Abstract
Few advances in GBM treatment have been made since the initiation of the Stupp trials in 2005. Experimental studies on immunotherapy drugs, molecular inhibitors, radiation dosage escalation and vascular growth factor blockers have all failed to provide satisfactory outcomes. TTFields therapy, on the other hand, have emerged as a viable substitute to therapies like radiation in GBM patients having a highly immunosuppressive tumor microenvironment. To enhance the biofunctional impacts, we explored the combination events with TTFields and proton treatment in this study. We conducted a cell viability test, a cell death detection evaluation, a ROS analysis, a three-dimensional (3D) culture system, and a migration assay. The combination of proton radiation and TTFields therapy laid a substantial anticancer impact on the F98 and U373 as compared to the consequences of either of these therapies used separately. The combination proton beam therapy used by TTFields was very successful in curbing GBM from migrating. GBM cell metastasis is restricted by TTFields combined proton by downregulating the MAPK, NF-κB, and PI3K/AKT indicating pathways, caused by reduced EMT marker expression. These findings furnish biological proof for the molecular grounds of TTFields in combination with proton used for GBM therapy.
Keywords: Tumor-treating fields, proton beam, glioblastoma, apoptosis
Introduction
Principally, proton therapy entails a significant clinical advantage vis-à-vis traditional photon therapy due to the protons’ unrivaled depth-dose attributes [1]. It is possible to take advantage of these characteristics to gain considerable reductions in normal tissue doses distally and proximally to the target volume [2]. In tumor patients, this allows tumor doses’ escalation and enhanced sparing as far as normal tissues are concerned, thereby possibly making improvements in survival/local control, improving the overall quality of life, and lowering toxicity [3]. Notwithstanding the promise shown by proton therapy, the extensive use of protons is yet to receive support. However, the overall consensus is that proton therapy remains an effective and safe treatment option for various kinds of ocular melanomas, pediatric cancers, chondrosarcomas, and chordomas [4]. When it comes to treating Glioblastoma (GBM), proton therapy’s therapeutic benefit is the possible elimination or reduction of the vulnerability to non-tumor brain tissues.
Recently, tumor-treating fields (TTFields) have exhibited promise in intermediate-frequency/ low-intensity, alternating electric fields delivered via noninvasive transducer arrays inserted all over the tumor [5]. Notably, TTFields have elicited FDA clearance for both new cases of GBM and recurrent ones after surgery and in combination with radiotherapy [6]. Furthermore, the latest National Comprehensive Cancer Network guidelines proposed TTFields in GBM as a viable treatment model for patients with tumors [7]. As per previous findings, their clinical efficiency is not convincing, which is why their combination with chemotherapy is shown to offer more promising results in new or recurrent cases of GBM [8]. While these results do demonstrate TTFields’ efficacy for treating cancers, other investigations are necessary to arrive at better-informed findings. Their scope in the future is likely to cover tumors that have not been covered previously. In the recent past, TTFields’ preclinical investigations have been made for colorectal, brain, gastric, survival, urinary, lung, and hepatocellular cancers, among others.
TTFields are capable of acting on rapidly dividing glioma as well as other types of cancer cells, particularly during these stages of mitotic cell division: metaphase, anaphase, and telophase stages [9]. By applying alternating electric fields, molecules are charged within cancerous cells [10]. Since these molecules are the polymers generated in a mitotic cell during metaphase, the TTFields disrupt microtubule spindle formation and the localization of septin fibers, leading to mitotic catastrophe and ultimately mitotic cell death [11]. The TTFields disturb the formation of microtubule spindle and septin fibers’ localization. Eventually, this leads to mitotic cell death [12]. An alternative electric field disrupts this new shape and causes the components to move toward the furrow (dielectrophoresis), eventually disrupting the mitosis process.
Therefore, applying TTFields can lead to the death of abnormal dividing cells with an unusual number of chromosomes [10,13]. Increased sensitivity to chemotherapy was observed in preclinical studies when it was used in conjunction with TTFields [14]. As per preclinical studies, there is heightened sensitivity to the combined use of chemotherapy and TTFields, as shown in animal tumor models as well as human glioblastoma cell lines [10,13,15]. Moreover, the synergy between TTFields and other forms of therapy, including radiotherapy, has been shown to yield encouraging results for GBM patients [16,17]. Clinical trials have also shown that patients with recurrent GBM can obtain a treatment benefit with TTFields alone in terms of prolonging overall survival without complications [18]. Further, common TTFields side effects did not show except included medical device site reaction, headache, and muscle twitching [19].
TTFields technology has evolved in recent years and modified to achieve better results, leading to its sanctioning by the authorities of the US Food and Drug Administration (FDA). Currently, TTFields is regarded as an alternative to the standard treatment for patients with recurrent GBM designated as NCCN (National Comprehensive Cancer Network) category 1. It is our suggestion that combining TTFields with other forms of therapy promotes apoptosis as well as autophagic cell death by resulting in inhibited survival invasion, proliferation, and migration in various kinds of cancer. We expect these findings to offer valuable experimental evidence and insights to support supporting multimodal treatment of tumors as previously shown in TTFields + sorafenib combination therapy. In this reference, the current study focused on combining TTFields plus proton beams on GBM.
Materials and methods
Experimental setup of the electric fields
A pair of insulated cords linked to an operational generator and an amplifier of high-voltage generated TTFields [20], which, in turn, emitted sine-wave transmissions spanning from 0 V to 800 V, causing the production of the applied electric field having the strength and frequency of 0.9 V/cm and 150 kHz. Due to its common usage in clinical settings, the field intensity of 1.0 V/cm has been chosen for this study. Cells were kept between 100-mm plates and cultured at 37°C under humid conditions with 5% atmospheric CO2 until they attained 70-80% confluence for irradiation treatment.
Photon and proton beam irradiations
The X-Rad 320 (Precision X-ray, Inc., USA) was used for irradiation by operating at 225 kV, 13 mA, a dose rate of 3.45 Gy/min, and irradiating at an intensity of 1 to 5 Gy. Proton beam irradiation was performed using a KOMAC TR102 (Kyungju, South Korea). Cells were irradiated at the position within the center of the SOBP.
The cells were seeded in 25-cm2 flasks (Corning Life Sciences, Corning, NY) placed on polyethylene plates during irradiation, and then immediately returned to an incubator maintained at 37°C. All experiments were performed in triplicate.
Cell culture
The American Type Culture Compilation (ATCC) provided rat glioma cells (F98) (Manassas, Virginia). F98 cells were cultured as single layers with the aid of 5% CO2 at 37°C in Dulbecco’s altered Eagle media containing 10% fetal bovine serum, penicillin (100 Uml-1100 Uml-1), 25 mM HEPES buffer (pH 7.4), and streptomycin (100 μgml-1100 μgml-1). U373 cells were cultivated at 37°C in a humidified incubator under 5% CO2 and DMEM reinforced with 10% FBS, HEPES, glutamine, and antibiotics.
Cell viability test
The exclusion analysis of trypan blue assisted in determining cell viability, wherein a triplicate analysis was performed by mixing trypan blue reagent and the cell suspension in equal volumes to examine the ratio of workable cells. To quantify cell viability, an equal volume of culture medium containing EZ-Cytox reagent (EZ3000, Daeillab Service, Chungcheongbuk-do, Republic of Korea) was added to the cells, and the mixture was incubated for 4 h. Cell viability was determined by measuring the absorbance at 450 nm using a Multiskan EX (Thermo Fisher Scientific; Waltham, MA, US).
Colony-forming assay
TTFields was added to cells 48 hr after proton exposure and the cells were then incubated. After 10-14 days, colonies were stained with 0.5% crystal violet (Sigma, St. Louis, MO, USA) in line with the manufacturer’s instructions [21].
ROS assay
Cells were grown and collected at the periods specified by the producer and ROS fluorescence measurements were the FLUOStar OPTIMA plate reader (BMG Lab technologies, Offenburg, Germany) at excitation 485 nm and emission of 535 nm with the manufacturer’s protocols [22].
Three-dimensional (3D) culture system
96-well plates in the 1 × 104 cells/well-formed the base for seeding the cells. Matrigel was used as a basement layer to pre-coat the 96-well plates in the 3D culture model via the addition of 40 ul of Matrigel in each well, after which it was incubated for a duration of 30 minutes at 37°C. Cells were laid on the gel in the best-fit culture medium, and wells were imaged at a span of 7 days.
Flow cytometry
Cells were cultured and treated with proton or TTFields. They were harvested at the indicated times, stained with propidium iodide (1 μg/mL, Sigma) according to the manufacturer’s protocol, and then analyzed using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). A minimum of 10,000 cells was counted for each sample, and data analysis was performed with the use of CellQuest software (BD Biosciences).
Autophagy assay
Cells were treated, harvested, stained with Cyto-ID® Green detection reagent (Cyto-ID® Autophagy Detection Kit 2.0, Enzo Life Science, Farmingdale, NY, US) and Hoechst 33342 in accordance with the manufacturer’s protocols, and observed under a confocal laser scanning microscope (LSM 880).
Analysis of transwell chamber
Transwell chambers assisted in measuring migration in vitro in alignment with the manufacturer’s recommendations. Cells were cultured on the layers of transwell upper chamber at 4 × 105 cells/ml in 150 μl of milieu and were treated or untreated with TTFields for a duration of 24 hours. The upper chamber’s medium had no serum, whereas the bottom chamber’s media included 10% FBS as a chemical allure. Imaging of such cells was done after 24 hours of incubation that transferred through Gelatin-coated layers and were dyed with a crystal violet solution provided in the transwell chamber test kit (Chemicon, Millipore, Billerica, MA, USA).
Western blot analysis
The Bradford technique was used to quantify total proteins isolated from GBM cells in RIPA buffer (50 mM Tris-Cl, pH 7.4; 1% NP-40; 150 mM NaCl, and 1 mM EDTA) treated with protease inhibitors (1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 mM Na3VO4). SDS/polyacrylamide gel electrophoresis segregated 30 g of protein samples, which were then deposited on a nitrocellulose membrane. The layer was treated overnight with primary antibodies at 4°C after obstructing non-specific antibody affinity sites. The protein bands were hatched with secondary antibodies combined with peroxidase for a span of 1 hour at 37°C, after which they were picturized with the help of chemiluminescence reagent (GE Healthcare Biosciences, Pittsburgh, PA, USA) and were identified through Amersham Imager 680 (GE Healthcare Biosciences).
Tumor xenografts of nude mice
Subcutaneous injections of single-cell solution (1 × 106 cells) were administered into the haunches of BALB/c nude mice that were 5 weeks old (Nara Biotech; Gyeonggi-do, Republic of Korea). In case of a tumor of volume 100-200 mm3, either 1 V/cm TTFields or 3 Gy proton therapy were used individually, or a combination (3 Gy proton pretreatment + TTFields) of both therapies could be exercised. Proton beam was needed to be administered for just a single fraction, whereas the treatment through 1 V/cm TTFields was extended to a week’s duration. The volume of a tumor can be calculated by using the formula (L × l2)/2 and by using a caliper to measure the tumor length (L) and breadth (l). To achieve the optimal standards of students’ t-test, including the competent size of 0.85, power of ≥ 80%, and significance level of ≤ 5%, the minimal sample size was set as 5 mice per group.
Acquisition technique of positron emission tomography (PET)/computed tomography (CT)
A Siemens Inveon PET scanner (Siemens Medical Solutions, Erlangen, Germany) was used for acquiring PET images. The mice were heated mildly through a steaming pad before ingesting 18F-fluoro-2-deoxy-d-glucose ([18F]-FDG), followed by the injection of 200 µCi of [18F]-FDG in their tail veins, along with subjecting the mice to the anasthesia made up of 200 µCi of [18F]-FDG (Forane solution, ChoongWae Pharma, Seoul, Korea). The acquisition of anatomical images was enabled by collecting the X-Ray CT information of mice through the use of Inveon system with 80 degree projection and complete rotation. The exposure duration for X-ray CT was 200 milliseconds, and its scan time was predicted to be 504 seconds. The reformation of X-ray CT data was possible through the Feldkamp reconstruction that uses Shepp and Logan filters (L.A. Feldkamp et al., Dearborn, MI, US). The rebuilt X-ray CT image had the potential pixel size of 109.69 µm × 109.69 µm. Post 30 minutes of tracer intake and collection of data linked with X-ray CT, the acquisition of PET data was done for 15 minutes within the energy span of 350-650 keV. The assortment of emission list-mode PET data was done to get 3D sinograms and their reconstruction was enabled through OSEM2D techniques. The rebuilt images had the pixel size of 0.38 × 0.38 × 0.79 mm3. The necessary amendments were applied to all datasets, including normalization, random rectification, and dead-time correction. The necessary amendments, including normalization, random rectification, and dead-time correction were applied to all databases. The Region of Interest (ROI) was defined by using X-ray CT data. The coregistration of CT and PET images was assisted by Inveon Research Workplace (version 2.0, Erlangen, Germany) (Siemens Medical Solutions). With the help of a preset conversion criterion, the highest pixel values inside the ROI on the PET image were assessed and converted to radioactive cpm values.
Study of CBC parameters in whole blood
CBC parameters were measured at the DKKorea (Seoul, Korea). CBC parameters considered for the current included hemoglobin (HGB) count, hematocrit (HCT), red blood cell (RBC) count, white blood cell (WBC) count, neutrophil (NEUT) count, lymphocyte (LYMPH) count, monocyte (MONO) count, eosinophil (EO) count, basophil (BASO) count, platelets (PLT) count.
Statistical analysis
Statistical significance was determined using ANOVA statistical test followed by Prism 6 software (La Jolla, California, USA). Differences were considered significant if the P-value was lesser than 0.05 or 0.001 (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Joint effect of TTFields and proton on proliferation of glioblastoma tumor cells
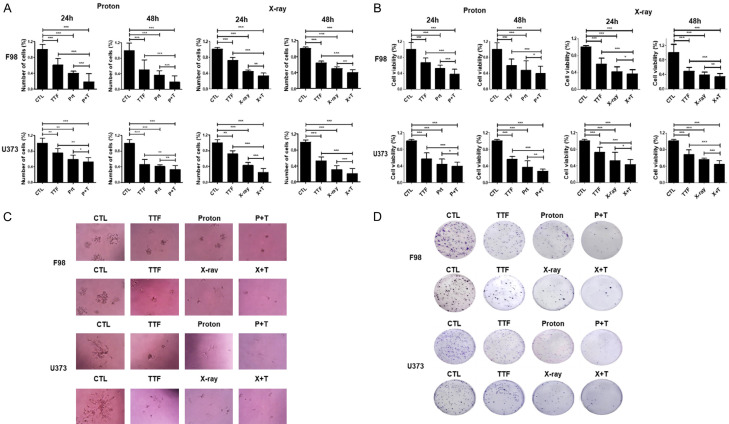
Treating F98 cells with variable doses of proton assists in analyzing the collegial impact of the proton beam and TTFields on GBM with the help of MTT analysis. As evidenced by Trypan Blue and MTT cell viability tests, the mixture of the proton beam and TTFields therapy had a considerably higher anticancer impact on F98 and U373 cells rather than either of the interventions used individually (Figure 1A and 1B). Furthermore, mono-treated 3D cultures produced bigger colonies as compared to the ones created by combination therapy (Figure 1C). In a colony formation experiment, cells administered with TTFields and proton beam combinedly displayed reduced survival fractions as compared to the cells treated with either of these treatments (Figure 1D). These study findings revealed that a proton beam or X-ray could make glioblastoma cells more sensitive to TTFields.
Figure 1.
Effect of TTFields plus proton on Glioblastoma cell proliferation. (A, B) F98 and U373 cells were exposed to TTFields for 24 h or 48 h and/or indicated dose of pretreated proton beam for cell counting (A), the MTT assay (B). *P < 0.05, **P < 0.01, ***P < 0.001. (C) 3D colony cultures of F98 cells treated as indicated. (D) The sensitivity of F98 and U373 cells treated with proton and TTFields was measured via a colony formation assay. The survival fraction, which was expressed as a function of the irradiation dose, was calculated as follows: survival fraction = colonies counted/(cells seeded × plating efficiency/100). *P < 0.05, **P < 0.01, ***P < 0.001.
TTFields mixed proton beam increases the apoptosis of GBM cells
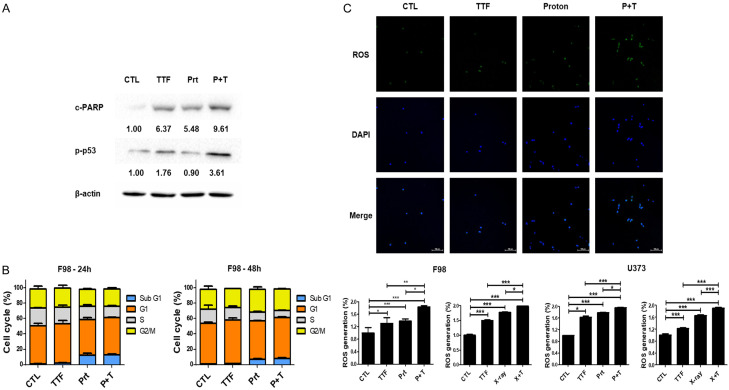
This step was to examine if the TTFields and proton beam-boosted cellular toxicity were caused due to greater PARP activation, which resulted in higher apoptotic cellular death. In fact, as compared to the control group, TTFields in conjunction with proton beam therapy caused a higher rate of PARP activation (Figure 2A). We found that enhanced levels of p-p53 (Ser-15) were detected after TTFields or proton treatment (Figure 2A), indicating that TTFields combined proton-induced apoptosis involves the p53-dependent pathway. Additionally, we next examined the effects of proton beam alone or in combination with TTFields on cell cycle progression using flow cytometry (Figure 2B). Sub-G1 cells, which represent apoptotic cells, were moderately increased by TTFields, but markedly increased by the combined treatment of TTFields and proton beam irradiation. To ascertain between the production of reactive oxygen species (ROS) and enhancement of proton-induced apoptosis, we examined the effects of combination on ROS production in GBM cells. The TTFields and proton radiation therapy enhanced the formation of ROS in the GBM cancer cells (Figure 2C), pointing out that ROS produced by the TTFields and proton beam treatment boosts intracellular caspase signaling thus, causing apoptosis.
Figure 2.
TTFields plus proton enhance the apoptosis of Glioblastoma through increased ROS. A. Cell lysates prepared from TTFields, proton, and TTFields + proton-treated cells were immunoblotted with the indicated antibodies. B. F98 cell was treated with proton and/or TTFields for 24 h or 48 h. Cell cycle distribution was analyzed quantitatively by flow cytometry. C. Analysis of ROS generation in F98 cell line 72 h after treatment with TTFields by a ROS detection kit. *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of proton and TTFields on autophagic cell
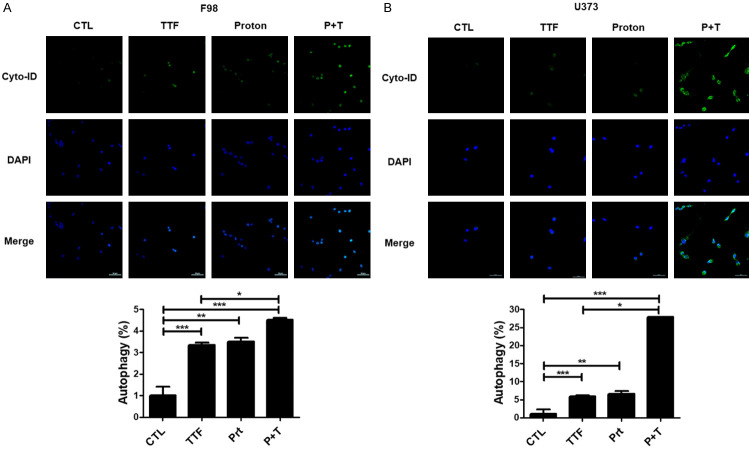
DeathTo study more about proton and TTFields’ antitumor effects, we looked into additional cellular responses linked with cell apoptosis caused by proton or TTFields. Precisely, we inspected the effects on autophagy which is triggered by TTFields, as well as proton treatment [23]. Increased deposition of Cyto-ID Green, which is an autophagy marker, was detected in the surroundings of combination-treated U373 and F98 cells, as illustrated in Figure 3A, 3B. Our finding demonstrates the contribution of autophagy to glioblastoma apoptosis after being subjected to in vitro.
Figure 3.
TTFields plus proton increases the autophagic cell death of Glioblastoma. A, B. cyto-ID staining of F98 and U373 cells with and without proton beam or with and without TTFields treatment by a cyto-ID kit. *P < 0.05, **P < 0.01, ***P < 0.001.
The combined therapy of TTFields and proton beam subdues cell migration via NF-κB, MAPK, and PI3K/AKT signaling
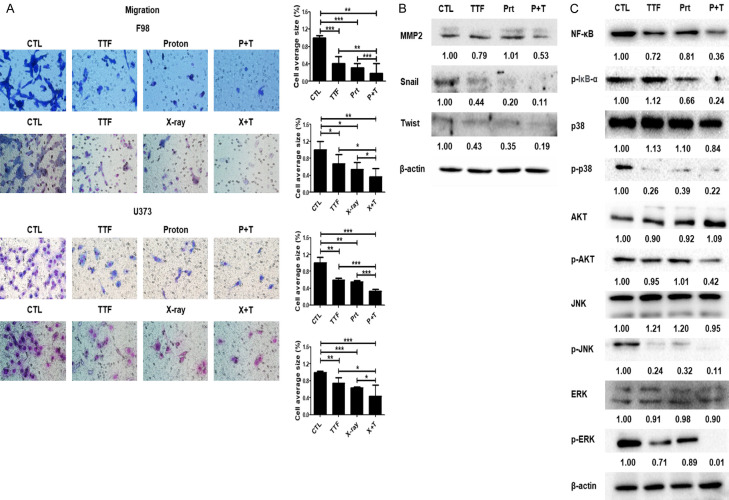
Treatment with TTFields has been found to reduce the mobility and invasion of tumor cells to a large extent [24]. As a result, we used Matrigel chamber tests to assess the impact of TTFields mixed proton beam on the intrusive and migratory capabilities of GBM cells and explored that TTFields combined proton beam therapy greatly decreased cell migration as compared to the control group (Figure 4A). Western blotting was conducted to determine the EMT modulation by TTFields (Figure 4B). TTFields suppressed the expression of MMP-2, which would lead to invasion and decreased the level of transcription factors regulating EMT (Snail, Twist). The results showed that the effects were significantly enhanced by the application of TTFields + proton.
Figure 4.
Effect of combinatorial treatment with proton beam and TTFields on the migration of glioblastoma cells. A. Tumor cell migration was assessed using a Transwell chamber assay. *P < 0.05, **P < 0.01, ***P < 0.001, bar = 500 µm; B, C. Cell lysates prepared from TTFields, proton, and TTFields + proton-treated cells were immunoblotted with the indicated antibodies.
Effective inhibition of IkBα phosphorylation and NF-κB p65 (p65) in F98 cells has been depicted through western blotting outcomes (Figure 4C). TTFields coupled proton reduced IκBα degradation and NF-κB p65 level, according to the outcomes of this research. Besides, TTFields coupled proton suppressed phosphorylation of p38, ERK, JNK, and AKT while leaving total protein levels of these MAPKs unaltered (Figure 4C). Altogether, our findings highlight the downregulation of the NF-κB, MAPK, and PI3K/AKT signaling pathways resulting in reduced EMT indicator expression, which tends to limit GBM cell metastasis due to the TTFields coupled proton (Figure 4C).
TTFields promotes proton therapy sensitivity in vivo
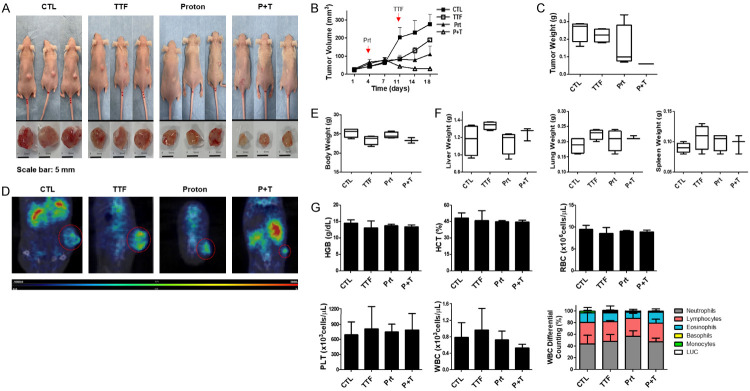
We employed a subcutaneous glioblastoma prototype created by injecting human F98 cells into mice to examine the effect of TTFields coupled with proton on in vivo development of glioblastoma. In comparison to the control group and the groups receiving any one of the treatments, xenografts administered with a blend of TTFields and proton grew at a slower pace, as indicated in Figure 5A. As a result, tumors’s volume of solo-treated groups was considerably bigger than the ones in the combined-intervention group (Figure 5B). Moreover, low-weight tumors were witnessed in mice that were given combined treatment rather than the mice subjected to any one of these therapies (Figure 5C). Figure 5D shows that tumors subjected to TTFields plus proton therapy had lower uptake of [Fluorine-18(18F)]-fluorodeoxyglucose (FDG) in comparison to cancers treated with any of these treatments. The paucity of variations in body mass and the weights of different organs, such as the spleen, lungs, and liver, indicated that there were no apparent symptoms of toxic effects due to TTFields or proton treatment in the mice (Figure 5E, 5F). No significantly apparent variations were noticed between the weights of the organs in control and treatment groups, along with the results of the Complete Blood Count (CBC) tests of these classes (Figure 5G). These findings revealed that TTFields in combination with proton suppress glioblastoma’s in vivo development.
Figure 5.
TTFields-sensitizing effects of the proton on glioblastoma in vivo. A. Image of isolated tumors derived from control or TTFields or proton or combination-treated mice. B. Nude mice were inoculated with F98 cells and treated with TTFields, proton, or a combination. Tumor volumes were measured at the indicated time points, using the formula: volume = (length × width2 × 3.14)/6 (n = 8). C. Tumors were excised and weighed at the end of the experiment (seven days). D. Representative PET/CT images of F98 tumor-bearing mice after injection of [18F]-fluorodeoxyglucose (FDG). E. The body weights of the mice were not significantly different among the proton-, TTFields-, and combination-treated groups, n = 4; F. The spleen, liver, and lung tissues of the mice were excised and weighed at the end of the experiment (seven days), n = 4. G. CBC test results in blood samples from control and treated groups in vivo.
Discussion
Since the publication of the Stupp trial in 2005, minimal advancements have been made in GBM therapy. Trials studying immunotherapy agents, molecular inhibitors, radiotherapy dose escalation, and vascular growth factor inhibitors have not yielded optimal results. However, TTFields therapy has emerged as a feasible alternative to current treatments such as RT and proton therapy in patients with GBM whose tumor microenvironment is regarded as extremely immunosuppressive. The tolerability and clinical efficacy of TTFields, which are linked with higher survival outcomes as well as minimal AEs, for GBM were proven in two phases of 3 trials besides being complemented by real-world data. EF-14 trial pointed out an enhanced overall medium survival of 21 months in the TTFields plus TMZ group as opposed to 16 months in the group that only used TMZ. Since clinical implementation, many reviews have been published on TTFields therapy for GBM [25,26].
Mild-to-moderate array-associated skin AEs are TTFields-linked AEs [27,28] which can be handled by moving the arrays and using topical corticosteroids that do not necessitate significant breaks in the treatment involving proton therapy as well [29]. While an inconvenience for patients may be in the form of regularly getting their head shaved while complying with the mandated 75% of treatment duration, the usage level appears to be positively correlated with survival benefits [30]. TTFields’ ability to disrupt/target the division of cancer cells at various cell cycle phases permits combinations with other therapies for additive/synergistic effects. Cell cycle agents and inhibitors that aim at cell migration/proliferation and DNA replication are being evaluated in conjunction with TTFields [31].
The combination of proton therapy and TTFields has polarized neuro-oncology experts. Some remain buoyant, while others are predictably skeptical. There is no consensus on the action mechanism of TTFields devices beyond the cellular level. Notably, the lack of a placebo-control device within trials prevents some experts from recommending this therapy to patients, although they are more optimistic about combining proton and TTFields. Moreover, there is more lenient precedence as far as medical devices are concerned: they can seek approval without placebo-controlled/randomized studies. It is notable that Novocure sponsored EF-11 as well as EF-14 trials. However, the EF-14 trial yielded encouraging findings regarding survival outcomes.
Other GBM treatments being examined include various RT methods such as proton beam, photon intensity-modulated as well as low-dose whole-brain rather than standard-dose RT and after combining with targeted agents such as and neurotrophin receptor kinase inhibitors and hypoxia-related growth factor receptor inhibitors proteasome inhibitors. One of TTFields therapy’s positive attributes is its minimally invasive process and reduced side effects.
This assumes great significance in treating recurrent ailments wherein patients typically are made to undergo a combination of treatment modalities such as chemotherapy, re-irradiation, and/or surgery. It is also reassuring to observe that the majority of studies on quality of life have yielded similar findings (to ours) between those who were provided TTFields/proton and those who were not. The use of TTFields has not been shown to change functional status, quality of life-related to health, overall wellbeing, or cognitive functioning. Despite accumulating data to clarify the TTFields’ MOA, current studies must explain their effect across different types of cancer. Early-phase clinical trial information indicates potential benefits of survival in other types of tumor, whereas clinical trials are underway to evaluate the safety/efficacy of TTFields in conjunction with other forms of therapies.
Acknowledgements
The authors thank to Novocure for reporting the study. This work was supported by a National Research Foundation of Korea (NRF) grant (No. NRF-2019M2A2B4095150) and by a grant of the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by Ministry and Science and ICT (MSIT), Republic of Korea (No. 50539-2020) and by the Rare Isotope Science Project of Institute for Basic Science funded by Ministry of Science and ICT and NRF of Korea (2013M7A1A1075764).
Disclosure of conflict of interest
None.
References
- 1.Harrabi SB, Bougatf N, Mohr A, Haberer T, Herfarth K, Combs SE, Debus J, Adeberg S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther Onkol. 2016;192:759–769. doi: 10.1007/s00066-016-1005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin WP, Kooy H, Loeffler JS, DeLaney TF. Proton beam therapy. Br J Cancer. 2005;93:849–854. doi: 10.1038/sj.bjc.6602754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi A, Nguyen NP, Welsh JS, Tse W, Monga M, Oduntan O, Almubarak M, Rogers J, Remick SC, Gius D. Strategies of dose escalation in the treatment of locally advanced non-small cell lung cancer: image guidance and beyond. Front Oncol. 2014;4:156. doi: 10.3389/fonc.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan R, Grosshans D. Proton therapy - present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenger C, Miranda PC, Salvador R, Thielscher A, Bomzon Z, Giladi M, Mrugala MM, Korshoej AR. A review on tumor-treating fields (TTFields): clinical implications inferred from computational modeling. IEEE Rev Biomed Eng. 2018;11:195–207. doi: 10.1109/RBME.2017.2765282. [DOI] [PubMed] [Google Scholar]
- 6.Rominiyi O, Vanderlinden A, Clenton SJ, Bridgewater C, Al-Tamimi Y, Collis SJ. Tumour treating fields therapy for glioblastoma: current advances and future directions. Br J Cancer. 2021;124:697–709. doi: 10.1038/s41416-020-01136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabian D, Guillermo Prieto Eibl MDP, Alnahhas I, Sebastian N, Giglio P, Puduvalli V, Gonzalez J, Palmer JD. Treatment of glioblastoma (GBM) with the addition of Tumor-Treating Fields (TTF): a review. Cancers (Basel) 2019;11:174. doi: 10.3390/cancers11020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20:S2–8. doi: 10.1188/16.CJON.S1.2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson KD, Lok E, Wong ET. An overview of alternating electric fields therapy (NovoTTF Therapy) for the treatment of malignant glioma. Curr Neurol Neurosci Rep. 2016;16:8. doi: 10.1007/s11910-015-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirson ED, Dbaly V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S, Gurvich Z, Schneiderman R, Wasserman Y, Salzberg M, Ryffel B, Goldsher D, Dekel E, Palti Y. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152–10157. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gera N, Yang A, Holtzman TS, Lee SX, Wong ET, Swanson KD. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One. 2015;10:e0125269. doi: 10.1371/journal.pone.0125269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durand DM, Bikson M. Suppression and control of epileptiform activity by electrical stimulation: a review. Proc IEEE. 2001;89:1065–1082. [Google Scholar]
- 13.Giladi M, Schneiderman RS, Voloshin T, Porat Y, Munster M, Blat R, Sherbo S, Bomzon Z, Urman N, Itzhaki A, Cahal S, Shteingauz A, Chaudhry A, Kirson ED, Weinberg U, Palti Y. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015;5:18046. doi: 10.1038/srep18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo Y, Kim EH, Sai S, Kim JS, Cho JM, Kim H, Baek JH, Kim JY, Hwang SG, Yoon M. Functional biological activity of sorafenib as a tumor-treating field sensitizer for glioblastoma therapy. Int J Mol Sci. 2018;19:3684. doi: 10.3390/ijms19113684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirson ED, Schneiderman RS, Dbaly V, Tovarys F, Vymazal J, Itzhaki A, Mordechovich D, Gurvich Z, Shmueli E, Goldsher D, Wasserman Y, Palti Y. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields) BMC Med Phys. 2009;9:1. doi: 10.1186/1756-6649-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim EH, Kim YH, Song HS, Jeong YK, Lee JY, Sung J, Yoo SH, Yoon M. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget. 2016;7:62267–62279. doi: 10.18632/oncotarget.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giladi M, Munster M, Schneiderman RS, Voloshin T, Porat Y, Blat R, Zielinska-Chomej K, Haag P, Bomzon Z, Kirson ED, Weinberg U, Viktorsson K, Lewensohn R, Palti Y. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017;12:206. doi: 10.1186/s13014-017-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bonis P, Doglietto F, Anile C, Pompucci A, Mangiola A. Electric fields for the treatment of glioblastoma. Expert Rev Neurother. 2012;12:1181–1184. doi: 10.1586/ern.12.112. [DOI] [PubMed] [Google Scholar]
- 19.Jo Y, Hwang SG, Jin YB, Sung J, Jeong YK, Baek JH, Cho JM, Kim EH, Yoon M. Selective toxicity of tumor treating fields to melanoma: an in vitro and in vivo study. Cell Death Discov. 2018;4:46. doi: 10.1038/s41420-018-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong H, Sung J, Oh SI, Jeong S, Koh EK, Hong S, Yoon M. Inhibition of brain tumor cell proliferation by alternating electric fields. Appl Phys Lett. 2014:105. [Google Scholar]
- 21.Liu C, Zhu Y, Lou W, Cui Y, Evans CP, Gao AC. Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells. Prostate. 2014;74:201–209. doi: 10.1002/pros.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji WO, Lee MH, Kim GH, Kim EH. Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci Rep. 2019;9:19837. doi: 10.1038/s41598-019-56160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schade AE, Oser MG, Nicholson HE, DeCaprio JA. Cyclin D-CDK4 relieves cooperative repression of proliferation and cell cycle gene expression by DREAM and RB. Oncogene. 2019;38:4962–4976. doi: 10.1038/s41388-019-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim EH, Song HS, Yoo SH, Yoon M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget. 2016;7:65125–65136. doi: 10.18632/oncotarget.11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta M, Wen P, Nishikawa R, Reardon D, Peters K. Critical review of the addition of tumor treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit Rev Oncol Hematol. 2017;111:60–65. doi: 10.1016/j.critrevonc.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Halasz LM, Mitin T. Tumor-treating fields: answering the concern about quality of life. JAMA Oncol. 2018;4:504–505. doi: 10.1001/jamaoncol.2017.5062. [DOI] [PubMed] [Google Scholar]
- 27.Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F, Dbaly V, Ram Z, Villano JL, Rainov N, Weinberg U, Schiff D, Kunschner L, Raizer J, Honnorat J, Sloan A, Malkin M, Landolfi JC, Payer F, Mehdorn M, Weil RJ, Pannullo SC, Westphal M, Smrcka M, Chin L, Kostron H, Hofer S, Bruce J, Cosgrove R, Paleologous N, Palti Y, Gutin PH. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomized phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–2202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Mrugala MM, Engelhard HH, Dinh Tran D, Kew Y, Cavaliere R, Villano JL, Annenelie Bota D, Rudnick J, Love Sumrall A, Zhu JJ, Butowski N. Clinical practice experience with NovoTTF-100A system for glioblastoma: the patient registry dataset (PRiDe) Semin Oncol. 2014;41(Suppl 6):S4–S13. doi: 10.1053/j.seminoncol.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015;17(Suppl 7):vii9–vii14. doi: 10.1093/neuonc/nov151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanner AA, Wong ET, Villano JL, Ram Z, Investigators EF. Post Hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A system versus best physician’s choice chemotherapy. Semin Oncol. 2014;41(Suppl 6):S25–34. doi: 10.1053/j.seminoncol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Mantica M, Pritchard A, Lieberman F, Drappatz J. Retrospective study of nivolumab for patients with recurrent high grade gliomas. J Neurooncol. 2018;139:625–631. doi: 10.1007/s11060-018-2907-4. [DOI] [PubMed] [Google Scholar]