Abstract
Non-small cell lung cancer (NSCLC) is a major type of lung cancer. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), represented by gefitinib (Gef), are targeted drugs used for the treatment of NSCLC. However, NSCLC patients often develop resistance to tyrosine kinase inhibitors, which limits their efficacy. Homeobox gene HOXC6 is dysregulated in many cancers and contributes to chemoresistance in cancer cells. However, the role and mechanism of HOXC6 in the development of Gef resistance in NSCLC remains unclear. In the present study, we found that HOXC6 was highly expressed in Gef-resistant NSCLC cells. Further experiments showed that silencing of HOXC6 ameliorated Gef resistance in PC9/G cells whereas overexpression of HOXC6 promoted Gef resistance in PC9 cells. HOXC6 influenced Gef sensitivity in NSCLC cells by regulating cell proliferation, colony formation, cell apoptosis, cell cycle, cell mobility and other related signaling molecules or pathways. HOXC6 was also found to be a direct target of miR-27a. As expected, overexpression of miR-27a ameliorated Gef resistance by inhibiting HOXC6 expression in vitro and in vivo. Clinical analysis revealed that high HOXC6 levels and low miR-27a levels were significantly correlated with more malignant clinical features and poorer survival of NSCLC patients. In summary, the present study demonstrates that HOXC6 may be a potential therapeutic target for overcoming Gef resistance in NSCLC patients. A combination of Gef and miR-27a agomirs may be an effective intervention for Gef-resistant NSCLC.
Keywords: HOXC6, miR-27a, gefitinib resistance, NSCLC
Introduction
Lung cancer is the second most prevalent cancer worldwide with the highest mortality [1]. In 2020, an estimated 0.82 million new cases and 0.72 million deaths were reported in China [2]. Non-small cell lung cancer (NSCLC), a major type of lung cancer, is generally characterized by rapid progression, easy relapse and poor prognosis. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), represented by gefitinib (Gef), have been widely used for the treatment of NSCLC with favorable effects in non-smokers and Asian patients with active EGFR mutation [3]. Unfortunately, most patients with EGFR mutation develop acquired resistance after 10-14 months of treatment with EGFR TKIs [4], which leads to treatment failure. The reported mechanisms of Gef resistance included T790M mutation, HER2 amplification, MET amplification, epithelial-mesenchymal transition (EMT), small cell lung cancer (SCLC) transformation and so on [5,6]. However, there is still 30% of resistant mechanism remains unknown [7].
HOXC6, a member of the Homeobox family, is abnormally expressed in carcinomas of the lung, liver, prostate, glioblastoma and breast cancer, and serves as a biomarker for the diagnosis and prognosis of malignant tumors [8-12]. In glioblastoma, HOXC6 promotes cell proliferation and migration by regulating the MAPK signaling pathway [10]. Besides, HOXC6 plays a role in development of chemoresistance of cancer cells to 5-Fluorouracil, paclitaxel and tamoxifen [13-15]. However, the specific role and mechanism of HOXC6 in the development of Gef resistance in NSCLC remains unclear.
MicroRNAs (miRNAs) are a class of endogenous small non-coding RNAs that specifically bind to 3’-UTR of target mRNAs thereby inhibiting their expression [16]. Several studies have reported the interactions between miRNAs and HOXC6 in cancer. For example, Chen et al. found that overexpression of miR-141 promoted epithelial-mesenchymal transition (EMT) and lymph node metastasis in laryngeal cancer by inhibiting the HOXC6/TGF-β signaling pathway [17]; You et al. found that miR-495 inhibited cell proliferation, migration, and invasion while promoting apoptosis of cancer stem cells in oral squamous cell carcinoma by targeting HOXC6 [18]. However, few studies have investigated the correlation between miRNAs and HOXC6 in drug resistance. MiR-27a has been proposed to target HOXC6. Though miR-27a can act as either an oncomiR or a tumor suppressor depending on the cancer type [19-21], its effects on Gef resistance of lung cancer have not been reported.
In this study, we demonstrated that HOXC6 promoted Gef resistance in NSCLC cells by regulating cell proliferation, colony formation, cell apoptosis, cell cycle, cell mobility and other related signaling molecules or pathways. Furthermore, we identified HOXC6 as a direct target of miR-27a. Overexpression of miR-27a ameliorated Gef resistance in vitro and reinforced the anti-tumor effect of Gef in vivo. Importantly, HOXC6 was upregulated and miR-27a was downregulated in human lung adenocarcinoma, both of which correlated with more aggressive clinical features and poorer prognosis. Taken together, our findings reveal that HOXC6 is a novel target for overcoming Gef resistance in NSCLC, and a combination of Gef and miR-27a agomirs may be an effective intervention for Gef-resistant NSCLC.
Material and methods
Cell culture
The human NSCLC cell lines HCC827, A549, H1299 and H1975 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The PC9 cell line was obtained from the RIKEN Cell Bank (Tsukuba, Japan). The Gef-resistant cell line PC9/G was established by culturing PC9 cell lines with increasing concentrations of Gef (0.005 μM-1 μM) for over 3 months. After that, cells were washed and cultured in drug-free medium for 14 days. Then cells were seeded in medium containing 1 μM Gef on 96-well plates for subcloning. After 30 days, the colonies were harvested and a single clone was obtained. CCK-8 assay was used to determine the extent of Gef resistance in these colonies. The stable resistant phenotype under drug-free conditions was picked out, as described previously [22]. All cells were cultured with 10% fetal bovine serum and 1% penicillin-streptomycin under a humidified atmosphere of 5% CO2 at 37°C.
Cell transfection and cotransfection
The HOXC6 siRNA (si-HOXC6), miR-27a mimics, miR-27a inhibitors, and negative control (si-NC, miR-NC) were designed and synthesized by GenePharma (Shanghai, China). The recombinant plasmid expression vector pcDNA3.1-HOXC6 and control vector (pcDNA3.1-NC) were purchased from GeneCopoeia (MD, USA). The PC9/G cells were cultured in 6-well plates to 30-50% confluence. For each transfection, siRNA (100 nM) or miR-27a mimics (100 nM) was mixed with Lipofectamine 3000 (Invitrogen), and then added into the 6-well plates according to the manufacturer’s instructions. The PC9 cells were cultured in 6-well plates to 70-90% confluence, after which the plasmid DNA (2 μg) was transfected into cells using Lipofectamine 3000. For the cotransfection, PC9/G cells were cotransfected with miR-27a mimics plus pcDNA3.1-HOXC6 or pcDNA3.1-NC, while PC9 cells were cotransfected with miR-27a inhibitors plus si-HOXC6 or si-NC.
Real-time PCR
Total RNA samples were extracted from the cultured cells using Trizol reagent and then reverse-transcribed into cDNA. Real-time PCR analysis of the cDNA was quantified with the ABI 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific, MA, USA). The reaction system was heated to 95°C for 10 min followed by 40 cycles, denaturing the mixture at 95°C for 11 s, annealing at 60 °C for 30 s, and extension at 72°C for 32 s. The primers of HOXC6 were 5’-TGAATTCGCACAGTGGGGT-3’ and 5’-TTGATCTGTCGCTCGGTCAG-3’, respectively. The primers of β-actin were 5’-TTGCCGACAGGATGCAGAA-3’ and 5’-GCCGATCCACACGGAGTACT-3’. At the end of the assay, melting curve analysis was performed. A standard curve with serial dilution control points was prepared for each assay. The mRNA levels were calculated by the 2-ΔΔCT method. All the experiments were repeated three times and each sample was assayed in triplicate.
Dual-luciferase reporter assay
PC9/G cells were plated at a density of 1 × 105 cells/well in a 24-well plate, transfected with the HOXC6-3’UTR-wt or HOXC6-3’UTR-mut, together with miR-27a mimics or miR-NC using Lipofectamine 3000. Luciferase activity was measured 24 h after transfection using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) and normalized to Renilla luciferase activity.
CCK-8 assay
The cells were seeded into 96-well plates at a density of 3 000 per well (100 μL). After overnight incubation, cells were transfected with HOXC6 siRNA or miR-27a mimics, alone or in combination with pcDNA3.1-HOXC6 plasmid. A further 24 h later, different concentrations of Gef (AstraZeneca, Cambridge, UK) were added to each well and incubated for 72 h. Subsequently, 10% CCK-8 reagent was added to each well and incubated for an additional 2 h. The absorbance was measured spectrophotometrically at 450 nm.
Colony formation assay
Cells were incubated with culture medium containing Gef (10 μM in PC9/G cells and 10 nM in PC9 cells) or vehicle control (0.05% DMSO) for 48 h after transfection. Then the cells were trypsinized, counted, diluted and replated at a density of 1000 cells per six-well plates. After 10 days, colonies were stained with 0.1% crystal violet and counted. Colonies containing at least 50 cells were scored.
Flow cytometry
Cells were incubated with a culture medium containing Gef or vehicle control (0.05% DMSO) for 48 h after transfection. For apoptosis analysis, cells were collected, washed with PBS twice, resuspended in 100 μL of 1 × binding buffer and stained with 5 μL Annexin V-FITC and 5 μL of PI for 10 min at room temperature in the dark. For cell cycle analysis, cells were washed with ice-cold PBS and fixed with 75% ethanol overnight at -20°C. Fixed cells were then rehydrated in PBS for 15 min and subjected to PI staining. The samples were analyzed by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ), and the acquired data were analyzed with the CellQuest software (BD Biosciences, San Jose, CA, USA).
Cell migration and invasion assay
After transfection, cells suspended in a medium containing 0.5% FBS in the presence of Gef or vehicle control were seeded onto either uncoated Transwell inserts (Corning, NY, USA) for migration assays or Matrigel-coated Transwell inserts for invasion assays, and the bottom chamber contained normal culture medium supplemented with 10% FBS. After incubation for 48 h, the cells on the upper surface were carefully removed and those invaded cells on the bottom surface were stained with 0.1% crystal violet and photographed under a microscope (Olympus, Tokyo, Japan). Cell numbers were counted under a light microscope at × 400 magnification.
Western blot analysis
After transfection, the cells were harvested and lysed with RIPA buffer. Equal amounts of protein (20-80 μg/lane) were subjected to SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked and then incubated overnight with the corresponding primary antibodies. Next, membranes were washed and subsequently incubated with secondary antibodies for 1 h. Protein bands were visualized using an enhanced chemiluminescence system (Pierce Biotechnology, Rockford, IL, USA). Quantitative analysis was carried out using Gel-Pro Analyser (Media Cybernetics Inc., Rockville, MD, USA).
In vivo assay
Balb/c nude mice (female, 5 weeks old) were obtained from the Hunan SJA Laboratory Animal Co., Ltd. and maintained under SPF condition. PC9/G cells (5 × 106) were subcutaneously injected into the right dorsal flank area of the mice. Tumor volume was measured with caliper and calculated by the formula (width2 × length)/2. Following the establishment of sizeable tumors, all nude mice were randomly divided into four groups. MiRNA agomirs were obtained from the Gene Pharma (Shanghai, China). MiR-27a agomirs (10 nmol) was injected into the tumor in a multi-site injection manner two times/week, and Gef (15 mg/kg) was administered intragastrically five times/week for three weeks. At the end of the treatment, the mice were sacrificed and their tumors were excised, weighed, fixed in 4% paraformaldehyde and embedded in paraffin for immunohistochemical staining of HE, Ki-67 and HOXC6 as previously described [23,24]. All animal experiments were approved by the Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology.
Tissue specimens
Lung adenocarcinoma tissue microarrays (TMAs) (Cat. No. HLugA180Su02, Shanghai Outdo Biotech, China) were constructed using 93 formalin-fixed, paraffin-embedded lung adenocarcinoma tissues and 87 corresponding non-tumor adjacent tissues (NATs). All samples used in this study were approved by the Institutional Review Board of Tongji Medical College of Huazhong University of Science and Technology, with informed consent from all patients. Immunohistochemistry (IHC) analysis of HOXC6 protein was performed as previously described [24]. The in-situ hybridization (ISH) detection of miR-27a was performed using digoxygenin (DIG)-labeled miRCURY Locked Nucleic Acid (LNA) microRNA Detection Probes (Exiqon, Vedbaek, Denmark). The IHC results of HOXC6 staining and ISH results of miR-27a staining in epithelial cells obtained from the samples were scored using a semi-quantitative evaluation as previously described [25].
Statistical analysis
All the experiments were repeated at least three times and values are presented as mean ± SD. Statistical analysis was performed using SPSS 22.0 (IBM SPSS, Armonk, NY, USA). χ2 test, two-tailed Student’s t-test, and one-way ANOVA test were applied to determine statistical significance. Survival analysis was estimated by the Kaplan-Meier method. P<0.05 was considered statistically significant.
Results
HOXC6 influences Gef resistance by regulating cell proliferation, apoptosis, cell cycle and cell mobility in NSCLC cells
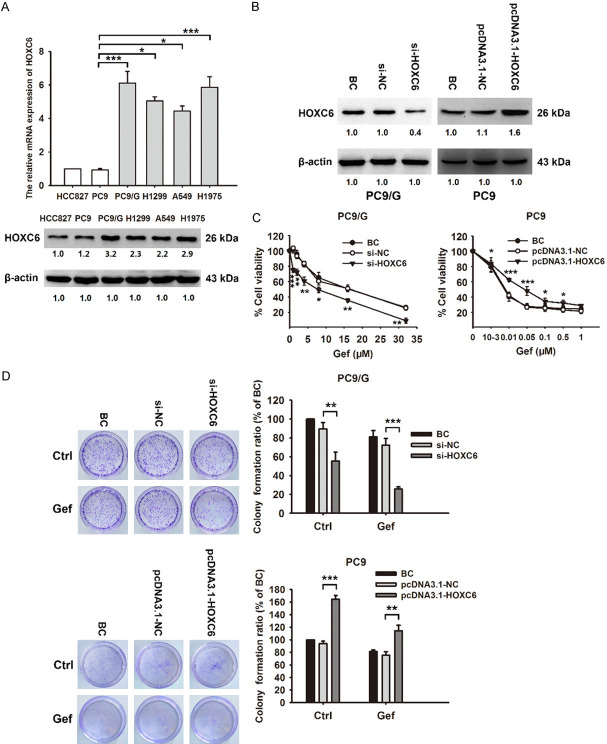
The cell viability of Gef-sensitive cell lines (HCC827 and PC9) and Gef-resistant cell lines (PC9/G, A549, H1299 and H1975) after Gef treatment was determined using CCK-8 assay. The IC50 values of Gef in these cell lines are shown in Table 1. The IC50 value of Gef on PC9/G cells was 600-fold higher than that on PC9 cells (12.9 ± 0.58 μM vs 0.021 ± 0.005 μM). We also found remarkably high levels of HOXC6 in Gef-resistant cell lines, especially in PC9/G, using qRT-PCR and western blot (Figure 1A), suggesting that HOXC6 might be involved in the development of Gef resistance in NSCLC cells. Therefore, we chose PC9 and PC9/G cell lines for further study.
Table 1.
The characteristics of NSCLC cells
| Gef sensitivity | Cell lines | EGFR gene | Met gene | K-Ras gene | Gef IC50 (μM) |
|---|---|---|---|---|---|
| sensitive | HCC827 | Exon19 (E746-A750)del | ± | WT | 0.014 ± 0.008 |
| sensitive | PC9 | Exon19 (E746-A750)del | ± | WT | 0.021 ± 0.005 |
| resistant | PC9/G | Exon19 (E746-A750)del | ± | WT | 12.9 ± 0.58 |
| resistant | H1299 | WT | unknown | WT | 7.08 ± 1.23 |
| resistant | A549 | WT | ± | G12S | 8.24 ± 0.25 |
| resistant | H1975 | Exon19 T790M-L858R | ± | WT | 12.35 ± 2.93 |
Figure 1.
HOXC6 plays a crucial role in Gef resistance in NSCLC. A. The relative mRNA and protein expression levels of HOXC6 in NSCLC cells (Gef-sensitive cell lines: HCC827, PC9; Gef-resistant cell lines: PC9/G, H1299, A549, H1975) are determined by qRT-PCR and western blot. B. PC9/G cells were transfected with HOXC6 siRNA; PC9 cells were transfected with HOXC6 overexpression plasmid. The level of HOXC6 protein was analyzed by western blot. C. Transfected cells were treated with increasing concentrations of Gef for 72 h. Cell viability was assessed by CCK-8 assay. D. Colony-forming efficiency was detected in PC9/G transfected with HOXC6 siRNA, or PC9 transfected with HOXC6 overexpression plasmid followed by exposure to Gef for 48 h. Data are presented as the mean ± SD of three independent experiments. P-values are estimated using a two-tailed Student’s t-test. *P<0.05, **P<0.01, ***P<0.001.
To assess the role of HOXC6 in the development of Gef resistance, we examined the effect of HOXC6 silencing or overexpression combined with Gef treatment on proliferation, apoptosis, cell cycle, migration and invasion of NSCLC cells. Results of HOXC6 knockdown in PC9/G cells and HOXC6 overexpression in PC9 cells are shown in Figure 1B. CCK-8 assay showed that HOXC6 silencing markedly promoted Gef-induced inhibition of the viability of PC9/G cells, while HOXC6 overexpression attenuated Gef-induced inhibition of the viability of PC9 cells (Figure 1C). Furthermore, silencing of HOXC6 in PC9/G cells inhibited cell colony formation, increased cell apoptosis, arrested cell at G2/M phase, and inhibited cell migration and invasion, which enhanced Gef efficacy and ameliorated Gef resistance (Figures 1D, 2A-D). Of note, HOXC6 overexpression in PC9 cells showed the opposite results (Figures 1D, 3A-D). These results indicate that HOXC6 promotes Gef resistance in NSCLC cells by regulating multiple cellular processes, including cell proliferation, cell apoptosis, cell cycle and cell mobility.
Figure 2.
Silencing of HOXC6 induced cell apoptosis and cell cycle arrest, and suppressed cell mobility, thereby enhancing sensitivity to Gef in NSCLC cells. PC9/G cells were transfected with HOXC6 siRNA for 24 h and then treated with Gef for 48 h. (A) Cell apoptosis and (B) Cell cycle were determined by flow cytometric analysis. (C) Cell migration and (D) Cell invasion capacity were measured by transwell assays. Data are presented as the mean ± SD of three independent experiments. P-values are estimated using a two-tailed Student’s t-test. *P<0.05, **P<0.01, ***P<0.001.
Figure 3.
HOXC6 overexpression attenuates cell apoptosis, reverses cell cycle arrest and enhances cell mobility, thereby reducing sensitivity to Gef in NSCLC cells. PC9 cells were transfected with HOXC6 overexpression plasmid for 24 h and then treated with Gef or vehicle for 48 h. (A) Cell apoptosis and (B) Cell cycle were determined by flow cytometric analysis. (C) Cell migration and (D) Cell invasion capacity were measured by transwell assays. Data are presented as the mean ± SD of three independent experiments. P-values are estimated using a two-tailed Student’s t-test. *P<0.05, **P<0.01, ***P<0.001.
HOXC6 regulates signaling molecules involved in cell apoptosis, cell cycle, cell mobility as well as ABC transporters
To determine the mechanisms by which HOXC6 promoted Gef resistance, we investigated the effects of HOXC6 silencing or overexpression on key proteins associated with cell apoptosis, cell cycle, cell mobility as well as ABC transporters. As shown in Figure 4A, silencing of HOXC6 increased Bim expression but decreased survivin and Bcl-2 expression in PC9/G cells, whereas overexpression of HOXC6 in PC9 cells showed opposite effects. This indicated that HOXC6 may impede cell apoptosis by upregulating Bim and downregulating survivin and Bcl-2. Cyclin D1 and cyclin B1 are known key regulators of cell cycle progression at the G1/S and G2/M transition [26,27]. Our study showed that silencing of HOXC6 increased cyclin B1 expression and decreased cyclin D1 expression in PC9/G cells, whereas overexpression of HOXC6 in PC9 cells produced opposite effects (Figure 4B). Therefore, HOXC6 may affect cell cycle progression by regulating cyclin B1 and cyclin D1.
Figure 4.

The influence of HOXC6 on the expression of proteins associated with apoptosis, cell cycle, cell mobility as well as proteins of ABC transporter family. PC9/G cells were transfected with HOXC6 siRNA for 72 h whereas PC9 cells were transfected with HOXC6 overexpression plasmid for 72 h. The expression level of (A) Apoptosis-associated proteins, (B) Cell cycle-associated proteins, (C) Migration- and invasion-associated proteins, (D) ABC transporter proteins were determined by western blot. β-actin or β-tubulin was used as an internal control.
The EMT phenotype has been linked high cancer cell motility, which contributes to Gef resistance in NSCLC [28,29]. Our results showed that silencing of HOXC6 in PC9/G cells promoted the EMT process accompanied by increased E-cadherin expression, decreased N-cadherin and β-catenin expression, while overexpression of HOXC6 in PC9 cells produced opposite effects (Figure 4C). However, HOXC6 did not affect the expression of vimentin. It has been reported that activation of Akt/GSK3β/β-catenin pathway promotes cancer cell mobility [30]. Here we found that silencing of HOXC6 in PC9/G cells decreased Akt expression, increased GSK3β expression, whereas overexpression of HOXC6 in PC9 cells yielded the opposite results (Figure 4C). Therefore, we speculate that HOXC6 may activate EMT via the Akt/GSK3β/β-catenin pathway, and promote the migration and invasion of NSCLC cells. ABC transporters are considered important promoters of drug resistance because they can reduce intracellular drug accumulation by pumping the drug out of the cell in various cancers [31]. Here we found that silencing of HOXC6 in PC9/G cells decreased ABCG2, MDR1, and MRP1 expression and increased ABCB5 expression. Overexpression of HOXC6 in PC9 cells exhibited opposite effects (Figure 4D). This observation indicates that ABC transporters may facilitate HOXC6-induced Gef resistance.
HOXC6 is a direct target of miR-27a
Currently, there are no commercially available HOXC6 inhibitors. Hence, we utilized bioinformatic tools to search for miRNAs targeting HOXC6 3’-UTR and identified miR-27a. By using western blot assay, we found that overexpression of miR-27a decreased HOXC6 expression in a dose-dependent manner (Figure 5A). Luciferase reporters containing specific mutations at the putative miR-27a binding site of HOXC6 3’-UTR were constructed. PC9/G cells cotransfected with the wild type HOXC6 3’-UTR and miR-27a mimics showed decreased luciferase activity; in contrast, there was no significant change in luciferase activity in PC9/G cells cotransfected with the mutant type HOXC6 3’-UTR and miR-27a mimics (Figure 5B). Taken together, these data strongly indicate that HOXC6 is a direct and functional target of miR-27a.
Figure 5.
miR-27a overexpression phenocopies HOXC6 silencing in vitro. (A) PC9/G cells were transfected with miR-27a mimics (50 nM or 100 nM) for 72 h. Protein level of HOXC6 was determined by western blot. (B) The wild type and mutant type binding site of HOXC6-3’-UTR for miR-27a are shown in the upper panel. Relative luciferase activity of reporter plasmid carrying wild-type or mutant HOXC6 3’-UTR in PC9/G cells co-transfected with miR-27a mimics are shown in the lower panel. (C) PC9/G cells transfected with miR-27a mimics with or without HOXC6 cotransfection were treated with increasing concentrations of Gef for 72 h. Cell viability was assessed by the CCK-8 assay. (D) Colony formation by PC9/G cells transfected with miR-27a mimics after exposure to Gef for 48 h. (E-H) PC9/G cells were transfected with miR-27a mimics for 24 h and then treated with Gef for 48 h. (E) Cell apoptosis and (F) Cell cycle were determined by flow cytometric analysis. (G) Cell migration and (H) Cell invasion capacity were measured by transwell assays. Data are presented as the mean ± SD of three independent experiments. P-values are estimated using a two-tailed Student’s t-test. *P<0.05, **P<0.01, ***P<0.001 vs. NC; #P<0.05, ## <0.01 vs. miR-27a+HOXC6.
MiR-27a overexpression phenocopies HOXC6 silencing in vitro
We next investigated whether overexpression of miR-27a has similar effects on Gef resistance as HOXC6 silencing. The results showed that ectopic overexpression of miR-27a reinforced Gef-induced inhibition on the viability of PC9/G cells, and this effect was partially counteracted by overexpression of HOXC6 (Figure 5C). Moreover, ectopic overexpression of miR-27a inhibited cell colony formation, increased cell apoptosis, arrested cell at G2/M phase, and inhibited cell migration and invasion, all of which enhanced Gef effectiveness and ameliorated Gef resistance in PC9/G cells (Figure 5D-H). These results suggest that miR-27a ameliorates Gef resistance by modulating cell proliferation, apoptosis, cell cycle and cell mobility through targeting HOXC6.
Subsequently, rescue experiments were performed to explore how the downstream markers responsible for Gef resistance were affected by miR-27a and the role of HOXC6 in it. Western blot analysis showed that HOXC6 expression was increased by miR-27a inhibitors, which was counteracted in PC9 cells when cotransfected with si-HOXC6. Cyclin B1 expression was decreased by miR-27a inhibitors, which was also recovered when cotransfected with si-HOXC6. Meanwhile, Bcl-2, N-cadherin and ABCG2 expression levels were increased by miR-27a inhibitors, which were abolished when cotransfected with si-HOXC6 (Supplementary Figure 1). These results indicate that miR-27a regulates downstream markers Cyclin B1, Bcl-2, N-cadherin and ABCG2 that are responsible for Gef resistance by targeting HOXC6.
miR-27a overexpression reinforces the anti-tumor effect of Gef through downregulation of HOXC6 in vivo
To further assess the effects of miR-27a on Gef resistance in vivo, we generated subcutaneous tumors in nude mice using PC9/G cells. As shown in Figure 6A-D, miR-27a agomirs combined with Gef markedly reduced the tumor volume and weight. IHC staining demonstrated significant decreases in Ki-67 and HOXC6 expression in tumor sections harvested from mice treated with miR-27a agomirs and Gef combined (Figure 6E). These results indicate that miR-27a overexpression in NSCLC ameliorated Gef resistance through targeting HOXC6 in vivo.
Figure 6.

miR-27a overexpression enhances the anti-tumor effects of Gef in vivo. Mice bearing established subcutaneous PC9/G xenografts were treated miR-27a agomir (10 nmol, intratumoral injection, two times/week), alone or combined with Gef (15 mg/kg, intragastric administration, five times/week) (n=5). A. Tumor growth curves of mice are shown. B. Total body weight was measured every 3 days during the study. C. The final weight of xenograft tumors measured once mice were sacrificed. D. Representative images of the dissected tumors at the end of the experiment. E. Immunohistochemical (IHC) staining of H&E, Ki-67 and HOXC6 of tumors from all treatment groups. Scale bar, 100 μm. All images at × 200 magnification. Statistical analysis was conducted using one-way ANOVA. *P<0.05, **P<0.01, ***P<0.001.
HOXC6 and miR-27a expression correlate with clinical stage and prognosis of NSCLC
Having explored the mechanisms by which HOXC6 and miR-27a affected Gef resistance and tumor growth, we further investigated the clinical significance of HOXC6 and miR-27a in human NSCLC. Higher expression of HOXC6 was observed in NSCLC compared to normal tissues, while miR-27a levels were lower in NSCLC (Figure 7A, 7B). We next investigated the association between clinicopathological characteristics of NSCLC patients and HOXC6, miR-27a expression (Table 2). HOXC6 expression was positively correlated with age, lymphatic invasion and TNM stage. The correlation between HOXC6 level and age might be attributed to the limited sample size of NSCLC patients enrolled, large sample sizes are needed to verify this conclusion. In addition, miR-27a expression was negatively correlated with gender, lymphatic invasion and TNM stage. Consistent with a report that females expressed lower levels of some miRNAs [32], our results showed that miR-27a was expressed at lower levels in females compared to males, suggesting a possible role of miR-27a in gender differences in NSCLC. Kaplan-Meier analysis indicated that patients with high HOXC6 or low miR-27a displayed poorer survival (Figure 7C).
Figure 7.

High HOXC6 or low miR-27a expression correlate with poor clinical outcomes in NSCLC patients. A. Representative images of HOXC6 and miR-27a expression in tumor tissues (Tumor) and the paired non-tumor adjacent tissues (NAT) from two NSCLC patient samples detected by immunohistochemistry (IHC) and in situ hybridization (ISH). Scale bar, 100 μm. All images 200 × magnification. B. Box plot depiction of IHC and ISH data. IHC and ISH score are indices of HOXC6 and miR-27a level, respectively, and were computed based on the intensity and tissue area of positive staining. C. Overall survival curves of NSCLC patients according to HOXC6 or miR-27a expression level.
Table 2.
Association between HOXC6 or miR-27a expression and the clinicopathological characteristics of patients with NSCLC
| Clinical Characteristics | n | HOXC6 | P | miR-27a | P | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Low (n=52) | High (n=41) | Low (n=57) | High (n=36) | ||||
| Gender | 0.412a | 0.047a * | |||||
| Male | 50 | 26 | 24 | 26 | 24 | 0.049b * | |
| Female | 43 | 26 | 17 | 31 | 12 | ||
| Age (year) | 0.015a * | 0.501a | |||||
| >60 | 45 | 31 | 14 | 0.015b * | 26 | 19 | |
| ≤60 | 48 | 21 | 27 | 31 | 17 | ||
| Location | 0.69a | 0.842a | |||||
| Left | 38 | 22 | 16 | 24 | 14 | ||
| Right | 54 | 29 | 25 | 33 | 21 | ||
| Unknown | 1 | 1 | 0 | 0 | 1 | ||
| Tumor size(cm) | 0.763a | 0.934a | |||||
| >3 | 46 | 25 | 21 | 28 | 18 | ||
| ≤3 | 47 | 27 | 20 | 29 | 18 | ||
| Lymphatic invasion | 0.005a * | 0.042a * | |||||
| 0.005b * | 0.043b * | ||||||
| Present | 14 | 3 | 11 | 12 | 2 | ||
| Absent | 79 | 49 | 30 | 45 | 34 | ||
| AJCC stage | 0.007a * | 0.043a * | |||||
| 1~2 | 45 | 30 | 15 | 0.007b * | 23 | 22 | 0.044b * |
| 3~4 | 31 | 11 | 20 | 23 | 8 | ||
| Unknown | 17 | 11 | 6 | 11 | 6 | ||
| Pathological grade | 0.765a | 0.316a | |||||
| I~II | 65 | 37 | 28 | 42 | 23 | ||
| III | 28 | 15 | 13 | 15 | 13 | ||
| Status | 0.258a | 0.808a | |||||
| Survival | 22 | 10 | 12 | 13 | 9 | ||
| Death | 71 | 42 | 29 | 44 | 27 | ||
Pearsoν χ2 test;
Fisher’s Exact Test;
P<0.05.
Discussion
For patients with EGFR mutation-positive advanced/metastatic NSCLC, Gef treatment is superior to chemotherapy regimens. Its application has been associated with prolonged progression-free survival, improved quality of life, and fewer hematological and neurological adverse effects [33]. However, almost all patients receiving Gef treatment develop resistance and eventually fail to respond to initial treatment. About 50% of Gef resistance is due to EGFR T790M secondary mutation, which can be controlled by Osimertinib (AZD9291) [34]. Nevertheless, the mechanisms for the remaining resistance are not well defined.
It has been reported that HOXC6 plays an important oncogenic role in NSCLC cells. For instance, overexpression of HOXC6 promoted cell proliferation, migration, and invasion in NSCLC [12]. Here we showed that HOXC6 not only stimulated cell proliferation and inhibited cell apoptosis, but also induced cell cycle arrest and promoted cell migration and invasion, thereby enhancing Gef resistance. We further investigated the functional targets of HOXC6. The results showed that HOXC6 altered expression of multiple signaling molecules, including survivin, Bim, Bcl-2, cyclin B1, cyclin D1, E-cadherin, N-cadherin, β-catenin, Akt, GSK3β, ABCG2, MDR1, MRP1, and ABCB5. To our knowledge, this is the first experimental study to investigate the functional roles of HOXC6 in Gef resistance. Since there are currently no commercially available HOXC6 inhibitors, our results support the prospect of developing HOXC6 inhibitors to control Gef resistance in NSCLC.
Tumor metastasis is the main cause of death in NSCLC patients, and many metastases are associated with poor overall survival [35]. The EMT process plays an important role in NSCLC metastasis. Li et al. [8] found that HOXC6 promoted metastasis of hepatocellular carcinoma (HCC) by driving EMT. Knockdown of HOXC6 significantly decreased the migration and invasion of HCC cells. In this study, we also found that HOXC6 promoted the migration and invasion of Gef-resistant NSCLC cells by driving EMT. Besides, HOXC6 regulated the Akt/GSK3β/β-catenin pathway. Downregulation of HOXC6 might inhibit Akt expression and therefore enhance ubiquitin-mediated degradation of β-catenin by upregulating GSK3β. Taken together, these findings show that HOXC6 promotes migration and invasion of NSCLC cells through regulating the EMT process via the Akt/GSK3β/β-catenin pathway.
Overexpression of ABC family transporters impedes drug uptake and retention, resulting in diminished intracellular drug accumulation, which is an important mechanism of resistance to EGFR TKIs [36]. Gef is a dual substrate for MDR1 and ABCG2. Concurrent knockout of MDR1 and ABCG2 increased the intracellular accumulation of Gef [37]. Kim et al. [14] found that silencing of HOXC6 downregulated MDR1 expression and increased accumulation of paclitaxel in paclitaxel-resistant human pharyngeal squamous cell carcinoma. Our results revealed that HOXC6 increased MDR1, ABCG2, and MRP1 expression, indicating that HOXC6 enhances Gef resistance and impairs Gef efficacy by affecting the intracellular Gef accumulation via upregulation of ABC transporters expression. However, ABCB5 was upregulated following HOXC6 silencing, this might be explained by the co-expression phenomenon of members of ABC transporters family, which implies that the dysfunction in some transporters may be compensated by the enhanced expression of other transporters [38].
Several papers have documented that miR-27a are key determinants of the diagnosis, prognosis, chemosensitivity and radiosensitivity of many cancers, such as gastric cancer, colorectal cancer, and ovarian cancer [39-42]. In this study, we confirmed that overexpression of miR-27a phenocopied the effect of HOXC6 silencing, ameliorated Gef resistance by inhibiting HOXC6 in vitro and in vivo. Besides, miR-27a regulated the expression of downstream markers (Cyclin B1, Bcl-2, N-cadherin and ABCG2) responsible for Gef resistance by targeting HOXC6. Moreover, low miR-27a level was correlated with more malignant prognostic features and poorer survival of NSCLC patients. These results indicated that miR-27a serves as a tumor suppressor miRNA in NSCLC, making it an important molecular marker for the diagnosis, treatment and prognosis prediction of NSCLC patients.
Recently, miRNAs have also been reported to mediate the chemopreventive effects of natural compounds on tumors. Resveratrol is a natural polyphenol compound that modulates various miRNAs in cancer and inflammatory disorder [43]. We previously showed that resveratrol overcame Gef resistance by increasing intracellular Gef concentration and triggering apoptosis, autophagy and senescence in NSCLC cells [22]. Interestingly, Partha et al. [44] found that resveratrol normalized and upregulated miR-27a expression by 5.5-fold in the ischemic heart. Given that restoration of miR-27a expression enhanced Gef efficacy, we hypothesized that upregulation of miR-27a might be one of the mechanisms by which resveratrol overcomes Gef resistance in NSCLC cells. However, this hypothesis needs to be confirmed.
In summary, our results demonstrate that HOXC6 promotes Gef resistance by regulating cell proliferation, colony formation, cell apoptosis, cell cycle, cell mobility and related signaling molecules or pathways. MiR-27a ameliorated Gef resistance by targeting HOXC6 in vitro and in vivo. Besides, high HOXC6 levels and low miR-27a levels were significantly correlated with more malignant prognostic features and poorer survival of NSCLC patients. These findings show that HOXC6 and miR-27a are potentially new targets for developing strategies to improve clinical outcomes in NSCLC patients with Gef resistance.
Acknowledgements
We thank Shunchang Zhou from the Experimental Animal Center of Tongji Medical College for valuable guidance and advice on animal experiments. This work was supported by grants from the National Natural Science Foundation of China (NSFC) (grant number: 81974450, 81672947, and 30973586); Natural Science Foundation of Hubei Province for Distinguished Young Scholars (grant number: 2018CFA032); Wuhan Science and Technology Research Project (grant number: 2017060201010149); and the Medical Research Fund of Wuhan Municipal Health Commission (grant number: WX21Q14).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134:783. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) Lung Cancer. 2019;137:113–122. doi: 10.1016/j.lungcan.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38. doi: 10.1186/s12943-018-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y, Wang X, Jin H. EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am J Cancer Res. 2014;4:411. [PMC free article] [PubMed] [Google Scholar]
- 6.Nagano T, Tachihara M, Nishimura Y. Mechanism of resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and a potential treatment strategy. Cells. 2018;7:212. doi: 10.3390/cells7110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai CS, Liam CK, Poh ME, Ong DBL, Pang YK, Cheah PL, Ho GF, Alip A. Predictors of acquired T790M mutation in patients failing first-or second-generation epidermal growth factor receptor-tyrosine kinase inhibitors. Cancer Manag Res. 2020;12:5439. doi: 10.2147/CMAR.S253760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li PD, Chen P, Peng X, Ma C, Zhang WJ, Dai XF. HOXC6 predicts invasion and poor survival in hepatocellular carcinoma by driving epithelial-mesenchymal transition. Aging (Albany NY) 2018;10:115–130. doi: 10.18632/aging.101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Yang X, Song P, Wang H, Wang X. HOXC6 in the prognosis of prostate cancer. Artif Cells Nanomed Biotechnol. 2019;47:2715–2720. doi: 10.1080/21691401.2019.1635136. [DOI] [PubMed] [Google Scholar]
- 10.Yang P, Kang W, Pan Y, Zhao X, Duan L. Overexpression of HOXC6 promotes cell proliferation and migration via MAPK signaling and predicts a poor prognosis in glioblastoma. Cancer Manag Res. 2019;11:8167–8179. doi: 10.2147/CMAR.S209904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Chen T, Zhang Y, Zhang N, Li C, Li Y, Liu Y, Zhang H, Zhao W, Chen B, Wang L, Yang Q. Long noncoding RNA Linc00339 promotes triple-negative breast cancer progression through miR-377-3p/HOXC6 signaling pathway. J Cell Physiol. 2019;234:13303–13317. doi: 10.1002/jcp.28007. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Tang X, Song X, Tang L, Cao Y, Liu X, Wang X, Li Y, Yu M, Wan H, Chen F. Evidence for an oncogenic role of HOXC6 in human non-small cell lung cancer. PeerJ. 2019;7:e6629. doi: 10.7717/peerj.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sui CJ, Xu F, Shen WF, Dai BH, Lu JJ, Zhang MF, Yang JM. MicroRNA-147 suppresses human hepatocellular carcinoma proliferation migration and chemosensitivity by inhibiting HOXC6. Am J Cancer Res. 2016;6:2787–2798. [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K, Moon S, Kim S, Kang K, Yoon J, Ahn S. Transcriptional regulation of MDR-1 by HOXC6 in multidrug-resistant cells. Oncogene. 2013;32:3339–3349. doi: 10.1038/onc.2012.354. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Lee H, Jeong D, Ham J, Park S, Choi EH, Kim SJ. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF-7 breast cancer cell. Free Radic Biol Med. 2017;110:280–290. doi: 10.1016/j.freeradbiomed.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Sun DZ, Fu YG, Yang PZ, Lv HQ, Gao Y, Zhang XY. Upregulation of microRNA-141 suppresses epithelial-mesenchymal transition and lymph node metastasis in laryngeal cancer through HOXC6-dependent TGF-beta signaling pathway. Cell Signal. 2020;66:109444. doi: 10.1016/j.cellsig.2019.109444. [DOI] [PubMed] [Google Scholar]
- 18.You X, Zhou Z, Chen W, Wei X, Zhou H, Luo W. MicroRNA-495 confers inhibitory effects on cancer stem cells in oral squamous cell carcinoma through the HOXC6-mediated TGF-beta signaling pathway. Stem Cell Res Ther. 2020;11:117. doi: 10.1186/s13287-020-1576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng L, Shen F, Zhou J, Li Y, Jiang R, Chen Y. Hypoxia-induced up-regulation of miR-27a promotes paclitaxel resistance in ovarian cancer. Biosci Rep. 2020;40:BSR20192457. doi: 10.1042/BSR20192457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Tong JL, Zhang CP, Xiao Q, Lin XL, Xiao XY. miR-27a induced by colon cancer cells in HLECs promotes lymphangiogenesis by targeting SMAD4. PLoS One. 2017;12:e0186718. doi: 10.1371/journal.pone.0186718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun YP, Lu F, Han XY, Ji M, Zhou Y, Zhang AM, Wang HC, Ma DX, Ji CY. MiR-424 and miR-27a increase TRAIL sensitivity of acute myeloid leukemia by targeting PLAG1. Oncotarget. 2016;7:25276–25290. doi: 10.18632/oncotarget.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, He W, Gao X, Li B, Mei C, Xu R, Chen H. Resveratrol overcomes gefitinib resistance by increasing the intracellular gefitinib concentration and triggering apoptosis, autophagy and senescence in PC9/G NSCLC cells. Sci Rep. 2015;5:17730. doi: 10.1038/srep17730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoyama T, Koizumi F, Fukumoto H, Kiura K, Tanimoto M, Saijo N, Nishio K. Effects of different combinations of gefitinib and irinotecan in lung cancer cell lines expressing wild or deletional EGFR. Lung Cancer. 2006;53:13–21. doi: 10.1016/j.lungcan.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Ji M, Feng Q, He G, Yang L, Tang W, Lao X, Zhu D, Lin Q, Xu P, Wei Y. Silencing homeobox C6 inhibits colorectal cancer cell proliferation. Oncotarget. 2016;7:29216–27. doi: 10.18632/oncotarget.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang L, Li H, Wang L, Hu J, Jin T, Wang J, Yang BB. MicroRNA-17-5p promotes chemotherapeutic drug resistance and tumour metastasis of colorectal cancer by repressing PTEN expression. Oncotarget. 2014;5:2974. doi: 10.18632/oncotarget.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Xiao X, Chen H, Chen Z, Hu K, Yin D. Transcription factor NFYA promotes G1/S cell cycle transition and cell proliferation by transactivating cyclin D1 and CDK4 in clear cell renal cell carcinoma. Am J Cancer Res. 2020;10:2446–2463. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Fan M, Candas D, Zhang TQ, Qin L, Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, Duru N, He F, Chen M, Finkel T, Weinstein LS, Li JJ. Cyclin B1/Cdk1 coordinates mitochondrial respiration for cell-cycle G2/M progression. Dev Cell. 2014;29:217–232. doi: 10.1016/j.devcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II, Yang SH, Kim CH, Lee JC. Epithelial to mesenchymal transition derived from repeated exposure to gefitinib determines the sensitivity to EGFR inhibitors in A549, a non-small cell lung cancer cell line. Lung Cancer. 2009;63:219. doi: 10.1016/j.lungcan.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Wang L, Sun Z, Zhang J, Wang X. Targeting c-kit inhibits gefitinib resistant NSCLC cell growth and invasion through attenuations of stemness, EMT and acquired resistance. Am J Cancer Res. 2020;10:4251–4265. [PMC free article] [PubMed] [Google Scholar]
- 30.Hu W, Xiao L, Cao C, Hua S, Wu D. UBE2T promotes nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by activating the AKT/GSK3beta/beta-catenin pathway. Oncotarget. 2016;7:15161–15172. doi: 10.18632/oncotarget.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher JI, Williams RT, Henderson MJ, Norris MD, Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resistance Updates. 2016;26:1–9. doi: 10.1016/j.drup.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Lamba V, Ghodke Y, Guan W, Tracy TS. microRNA-34a is associated with expression of key hepatic transcription factors and cytochromes P450. Biochem Biophys Res Commun. 2014;445:404–411. doi: 10.1016/j.bbrc.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanford M, Scott LJ. Gefitinib: a review of its use in the treatment of locally advanced/metastatic non-small cell lung cancer. Drugs. 2009;69:2303–2328. doi: 10.2165/10489100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Palakurthi S, Xu M, Redig AJ, Dills M, Gokhale P, Choi J, Ogino A, Kuang Y, Feeney N, Paweletz C. Abstract 5192: Utilizing NSCLC PDXs derived from patients on osimertinib (AZD9291) clinical trials to further refine therapeutic strategies. Cancer Res. 2016;76:5192–5192. [Google Scholar]
- 35.He YY, Zhang XC, Yang JJ, Niu FY, Zeng Z, Yan HH, Xu CR, Guan JL, Zhong WZ, Yang LL, Guo LH, Wu YL. Prognostic significance of genotype and number of metastatic sites in advanced non-small-cell lung cancer. Clin Lung Cancer. 2014;15:441–447. doi: 10.1016/j.cllc.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Juchum M, Günther M, Laufer SA. Fighting cancer drug resistance: opportunities and challenges for mutation-specific EGFR inhibitors. Drug Resist Updat. 2015;20:12. doi: 10.1016/j.drup.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Neul C, Schaeffeler E, Sparreboom A, Laufer S, Schwab M, Nies AT. Impact of membrane drug transporters on resistance to small-molecule tyrosine kinase inhibitors. Trends Pharmacol Sci. 2016;37:904–932. doi: 10.1016/j.tips.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Massey PR, Fojo T, Bates SE. ABC transporters: involvement in multidrug resistance and drug disposition. New York: Springer; 2014. [Google Scholar]
- 39.Zhou L, Liang X, Zhang L, Yang L, Nagao N, Wu H, Liu C, Lin S, Cai G, Liu J. MiR-27a-3p functions as an oncogene in gastric cancer by targeting BTG2. Oncotarget. 2016;7:51943–51954. doi: 10.18632/oncotarget.10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Qian K, Wei X, Deng H, Zhao B, Chen Q, Zhang J, Liu H. miR27a promotes proliferation, migration, and invasion of colorectal cancer by targeting FAM172A and acts as a diagnostic and prognostic biomarker. Oncol Rep. 2017;37:3554–3564. doi: 10.3892/or.2017.5592. [DOI] [PubMed] [Google Scholar]
- 41.Feng L, Shen F, Zhou J, Li Y, Jiang R, Chen Y. Hypoxia-induced up-regulation of miR-27a promotes paclitaxel resistance in ovarian cancer. Biosci Rep. 2020;40:BSR20192457. doi: 10.1042/BSR20192457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge Y, Jin F, Zhang D. Radio-sensitizing effects of microRNA-27a elevation in lung cancer cells by inhibiting ZEB1 expression and activating DNA damage repair pathway. J Biol Regul Homeost Agents. 2021;35:45–57. doi: 10.23812/20-502-A. [DOI] [PubMed] [Google Scholar]
- 43.Amiri A, Tehran MM, Asemi Z, Shafiee A, Hajighadimi S, Moradizarmehri S, Mirzaei HR, Mirzaei H. Role of resveratrol in modulating microRNAs in human diseases: from cancer to inflammatory disorder. Curr Med Chem. 2021;28:360–376. doi: 10.2174/0929867326666191212102407. [DOI] [PubMed] [Google Scholar]
- 44.Mukhopadhyay P, Mukherjee S, Ahsan K, Bagchi A, Pacher P, Das DK. Restoration of altered microRNA expression in the ischemic heart with resveratrol. PLoS One. 2010;5:e15705. doi: 10.1371/journal.pone.0015705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.