Abstract
Introduction
Dual antiplatelet therapy (DAPT), primarily the combination of aspirin with a P2Y12 inhibitor, in patients undergoing intravascular stent or flow diverter placement remains the primary strategy to reduce device-related thromboembolic complications. However, selection, timing, and dosing of DAPT is critical and can be challenging given the existing significant inter- and intraindividual response variations to P2Y12 inhibitors.
Methods
Assessment of indexed, peer-reviewed literature from 2000 to 2020 in interventional cardiology and neuroendovascular therapeutics with critical, peer-reviewed appraisal and extraction of evidence and strategies to utilize DAPT in cardio- and neurovascular patients with endoluminal devices.
Results
Both geno- and phenotyping for DAPT are rapidly and conveniently available as point-of-care testing at a favorable cost-benefit ratio. Furthermore, systematic inclusion of a quantifying clinical risk score combined with an operator-linked, technical risk assessment for potential adverse events allows a more precise and individualized approach to new P2Y12 inhibitor therapy.
Conclusions
The latest evidence, primarily obtained from cardiovascular intervention trials, supports that combining patient pharmacogenetics with drug response monitoring, as part of an individually tailored, precision medicine approach, is both predictive and cost-effective in achieving and maintaining individual target platelet inhibition levels. Indirect evidence supports that this gain in optimizing drug responses translates to reducing main adverse events and overall treatment costs in patients undergoing DAPT after intracranial stent or flow diverting treatment.
Keywords: Precision medicine, hemodynamics, thrombolysis, neurosurgery, antiplatelet
Introduction
Neuroendovascular interventions with the placement of intracranial stents and flow diverters are increasingly performed worldwide, a rise which is closely linked to increased utilization of brain imaging, improvements in interventional devices and techniques, and reduction in thromboembolic events (TEE) by combining a P2Y12 receptor inhibitor with aspirin, termed dual antiplatelet therapy (DAPT). With the growing complexity of neuroendovascular therapeutics, the risk profiles for adverse events have also changed and antiplatelet strategies driven by patient-specific characteristics have gained importance. Most of the evidence for DAPT effectiveness originates from extensive, randomized studies performed in high-risk cardiac patients which consistently showed substantial reductions of in-stent thrombosis and thromboembolic events employing various DAPT regimens, predominantly a combination of clopidogrel and aspirin. However, the newer oral P2Y12 inhibitors prasugrel and ticagrelor, together with the recent addition of the intravenous P2Y12 inhibitor cangrelor1 and the anticipated availability of a specific reversal agent for ticagrelor,2 provide increasing variety, and at times ambiguity, in addressing specific treatment scenarios.
The rationale to employ individualized P2Y12 inhibitor strategies is based on differences in genetic susceptibility, unpredictable pharmacokinetics, and clinical factors that introduce substantial inter- and intra-patient response variability in platelet reactivity (PR). For example, CYP gene polymorphisms cause significant variances in active clopidogrel metabolites, and genetic variation in high on-treatment platelet reactivity (HPR) is a consistent risk factor for stent thrombosis and TEE in both cardiac and neuroendovascular interventions. This complexity increases even further with the inclusion of clinical factors such as care setting (elective versus emergent), concomitant therapies, costs, side effects, medication adherence, and patient/physician preference. The integration of such factors with the ability to detect genetic polymorphism (genotype) and degree of PR (phenotype) at the bedside encourages a patient-specific, individualized therapeutic approach, also referred to as precision medicine. Precision medicine is a new frontier combining genomics, data analytics, and population health to develop “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person.”3 The difference between traditional clinical assessments and precision medicine can be expressed as the degree of reliance on objective data to identify personalized treatments. As discussed in this review, adding genetic or molecular data to established laboratory and clinical risk profiles, allows neuroendovascular surgeons and interventionalists to tailor an antiplatelet regimen to an individual patient uniquely. Here, we integrate the pharmacogenetics of P2Y12 platelet inhibitors, neuroendovascular DAPT experience gained from the investigation of flow diverters, and pertinent cardiovascular evidence to conceptualize a precision medicine approach to DAPT.
Methods
We identified records through database searching, including MEDLINE (http://www.ncbi.nlm.nih.gov/pubmed/); EMBASE (Excerpta Medica Database; http://www.embase.com/); Cochrane CENTRAL (The Cochrane Central Register of Controlled Trials; http://uscc.cochrane.org/); AHRQ (Agency for Healthcare Research and Quality; http://www.ahrq.gov/); Lexicomp Clinical Drug Information; and MICROMEDEX Healthcare Series. When appropriate, Medical subject headings (MeSH) and keywords pertaining to neuroendovascular interventions, intracranial flow diversion, intracranial stent, DAPT, percutaneous coronary intervention; myocardial infarction, acute coronary syndrome; antithrombotic therapy; P2Y12 inhibitor, aspirin, clopidogrel, ticagrelor, prasugrel, ischemic stroke, hemorrhage, thrombosis, platelet reactivity, platelet function test, pharmacogenetic, polymorphism, outcome adverse events, and cost were used. Key publications were searched for additional citations. Identified publications were screened at the title and abstract level, and selected references independently evaluated by all authors for eligibility and discrepancies.
Results
The need to improve on current antiplatelet therapy
Improved antiplatelet therapy is ever more pressing with the emergence of flow diversion as a first-line treatment for many intracranial aneurysms in the last decade. Flow diversion represents a conceptual shift in aneurysm treatment from packing the aneurysm to reconstructing the parent artery and the arising aneurysm using minimal porosity endoluminal devices. Two mechanisms primarily obtain occlusion: cutting off blood supply to the aneurysm which leads to thrombosis and ultimately, scar formation, providing a scaffold for neointimal neck overgrowth. Flow diverters, however, are thrombogenic, especially before completion of neointimal coverage. Thromboembolic rates with the Pipeline embolization device (PED) and DAPT have been reported to range from 8% to 12%,4–6 and 6-month in-stent stenosis from 3.5% to 16%.4–6 Small sample sizes explain this relatively wide distribution, retrospective study design, lack of anatomical stratification, and variation in DAPT between existing studies. Given these limitations, Saber et al.7 reported in their pooled analysis of 2,002 patients undergoing PED placement (2009 to 2017) an overall 7% (95% CI 6-9%) rate for thromboembolism and 5% (95% CI 4-6%) for hemorrhage For a concise overview, Table 1 summarizes ischemic and hemorrhagic event rates, as well as overall mortality rates for the respective DAPT regimens employed across six major prospective multicenter flow diversion trials for unruptured aneurysms.
Table 1.
Various DAPT regimens and reported ischemic and hemorrhagic complications of selected flow diversion publications.
| PITA68 | PUFS69 | PREMIER70 | SAFE71 | SCENT72 | PARAT73 | Adeeb et al.29 | Atallah et al.74 | Griessenauer et al.75 | Moore et al.76 | Soize et al.77 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study type | P / MC | P / MC | P / MC | P / MC | P / MC | P / RCT | R | R | R | R | R |
| Aneurysms | 31 | 109 | 141 | 103 | 180 | 144 | 402 | 396 | 114 | 103 | 80 |
| PreTx AntiplateletDosage | Not reported | ASA 325 mg/d x2 ds AND CLO 75 mg/d x7 dsOR CLO 600 mg x1d | ASA min 81 mg/d x7 dsANDCLO min 75 mg/d x7 ds | Not reported | ASA min 75 mg/d AND CLO 75 mg/d x5 ds | ASA 300 mg/d AND CLO 75 mg/d x3 ds | ASA 325 mg/d AND CLO 75 mg/d daily3 to 14 dsALT: CLO 600mgx1 or ticagrelor 90 mg/BID for nonresponders | ASA min 75 mg/d AND CLO 75 mg/d x10 ds ALT: ASA 325-650mg x1 AND CLO 600 mg x1 OR PRA 40–60 mg x1 OR TIC 90 mg x1 | ASA min 81 mg/d AND CLO 75 mg/d min x10 ds | ASA 325 mg/d AND CLO 75 mg/d daily x14 ds OR Nonresponders TIC 180 mg x1 ∼3 h prior | ASA 325 mg/d AND CLO 75 mg/d x5 ds OR ASA 325 mg/d AND TIC 90 mg/BID x2ds |
| PostTx AntiplateletDosage | ASA 100 mg/d x180 dsCLO 75 mg/d x30 ds | ASA 325 mg d x 6 msCLO 75 mg/d X3 ms | ASA min 81 mg/dx6 msCLO 75 mg/d x3 ms | Not reported | ASA min 75 mg/d indefinitelyAND CLO 75 mg/d or equivalent x6 ms | <6 wks: ASA 300 mg/d AND CLO 75 mg/d; 6 wks to 3 ms: ASA 100 mg/d AND CLO 75 mg/d; then only ASA | DAPT x3 msthen only ASA | CLO 75 mg/d OR PRA 10 mg/d OR TIC 90 mg/BIDx6 to 12 ms | DAPT x3-6 ms | DAPT x3 msthen only ASA | DAPT x3 ms |
| PhenotypingTargets | None | None | VerifyNow PRU 60 to 200 | None | Platelet aggregometry30% to 90% | None | Nonresponders if PRU >208 | Nonresponders if PRU <30% inhibition | None | Aggregometry >50% with 5 μM ADP OR VerifyNow PRU >208 | None |
| GenotypingAlternative | Not done | Not done | Not done | Not done | Not done | Not done | Not done | Not done | Not done | Not done | Not done |
| Follow-up | 6 mo | 6 ms and 5 yr | 1 yr | 14.7 ms | 12 ms | 6 ms | Not specified | 15.8 ms | 6.5 ms | 7.2 ms | 1 m |
| Thromboembolism | 6.7% | 2.8% | 0.7% | 6.8% | 6.1% | Tubridge 10% SAC 10% | ASA CLO 5.6%ASA CLO plus BOOST 9.8%ASA TIC 2.7% | ASA CLO 7.5%ASA PRA OR TIC 4.5% | All: 8.5% | ASA CLO 6%ASA TIC 4.2% | ASA CLO 5%ASA TIC 12.5% |
| Hemorrhage | 0% | 1.9% | 1.4% | 1% | 2.8% | Tubridge 6%SAC 3% | ASA CLO 3.2%ASA CLO plus BOOST 2%ASA TIC 0% | ASA CLO 5.6%ASA PRA OR TIC 0% | All: 1 patient | ASA CLO 1.9%ASA TIC 0% | ASA CLO 5%ASA TIC 5% |
The need to further optimize current DAPT strategies is also identified in imaging patients undergoing neurointerventional procedures. In a prospective cohort study of 164 patients treated for unruptured aneurysms, new DWI-positive lesions on 3 T MRI appeared in 64% to 75% despite preprocedural 75 mg clopidogrel and 160 mg aspirin daily. Age and procedure duration were independent risk factors, and lesion diameter correlated with discharge Rankin scale.8 Yang et al. identified DWI-positive lesions in 54% of 193 aneurysm patients undergoing stent treatment while on daily 75 mg clopidogrel and 100 mg aspirin (ruptured aneurysms were preloaded with 300 mg of each clopidogrel and aspirin.) TEE incidence correlated with platelet ADP inhibition percentage, and 82% of events during an 8-month follow up were associated with clopidogrel resistance.9 A meta-analysis including 2,268 aneurysms identified that, depending on treatment type, 43% to 67% (95% CI 46-85%) had new DWI lesions that did not relate to rupture status, location, or size. Flow diversion as compared to other treatments showed a trend towards a higher lesion rate (67% vs. 45%).10 The neurocognitive impact of such brain lesions remains uncertain; however, one investigation assessed 51 patients undergoing flow diverter treatment for unruptured aneurysms and found no difference in Montreal Cognitive Assessment scoring between baseline and 1-month post-procedure.11
Aspirin
Aspirin produces acetylation of the critical residue serine 529 of cyclooxygenase 1 (COX-1) leading to its inhibition and, consequently, reduced conversion of arachidonic acid to prostaglandin G2. Diminished prostaglandin substrate for thromboxane synthase in turn reduces thromboxane A2, which acts as a potent vasoconstrictor and platelet activator. As a result, both platelet aggregation and endothelial inflammatory factors are diminished. Low aspirin doses (75–300 mg) solely inhibit COX-1; nevertheless, PR can still be elevated due to competing for coactivating mechanisms. About 2–57% of aspirin users exhibit a suboptimal response, with some experiencing a cardiovascular event indicative of clinical aspirin failure. For others, in vitro platelet reactivity is not adequately blocked, even after aspirin addition (laboratory resistance). Pharmacokinetic resistance, or limited active drug at the target site, is commonly multifactorial and should be differentiated from pharmacodynamic resistance such as genetic polymorphism. While various laboratory assays quantify ranges for normal and therapeutic platelet reactivity or metabolites, these are mostly nonspecific and inconsistent, and assays are not considered to guide clinical decisions exclusively.12 Genotyping aspirin resistance, however, has gained attention, i.e., for the genetic polymorphism of the PTGS1 gene that encodes COX-1, as specific short nucleotide polymorphisms and haplotypes dysregulate arachidonic acid-induced thromboxane production leading to inadequate aspirin responses.13
Aspirin HPR, a risk factor for major adverse cardiac events (MACE) after percutaneous coronary intervention (PCI),14 is observed in up to 15% of patients undergoing neuroendovascular interventions; however, in alignment with cardiovascular studies, a consistent association of aspirin PR and clinical outcomes is not evident. However, two recent meta-analyses suggest that in DAPT, low-dose (≤150 mg daily) as compared to high-dose aspirin given either before or after the neuroendovascular intervention increases late (>6 months) thromboembolism by about 2.5 times without differences in hemorrhagic events.7,15 Furthermore, observational cardiac studies identified a roughly 4-fold increase in ischemic events in cardiovascular patients deemed resistant to aspirin.16 In an updated meta-analysis of 11,857 coronary patients, any aspirin resistance correlated with an increased risk of all-cause death (OR 2.42, 95% CI 1.86–3.15) and target vessel revascularization (OR 2.20, 95% CI 1.19–4.08).17 Low PR (LPR) on-aspirin-specific testing has been associated with an increased bleeding risk in one registry (HR 0.65, 95% CI 0.43–0.99) but not in another.18
P2Y12 inhibitors and genetic polymorphism
Clopidogrel and prasugrel are prodrugs biotransformed by multiple hepatic cytochrome P450 (CYP) enzymes to active metabolites that irreversibly inhibit the ADP P2Y12 platelet receptor. Of ingested clopidogrel, only 15% is transformed into active metabolites, an action predominantly promoted by the CYP2C19 subtype. Functional relevant polymorphisms of the CYP2C19 gene exist and expressions of different allelic combinations dictate active clopidogrel metabolite levels resulting in substantial inter-patient variabilities in platelet inhibition. However, the CYP2C19 genotype does not influence the clinical effectiveness of prasugrel and ticagrelor which exhibit more prompt, potent, and consistent platelet inhibition compared with clopidogrel. Prasugrel activation by CYP3A4 and CYP2B6, and to a lesser extent CYP2C19, is not affected by genetic polymorphisms. Ticagrelor, a reversible, non-competitive P2Y12 inhibitor, does not require activation and, moreover, 30% is metabolized by CYP3A4 into the equipotent antiplatelet metabolite AR-C124910XX.
Clopidogrel metabolism can be categorized by its underlying, genetically determined CYP metabolism using fast and reliable bedside assays, as well as laboratory-based methods, to detect CYP polymorphism and guide P2Y12 inhibitor therapy (Table 2). Based on the specific CYP2C19 allelic combination, five activity phenotypes with resultant clopidogrel metabolic responses are clinically recognized: ultrarapid, rapid, normal, intermediate (IM), and poor metabolizers (PM) (Table 3).19 Poor metabolizers have homozygous polymorphism CYP2C19 no-function (NF; formerly loss-of-function, LOF), both CYP2C19 wild-type (*1) alleles are replaced, for example, by *2, *3, or other combinations of no function alleles. The absence of any active clopidogrel metabolites is seen in about 5% of the US and 12% of Asian populations, though on average approximately 30% of the populations throughout the US, Africa, Middle East, and Europe are IMs/PMs; this prevalence increases to about 57% in Asian and 94% in Oceanian populations.20,21 Intermediate metabolizers have reduced amounts of active clopidogrel. They are either heterozygous with one normal *1 and one NF allele leading to *1/*2, *1/*3, or show mixed *2/*17, *3/*17 polymorphism by replacing both *1 alleles with the combination of a single no function and gain-of-function allele (*17). In contrast, CYP2C19*17 is a gain-of-function (GoF) allelic variant that increases clopidogrel metabolic activity. Individuals with one or two of the GoF allelic variant *17 have rapid and ultrarapid clopidogrel metabolisms, respectively. Increased bleeding risk due to GoF has been reported in some,22 but not all, cardiovascular studies. Additional genetic variants with insignificant impact on clopidogrel metabolism have been described,23 and there is no data to support genotyping in prasugrel- or ticagrelor-treated patients. Overall, *17 polymorphism outcome data are inconsistent and insufficient to support its role in treatment decisions. As discussed further below, reproducible correlations do exist between CYP2C19 *2 and *3 NF, reduced capacity for clopidogrel bioactivation, impaired platelet inhibition, and significantly higher risks of TEE substantiate CYP genotyping to assess inter-patient variability.
Table 2.
Pharmacology of oral P2Y12 inhibitors and expected clopidogrel bioactivation frequencies within the U.S. population.
| Clopidogrel (Plavix, Bristol-Myers Squibb/Sanofi) | Prasugrel (Effient, Eli Lilly) | Ticagrelor (Brilinta, AstraZeneca) | Cangrelor (Kengreal, Chiesi) | |
|---|---|---|---|---|
| Drug class | Thienopyridine | Thienopyridine | Cyclopentyl-triazolopyrimidine | Adenosine triphosphate analogue |
| Receptor blockade | Irreversible | Irreversible | Reversible | Reversible |
| Prodrug | Yes | Yes | No but active metabolite | No |
| Half-life parent drug | ≈6 h | <5 min | 6–12 h | 3–6 min |
| Half-life active metabolite | 30 mins | Distribution 30-60 mins Elimination 2–15 h | 8–12 h | 1-2 mins |
| Binding site1 | ADP | ADP | Allosteric, non-ADP | site uncertain |
| Route, dosage, frequency | oral, 75- and 300-mg tablets, once daily | oral, 5- and 10-mg tablets, once daily | oral, 60- and 90-mg tablets, twice daily | 10 mL vial containing 50 mg |
| Loading dose | 300 mg or 600 mg | 60 mg | 180 mg | 30 mcg/kg IV bolus |
| Maintenance dose | 75 mg daily | 10 mg daily(5 mg if <60 kg) | 90 mg twice daily | mcg/kg/min IV for ≥2 h |
| Onset2-offset of action | 2–8 h; 5–10 d | 0.5–4 h; 7–10 d | 0.5–4 h; 3–5 d | ≈2 min; <1 h |
| CYP drug interaction3 | CYP2C19 | no | CYP3A | no |
| Use in renal/hepatic impairment | no dose adjustment | caution in moderate-to-severe renal or severe hepatic impairment | caution in moderate and avoid in severe hepatic impairment | no dose adjustment |
| Nonbleeding side effects | none | none | dyspnea; elevated uric acid; elevated creatinine | dyspnea |
| Average wholesale price 30-day supply at maintenance dose | $232 (brand) $184 (lowest generic) | $551 (brand) $495 (lowest generic) | $236 (brand) no generic | $899 (brand) for 50 mg solutionno generic |
1Bind to ADP P2Y12 receptor preventing ADP from binding and activating GPIIb/IIIa complex.
2After loading dose-bolus.
3Indicates clinically significant drug interactions.
ADP = adenosine diphosphate; CYP = cytochrome P450; IV = intravenous.
Table 3.
Five activity phenotypes of clopidogrel metabolic responses.
| Metabolizer Phenotype | Genotype with Examples | Frequency | Antiplatelet Response |
|---|---|---|---|
| Poor | 2 LOF alleles(*2*2, *2/*3, *3/*3) | 1%–5%Highest in Asia: 12.2% | Effectively absent |
| Intermediate | 1 LOF/NF allele (*1/*2, *1/*3, *2/*17, *3/*17) | 20%–30%Highest in Asia: 45.5% | Reduced |
| Normal | 2 NF alleles (*1/*1) | 35%–50% | Normal |
| Rapid | 1 GOF allele (*1/*17) | 20%–30%Highest in Middle East: 32% | Normal to increased |
| Ultrarapid | 2 GOF alleles (*17/*17) | 1%–5%Highest in Americas: 4.2% | Normal to increased |
LOF/NF = Loss of/No Function; GOF = Gain of Function; NF = Normal Function.
Determining platelet reactivity
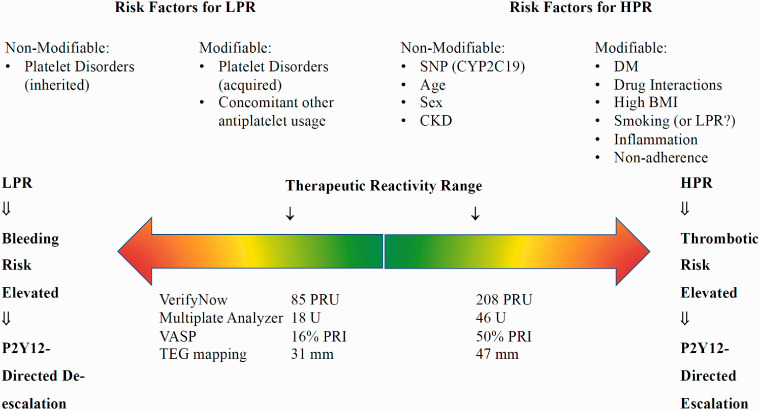
Assessment of platelet function with reliable and reproducible tests is crucial to identify patients not or over-responding to ongoing antiplatelet treatment. Because platelets react to a plethora of stimuli, different methodologies were developed to investigate certain platelet function aspects. Commonly employed test batteries include assessing platelet adhesion and aggregation, exposure of platelets to shear conditions, analysis of clot properties or measuring platelet compounds after activation. Light transmission platelet aggregometry (LTA) studies the changes of light penetration through an optically dense sample of plasma-rich platelets in response to agonists such as collagen, ADP, thrombin, ristocetin, epinephrine, and arachidonic acid. The photometer results are graphically depicted as the ratio of patient-to-standard (platelet-poor plasma or transparent) transmission during aggregation and expressed as the velocity of aggregation (slope of the curve), the maximal extent of aggregation (%), and latency time (lag phase). It is considered the gold standard to evaluate platelet function; however, limitations include aggregation testing without the presence of other blood cells, the need for trained operators, and sensitivity to pre-analytical (hemolysis, low platelet count, anticoagulant presence, etc.) and procedural conditions (reagent preparation, variations in agonists, etc.). In contrast, VerifyNow measures platelet aggregation in whole blood by a turbidimetric-based optical detection using a cartridge containing fibrinogen-coated beads and platelet agonists. Agonists, such as arachidonic acid, ADP, prostaglandin E1, or thrombin receptor activating peptide (iso-TRAP) aggregated platelets, and changes in optical transparency are quantified in the whole blood specimen. This primarily evaluates arachidonic acid resistance, with results expressed as Aspirin Reaction Units (ARU) and resistance to ADP/PGE1 P2Y12 inhibitors as P2Y12 Reaction Units (PRU). VerifyNow is widely used as a point-of-care device; its advantages and limitations are outlined in Figure 1, and platelet function tests in clinical use.
Figure 1.
Platelet reactivity and therapeutic range of P2Y12 inhibition.
Phenotyping and clinical outcome
Two neurointerventional studies applied receiver operating characteristic analyses to identify the best on-clopidogrel PR cutoffs for predicting adverse events. Kang et al.24 derived from an elective cohort with 209 aneurysms a PRU ≥295 as the strongest significant cutoff to predict TEE (sensitivity 75%, specificity 57%). Nishi et al.25 reported intracranial bleeding in 5.26% vs. 2.56% of 174 patients undergoing elective coil embolization applying a PRU cutoff ≤175. Smaller, retrospective studies reported PRU values of <70 and >150,4 >208,26 <60 and >24027 for an increase in hemorrhagic and thromboembolic rates, respectively (Figure 1). Evidence indeed exists to support a modified treatment strategy based on PRU testing. Using the ARU and PRU cutoff of 550 and 213, respectively, to define HTPR, Hwang et al.28 prospectively identified among 228 patients undergoing elective coil embolization 126 with and 102 without HTPR. Patients with HTPR were randomized to either standard (100 mg ASA and 75 mg clopidogrel daily) or modified regimen (additional 300 mg aspirin if ARU ≥550 and 200 mg cilostazol if PRU >213). All non-HTPR patients also received standard treatment. Thromboembolism was defined as TIA or stroke within seven days and bleeding within 30 days after coiling. The TEE rate in HPR patients receiving standard treatment was significantly worse than those without HTPR, 16.3% and 1%, respectively. However, patients with HTPR receiving the modified regimen had a significant decrease in TEE (1.6% vs. 11.1%) compared to HTPR patients on a standard regimen without a detectable change in bleeding rate among groups. Further, Kang et al.24 demonstrated a positive relationship between increasing PRU quartiles with 17% TEE and 21% procedure-related adverse events in the 4th quartile. Adeeb et al.29 retrospectively evaluated 414 PED procedures and reported significantly lower TEE between clopidogrel responders and non-responders (5.6% vs. 17.4%), and among non-responders switched to ticagrelor rather than remaining on clopidogrel (2.7% vs. 24.4%). Podlasek et al.,30 in a meta-analysis of five retrospective studies with control comparison groups, included 1,005 patients on DAPT undergoing flow diversion treatment of an unruptured or ruptured aneurysm. In addition to aspirin, 832 (82.8%) of all analyzed patients received clopidogrel while the ticagrelor and prasugrel comparison groups represented only 137 (13.6%) and 36 (3.6%) patients, respectively. The authors summarized a significant mortality risk reduction with ticagrelor or prasugrel (4.57, CI 1.23-16.99), similar TEE prevention rates, and in 2 studies, an insignificant increase in intracerebral hemorrhage among patients receiving ticagrelor.31,29
In acute coronary syndromes, DAPT with aspirin and a P2Y12 receptor inhibitor significantly reduces the RR of MACE by about 20% at 1 year, and an additional 20% when prasugrel or ticagrelor are used instead. Depending on the clinical setting, DAPT duration commonly ranges between 1 month to >1 year. While on DAPT, approximately 10% experience thrombotic and 2% hemorrhagic events within one year. Current cardiology guidelines define LPR, optimal PR (OPR), and HPR categories as <95, 95–208, and >208 PRU for VerifyNow testing.32 Similar to aspirin treatment,33 the presence of risk factors seems a significant contributor to the clinical relevance of HPR and outcome.34 A meta-analysis of 6,000 individual on-clopidogrel patient data35 showed that HPR was prognostic for MACE in a dose-dependent fashion. Low-risk cardiovascular patients had a 2% yearly risk of TEE; however, with ≥2 risk factors (including age >75, hypertension, and diabetes) the annual TEE increased from 2.5% to 8% in those with lower and higher PR, respectively. A meta-analysis including individual data from >20,000 on-clopidogrel patients associated LPR with a 1.74 RR (95% CI 1.47–2.06) of bleeding. Also constrained by statistical heterogeneity, a recent meta-analysis of 13 RCTs including >7,000 patients showed PFT-driven therapy decreases the incidence of cardiovascular events without increasing the risk of bleeding.36
Genotyping and clinical outcome
Studies evaluating the role of genotyping in neuroendovascular patients are few. Ge et al.37 analyzed 215 patient undergoing stent-assisted coiling for intracranial aneurysms and treated with 75 mg clopidogrel and 100 mg aspirin daily. In multivariate analysis carriage of CYP2C19 NF alleles and clopidogrel resistance were predictors of TEE, while normal (formerly referred to as ‘extensive') metabolizers were significantly associated with bleeding at 3-month follow up. Limited by small and heterogeneous subgroup sizes, Lin et al. suggested, in contrast to previously reported findings, an increase in TEE in 108 neuroendovascular patients with the allele CYP2C19*17, which did not correlate to the level of PR.38
A meta-analysis supported by clinical trials showed that poor and intermediate clopidogrel responders have an increased HR of 1.57 (95% CI, 1.13–2.16) for MACE and 2.81 (95% CI, 1.81–4.37) for stent thrombosis.39,40 Notably, increasing clopidogrel dosages did not reliably overcome the incomplete platelet inhibition seen in IM and PMs.41 For example, in patients with acute CAD, the presence of *2 polymorphism has been shown to increase the risks for MACE and stent thrombosis despite high-dose 600/900 mg clopidogrel loading followed by 150 mg daily.42 In clopidogrel IMs/PMs, prasugrel or ticagrelor (both unaffected by CYP2C19 genotype) significantly reduce MACE with a HR of 0.98 (95% CI, 0.80–1.20) and 0.77 (95% CI, 0.60–0.99), respectively.43,44 The majority of studies using a genotype-targeted switch from clopidogrel to prasugrel or ticagrelor alternatives identified a significant reduction in MACE without a change in bleeding risks. The largest benefit of genotype-guided therapy is seen in PCI, in which IMs/PMs with clopidogrel increased risks of MACE 2-3 times compared to prasugrel or ticagrelor.45 In the genotype-driven de-escalation study, POPULAR-GENETICS, 2,488 ST-elevation myocardial infarction (STEMI) patients were randomized after PCI either to a group receiving standard prasugrel/ticagrelor or a group guided by genotype such that *2 carriers received either prasugrel or ticagrelor while non-carriers received clopidogrel instead. Using the composite ischemic and hemorrhagic outcome, genotyping was not inferior and displayed fewer bleeding events (9.8% vs. 12.5%; HR = 0.78, 95% CI 0.61–0.98, p = 0.04). Furthermore, the PHARMCLO trial screened for three CYP2C19 and ABCB1 gene polymorphisms and included clinical parameters to select the P2Y12 inhibitor after PCI. The composite outcome occurred in 15.9% of patients with genetic testing and 25.9% with standard care (HR = 0.58, 95% CI 0.43–0.78, p < 0.001).46 However, a subpart of TROPICAL-ACS study inferred no added benefits from using genotyping to predict ischemic and bleeding risks among patients who had undergone a de-escalation treatment guided by a PFT.47 Recently, the TAILOR-PCI followed genotype-guided, oral P2Y12 inhibitor selection in ACS and stable CAD patients undergoing PCI, where a CYP2C19 LOF (NF) finding triggered in ticagrelor instead of clopidogrel therapy.48 The authors concluded that there was no significant difference between groups for the composite end point of cardiovascular death, MI, stroke, stent thrombosis, and severe recurrent ischemia at 12 months. However, 15% of CYP2C19 LOF carriers still received clopidogrel; since the study enrollment in 2012 coronary stents and delivery techniques have improved; and genotype-guided therapy had a 34% lower occurrence of major cardiovascular events as well the inclusion of multiple end points significantly favored genotype-based therapy (HR, 0.60 [95% CI, 0.41-0.89]; P = .01). Furthermore, consistent with other studies,49 post hoc analysis showed an about 80% reduction with genotype guidance during the first 3 months (HR, 0.21 [95% CI, 0.08-0.54]; P = .001).
Costs of genotyping
Over 3 million people annually in the United States receive prescriptions for clopidogrel after PCI, and in 2010 clopidogrel sales of US$9.4 billion were the second highest selling medication worldwide.50,51 Cost prediction remains challenging, and cost-effectiveness models suffer from price changes in generic and branded medication and associated test kits. These models must also consider diverse estimates of adverse risk and target populations (i.e., ethnicity, age, principal diagnosis, and comorbidities). For example, cost-effectiveness changes rapidly with the incidence of cerebral ischemia and hemorrhage due to high management cost, significant socioeconomic long-term impact, and a drastic reduction in the patient’s quality of life. While the incidences for various treatment and medication regimens are relatively well-defined in patients with acute coronary syndromes, data from neuroendovascular trials are sparse. Cost-effectiveness studies provide insight into the tradeoffs and consequences of individual management choices and are helpful for clinical guidelines, but they are not well suited for individual decision-making, incorporation into institutional values, or for the complex determination of resource allocation.52
Data on medication cost-effectiveness in neuroendovascular patients are lacking. Kim et al.53 compared the cost-effectiveness of both genotype- and phenotype-guided strategies for selecting P2Y12 inhibitors in patients with ACS. Incremental cost-effectiveness ratios (ICER), or the ratio of extra cost per extra unit of health benefit, were most acceptable for a clopidogrel + phenotype and a ticagrelor + genotype with $12,119 per quality-adjusted life-year (QALY) and $29,412/QALY, respectively; the ICER of universal ticagrelor was cost-prohibitive $1,42,456/QALY. Another decision model in which intermediate metabolizers (those with persistent HTPR despite high-dose clopidogrel at 225 mg daily) were switched to an alternative inhibitor, found that combining both genotype and phenotype strategies was less costly and more effective.54 Limdi et al.55 used data derived from trials, observations, US life tables, Medicare claims, and guidelines to estimate the incidence of MACE events in ACS patients and, compared with universal clopidogrel, both universal ticagrelor and genotype-guided strategy were superior in QALY. However, only the genotype strategy was cost-effective at US$42,365/QALY. Additionally, AlMukdad et al.51 concluded in a summary analysis of 13 published studies of genotype-guided versus universal antiplatelet therapy in PCI patients that genotype-guided escalation was cost-effective. Johnson et al.56 reasoned in 2015 that changing from to an all-genotyping approach in 1,000 ACS patients leads to savings approaching US$5,00,000 per year. Taken together, recent evidence identifies genotyping as a cost-effective strategy to determine DAPT therapy for ACS. Single gene testing, however, is falling out of favor as the costs for single gene vs. multi-gene (average price of US$250, range US$146-386, for a 4-gene panel) are comparable and multi-gene diagnostics will add lifelong value to the patient’s care with predicted cost-savings.57
Bergmeijer et al.58 studied the clinical feasibility of two genotyping devices, TaqMan StepOnePlus and Spartan RX POC. Test results of the Spartan RX POC, processing a single patient at a time, were available within 1 h after collecting a buccal swab allowing quick genotyping. Together with its POC feature, this is appealing for same-day surgery, including interventional or advanced care settings. Despite these benefits, the difficulties presented with capturing POC data in the medical record may make this option less desirable to some institutions due to the lifelong value of germline genetic data. TaqMan genotyping relies on blood sampling and trained laboratory personnel, which increase turnaround times. However, in addition to the basic to CYP2C19*2, *3, and *17 genotyping obtainable from Spartan RX POC, TaqMan types more polymorphisms allowing simultaneous batch-testing, which may present a cost advantage. Cost data on laboratory-based CYP2C19 genotyping vary and are estimated at US$100 (range US$50-250) per test, including reagent and personnel costs but excluding equipment. For example, utilizing the Spartan RX CYP2C19 system costs about $200 per test kit and approximately US$15,000 for the POC device.
Discussion
Antiplatelet therapy is the foundation of medical treatment preventing TEE and hemorrhagic events in neuroendovascular patients. Current antiplatelet treatment strategies rely on evidence-based medicine primarily obtained from cardiovascular RCT. Similar, level 1 and 2 neuroendovascular-specific results are currently unavailable due to sample size limitations, anatomical and clinical heterogeneity, device advancements, among others. The lack of large-scale neuroendovascular data and the unavailability of statistical assurance to select the optimal antiplatelet therapy in specific treatment scenarios encourages the application of precision medicine concepts. Recent and expected developments increasingly allow concurrent integration of pharmacodynamic and genomic data, promptly monitored platelet reactivity, and clinical factors (i.e. employing risk scores) to facilitate individualized risk mitigation.
In the traditional treatment paradigm, an antiplatelet agent is prescribed without the weighted integration of all available data. An ineffective antiplatelet agent is switched only in response to an adverse event. This is the cornerstone of reactive medicine, an approach intrinsically inefficient concerning safety, outcomes, and costs. The process can repeat itself several times over without statistical assurance of its effectiveness. The critical point of precision medicine in antiplatelet strategies is to increase the likelihood of prescribing the individually efficient drug right from the beginning. Employing global risk algorithms for personalized antiplatelet therapy that integrate clinical, biological, and genetic data are increasingly utilized in cardiac patients, but not yet neuroendovascular patients. For example, Angiolillo et al. recently introduced the ABCD-GENE hybrid score incorporating age, BMI, renal impairment, DM, and *2 genotyping. With a c-statistic of 0.66, higher than seen in VerifyNow or LTA testing, good discrimination and calibration in identifying HPR status and predicting adverse ischemic events following PCI was demonstrated. However, an essential step towards individualized neurointerventional decision-making could be done by integrating univariate clinical, biological, and genetic associations, nowadays quickly available at the bedside. Furthermore, genetic point-of-care testing is increasingly available, reducing the clinical uncertainties in P2Y12 therapy both at therapy initiation and hypo- or hyper-responders. The RAPID GENE study, which randomized verified hypometabolizers to prasugrel instead of clopidogrel post PCI, represents one example of successful integration of genetic point-of-care genetic testing, as one week post-procedure the genetic testing group was significantly less likely to have HTPR compared to no genetic testing.59
CYP2C19 genotyping tests are available and results have become as fast as PR levels. However, genotype determination is not a perfect surrogate for the phenotype. In contrast to homozygous NF allele carriage, heterozygotes express a more intermediate and variable response to clopidogrel, although still significantly worse than that observed in wild-type patients.60 At the same time, carriers of *2/*17 and *3/*17 have largely unpredictable reactivities. Hence, ascertainment of the individual gene-dose effect, that is, correlation of geno- and phenotype, remains vital as identified in the ELEVATE-TIMI 56 trial. Here, tripling of clopidogrel maintenance dose in 1-NF allele patients achieved levels of platelet reactivity similar to those seen with the standard 75-mg dosing in non-carriers; however, in 2-NF allele patients with doses as high as 300 mg daily, this did not result in comparable PR.61
It is essential to realize that antiplatelet therapy failure is commonly multifactorial and only targeted testing may provide real-time, objective data. The prevalence of cardiovascular medication non-adherence in high-income countries is estimated at 50%, with rates greater still among marginalized groups.62 Recently, Fanaroff et al. presented a post hoc analysis of the cluster randomized clinical trial ARTEMIS where 8,373 patients with MI were followed up for 1 year at 287 US hospitals with measurement of P2Y12 inhibitor presence. Medication compliance rates were 48% using pharmacy fills compared with 15% patient self-reported, and agreement between P2Y12 inhibitor drug levels and patient-reported or fill-based persistence was low. Patients who were nonpersistent by both pharmacy fills and self-report had the highest 1-year adverse cardiac event rates.63 To that effect, a promising intervention was described by Griessenauer et al.64 demonstrating that a pharmacy-supervised antiplatelet management protocol with closed-loop feedback for patients undergoing PED implantation is safe and efficacious.
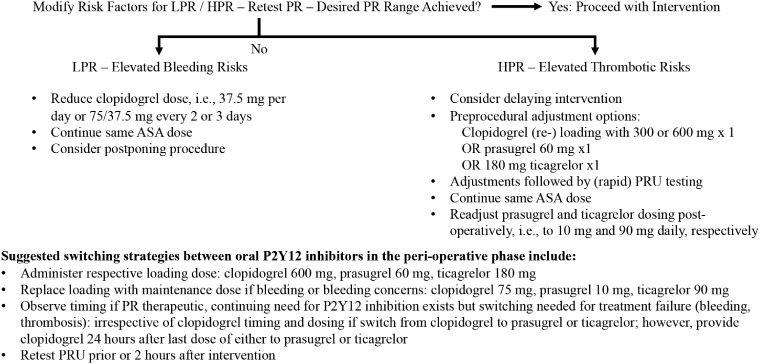
Nevertheless, platelet function results are not the holy grail for thromboembolic and hemorrhagic risk assessments, and results must be weighed and examined within the clinical setting (Figure 2). High PR cutoff values have high negative predicative value for TEE, but their positive predictive value is low,65 the latter caused by very low event rates; clinical event rates are dependent on multiple factors in addition to HPR. HPR is a significant and modifiable, though not stand-alone, risk factor for TEE, but rather should be part of a risk algorithm and other data points. Multiple risk factors and covariates such as diabetes, old age, previous myocardial infarction, renal insufficiency, and smoking status influence platelet physiology. Kirtane et al. analyzing PR data from an observational study of 8,582 acute coronary patients (ADAPT-DES) found that a monotonic increase in PRU was independently associated with cardiac stent thrombosis within the highest PRU quintile (median 317), and a threshold set at >208 PRUs showed a 2.3-fold increase of 2-year stent thrombosis. In contrast, clinically relevant bleeding was independently associated with the lowest PRU quintile (median 57) without an identifiable threshold.66
Figure 2.
Perioperative on-treatment platelet reactivity-based de-escalation and escalation strategies.
Lastly, it is essential to appreciate that CYP genotyping results may affect other treatments beyond antiplatelet therapy. The CYP2C19 enzyme is also involved in the metabolism of selective serotonin reuptake inhibitors, tricyclic antidepressants, proton pump inhibitors, and voriconazole.67 To further enhance overall patient care, predefined protocols to regulate access of obtained genotyping results, i.e. to the clinical pharmacist, are available. Genotyping results should also be shared with the local pharmacy and general practitioner.
Conclusion
In conclusion, both geno- and phenotyping are rapidly and conveniently available as point-of-care testing at a favorable cost-benefit ratio allowing detailed, real-time assessment of expected and actual platelet reactivity in response to antiplatelet agents. When taken together with a risk factors assessment for thrombotic or hemorrhagic adverse events, most reliably in the context of a quantifying clinical risk score and a technical risk assessment for potential adverse events, a more precise and individualized approach to modern-day P2Y12 inhibitor therapy can be achieved.
Footnotes
Author’s Note: Philipp Hendrix is also affiliated with Department of Neurosurgery, Saarland University Medical Center and Saarland University, Faculty of Medicine, Homburg/Saar, Germany.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Malie K Collins https://orcid.org/0000-0002-9553-3824
Ryley Uber https://orcid.org/0000-0003-0448-7584
Abhi Jain https://orcid.org/0000-0001-7398-1039
Christoph J Griessenauer https://orcid.org/0000-0002-2952-3812
References
- 1.Rollini F, Franchi F, Angiolillo DJ.Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat Rev Cardiol 2016; 13: 11–27. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Pollack CV, Weitz JI, et al. Antibody-Based Ticagrelor Reversal Agent in Healthy Volunteers. N Engl J Med 2019; 380: 1825–1833. [DOI] [PubMed] [Google Scholar]
- 3.White House Precision Medicine Initiative. The White House, https://obamawhitehouse.archives.gov/node/333101 (accessed 12 September 2020).
- 4.Daou B, Starke RM, Chalouhi N, et al. P2Y12 Reaction Units: Effect on Hemorrhagic and Thromboembolic Complications in Patients With Cerebral Aneurysms Treated With the Pipeline Embolization Device. Neurosurgery 2016; 78: 27–33. [DOI] [PubMed] [Google Scholar]
- 5.Kan P, Siddiqui AH, Veznedaroglu E, et al. Early Postmarket Results After Treatment of Intracranial Aneurysms With the Pipeline Embolization DeviceA US Multicenter Experience. Neurosurgery 2012; 71: 1080–1088. [DOI] [PubMed] [Google Scholar]
- 6.Lin N, Lanzino G, Lopes DK, et al. Treatment of Distal Anterior Circulation Aneurysms With the Pipeline Embolization Device: A US Multicenter Experience. Neurosurgery 2016; 79: 14–22. [DOI] [PubMed] [Google Scholar]
- 7.Saber H, Kherallah RY, Hadied MO, et al. Antiplatelet therapy and the risk of ischemic and hemorrhagic complications associated with Pipeline embolization of cerebral aneurysms: a systematic review and pooled analysis. J NeuroInterventional Surg 2019; 11: 362–366. [DOI] [PubMed] [Google Scholar]
- 8.Iosif C, Lecomte J-C, Pedrolo-Silveira E, et al. Evaluation of ischemic lesion prevalence after endovascular treatment of intracranial aneurysms, as documented by 3-T diffusion-weighted imaging: a 2-year, single-center cohort study. J Neurosurg 2018; 128: 982–991. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Li Y, Jiang Y.Insufficient platelet inhibition and thromboembolic complications in patients with intracranial aneurysms after stent placement. J Neurosurg 2016; 125: 247–253. [DOI] [PubMed] [Google Scholar]
- 10.Bond KM, Brinjikji W, Murad MH, et al. Diffusion-Weighted Imaging–Detected Ischemic Lesions following Endovascular Treatment of Cerebral Aneurysms: A Systematic Review and Meta-Analysis. Am J Neuroradiol 2017; 38: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner K, Srivatsan A, Mohanty A, et al. Cognitive outcomes after unruptured intracranial aneurysm treatment with flow diversion. J Neurosurg 2019; 1: 1–6. [DOI] [PubMed] [Google Scholar]
- 12.Lordkipanidzé M, Pharand C, Schampaert E, et al. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J 2007; 28: 1702–1708. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira M, Freitas-Silva M, Assis J, et al. The emergent phenomenon of aspirin resistance: insights from genetic association studies. Pharmacogenomics 2020; 21: 125–140. [DOI] [PubMed] [Google Scholar]
- 14.Dannenberg L, Metzen D, Zako S, et al. Enhanced Platelet Reactivity under Aspirin Medication and Major Adverse Cardiac and Cerebrovascular Events in Patients with Coronary Artery Disease. Pharmacology 2020; 105: 118–122. [DOI] [PubMed] [Google Scholar]
- 15.Skukalek SL, Winkler AM, Kang J, et al. Effect of antiplatelet therapy and platelet function testing on hemorrhagic and thrombotic complications in patients with cerebral aneurysms treated with the pipeline embolization device: a review and meta-analysis. J Neurointerventional Surg 2016; 8: 58–65. [DOI] [PubMed] [Google Scholar]
- 16.Snoep JD, Hovens MMC, Eikenboom JCJ, et al. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysis. Arch Intern Med 2007; 167: 1593–1599. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Liu J, Zhou Y, et al. Association among PlA1/A2 gene polymorphism, laboratory aspirin resistance and clinical outcomes in patients with coronary artery disease: An updated meta-analysis. Sci Rep; 9. Epub ahead of print 11 September 2019. DOI: 10.1038/s41598-019-49123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai G, Zhou W, Lu Y, et al. Aspirin resistance and other aspirin-related concerns. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 2016; 37: 181–189. [DOI] [PubMed] [Google Scholar]
- 19.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med Off J Am Coll Med Genet 2017; 19: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine GN, Jeong Y-H, Goto S, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol 2014; 11: 597–606. [DOI] [PubMed] [Google Scholar]
- 21.Fricke-Galindo I, Céspedes-Garro C, Rodrigues-Soares F, et al. Interethnic variation of CYP2C19 alleles, ‘predicted’ phenotypes and ‘measured’ metabolic phenotypes across world populations. Pharmacogenomics J 2016; 16: 113–123. [DOI] [PubMed] [Google Scholar]
- 22.Sibbing D, Koch W, Gebhard D, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation 2010; 121: 512–518. [DOI] [PubMed] [Google Scholar]
- 23.Moon JY, Franchi F, Rollini F, et al. Role of genetic testing in patients undergoing percutaneous coronary intervention. Expert Rev Clin Pharmacol 2018; 11: 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang H-S, Kwon BJ, Kim JE, et al. Preinterventional clopidogrel response variability for coil embolization of intracranial aneurysms: clinical implications. AJNR Am J Neuroradiol 2010; 31: 1206–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishi H, Nakahara I, Matsumoto S, et al. Platelet reactivity and hemorrhage risk in neurointerventional procedures under dual antiplatelet therapy. J Neurointerventional Surg 2016; 8: 949–953. [DOI] [PubMed] [Google Scholar]
- 26.Tan LA, Keigher KM, Munich SA, et al. Thromboembolic complications with Pipeline Embolization Device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerventional Surg 2015; 7: 217–221. [DOI] [PubMed] [Google Scholar]
- 27.Almandoz JED, Crandall BM, Scholz JM, et al. Pre-procedure P2Y12 reaction units value predicts perioperative thromboembolic and hemorrhagic complications in patients with cerebral aneurysms treated with the Pipeline Embolization Device. J NeuroInterventional Surg 2013; 5: iii3–iii10. [DOI] [PubMed] [Google Scholar]
- 28.Hwang G, Huh W, Lee JS, et al. Standard vs Modified Antiplatelet Preparation for Preventing Thromboembolic Events in Patients With High On-Treatment Platelet Reactivity Undergoing Coil Embolization for an Unruptured Intracranial Aneurysm: A Randomized Clinical Trial. JAMA Neurol 2015; 72: 764–772. [DOI] [PubMed] [Google Scholar]
- 29.Adeeb N, Griessenauer CJ, Foreman PM, et al. Use of Platelet Function Testing Before Pipeline Embolization Device Placement: A Multicenter Cohort Study. Stroke 2017; 48: 1322–1330. [DOI] [PubMed] [Google Scholar]
- 30.Podlasek A, Al Sultan AA, Assis Z, et al. Outcome of intracranial flow diversion according to the antiplatelet regimen used: a systematic review and meta-analysis. J Neurointerventional Surg 2020; 12: 148–155. [DOI] [PubMed] [Google Scholar]
- 31.Moore JM, Adeeb N, Shallwani H, et al. A Multicenter Cohort Comparison Study of the Safety, Efficacy, and Cost of Ticagrelor Compared to Clopidogrel in Aneurysm Flow Diverter Procedures. Neurosurgery 2017; 81: 665–671. [DOI] [PubMed] [Google Scholar]
- 32.Gross L, Aradi D, Sibbing D.Platelet Function Testing in Patients on Antiplatelet Medications. Semin Thromb Hemost. Epub ahead of print 2016. DOI: 10.1055/s-0035-1570083. [DOI] [PubMed] [Google Scholar]
- 33.Pettersen A-ÅR, Seljeflot I, Abdelnoor M, et al. High On-Aspirin Platelet Reactivity and Clinical Outcome in Patients With Stable Coronary Artery Disease: Results From ASCET (Aspirin Nonresponsiveness and Clopidogrel Endpoint Trial). J Am Heart Assoc Cardiovasc Cerebrovasc Dis; 1. Epub ahead of print 22 June 2012. DOI: 10.1161/JAHA.112.000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rade JJ.Platelet function testing in patients with coronary artery disease: is the Who and the When any clearer than the What and the What then? Circulation 2012; 125: 3073–3075. [DOI] [PubMed] [Google Scholar]
- 35.Reny JL, Fontana P, Hochholzer W, et al. Vascular risk levels affect the predictive value of platelet reactivity for the occurrence of MACE in patients on clopidogrel: Systematic review and meta-analysis of individual patient data. Thromb Haemost 2016; 115: 844–855. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Wang Y, Wu Y, et al. Individualized dual antiplatelet therapy based on platelet function testing in patients undergoing percutaneous coronary intervention: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2017; 17: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge H, Lv X, Ren H, et al. Influence of CYP2C19 genetic polymorphisms on clinical outcomes of intracranial aneurysms treated with stent-assisted coiling. J Neurointerventional Surg 2017; 9: 958–962. [DOI] [PubMed] [Google Scholar]
- 38.Lin M, Todaro M, Chan J, et al. Association between CYP2C19 Polymorphisms and Outcomes in Cerebral Endovascular Therapy. AJNR Am J Neuroradiol 2016; 37: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorich Michael J., Rowland Andrew, McKinnon Ross A., et al. CYP2C19 Genotype Has a Greater Effect on Adverse Cardiovascular Outcomes Following Percutaneous Coronary Intervention and in Asian Populations Treated With Clopidogrel. Circ Cardiovasc Genet 2014; 7: 895–902. [DOI] [PubMed] [Google Scholar]
- 40.Mega JL, Simon T, Collet J-P, et al. Reduced-Function CYP2C19 Genotype and Risk of Adverse Clinical Outcomes Among Patients Treated With Clopidogrel Predominantly for PCI: A Meta-Analysis. JAMA J Am Med Assoc 2010; 304: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi JS, Cammarata M, Dharmavaram J, et al. Clopidogrel dose adjustment after outpatient screening for CYP2C19 variant alleles: a pilot study. Pharmacogenomics 2014; 15: 915–923. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Yang J, Zhu X, et al. Effect of high-dose clopidogrel according to CYP2C19*2 genotype in patients undergoing percutaneous coronary intervention- a systematic review and meta-analysis. Thromb Res 2015; 135: 449–458. [DOI] [PubMed] [Google Scholar]
- 43.Sorich MJ, Vitry A, Ward MB, et al. Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. J Thromb Haemost JTH 2010; 8: 1678–1684. [DOI] [PubMed] [Google Scholar]
- 44.Wallentin L, James S, Storey RF, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet Lond Engl 2010; 376: 1320–1328. [DOI] [PubMed] [Google Scholar]
- 45.Cavallari LH, Lee CR, Beitelshees AL, et al. Multisite Investigation of Outcomes With Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention. JACC Cardiovasc Interv 2018; 11: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notarangelo FM, Maglietta G, Bevilacqua P, et al. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients With Acute Coronary Syndromes: The PHARMCLO Trial. J Am Coll Cardiol 2018; 71: 1869–1877. [DOI] [PubMed] [Google Scholar]
- 47.Gross L, Trenk D, Jacobshagen C, et al. Genotype-Phenotype Association and Impact on Outcomes following Guided De-Escalation of Anti-Platelet Treatment in Acute Coronary Syndrome Patients: The TROPICAL-ACS Genotyping Substudy. Thromb Haemost 2018; 118: 1656–1667. [DOI] [PubMed] [Google Scholar]
- 48.Pereira NL, Farkouh ME, So D, et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes After Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020; 324: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou J-J, Xie H-G, Chen S-L, et al. Influence of CYP2C19 loss-of-function variants on the antiplatelet effects and cardiovascular events in clopidogrel-treated Chinese patients undergoing percutaneous coronary intervention. Eur J Clin Pharmacol 2013; 69: 771–777. [DOI] [PubMed] [Google Scholar]
- 50.Roden DM.Clopidogrel Pharmacogenetics — Why the Wait? N Engl J Med 2019; 381: 1677–1678. [DOI] [PubMed] [Google Scholar]
- 51.AlMukdad S, Elewa H, Al-Badriyeh D.Economic Evaluations of CYP2C19 Genotype-Guided Antiplatelet Therapy Compared to the Universal Use of Antiplatelets in Patients With Acute Coronary Syndrome: A Systematic Review. J Cardiovasc Pharmacol Ther 2020; 25: 201–211. [DOI] [PubMed] [Google Scholar]
- 52.Cohen DJ, Reynolds MR.Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol 2008; 52: 2119–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim K, Touchette DR, Cavallari LH, et al. Cost-Effectiveness of Strategies to Personalize the Selection of P2Y12 Inhibitors in Patients with Acute Coronary Syndrome. Cardiovasc Drugs Ther 2019; 33: 533–546. [DOI] [PubMed] [Google Scholar]
- 54.M J, Jh Y. CYP2C19 genotype plus platelet reactivity-guided antiplatelet therapy in acute coronary syndrome patients: a decision analysis. Pharmacogenet Genomics 2015; 25: 609–617. [DOI] [PubMed] [Google Scholar]
- 55.Limdi NA, Cavallari LH, Lee CR, et al. Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data. Pharmacogenomics J. Epub ahead of print 11 February 2020. DOI: 10.1038/s41397-020-0162-5. [DOI] [PMC free article] [PubMed]
- 56.Johnson SG, Gruntowicz D, Chua T, et al. Financial Analysis of CYP2C19 Genotyping in Patients Receiving Dual Antiplatelet Therapy Following Acute Coronary Syndrome and Percutaneous Coronary Intervention. J Manag Care Spec Pharm 2015; 21: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong OM, Wheeler SB, Cruden G, et al. Cost-Effectiveness of Multigene Pharmacogenetic Testing in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention. Value Health J Int Soc Pharmacoeconomics Outcomes Res 2020; 23: 61–73. [DOI] [PubMed] [Google Scholar]
- 58.Bergmeijer TO, Vos GJ, Claassens DM, et al. Feasibility and implementation of CYP2C19 genotyping in patients using antiplatelet therapy. Pharmacogenomics 2018; 19: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet Lond Engl 2012; 379: 1705–1711. [DOI] [PubMed] [Google Scholar]
- 60.Gurbel PA, Shuldiner AR, Bliden KP, et al. The relation between CYP2C19 genotype and phenotype in stented patients on maintenance dual antiplatelet therapy. Am Heart J 2011; 161: 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hochholzer W, Ruff CT, Mesa RA, et al. Variability of individual platelet reactivity over time in patients treated with clopidogrel: insights from the ELEVATE-TIMI 56 trial. J Am Coll Cardiol 2014; 64: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laba T-L, Bleasel J, Brien J, et al. Strategies to improve adherence to medications for cardiovascular diseases in socioeconomically disadvantaged populations: A systematic review. Int J Cardiol 2013; 167: 2430–2440. [DOI] [PubMed] [Google Scholar]
- 63.Fanaroff AC, Peterson ED, Kaltenbach LA, et al. Agreement and Accuracy of Medication Persistence Identified by Patient Self-report vs Pharmacy Fill: A Secondary Analysis of the Cluster Randomized ARTEMIS Trial. JAMA Cardiol 2020; 5: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Griessenauer CJ, Jain A, Enriquez-Marulanda A, et al. Pharmacy-Mediated Antiplatelet Management Protocol Compared to One-time Platelet Function Testing Prior to Pipeline Embolization of Cerebral Aneurysms: A Propensity Score-Matched Cohort Study. Neurosurgery 2019; 84: 673–679. [DOI] [PubMed] [Google Scholar]
- 65.Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56: 919–933. [DOI] [PubMed] [Google Scholar]
- 66.Kirtane AJ, Parikh PB, Stuckey TD, et al. Is There an Ideal Level of Platelet P2Y12-Receptor Inhibition in Patients Undergoing Percutaneous Coronary Intervention?: ‘Window’ Analysis From the ADAPT-DES Study (Assessment of Dual AntiPlatelet Therapy With Drug-Eluting Stents). JACC Cardiovasc Interv 2015; 8: 1978–1987. [DOI] [PubMed] [Google Scholar]
- 67.Research C for DE and. Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. FDA, https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers (2020, accessed 13 September 2020).
- 68.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Becske T, Kallmes DF, Saatci I, et al. Pipeline for Uncoilable or Failed Aneurysms: Results from a Multicenter Clinical Trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 70.Hanel RA, Kallmes DF, Lopes DK, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J NeuroInterventional Surg 2020; 12: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pierot L, Spelle L, Berge J, et al. SAFE study (Safety and efficacy Analysis of FRED Embolic device in aneurysm treatment): 1-year clinical and anatomical results. J NeuroInterventional Surg 2019; 11: 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meyers Philip M., Coon Alexander L., Kan Peter T., et al. SCENT Trial. Stroke 2019; 50: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y, Yang P-F, Fang Y-B, et al. Parent artery reconstruction for large or giant cerebral aneurysms using a Tubridge flow diverter (PARAT): study protocol for a multicenter, randomized, controlled clinical trial. BMC Neurol 2014; 14: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atallah E, Saad H, Bekelis K, et al. The use of alternatives to clopidogrel in flow-diversion treatment with the Pipeline embolization device. J Neurosurg 2018; 129: 1130–1135. [DOI] [PubMed] [Google Scholar]
- 75.Griessenauer CJ, Ogilvy CS, Foreman PM, et al. Pipeline Embolization Device for Small Intracranial Aneurysms: Evaluation of Safety and Efficacy in a Multicenter Cohort. Neurosurgery 2017; 80: 579–587. [DOI] [PubMed] [Google Scholar]
- 76.Moore JM, Adeeb N, Shallwani H, et al. A Multicenter Cohort Comparison Study of the Safety, Efficacy, and Cost of Ticagrelor Compared to Clopidogrel in Aneurysm Flow Diverter Procedures. Neurosurgery 2017; 81: 665–671. [DOI] [PubMed] [Google Scholar]
- 77.Soize S, Foussier C, Manceau P-F, et al. Comparison of two preventive dual antiplatelet regimens for unruptured intracranial aneurysm embolization with flow diverter/disrupter: A matched-cohort study comparing clopidogrel with ticagrelor. J Neuroradiol 2019; 46: 378–383. [DOI] [PubMed] [Google Scholar]