Abstract
Background
The objective of the present study is to analyze the outcomes of patients with subarachnoid hemorrhage (SAH) in the acute phase after treatment with Y-stent-assisted coiling (YSAC) embolization.
Methods
This retrospective study assessed of 30 patients with acutely ruptured wide-neck aneurysms following YSAC treatment between April 2013 and October 2019. The demographic data, aneurysm occlusion grade, procedural and periprocedural complications, and clinical outcomes were assessed.
Results
The procedure was completed in 30 cases (90.1%) and technical failure occurred in 3 cases (9.1%). Immediate control angiography revealed that total occlusion Raymond-Ray Class 1 (RR1) was achieved in 21 (70%), neck filling (RR2) in eight (26.6%) and sac filling (RR1) in one (3.3%) aneurysm. Upon angiographic follow-up, RR1 occlusion was observed in 15 (71.4%) patients, RR2 in three (14.3%) patients and RR3 in three (14.3%) patients. In-stent thrombus developed in five (16.6%) patients; procedural ischemic events were observed in four (13.3%) patients; and two (6.6%) patients were symptomatic. A periprocedural asymptomatic intracranial hemorrhage was detected in two patients. At discharge, 17 (56.6%) patients were in good clinical condition, six (20%) were in a severe disability condition, and seven (23.3%) patients had died. At the final follow-up visit (mean: 18.9 months), 16 (76,2%) of 21 patients were in a good clinical condition and five (23.8%) had severe disabilities.
Conclusions
Y-stent assisted coiling in might be a feasible and promising option for treatment in acute phase in selected wide-necked ruptured intracranial aneurysms.
Keywords: Aneurysm, ruptured, stents
Introduction
There have been considerable developments in the endovascular treatment approach to intracranial aneurysms. The technical success of the simple coiling of wide-neck aneurysms varies based on aneurysmal morphology,1 and a growing number of patients can undergo endovascular treatment using balloon-assisted, multiple catheter techniques and neck bridge devices.2–6
The endovascular treatment of wide-neck aneurysms with unfavorable geometric configurations remains challenging. When the patency of the parent artery and efferent branches of the aneurysm cannot be maintained during coiling, especially in patients with complex bifurcation aneurysms, the Y-stent-assisted coiling (YSAC) technique offers an alternative treatment approach.7 However, anticoagulation and antiplatelet therapies are necessary to avoid procedure-related thromboembolic complications during coiling using dual self-expandable stents in ruptured aneurysms. Currently, neurointerventional radiologists and neurosurgeons are reluctant to use antiplatelet therapy in ruptured aneurysms, particularly when YSAC is applied, because of the potential for periprocedural hemorrhagic complications and the likelihood of additional surgical procedures (e.g., extraventricular drainage or decompressive craniectomy).4,8
Here, we report the immediate treatment outcomes, as well as midterm follow-up angiographic and clinical data, for 30 ruptured wide-neck bifurcation aneurysms that were treated with the YSAC technique in the acute period.
Methods
Patient selection
This retrospective study assessed the clinical and angiographic results of 30 patients with acutely ruptured wide-neck aneurysms who underwent YSAC treatment between April 2013 and October 2019. Our institutional review board approved the study design (approval no. 2020/1270) and all included patients provided written informed consent to participate. All patients were administered endovascular treatment within the first 72 h of a subarachnoid hemorrhage (SAH), and each patient was evaluated individually by neurointerventional radiologists and neurosurgeons.
The YSAC was performed on wide-neck complex bifurcation aneurysms. Wide-neck aneurysms were defined as aneurysms with a dome-to-neck ratio of <2 or a neck size >4 mm. Complex bifurcation aneurysms were defined as wide-neck bifurcation aneurysms that incorporated at least one side branch of the bifurcation. The patients’ demographic data, aneurysm occlusion grade, procedural and periprocedural complications, and clinical outcomes were retrospectively assessed by two independent reviewers.
Endovascular procedure
YSAC technique
The procedure was performed while each patient was under general anesthesia, and was guided by biplane or monoplane flat panel angiography systems using a unilateral femoral approach. The procedures involved a 90-cm 6-Fr or 7-Fr long introducer sheath, combined with a 6-Fr guiding catheter. These were placed in the petrous segment of the internal carotid artery for anterior circulation aneurysms, and in the distal vertebral artery for posterior circulation aneurysms. In the present study, closed-cell Enterprise stents (Codman Neurovascular, Raynham, MA, USA), low-profile braided LEO Baby stents (Balt Extrusion, Montmorency, France), and open-cell low-profile Neuroform Atlas stents (Stryker, Los Angeles, CA, USA) were used. A transport microcatheter with an internal diameter of 0.021 inches (Prowler Select Plus; Codman, Miami Lakes, FL, USA) was used for Enterprise stents and a microcatheter with an internal diameter of 0.0165 inches (Excelsior SL 10; Stryker) was used for Neuroform Atlas and LEO Baby stents.
The first vessel to stent was selected on the basis of two requirements. The first requirement was to stent the sharply angled branch diverging from the proximal artery or the aneurysm before stenting the wide angled branch. The second requirement was to stent the segment with the maximum coverage of the aneurysm neck. A microcatheter was inserted into the aneurysm sac before placement of the first stent, and the first stent was placed inside the vessel diverging from the parent vessel to partially cover the aneurysm neck (Supplementary Video). An appropriate size of coiling was attempted after the coil microcatheter had been jailed in some patients. However, this procedure was abandoned in patients with coil protrusion into the parent artery, and the stent was placed in the other branch by navigating the microcatheter through the first stent struts and then by coiling.
In this study, the selection of stents for YSAC was generally based on the aneurysmal morphology, parent artery anatomy, and treatment goal. Stent physical properties such as pore density, trackability, radial force, and foreshortening were important this decision-making process. Operator experience was also an important factor in stent selection. In patients with a more acute and sharp aneurysm angle, and where the aneurysm vessel branching was smaller in diameter, low-profile stents (i.e., Neuroform Atlas or LEO Baby stents) were used because they could be delivered via low-profile microcatheters. In some of these patients, the braided LEO Baby stent was avoided because of potential stent shortening-related problems. Enterprise stents were used when easy vessel catheterization was expected because of larger size and aneurysm branching. Immediately after the procedure, a complete neurological examination of all patients was carried out by a neurosurgeon, and non-contrast computed tomography scanning was performed to identify potential hemorrhagic complications.
Anticoagulation/antiplatelet regimen
After insertion of the femoral sheath, a bolus of 50–70 U/kg heparin was administered. The activated clotting time was maintained at 2–3-fold above baseline. After the first stent had been successfully placed, tirofiban was intravenously administered at a bolus dose of 8.0 µg/kg within the first few minutes, then with a maintenance dose of 0.10 µg/min/kg. Following placement of the second stent, the first coil packing was performed and loading doses of 300 mg clopidogrel and 300 mg aspirin were administered through a nasogastric tube. The duration of tirofiban maintenance was 4–6 h after the bolus dose. Clopidogrel was discontinued 6 months after the procedure, but aspirin was prescribed indefinitely. In the present study, responsiveness to antiplatelet therapy was not tested due to the emergent nature of the aneurysm. In the event of an intraprocedural stent thrombosis, the aneurysm was coiled immediately without dense packing if it was not already coiled, followed immediately by the intra-arterial administration of 0.5–1.5 mg tirofiban (12.5 mg/50 mL) near the thrombosis through a microcatheter. The tirofiban bolus was administered using 2-mL (0.5-mg) syringes within a few minutes. The tirofiban administration was repeated with follow-up angiograms taken at intervals of 5–10 min. The procedure duration was no more than 30 min. Clopidogrel was discontinued, but aspirin therapy was maintained to reduce the risk of postoperative bleeding in patients with extraventricular drains. The dual antiplatelet regimen was restarted 3 days after extraventricular drain insertion. Because the risk of aneurysm re-bleeding was greater in patients with preoperative extraventricular drain insertion, endovascular treatment was performed immediately after the extraventricular drain procedure.
Data collection and analysis
Patient data collection included age, sex, technical success and severity of SAH (both Fisher grading and Hunt–Hess grading), timing of treatment, external ventricular drainage, aneurysm localizations and sizes (small, 2–7 mm; medium, 7–12 mm; large, 12–24 mm; and giant, >24 mm), and dome-to-neck ratio. The initial occlusions were graded in accordance with the Raymond–Roy occlusion classification (grade 1, complete; grade 2, residual neck; and grade 3, residual aneurysm). The first follow-up visit of the patients was performed at 6–12 months postoperatively, involving magnetic resonance angiography or digital subtraction angiography examinations. Retreatment was advised for patients with aneurysms that exhibited recanalization at follow-up.
Treatment-related complications were classified into intraprocedural complications, periprocedural complications (within 30 days), and delayed complications (after 30 days). All ischemic and hemorrhagic events were recorded. Clinical outcomes were evaluated using the Glasgow Outcome Scale at discharge and at the final follow-up. A Glasgow Outcome Scale score of 4–5 was regarded as good and a score of 0–3 was regarded as poor.
Results
Baseline characteristics
In total, 30 patients (19 women and 11 men) with wide-neck ruptured aneurysms underwent YSAC. The mean patient age was 55.8 years (range, 39–70 years) and the mean aneurysm size was 8.8 mm (range, 4–28 mm). The types of aneurysms were 13 small, 11 medium, five large, and one giant. The dome-to-neck ratio of all aneurysms was 1.3 (range, 0.95–1.77). The aneurysm was located at the bifurcation of the middle cerebral artery in 18 patients (60%), at the anterior communicating artery in six patients (20%), at the top of the basilar artery in five patients (16.6%), and at the bifurcation of internal carotid artery in one patient (3.3%). At the time of admission, five patients (16.6%) had SAH severity of Fisher grades 1–2, 13 patients (43.3%) had SAH severity of Fisher grade 3, and 12 patients (40%) had SAH severity of Fisher grade 4. Hunt–Hess assessment showed that 14 patients (46.6%) had SAH grades 1–2 and 16 patients (53.3%) had SAH grades 3–5. Following SAH, endovascular treatment was administered to 22 patients (73.3%) within the first 48 h and to eight patients (26.6%) within 48–72 h. An extraventricular drain was placed in four patients: preoperatively in two and postoperatively in two (Table 1).
Table 1.
Baseline characteristics.
| Age (years)(mean/range) | 55.8 (39–70) |
| Sex (men/women) (n, %) | 11/19 (36.6/64.4) |
| Admission Fisher grade | |
| (n, %) | |
| Grade 1–2 | 5 (16.6) |
| Grade 3 | 13 (43,3) |
| Grade 4 | 12 (40) |
| Admission Hunt-Hess Grade | |
| (n, %) | |
| Grades 1–2 | 14 (46.6) |
| Grades 3–5 | 16 (53.3) |
| Aneurysms location | |
| (n, %) | |
| Anterior communication artery | 6 (20) |
| Middle cerebral artery | 18 (60) |
| Basillar top | 5 (16.6) |
| Internal carotid artery bifurcation | 1 (3.3) |
| Aneurysms size | |
| Dome size mm (mean/range) | 8.8 (4.1–28) |
| Dome to neck ratio mm (mean/range) | 1.3 (0.95–1.77) |
| Treatment time | |
| 0–48 h (n, %) | 22 (73.3) |
| 48–72 h (n, %) | 8 (26.6) |
| External ventriculer dranaige | |
| Preoperative (n) | 2 |
| Postoperative (n) | 2 |
Immediate angiographic results
Among the 30 patients who underwent YSAC, no branch catheterization could be performed in three wide-neck and sharp-angle branching aneurysms, resulting in technical failure. In nine patients (30%), initial coiling was attempted after placement of a single stent. However, a second “salvage” stent was placed following determination that the patency of the other branch from the aneurysm could not be maintained. In 21 patients (70%), dual stents were placed based on previous plans. In the 30 patients, dual Enterprise stents, dual Neuroform Atlas stents, and dual LEO Baby stents were used in six (20%), 18 (60%), and six (20%) aneurysms, respectively. Among patients with SAH, four unruptured aneurysms were identified incidentally, in addition to the ruptured aneurysms. Two of the four unruptured aneurysms (i.e., paraophthalmic aneurysms) were treated with stent-assisted coil embolization and one (associated with the anterior choroidal artery) was treated with simple coil embolization. Digital subtraction angiography revealed that total aneurysm occlusion (RR1) had been achieved in 21 patients (70%), neck filling (RR2) in eight patients (26.6%), and aneurysm and sac filling in one patient (3.3%) (Table 2).
Table 2.
Angiographic occlusion rates.
| Initial angiographic rates n:30 | |
| RR1 | n:21 (70%) |
| RR2 | n:8 (26.6) |
| RR3 | n:1 (3.3%) |
| Last follows-up occlusion rates n:21 (MRA or angiography) | |
| RR1 | n:15 (71.4%) |
| RR2 | n:3 (14.3%) |
| RR3 | n:3 (14.3%) |
| Mean follow up (18.9 months) | |
‘RR’ Raymond-Roy occlusion classification.
‘MRA’Magnetic resonance angiograph.
Complications
Of the 30 patients, five (16.6%) developed intraprocedural stent thrombosis. Four of these five had unilateral stent thrombosis, but the remaining patient developed stent thrombosis in two branches from the aneurysm. Only one patient had partial occlusion, and the other four patients had total occlusion. In all five patients, thrombosis developed in the branch where the first stent was placed, which had an acute angle and a vessel diameter smaller than that of the other branch. For all thromboses, intra-arterial injections of tirofiban were administered near the thrombosis through a microcatheter. After each 0.5-mg (2-mL) infusion, arterial flow was evaluated at intervals of 5–10 min. A maximum tirofiban infusion dose of 1.5 mg (6 mL) was used, and a complete lysis of the in-stent thrombosis was achieved in four patients. Thrombolysis in cerebral infarction grade 2b patency was achieved in one patient. However, two patients developed periprocedural symptomatic infarcts (Figure 1), and two patients had clinically silent infarcts. No patients experienced intraprocedural aneurysm ruptures.
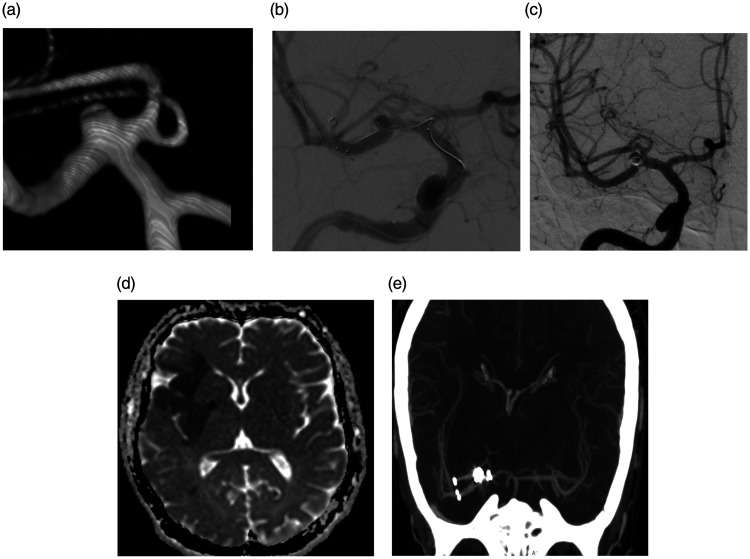
Figure 1.
(a–e) 3D image (a) shows a wide-neck small aneurysm at the MCA bifurcation. Digital subtraction angiography (b and c) shows stent trombosis during YSAC procedure and patency provided after intra-arterial tirofiban administration. Follow-up postoperatif diffusion-weighted image (d) shows an acute infarct in the MCA superior division territory and this patient was discharged with GOS 3. 1 year later this patient was in good clinical condition and CT angiography shows (e) patency of both stents.
Because of periprocedural regressions in their clinical statuses, three patients underwent follow-up digital subtraction angiography examinations, which revealed diffuse vasospasms in the distal segments of the middle and anterior cerebral arteries. Thus, intra-arterial phosphodiesterase inhibitor (milrinone) was administered at a total dose of 5–10 mg per session in two sessions (two patients) or in one session (one patient).9 Sufficient arterial calibration was achieved with intra-arterial milrinone, and the patients were followed up with medical vasospasm therapy. Vasospasm continued in two patients, and an infarct developed in the middle cerebral artery territory.
In total, four patients developed hemorrhagic complications, two of which were intracranial hemorrhages. In one patient with an intracranial hemorrhage, an additional total dose of 1.5 mg tirofiban was used for stent thrombosis at the middle cerebral artery inferior trunk. No additional dose of tirofiban was administered to the other patient. There were no symptomatic findings secondary to the hematomas, which were resorbed during follow-up. Upon observations of periprocedural reductions in hemoglobin levels in two patients during follow-up, a hemorrhage was detected due to an abdominal wall hematoma (16 × 20 cm) in one patient, and a pseudoaneurysm was detected at the entry site in the groin in another patient. The patient with the abdominal wall hematoma was instructed to discontinue clopidogrel and undergo close monitoring of their hemoglobin levels. Clopidogrel was reinitiated after 1 week in that patient, who exhibited laboratory and clinical stability during subsequent follow-up. The patient bleeding from the entry site in the groin underwent emergent placement of a covered stent, and then continued dual antiplatelet therapy. Notably, no delayed ischemic or hemorrhagic complications were observed in any patients (Table 3).
Table 3.
Complications.
| Intraprosedurel | |
| Stent thrombus | n:5 (16.6%) |
| Aneurysm rupture | – |
| Periprosedurel | |
| Prosedure related ischemic event | n:4 (13.3%) |
| Asymptomatic | n:2 (6.6%) |
| Symptomatic | n:2 (6.6%) |
| Intracranial hemorrhage | n:2 (6.6%) |
| Asymptomatic | n:2 (6.6%) |
| Symptomatic | – |
| Other events | n:2 (6.6%) |
| Angiographically demonstrated vasospasm | n:3 (10%) |
| Abdominal wall hematoma | n:1 (3.3%) |
| Puncture site hematoma | n:1 (3.3%) |
| Delayed | – |
Clinical and radiological outcomes
At discharge, 17 patients (56.6%) were in good clinical condition (Glasgow Outcome Scale scores of 4 and 5), six patients (20%) exhibited severe disability (Glasgow Outcome Scale score of 3), and seven patients (23.3%) had died. Among the patients who died, one died of a treatment-related thromboembolic complication (Hunt–Hess grade 3 at admission) and six patients died of SAH-related complications. Of the remaining 23 patients, two who exhibited good clinical conditions at admission were lost to follow-up. Therefore, outcomes were assessed in 21 patients. The mean duration of clinical follow-up was 17.8 months (range, 6–48 months). At the final follow-up, one of the six patients with severe disability had improved to a good clinical condition (Figure 1), and the remaining five showed no clinical changes. There were no changes in the clinical conditions of the other patients (Table 4).
Table 4.
Clinical outcomes.
| At discharge (n:30) | |
| GOS 4-5 | n:17(56.6%) |
| GOS 3 | n:6 (20%) |
| Death | n:7 (23.3) |
| At last follows up visit (n:21) (mean 18.9 months) | |
| GOS 4-5 | n:16 (76.2%) |
| GOS 3 | n:5 (23.8%) |
| GOS 0-2 | – |
A mean angiographic follow-up duration of 18.9 months (range, 12–48 months) was achieved for 21 patients. During angiographic follow-up, an RR1 occlusion was observed in 15 patients (71.4%), an RR2 occlusion was observed in three patients (14.3%), and an RR3 occlusion was observed in three patients (14.3%). The three patients with RR3 (two with basilar top aneurysm and one with middle cerebral artery aneurysm) were advised to undergo retreatment. Neuroform Atlas stents were fitted in these three patients with recanalization (Figures 2 and 3), although endovascular treatment was administered at different centers. No stent stenosis was observed in any parent arteries or branch arteries (Table 4).
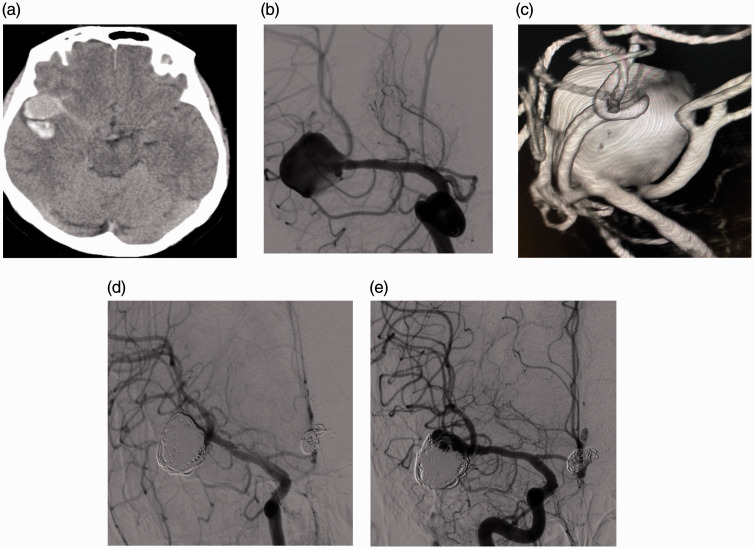
Figure 2.
(a–e) (A) patient with Fisher grade 3/Hunt & Hess grade 2 subarachnoid hemorrhage. Non-Contrast CT image show (a) a large localized clot adjacent to the aneurysm. DSA and 3D image (b and c) show a complex wide-neck aneurysm at the right MCA bifurcation. Immediate angiography (d) shows RR1 total occlusion of the aneurysm after a YSAC (Neuroform Atlas) procedure. 1-year follow-up angiography shows (e) RR3 recanalization of the aneurysm.
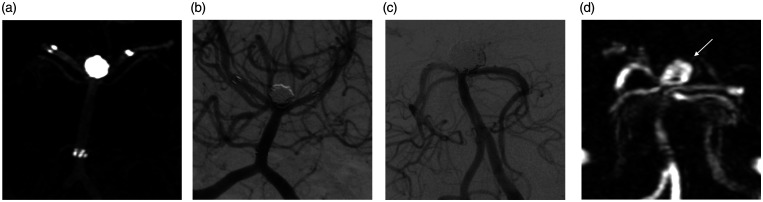
Figure 3.
(a–d) (A) A patient with Fisher grade 3, Hunt & Hess grade 2 subarachnoid hemorrhage. Wide-neck basilar top aneurysm treated with embolization using dual Enterprice stents VasoCT image shows (a) stent position and RR1 total occlusion of aneurysm and 1-year follow-up angiography (b) shows that recanalization is not observed in the aneurysm. (B) A another patient with Fisher grade-4, H&H grade-3 patient. A 12-mm wide-neck basilar top aneurysm treated with embolization using dual Neuroform Atlas stents. Immediate angiography (c) shows RR1 occlusion of aneurysm and 1-year follow-up Magnetic Resonance angiography (d) shows recanalization of the aneurysm (white arrow).
Discussion
Although there are various approaches to the acute treatment of patients with SAH, the prevailing opinion is that early intervention reduces mortality and leads to good clinical outcomes10,11; however, the techniques and medications for the treatment of wide-neck aneurysms should carefully selected. Wide-neck bifurcation aneurysms are particularly difficult to treat because of the need to protect both daughter vessels. In wide-neck aneurysms, assistive devices are usually necessary to prevent coil herniation. Recently, there has been improved use of devices such as the web, PulseRider, and pCONUS.12–15 It is promising that the PulseRider and web devices reduce the requirement for antiplatelet therapy in patients with ruptured aneurysms. Similar to the PulseRider, the pCONUS device bridges the aneurysm neck, but its recommended use involves dual antiplatelet therapy. The pCONUS device is presumed to have a lower risk of thromboembolic complications compared with a Y-stent.16 In a study involving use of the pCONUS device in 21 acute ruptured aneurysms, Perez et al.17 observed intraoperative complications in two patients (one thromboembolic and one hemorrhagic).
Temporary stent-assisted coiling is an emerging technical approach. The absence of dual antiplatelet maintenance therapy is the most important advantage of this technique.18,19 Sirlov et al.20 treated acutely ruptured basilar artery bifurcation aneurysms with simultaneous Cascade and Comaneci temporary-assisted coiling. Their findings suggested that this method may be an alternative treatment to YSAC, especially in patients with acute ruptured aneurysms. The YSAC is regarded as an appropriate alternative approach in such instances, whereby a scaffold is created by closing the aneurysm neck with stents and preventing coil protrusion.7
Use of the YSAC method for the treatment of patients with SAH, especially in the acute phase, carries several challenges. Despite various practices related to the timing and maintenance of premedication for stent-assisted coiling procedures in patients with ruptured aneurysms, there remains a lack of consensus concerning when this method is appropriate. Furthermore, there is a thrombosis concern associated with Y-stent procedures due to the increased metal load. The hypercoagulable state during the acute phase in patients with SAH is presumed to enhance the risk of stent thrombosis.21 In this sense, the timing and maintenance of the antiplatelet therapy is critical in patients with acute SAH. Another challenge associated with the YSAC procedure is the unpredictability of its technical success in aneurysms, prior to treatment. Notably, preprocedural dual antiplatelet therapy may leave the aneurysm vulnerable to re-bleeding. Furthermore, it may be difficult to catheterize the acutely angled branches of the aneurysms and perform stent placement. In the present study, branches with acute sharp angles could not be catheterized in three patients, and this uncertainty prevented the administration of a loading dose of preoperative dual antiplatelet therapy at our center. As an alternative, maintenance of patency for both stents was attempted with an infusion of rapid-acting tirofiban following the placement of the first stent. The loading dose of dual antiplatelet therapy was then administered through a nasogastric tube.
A comprehensive meta-analysis by Ryu et al.22 compared three methods of antiplatelet administration: preprocedural, postprocedural, and a modified approach involving IV glycoprotein IIA/3a inhibitors in the stent-assisted coil embolization of ruptured aneurysms. No significant differences were evident in thromboembolic complications between patients receiving preprocedural antiplatelet administration and those receiving modified antiplatelet administration. Another point of uncertainty constitutes the optimal times of the tirofiban bolus dose and maintenance infusions in patients with SAH who have ruptured aneurysms.23 In a similar study, Lee et al.24 administered tirofiban for 24 h in patients with ruptured aneurysms, and initiated dual antiplatelet therapy 6 h before the cessation of tirofiban. They reported the development of asymptomatic stent thrombosis in five patients (10%). In the present study, maintenance tirofiban therapy was administered for 4–6 h after the loading dose of aspirin and clopidogrel. Notably, the inhibition of adenosine diphosphate-induced platelet aggregation can decrease to 38–54% at 2–4 h after the administration of a 300-mg loading dose due to the pharmacokinetic properties of clopidogrel.25 This overlapping of maintenance tirofiban administration after 6 h and antiplatelet therapy can enhance the risk of hemorrhagic complications in patients with acute SAH. The Delphi consensus statement26 emphasized the importance of recommendations regarding stent placement time and antiplatelet therapy management in ruptured aneurysms. The consensus recommendations for the loading dose of GPIIb/IIIa receptor inhibitors were 12 mcg/kg for 30 min and 0.1 mcg/kg/min (if necessary, IV infusion for 12–24 h) for the maintenance dose. In our study, in accordance with the consensus recommendations, a lower loading dose of tirofiban (8 µg/kg) was used and the maintenance time was shorter (4–6 h). Furthermore, in our study clopidogrel loading dose was lower than consensus recommendations. In this study, the authors consider that it is important to evaluate the clopidogrel response, especially when lower loading dose of clopidogrel is used in the YSAC procedure. Unknown response to clopidogrel and relatively low trofiban dose may have caused stent thrombosis in our study. Importantly, our study revealed that catheterization for the first stent placement was performed through relatively more challenging branches in patients who developed stent thrombosis. Thus, thrombosis may result from inadequate medication, as well as from vascular endothelial damage caused by microwire and microcatheter manipulations. In the present study, four patients (13.3%) developed procedural ischemic events and two patients developed symptomatic infarcts. Although acute ruptured aneurysms should be avoided in patients with dual antiplatelet therapy, this therapy in patients with Y stenting is important for the prevention of delayed cerebral ischemia. In our study, no patients developed delayed cerebral ischemia.
A meta-analysis by Cagnozzo et al.27 included 750 wide-neck aneurysms treated with the YSAC method. Although acutely ruptured aneurysms were present in 11% of the affected patients, the authors reported that the rate of ischemic thromboembolic events was 6.5%. Chalouhi et al.28 noted that the risk of complications with stent-assisted procedures was greater in patients with ruptured aneurysms than in those with unruptured aneurysms. Furthermore, Chung et al.29 reported that the rates of asymptomatic and symptomatic thromboembolic complications were 5.6% and 13.6%, respectively, after the stent-assisted coiling of ruptured aneurysms. Another study reported that thromboembolic complications were present in 20% of ruptured aneurysms.30 Although a review of the literature accumulated insufficient data concerning YSAC treatment alone in the acute period of ruptured aneurysms, the thromboembolic complication rates in our study appeared tolerable compared with previously published data.
No intraprocedural hemorrhagic complications were observed in the present study, although a periprocedural intracranial hemorrhage was detected in two patients (6.6%), both of whom were asymptomatic. Follow-up computed tomography scans showed that these hematomas had resorbed uneventfully. However, an extracranial hemorrhage was detected in two patients, one of whom exhibited a puncture site hematoma in which the bleeding was stopped with a covered stent. The other patient had a large (16 × 20 cm) abdominal wall hemorrhage that was likely associated with tirofiban administration. Thus, clopidogrel was discontinued and aspirin therapy was administered alone. Although rare in patients undergoing YSAC embolization, intracranial hemorrhagic complications may occur because of aneurysmal or vascular perforations related to the intraprocedural technique.31 A systematic review of 339 patients revealed a significant intracranial complication rate of 8% after the stent-assisted coil embolization of ruptured aneurysms.4 Similarly, this rate was 6.6% in the present study, with no bleeding-related morbidity or mortality.
Another problem with endovascular treatments is the recanalization of aneurysms, which may be affected by patient factors, aneurysm morphology, hemodynamics, and treatment-related features. There are stent variations such as nonoverlapping, kissing, and crossing in the YSAC procedure. In an experimental study, kissing- and crossing-Y stents showed the greatest reduction in aneurysm flow velocity because of the diminished stent porosity and redirection of impingement flow.32 Furthermore, various structural features of stents may also influence aneurysm recanalization.33 In a comprehensive meta-analysis conducted by King et al.,34 recanalization rates were lower in aneurysms using Enterprise stents than in those using Neuroform Atlas stents. In another study, the rate of aneurysm recanalization was lower in patients using braided stents than in those using laser-cut closed design stents.35 In the present study, the initial (70%) and follow-up (71.4%) RR1 occlusion rates were acceptable, considering the findings of previous studies. At follow-up, recanalization was observed in three patients (14.3%; two with basilar top aneurysm and one with middle cerebral artery aneurysm). The initial angiographic occlusion grade of these patients was RR1 and all patients had received Neuroform Atlas stents (Figures 2 and 3). Although recanalization was identified in aneurysms that were relatively larger and treated with Neuroform Atlas stents,the present study lacked sufficient patients for a comparison of the recanalization rates of different stents.
Notably, the present study evaluated the YSAC procedure only in ruptured aneurysms. There have been several studies in which subgroup analysis of the YSAC procedure was performed in patients with ruptured aneurysms, although there are insufficient published data concerning the clinical outcomes in these patients. However, the post-treatment clinical outcome of ruptured aneurysms is presumed to be closely associated with the patient’s clinical status at admission. In a systematic review, Bodilly et al.4 reported that the clinical outcomes after stent-assisted coiling of ruptured aneurysms were favorable in 81% of patients and poor in 6% of patients with favorable presentation at admission, but the remaining 13% died. Among the patients with poor presentation at admission, the clinical outcomes were favorable in 29% and poor in 34%, but the remaining 37% died. Of all patients in that review, 67% had favorable clinical outcomes, 14% had poor clinical outcomes, and 19% died. In the present study, 17 patients (56.6%) were in good clinical condition and six patients (20%) exhibited severe disability at discharge, but seven patients (23.3%) died. Of the seven patients who died, six had poor presentation at admission. No patient died after discharge, and the poor outcome rate was 23.8% at the final follow-up.
This study had a few limitations. First, it used a retrospective design and small sample size. Second, it did not report long-term outcomes. Finally, the ruptured aneurysms treated in this study were mostly anterior circulation aneurysms, and clopidogrel response was not evaluated prior to treatment.
Conclusion
The treatment of ruptured complex wide-neck aneurysms in patients with SAH during the acute phase, as well as post-treatment management, can be challenging. The findings in this study suggest that the YSAC procedure may offer a feasible, safe, and effective method for the treatment of such patients, although the results should be confirmed in additional studies with larger numbers of patients and longer follow-up duration.
Footnotes
Authors’ Note: Our institutional review board approved the study design (No: 2020/1270).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mehmet Akif Durak https://orcid.org/0000-0003-0827-2708
Supplemental material: Supplemental material for this article is available online.
References
- 1.Debrun GM, Aletich VA, Kehrli P, et al. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery 1998; 43: 1281–1295. [DOI] [PubMed] [Google Scholar]
- 2.Cekirge HS, Yavuz K, Geyik S, et al. HyperForm balloon remodeling in the endovascular treatment of anterior cerebral, middle cerebral, and anterior communicating artery aneurysms: clinical and angiographic follow-up results in 800 consecutive patients. J Neurosurg 2011; 114: 944–953. [DOI] [PubMed] [Google Scholar]
- 3.Mocco J, Snyder KV, Albuquerque FC, et al. Treatment of intracranial aneurysms with the enterprise stent: a multicenter registry. J Neurosurg 2009; 110: 35–39. [DOI] [PubMed] [Google Scholar]
- 4.Bodily KD, Cloft HJ, Lanzino G, et al. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. AJNR Am J Neuroradiol 2011; 32: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon O-K, Kim SH, Oh CW, et al. Embolization of wide-necked aneurysms with using three or more microcatheters. Acta Neurochir (Wien) 2006; 148: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 6.Raymond J, Guilbert F, Roy D.Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology 2001; 221: 318–326. [DOI] [PubMed] [Google Scholar]
- 7.Chow MM, Woo HH, Masaryk TJ, et al. A novel endovascular treatment of a wide-necked basilar apex aneurysm by using a Y-configuration, double-stent technique. AJNR Am J Neuroradiol 2004; 25: 509–512. [PMC free article] [PubMed] [Google Scholar]
- 8.Kung DK, Policeni BA, Capuano AW, et al. Risk of ventriculostomy-related hemorrhage in patients with acutely ruptured aneurysms treated using stent-assisted coiling. J Neurosurg 2011; 114: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 9.Duman E, Karakoç F, Pinar HU, et al. Higher dose intra-arterial milrinone and intra-arterial combined milrinone-nimodipine infusion as a rescue therapy for refractory cerebral vasospasm. Interv Neuroradiol 2017; 23: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Z, Hu X, Ma L, et al. Timing of surgery for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Int J Surg 2017; 48: 266–274. [DOI] [PubMed] [Google Scholar]
- 11.Phillips TJ, Dowling RJ, Yan B, et al. Does treatment of ruptured intracranial aneurysms within 24 hours improve clinical outcome? Stroke 2011; 42: 1936–1945. [DOI] [PubMed] [Google Scholar]
- 12.Folzenlogen Z, Seinfeld J, Kubes S, et al. Use of the PulseRider device in the treatment of ruptured intracranial aneurysms: a case series. World Neurosurg 2019; 127: e149–e154. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor KP, Strickland AE, Bohnstedt BN, et al. PulseRider use in ruptured basilar apex aneurysms. World Neurosurg 2019; 127: 346–349. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar Perez M, Hellstern V, Serna Candel C, et al. Use of pCONUS HPC for the treatment of unruptured wide-necked bifurcation aneurysms: early clinical experience using single antiplatelet therapy. Stroke Vasc Neurol. Epub ahead of print 12 September 2020. DOI: 10.1136/svn-2020-000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhogal P, Lenz-Habijan T, Bannewitz C, et al. The pCONUS HPC: 30-day and 180-day in vivo biocompatibility results. Cardiovasc Intervent Radiol 2019; 42: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilar Perez M, AlMatter M, Hellstern V, et al. Use of the pCONus HPC as an adjunct to coil occlusion of acutely ruptured aneurysms: early clinical experience using single antiplatelet therapy. J Neurointerv Surg 2020; 12: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez MA, Bhogal P, Moreno RM, et al. Use of the pCONus as an adjunct to coil embolization of acutely ruptured aneurysms. J Neurointerv Surg 2017; 9: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Signorelli F, Gory B, Turjman F.Temporary solitaire stent-assisted coiling: a technique for the treatment of acutely ruptured wide-neck intracranial aneurysms. AJNR Am J Neuroradiol 2014; 35: 984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heo HY, Ahn JG, Ji C, et al. Selective temporary stent-assisted coilembolization for intracranial wide-necked small aneurysms using solitaire AB retrievable stent. J Korean Neurosurg Soc 2019; 62: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirakov A, Raychev R, Bhogal P, et al. Acutely ruptured basilar artery bifurcation aneurysm, treated with simultaneous Cascade and Comaneci temporary-assisted coiling. J Neurointerv Surg 2020; 13: 196. [DOI] [PubMed] [Google Scholar]
- 21.Rajajee V, Brown DM, Tuhrim S.Coagulation abnormalities following primary intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2004; 13: 47–51. [DOI] [PubMed] [Google Scholar]
- 22.Ryu C-W, Park S, Shin HS, et al. Complications in stent-assisted endovascular therapy of ruptured intracranial aneurysms and relevance to antiplatelet administration: a systematic review. AJNR Am J Neuroradiol 2015; 36: 1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang X-D, Wang Z-L, Li T-X, et al. Safety and efficacy of a new prophylactic tirofiban protocol without oral intraoperative antiplatelet therapy for endovascular treatment of ruptured intracranial aneurysms. J Neurointerv Surg 2016; 8: 1148–1153. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Park IS, Lee JM, et al. Stent-assisted coil embolization using only a glycoprotein IIb/IIIa inhibitor (tirofiban) for ruptured wide-necked aneurysm repair. J Cerebrovasc Endovasc Neurosurg 2018; 20: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller I, Seyfarth M, Rüdiger S, et al. Effect of a high loading dose of clopidogrel on platelet function in patients undergoing coronary stent placement. Heart 2001; 85: 92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ospel JM, Brouwer P, Dorn F, et al. Antiplatelet management for stent-assisted coiling and flow diversion of ruptured intracranial aneurysms: a DELPHI consensus statement. AJNR Am J Neuroradiol 2020; 41: 1856–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cagnazzo F, Limbucci N, Nappini S, et al. Y-stent-assisted coiling of wide-neck bifurcation intracranial aneurysms: a meta-analysis. AJNR Am J Neuroradiol 2019; 40: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalouhi N, Jabbour P, Singhal S, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke 2013; 44: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 29.Chung J, Lim YC, Suh SH, et al. Stent-assisted coil embolization of ruptured wide-necked aneurysms in the acute period: incidence of and risk factors for periprocedural complications. J Neurosurg 2014; 121: 4–11. [DOI] [PubMed] [Google Scholar]
- 30.Lessne ML, Shah P, Alexander MJ, et al. Thromboembolic complications after neuroform stent-assisted treatment of cerebral aneurysms: the duke cerebrovascular center experience in 235 patients with 274 stents. Neurosurgery 2011; 69: 369–375. [DOI] [PubMed] [Google Scholar]
- 31.Limbucci N, Renieri L, Nappini S, et al. Y-stent assisted coiling of bifurcation aneurysms with enterprise stent: long-term follow-up. J Neurointerv Surg 2016; 8: 158–162. [DOI] [PubMed] [Google Scholar]
- 32.Kono K, Terada T.Hemodynamics of 8 different configurations of stenting for bifurcation aneurysms. AJNR Am J Neuroradiol 2013; 34: 1980–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voigt P, Schob S, Jantschke R.Stent-assisted coiling of ruptured and incidental aneurysms of the intracranial circulation using moderately flow-redirecting, Braided Leo stents-initial experience in 39 patients. Front Neurol 2017; 8: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King B, Vaziri S, Singla A, et al. Clinical and angiographic outcomes after stent-assisted coiling of cerebral aneurysms with enterprise and neuroform stents: a comparative analysis of the literature. J NeuroIntervent Surg 2015; 7: 905–909. [DOI] [PubMed] [Google Scholar]
- 35.Lim J, Cho YD, Hong N, et al. Follow-up outcomes of intracranial aneurysms treated using braided or laser-cut stents with closed-cell design: a propensity score-matched case-controlled comparison. J Neurointerv Surg. Epub ahead of print 18 August 2020. DOI:10.1136/neurintsurg-2020-016165 [DOI] [PubMed] [Google Scholar]