Abstract
Drug resistance is one of the main causes of chemotherapy failure. Although several factors are involved in cancer drug resistant, the exporter pumps overexpression that mediates the drugs flow to outside the cells and reduces both the drugs intracellular concentration and effectiveness, has been one of the most important challenges. Overexpression of ABCC3, a member of the ABCC subfamily, has been strongly associated to the resistance to multiple drugs. ABCC3 has been found highly expressed in different types of cancers and is associated with poor prognosis and resistance to treatments. In this review, we summarize the molecular mechanisms involved in cancer drug resistance and discuss the current knowledge about the structure, function and role of ABCC3 in drug resistance, as well as, the expression status of ABCC3 in different types of cancer. We also provide evidences that place ABCC3 as a potential therapeutic target for improving the cancer treatment by focusing on the need of developing more effective cancer therapies to target ABCC3 in translational researches.
Keywords: Cancer, drug resistance, transporters, ABCC3, therapeutic target
Introduction
Resistance to antineoplastic drugs is one of the main challenges for chemotherapy treatments. Several factors have been associated to drugs resistance such as anti-apoptotic mechanisms, survival, genetic mutations, absorption deficiency, and drugs over-export from cells [1]. ABC transporter proteins are pumps that export different compounds and some drugs out of cells by using ATP hydrolysis, which provide them drugs resistance [2,3]. Within ABC family, nine members of ABCC subfamily are well-known as multidrug resistance proteins (MRP) since have shown chemoresistance in different cancers [3]. The organic anion transporter 3 (ABCC3) or multi-drug resistance associated protein 3 (MRP3), is a protein mainly expressed in the basolateral part and cytoplasm of hepatocytes; however, its overexpression has also been reported in different organs [4-6]. This protein is responsible for the transport of bile salts, different compounds, and drugs such as etoposide, cefadroxil, and methotrexate [7-9]. The ABCC3 elevated expression has been associated to both poor treatments response and prognosis in different types of cancer; in addition, the inhibition of its activity increases the cells sensitivity to different drugs against cancer [10-12].
In this review, we summarize the molecular mechanisms involved in cancer drug resistance, current knowledge about the structure, function and expression status of ABCC3 in different cancers; as well as, its role in drug resistance. We also propose that ABCC3 might be a potential therapeutic target in cancer treatment.
Drug resistance
Cancer treatment failures are closely associated to multidrug resistance. Many tumors respond satisfactorily to initial medication although prolonged use of drugs may lead to drug resistance. To date, several cellular mechanisms associated to drugs resistance such as drug inactivation, apoptosis evasion, DNA repair, decreased absorption and overexpression of export pumps have been determined [13]. Overexpression of ABC transporters family is one of the most important mechanisms involved in resistance to multiple drugs. These transmembrane proteins actively export drugs from inside to outside of cells, limiting their accumulation within target cells. Three transporters involved in multidrug resistance have been mainly studied, the P-glycoprotein (P-gp), MRP1, and breast cancer resistance protein (BCRP). The expression of these transporters increases after chemotherapy and they mainly expel hydrophobic drugs such as taxanes, vinca alkaloids, anthracyclines, doxorubicin, daunorubicin, vinblastine, vincristine, and taxol [14,15]. Additionally, genetic alterations of these transporters have been associated to the modification of pharmacokinetics and response to treatments [16].
These evidences have led to investigate the ABC family transporters and their participation in resistance to multiple drugs such as the ATPase pump ABCC3, which has recently been reported in several types of cancer [17,18].
ABCC3
Structure
The ABCC3 protein consists of 1527 amino acids, made up of five domains, three transmembrane domains (TMD) that form a membrane crossover channel through which substrates are translocated, and two highly conserved hydrophilic nucleotide-binding domains (NBD) that are responsible for the binding, hydrolysis and ATP release for molecules transport (Figure 1) [19,20].
Figure 1.
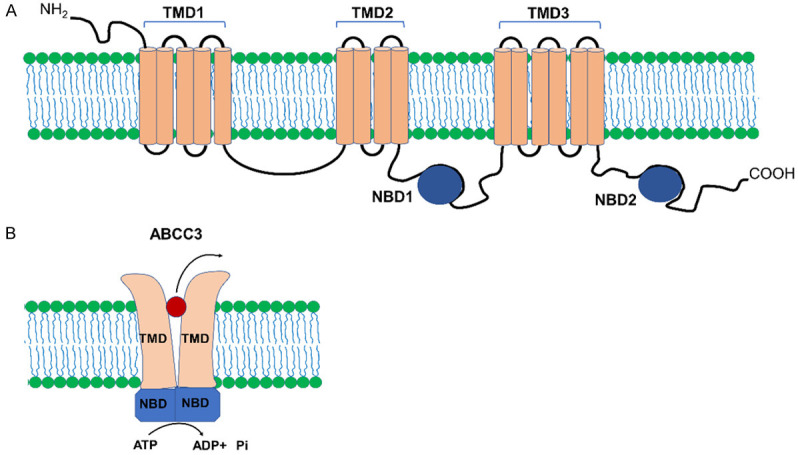
Structure of ABCC3 transporter. A. ABCC3 consists of three TMD domains and two NBD domains. B. In the ABCC3 transporter, the NBD domains are responsible for binding and hydrolyzing ATP in the molecules transport process. TMD: Transmembrane Domain, NBD: Nucleotide Binding Domain, NH2: Amino, COOH: Carboxyl.
The NBD domains consist of two subdomains namely the catalytic core made up of the Walker A motif that binds to phosphate groups of nucleotides and the Walker B motif bearing an aspartate residue that forms a bond with the Mg2+ ion and a glutamate that acts as the catalytic basis for ATP hydrolysis. The α-helical domain contains a signature sequence, known as the ABC characteristic motif that forms the catalytic site of the protein [2,21].
Expression of ABCC3
ABCC3 is expressed in various tissues but mainly in liver, pancreas, adrenal glands and to a lesser extent in colon, kidney and lung, as well as, in some cell lines [5].
ABCC3 is expressed in the liver at low levels, mainly in the basolateral membrane and hepatocytes cytoplasm, cholangiocytes and epithelial cells of the intrahepatic bile ducts, although this expression may vary between individuals [4,5,22]. Kurzawski and collaborators (2019) have reported that ABCC3 mRNA and protein expression in both healthy donors and metastatic tumors showed no significant differences when analyzed by LC-MS/MS [23]. It has been also shown that ABCC3 is highly regulated by miR-378a-5p in cholestatic liver tissues showing high expression levels in cholestatic rat livers [24,25].
The study of ABCC3 in liver diseases and cancer has been of relevant since this protein has the potential to be classified as a liver disease biomarker. It has been reported that the ABCC3 protein level increases as the carcinogenesis progresses in a hepatocellular carcinoma (HCC) model; as well as, high protein level has been observed in both HepG2 cell line and liver biopsies obtained from patient bearing HCC [4,26]. Cirqueira and collaborators (2019) have demonstrated that ABCC3 is expressed in 18% of tissues from patients with HCC and in 45% of patients with cholangiocarcinoma [17]. ABCC3 mRNA and protein expression is increased 3.4 and 1.4 fold in an obese and steatosis mouse model, respectively [27]; as well as, its protein level is also increased in alcoholic cirrhosis, diabetic, cirrhosis, and nonalcoholic steatohepatitis patients [28,29].
ABCC3 mRNA and protein expression has been detected in the basolateral membranes of pancreatic duct epithelial cells, as well as, in the membrane of acinar cells of normal pancreatic tissue but it is increased in pancreatic cancer associated to poor prognosis [5,30]; additionally, its expression is also elevated in pancreatic cancer cell lines [18].
The expression of ABCC3 has also been detected in the adrenal cortex of normal adrenal glands and in cells located in both fasciculata and reticularis zone [5,31], however, in samples of cortical adrenal adenocarcinoma, its presence at protein level has been undetected. Moreover, it has been highly expressed in the basolateral membrane of epithelial cells of gallbladder [5,32].
ABCC3 mRNA and protein are normally expressed in healthy tissues mainly located in the epithelial cell membranes [5], however, a study carried out by Kobayashi and collaborators (2016), has shown that ABCC3 mRNA and protein expressions are downregulated in samples from colon cancer patients compared to that from healthy individuals [33].
In the kidney, ABCC3 expression has been found relatively low in tissues obtained from healthy donors, as well as, in healthy tissues adjacent to the tumor [34,35]. On the other hand, Scheffer and collaborators (2002) have reported that ABCC3 protein is located in the basolateral membrane of the distal convoluted tubules and in the ascending loops of Henle but it is absent in the glomeruli, the proximal convoluted tubules and the collecting ducts in healthy tissue samples and a weak signal has been observed in Grawitz tumors [5]. ABCC3 gene and protein expressions are significantly reduced in the kidneys of patients with polycystic kidney disease, as well as, in mouse polycystic kidneys which are located in both the proximal and distal tubules. These observations were similar than in the cell line of human renal cysts WT9-12 [36].
In healthy lungs, ABCC3 expression is very low [37], however, mRNA expression is increased in cell lines and samples from patients with non-small cell lung cancer [38,39].
Function
Although the biological functions of ABCC3 have not been fully elucidated as has been clarified for other transporters, current evidence indicates that ABCC3 plays an important role in the flow and regulation of different organic and toxic compounds in the cell. For example, elimination of ABCC3 causes a high accumulation of liver bile acids, reduction in serum levels of bilirubin glucuronide, and an increased expression under cholestatic conditions, suggesting that ABCC3 is involved in the transport, excretion and enterohepatic regulation of bile salts [40,41]. In addition to function as an alternative detoxification pathway of bilirubin, its participation in drugs efflux outside of cells, ABCC3 is involved in treatment resistance [42].
Substrates
ABCC3 has high affinity to numerous substrates and mediates mainly the transport of glucuronates, sulfates or glutathione. Studies that determined the substrate specificity of ABCC3 in rats, have shown that this protein mediates the transport of glucuronides such as E2 17βG and E3040 and methotrexate, non-sulfated bile salts (TC and GC) and sulfated (TLC-S and TCDC-S) [7,43]. Other studies have reported that ABCC3 transports bilirubin diglucuronide, monoglucuronosyl bilirubin, bisglucuronosyl bilirubin, leukotriene C4, dehydroepiandrosterone-3-sulphate, and 17 beta-glucuronosyl estradiol [44], in addition to resveratrol glucuronide [45].
ABCC3 as a drug exporter
Besides its role in anions transport and other organic molecules, current evidence has shown the role of ABCC3 as drugs exporter and their metabolites. By using ABCC3 transfected cells a study showed that this transporter is responsible for the cell resistance to etoposide since it actively provoked the efflux of the drug. ABCC3 overexpression decreases the sensitivity to vincristine and inhibit the accumulation of etoposide in embryonic kidney cells [46]. Furthermore, it has been reported that in the absence of ABCC2, ABCC3 is responsible for transporting methotrexate and 7OH-MTX from liver cells to the circulation [47,48].
This protein also participates in the hepatic excretion of glucuronide and sulfate metabolites of acetaminophen, 4-methylumbelliferone and harmol [49], as well as, promotes the transport and efflux of other drugs such as cefadroxil [9], folic acid, leucovorin [50] and omeprazole [51]; additionally, it is involved in the sinusoidal efflux of fexofenadine and sorafenib-glucuronide [52,53].
ABCC3 polymorphisms
Genetic diversity among same species is reflected by molecular specific markers such as polymorphisms, defined as the existence of one or more variants in a DNA sequence. The most common polymorphism affects a single nucleotide known as SNP (single-nucleotide polymorphism) [54]. SNP can determine the regulation of both expression and function of the encoded proteins. Polymorphisms in ABCC genes alter the expression and function of ABCC transporter proteins, affecting their substrate specificity and activity [16].
Around 100 synonymous and nonsynonymous SNPs have been discovered for ABCC3 transporter, but the 211C>T (rs4793665) polymorphism alters ABCC3 mRNA expression which is located in its promoter region. From 51 mutations found in 103 individuals, including 16 exons, 25 intronic, and 10 promoters, the 211C>T polymorphism is the one that correlated with the down-expression of ABCC3 mRNA and protein and affected the binding of nuclear proteins to its promoter in the liver [6].
It has been reported that some polymorphisms associated with the drug pharmacokinetics affect metabolism, drug disposition, and the success of cancer therapies [55]. In acute myeloid leukemia (AML), the ABCC3 211C>T polymorphism is related to treatment failure and decreased overall survival and as a result, only 7.3% of Israeli patients have achieved remission after the first chemotherapy [56]. Furthermore, Yee et al. (2013) have shown that rs4148405 polymorphism in the first intron of ABCC3, decreases disease-free survival and the time of relapse in patients with AML treated with high dose of cytarabine, etoposide and busulfan; this has associated the latter to its low distribution [57].
Several genetic variations have been identified in patients with acute lymphoblastic leukemia, including the A-189 T polymorphism of ABCC3 which affects output and bioavailability, reduced toxicity and response to methotrexate [55].
On the other hand, G1013G polymorphism in exon 2 of ABCC3 is associated with a poor prognosis and decreased survival of patients with osteosarcoma after treatment with cisplatin, adriamycin, and methotrexate [58]. The identification of these and other polymorphisms could be prognostic markers for cancer treatments.
ABCC3 and resistance to cancer treatments
The export of this variety of antineoplastic drugs such as etoposide, vincristine, methotrexate and sorafenib, reduces their intracellular concentration and as a result, cancer cells show resistance and causing treatments failure in different types of cancers [8,12,47].
The first studies focused on the role of ABCC3 and its association with drug resistance in cancer, demonstrated that overexpression of ABCC3 induced resistance against epipodophyllotoxins: etoposide resistance factor (RF) 3.3 ± 0.8 and teniposide (RF 2.7 ± 0.4), as well as, resistance to methotrexate after prolonged high concentration treatment in ABCC3 transfected ovarian cancer cell lines [59].
Studies on ABCC3 participation in the resistance to antineoplastic drugs have shown that ABCC3 functions as a predictive protein for treatments sensitivity since its expression in both drug resistant and sensitive to treatment showed higher expression in the resistant group as compared to the sensitive one in non-small cell lung cancer (NSCLC) tumors. The high ABCC3 expression was correlated with an advanced stage and aggressiveness of the disease. Additionally, ABCC3 overexpression has correlated with the sensitivity reduction of five drugs namely paclitaxel, docetaxel, gemcitabine, vinorelbine and cisplatin [39]. Another study showed that the high expression of ABCC3 mRNA and protein correlated with lower sensitivity to doxorubicin, vincristine, VP-16, and cisplatin in NSCLC [60].
ABCC3 has been found highly expressed in patients who have poor response to treatment with prednisone, a corticosteroid used in patients with leukemia, lymphoma and other types of cancer, which is associated with a worse prognosis and survival rates [61] and it contributes to the resistance to daunorubicin in patients with AML [62].
ABCC3 is regulated by Wnt pathway activation; thus, inhibition of Wnt signaling increases ABCC3 expression which in turn decreases the sensitivity to etoposide and teniposide in colon cancer [33]. 5-fluorouracil (5-FU) is the most used treatment for colorectal cancer; however, ABCC3 provides resistance to this treatment. ABCC3 mRNA is mostly expressed in human colon cancer cells which are resistant to 5-FU [63]. In patients with advanced rectal cancer, a high expression of this transporter correlates with a poor response to chemoradiotherapy and an unfavorable prognosis; in addition, its inhibition reduces reactive oxygen species and increases the cells sensitive after treatment with 5-FU [64]. It has been shown that pregnane X receptor (PXR) overexpression induces ABCC3 transcription. This receptor binds to the promoter of ABCC3 gene and upregulates its expression, increases the outflow of oxaliplatin from tumor cells and thus generates drug resistance. The protein is also overexpressed in tumor tissues [65].
On the other hand, ABCC3 expression in urinary bladder cancer is higher as compared to healthy tissues, showing a positive correlation with the severity of the disease and low survival. In this study was also showed that ABCC3 silencing increases the cell sensitivity and decreases their proliferation when exposed to cisplatin [11]. Another study has demonstrated a positive correlation of ABCC3 with the tumor size and cancer severity and confers resistance to treatment by inducing a positive regulation of aerobic glycolysis in cancer cells. Inhibition of ABCC3 reduces lactate dehydrogenase A (LDHA) activity, blocks the glycolytic capability and sensitizes the cells to the treatment with cis-diamine dichloroplatin [11].
Other study has revealed that 40% of patients with chronic myeloid leukemia (CML) develop resistance to imatinib, demonstrating that his drug is transported by ABCC3 in chronic- phase of CML patients which predispose to have low response to the drug [66].
ABCC3 mRNA is overexpressed in samples from breast cancer patients who have received chemotherapy. The treatment of cell lines with doxorubicin, a drug widely used for the treatment of breast cancer, increases ABCC3 expression and decreases the retention of this drug; contrary, ABCC3 deletion increases retention and sensitivity to the drug [10]. Also, amplification of ABCC3 reduces cell sensitivity to paclitaxel and monomethyl auristatin-E-reflected in the IC50 index. Furthermore, ABCC3 gene silencing reduces resistance and increases the mitotic rate of cells treated with these drugs [67].
On the other hand, treatment with tamoxifen induces oxidative stress that increases the activation of the transcription factor Nfr2 and the overexpression of transporters such as ABCC3 in breast cancer. This phenomenon protects cancer cells and generates resistance to tamoxifen after continuous use. ABCC3 functions as a predictive marker in response to this drug [68]. In addition, ABCC3 overexpression in breast cancer cells induces resistance to methotrexate and its silencing restores the cell sensitivity to the drug which suggests an important role of ABCC3 in the resistance to drug in breast cancer [69].
ABCC3 has been shown to be highly expressed in NK cells in a malignant glioma model treated with temozolomide (TMZ), but the inhibition of this transporter increases intracellular retention of the drug, and its overexpression confers resistance to NK cells and reduces apoptosis that is associated with the activation of Akt pathway in response to the cytotoxic capability of treatment [70]. Furthermore, another study has confirmed that ABCC3 protein is expressed preferentially in CD56dim CD16+-positive NK cells in patients with glioblastoma, and this transporter is functionally active when the cells are treated with TMZ, conferring chemoresistance. In this mechanism, the nuclear factor erythroid 2-related factor 2 (Nrf2) was responsible for the ABCC3 induction under an oxidative stress condition [71].
Sorafenib is one of the most used drugs for the treatment of HCC, however, after a while, patients may become resistant to this drug [72]. A study showed that in enriched subpopulations of cancer stem cells CD44+ and CD133+, a higher ABCC3 mRNA expression was observed which increased drug resistance and metastatic capability of the cells [72]. Likewise, in PLC-PRF5-R2 cells resistant to sorafenib, the expression of ABCC3 is high and its silencing restores their sensitivity [12].
Chemotherapy induces ABCC3 expression
Several studies have shown that the expression of ABCC3 could be transcriptionally induced by antineoplastic agents. Drugs used in chemotherapy induce oxidative stress in cells and activate antioxidant response elements. Nrf2 is the transcription factor that activates the response to oxidative stress. When Nrf2 translocates into the nucleus, it binds to the putative electrophile response elements (EpRE), also called antioxidant response elements (ARE) [73].
Mahaffey et al. (2009) reported that the promoter region of ABCC3 gene contains a EpRE sequences, suggesting that ABCC3 could be regulated by Nrf2. In lung cancer cells, the expression of ABCC3 is increased by the activation of Nrf2 [74]. In addition, it has been shown that Nrf2 directly regulates the expression of ABCC3 in NSCLC cell lines through the binding of Nrf2 on the EpRE element of ABCC3 promoter [75]. Furthermore, Canet et al. (2015) identified the presence of an ARE in the eighth intron of ABCC3 where Nrf2 might be interacting [76].
Nrf2 must be activated to be translocated into the nucleus, bind to the EpRE sequences and thus to promote gene transcription. Temozolomide treatment activates Nrf2 leading to the expression of ABCC3 in NK cells [71]. Bekele et al. (2016) have demonstrated that tamoxifen treatment increases oxidative stress in breast cancer cells, which promotes the expression of Nrf2 and its translocation into the nucleus and as a result leads to the increase of gene transcription through ARE binding [68].
Additionally, it has been reported that the transcription factor Y-box binding protein-1 (YB-1) is associated to drug resistance since it can positively regulate the expression of ABC transporters. Also, it has been shown that doxorubicin increases the expression of YB-1 by inducing its translocation into the nucleus in the gastric adenocarcinoma cell line NUGC3; thus, YB-1 might initiate the gene transcription of ABCC3 once internalized into the nucleus [77].
Chen et al. (2017) have also reported that the transcription factor p53 is involved in the regulation of ABC transporters expression. In hepatocytes, doxorubicin causes both the expression and activation of p53 and in turn positively regulates the expression of ABCC3. Since ABCC3 has sequences of p53 response elements in its promoter region, this data suggests that the activation of the transcription occurs by the direct binding of p53 to the promoter region of ABCC3 [78].
ABCC3 expression can also be regulated by PXR, a key regulator of genes involved in the transport of drugs. Dong et al. (2017) demonstrated a positive correlation between the expression of PXR and ABCC3, where the transcriptional activation of ABCC3 is mediated by direct binding of PXR to the ABCC3 promoter in colorectal cancer which results in resistance to chemotherapy [65].
Undoubtedly, there is sufficient evidence to show that current chemotherapy treatments induce transcription factors that mediate the transcription of ABCC3, which might promote the export of anti-neoplastic drugs that ultimately leads the chemoresistance (Figure 2).
Figure 2.
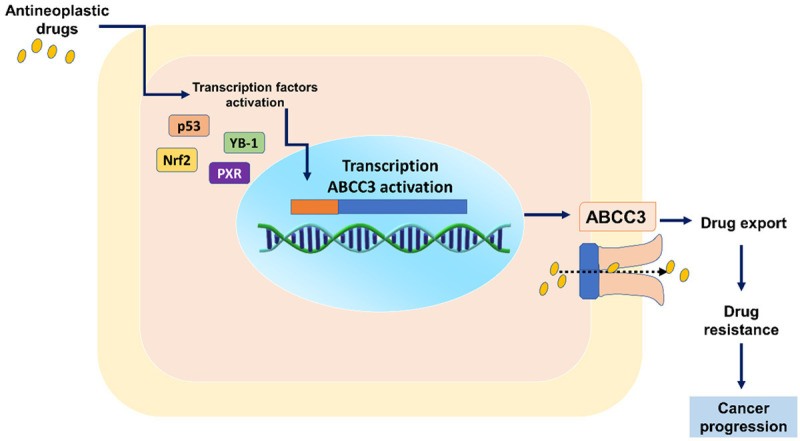
Expression of ABCC3 and chemoresistance. ABCC3 can be positively regulated by different transcription factors targeted by antineoplastic drugs. ABCC3 contributes to chemoresistance by increasing the export of antineoplastic drugs outside the cells, which ultimately leads to cancer progression. PXR: pregnane X receptor, Nrf2: nuclear factor erythroid 2-related factor 2, YB-1: Y-box binding protein-1.
ABCC3 as a therapeutic target
The increasing evidence of ABCC3 overexpression in different tumors and its association with the decrease in response to treatments and severity of the disease, place this molecule as a putative biomarker and a possible therapeutic target for the treatment of cancer. It has been suggested that blocking this transporter at the genetic and functional level could reduce resistance and improve the response to antineoplastic drugs [10]. Currently, different technologies have been used in cancer therapy.
Interference RNAs (siRNAs) are small RNA sequences that have been widely used for the specific and efficient silencing of molecules at gene and protein level. The use of siRNA against ABCC3 as a therapeutic target has been one of the most widely used techniques that has been shown to reverse drug resistance [10]. Su and collaborators (2016) have demonstrated that ABCC3 silencing by the lentivirus stable transfection of siRNA reduces the expression of ABCC3 ≥40% in human HCC cells resistant to doxorubicin which increases the sensitivity to doxorubicin, 5-FU, vinblastine and oxaliplatin, reducing the average inhibitory concentration (IC50) and increasing 8% cell apoptosis. ABCC3 silencing had no detrimental effects in animal models but inhibited tumor growth in vivo [79].
Likewise, colorectal cancer cells have been transfected with siRNA against ABCC3, with an efficiency of 90%, the inhibition of mRNA and protein increases the chemosensitivity to 5-FU, increases its IC50 and decreases cell proliferation and also increases sensitivity radiation and attenuates tumor growth in an in vivo model [64]. The interference of ABCC3 expression showed to significantly reduce drug resistance and growth inhibition of bladder cancer cells [11].
The use of siRNA-ABCC3 in bladder cancer and liver cancer resistant to doxorubicin and sorafenib, respectively, has shown ≥60% efficiency and stable inhibition that restores retention and sensitivity of cells to treatments [11,12]. Recently, it has also been described that the combination of siRNA with chemotherapy/radiotherapy, re-sensitizes cancer cells, also reduces side effects by reducing the dose of chemo/radiotherapy. This type of combined treatment consists of placing anticancer drugs and siRNA in nanoparticles and administering them simultaneously. This strategy can be used to reverse drug resistance in cancer therapy [80].
On the other hand, natural compounds have been used for the treatment of cancer. Its inhibitory effect on transporters such as ABCC3 is a promising strategy to improve the sensitivity of cancer cells. The effect of these compounds lies in the down-regulation of the mRNA of the transporter and protein or competitive binding with their substrates.
The administration of flavonoids, curcuminoid derivatives, among others, has been reported to inhibit drug export by these transporters. Tetramethylpyrazine acts as a transporter modulator. At concentrations of 400 and 600 µM, it reduces ABCC3 mRNA and protein expression and reduces adriamycin resistance 9.23-fold in HCC cells [81]. In addition, combinatorial treatments have been proposed to target ABCC3 and to reduce its activity, which together with an antineoplastic drug, improve the anticancer response and inhibit tumor progression. Curcumol, a major component of the essential oil of Rhizoma Curcumae, has been administered in combination with doxorubicin to MDA-MB-231/ADR breast cancer cells resistant to this drug. Treatment with curcumol increases the cytotoxic effect of doxorubicin by negatively regulating the expression of ABCC3, through the up-regulation of miR-181b-2-3p, which confirms its role as a therapeutic target to increase the sensitivity to antineoplastic drugs. These results indicate that the identification of the discovery of novel compounds that negatively regulate the expression and function of ABCC3 might be an interesting anticancer strategy [82].
Although ABCC3 is the transporter of several substrates, it lacks specific pharmacological inhibitors. Recently, a computational prediction study used the Bayesian model to identify possible inhibitors of the function of this transporter. In this study, 86 structurally different drugs were analyzed: fidaxomicin, suramin, and dronedarone were the most promising by showing the following IC50 = 1.83 ± 0.46 μM, IC50 of 3.33 ± 0.41 and 47.44 ± 4.41 μM, respectively [83], however, they have not been used in cancer treatments yet; thus, their subsequent application still need to be tested.
To analyze the effect of the pharmacological inhibition of ABCC3 on the development of pancreatic cancer, Adamska et al. (2019), designed a synthetic ABCC3 inhibitor called MCI-715, which effectively inhibits its transport activity. Its inhibition reduces cell proliferation in vitro and PDAC tumor growth in xenografted mice and xenografts derived from patients, increasing the survival rate [84].
Monoclonal antibodies directed at the functional blockade of key proteins in cancer development have been used for the treatment of various tumors. For example, treatment with anti-programmed cell death ligand-1 (PD-L1) antibodies improves the effector immune function of T cells in gastric cancer [85], as well as, bispecific antibodies have been synthesized to bind to the epithelial cell mesenchymal transition factor, towards the molecule PD-1 and induce its degradation in malignant cells, and inhibit the tumor growth [86]. Antibodies directed to block the prolactin receptor were successful in treating breast and prostate cancer in vitro, however, they showed safety but low efficiency in clinical trials [87,88].
The neutralization of transporter function by antibodies is still poorly explored. The use of Fv fragments for therapy directed against malignant cells has been used for the synthesis of recombinant human scFv antibodies, specific against the extracellular N-terminus of human ABCC3 expressed in glioma. Its size allowed the tumor to penetrate with an internalization capacity greater than 20%. This type of antibody could be used for tumor immunotherapy and attack cancer cells that overexpress ABCC3 [89].
Another approach based on immunotherapy is the administration of natural antibodies from human plasma for tumor cells destruction. It was recently shown that treatment with anti-ABCC3 IgG positive plasma inhibits the proliferation of oral squamous carcinoma cells compared to negative plasma, however, its administration and effects in patients has not been demonstrated yet [90].
MicroRNAs (miRNAs) are small non-coding RNA sequences that regulate gene expression. They can modulate the expression of oncogenes or tumor suppressor genes. Furthermore, the use of these non-coding RNAs against molecules responsible for drug resistance is a promising tool to improve cancer therapy [91].
Successful downregulation of transporters ABCB1 and ABCG2, ABCC1, ABCC2 has been demonstrated using miRNAs that improve the response to drugs such as vincristine, doxorubicin, methotrexate, and 5-FU in different cancers [92-95]. However, the use of these sequences targeting ABCC3 is poorly explored. The miRNAs that regulate ABCC3 expression and confer drug resistance need to be identified and selected by bioinformatic and functional analysis.
The use of ABCC3 as a therapeutic target through these tools raises broad expectations to increase the chemosensitivity of cells and increase the success of treatments, however, this strategy still needs to be considered for preclinical and clinical trials to definitively establish ABCC3 as a therapeutic target in patients.
Conclusion
Drug resistance involves complex mechanisms. The overexpression of exporting pumps has been one of the most important. In recent years, the functions of three transporters have been analyzed, the P-gp, the MRP1 transporter and BCRP. In this review we have focuses on ABCC3 transporter, a protein involved in the export of various anticancer drugs used in conventional therapies; as a result, we have concluded that the follow up in its investigation as both biomarker and target in the failure of anticancer treatments, it might be relevant for improving clinical interventions in a short future since its overexpression indicates a poor prognosis in different types of cancer. Moreover, this molecule could function as both potent biomarker and prognostic factor for drug resistance. Thus, the development of therapies targeting this transporter may be promising for both improving cancer treatments and increasing patient survival rates.
Perspectives
The high expression of drug exporting pumps in different cancers is a problem that affects treatments efficiency and patient survival. Thus, to investigate the functioning of these transporters is an attractive challenge that needs to be addressed; as a result, this will allow the development of novel therapeutic strategies to be tested in preclinical and clinical studies for the final identification of ABCC3 as a specific target against resistance to anticancer drugs.
Acknowledgements
This work was granted by CONACYT, project FOSISS-2017-2-290194 to Verónica R Vásquez-Garzón.
Disclosure of conflict of interest
None.
References
- 1.Hoffmann EK, Lambert IH. Ion channels and transporters in the development of drug resistance in cancer cells. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130109. doi: 10.1098/rstb.2013.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkens S. Structure and mechanism of ABC transporters. F1000Prime Rep. 2015;7:14. doi: 10.12703/P7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco-Torres G, Fattel-Fazenda S, Lopez-Alvarez GS, Garcia-Roman R, Villa-Trevino S, Vasquez-Garzon VR. The transmembrane transporter ABCC3 participates in liver cancer progression and is a potential biomarker. Tumour Biol. 2016;37:2007–2014. doi: 10.1007/s13277-015-3999-5. [DOI] [PubMed] [Google Scholar]
- 5.Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82:193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- 6.Lang T, Hitzl M, Burk O, Mornhinweg E, Keil A, Kerb R, Klein K, Zanger UM, Eichelbaum M, Fromm MF. Genetic polymorphisms in the multidrug resistance-associated protein 3 (ABCC3, MRP3) gene and relationship to its mRNA and protein expression in human liver. Pharmacogenetics. 2004;14:155–164. doi: 10.1097/00008571-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J Biol Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- 8.Lagas JS, Fan L, Wagenaar E, Vlaming ML, van Tellingen O, Beijnen JH, Schinkel AH. P-glycoprotein (P-gp/Abcb1), Abcc2, and Abcc3 determine the pharmacokinetics of etoposide. Clin Cancer Res. 2010;16:130–140. doi: 10.1158/1078-0432.CCR-09-1321. [DOI] [PubMed] [Google Scholar]
- 9.de Waart DR, van de Wetering K, Kunne C, Duijst S, Paulusma CC, Oude Elferink RP. Oral availability of cefadroxil depends on ABCC3 and ABCC4. Drug Metab Dispos. 2012;40:515–521. doi: 10.1124/dmd.111.041731. [DOI] [PubMed] [Google Scholar]
- 10.Balaji SA, Udupa N, Chamallamudi MR, Gupta V, Rangarajan A. Role of the Drug Transporter ABCC3 in Breast Cancer Chemoresistance. PLoS One. 2016;11:e0155013. doi: 10.1371/journal.pone.0155013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Yao D, Liu C, Cao Y, Yang Q, Sun Z, Liu D. Overexpression of ABCC3 promotes cell proliferation, drug resistance, and aerobic glycolysis and is associated with poor prognosis in urinary bladder cancer patients. Tumour Biol. 2016;37:8367–8374. doi: 10.1007/s13277-015-4703-5. [DOI] [PubMed] [Google Scholar]
- 12.Tomonari T, Takeishi S, Taniguchi T, Tanaka T, Tanaka H, Fujimoto S, Kimura T, Okamoto K, Miyamoto H, Muguruma N, Takayama T. MRP3 as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget. 2016;7:7207–7215. doi: 10.18632/oncotarget.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF. Molecular mechanisms for tumour resistance to chemotherapy. Clin Exp Pharmacol Physiol. 2016;43:723–737. doi: 10.1111/1440-1681.12581. [DOI] [PubMed] [Google Scholar]
- 14.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 15.Bogman K, Peyer AK, Torok M, Kusters E, Drewe J. HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br J Pharmacol. 2001;132:1183–1192. doi: 10.1038/sj.bjp.0703920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruhn O, Cascorbi I. Polymorphisms of the drug transporters ABCB1, ABCG2, ABCC2 and ABCC3 and their impact on drug bioavailability and clinical relevance. Expert Opin Drug Metab Toxicol. 2014;10:1337–1354. doi: 10.1517/17425255.2014.952630. [DOI] [PubMed] [Google Scholar]
- 17.Cirqueira CS, Felipe-Silva AS, Wakamatsu A, Marins LV, Rocha EC, de Mello ES, Alves VAF. Immunohistochemical assessment of the expression of biliary transportation proteins MRP2 and MRP3 in hepatocellular carcinoma and in cholangiocarcinoma. Pathol Oncol Res. 2019;25:1363–1371. doi: 10.1007/s12253-018-0386-8. [DOI] [PubMed] [Google Scholar]
- 18.Adamska A, Ferro R, Lattanzio R, Capone E, Domenichini A, Damiani V, Chiorino G, Akkaya BG, Linton KJ, De Laurenzi V, Sala G, Falasca M. ABCC3 is a novel target for the treatment of pancreatic cancer. Adv Biol Regul. 2019;73:100634. doi: 10.1016/j.jbior.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Beis K. Structural basis for the mechanism of ABC transporters. Biochem Soc Trans. 2015;43:889–893. doi: 10.1042/BST20150047. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi H, Parton A, Czechanski A, Durkin C, Kong CC, Barnes D. Multidrug resistance-associated protein 3 (Mrp3/Abcc3/Moat-D) is expressed in the SAE Squalus acanthias shark embryo-derived cell line. Zebrafish. 2007;4:261–275. doi: 10.1089/zeb.2007.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohl M, Briand C, Grutter MG, Seeger MA. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat Struct Mol Biol. 2012;19:395–402. doi: 10.1038/nsmb.2267. [DOI] [PubMed] [Google Scholar]
- 22.Konig J, Rost D, Cui Y, Keppler D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology. 1999;29:1156–1163. doi: 10.1002/hep.510290404. [DOI] [PubMed] [Google Scholar]
- 23.Kurzawski M, Szelag-Pieniek S, Lapczuk-Romanska J, Wrzesinski M, Sienko J, Oswald S, Drozdzik M. The reference liver - ABC and SLC drug transporters in healthy donor and metastatic livers. Pharmacol Rep. 2019;71:738–745. doi: 10.1016/j.pharep.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Song CW, Qiu W, Zhou XQ, Feng XC, Chen WS. Elevated hepatic MDR3/ABCB4 is directly mediated by MiR-378a-5p in human obstructive cholestasis. Eur Rev Med Pharmacol Sci. 2019;23:2539–2547. doi: 10.26355/eurrev_201903_17402. [DOI] [PubMed] [Google Scholar]
- 25.Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology. 2001;33:783–791. doi: 10.1053/jhep.2001.23501. [DOI] [PubMed] [Google Scholar]
- 26.Zuniga-Garcia V, Chavez-Lopez Mde G, Quintanar-Jurado V, Gabino-Lopez NB, Hernandez-Gallegos E, Soriano-Rosas J, Perez-Carreon JI, Camacho J. Differential expression of ion channels and transporters during hepatocellular carcinoma development. Dig Dis Sci. 2015;60:2373–2383. doi: 10.1007/s10620-015-3633-9. [DOI] [PubMed] [Google Scholar]
- 27.More VR, Slitt AL. Alteration of hepatic but not renal transporter expression in diet-induced obese mice. Drug Metab Dispos. 2011;39:992–999. doi: 10.1124/dmd.110.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.More VR, Cheng Q, Donepudi AC, Buckley DB, Lu ZJ, Cherrington NJ, Slitt AL. Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug Metab Dispos. 2013;41:1148–1155. doi: 10.1124/dmd.112.049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2011;39:2395–2402. doi: 10.1124/dmd.111.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konig J, Hartel M, Nies AT, Martignoni ME, Guo J, Buchler MW, Friess H, Keppler D. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int J Cancer. 2005;115:359–367. doi: 10.1002/ijc.20831. [DOI] [PubMed] [Google Scholar]
- 31.Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4) Biochem J. 2003;371:361–367. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rost D, Konig J, Weiss G, Klar E, Stremmel W, Keppler D. Expression and localization of the multidrug resistance proteins MRP2 and MRP3 in human gallbladder epithelia. Gastroenterology. 2001;121:1203–1208. doi: 10.1053/gast.2001.28648. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi M, Funayama R, Ohnuma S, Unno M, Nakayama K. Wnt-beta-catenin signaling regulates ABCC3 (MRP3) transporter expression in colorectal cancer. Cancer Sci. 2016;107:1776–1784. doi: 10.1111/cas.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li CY, Basit A, Gupta A, Gaborik Z, Kis E, Prasad B. Major glucuronide metabolites of testosterone are primarily transported by MRP2 and MRP3 in human liver, intestine and kidney. J Steroid Biochem Mol Biol. 2019;191:105350. doi: 10.1016/j.jsbmb.2019.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oswald S, Muller J, Neugebauer U, Schroter R, Herrmann E, Pavenstadt H, Ciarimboli G. Protein abundance of clinically relevant drug transporters in the human kidneys. Int J Mol Sci. 2019;20:5303. doi: 10.3390/ijms20215303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang E, Park EY, Woo Y, Kang DH, Hwang YH, Ahn C, Park JH. Restoring multidrug resistance-associated protein 3 attenuates cell proliferation in the polycystic kidney. Am J Physiol Renal Physiol. 2015;308:F1004–1011. doi: 10.1152/ajprenal.00159.2014. [DOI] [PubMed] [Google Scholar]
- 37.Kiuchi Y, Suzuki H, Hirohashi T, Tyson CA, Sugiyama Y. cDNA cloning and inducible expression of human multidrug resistance associated protein 3 (MRP3) FEBS Lett. 1998;433:149–152. doi: 10.1016/s0014-5793(98)00899-0. [DOI] [PubMed] [Google Scholar]
- 38.Young LC, Campling BG, Voskoglou-Nomikos T, Cole SP, Deeley RG, Gerlach JH. Expression of multidrug resistance protein-related genes in lung cancer: correlation with drug response. Clin Cancer Res. 1999;5:673–680. [PubMed] [Google Scholar]
- 39.Zhao Y, Lu H, Yan A, Yang Y, Meng Q, Sun L, Pang H, Li C, Dong X, Cai L. ABCC3 as a marker for multidrug resistance in non-small cell lung cancer. Sci Rep. 2013;3:3120. doi: 10.1038/srep03120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, Lerro A, Kruh GD. Analysis of the in vivo functions of Mrp3. Mol Pharmacol. 2005;68:160–168. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- 41.Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology. 2001;34:351–359. doi: 10.1053/jhep.2001.26213. [DOI] [PubMed] [Google Scholar]
- 42.Keppler D. The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab Dispos. 2014;42:561–565. doi: 10.1124/dmd.113.055772. [DOI] [PubMed] [Google Scholar]
- 43.Hirohashi T, Suzuki H, Sugiyama Y. Characterization of the transport properties of cloned rat multidrug resistance-associated protein 3 (MRP3) J Biol Chem. 1999;274:15181–15185. doi: 10.1074/jbc.274.21.15181. [DOI] [PubMed] [Google Scholar]
- 44.Lee YM, Cui Y, Konig J, Risch A, Jager B, Drings P, Bartsch H, Keppler D, Nies AT. Identification and functional characterization of the natural variant MRP3-Arg1297His of human multidrug resistance protein 3 (MRP3/ABCC3) Pharmacogenetics. 2004;14:213–223. doi: 10.1097/00008571-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 45.van de Wetering K, Burkon A, Feddema W, Bot A, de Jonge H, Somoza V, Borst P. Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol Pharmacol. 2009;75:876–885. doi: 10.1124/mol.108.052019. [DOI] [PubMed] [Google Scholar]
- 46.Zeng H, Bain LJ, Belinsky MG, Kruh GD. Expression of multidrug resistance protein-3 (multispecific organic anion transporter-D) in human embryonic kidney 293 cells confers resistance to anticancer agents. Cancer Res. 1999;59:5964–5967. [PubMed] [Google Scholar]
- 47.Vlaming ML, Pala Z, van Esch A, Wagenaar E, van Tellingen O, de Waart DR, Oude Elferink RP, van de Wetering K, Schinkel AH. Impact of Abcc2 (Mrp2) and Abcc3 (Mrp3) on the in vivo elimination of methotrexate and its main toxic metabolite 7-hydroxymethotrexate. Clin Cancer Res. 2008;14:8152–8160. doi: 10.1158/1078-0432.CCR-08-1609. [DOI] [PubMed] [Google Scholar]
- 48.Kitamura Y, Hirouchi M, Kusuhara H, Schuetz JD, Sugiyama Y. Increasing systemic exposure of methotrexate by active efflux mediated by multidrug resistance-associated protein 3 (mrp3/abcc3) J Pharmacol Exp Ther. 2008;327:465–473. doi: 10.1124/jpet.108.140475. [DOI] [PubMed] [Google Scholar]
- 49.Zamek-Gliszczynski MJ, Nezasa K, Tian X, Bridges AS, Lee K, Belinsky MG, Kruh GD, Brouwer KL. Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3-/- and Abcc4-/- mice. J Pharmacol Exp Ther. 2006;319:1485–1491. doi: 10.1124/jpet.106.110106. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura Y, Kusuhara H, Sugiyama Y. Basolateral efflux mediated by multidrug resistance-associated protein 3 (Mrp3/Abcc3) facilitates intestinal absorption of folates in mouse. Pharm Res. 2010;27:665–672. doi: 10.1007/s11095-009-0047-4. [DOI] [PubMed] [Google Scholar]
- 51.Pan YQ, Mi QY, He BS, Zhao SL, Tai T, Xie HG. The molecular mechanism underlying the induction of hepatic MRP3 expression and function by omeprazole. Biopharm Drug Dispos. 2015;36:232–244. doi: 10.1002/bdd.1936. [DOI] [PubMed] [Google Scholar]
- 52.Matsushima S, Maeda K, Hayashi H, Debori Y, Schinkel AH, Schuetz JD, Kusuhara H, Sugiyama Y. Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol. 2008;73:1474–1483. doi: 10.1124/mol.107.041459. [DOI] [PubMed] [Google Scholar]
- 53.Vasilyeva A, Durmus S, Li L, Wagenaar E, Hu S, Gibson AA, Panetta JC, Mani S, Sparreboom A, Baker SD, Schinkel AH. Hepatocellular Shuttling and Recirculation of Sorafenib-Glucuronide Is Dependent on Abcc2, Abcc3, and Oatp1a/1b. Cancer Res. 2015;75:2729–2736. doi: 10.1158/0008-5472.CAN-15-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunderson KL, Kuhn KM, Steemers FJ, Ng P, Murray SS, Shen R. Whole-genome genotyping of haplotype tag single nucleotide polymorphisms. Pharmacogenomics. 2006;7:641–648. doi: 10.2217/14622416.7.4.641. [DOI] [PubMed] [Google Scholar]
- 55.Ansari M, Sauty G, Labuda M, Gagne V, Rousseau J, Moghrabi A, Laverdiere C, Sinnett D, Krajinovic M. Polymorphism in multidrug resistance-associated protein gene 3 is associated with outcomes in childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2012;12:386–394. doi: 10.1038/tpj.2011.17. [DOI] [PubMed] [Google Scholar]
- 56.Muller P, Asher N, Heled M, Cohen SB, Risch A, Rund D. Polymorphisms in transporter and phase II metabolism genes as potential modifiers of the predisposition to and treatment outcome of de novo acute myeloid leukemia in Israeli ethnic groups. Leuk Res. 2008;32:919–929. doi: 10.1016/j.leukres.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Yee SW, Mefford JA, Singh N, Percival ME, Stecula A, Yang K, Witte JS, Takahashi A, Kubo M, Matsuda K, Giacomini KM, Andreadis C. Impact of polymorphisms in drug pathway genes on disease-free survival in adults with acute myeloid leukemia. J Hum Genet. 2013;58:353–361. doi: 10.1038/jhg.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caronia D, Patino-Garcia A, Perez-Martinez A, Pita G, Moreno LT, Zalacain-Diez M, Molina B, Colmenero I, Sierrasesumaga L, Benitez J, Gonzalez-Neira A. Effect of ABCB1 and ABCC3 polymorphisms on osteosarcoma survival after chemotherapy: a pharmacogenetic study. PLoS One. 2011;6:e26091. doi: 10.1371/journal.pone.0026091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ, Elferink RP, Baas F, Borst P. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A. 1999;96:6914–6919. doi: 10.1073/pnas.96.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young LC, Campling BG, Cole SP, Deeley RG, Gerlach JH. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res. 2001;7:1798–1804. [PubMed] [Google Scholar]
- 61.Steinbach D, Wittig S, Cario G, Viehmann S, Mueller A, Gruhn B, Haefer R, Zintl F, Sauerbrey A. The multidrug resistance-associated protein 3 (MRP3) is associated with a poor outcome in childhood ALL and may account for the worse prognosis in male patients and T-cell immunophenotype. Blood. 2003;102:4493–4498. doi: 10.1182/blood-2002-11-3461. [DOI] [PubMed] [Google Scholar]
- 62.Varatharajan S, Abraham A, Karathedath S, Ganesan S, Lakshmi KM, Arthur N, Srivastava VM, George B, Srivastava A, Mathews V, Balasubramanian P. ATP-binding casette transporter expression in acute myeloid leukemia: association within vitrocytotoxicity and prognostic markers. Pharmacogenomics. 2017;18:235–244. doi: 10.2217/pgs-2016-0150. [DOI] [PubMed] [Google Scholar]
- 63.Boyer J, Allen WL, McLean EG, Wilson PM, McCulla A, Moore S, Longley DB, Caldas C, Johnston PG. Pharmacogenomic identification of novel determinants of response to chemotherapy in colon cancer. Cancer Res. 2006;66:2765–2777. doi: 10.1158/0008-5472.CAN-05-2693. [DOI] [PubMed] [Google Scholar]
- 64.Yu Z, Zhang C, Wang H, Xing J, Gong H, Yu E, Zhang W, Zhang X, Cao G, Fu C. Multidrug resistance-associated protein 3 confers resistance to chemoradiotherapy for rectal cancer by regulating reactive oxygen species and caspase-3-dependent apoptotic pathway. Cancer Lett. 2014;353:182–193. doi: 10.1016/j.canlet.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 65.Dong Y, Wang Z, Xie GF, Li C, Zuo WW, Meng G, Xu CP, Li JJ. Pregnane X receptor is associated with unfavorable survival and induces chemotherapeutic resistance by transcriptional activating multidrug resistance-related protein 3 in colorectal cancer. Mol Cancer. 2017;16:71. doi: 10.1186/s12943-017-0641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giannoudis A, Davies A, Harris RJ, Lucas CM, Pirmohamed M, Clark RE. The clinical significance of ABCC3 as an imatinib transporter in chronic myeloid leukaemia. Leukemia. 2014;28:1360–1363. doi: 10.1038/leu.2014.38. [DOI] [PubMed] [Google Scholar]
- 67.O’Brien C, Cavet G, Pandita A, Hu X, Haydu L, Mohan S, Toy K, Rivers CS, Modrusan Z, Amler LC, Lackner MR. Functional genomics identifies ABCC3 as a mediator of taxane resistance in HER2-amplified breast cancer. Cancer Res. 2008;68:5380–5389. doi: 10.1158/0008-5472.CAN-08-0234. [DOI] [PubMed] [Google Scholar]
- 68.Bekele RT, Venkatraman G, Liu RZ, Tang X, Mi S, Benesch MG, Mackey JR, Godbout R, Curtis JM, McMullen TP, Brindley DN. Oxidative stress contributes to the tamoxifen-induced killing of breast cancer cells: implications for tamoxifen therapy and resistance. Sci Rep. 2016;6:21164. doi: 10.1038/srep21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Zhang X, Zhao B, Xu Z, Lv Y. Suspension State Promotes Drug Resistance of Breast Tumor Cells by Inducing ABCC3 Overexpression. Appl Biochem Biotechnol. 2020;190:410–422. doi: 10.1007/s12010-019-03084-0. [DOI] [PubMed] [Google Scholar]
- 70.Pessina S, Cantini G, Kapetis D, Cazzato E, Di Ianni N, Finocchiaro G, Pellegatta S. The multidrug-resistance transporter Abcc3 protects NK cells from chemotherapy in a murine model of malignant glioma. Oncoimmunology. 2016;5:e1108513. doi: 10.1080/2162402X.2015.1108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pellegatta S, Di Ianni N, Pessina S, Paterra R, Anghileri E, Eoli M, Finocchiaro G. ABCC3 expressed by CD56(dim) CD16(+) NK cells predicts response in glioblastoma patients treated with combined chemotherapy and dendritic cell immunotherapy. Int J Mol Sci. 2019;20:5886. doi: 10.3390/ijms20235886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chow AK, Ng L, Lam CS, Wong SK, Wan TM, Cheng NS, Yau TC, Poon RT, Pang RW. The Enhanced metastatic potential of hepatocellular carcinoma (HCC) cells with sorafenib resistance. PLoS One. 2013;8:e78675. doi: 10.1371/journal.pone.0078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mahaffey CM, Zhang H, Rinna A, Holland W, Mack PC, Forman HJ. Multidrug-resistant protein-3 gene regulation by the transcription factor Nrf2 in human bronchial epithelial and non-small-cell lung carcinoma. Free Radic Biol Med. 2009;46:1650–1657. doi: 10.1016/j.freeradbiomed.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahaffey CM, Mahaffey NC, Holland W, Zhang H, Gandara DR, Mack PC, Forman HJ. Aberrant regulation of the MRP3 gene in non-small cell lung carcinoma. J Thorac Oncol. 2012;7:34–39. doi: 10.1097/JTO.0b013e318233d753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Canet MJ, Merrell MD, Harder BG, Maher JM, Wu T, Lickteig AJ, Jackson JP, Zhang DD, Yamamoto M, Cherrington NJ. Identification of a functional antioxidant response element within the eighth intron of the human ABCC3 gene. Drug Metab Dispos. 2015;43:93–99. doi: 10.1124/dmd.114.060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chua PJ, Lim JP, Guo TT, Khanna P, Hu Q, Bay BH, Baeg GH. Y-box binding protein-1 and STAT3 independently regulate ATP-binding cassette transporters in the chemoresistance of gastric cancer cells. Int J Oncol. 2018;53:2579–2589. doi: 10.3892/ijo.2018.4557. [DOI] [PubMed] [Google Scholar]
- 78.Chen P, Li D, Chen Y, Sun J, Fu K, Guan L, Zhang H, Jiang Y, Li X, Zeng X, Chen X, Huang M, Bi H. p53-mediated regulation of bile acid disposition attenuates cholic acid-induced cholestasis in mice. Br J Pharmacol. 2017;174:4345–4361. doi: 10.1111/bph.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su Z, Liu G, Fang T, Wang Y, Zhang H, Yang S, Wei J, Lv Z, Tan L, Liu J. Silencing MRP1-4 genes by RNA interference enhances sensitivity of human hepatoma cells to chemotherapy. Am J Transl Res. 2016;8:2790–2802. [PMC free article] [PubMed] [Google Scholar]
- 80.Cao S, Lin C, Liang S, Er Saw P, Xu X. Enhancing chemotherapy by RNA interference. BIO Integration. 2020 [Google Scholar]
- 81.Wang XB, Wang SS, Zhang QF, Liu M, Li HL, Liu Y, Wang JN, Zheng F, Guo LY, Xiang JZ. Inhibition of tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug resistant human hepatocellular carcinoma cells. Oncol Rep. 2010;23:211–215. [PubMed] [Google Scholar]
- 82.Zeng C, Fan D, Xu Y, Li X, Yuan J, Yang Q, Zhou X, Lu J, Zhang C, Han J, Gu J, Gao Y, Sun L, Wang S. Curcumol enhances the sensitivity of doxorubicin in triple-negative breast cancer via regulating the miR-181b-2-3p-ABCC3 axis. Biochem Pharmacol. 2020;174:113795. doi: 10.1016/j.bcp.2020.113795. [DOI] [PubMed] [Google Scholar]
- 83.Ali I, Welch MA, Lu Y, Swaan PW, Brouwer KLR. Identification of novel MRP3 inhibitors based on computational models and validation using an in vitro membrane vesicle assay. Eur J Pharm Sci. 2017;103:52–59. doi: 10.1016/j.ejps.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adamska A, Domenichini A, Capone E, Damiani V, Akkaya BG, Linton KJ, Di Sebastiano P, Chen X, Keeton AB, Ramirez-Alcantara V, Maxuitenko Y, Piazza GA, De Laurenzi V, Sala G, Falasca M. Pharmacological inhibition of ABCC3 slows tumour progression in animal models of pancreatic cancer. J Exp Clin Cancer Res. 2019;38:312. doi: 10.1186/s13046-019-1308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou W, Yuan Q, Yuan X, Wang Y, Mo W, Wang H, Yu M. A novel tetravalent bispecific antibody targeting programmed death 1 and tyrosine-protein kinase Met for treatment of gastric cancer. Invest New Drugs. 2019;37:876–889. doi: 10.1007/s10637-018-0689-3. [DOI] [PubMed] [Google Scholar]
- 86.Sun ZJ, Wu Y, Hou WH, Wang YX, Yuan QY, Wang HJ, Yu M. A novel bispecific c-MET/PD-1 antibody with therapeutic potential in solid cancer. Oncotarget. 2017;8:29067–29079. doi: 10.18632/oncotarget.16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agarwal N, Machiels JP, Suarez C, Lewis N, Higgins M, Wisinski K, Awada A, Maur M, Stein M, Hwang A, Mosher R, Wasserman E, Wu G, Zhang H, Zieba R, Elmeliegy M. Phase I study of the prolactin receptor antagonist LFA102 in metastatic breast and castration-resistant prostate cancer. Oncologist. 2016;21:535–536. doi: 10.1634/theoncologist.2015-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Sullivan CC, Bates SE. Targeting prolactin receptor (PRLR) signaling in PRLR-positive breast and prostate cancer. Oncologist. 2016;21:523–526. doi: 10.1634/theoncologist.2016-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuan CT, Srivastava N, McLendon RE, Marasco WA, Zalutsky MR, Bigner DD. Recombinant single-chain variable fragment antibodies against extracellular epitopes of human multidrug resistance protein MRP3 for targeting malignant gliomas. Int J Cancer. 2010;127:598–611. doi: 10.1002/ijc.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Huang Z, He Z, Meng Q, Wang X, Hu Y. A study of natural IgG antibodies against ATP-binding cassette subfamily C member 3 in oral squamous cell carcinoma. J Cancer Res Ther. 2019;15:921–926. doi: 10.4103/jcrt.JCRT_150_18. [DOI] [PubMed] [Google Scholar]
- 91.Si W, Shen J, Zheng H, Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clinical Epigenetics. 2019;11:25. doi: 10.1186/s13148-018-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bao L, Hazari S, Mehra S, Kaushal D, Moroz K, Dash S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am J Pathol. 2012;180:2490–2503. doi: 10.1016/j.ajpath.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma MT, He M, Wang Y, Jiao XY, Zhao L, Bai XF, Yu ZJ, Wu HZ, Sun ML, Song ZG, Wei MJ. MiR-487a resensitizes mitoxantrone (MX)-resistant breast cancer cells (MCF-7/MX) to MX by targeting breast cancer resistance protein (BCRP/ABCG2) Cancer Lett. 2013;339:107–115. doi: 10.1016/j.canlet.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 94.Xu K, Liang X, Shen K, Cui D, Zheng Y, Xu J, Fan Z, Qiu Y, Li Q, Ni L, Liu J. miR-297 modulates multidrug resistance in human colorectal carcinoma by down-regulating MRP-2. Biochem J. 2012;446:291–300. doi: 10.1042/BJ20120386. [DOI] [PubMed] [Google Scholar]
- 95.Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, Wagar N, Yoon Y, Cho HT, Scala S, Shim H. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol. 2010;79:817–824. doi: 10.1016/j.bcp.2009.10.017. [DOI] [PubMed] [Google Scholar]


