Abstract
Introduction
Angio-Seal is a commonly used device for femoral hemostasis in neuroendovascular procedures. This meta-analysis investigates of the safety and efficacy of Angio-Seal in patients undergoing endovascular neurointerventional procedures.
Methods
A systematic review and meta-analysis on all studies evaluating the Angio-Seal device in neurointerventional procedures from inception through 2020 were performed. We studied rates of groin hematoma, retroperitoneal hematoma, pseudoaneurysm, ipsilateral DVT, and ischemic complications. Meta-analysis was performed using the random-effects model.
Results
13 studies were included in our analysis. 2250 patients with 104 complications were found {4.5% (95% CI, 2.7%–6.3%)}. Of these complications, groin hematoma was the most common with a rate of 2.4% (95% CI, 1.1%–3.6%). Retroperitoneal hematoma {0.3% (95% CI, 0%–0.5%)}, pseudo-aneurysm {0.5% (95% CI, 0.2%–0.8%), and ipsilateral DVT {0.3% (95% CI, 0.1%–0.7%) were also not in negligible rate. The rate of other complications were as follows: vessel occlusion/stenosis; 0.2% (95% CI, 0%–0.4%), vascular surgery; 0.2% (95% CI, 0%–0.5%), and infection; 0.2% (95% CI, 0%–0.5%). One patient died as result of hemorrhagic complications {0.1% (95% CI, 0%–0.3%)}. Use of anticoagulant/antiplatelet therapy was found to be positively correlated with high risk of any groin complication and groin hematoma (p ≤ .05). Female gender was associated with high risk of ipsilateral DVT (p ≤ .05). Interestingly, large sheath size was associated with low risk of groin hematoma (p ≤ .05).
Conclusion
The safety and efficacy rate of Angio-Seal was approximately 95%. The most common complication was groin hematoma. Serious complications including retroperitoneal hematoma and femoral artery occlusion were rare.
Keywords: Angio-Seal, vascular closure devices, transfemoral access, transradial access
Introduction
Vascular closure devices were introduced in the early 1990s as an alternative to manual compression. These medical devices aimed mainly at decreasing complications associated with delayed hemostasis in manual compression applied to patients by reducing the time to hemostasis. One of the more commonly used closure devices in neurointerventional suites is the Angio-Seal. The Angio-Seal consists of a collagen plug, a small rectangular anchor, and a connecting polyglycolic suture and was first introduced in 2006.
Understanding the safety and efficacy of the Angio-Seal as it relates to neuroendovasuclar procedures is important given the fact that neurointerventional patients are frequently fully anticoagulated, on dual antiplatelet therapy, and, in the setting of stroke, receive tPA. A comprehensive meta-analysis of Angio-Seal related complications is also important for benchmarking femoral artery access complication rates as well. In this meta-analysis, we investigate important femoral artery access-site complications of Angio-Seal vascular closure device in the patients who treated with interventional neuro-endovascular procedures.
Materials and methods
Search strategy
A comprehensive search of several databases from inception to September 24, 2020, limited to English language and excluding animal studies, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The key words “Angioseal ““Angio-Seal” “Vascular closure device” “neuro-interventional” “neurovascular” “cerebrovascular accident” “stroke” “cerebral Angioplasty” “aneurysm rupture” “cerebral angiography” were used in both “AND” and “OR” combinations. The search strategy was designed and conducted by an experienced librarian with input from the study’s principal investigator. Controlled vocabulary supplemented with keywords was used to search for studies describing the Angio-Seal device in neurointerventional patients. (Full search strategy is available in Appendix 1)
Eligibility criteria
Inclusion criteria were the following: 1) Study reporting a consecutive series of patients, 2) The patients were treated with interventional neuro-endovascular procedures with clear reporting of outcomes, 3) common femoral artery access with hemostasis achieved using the Angio-Seal vascular closure device, 4) Series of at least 20 patients reporting clearly the complications of the Angio-Seal vascular closure device. Review articles, guidelines, technical notes, comments, and editorials were excluded.
Study selection process
Titles and abstracts were screened for inclusion by one author using EndNote (Clarivate Analytics, Philadelphia, United States). Full-text articles were retrieved for the included abstracts and screened by the same author and were reviewed by the senior author.
Data extraction and outcome measures
We extracted the baseline characteristics of the patients from each study including patient number, gender, mean age, mean BMI, history of co-morbidities like stroke, hypertension. DM, cardiac diseases, and dyslipidemia. Information of the procedures included the sheath size, the use of tPA, anticoagulant therapy (ACT), or antiplatelet drugs (APT) were also extracted.
The number of any complication associated with Angio-Seal vascular closure device use to facilitate the hemostasis in the femoral arteriotomy in the patients who treated with interventional neuro-endovascular procedures was extracted. Nine complications were considered for extraction included; groin hematoma (any size if reported), retroperitoneal hematoma, pseudoaneurysm, ipsilateral DVT, ipsilateral limb ischemia, vessel occlusion/stenosis, vascular surgery, infection, and death. All these complications were counted under “Any groin complication”. Thus, the goal of this meta-analysis is to investigate the complications and safety of the Angio-Seal vascular closure device in the neuro-interventional procedures.
Study risk of bias
Newcastle-Ottawa Quality Assessment Scale for Case Control Studies was designed to assess the risk of bias in comparative studies. However, because none of our included studies was controlled, we selected specific items from this tool and modified it to assess the risk of bias in our included studies in this meta-analysis focusing on these questions: 1) did the study include all patients or consecutive patients versus a selected sample?; 2) was the study prospective or retrospective?; 3) were outcomes clearly reported?; and, 4) were the interventionalists treating the patients and those who assessed angiographic and clinical outcomes same?.
Statistical analysis
The cumulative incidence (event rate per patient at the end of the study) for each study was estimated and 95% CI. Because we anticipated marked heterogeneity in the populations and interventions across the various included studies, a random-effects model was used to pool incidence rates across studies.1 The I2 statistic was used to express the proportion of inconsistency that is not attributable to chance.2 Meta-analysis for all outcomes, meta regression for outcomes of interest and a funnel plot for each outcome -to investigate any probable publication bias- were conducted using Jamovi 1.2 (The Jamovi Project, Sydney, Australia, 2020),3 and OpenMeta[Analyst] open source Statistical Software.4 Outcomes or variables which were reported in limited number were not included in meta regression according to Cochrane recommendations.5
Results
Study selection and characteristics
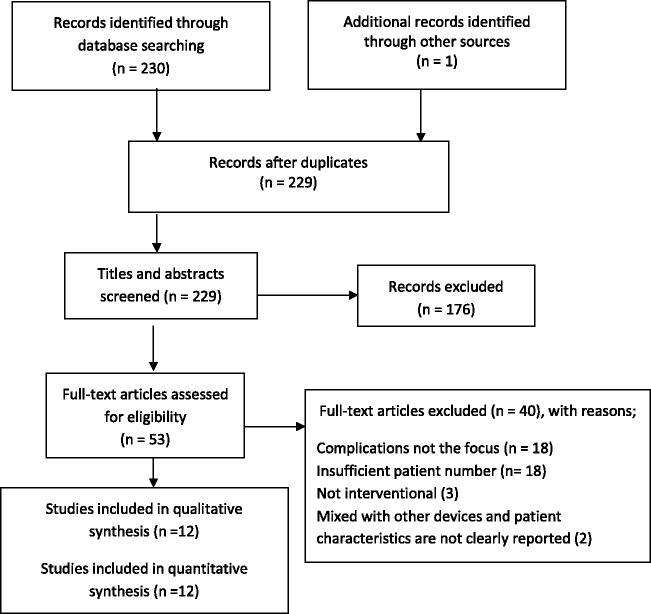
230 articles were found by the librarian search and one article was added after hand-search. 229 articles were selected for review after removing the duplicates. Upon the review of the title and abstract, 176 articles were excluded. The full text of 53 articles was screened and 12 articles6–17 with 2478 patients were included in the qualitative synthesis. 1224 (49.4%) and 1254 (50.6%) of these patients were male and female, respectively and their mean age was 60.4 years. The mean BMI of 538 patients in 4 studies was 27.67 kg/m2. 2250 patients treated with interventional neuro-endovascular procedures were included in the quantitative synthesis. The baseline characteristics of the patients including the reported co-morbidities and the procedure information are listed in Table 1.
Table 1.
Baseline characteristics.
|
Study |
Patient characteristics |
Procedure information |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, date, (total patients no.) | Study design | Risk of bias | Patients who underwent neuro-interventional procedures with Angio-Seal device | Female gender (%) | Mean age | Mean BMI (kg/m²) | Stroke or SAH (%) | HTN (%) | DM (%) | Cardiac morbidities (%) | Dyslipidemia (%) | Sheath size -Fr- (mean) | No. of patients on tPA (%) | No. of patients on Anticoagulant (%) | No. of patients on Antiplatelet (%) |
| Pierot et al.6 | P | Low | 119 | 53 | 51.60 | 4 pt >30 | NR | 19.3 | 9.2 | NR | NR | 6.27 | NR | 67.2 | NR |
| Geyik et al.15 | R | Mod | 640 | 46.4 | 54.50 | NR | NR | NR | NR | NR | NR | 6.42 | NR | 21.25 | 48.75 |
| Wong et al.12 (153) | P | Low | 90 | 43.8 | 56.10 | NR | NR | NR | NR | NR | NR | 6 or 8 | NR | NR | NR |
| Shah et al.8 (472) | R | Mod | 443a | 50 | 70.00 | NR | NR | NR | NR | NR | NR | 8.05 | 55.1 | 12.7 | 2.1 (DAPT) |
| Shah et al.10 (234) | R | High | 98b | 61.5 | 52.50 | NR | NR | NR | NR | NR | 10.25 | 8 | NR | 40.6 | 17.1 |
| Sato et al.16 | P | Low | 119 | 47.06 | 62.10 | 34.00 | NR | 52.1 | 13.4 | NR | 30.25 | 7.36 | 2.5 | 7.5 | 89.1 |
| Aida et al.11 (141) | R | Low | 141 | 54.6 | 63.07 | 22.99 | NR | 64.5 | 18.4 | NR | 31.2 | 6.3 | NR | NR | 77.3 |
| Janssen et al.6 (72) | R | High | 72 | 52.8 | 70.60 | NR | 100 | 86.1 | 29.2 | 48.6 | 61.1 | 9 | 63.9 | 20.8 | 33.3 |
| Wareham et al.14 (145)] | R | Mod | 145 | 51 | 69.10 | NR | 100 | 33.1 | 7.6 | 25.5 | NR | 8 | 48.3 | 11.7 | 4.8 |
| Addepalli et al.13 (459) | R | Low | 197 | 68.5 | 53.50 | 29.95 | 100 | 62.9 | 16.75 | NR | NR | NR | NR | 60.9 | NR |
| Ishida et al.17 (60) | R | Mod | 60 | 13.3 | 71.10 | NR | NR | 81.7 | 41.7 | NR | 88.3 | NR | NR | 100 | 100 |
| Ozono et al.9 (116) | R | Mod | 126 | 46.8 | 68 | 23.35 | 19 | 65.9 | 24.6 | 15.1 | 44.4 | NR | NR | 100 | NR |
HTN: Hypertension, DM: Diabetes Mellitus, P: Prospective, R: Retrospective, NR: Not reported, DAPT: Dual Antiplatelet Therapy, Pt: Patient, Mod: Moderate.
aThe baseline characteristics of the Angio-Seal group were mixed with the data of patients with other vascular closure devices but the outcomes of each group were reported clearly.
bThe baseline characteristics of the patients who underwent interventional procedures were mixed with the ones who underwent diagnostic procedures but the outcomes of each group were reported clearly.
Overall outcomes
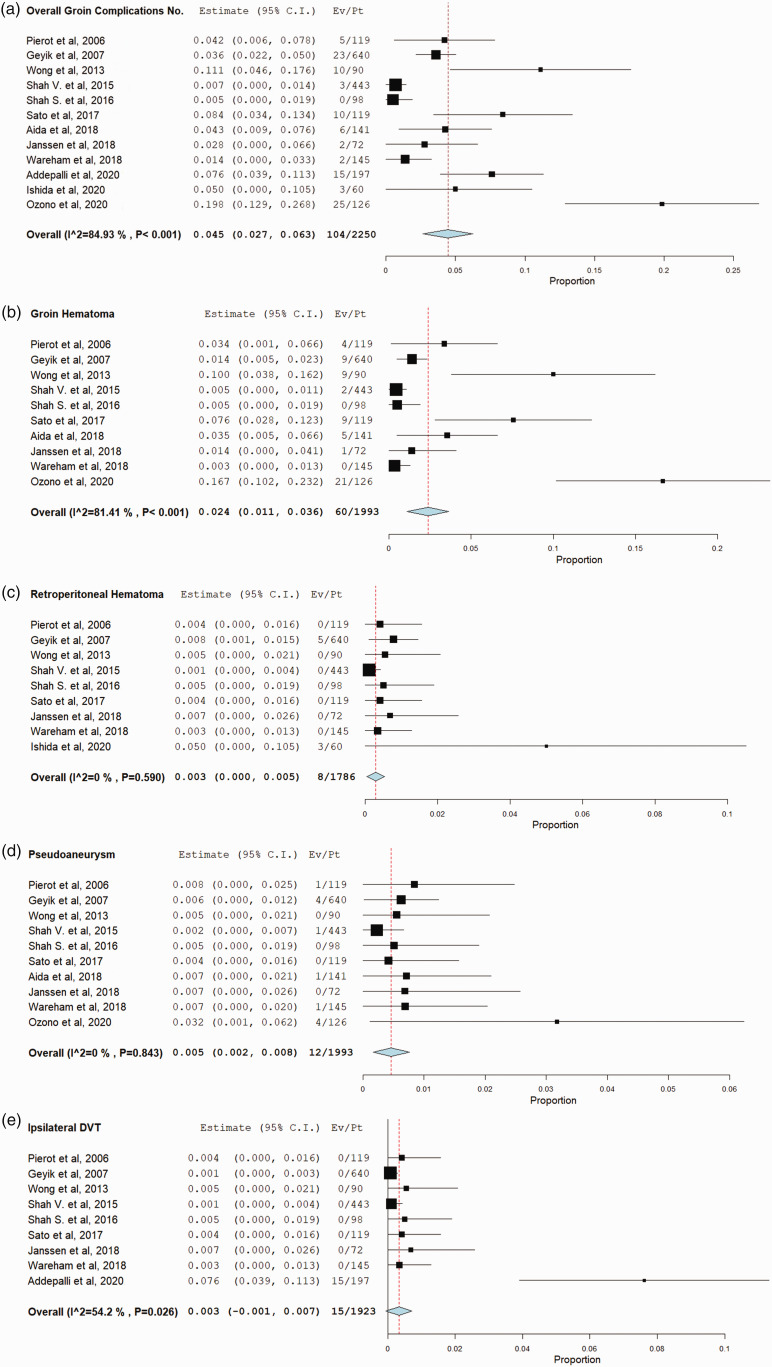
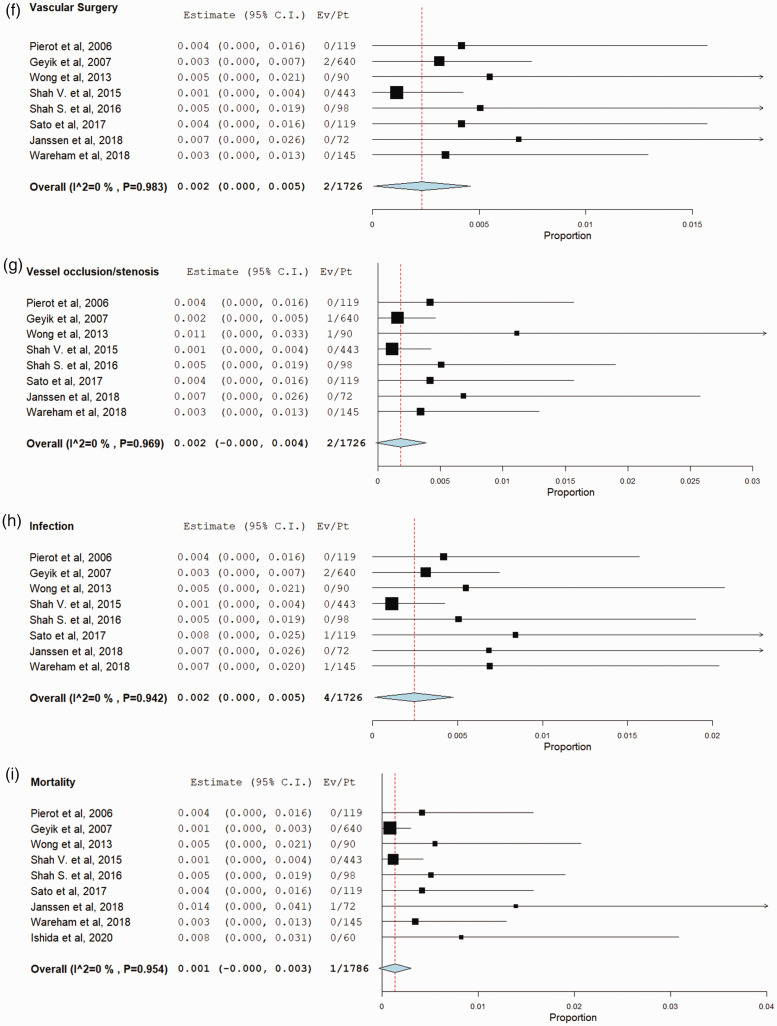
Total of 104 {4.5% (95% CI, 2.7%–6.3%)} groin complications in 2250 patients were reported in 12 studies. The most common complication was groin hematoma, which occurred in 60 patients {2.4% (95% CI, 1.1%–3.6%), in 1993 patients and 10 studies. The rate of other complications was as follows: of pseudoaneurysm was 0.5% (95% CI, 0.2%–0.8%), retroperitoneal hematoma was 0.3% (95% CI, 0%–0.5%), ipsilateral DVT was 0.3% (95% CI, 0.1%–0.7%), vessel occlusion/stenosis was 0.2% (95% CI, 0%–0.4%), vascular surgery was 0.2% (95% CI, 0%–0.5%), infection was 0.2% (95% CI, 0%–0.5%), and death was 0.1% (95% CI, 0%–0.3%). No complication of ipsilateral limb ischemia was reported in the included studies. According to our meta regression results, the larger the proportion of patients who received ACT/APT, the higher the risk of any groin complication and groin hematoma was found to be exist (p < .05). Interestingly, the larger sheath size was associated with less risk of groin hematoma (p < .05). A significant correlation was found between female gender and high risk of ipsilateral DVT (p < .05). All complications rates are provided in Table 2 and Figure 2.
Table 2.
Complication rates.
|
Complications of Angio-Seal device in transfemoral neuroendovascular procedures | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, date. | Patients no. | Any groin complication | Groin hematoma | Retroperitoneal hematoma | Pseudo-aneurysm | DVT (ipsilateral) | Ipsilateral limb ischemia | Vessel occlusion/stenosis | Vascular surgery | Infection | Complication-associated mortality |
| Pierot et al.6 | 119 | 5 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Geyik et al.15 | 640 | 23 | 9 | 5 | 4 | 0 | 0 | 1 | 2 | 2 | 0 |
| Wong et al.12 | 90 | 10 | 9 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Shah et al.8 | 443 | 3 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Shah et al.10 | 98 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sato et al.16 | 119 | 10 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Aida et al.11 | 141 | 6 | 5 | NR | 1 | NR | NR | NR | NR | NR | NR |
| Janssen et al.6 | 72 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Wareham et al.14 | 145 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Addepalli et al.13 | 197 | 15 | NR | NR | NR | 15 | NR | NR | NR | NR | NR |
| Ishida et al.17 | 60 | 3 | NR | 3 | NR | NR | NR | NR | NR | NR | 0 |
| Ozono et al.9 | 126 | 25 | 21 | NR | 4 | NR | NR | NR | NR | NR | NR |
NR: Not reported.
Figure 2.
Forest plots with random-effect model demonstrate the risk of following complications after use of Angio-Seal closure device in transfemoral neuroendovascular procedures; (a) Overall Groin complication. (b) Groin hematoma. (c) Retroperitoneal hematoma. (d) Pseudoaneurysm formation. (e) Ipsilateral DVT. (f) Vascular surgery. (g) vascular occlusion/stenosis. (h) Infection. (i) Complication-associated mortality.
Figure 1.
The Flow diagram of study selection process.
Heterogeneity & risk of bias
High heterogeneity was found in two outcomes; any groin complication (84.93%) and groin hematoma (81.41%). Outcome of ipsilateral DVT was associated with moderate heterogeneity (54.2%). A publication bias was found to be in all outcomes. Funnel plots of the outcomes and all the results of meta-regression are provided in Appendix 2.
Of 12 studies, the risk of bias was low in 5, moderate in 5, and high in 2 studies. The smallest study had 60 patients and the largest study had 640 patients. The included studies’ data are summarized in Table 1.
Discussion
Our meta-analysis of over 2000 patients undergoing femoral hemostasis for neurointerventional procedures using the Angio-Seal demonstrated a number of interesting findings. First, Angio-Seal resulted in complete femoral hemostasis without hematoma or other complication in over 95% of cases. Overall, this efficacy rate is satisfactory given the large sheath size and use of anti-platelet agents and anticoagulation during these neurointerventional procedures (bearing in mind that patients undergoing diagnostic angiography were excluded). The most common complication was groin hematoma, occurring in 2.5% of cases. Major complications including pseudoaneurysms, retroperitoneal hematoma, DVT, vessel occlusion, and infection occurred in a total of 2.5% of cases as well. The rate of these complications is not negligible however. These findings are important as they 1) highlight some of the complications associated with Angio-Seal that physicians should be vigilant of and 2) demonstrate that with the use of Angio-Seal, major and minor complications can occur in up to 5% of patients.
We selected specific complications because of their important impact on the clinical prognosis of the patients. Among these complications, groin hematoma was found to be the most common complication. However, groin hematoma was associated with low morbidity and usually resolved after additional treatments in most of the studies. Groin hematomas can be fatal however, as demonstrated in a study published by Janssen et al which included the only mortality in our study.6 Twelve pseudo-aneurysms and eight retroperitoneal hematomas were also noted. Two vascular occlusions (or stenosis) were also reported. Two surgical interventions were reported; the first was to repair a stenotic femoral artery and the second was to resect a local infection associated with groin hematoma. Four infections were seen in 1726 patients.14–16
On review of the literature, low BMI and large sheath size were reported to be associated with a high risk of complications in the neurovascular literature. Aida et al. attributed the high risk of bleeding in patients with low BMI to the low subcutaneous tissue to cover the collagen sponge and the effect of antiplatelet therapy that they assumed to be more powerful in patients with low BMI.11 However, only four studies in our systematic review reported the mean BMI. Larger sheath size was reported as an independent predictor of access site complications in the percutaneous coronary interventions.18,19 However, in our included studies, 900 patients with sheath size smaller or equal to 7 Fr suffered from 34 (3.8%) complications while only 7 (0.9%) complications were seen in 758 patients with large size sheath (≥ 8 Fr). Meta regression of outcome of groin hematoma concerning mean sheath size also signified the correlation between the large mean sheath size and low rate of groin hematoma (p < .05).
Concomitant anticoagulant therapy, peripheral vascular disease (PVD), and older age were found to be risk factors for many complications in the Angio-Seal use on the cardiac patients.20,21 However, in this study, the results did not show a statistically significant correlation between the mean age and any groin complication. The association between PVD and Angio-Seal complications were assessed in only a few neuro-interventional studies. Female sex, prothrombotic diseases (e.g. DM, dyslipidemia, cardiac diseases) and morbidities like stroke, hypertension were reported as predictors for access site complications in the cardiac literature.22 In our review, many of the patients were suffering from these diseases suggesting that our meta-analysis had adequate representation of these comorbidities. The female gender was found to be associated with higher risk of ipsilateral DVT according to our meta regression results. However, female gender was associated with lower risk of groin hematoma, but the results were not statistically significant (p=0.061).
The patients in the neuro-interventional procedures generally receive high doses of heparin and antiplatelet drugs before and after the procedure and occasionally lifelong. In addition, those with ischemic stroke and thromboembolic cerebrovascular diseases are often started on tPA and thrombolytic agents. Many trials and cohort studies in the cardiologic literature have drawn the attention to the complications related to high heparin doses, thrombolytic agents, and prolonged heparin treatment.23–25 Since puncture site complications were seen to increase significantly in the patients who receive those treatments, some authors recommended to cease or reverse these drugs prior to sheath removal.6,15 In our systematic review, most of the studies reported that their patients received anticoagulant or antiplatelet treatments. However, only four studies mentioned the use of tPA.6,8,14,16 Geyik et al. showed in their retrospective study that the vascular closure device related complications rate in the interventional procedures is around 5 times more than in the diagnostic procedures where there is no need for the activated clotting time measurements, generally.15 However, Wong et al. reported an acceptable low level of VCD complications in patients with prolonged elevated activated clotting time (range 250–500 seconds).12 Shah et al. evaluated the Angio-Seal device complications in non-ideal circumstances to use the device and reported no complication in the interventional group.10 In our study, a meta regression of the outcomes “any groin complication” and “groin hematoma” regarding ACT and APT use showed that the larger the proportion of patients who received ACT or APT, the higher the risk of groin hematoma.
The high heterogeneity among the included studies in outcomes of any groin complication, groin hematoma, and moderate heterogeneity in ipsilateral DVT can be referred to the variation in sheath size, use of ACT/APT, segments of femoral artery where the device has been applied, and the experience of the interventionist.
Limitations
The main limitation of this meta-analysis is that all the included studies were uncontrolled and many were retrospective and the results have high heterogeneity. There might be variability in practice patterns and operator and medical center expertise. This is evident by the wide range of antiplatelet therapy regimens that were administered to patients before and after the neurovascular interventions. Groin hematoma was listed as a complication, but many of these hematomas were clinically inconsequential. There was no reported follow-up period to assess any possible or delayed Angio-Seal device complication like DVT in most of the included studies. Another limitation is the lack of stratification of outcomes based on important variables such as the type of the neuro-endovascular intervention. Publication bias is obvious among the studies, and the role of the device manufacturer in the research is not fully clear. Lastly, we do not have specific data on baseline patient morbidity. Thus, we are unable to determine what proportion of patients with poor medical history developed the complication after procedure. Therefore, the overall certainty in the evidence at present is rated very low.26
Conclusion
The complication rates associated with the use of the Angio-Seal vascular closure device in neurointerventional patients are not negligible, at around 5%. A majority of the complications was due to groin hematomas; however, complications such as pseudoaneurysm, infection and arterial stenosis occurred in slightly over 2% of cases. These findings are important to consider when benchmarking femoral artery complication rates.
Footnotes
Authors’ Contribution: All authors contributed equally to the manuscript, read, and approved the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Muhammed Amir Essibayi https://orcid.org/0000-0001-8325-2382
Waleed Brinjikji https://orcid.org/0000-0001-5271-5524
References
- 1.DerSimonian R, Laird N.Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 2.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The jamovi project. jamovi (Version 1.2) [Computer Software]. https://www.jamovi.org (2020, accessed February 2021)
- 4.Wallace BC, Dahabreh IJ, Trikalinos TA, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw 2012; 49: 1–15. [Google Scholar]
- 5.Deeks JJ, Higgins JPT, Altman DG. (eds). Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT and Green S (eds) Cochrane handbook for systematic reviews of interventions (Version 5.1.0). London: The Cochrane Collaboration, 2011. pp. 9.33–9.34. [Google Scholar]
- 6.Pierot L, Herbreteau D, Bracard S, et al. An evaluation of immediate sheath removal and use of the Angio-Seal vascular closure device in neuroradiological interventions. Neuroradiology 2006; 48: 45–49. [DOI] [PubMed] [Google Scholar]
- 7.Janssen H, Killer-Oberpfalzer M, Lange R.Closure of large bore 9 F arterial puncture sites with the AngioSeal STS device in acute stroke patients after intravenous recombinant tissue plasminogen activator (rt-PA). J Neurointerv Surg 2019; 11: 28–30. [DOI] [PubMed] [Google Scholar]
- 8.Shah VA, Martin CO, Hawkins AM, et al. Groin complications in endovascular mechanical thrombectomy for acute ischemic stroke: a 10-year single center experience. J Neurointerv Surg 2016; 8: 568–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozono I, Sakamoto S, Okazaki T, et al. Management of post-puncture bleeding after neurointerventional procedures performed with a large-bore sheath introducer. J Clin Neurosci 2020; 74: 61–64. [DOI] [PubMed] [Google Scholar]
- 10.Shah SS, Perez G, Snelling BM, et al. Off-label use of the Angioseal vascular closure device for femoral arteriotomy: retrospective analysis of safety and efficacy. J Neurointerv Surg 2017; 9: 982–985. [DOI] [PubMed] [Google Scholar]
- 11.Aida Y, Misaki K, Kamide T, et al. Physical risk factors of hemorrhagic complications associated with Angio-Seal closure device use in neurointerventional procedures. World Neurosurg 2018; 111: e850–e855. [DOI] [PubMed] [Google Scholar]
- 12.Wong HF, Lee CW, Chen YL, et al. Prospective comparison of Angio-Seal versus manual compression for hemostasis after neurointerventional procedures under systemic heparinization. AJNR Am J Neuroradiol 2013; 34: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addepalli A, Benton J, Zhu S, et al. Risk of ipsilateral deep vein thrombosis after use of angioseal closure device in aneurysmal subarachnoid hemorrhage patients. World Neurosurg 2020; 134: e162–e165. [DOI] [PubMed] [Google Scholar]
- 14.Wareham J, Luppe S, Youssef A, et al. Safety profile of an 8F femoral arteriotomy closure using the Angio-Seal device in thrombolysed acute stroke patients undergoing thrombectomy. Interv Neuroradiol 2018; 24: 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geyik S, Yavuz K, Akgoz A, et al. The safety and efficacy of the Angio-Seal closure device in diagnostic and interventional neuroangiography setting: a single-center experience with 1,443 closures. Neuroradiology 2007; 49: 739–746. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Matsumaru Y, Sakai N, et al. Usefulness of an access-site hemostasis device in neuroendovascular treatment. Acta Neurochir (Wien) 2017; 159: 2331–2335. [DOI] [PubMed] [Google Scholar]
- 17.Ishida A, Asakuno K, Shiramizu H, et al. Very low rate of new brain lesions after vulnerable carotid artery stenting cases using only FilterWire EZ as distal embolic protection. World Neurosurg 2020; 141: e145–e150. [DOI] [PubMed] [Google Scholar]
- 18.Frank JJ, Kamalakannan D, Kodenchery M, et al. Retroperitoneal hematoma in patients undergoing cardiac catheterization. J Interv Cardiol 2010; 23: 569–574. [DOI] [PubMed] [Google Scholar]
- 19.Trimarchi S, Smith DE, Share D, et al. Retroperitoneal hematoma after percutaneous coronary intervention: prevalence, risk factors, management, outcomes, and predictors of mortality: a report from the BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) registry. JACC Cardiovasc Interv 2010; 3: 845–850. [DOI] [PubMed] [Google Scholar]
- 20.Rabah M, Mason D, Muller DW, et al. Heparin after percutaneous intervention (HAPI): a prospective multicenter randomized trial of three heparin regimens after successful coronary intervention. J Am Coll Cardiol 1999; 34: 461–467. [DOI] [PubMed] [Google Scholar]
- 21.Applegate RJ, Grabarczyk MA, Little WC, et al. Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J Am Coll Cardiol 2002; 40: 78–83. [DOI] [PubMed] [Google Scholar]
- 22.Piper WD, Malenka DJ, Ryan TJ, Jr, et al. Predicting vascular complications in percutaneous coronary interventions. Am Heart J 2003; 145: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 23.Blankenship JC, Balog C, Sapp SK, et al. Reduction in vascular access site bleeding in sequential abciximab coronary intervention trials. Catheter Cardiovasc Interv 2002; 57: 476–483. [DOI] [PubMed] [Google Scholar]
- 24.Chevalier B, Lancelin B, Koning R, et al. Effect of a closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter Cardiovasc Interv 2003; 58: 285–291. [DOI] [PubMed] [Google Scholar]
- 25.Ouriel K, Gray B, Clair DG, et al. Complications associated with the use of urokinase and recombinant tissue plasminogen activator for catheter-directed peripheral arterial and venous thrombolysis. J Vasc Interv Radiol 2000; 11: 295–298. [DOI] [PubMed] [Google Scholar]
- 26.Murad MH, Montori VM, Ioannidis JP, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users' guides to the medical literature. J Am Med Assoc 2014; 312: 171–179. [DOI] [PubMed] [Google Scholar]