Abstract
Non-small cell lung cancer (NSCLC) is a malignant tumor that accounts for the most new cancer cases and cancer-related deaths worldwide, and the proliferation and metastasis of NSCLC are the main reasons for treatment failure and patient death. Traditional chemotherapeutic drugs have low selectivity, which can kill cancer cells and cause damage to normal cells at the same time. Therefore, it is particularly important to study therapies that target cancer cells and to find low-toxicity, high-efficiency anticancer drugs. Cyy260 is a novel small molecule inhibitor that we synthesized for the first time. Here, we investigated the in vitro and in vivo antitumor activities of Cyy260 and explored the underlying mechanisms in NSCLC. Cyy260 had a concentration- and time-dependent inhibitory effect on NSCLC cells, but it was less toxic to normal cells. Cyy260 regulated apoptosis through intracellular and extracellular apoptotic pathways. In addition, Cyy260 could also induce cell cycle arrest, thereby inhibiting cell proliferation. Further analysis of molecular mechanisms showed that the JAK2/STAT3 signaling pathway was involved in the antitumor effect mediated by Cyy260. Analysis of subcutaneously transplanted tumors in mice showed that Cyy260 suppressed tumor growth in vivo. Our results proved that Cyy260 is a novel inhibitor of the JAK2/STAT3 pathway thus may have potential in therapy of NSCLC and other cancers.
Keywords: Cyy260, JAK2, NSCLC, STAT3, inhibitor
Introduction
Lung cancer has become the most widely diagnosed cancer worldwide with the morbidity and mortality increasing [1]. Since the 1990s, the treatment of lung cancer has made significant progress and the survival rate has improved [2]. In particular, chemotherapy and molecular targeted therapy have strengthened the standardized and individualized treatment of lung cancer patients, with significant results [3,4]. About 85% of lung cancer patients have a group of histological subtypes collectively called non-small cell lung cancer (NSCLC) [5]. The standard treatment for advanced NSCLC typically includes platinum-based doublet chemotherapy. For example, platinum therapy with pemetrexed is the first choice for the treatment of adenocarcinoma, whereas platinum plus gemcitabine or taxane is more commonly used to treat squamous cell carcinoma [6]. However, the adverse effects and drug resistance of chemotherapeutic drugs limit the application, and it is difficult for many patients with advanced lung cancer to complete chemotherapy [7]. Although there have been great breakthroughs in the treatment of NSCLC in the past few decades, and although surgical treatment methods are constantly being updated, when it comes to drug treatment, traditional chemotherapeutic drugs still lack specific targeting for NSCLC [8-10]. For adenocarcinoma patients with epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), reactive oxygen species 1 (ROS1), and serine/threonine kinase mutations, tyrosine kinase inhibitors can effectively be used for treatment, and therapies targeting the respective encoding genes are more effective than traditional chemotherapy and radiotherapy strategies [6]. Many newly discovered cancer-promoting changes, such as overexpression of the transcription factors SOX2 and MYC, pose clear challenges to current treatment methods [11,12]. According to current understanding, more effective methods may need to target tumor cells as well as other components of the tumor.
With the in-depth study of the JAK2/STAT3 signaling pathway, it is found that the JAK2/STAT3 signaling pathway plays a very critical role in the occurrence and development of many tumors [13,14]. JAK genes, especially JAK2, often mutate in myeloproliferative tumors, resulting in constitutive activation of JAK2/STAT3 signaling [15]. STAT3 is an important functional protein involved in tyrosine phosphorylation signaling in the cytoplasm, and it regulates multiple oncogenic signaling pathways. In lung cancer, breast cancer, prostate cancer, melanoma, and so on, the majority of tumor tissues continue to show activated phosphorylation of STAT3 [13,16-18]. STAT3 and STAT5 promote the growth and progression of breast cancer [19]. Targeted therapy for the JAK2/STAT3 signaling pathway has great application potential, and the JAK2/STAT3 signaling pathway can become a key point in regulating cell life activities, and it will also become a targeted site for tumor treatment [20,21]. The JAK2/STAT3 pathway is being explored as a potential therapeutic target for breast cancer patients [22]. However, its role in NSCLC remains unclear.
In this study, we investigated the antitumor effect of the newly synthesized compound Cyy260. Cyy260 was found to inhibit the growth and migration of tumor cells both in vitro and in vivo, and it blocked the cell cycle and promoted apoptosis through the JAK2/STAT3 pathway. The results suggest that Cyy260 has great potential as a new drug for the treatment of NSCLC.
Materials and methods
Cells and cell culture
Human NSCLC cells (A549, H1975, and PC-9), normal human lung epithelial cells (Beas-2B), and normal human liver cells (LO2) were obtained from the Cell Resource Center of the Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. The A549 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco) and 10% fetal bovine serum. The H1975 and PC-9 cells were cultured in high-glucose DMEM supplemented with 10% fetal bovine serum. All cells were cultured at 37°C in an incubator containing 5% carbon dioxide.
Reagents and antibodies
A stock solution of Cyy260 (20 mM) was prepared by using dimethyl sulfoxide (DMSO) purchased from Sigma-Aldrich Co. The Annexin V-Fluorescein Isothiocyanate Apoptosis Detection Kit I and propidium iodide were purchased from BD Pharmingen (Franklin Lakes, New Jersey, USA). The phosphatase inhibitor used in this study was a protease inhibitor patented by the People’s Republic of China. Acrylamide (30%), Coomassie Brilliant Blue, tetramethylethylenediamine, triglycine, sodium lauryl sulfate, protein markers, and skimmed milk powder were all obtained from Bio-Rad Laboratories. The antibodies used in this experiment were: P-JAK2 (Cell Signaling Technology, #3774S), JAK2 (Cell Signaling Technology, #3230S), P-PI3K (Cell Signaling Technology, #4228S), P-AKT (Cell Signaling Technology, #4060S), AKT (Cell Signaling Technology, #4691S), P-mTOR (Cell Signaling Technology, #5536S), mTOR (Cell Signaling Technology, #2983S), P-STAT3 (Abcam, EP2147Y), STAT3 (Cell Signaling Technology, #12640S), MCL-1 (Cell Signaling Technology, #4572S), BAX (Abcam, E63), CL-caspase3 (Abcam, E83-77), CDC2 (Cell Signaling Technology, #28439S), Cyclin B1 (Cell Signaling Technology, #4135S0), Cyclin A2 (Cell Signaling Technology, #4656S), and GAPDH (Cell Signaling Technology, #5174S).
MTT cell viability assay
The A549, H1975, and PC-9 cells were implanted into 96-well plates, and 4×103 cells were cultured overnight. After sticking to the wall, Cyy260 was added according to the set drug concentrations, and the MTT solution was added (25 μL/well) after 48 h. This was placed in the incubator for 4-6 h, then dimethyl sulfoxide was added (150 μL/well). The absorbance was measured at 490 nm with a microplate reader.
Colony formation assay
The human NSCLC cells were inoculated on a 6-well plate (1000 cells/well) and incubated overnight at 37°C in an incubator containing 5% carbon dioxide. When the cells were treated with different concentrations of drugs (1, 2, and 4 μM), the medium was discarded when the cells were dead. The medium was then replaced every 2 days, and the cells were left to grow for 7 days. The cells were washed with phosphate-buffered saline (PBS) for 3 times, fixed with 4% paraformaldehyde for 10 min at room temperature, then washed with PBS for 3 times, and then stained with crystal violet for 20 min.
Cell apoptosis assay
The NSCLC cells were planted on a 6-well plate and incubated overnight. The cells were treated with different concentrations of Cyy260 for 48 h (1, 2, and 4 μM). The treated cells were simultaneously incubated with fluorescein-labeled annexin V and propidium iodide. All samples were analyzed on a NovoCyte (ACEA Biosciences Inc.), and the data were evaluated by using NovoExpressTM software.
Cell cycle assay
After treating the cells with different drug concentrations for 24 h (1, 2, and 4 μM), the cells were collected, washed with PBS for twice, and fixed with ice-cold absolute ethanol at -20°C for more than 8 h. Then, the cells were washed with PBS twice at room temperature. They were then stained with propidium iodide for 10 min and analyzed by flow cytometry.
Hoechst 33342 staining
After the cells were spread overnight on a 6-well plate and adhered to the wall, they were treated with different drug concentrations for 24 h (1, 2, and 4 μM), washed twice with PBS, fixed with 4% paraformaldehyde for 15 min, and washed with PBS for 3 times, 5 min each time. The cells were stained with Hoechst 33342 for 20 min, then observed with a fluorescence microscope.
Western blot analysis
The cancer cells were collected 24 h after the drug was applied. The cells were lysed on ice with a lysis buffer supplemented with protease/phosphatase inhibitors for 10 min, then the total protein was extracted. The protein was separated by using 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane, then the blot was blocked with 10% non-fat milk at room temperature for 90 min. This was incubated with the primary antibody at 4°C overnight. After washing with PBS for 3 times, the membrane was incubated with the secondary antibody for 1 h, and the protein bands were visualized by using an enhanced chemiluminescence detection kit (Pierce Biotechnology).
Transient transfection of small interfering RNA (siRNA)
The siRNA targeting human JAK2 was purchased from Genepharma (Shanghai, China) with the following sequence: siJAK2-1: 5-GGAUGGCAGUGUUAGAUAUTT-3, siJAK2-2: 5-CCACCUGAA UGCAUUGAAATT-3, siJAK2-3: 5-CCUGGUGAAAGUCCCAUAUTT-3. Cells were transfected with 50 nM siRNA using Lipofectamine 3000 (Invitrogen, CA, USA) for 48 h. The overexpression vectors JAK2 was purchased from Takara Biotechnology Co., Ltd. (Dalian, China).
Immunofluorescent staining
A small amount of cancer cells was evenly distributed at the bottom of the 6-well plate and cultured overnight in an incubator at 37°C. After adhering to the wall, they were fixed with 4% paraformaldehyde for 15 min, then soaked and washed 3 times with PBS. After 30 min of infiltration, they were washed with PBS for 3 times, then sealed with sealing solution for 1 h. Each well was supplied with sufficient diluted primary antibody and incubated at 4°C overnight. Then, goat anti-rabbit antibody IgG was used as the secondary antibody. The antibody was incubated at 37°C for 1 h, and images were observed by using a confocal microscope.
Xenograft model
Twenty-six immunodeficient BALB/c nude female mice (4-6 weeks old) were kept in the SPF animal room of Wenzhou Medical University for in vivo experiments. All mice were purchased and raised uniformly by the Animal Experiment Center of Wenzhou Medical University. In the lung cancer xenograft model, the A549 cancer cells (5×105 cells/0.1 mL) were subcutaneously implanted into the axillae of the mice. Mung bean sized tumors could be seen or felt within 10-15 days after tumor cell implantation. The mice were divided into 4 groups (2 mice without tumor formation), and the average body weight and tumor volume of each group showed no significant differences. The groups included the control group (6 mice in each group), the low concentration group (6 mice in each group, 1 mg/kg), the high concentration group (6 mice in each group, 4 mg/kg), and the positive control group (Ruxolitinib, 6 mice in each group, 4 mg/kg). The treatment groups were intraperitoneally administered Cyy260 or Ruxolitinib every three days. Before each injection, we measured the length (L), width (W), and volume (V=0.5×L×W2) of the tumor. On the 16th day of drug injection, the animals were executed in anesthesia with CO2 and sacrifced at the end of study. Their corpses were unified with harmless treatment. All animal experiments were conducted using protocols approved by The Wenzhou Medical University Animal Policy and Welfare Committee.
Statistical analysis
Except for the animal model experiment, all experiments were repeated at least 3 times independently. The data were expressed as the standard deviation (SD) of the mean. All statistical data were calculated by using GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA), and the student’s t-test or one-way analysis of variance was used to determine statistical differences between groups. P<0.05 was considered statistically significant. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used for western blot analysis quantification. The half-inhibitory concentration (IC50) value was also calculated by using GraphPad Prism 8.
Results
Cyy260 inhibits the growth and proliferation of NSCLC cells
First, we investigated the biological effects of the newly synthesized organosilicon compound Cyy260 on NSCLC cells in vitro (Figures 1A and S1). The results showed that different drug concentrations and treatment time inhibited NSCLC cell proliferation (Figure 1B, 1C). The IC50 values obtained at 48 h were 1.007 μM for the A549 cells, 0.651 μM for the PC-9 cells and 2.117 μM for the H1975 cells (Figure 1B). The colony formation assay also showed that Cyy260 inhibited colony formation (Figure 1D). In order to verify the anti-tumor activity of Cyy260, we selected the JAK2 inhibitors Ruxolitinib (Rux) and Momelotinib (Mom), which have been proven to have anti-tumor activity in NSCLC [23-25], and compared the anti-tumor activity with Cyy260. MTT cell viability assay showed that Cyy260 inhibited tumor cell activity more significantly than Rux and Mom (Figure 1E). Colony formation assay also showed that Cyy260 inhibited tumor cell proliferation more significantly (Figure 1F). In addition, the MTT and colony formation assay showed that Cyy260 had low toxicity toward Beas-2B and LO2 (Figure 1G, 1H). These results suggest that Cyy260 is safe for normal cells and has strong cytotoxicity toward NSCLC cells.
Figure 1.

Cyy260 inhibits the growth and proliferation of NSCLC cells. A. Schematic diagram of the structure of Cyy260. B. The MTT method was used to detect the rate of cell survival. Lung cancer cells were treated with different concentrations of Cyy260 for 48 h (0, 0.01, 0.05, 0.1, 0.5, 1, 2.5, 5, 10, 20 μM). The absorbance of live cells was measured by using a microplate reader, and the IC50 value was calculated by using GraphPad Prism 8. C. NSCLC cells were treated with different concentrations of Cyy260 for 24, 48, and 72 h. The absorbance of live cells was measured by using a microplate reader, and graphs were created by using GraphPad Prism 8 to show the effect of Cyy260 on lung cancer cells at different times. D. The colony formation assay was used to detect the effect of Cyy260 on the viability of NSCLC cells. Lung cancer cells were treated with different concentrations of Cyy260 (1, 2, and 4 μM) for 24 h, incubated in an incubator for 1 week, fixed with 4% paraformaldehyde, stained with crystal violet, and photographed under a microscope. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. E. The MTT method was used to detect the rate of cell survival. PC-9 and A549 cells were treated with different concentrations of Cyy260, Rux and Mom for 48 h (0, 0.01, 0.05, 0.1, 0.5, 1, 2.5, 5, 10, 20 μM). The absorbance of live cells was measured by using a microplate reader, and graphs were created by using GraphPad Prism 8. F. The colony formation assay was used to detect the effect of Cyy260 on the viability of PC-9 and A549 cells. NSCLC cells were treated with same concentrations of Cyy260, Rux and Mom (4 μM) for 24 h, incubated in an incubator for 1 week, fixed with 4% paraformaldehyde, stained with crystal violet, and photographed under a microscope. G. The MTT method was used to detect the rate of cell survival. Beas-2B and LO2 cells were treated with different concentrations of Cyy260 for 48 h (0, 0.01, 0.05, 0.1, 0.5, 1, 2.5, 5, 10, 20 μM). The absorbance of living cells was detected by using a microplate reader, and the IC50 value was calculated by using GraphPad Prism 8. H. The colony formation assay was used to detect the effect of Cyy260 on the viability of Beas-2B and LO2 cells. Beas-2B and LO2 cells were treated with different concentrations of Cyy260 (1, 2, and 4 μM) for 24 h, incubated in an incubator for 1 week, fixed with 4% paraformaldehyde, stained with crystal violet, and photographed under a microscope. The statistical data are presented as the mean ± standard deviation from 3 independent experiments.
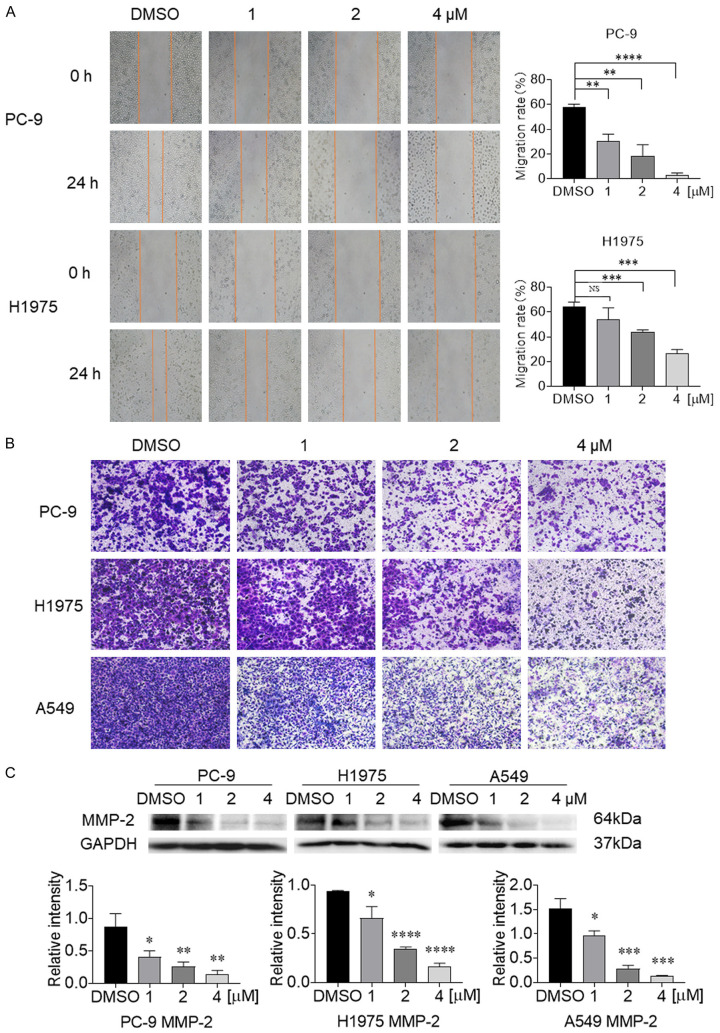
Cyy260 inhibits the invasion and migration of NSCLC cells
In addition to its direct antitumor effect, we also evaluated the effect of Cyy260 on NSCLC cell migration and invasion. We tested the inhibitory effect of Cyy260 on the migration and movement of PC-9 and H1975 cells by using scratch healing assay. The results showed that the migration ability of the NSCLC cells decreased in a concentration-dependent manner after treatment with Cyy260 for 24 h (Figure 2A). Next, we evaluated the invasiveness of NSCLC cells by using transwell assay. Cyy260 reduced the invasiveness of PC-9, H1975 and A549 cells in a concentration-dependent manner (Figure 2B). Similarly, we performed transwell assay to compare the inhibitory effects of Cyy260, Rux, and Mom on NSCLC cell invasion. Transwell assay results show that Cyy260 has a more significant inhibitory effect on NSCLC cell invasion (Figure S2). MMP-2, which is an important enzyme that degrades the extracellular matrix, plays an important role in mediating tumor angiogenesis, metastasis, and invasion [26]. Western blot analysis found that MMP-2 also decreased in a concentration-dependent manner in the NSCLC cells (Figure 2C). Our results suggest that Cyy260 has a good ability to inhibit the migration and invasion of NSCLC cells.
Figure 2.
Cyy260 inhibits the invasion and migration of lung cancer cells. A. The cell scratch assay was used to determine the effect of Cyy260 on tumor cell migration. After PC-9 and H1975 cells adhered to the wall, a small pipette tip was used to gently draw a hollow mark on the cells. When the drug was added, the cell migration was inhibited, while the cells without the drug maintained their original migration ability; after 24 h, the scratches were partially covered by migrated cells. **P<0.01, ***P<0.001, ****P<0.0001. B. The transwell assay was used to measure the effect of Cyy260 on tumor cell invasion. NSCLC cells were added to the chamber and incubated at 37°C for 24 h. After the cells adhered to the wall, the cells that invaded the chamber were fixed, stain with crystal violet, and washed with PBS for 3 times. The quantitative number of cells was checked under a microscope (40× magnification). C. Western blot analysis results showed that MMP-2 decreased in a drug concentration-dependent manner. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Cyy260 induces cell cycle arrest in NSCLC cells
We next explored whether Cyy260 could induce cell cycle arrest. We treated the A549, PC-9, and H1975 cells with different concentrations of Cyy260 for 24 h, then we analyzed the cells by flow cytometry. The results showed that when the drug concentration increased, the ratio of cells in the G2/M phase increased significantly (Figure 3A). This suggested that Cyy260 has a blocking effect on the G2/M phase of the cell cycle. We then detected the levels of cell cycle-related proteins, including Cyclin B1, Cyclin A2, and CDC2. After Cyy260 treatment, these cell cycle-related proteins were significantly reduced (Figure 3B). This result was consistent with the cell cycle arrest of NSCLC cells in the G2/M phase induced by Cyy260.
Figure 3.
Cyy260 induces cell cycle arrest in NSCLC cells. A. Flow cytometry analysis was used to show the effect of Cyy260 on the cell cycle of lung cancer cells. After Cyy260 treatment with different concentrations for 24 h, the cell cycle analysis results showed that Cyy260 had a blocking effect on the G2/M phase. The experiment was repeated 3 times independently. B. Western blot analysis of the cell cycle-related proteins CDC2, Cyclin B1, and Cyclin A2 in PC-9, H1975 and A549 cells was performed.
Cyy260 induces the apoptosis of NSCLC cells
To determine whether apoptosis was related to the inhibition of tumor cell growth induced by Cyy260, the annexin V and propidium iodide staining method was used to detect the apoptosis-inducing effect of Cyy260. We treated the NSCLC cells with different concentrations of Cyy260 for 48 h. The PC-9 and H1975 cells all exhibited dose-dependent apoptosis (Figure 4A). Cyy260 significantly induced cell apoptosis at high concentrations, while it induced less apoptosis at low concentrations. We also used the same concentration (4 μM) of Cyy260, Rux and Mom to act on NSCLC cells. The results of flow cytometry assay showed that Cyy260 has a stronger effect on promoting cell apoptosis (Figure S3). Hoechst 33342 is a specific DNA dye. This dye can stain dead cells immediately, while the staining of living cells is progressive. The living cell nuclei showed diffuse and uniform fluorescence, and when the drug-treated cells exhibited apoptosis, the nuclei exhibited dense staining, with granular and clumped fluorescence (Figure 4B). We further determined the levels of apoptosis-related proteins CL-Caspase-3 and BAX in the NSCLC cells after Cyy260 treatment. Consistent with the flow cytometry results, we found that Cyy260 increased the levels of CL-Caspase-3 and BAX in a concentration-dependent manner after 48 h (Figure 4C). These data suggest that Cyy260 promotes NSCLC cell apoptosis in vitro.
Figure 4.
Cyy260 induces the apoptosis of NSCLC cells. A. Flow cytometry analysis was used to show the effect of Cyy260 on lung cancer cell apoptosis. After treating PC-9 and H1975 cells with different concentrations of Cyy260 for 48 h, they were stained with annexin V and propidium iodide and analyzed by flow cytometry. The experiment was repeated 3 times independently. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. B. H1975 and A549 cells were treated with Cyy260 for 24 h, fixed with 4% paraformaldehyde, washed with PBS for 3 times, and stained with Hoechst 33342. The morphological characteristics of apoptosis were observed under a fluorescence microscope. ***P<0.001. C. Western blot analysis of the apoptosis-related proteins BAX and CL-Caspase3 in NSCLC cells was performed.
Cyy260 inhibits NSCLC cells by blocking the JAK2/STAT3 pathway
High-throughput screening of our kinase-focused libraries identified Cyy260 as a potent JAK2 inhibitor, with a biochemical IC50 value of 7.0 nM. To understand the mechanism of Cyy260 selectively inhibiting the JAK2 signaling pathway, we studied the effect of Cyy260 on JAK2 and its downstream proteins. We used different drug concentrations to act on the A549 and PC-9 cells (1, 2, and 4 μM). The results showed that the content of P-JAK2, P-PI3K, P-AKT, P-mTOR, and P-STAT3 decreased significantly in a concentration-dependent manner, while the total protein level had no change (Figure 5A). This suggested that Cyy260 can inhibit the activation state of JAK2 and its downstream proteins. Furthermore, Cyy260 was more effective than Ruxolitinib (4 μM), which is a JAK2 inhibitor (Figure 5B). At the same time, we detected the upstream of JAK2. The same Cyy260 concentration gradient affects H1975 and PC-9 cells. The results of western blot analysis show that Cyy260 has no obvious inhibitory effect on the GP130 and IL-6R proteins in the upstream of JAK2 (Figure 5C). To further confirm the inhibitory effect of Cyy260 on JAK2, we used interleukin-6 (IL-6), which is a JAK2 upstream cytokine, to activate JAK2 for 30 min. The results showed that Cyy260 directly inhibited JAK2 and downstream STAT3 phosphorylation induced by IL-6 (Figure 5D). Further, to explore the effect of Cyy260 on the JAK2/STAT3 pathway, we used IL-6 to stimulate the phosphorylation of STAT3 into the nucleus. After the action of Cyy260, the nuclear translocation of STAT3 induced by IL-6 was blocked in a concentration-dependent manner (Figure 5E, 5F). Immunofluorescence showed the same results (Figure 5G). These results suggest that Cyy260 may induce cell cycle arrest and activate apoptosis by inhibiting the JAK2/STAT3 pathway.
Figure 5.

Cyy260 inhibits NSCLC cells by blocking the JAK2/STAT3 signaling pathway. A. Western blot analysis was used to analyze the inhibitory effect of Cyy260 on JAK2 and its downstream proteins. B. The JAK2 inhibitor ruxolitinib (Rux) was used as a positive control to compare the inhibitory effect of Cyy260 on JAK2 and its downstream proteins. C. Western blot analysis was used to analyze the inhibitory effect of Cyy260 on JAK2 upstream proteins GP130 and IL-6R. D. The cells were pretreated with different concentrations of Cyy260 for 24 h, then the cells were stimulated with IL-6 (50 ng/mL) for 30 min. The total cell protein was extracted for Western blot analysis. E. H1975 and PC-9 cells were treated with Cyy260 without serum for 24 h, and IL-6 (50 ng/mL) was used for stimulation for 30 min. The NE-PER™ Nuclear and Cytoplasmic Extraction Reagent Kit was used to isolate cytoplasmic nucleoprotein, and the distribution of STAT3 in cells was determined by using Western blot analysis. F. A statistical graph of STAT3 protein distribution in the cytoplasm and nucleus was created. *P<0.05, **P<0.01. G. For the immunofluorescence experiment, PC-9 lung cancer cells were pretreated with Cyy260 for 24 h, then stimulated with IL-6 (25 ng/mL) for 30 min. STAT3 nuclear translocation was detected by using a confocal microscope. *P<0.05, **P<0.01, ***P<0.001.
JAK2/STAT3 pathway is involved in the antitumor effect of NSCLC cells mediated by Cyy260
To further study whether Cyy260 regulates the occurrence and development of NSCLC by JAK2 pathway, we downregulated JAK2 (si-JAK2) and overexpressed JAK2 (JAK2-OE) in NSCLC cells to artificially regulate JAK2 levels. We transfected the A549, H1975, and PC-9 cells with siRNA #1, siRNA #2, siRNA #3, and JAK2-OE for 48 h (Figure 6A, 6B). Western blot analysis results showed that in H1975 cells, the silencing effect of siRNA #2 and siRNA #3 was better than that of siRNA #1, so we chose siRNA #2 and siRNA #3 for the following experiment. The MTT results showed that compared with the cells treated with Cyy260 alone, the viability of the H1975 and PC-9 cells with JAK2 silencing was not significantly reduced after Cyy260 treatment (Figure 6C). At the same time, JAK2 overexpression made the cells more sensitive to Cyy260, and their viability were significantly reduced (Figure 6D). The colony formation assay had the same results (Figure 6E). Flow cytometry results showed that silencing JAK2 reduced the apoptosis rate of the A549 and PC-9 cells treated with Cyy260, while overexpressing JAK2 increased the apoptosis rate of the cells treated with Cyy260 (Figure 6F). This suggested that JAK2 is involved in the apoptosis of NSCLC cells treated with Cyy260. After silencing JAK2, the phosphorylation level of JAK2 and its downstream proteins was reduced to the same level as in cells treated with Cyy260. However, the levels of the apoptotic proteins CL-Caspase-3 and BAX in the group with JAK2 silencing and Cyy260 treatment were lower than those in the Cyy260 alone group (Figure 6G). To further study the relationship between JAK2 pathway and Cyy260, we established a cell population containing a JAK2 overexpression vector (JAK-OE). The results showed that compared with the Cyy260 alone group, the levels of CL-Caspase-3 and BAX in the group with JAK2-OE and Cyy260 treatment were significantly increased (Figure 6H). The above results suggest that JAK2 may be the target of Cyy260. In addition, similar to silencing STAT3 (Figure 6I), Cyy260 downregulated the level of the MCL-1 protein downstream of STAT3 (Figure 6J), partially showed that Cyy260 mediated the tumor suppressor phenotype.
Figure 6.
JAK2/STAT3 pathway is involved in the antitumor effect of NSCLC cells mediated by Cyy260. A. Western blot analysis was used to detect the effect of JAK2 protein silencing. B. Western blot analysis was used to detect the effect of JAK2 protein overexpression. C. After silencing the JAK2 protein (si-JAK2) in the cells for 24 h, the cells were digested and spread on a 96-well plate (4000 cells/well). Then, the cells were treated with different concentrations of Cyy260 (1, 2, and 4 μM) for 48 h. The MTT method was used to detect the effect of Cyy260 on lung cancer cell viability after JAK2 silencing. **P<0.01, ***P<0.001, ****P<0.0001. D. After overexpressing the JAK2 protein (JAK2-OE), the cells were spread on a 96-well plate (4000 cells/well). Then, the cells were treated with different concentrations of Cyy260 (1, 2, and 4 μM) for 48 h. The MTT method was used to detect drug resistance. The effect on lung cancer cell viability after overexpressing the JAK2 protein was observed. ***P<0.001, ****P<0.0001. E. H1975 and PC-9 cells were treated with si-JAK2 or JAK2-OE combined with Cyy260 for 24 h, and the effect on colony formation and cell proliferation was assessed. F. A549 and PC-9 cells were treated with si-JAK2 or JAK2-OE combined with Cyy260 for 48 h. The cells were double stained with annexin V and propidium iodide, then analyzed by flow cytometry. **P<0.01, ****P<0.0001. G. The JAK2 protein was silenced in A549 and PC-9 cells for 24 h, then the cells were treated with different concentrations of Cyy260 for 24 h. Western blot analysis was used to detect the effect of JAK2 silencing plus Cyy260 treatment in lung cancer cells on JAK2 and its downstream proteins. H. After A549 and PC-9 cells overexpressed the JAK2 protein for 24 h, they were treated with different concentrations of Cyy260 for 24 h. Western blot analysis was performed to detect the effect of JAK2 overexpression plus Cyy260 treatment in lung cancer cells on JAK2 and its downstream proteins. I. Western blot analysis was used to detect the effect of silencing STAT3. J. Western blot analysis was used to detect changes in the downstream protein MCL-1 after STAT3 silencing and after Cyy260 treatment. GraphPad Prism 8 was used to make a statistical chart. **P<0.01, ***P<0.001.
Cyy260 inhibits the growth of lung cancer cells in nude mice
Given the significant toxicity of Cyy260 toward NSCLC cells in vitro, we further studied the efficacy of Cyy260 in xenograft tumor models. BALB/c nude mice were subcutaneously inoculated with the A549 lung cancer cells to create a tumor model to evaluate the antitumor activity of Cyy260 in vivo. Intraperitoneal injections were administered every three days, and the JAK2-targeted therapy drug Ruxolitinib was used as a positive control (4 mg/kg). Cyy260 caused decreased tumor growth in the xenograft model. The results showed that the tumor volume and size of the mice in the Cyy260 treatment groups were significantly smaller than those in the positive control group (Figure 7A-D), and the weight of the nude mice was stable. The hematoxylin eosin (H&E) staining analysis was used to detect the toxic effects of Cyy260 on the normal tissues of the hearts, livers, lungs and kidneys. The results of the experiment showed no inflammation or necrosis in the tissues, which proved that Cyy260 has no toxic effect on normal tissues in the body (Figure S4A). Regarding the mechanism, we found that as the concentration of Cyy260 increased, the phosphorylation of JAK2 and its downstream proteins were significantly inhibited, and the apoptotic proteins CL-Caspase-3 and BAX were significantly activated. Moreover, the cell cycle-related proteins Cyclin B1, Cyclin A2, and CDC2 were inhibited (Figure 7E). Immunohistochemical analysis also showed that Cyy260 can inhibit the phosphorylation of JAK2 and STAT3, inhibit the proliferation-related protein Ki67, and activate the apoptotic protein CL-Caspase-3 (Figure S4B). The above results suggest that Cyy260 has a strong antitumor effect in NSCLC cells in xenograft tumor models, and that Cyy260 is a promising nontoxic drug for the treatment of NSCLC in vivo and in vitro.
Figure 7.

Cyy260 inhibits the growth of lung cancer cells in nude mice. A. Tumor volume was measured every 3 days. *P<0.05, ***P<0.001. B. Tumor weight after death in the nude mice was measured. **P<0.01, ***P<0.001, ****P<0.0001. C. The body weight of the mice was measured every 3 days. D. A graph of the tumors in the different groups was created. E. Western blot analysis was performed on the target proteins after lysis by tumor cell lysis buffer.
Discussion
Research increasingly shows that the JAK2/STAT3 pathway is involved in tumor progression and metastasis [13,14]. Activation of the JAK2/STAT3 pathway promotes pancreatic cancer growth and chemotherapy tolerance [27]. CpG-B inhibits activation of the JAK2/STAT3 pathway to counter immune suppression [13]. Macrophages stimulate the migration and invasion of ovarian cancer cells by mediating activation of the JAK2/STAT3/MMP-9 pathway, and autophagy regulates the JAK2/STAT3 pathway in B-cell lymphoma and glioblastoma [28-30]. After years of research, the tumor-promoting effect of the JAK2/STAT3 pathway in other malignant tumors has been partially verified [31]. However, the mechanism of the development of NSCLC mediated by the JAK2/STAT3 pathway needs further research. These studies prompted us to further explore the role of the JAK2/STAT3 pathway in NSCLC.
The small molecule compounds are being widely studied as effective anticancer drugs due to their low cytotoxicity toward normal cells, wide therapeutic range, and high-efficiency tumor inhibitory effect [32]. Currently, a variety of small molecule JAK2 inhibitors have entered into clinical research, including the JAK1/2 inhibitor Ruxolitinib, which was approved for use in patients with myelofibrosis in 2011 and for use in hydroxyurea-resistant or -intolerant polycythemia vera patients in 2014 [33]. Our research group used Ruxolitinib as a positive control. Our experiments showed that Cyy260 had a stronger inhibitory effect than Ruxolitinib on the activation of JAK2. There are other JAK2 inhibitors, but many have been discontinued, mainly because of strong side effects. A prominent example is Fedratinib. In a randomized trial with a placebo as an active control, Fedratinib was associated with Wernicke encephalopathy in 4 patients. Further, Fedratinib may cause severe vitamin B1 deficiency-induced encephalopathy, and 1 person died in clinical trials [34]. The JAK2/Src inhibitor NS-018 is currently undergoing phase II trials in myelofibrosis patients. In vitro experiments have shown that the preparation is 4.3 times more selective for JAK2’s single somatic mutation V617F than for wild-type JAK2. However, the second stage results showed 3 cases of persistent splenic reactions, and side effects, such as nausea, diarrhea, fatigue, and adverse neurological events (mainly dizziness), occurred in 17% of patients [35]. Therefore, the potential of NS-018 as a clinical drug needs further exploration. SB1317 is an inhibitor of CDK2, JAK2, and FLT3. High doses of SB1317 can significantly inhibit tumor growth, but low doses have little effect [36]. Gandotinib is a small molecule inhibitor that is more selective for JAK2 than for JAK1, but it was shown to be less potent in IL-3-treated wild-type JAK2-dependent cellular assays, suggesting that Gandotinib influence on IL-3-dependent erythroid lineage development may be small [37].
Cyy260 is our newly synthesized small molecule inhibitor. In the current study, we investigated the biological effects of Cyy260 on NSCLC cells both in vivo and in vitro. We proved that Cyy260 can inhibit the proliferation, migration, and invasion of NSCLC cells. Cell cycle regulation includes several checkpoints, most of which are related to the activation of cyclins. The G2/M checkpoint is the last checkpoint to prevent cells with DNA damage from entering the mitotic phase, and the CDC2-Cyclin B complex is a key factor in the regulation of the G2/M checkpoint [38]. In our study, Cyy260 induced cell cycle arrest in the G2/M phase. One of the reasons why malignant tumors can grow indefinitely is damage to the apoptosis program, so regulating apoptosis-related pathways is a promising tumor treatment strategy [39]. Apoptosis is strictly and complicatedly regulated. Its regulation can be achieved through death receptors, mitochondria, apoptosis inhibitor proteins, caspases, and other factors, and it can also be regulated by related signal transduction pathways [40]. In this study, flow cytometry data showed that Cyy260 promoted cell apoptosis and activated internal and external apoptotic pathways via activation of proapoptotic protein CL-Caspase-3 and BAX. Interestingly, although our cell proliferation experiment results showed that the inhibitory effect of Cyy260 on NSCLC cells was time- and concentration-dependent, our flow cytometry analysis results only showed concentration-dependent inhibition. This difference may be due to Cyy260-activated non-apoptotic cell death pathways, such as autophagy and iron effects. Detailed exploration is necessary in future research. Both JAK2 and STAT3 are composed of several subunits, and activation of JAK2 and STAT3 by phosphorylation of specific tyrosine residues is closely related to tumor progression. In this study, Cyy260 reduced the levels of phosphorylated JAK2 and STAT3, providing new insights into the antitumor effect of this new drug on NSCLC cells. It has been reported that mTOR directly upregulates the activity of STAT3 by enhancing the phosphorylation of s727 residues [41]. In our follow-up studies, we explored other proteins that could be involved in the activation of the JAK2/STAT3 pathway. We found that Cyy260 inhibited the phosphorylation of PI3K, AKT, and mTOR. Therefore, inhibitors such as JAK2 and mTOR inhibitors can inhibit the activity of STAT3 and be used in the treatment of NSCLC caused by STAT3 activation. To determine the possible target of Cyy260, we artificially downregulated or upregulated JAK2 levels in NSCLC cells. Although the signaling pathway was still blocked, JAK2 knockdown partly abolished the effect of Cyy260-mediated tumor suppression on cell proliferation and apoptosis. However, the pharmacokinetic characteristics and side effects of Cyy260 are still unclear. The exact cellular mechanism and true clinical value need to be further studied.
In conclusion, this study confirmed the antitumor effect of Cyy260 on NSCLC cells in vivo and in vitro. Our results suggest that Cyy260 can promote the apoptosis of NSCLC cells and inhibit the proliferation of NSCLC cells by blocking the G2/M phase, and it can inhibit the progression of NSCLC cells by inhibiting the JAK2/STAT3 pathway (Figure 8). In summary, Cyy260 is a potential drug candidate for the treatment of NSCLC.
Figure 8.

Schematic diagram of anti-tumor mechanism of Cyy260.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (82173856 and 81973168), Natural Science Foundation of Zhejiang Province (LY21H300005 and LQ21H310007) and Wenzhou Municipal Science and Technology Bureau (ZY2020025 and Y20190056). The disclosed funders were independent from the study design, data collection and analysis, interpretation, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Liang W, Liu J, He J. Driving the improvement of lung cancer prognosis. Cancer Cell. 2020;38:449–451. doi: 10.1016/j.ccell.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Singh A, Venkannagari S, Oh KH, Zhang YQ, Rohde JM, Liu L, Nimmagadda S, Sudini K, Brimacombe KR, Gajghate S, Ma J, Wang A, Xu X, Shahane SA, Xia M, Woo J, Mensah GA, Wang Z, Ferrer M, Gabrielson E, Li Z, Rastinejad F, Shen M, Boxer MB, Biswal S. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem Biol. 2016;11:3214–3225. doi: 10.1021/acschembio.6b00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KT, Gray RJ, Chen AP, Li S, McShane LM, Patton D, Hamilton SR, Williams PM, Iafrate AJ, Sklar J, Mitchell EP, Harris LN, Takebe N, Sims DJ, Coffey B, Fu T, Routbort M, Zwiebel JA, Rubinstein LV, Little RF, Arteaga CL, Comis R, Abrams JS, O’Dwyer PJ, Conley BA NCI-MATCH team. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: national cancer institute molecular analysis for therapy choice (NCI-MATCH) J. Clin. Oncol. 2020;38:3883–3894. doi: 10.1200/JCO.19.03010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao C, Yang L, Zhou F, Yu Y, Du X, Xiang Y, Li C, Huang X, Xie C, Liu Z, Lin J, Wang L, Liang G, Cui R. Feedback activation of EGFR is the main cause for STAT3 inhibition-irresponsiveness in pancreatic cancer cells. Oncogene. 2020;39:3997–4013. doi: 10.1038/s41388-020-1271-y. [DOI] [PubMed] [Google Scholar]
- 5.Qin N, Ma Z, Wang C, Zhang E, Li Y, Huang M, Chen C, Zhang C, Fan J, Gu Y, Xu X, Yang L, Wei X, Yin R, Jiang Y, Dai J, Jin G, Xu L, Hu Z, Shen H, Ma H. Comprehensive characterization of functional eRNAs in lung adenocarcinoma reveals novel regulators and a prognosis-related molecular subtype. Theranostics. 2020;10:11264–11277. doi: 10.7150/thno.47039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C, Giaccone G. MEK inhibitors under development for treatment of non-small-cell lung cancer. Expert Opin Investig Drugs. 2018;27:17–30. doi: 10.1080/13543784.2018.1415324. [DOI] [PubMed] [Google Scholar]
- 7.Yao G, Tang J, Yang X, Zhao Y, Zhou R, Meng R, Zhang S, Dong X, Zhang T, Yang K, Wu G, Xu S. Cyclin K interacts with β-catenin to induce Cyclin D1 expression and facilitates tumorigenesis and radioresistance in lung cancer. Theranostics. 2020;10:11144–11158. doi: 10.7150/thno.42578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández LP, Merino M, Colmenarejo G, Moreno-Rubio J, Sánchez-Martínez R, Quijada-Freire A, Gómez de Cedrón M, Reglero G, Casado E, Sereno M, Ramírez de Molina A. Metabolic enzyme ACSL3 is a prognostic biomarker and correlates with anticancer effectiveness of statins in non-small cell lung cancer. Mol Oncol. 2020;14:3135–3152. doi: 10.1002/1878-0261.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu J, Mei Q, Chen L, Zhou J. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother. 2020;70:619–631. doi: 10.1007/s00262-020-02735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daemen A, Cooper J, Myrta S, Wongchenko MJ, Lin E, Long JE, Foreman O, Modrusan Z, Tremayne JR, de la Cruz CC, Merchant M, Martin SE, Yan Y, Junttila MR. Transcriptional subtypes resolve tumor heterogeneity and identify vulnerabilities to MEK inhibition in lung adenocarcinoma. Clin Cancer Res. 2020;27:1162–1173. doi: 10.1158/1078-0432.CCR-20-1835. [DOI] [PubMed] [Google Scholar]
- 11.Kwon OJ, Zhang L, Jia D, Zhou Z, Li Z, Haffner M, Lee JK, True L, Morrissey C, Xin L. De novo induction of lineage plasticity from human prostate luminal epithelial cells by activated AKT1 and c-Myc. Oncogene. 2020;39:7142–7151. doi: 10.1038/s41388-020-01487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Ji W, Yu Y, Li Z, Niu X, Xia W, Lu S. Correction: FGFR1-ERK1/2-SOX2 axis promotes cell proliferation, epithelial-mesenchymal transition, and metastasis in FGFR1-amplified lung cancer. Oncogene. 2020;39:6619–6620. doi: 10.1038/s41388-020-01441-6. [DOI] [PubMed] [Google Scholar]
- 13.van Pul KM, Vuylsteke RJCLM, de Beijer MTA, van de Ven R, van den Tol MP, Stockmann HBAC, de Gruijl TD. Breast cancer-induced immune suppression in the sentinel lymph node is effectively countered by CpG-B in conjunction with inhibition of the JAK2/STAT3 pathway. J Immunother Cancer. 2020;8:e000761. doi: 10.1136/jitc-2020-000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge X, Liu W, Zhao W, Feng S, Duan A, Ji C, Shen K, Liu W, Zhou J, Jiang D, Rong Y, Gong F, Wang J, Xu Z, Li X, Fan J, Wei Y, Bai J, Cai W. Exosomal transfer of LCP1 promotes osteosarcoma cell tumorigenesis and metastasis by activating the JAK2/STAT3 signaling pathway. Mol Ther Nucleic Acids. 2020;21:900–915. doi: 10.1016/j.omtn.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y, Park J, Jo I, Lee GD, Kim J, Kwon A, Choi H, Jang W, Chae H, Han K, Eom KS, Cho BS, Lee SE, Yang J, Shin SH, Kim H, Ko YH, Park H, Jin JY, Lee S, Jekarl DW, Yahng SA, Kim M. Genetic-pathologic characterization of myeloproliferative neoplasms. Exp Mol Med. 2016;48:e247. doi: 10.1038/emm.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Yang L, Yao Y, Xu L, Xiang Y, Zhao H, Wang L, Zuo Z, Huang X, Zhao C. Flubendazole demonstrates valid antitumor effects by inhibiting STAT3 and activating autophagy. J Exp Clin Cancer Res. 2019;38:293. doi: 10.1186/s13046-019-1303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. 2020;19:145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J, Wang K, Yeh S, Sun Y, Liang L, Xiao Y, Xu W, Niu Y, Cheng L, Maity SN, Jiang R, Chang C. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat Commun. 2019;10:2571. doi: 10.1038/s41467-019-09784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Blaskovich MA, Forinash KD, Sebti SM. Withacnistin inhibits recruitment of STAT3 and STAT5 to growth factor and cytokine receptors and induces regression of breast tumours. Br J Cancer. 2014;111:894–902. doi: 10.1038/bjc.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correction: miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin Sci (Lond) 2020;134:1801–1804. doi: 10.1042/CS-20200039_COR. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Jiang Y, Zhao J, Zhang H, Fu J, Luo P, Ma Y, Zou D, Gao H, Hu J, Zhang Y, Jing Z. Dp44mT, an iron chelator, suppresses growth and induces apoptosis via RORA-mediated NDRG2-IL6/JAK2/STAT3 signaling in glioma. Cell Oncol (Dordr) 2020;43:461–475. doi: 10.1007/s13402-020-00502-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Wang F, Du C, Guo H, Ma L, Liu X, Kornmann M, Tian X, Yang Y. BRM/SMARCA2 promotes the proliferation and chemoresistance of pancreatic cancer cells by targeting JAK2/STAT3 signaling. Cancer Lett. 2017;402:213–224. doi: 10.1016/j.canlet.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Yu HA, Perez L, Chang Q, Gao SP, Kris MG, Riely GJ, Bromberg J. A Phase 1/2 trial of ruxolitinib and erlotinib in patients with EGFR-mutant lung adenocarcinomas with acquired resistance to erlotinib. J Thorac Oncol. 2017;12:102–109. doi: 10.1016/j.jtho.2016.08.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JS, Hong MH, Chun YJ, Kim HR, Cho BC. A phase Ib study of the combination of afatinib and ruxolitinib in EGFR mutant NSCLC with progression on EGFR-TKIs. Lung Cancer. 2019;134:46–51. doi: 10.1016/j.lungcan.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Dong XZ, Liu X, Liu P, Chen YB. Enhanced antitumor activity of cetuximab in combination with the jak inhibitor CYT387 against non-small-cell lung cancer with various genotypes. Mol Pharm. 2016;13:689–697. doi: 10.1021/acs.molpharmaceut.5b00927. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Yan L, Zhu W, Song X, Yang G, Wang S. MMP2/3 promote the growth and migration of laryngeal squamous cell carcinoma via PI3K/Akt-NF-κB-mediated epithelial-mesenchymal transformation. J Cell Physiol. 2019 doi: 10.1002/jcp.28242. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Lin S, Kang Y, Xiang Y, Xu L, Li J, Dai X, Liang G, Huang X, Zhao C. Rhein sensitizes human pancreatic cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. J Exp Clin Cancer Res. 2019;38:31. doi: 10.1186/s13046-018-1015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fogg KC, Olson WR, Miller JN, Khan A, Renner C, Hale I, Weisman PS, Kreeger PK. Alternatively activated macrophage-derived secretome stimulates ovarian cancer spheroid spreading through a JAK2/STAT3 pathway. Cancer Lett. 2019;458:92–101. doi: 10.1016/j.canlet.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukthavaram R, Ouyang X, Saklecha R, Jiang P, Nomura N, Pingle SC, Guo F, Makale M, Kesari S. Effect of the JAK2/STAT3 inhibitor SAR317461 on human glioblastoma tumorspheres. J Transl Med. 2015;13:269. doi: 10.1186/s12967-015-0627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Hu K, Sun H, Zhou J, Song D, Xu Z, Gao L, Lu Y, Cheng Y, Feng Q, Zhang H, Wang Y, Hu L, Lu K, Wu X, Li B, Zhu W, Shi J. A novel phosphoramide compound, DCZ0847, displays in vitro and in vivo anti-myeloma activity, alone or in combination with bortezomib. Cancer Lett. 2020;478:45–55. doi: 10.1016/j.canlet.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Jayavelu AK, Schnöder TM, Perner F, Herzog C, Meiler A, Krishnamoorthy G, Huber N, Mohr J, Edelmann-Stephan B, Austin R, Brandt S, Palandri F, Schröder N, Isermann B, Edlich F, Sinha AU, Ungelenk M, Hübner CA, Zeiser R, Rahmig S, Waskow C, Coldham I, Ernst T, Hochhaus A, Jilg S, Jost PJ, Mullally A, Bullinger L, Mertens PR, Lane SW, Mann M, Heidel FH. Splicing factor YBX1 mediates persistence of JAK2-mutated neoplasms. Nature. 2020;588:157–163. doi: 10.1038/s41586-020-2968-3. [DOI] [PubMed] [Google Scholar]
- 32.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 33.Bose P, Verstovsek S. JAK2 inhibitors for myeloproliferative neoplasms: what is next? Blood. 2017;130:115–125. doi: 10.1182/blood-2017-04-742288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talpaz M, Kiladjian JJ. Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia. 2021;35:1–17. doi: 10.1038/s41375-020-0954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verstovsek S, Talpaz M, Ritchie E, Wadleigh M, Odenike O, Jamieson C, Stein B, Uno T, Mesa RA. A phase I, open-label, dose-escalation, multicenter study of the JAK2 inhibitor NS-018 in patients with myelofibrosis. Leukemia. 2017;31:393–402. doi: 10.1038/leu.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goh KC, Novotny-Diermayr V, Hart S, Ong LC, Loh YK, Cheong A, Tan YC, Hu C, Jayaraman R, William AD, Sun ET, Dymock BW, Ong KH, Ethirajulu K, Burrows F, Wood JM. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia. 2012;26:236–243. doi: 10.1038/leu.2011.218. [DOI] [PubMed] [Google Scholar]
- 37.Ma L, Clayton JR, Walgren RA, Zhao B, Evans RJ, Smith MC, Heinz-Taheny KM, Kreklau EL, Bloem L, Pitou C, Shen W, Strelow JM, Halstead C, Rempala ME, Parthasarathy S, Gillig JR, Heinz LJ, Pei H, Wang Y, Stancato LF, Dowless MS, Iversen PW, Burkholder TP. Discovery and characterization of LY2784544, a small-molecule tyrosine kinase inhibitor of JAK2V617F. Blood Cancer J. 2013;3:e109. doi: 10.1038/bcj.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yam CH, Fung TK, Poon RY. Cyclin A in cell cycle control and cancer. Cell Mol Life Sci. 2002;59:1317–1326. doi: 10.1007/s00018-002-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, Bertrand MJ. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 40.Conlon TM, John-Schuster G, Heide D, Pfister D, Lehmann M, Hu Y, Ertüz Z, Lopez MA, Ansari M, Strunz M, Mayr C, Ciminieri C, Costa R, Kohlhepp MS, Guillot A, Günes G, Jeridi A, Funk MC, Beroshvili G, Prokosch S, Hetzer J, Verleden SE, Alsafadi H, Lindner M, Burgstaller G, Becker L, Irmler M, Dudek M, Janzen J, Goffin E, Gosens R, Knolle P, Pirotte B, Stoeger T, Beckers J, Wagner D, Singh I, Theis FJ, de Angelis MH, O’Connor T, Tacke F, Boutros M, Dejardin E, Eickelberg O, Schiller HB, Königshoff M, Heikenwalder M, Yildirim A. Inhibition of LTβR signalling activates WNT-induced regeneration in lung. Nature. 2020;588:151–156. doi: 10.1038/s41586-020-2882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.