Abstract
Metastasis is the primary cause of death in lung cancer, one of the most prevalent and deadly neoplasms. The tumour-associated macrophages (TAMs) are crucial mediators to induce epithelial-mesenchymal transition (EMT) and promote lung metastasis via release of the cytokines. Matrine, a naturally occurring alkaloid, has been found with a variety of pharmacological effects, such as anti-cancer. In this study, an in vitro co-culture cell systems and a Lewis-bearing mouse model were employed to assay the potential effects of matrine on macrophages polarization, and its regulatory effects on EMT of Lewis lung cancer cells (LLCs). Our results clearly demonstrated that matrine inhibited M2-like RAW264.7 polarization, reducing the production of anti-inflammatory cytokines (IL-4, IL-10, and Arg-1), and M2 surface markers (CD206) were induced by LLCs via mTOR/PI3k/Akt signaling pathway, while it had no significant effect on M1 macrophages polarization. In vitro assays suggested that matrine partially blocked the metastasis of LLCs, and inhibited EMT induced by M2-like macrophages, which was evidenced by up-regulating the expression of E-cadherin and down-regulating the expression of N-cadherin, vimentin, and Snail. In vivo studies revealed that matrine decreased the ratio of CD206+/F4/80+, promoted the expression of CD4+ and CD8+ T cells, and inhibited the expression of Th2 in tumor and spleen tissues. Cell co-culture experiments revealed that Matrine promoted T-cell proliferation, which was impaired by tumour-derived CD11b+ myeloid cells. Collectively, our findings suggest that suppression of M2-like macrophages polarization of TAMs is a potential mechanism underlying the anti-metastasis effects of matrine in lung cancer.
Keywords: Macrophage differentiation, mTOR/PI3k/Akt pathway, matrine, EMT, metastasis
Introduction
Lung caner is the leading cause of cancer-relatede mortality in worldwide, the 5-year survival rate of which was less than 15%. Around 1.8 million lung cancer patients are diagnosed each year, and 80% of them suffer from an advanced stage disease [1]. Approximately 80% of total lung cancer cases fall into the category of non-small cell lung cancer (NSCLC). The prognosis of NSCLC patients remains poor despite several advances in the early detection and systematic therapies [2]. Metastasis is the secondary leading cause of cancer-related mortality, only after malignant tumours. Epithelial-mesenchymal transition (EMT) is a vital process of tumour invasion and metastasis. This change enhances the cell migration, metastasis of cancer cells, as well as establishment of the distant secondary tumors [3]. It is therefore crucial to gain deeper insights into the underlying mechanisms potentially involved in the progression and development of NSCLC, so as to find novel and effective strategies for NSCLC treatment.
Tumor-associated macrophages (TAMs) are a key class of inflammatory cells within the tumor stroma, and increasing evidence has indicated that TAMs are core regulators of the metastatic phenotype of cancer cells [4]. There tend to be two distinct subpopulations of activated macrophages coexisting in the tumour microenvironment, classically activated macrophages (M1-like macrophages), and the alternatively active macrophages (M2-like macrophages), which also display the Th1/Th2 differentiation paradigm [5]. M1-related cytokines release a high degree of proinflammatory cytokines, such as IL-6, IL-1β, TNF-α, and nitrogen species [6]. By comparison, IL-13 stimulates macrophages to differentiate into M2 phenotype, exerting anti-inflammatory and pro-tumorigenic. They are involved in stromal development, immunosuppression and tumour growth [7]. This pro-tumor function of TAMs is again confirmed by clinical trials, showing a link between poor patient prognosis and high macrophages numbers in tumor tissue [8]. While these two subpopulations have been well characterized [9-11], the roles of M1 and M2-polarized macrophages in the development and metastasis of lung cancer have not been clearly elucidated, or contradictory. TAMs are implicated in tumour development, and are associated with poor prognosis in several cancers, while EMT is one of the most important mechanisms involved in the tumour invasion and metastasis. M2 TAMs play a center role in the cancer metastasis, reinforcing the cell metastasis. Moreover, TMAs have been reported to be correlated with EMT. A number of substances secreted by TMAs stimulate the proliferation and metastasis of tumour cells [12].
The traditional medicinal herbs can exert significant anti-tumor effect through targeting the tumor microenvironment. Matrine (C15H24N2O, Figure S1), a naturally occurring alkaloid distributed in the Chinese herb Sophora flavescens Ait, has long been used for treating inflammation diseases and tumors, including lung cancer, breast cancer and so on [13]. It has been reported that matrine induces apoptosis by inhibiting PI3K.AKT pathway in human acute myeloid leukemia cells. Previous studies showed that matrine treatment exerted anti-tumor and anti-metastasis activities. Several proteins have been indicated to be involved in these activities, including protein kinase B/rapamycin (AKT/mTOR), epidermal growth factor receptor/matrix metalloproteinase-9 (EGFR/MMP9), extracellular signal-regulated kinase (Ras-ERK), T-cell-specific factor (TCF)/Smad [14,15]. In addition, clinical studies have shown that matrine significantly enhanced the immune function of patients and reduced the size of tumor [16,17]. Matrine displayed good saftey profiles and oral administration of high-dose matrine has no apparent side-effects [18]. While matrine has valid influence on the development and progression of different cancer types, whether and how matrine exerts anti-tumor effect for EMT induced by macrophages polarization remains poorly understood.
According to the gaps mentioned above, herein, IL-13 was used to co-stimulate macrophages polarizing to M2 phenotype. The M2 polarized macrophages were co-cultured Lewis lung carcinoma (LLC) cells to develop a M2-likeTAMs macroenvironment. The effects of matrine on the metastatic behavior of LLCs in the co-culture system were investigated. In addition, the in vivo anti-cancer effects of matrine were investigated in a subcutaneous and intravenous model. Our study provided a novel mechanism of disrupting the M2 polarization-induced signalling to reverse EMT process, which can be utilized to develop therapeutic applications of matrine as an anti-metastatic therapy for NSCLC.
Materials and methods
Regents and cell lines
H460, A549 human lung cancer cell lines, Lewis mouse lung cancer cell line and RAW264.7 the mouse macrophages, were purchased from by the Cell Bank of the China Academy of Sciences (Shanghai, China) and were maintained as suggested by the supplier. The macrophages were polarized into M2 by 20 ng/mL IL-13 for 24 h. M2 macrophages were stimulated with 20 μmol/L matrine for 24 h. The stock solution of Matrine (MCE) was diluted with dimethyl sulfoxide (DMSO) and stored in -20°C in the dark.
Bone marrow-derived macrophages (BMDMs) isolation and differentiation
Bone marrow cells from C57BL/6 male mice were cultured in DMEM containing 10% FBS and 50 ng/mL macrophage colony-stimulating factor (M-CSF) for three days to acquire BMDMs.
CCK-8 assays
RAW264.7, Lewis, H460 and A549 cells were used to measure cell proliferation. Cell Viability Assay was determined using CCK8 (Dojindo, Kumamato, Japan) according to the manufacture’s instruction.
Flow cytometry analysis
RAW264.7 cells and BMDMs were stained with the following fluorochrome-conjugated antibodies: anti-mouse FITC-CD86 and human/mouse PC7-CD206. Spleens were filtered through a 40 μm of nylon mesh strainer, and then were lysed for 5 min by using 1× Red Blood Cell Lysis Buffer. Lewis tumor tissues and lung tissues were digested into single cell suspension using mouse tumor dissociation kit (Miltenyi). Cells were stimulated with Cell Activation Cocktail (with Brefeldin A) (BioLegend., CA, USA) to measure the intracellular cytokine expression. Intracellular cytokine staining was employed using the Fixation/Permeabilization Solution Kit (BioLegend., CA, USA). Stained cells were analyzed by FACS. Data analysis was performed using FlowJo software.
RT-PCR analysis
Total RNA is cell extracted using (Sigma-Aldrich), and cDNA was reversed using a PrimeScript II 1st Strand cDNA Synthesis Kit (Takara, Tokyo, Japan). mRNA expression was conducted using SYBR Green PC Master Mix (Applied Biosystems, USA). qRT-PCR was then performed with SYBR Premix Ex Taqll (Takaro, Japan). The results were expressed as RQ=2-ΔΔCt. The primers used in the experiment are shown in Table 1.
Table 1.
Primer sequences for real-time polymerase chain reaction
| Primer | Sequence (5’-3’) | |
|---|---|---|
| IL-6 | Forward | TGAACAACGATGATGCACTT |
| Reverse | CGTAGAGAACAACATAAGTC | |
| TNF-α | Forward | AATGGCCTCCCTCTCATCAGTT |
| Reverse | CGAATTTTGAGAAGATGATCTGAGTGT | |
| IL-12 | Forward | AGAGGTGGACTGGACTCCCGA |
| Reverse | TTTGGTGCTTCACACTTCAG | |
| IL-10 | Forward | TGCACTACCAAAGCCACAAGGCAG |
| Reverse | AGTAAGAGCAGGCAGCATAGCAGT | |
| IL-4 | Forward | TACCAGGAGCCATATCCACGGATG |
| Reverse | TGTGGTGTTCTTCGTTGCTGTGAG | |
| Arg-1 | Forward | CCACAGTCTGGCAGTTGGAAG |
| Reverse | GGTTGTCAGGGGAGTGTTGATG | |
| CD206 | Forward | CTCGTGGATCTCCGTGACAC |
| Reverse | GCAAATGGAGCCGTCTGTGC | |
| β-actin | Forward | TGGAATCCTGTGGCATCCATGAAAC |
| Reverse | TAAAACGCAGCTCAGTAACAGTCCG |
Enzyme-linked immunosorbent assay (ELISA)
The release of cytokines in RAW264.7 and BMDMs were measured by enzyme-linked immunosorbent assay (ELISA) according to vendor’s instructions.
Griess test
After the incubation, NO assay kit (Sigma-Aldrich) measured Nitric Oxide (NO) of cell supernatants, and incubated for 10 min according to the manufacturer’s protocol.
Wound healing assay
LLCs were grown to 90-100% confluence in a 12-well plate and a wound was scratched by a 10 μl of pipette tip. Washed with PBS, the scratched cells were then incubated in M0-CM or M2-CM with or without matrine for 24 h, migrating cells at the wound front were photographed.
Migration and invasion assay
Cell migration and invasion assay was performed using a Trans-well cell culture chamber (Corning, NY, USA). Cell invasion assay needs that insert membranes were coated with diluted Matrigel (Corning, NY, USA). LLC cells were seeded into the upper chamber with 200 μl culture medium, and a volume of 0.6 mL CM was placed into the lower chamber for 24 h. Cells on the opposite side were fixed with 4% paraformaldehyde and stained with crystal violet.
Cell adhesion assay
Cell adhesion was analysed using a Vybrant Cell Adhesion Assay kit (Invitrogen Corp) and gelatin (2% solution type B)-coated plates. The results were quantified as the ratio of fluorescence after wash/fluorescence before wash.
Western blot analysis
RAW264.7, Lewis, H460 or A549 cells were lysed in RIPA buffer (Thermo Scientific). The primary against pAKT (Cat.4784, Lot:12) , AKT (Cat.9279, Lot:16), pPI3K (Cat.17366, Lot:5), PI3K (Cat.4249, Lot:13), pMTOR (Cat.5536, Lot:7), MTOR (Cat.2983, Lot:3), Vim (Cat.5741, Lot:4), E-Cad (Cat.14472, Lot:6), N-Cad (Cat.13116, Lot:8) and Snail (Cat.3879, Lot:11) were purchased from Cell Signaling Technology. The cell lysates were separated on 10% or 12.5% SDS-PAGE gels. The antibodies used were against GAPDH (Cell Signaling Technology).
Cell apoptosis assay
Cell apoptosis was determined using an Annexin V-FITC/PI Apoptosis Detection Kit (BioLegend., CA, USA) regarding to manufacturer’s instructions. Samples were measured using a CytExpert flow cytometer (Beckman Coulter, USA).
Animal experiments
Male C57BL/6 (6-weeks old) mice were obtained from Shanghai University of Traditional Chinese Medicine and approved (Approval No. P2SHUTCM201120008). Mice were subcutaneously injected with 8×105 LLC cells in the right flank. In the LLCs intravenous model, C57BL/6 mice were injected intravenously with 8×105 LLCs in 0.2 mL of PBS. In both models, after one day the mice were given an intragastric (i.g.) administration of 0.2 mL of Martine (40 and 80 mg/kg) for 16 consecutive days. On day 16, Mice were killed , and tumours and lung tissue were cut out and weighed.
T-lymphocyte proliferation assay
T cells were isolated with a MACS CD8+ isolation kit (Biolegend, CA, USA) from the spleen cells of C57BL/6 mice. T cells (1×105) were co-stimulated with anti-CD3 (BioLegend) and anti-CD28 (BioLegend) and stained with 2 μmol/L CFSE (BioLegend) in 96-well round-bottom plates. Afterward, T cells were co-cultured with CD11b+ myeloid cells isolated from tumors (Miltenyi Biotec) in a ratio of 2:1 CD8+ T cells to CD11b+ cells. After 72 h, T-cell proliferation was detected using CFSE dilution by FACS analysis (BioLegend) and analyzed by FlowJo software.
Immunohistochemistry (IHC) staining
Samples were cut into 10 μm slices with a rotary microtome. Slides were incubated with anti- E-cadherin antibody (Cat.14472, Lot:6) and anti-Vimentin antibody (Cat.5741, Lot:4).
Immunofluorescent staining
Tumour tissues and lung tissues were fixed by 4% paraformaldehyde for 10 min. Then, all tissues made the tissue into paraffin were cut at a thickness of 4 μm. The sections were the incubated with anti-F4/80 (1:300) and anti-CD206 (1:300) antibodies, followed by secondary antibody.
Statistical analysis
Results were analysed as the arithmetical Mean ± Standard Deviation (Mean ± SD) were analyzed by one-way analysis of variance (ANOVA) followed. The difference between groups was measured using Unpaired Student’s t-test (two-tailed) and one-way ANOVA test. Values with P<0.05 (*) was considered statistically significant.
Results
Matrine inhibited polarization of TAMs to M2 phenotype and inactivated M1 polarization in RAW264.7
CD206 is a crucial marker for M2 macrophages. RAW264.7 was co-stimulated with IL13 to polarize macrophages switching to M2 phenotype, and then treated with or without matrine. As shown in Figure S3A, matrine (20 μmol/L) could significantly down-regulate the expression of CD206 compared with M2 (P<0.05). By contrast, matrine had no toxic effect on macrophages at the same dosage (20 μmol/L), as depicted in Figure S3B. In this case, 20 μmol/L matrine was used for further investigation. As shown in Figure 1A, LLCs were treated with or without matrine, and then co-cultured with RAW264.7 to investigate the how matrine affected the distribution of M1/M2 polarization in TAMs. CD86, a co-stimulatory molecular, is a differentiated marker of M1-like macrophages polarization, while CD206 is a crucial marker for M2 macrophages. They were both detected through flow cytometry analysis. According to the Figure 1B, 1C, the expression of CD86 in RAW264.7 had no significant difference from LLCS-treated RAW264.7 (P>0.05), whilst the expression of CD206 markedly decreased (P<0.05). These results suggested that matrine may inhibit TAMs polarizing to M2 phenotype induced by LLCS cells, and have no effect on M1 macrophages polarization.
Figure 1.
Matrine inhibited polarization of TAMs to M2 phenotype and inactivated M1 polarization in RAW264.7. A. LLCs were treated with or without matrine, and then co-cultured with RAW264.7; B. The percentage of CD86 in co-cultured RAW264.7 macrophages treated with or without matrine; C. The percentage of CD206 in co-cultured RAW264.7 macrophages treated with or without matrine; D. The expression of IL-6, NO, TNF-α, and IL-12 in co-cultured RAW264.7 macrophages treated with or without matrine measure by ELISA; E. The expression of IL-10 and IL-4 measured by ELISA, and the relative mRNA expression of Arg-1 and CD206 measure by RT-PCR assay in co-cultured RAW264.7 macrophages treated with or without matrine (n=5), P<0.05 (*).
To further determine whether the suppression of macrophages M2 polarization induced by matrine was functional, the expression of the classic cytokines of M1-like (IL-6, TNF-α, and IL-12) was measured by ELISA, and the NO production was quantified by Griess assay. As shown in Figure 1D, LLCS significantly decreased the release of IL-6, TNF-α, and IL-12. No production n in LLCs-treated RAW264.7 was reduced to 1.5-time than in control group (P<0.05). However, the expression of these M1-related cytokines in matrine-treated RAW264.7 had no significant difference from the LLCs-treated RAW264.7 (P>0.05). Furthermore, the expression of M2-like (IL-10 and IL-4) macrophages, and the mRNA expression of M2-related markers, including Arg-1 and CD206, was detected by RT-PCR assay. By comparison with the expression of IL-10, IL-4, Arg-1, and CD206 marker in LLCs-treated RAW264.7 macrophages were upregulated considerably (P<0.05). Upon treating with matrine, the expression of M2-related cytokines in RAW264.7 macrophages was dramatically decreased (P<0.05) (Figure 1E). These observations were consistent with Figure 1B, illustrating that matrine could inhibit polarization of TAMs to M2 phenotype and inactivate M1 polarization in RAW264.7, which was reflected by the unchanged release of pro-inflammatory cytokines, and decreased release of anti-inflammatory cytokines induced by LLCs.
Matrine suppressed M2-like macrophages polarization via mTOR/PI3k/Akt signaling pathway
To further determine the underlying mechanisms involved in the suppression of macrophages M2 polarization by matrine, the expression of M2-like surface marker, CD206, in macrophages, was measured by flow cytometry analysis. BMDMs cells were differentiated into native inactivated macrophages by treatment with M-CFS. A sharp rise of Macrophage marker CD11b verified the differentiation of macrophage cells (Figure S2). Figure 2A, 2E shows that IL-13 increased the expression of CD206 in RAW264.7 and BMDMs significantly (P<0.05). After treating with matrine, CD206 expression decreased considerably in RAW264.7 and BMDMs (P<0.05). This result is in agreement with the mRNA expression of CD206 measured by RT-PCR (Figure 2C). To further determine whether IL-13 could stimulate macrophages polarizing to M2 phenotype, and the effects of matrine on macrophage M2 polarization, the expression of M2-related cytokines, including IL-4, IL-10, and Arg-1 was detected. According to Figure 2B, co-stimulation of IL-13 increased the expression of IL-4, IL-10, and the mRNA expression of Arg-1 markedly increased (P<0.05). However, matrine reversed this increased expression. Taken together, IL-13 stimulated macrophages polarizing to M2 phenotype. Matrine could inhibit macrophages M2 polarization, leading to the decreased level of anti-inflammatory cytokines and M2 surface marker.
Figure 2.
Matrine suppressed M2-like macrophages polarization via mTOR/PI3k/Akt signaling pathway. A. The percentage of CD206 in IL-13 co-stimulated RAW264.7 M2-like macrophages treated with or without matrine; B. The expression of IL-4 and IL-10 measured by ELISA, and the relative mRNA expression of Arg-1 and CD206 measure by RT-PCR assay in co-stimulated RAW264.7 M2-like macrophages treated with or without matrine; C. The relative protein expression of p-PI3K, p-AKT, and p-mTOR (relative to PI3K, AKT, and mTOR, respectively) in co-stimulated RAW264.7 M2-like macrophages treated with or without matrine determine by Western Blotting; D. The percentage of CD206+ F4/80+ in IL-13 co-stimulated BMDMs M2-like macrophages treated with or without matrine; E. BMDMs were treated with IL-13 and/or matrine for 24 h. Elisa and RT-PCR was carried out to analyze expression of M2 marker. (n=5), P<0.05 (*).
It is well-known that PI3K regulates macrophage polarizationPlays a crucial part affectes the downstream signalling molecules, Akt and mTOR, which regulates several biological processes, such as cell cycle, cell growth, and protein synthesis. To further determine whether PI3K signalling pathway involved in the suppression of M2 macrophages Plays a crucial part, herein, the expression of PI3K, Akt, and mTOR involved in PI3K signalling pathway was detected by western blotting. Figure 2C depicts that matrine significantly inhibited PI3K, Akt and mTOR phosphorylation levels in IL-13-stimulated RAW264.7 (P<0.05). Collectively, matrine inhibited M2 macrophages polarization induced by IL-13 through down-regulation of PI3K/Akt/mTOR signalling pathway in RAW264.7.
Matrine inhibited EMT of LLC induced by M2 polarization of TAMs
EMT is an important biological mechanism in the progression to metastasis of primary tumors [19]. EMT caused by inflammation has already been demonstrated. In addition to EMT, cancer cells display increased mobility and capacity of invasion. Therefore, it was speculated that M2-polarized TAMs would induce EMT in lung cancer. Herein, LLC was co-cultured with IL-13-stimulated RAW264.7, and then the capacity of cell migration and invasion, the Vimentin, E-cadherin, N-cadherin, and snail expression were detected by western blotting to investigate the effects of M2-polarized TAMs on capacity of migration and invasion of LLCs. The capacity of LLCs migration and invasion was detected by Transwell assay. The results suggest that M2 polarized TAMs promoted the cell migration (Figure 3A) and invasion abilities (Figure 3B) of LLCs, and matrine could attenuate these abilities of LLCs infiltrated with M2 polarized TAMs. The 24 h wounding healing assay was also performed to further confirm the migration ability of LLCs. Matrine gradually decreased the healing rate of LLCs infiltrated with M2 polarized TAMs (P<0.05) (Figure 3C). To explore the role of matrine in adhesion of LLCs, the qualitative and quantitative adhesion assays were conducted under static conditions. Findings showed that matrine significantly reduced the adhesion of LLCs (P<0.05) (Figure 3D) compared to the control group. This result suggested that matrine weakened adhesion ability of LLCs. Furthermore, the result of western blotting shows that matrine remarkably increased the expression of E-cadherin, and decreased the expression of vimentin, N-cadherin, and snail, alleviating the EMT progress induced by TAMs (Figure 3E). These results revealed that matrine could inhibit the LLCs migration and invasion capacity induced by TAMs through reversing EMT process.
Figure 3.

Matrine inhibited EMT of LLCs induced by M2 polarization of TAMs. A. The cell migration ability in co-stimulated RAW264.7 M2-like macrophages treated with or without matrine measured by transwell assay; B. The cell invasion ability in co-stimulated RAW264.7 M2-like macrophages treated with or without matrine measured by transwell assay; C. The cell migration ability determined by 24 h cell wounding healing assay in co-stimulated RAW264.7 M2-like macrophages treated with or without matrine; D. The cell adhesion relative calcenin fluorescence determined by a Vybrant Cell Adhesion Assay kit in co-stimulated RAW264.7 M2-like macrophages treated with or without matrine; E. The relative protein expression of Vim, E-card, N-card, and snail (relative to GAPDH) in co-stimulated RAW264.7 M2-like macrophages treated with or without matrine determine by Western Blotting (n = 5), p<0.05 (*).
Matrine targeted TAMs to inhibit metastasis of lung cancer cells
The data mentioned above clearly illustrated that matrine inhibited the cell metastasis of LLCs by reversing EMT process. In order to further confirm whether this anti-metastasis property of matrine was TAMs target, three types of lung cancer cells, including A549, H460, and LLCs, were all employed to evaluate the cell apoptosis and migration induced by matrine. As shown in Figure 4A, 4B, there was no significant difference between control groups and matrine treated groups regarding their apoptosis and migration (P>0.05). Furthermore, the protein expression of vimentin and E-cadherin in these three lung cancer cells was measured by western blotting. According to Figure 4C, there was no significant difference in the expression of vimentin and E-cadherin between control and matrine treated groups (P>0.05). Collectively, it was suggested that the anti-cancer effect of matrine might be targeted TAMs.
Figure 4.
Matrine targeted TAMs to inhibit tumour growth and metastasis of lung cancer. A. The cell apoptosis rate of A549, H460, and LLCs treated with or without matrine determined by flow cytometry assay; B. The cell migration ability of A549, H460, and LLCs treated with or without matrine determined by transwell assay; C. The relative protein expression of Vim and E-card (relative to GAPDH) of A549, H460, and LLCs treated with or without matrine determine by Western Blotting (n=5), P<0.05 (*).
Matrine inhibited the tumor growing and metastasis
To identify whether matrine had the same therapeutic functions in lung cancer in vivo, LLCs subcutaneous and intravenous model were employed. 40 and 80 mg/kg of matrine were selected to conduct the following in vivo study. Administration of 80 mg/kg matrine in Lewis-bearing mice significantly decreased the tumour volume (Figure 5A), and the tumour weight (Figure 5B) (P<0.05), but the mouse body weight didn’t change (Figure 5C). Additionally, molecular changes in tumour tissues associated with the reversal of EMT, including the expression of Vimentin and E-cadherin were determined by IHC staining. The results shown that after treating with matrine, the expression of vimentin was down-regulated (Figure 5D), while the expression of E-cadherin (Figure 5E) was up-regulated after treating with 40 and 80 mg/kg matrine when compared to control groups. Further wards, the metastatic nodes on the surface of lungs in mice were observed. As shown in Figure 5F, 5H, after administration of 40 and 80 mg/kg matrine, the number of metastatic nodes was significantly reduced compared to the control group (P<0.05). These results suggested that the progression of cell metastasis in matrine-treated groups was strongly attenuated.
Figure 5.

Matrine inhibited the tumor growing and metastasis in vivo. A. Administration of 40 and 80 mg/kg matrine decreased the tumour volume (mm3); B. Administration of 40 and 80 mg/kg matrine decreased the tumour weigh (g); C. Administration of 40 and 80 mg/kg matrine increased the weight (mg) of Lewis-bearing mice model; D. The expression of Vimentin measured by IHC staining in tumour tissues from Lewis-bearing mice model; The Vimentin-positive cells are stained brown. E. The expression of E-cadherin measured by IHC staining in tumour tissues from Lewis-bearing mice model; The E-cadherin-positive cells are stained brown. F, G. The number of metastatic nodes on the surface of lung from Lewis-bearing mice model; H. The images showed representative H&E stained lung sections in the two groups. Bars: 5 μm. (n=5), P<0.05 (*).
Matrine boosted T-cell-mediated anti-tumor immunity via inhibition of macrophages polarization
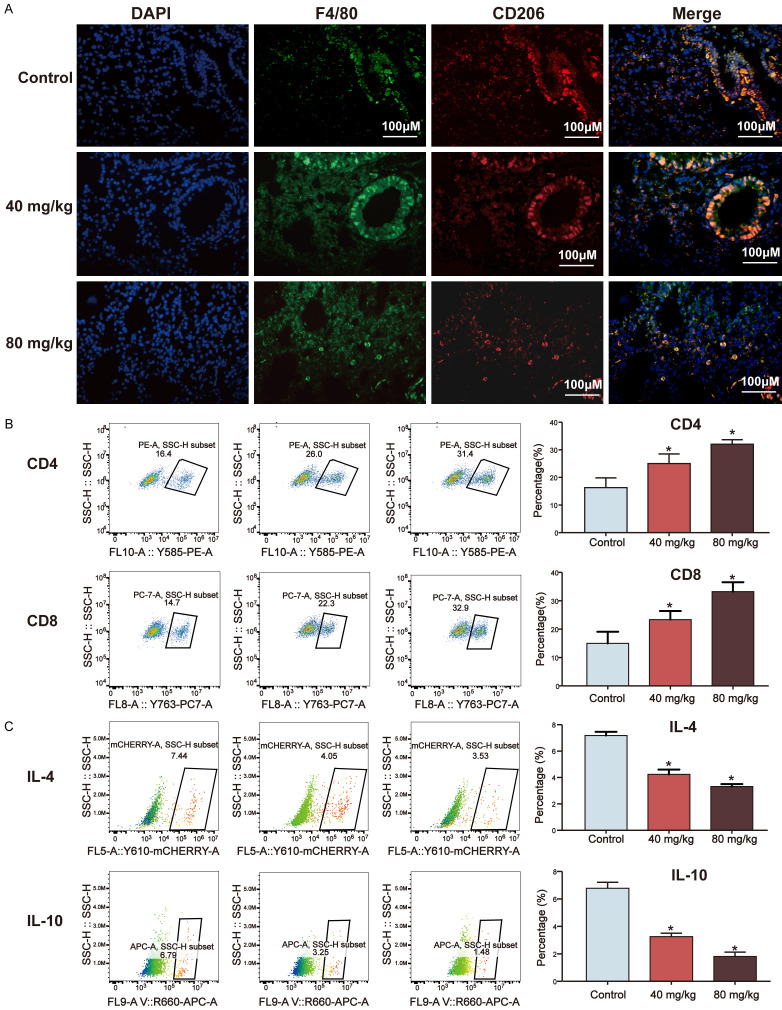
To further validate the in vivo results, double IF staining with the macrophage marker F4/80, a marker for the M0 phenotype, and either CD206, a marker for the M2 phenotype, in the tumor tissues and lung tissues from LLCs subcutaneous and intravenous model were performed to identify the distribution of M2 polarization. Whereas, in 40 mg/kg Matrine treated group, the percentage of F4/80+ CD206+ cells were decreased in the spleen from LLCs subcutaneous model (Figure S4A). As shown in Figures 6A, 7A, administration of matrine significantly decreased the expression of F4/80, and CD206, when compared to control groups. The staining results suggested that matrine could inhibit M2 macrophages polarization in tumor tissue. Macrophages also constitute essential regulators of initiating anti-tumor immune responses. M2 macrophage exhibits a Th2-like phenotype. CD4+ Th2 cytokines, such as IL-4, IL-10, have shown to promote M2 macrophage polarization [20]. To provide a better understanding of the effects of matrine on suppression of macrophages polarization into the M2 phenotype in long-term anti-tumor immune memory, the percentages of tumour tissues and splenic CD4+ and CD8+ T cells, and Th2 cytokines (IL-4 and IL-10) were analysed by flow cytometry. The percentage of splenic CD4+ and CD8+ T cells of 80 mg/kg treated matrine group was considerably higher than control group (P<0.05), whilst 40 mg/kg treated matrine group was not statistically different (P>0.05) in LLCs subcutaneous model (Figure S4B). After administration with 40 and 80 mg/kg matrine in both LLCs subcutaneous and intravenous model, the percentage of CD4+ and CD8+ T cells in tumour tissues increased significantly compared to control group (Figures 6B, 7B, P<0.05), while the expression of IL-10 and IL-4 in CD4+ T cells were less than control groups (Figures 6C and 6D, 7C, P<0.05). The data showed that the expression of IL-4 and IL-10 were no significant difference between control group and 40 mg/kg matrine treated group in the spleen, while 80 mg/kg matrine treated group were decreased (Figure S4C and S4E, P<0.05).
Figure 6.
Matrine boosted T-cell-mediated anti-tumor immunity via inhibition of macrophages polarization in LLCs subcutaneous mice model. (A) Administration of 40 and 80 mg/kg matrine decreased the expression of CD206 of the tumour tissues from LLCs subcutaneous mice model;, determined by double IF staining; (B) Administration of 40 and 80 mg/kg matrine increased the percentage of CD4+ and CD8+ T cells in primary tumour tissues from LLCs subcutaneous mice model; (C) Administration of 40 and 80 mg/kg matrine decreased the expression of IL-10 in primary tumour tissues from LLCs subcutaneous mice model determined by flow cytometry assay; (D) Administration of 40 and 80 mg/kg matrine decreased the expression of IL-4 in primary tumour tissues from LLCs subcutaneous mice model determined by flow cytometry assay (E) In vitro T-cell suppression activity of Lewis-tumor-infiltrating CD11b+ cells collected at 2 weeks post-treatment with control, 40 mg/kg and 80 mg/kg matrine. CD8+ T cells were labeled with CFSE and then stimulated with CD3/CD28 antibodies for 3 days in the absence or presence of tumor-infiltrating CD11b+ myeloid cells from control, 40 mg/kg and 80 mg/kg matrine-treated Lewis tumors. Representative flow cytometry histograms and percentages of proliferating CD8+ T cells when plated in a ratio of 2:1 CD8+ T cells to CD11b+ cells (n=3), P<0.05 (*).
Figure 7.
Matrine boosted T-cell-mediated anti-tumor immunity via inhibition of macrophages polarization in LLCs intravenous mice model. A. Administration of 40 and 80 mg/kg matrine decreased the expression of CD206 of the tumour tissues from LLCs intravenous mice model, determined by double IF staining; B. Administration of 40 and 80 mg/kg matrine increased the percentage of CD4+ and CD8+ T cells in primary tumour tissues from LLCs intravenous mice model; C. Administration of 40 and 80 mg/kg matrine decreased the expression of IL-4 and IL-10 in primary tumour tissues from LLCs subcutaneous mice model determined by flow cytometry assay (n=3), P<0.05 (*).
Subsequently, we evaluated the direct effect of macrophage derived from control, 40 or 80 mg/kg matrine-treated LLCs on CD8+ T cell proliferation in vitro. CD8+ T cells were isolated from normal C57BL/6 mice and were stained with CFSE. CD8+ T cells were stimulated with CD3/CD28 antibodies for 72 h. At the same time, T cell were co-cultured with tumor-infiltrating CD11b+ cells from control, 40 or 80 mg/kg matrine-treated Lewis tumors. Notably, CD11b+ cells from the 80 mg/kg matrine-treated group suppressed proliferation of CD8+ T cells to a lesser extent compared with those from the control vehicle-treated group (Figure 6E). All of these findings indicate that matrine suppressed M2 TAMs polarization and, probably contributing to the infiltration CD4+ and CD8+ T cells and the inhibition of the differentiation of CD4+ into Th2 cells.
Discussion
NSCLC is the dominant type of of lung cancers. Strategies for NSCLC prevention and treatment are limited due to high recurrence rates and metastases. It is documented that M2 macrophages such as TAMs, encourage several types of tumor development [21]. TAMs are the most abundant immune cells in tumor microenvironment which contribute to tumor malignancy [22]. Specifically, TAMs contribute greatly to aggressive cancer progression by cytokines release. Upon infiltrating through the tumor, macrophages preferentially differentiate into M2-like TAMs and promote tumor growth by anti-tumor immunity elimination, and promote the release of Th2 anti-inflammatory cytokines, such as IL-4 and IL-10, tumor invasion, as well as metastasis following the EMT process [12]. EMT processes, therefore, involve immune cells, such as the inflammatory microenvironments. The interplay between cancer cells and different tumor microenvironment components during carcinogenesis forms the physiology and phenotype of TAMs that can encourage or inhibit production of tumors. IL-13 have been shown to be strong inducers of M2-like macrophage polarization, and these macrophages are also known as M2a subset [23]. Herein, IL-13 were employed to co-stimulate RAW264.7 and BMDMs polarizing to M2 phenotype, and then LLCs were co-cultured with IL-13-stimualted RAW264.7 to mimic TAMs. The findings showed that lung cancer cells co-cultivated with M2 polarized RAW264.7, which had been stimulated with IL-13 retained macrophages as TAM-like phenotypes, forming a positive feedback loop, which in turn had polarized to M2-like TAMs, and induced EMTs in lung cancer cells. Matrine inhibited skewing of M2-like TAMs, but not M1 macrophages, and lowered the release of anti-inflammatory cytokine, such as IL-10, IL-4, Arg-1, and M2 surface marker CD206.
Local progression and metastasis of NSCLC has been associated with various mechanisms, but in particular by EMT. Snail is a transcription factor that is capable of stimulating the occurrence of EMT, which is substantially upregulated in many types of tumors and inhibits E-cadherin expression [24]. E-cadherin expression reduction and deletion can cause cell polarity to vanish and cell adhesion to decrease. It is a significant indicator of the processes involved in EMT [19]. The loss of epithelial cell polarity is coincides with an increase in the expression of mesenchymal cell markers vimentin, and N-cadherin, which causes the occurrence of EMT processes, leading to metastatic initiation [25]. After co-culture, EMT associated with protein expression has also been tested in LLCs. Matrine treated M2 macrophages increased the expression of E-cadherin in LLCs, and reduced the expression of Vimentin, and Snail. Three types of lung cancer cells without co-cultured with M2-like macrophages were treated with or without matrine. The finding suggested TAMs were targeted for the anti-cancer effect of matrine. LLCs subcutaneous and intravenous model were established to confirm the consistency of in vivo tumour growth and metastasis inhibitory effect of matrine with in vitro study. IHC staining results plays a crucial part the results of EMT related protein expression. The depth of the invasion, clinical process, and vascular invasion of cancer cells were reported to be correlated with the overexpression of Vimentin [26]. Hence, matrine inhibited the tumour metastasis via EMT, and this inhibitory effect could are targeted TAMs.
The presence of TAMs has also been reported to lead to a histologically more malignant phenotype, enhance the rate of tumor growth, and increase tumor microvascular density in vivo [27]. These findings were consistent with this study, demonstrating that M2 polarized macrophages, but not M1 macrophages, induced the cell invasion, migration and adhesion of LLCs. After co-culturing of LLCs with macrophages, M0 macrophages were polarized towards the M2 phenotype, and LLCs exhibited greater invasive and migrative potentials. Hagemann, et al. [28] also found that ovarian cancer plays a crucial part co-cultured macrophages towards a tumor-associated phenotype, leading to a TAM activation pattern, a finding similar to those in ovarian tumors in vivo. Therefore, it was suggested that macrophages and lung cancer cells mutually altered the behaviors between each other.
mTOR/PI3K/Akt signalling pathway involves in the cell growth and migration, and regulation of the macrophage phenotype activation. Activation of the PI3K/Akt/mTOR signalling pathway was reported to responsible for the M2 polarization in steady-state macrophages by skewing M1-like macrophages polarizing to M2-type macrophages. PI3K/Akt/mTOR inhibitors can prevent M2 macrophages polarization and redirect their differentiation [29]. PI3K activation is an essential step towards M2 macrophages activation in response to IL-13. Akt inhibition has been reported to abolish the upregulation of M2 genes [25]. Hence, PI3Kγ/AKT/mTOR signaling pathway is closely associated with M2 macrophages polarization. In addition, Akt isoforms are crucial for tumor migration and proliferation induced by TAMs. p-Akt is an extensively characterized anti-apoptotic protein, and it is also the primary downstream kinase of PI3K [30]. Cheng, et al. [31] reported that co-culture with TAMs enhanced EMT of lung cancer cells probably through mTOR/PI3K/Akt signaling pathway. Moreover, matrine has been reported to provide a novel therapeutic approach for treating tumors by suppressing EMT [32]. Accordingly, it was possible that matrine could inhibit M2-like macrophages polarization through inhibiting mTOR/PI3K/Akt signaling pathway, thus reversing the EMT process. The results showed that matrine inhibited the activation of PI3K, thus inhibiting the phosphorylation of Akt, and mTOR, leading to the inhibition of M2-like macrophages.
In vitro experiments have shown that TAMs not only accelerated the transformation of Lewis lung cancer epithelial cells into spindle-shaped mesenchymal cells, but also regulated the expression of EMT cell markers (E-cadherin, vimentin, and snail) at protein levels. The migration and invasion of LLCs were dramatically increased, implying that TAMs exerted vital biological functions in the promotion of invasion, metastasis and adhesion of LLCs. TAMs can generate certain cytokines which are involved in EMT of LLCs during this process. However, Plays a crucial part. The future work would evaluate the changes in cytokine levels in the co-culture framework to explore the underlying mechanisms of TAMs in promoting EMT in NSCLC.
Macrophages are the major immunoregulatory cells in tumors and take great part in immune responses in tumors. A previous study [33] reported that in human ovarian cancers, the production of CCL22 from TAMs regulated the influx of regulatory T cells that inhibit cytotoxic T cell responses. In addition, in mammary tumor xenografts, TAMs were responded to cytotoxic T cell by synthesis of PGE2 and TGF-β [34]. These pieces of evidence indicated the immunoregulation roles of TAMs in cancers. In this study, matrine inhibited TAMs skewing M2 polarization, and decreased the expression of IL-4 and IL-10 in splenic and neoplastic CD4+ T cells, respectively, while it increased the number of CD4+ and CD8+ T cells in both spleen and primary tumour tissues. Interestingly, TAM from matrine-treated C57BL/6 suppressed the proliferation of CD8+ T cells to a lesser degree, compared with control group. These results revealed that the inhibition of M2-like TAMs polarization could inhibit Th2 immune response and promote the expression of CD4+ and CD8+ T cells, Plays a crucial part Th2-cytokines.
Conclusion
Taken together, this study presented an important role of the positive feedback loop between lung cancer cells and TAMs in tumour growth and metastasis. The anti-cancer and anti-matastasis effect of matrine inhibited EMT induced by M2-like macrophages via AKT/PI3K signaling pathways, boosted T-cell-mediated anti-tumor immunity by targeting TAMs. This study provided a novel insight regarding the potential therapeutic application of matrine as an anti-metastatic therapy for NSCLC, and possibly for other cancers as well.
Acknowledgements
This work was financially supported by he Grants of NSF of China (81922070, 81973286, 81801818, 8170427), The National Key Research and Development Program of China (2020YFC0845400, 2017YFC1700200, 2017YFC1702000), the Three-year Action Plan of Shanghai TCM Development (ZY-(2018-2020)-CCCX-5001), Shanghai Talent Development Fund (2019093), Shuguang Program (18SG40) supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission, and The Health System Independent Innovation Science Foundation of Shanghai Putuo District (ptkwws201802), Clinical Characteristics of Health System in Putuo District, Shanghai (2020tszk01); Shanghai Key Medical Specialities (ZK2019B16).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Lawrenson R, Lao C, Brown L, Moosa L, Chepulis L, Keenan R, Kidd J, Middleton K, Conaglen P, de Groot C, Aitken D, Wong J. Management of patients with early stage lung cancer-why do some patients not receive treatment with curative intent? BMC Cancer. 2020;20:109. doi: 10.1186/s12885-020-6580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang SY, Song XY, Li Y, Ye LL, Zhou Q, Yang WB. Tumor-associated macrophages: a promising target for a cancer immunotherapeutic strategy. Pharmacol Res. 2020;161:105111. doi: 10.1016/j.phrs.2020.105111. [DOI] [PubMed] [Google Scholar]
- 5.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front Immunol. 2019;10:1084. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muraille E, Leo O, Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014;5:603. doi: 10.3389/fimmu.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:1801. doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larionova I, Cherdyntseva N, Liu T, Patysheva M, Rakina M, Kzhyshkowska J. Interaction of tumor-associated macrophages and cancer chemotherapy. Oncoimmunology. 2019;8:1596004. doi: 10.1080/2162402X.2019.1596004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V, Choinzonov E, Kzhyshkowska J. Tumor-associated macrophages in human breast, colorectal, lung, ovarian and prostate cancers. Front Oncol. 2020;10:566511. doi: 10.3389/fonc.2020.566511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway EM, Pikor LA, Kung SHY, Hamilton MJ, Lam S, Lam WL, Bennewith KL. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. 2016;193:116–130. doi: 10.1164/rccm.201508-1545CI. [DOI] [PubMed] [Google Scholar]
- 11.Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano H, Perry C, Hanaoka J, Fukuoka J, Chung JY, Hewitt SM. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18:443. doi: 10.1186/s12967-020-02618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:78. doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang C, Liu SP, Fang CH, He RS, Wang Z, Zhu YQ, Jiang SW. Effects of matrine on the proliferation of HT29 human colon cancer cend its antitumor mechanism. Oncol Lett. 2013;6:699–704. doi: 10.3892/ol.2013.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J, Li J, Song D, Li Q, Li L, Li B, Li L. Matrine exerts anti‑breast cancer activity by mediating apoptosis and protective autophagy via the AKT/mTOR pathway in MCF-7 cells. Mol Med Rep. 2020;22:3659–3666. doi: 10.3892/mmr.2020.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Hou G, Liu A, Xu H, Guan Y, Wu Y, Deng J, Cao X. Matrine inhibits the development and progression of ovarian cancer by repressing cancer associated phosphorylation signaling pathways. Cell Death Dis. 2019;10:770. doi: 10.1038/s41419-019-2013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Mei Q, Xu YC, Du J, Wei Y, Xu ZM. Effects of Matrine Injection on T-lymphocyte subsets of patients with malignant tumor after gamma knife radiosurgery. Zhong Xi Yi Jie He Xue Bao. 2006;4:78–9. doi: 10.3736/jcim20060121. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Fan W, Liu P, Tian J. Meta analysis of compound matrine injection combined with cisplatin chemotherapy for advanced gastric cancer. Zhongguo Zhong Yao Za Zhi. 2011;36:3198–202. [PubMed] [Google Scholar]
- 18.Wu L, Wang G, Liu S, Wei J, Zhang S, Li M, Zhou G, Wang L. Synthesis and biological evaluation of matrine derivatives containing benzo-α-pyrone structure as potent anti-lung cancer agents. Sci Rep. 2016;6:35918. doi: 10.1038/srep35918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen P, Saha M, Ghosh SS. Nanoparticle mediated alteration of EMT dynamics: an approach to modulate cancer therapeutics. Mater Adv. 2020;1:2614–2630. [Google Scholar]
- 20.DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remark R, Becker C, Gomez JE, Damotte D, Dieu-Nosjean MC, Sautès-Fridman C, Fridman WH, Powell CA, Altorki NK, Merad M, Gnjatic S. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;191:377–390. doi: 10.1164/rccm.201409-1671PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9:289–302. doi: 10.2217/imt-2016-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019;8:4709–4721. doi: 10.1002/cam4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13:963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Yan Y, Cheng Z, Hu Y, Liu T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018;4:26. doi: 10.1038/s41420-018-0026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka M, Kijima H, Shimada H, Makuuchi H, Ozawa S, Inokuchi S. Expression of podoplanin and vimentin is correlated with prognosis in esophageal squamous cell carcinoma. Mol Med Rep. 2015;12:4029–4036. doi: 10.3892/mmr.2015.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025. doi: 10.1038/npjbcancer.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Plüddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 29.Linton MF, Moslehi JJ, Babaev VR. Akt signaling in macrophage polarization, survival, and atherosclerosis. Int J Mol Sci. 2019;20:2703. doi: 10.3390/ijms20112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamcheu JC, Roy T, Uddin MB, Banang-Mbeumi S, Chamcheu RN, Walker AL, Liu YY, Huang S. Role and therapeutic targeting of the PI3K/Akt/mTOR signaling pathway in skin cancer: a review of current status and future trends on natural and synthetic agents therapy. Cells. 2019;8:803. doi: 10.3390/cells8080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng H, Shcherba M, Pendurti G, Liang Y, Piperdi B, Perez-Soler R. Targeting the PI3K/AKT/mTOR pathway: potential for lung cancer treatment. Lung Cancer Manag. 2014;3:67–75. doi: 10.2217/lmt.13.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Hou G, Liu A, Xu H, Guan Y, Wu Y, Deng J, Cao X. Matrine inhibits the development and progression of ovarian cancer by repressing cancer associated phosphorylation signaling pathways. Cell Death Dis. 2019;10:770. doi: 10.1038/s41419-019-2013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapp M, Wintergerst MWM, Kunz WG, Vetter VK, Knott MML, Lisowski D, Haubner S, Moder S, Thaler R, Eiber S, Meyer B, Röhrle N, Piseddu I, Grassmann S, Layritz P, Kühnemuth B, Stutte S, Bourquin C, von Andrian UH, Endres S, Anz D. CCL22 controls immunity by promoting regulatory T cell communication with dendritic cells in lymph nodes. J Exp Med. 2019;216:1170–1181. doi: 10.1084/jem.20170277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.