcinogenesis: emphasis on imaging diagnosis. Liver Cancer 2017;6:189-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pomfret 2010
- Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transplantation 2010;16(3):262-78. [DOI] [PubMed] [Google Scholar]
Quaglia 2018
- Quaglia A. Hepatocellular carcinoma: a review of diagnostic challenges for the pathologist. Journal of Hepatocellular Carcinoma 2018;5:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Managr 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Roberts 2018
- Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology (Baltimore, Md.) 2018;67(1):401-21. [DOI] [PubMed] [Google Scholar]
Ryerson 2016
- Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016;122(9):1312-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salameh 2020
- Salameh JP, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ (Clinical Research Ed.) 2020;370:m2632. [DOI] [PubMed] [Google Scholar]
SAS [Computer program]
- SAS Statistical Software. Version 9.4. Cary, NC: SAS Institute Inc.
Schirner 2004
- Schirner M, Menrad A, Stephens A, Frentzel T, Hauff P, Licha K. Molecular imaging of tumor angiogenesis. Annals of the New York Academy of Sciences 2004;1014:67-75. [DOI] [PubMed] [Google Scholar]
Schuetz 2012
- Schuetz GM, Schlattmann P, Dewey M. Use of 3x2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. BMJ (Clinical Research Ed.) 2012;345:e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ (Clinical Research Ed.) 2008;336(7653):1106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2016
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016;76:89-98. [DOI] [PubMed] [Google Scholar]
Schünemann 2020a
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [DOI] [PubMed] [Google Scholar]
Schünemann 2020b
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Inconsistency, Imprecision, publication bias and other domains for rating the certainty of evidence for test accuracy and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. [DOI] [PubMed] [Google Scholar]
Senkerikova 2014
- Senkerikova R, Frankova S, Sperl J, Oliverius M, Kieslichova E, Filipova H, et al. Incidental hepatocellular carcinoma: risk factors and long-term outcome after liver transplantation. Transplantation Proceedings 2014;46(5):1426-9. [DOI] [PubMed] [Google Scholar]
Shah 2014
- Shah S, Shukla A, Paunipagar B. Radiological features of hepatocellular carcinoma. Journal of Clinical and Experimental Hepatology 2014;4:63-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shirki 2015
- Shriki JE, Seyal AR, Dighe MK, Yeh MM, Jalikis FG, Andeen NK, et al. CT of atypical and uncommon presentations of hepatocellular carcinoma. American Journal of Roentgenology 2015;205(4):W411-23. [DOI] [PubMed] [Google Scholar]
Silva 2008
- Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 2008;57(11):1592-6. [DOI] [PubMed] [Google Scholar]
Stanaway 2016
- Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tao 1984
- Tao LC, Ho CS, McLoughlin MJ, Evans WH, Donat EE. Cytologic diagnosis of hepatocellular carcinoma by fine-needle aspiration biopsy. Cancer 1984;53:547-52. [DOI] [PubMed] [Google Scholar]
Thomsen 2014
- Thomsen H, Webb J. Contrast Media Safety Issues and ESUR Guidelines. 3rd edition. Berlin: Springer, 2014. [Google Scholar]
Van der Pol 2019
- Van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-a systematic review. Gastroenterology 2019;156(4):976-86. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
Xie 2011
- Xie L, Guang Y, Ding H, Cai A, Huang Y. Diagnostic value of contrast-enhanced ultrasound, computed tomography and magnetic resonance imaging for focal liver lesions: a meta-analysis. Ultrasound in Medicine and Biology 2011;37(6):854-61. [DOI] [PubMed] [Google Scholar]
Yang 2011
- Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, et al. Factors that affect the risk for hepatocellular carcinoma and effects of surveillance. Clinical Gastroenterology and Hepatology 2011;9(7):617-23. [DOI] [PubMed] [Google Scholar]
Ye 2015
- Ye F, Liu J, Ouyang H. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging and multidetector-row computed tomography for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Medicine 2015;94:e1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yokoyama 1990
- Yokoyama I, Todo S, Iwatsuki S, Starzl TE. Liver transplantation in the treatment of primary liver cancer. Hepato-gastroenterology 1990;37(2):188-93. [PMC free article] [PubMed] [Google Scholar]
Young 2012
- Young AL, Adair R, Prasad KR, Toogood GJ, Lodge JP. Hepatocellular carcinoma within a noncirrhotic, nonfibrotic, seronegative liver: surgical approaches and outcomes. Journal of the American College of Surgeons 2012;214(2):174-83. [DOI] [PubMed] [Google Scholar]
Zwinderman 2008
- Zwinderman AH, Glas AS, Bossuyt PM, Florie J, Bipat S, Stoker J. Statistical models for quantifying diagnostic accuracy with multiple lesions per patient. Biostatistics 2008;9(3):513-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Nadarevic 2019
- Nadarevic T, Giljaca V, Colli A, Fraquelli M, Casazza G, Miletic D, et al. Computed tomography for the diagnosis of hepatocellular carcinoma in chronic advanced liver disease. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD013362. [DOI: 10.1002/14651858.CD013362] [DOI] [PMC free article] [PubMed] [Google Scholar]


