Key Points
Question
Is flexible, response-driven deutetrabenazine dosing a safe and effective treatment for tics associated with Tourette syndrome in the pediatric and adolescent population?
Findings
In this randomized clinical trial of 119 children and adolescents receiving deutetrabenazine or placebo, no significant differences in tic severity were observed at the end of the study period. Treatment-emergent adverse events were generally mild or moderate in severity.
Meaning
In this study, deutetrabenazine did not demonstrate efficacy in treating tics associated with Tourette syndrome, and there were no new safety signals.
This randomized clinical trial examines whether deutetrabenazine is effective and safe when compared with placebo for the treatment of tics associated with Tourette syndrome in children and adolescents.
Abstract
Importance
Tourette syndrome is a neurodevelopmental disorder characterized by childhood onset of motor and phonic tics; treatments for tics are associated with safety concerns. Deutetrabenazine is a selective vesicular monoamine transporter 2 inhibitor approved for the treatment of chorea associated with Huntington disease and tardive dyskinesia in adults.
Objective
To examine whether deutetrabenazine is effective and safe for the treatment of Tourette syndrome in children and adolescents.
Design, Setting, and Participants
This phase 2/3, randomized, double-masked, placebo-controlled, parallel-group, dose-titration study included children and adolescents (aged 6-16 years) with Tourette syndrome with active tics causing distress or impairment (ie, Yale Global Tic Severity Scale–Total Tic Score [YGTSS-TTS] ≥20). The trial was conducted over 12 weeks, with 1 week of follow-up from February 2018 to November 2019 at 36 centers in the United States, Canada, Denmark, Russia, Serbia, and Spain. Data analysis was conducted from January 31 to April 22, 2020.
Intervention
Patients were randomized (1:1) to receive deutetrabenazine or placebo, titrated during 7 weeks to an optimal level, followed by a 5-week maintenance period. The maximum total daily deutetrabenazine dose was 48 mg/d.
Main Outcomes and Measures
The primary efficacy end point was change from baseline to week 12 in YGTSS-TTS. Key secondary end points included changes in Tourette Syndrome–Clinical Global Impression, Tourette Syndrome–Patient Global Impression of Impact, and Child and Adolescent Gilles de la Tourette Syndrome–Quality of Life Activities of Daily Living subscale score. Safety was assessed based on treatment-emergent adverse events, vital signs, questionnaires, and laboratory parameters.
Results
A total of 119 participants were randomized to deutetrabenazine (59 participants; mean [SD] age, 11.5 [2.5] years; 53 [90%] boys; 49 [83%] White; 3 [5%] Black) and placebo (60 participants; mean [SD] age, 11.5 [2.6] years; 51 [85%] boys; 53 [88%] White; 3 [5%] Black). At week 12, the difference in YGTSS-TTS score was not significant between deutetrabenazine and placebo (least squares mean difference, –0.7; 95% CI, –4.1 to 2.8; P = .69; Cohen d, –0.07). There were no nominally significant differences between groups for key secondary end points. Treatment-emergent adverse events were reported for 38 patients (66%) and 33 patients (56%) receiving deutetrabenazine and placebo, respectively, and were generally mild or moderate.
Conclusions and Relevance
In this study of deutetrabenazine in children and adolescents with Tourette syndrome, the primary efficacy end point was not met. No new safety signals were identified. These results may be informative for future studies of treatments for tics in Tourette syndrome.
Trial Registration
ClinicalTrials.gov Identifier: NCT03452943
Introduction
Tourette syndrome (TS) is a childhood-onset neurodevelopmental disorder characterized by motor and phonic tics.1,2 Tics are sudden, rapidly recurring, nonrhythmic movements or noises that can vary in intensity, location, duration, and complexity.1,3 Tic development typically starts with simple movements, such as eye blinking, facial twitching, coughing, or throat clearing. Over time, tics often become more complex and may include socially inappropriate words or phrases (coprolalia), gestures (copropraxia), or other movements or noises.4 Attention-deficit/hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), and other behavioral-emotional symptoms frequently co-occur and complicate clinical presentation.
Tic treatment should be individualized based on disease severity and burden, comorbid symptoms, and collaborative discussions among patients, caregivers, and clinicians.5,6,7 When treatment is required, recommended options include comprehensive behavioral intervention for tics, oral pharmacotherapy, botulinum toxin injections, or surgical treatment.8,9 While certain antipsychotics are available for the treatment of tics in TS,10,11 there are potential safety concerns (eg, cognitive impairment, weight gain, glucose intolerance, lethargy, tardive dyskinesia [TD]) associated with their use.7,10,12
In contrast to antipsychotics, vesicular monoamine transporter 2 (VMAT2) inhibitors have been found to be effective in a variety of hyperkinetic disorders without causing TD. Deutetrabenazine is a VMAT2 inhibitor approved by the US Food and Drug Association (FDA) to treat chorea associated with Huntington disease and TD in adults.13 In a pilot study in adolescents with TS, deutetrabenazine was generally well tolerated and significantly reduced tic severity.14 Based on these results, clinical development of deutetrabenazine was continued in the Alternatives for Reducing Tics in TS (ARTISTS) program. Here we report results from the ARTISTS 1 study, which evaluated the efficacy, safety, and tolerability of flexible, response-driven doses of deutetrabenazine in children and adolescents with TS.
Methods
ARTISTS 1 was a phase 2/3, randomized, double-masked, placebo-controlled, parallel-group, dose-titration study of deutetrabenazine in patients with TS. The study was conducted at 36 centers in the United States, Canada, Denmark, Russia, Serbia, and Spain from February 5, 2018, to November 12, 2019.
The study protocol (Supplement 1) was approved by appropriate national and local authorities and independent ethics committees or institutional review boards. The study was conducted in accordance with the International Council for Harmonisation Good Clinical Practice Tripartite Guidance E6, and applicable national and local laws and regulations. A parent or legal guardian for each patient provided written informed consent, and patients who were able (based on age) provided assent to participate. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Participants
Eligible patients were aged 6 to 16 years (inclusive); weighed at least 20 kg at baseline; met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for TS; and in the opinion of the investigator, patient, and caregiver, had active tics causing distress or impairment. Patients were required to have a Yale Global Tic Severity Scale–Total Tic Score (YGTSS-TTS) of at least 20 at screening and baseline. Complete inclusion and exclusion criteria are provided in the eAppendix in Supplement 2.
Study Design
The study consisted of a prescreening period for patients who required discontinuation of prohibited concomitant medications, a screening period for as long as 31 days, a 12-week treatment period followed by a 1-week washout period, and a 1-week follow-up period. Patients were randomized (1:1) to receive deutetrabenazine or placebo, with matching tablets and packaging, and stratified by age at baseline (6-11 or 12-16 years). During the treatment period, study medication was initiated at 6 mg/d for each patient and titrated based on body weight and cytochrome P450 2D6 (CYP2D6) impairment status over 7 weeks, followed by a 5-week maintenance period (eFigure 1 in Supplement 2). Weekly dose titration was permitted until optimal tic control was achieved and treatment was tolerated or until the maximum allowed dose was reached. Patients remained on stable doses attained before the end of titration throughout the maintenance period. Maximum deutetrabenazine dose was based on baseline body weight and CYP2D6 impairment status. Patients were classified as having CYP2D6 impairment if they were receiving a strong CYP2D6 inhibitor or had poor CYP2D6 metabolization based on a masked assessment of the CYP2D6 genotype.
Based on robust pharmacokinetic sampling data from a previous pilot study,14 a population pharmacokinetic model was developed to guide dose selection by modeling deutetrabenazine’s active metabolites and simulating total metabolite exposure across a range of body weights corresponding to a pediatric and adolescent population. Maximum total daily doses of deutetrabenazine were 48 mg/d (24 mg twice daily) for patients weighing 40 kg or more, 42 mg/d (21 mg twice daily) for patients weighing 30 to less than 40 kg, and 30 mg/d (15 mg twice daily) for patients weighing 20 to less than 30 kg. For patients who had CYP2D6 impairment, maximum daily doses were 36 mg/d for those weighing 40 kg or more, 24 mg/d for those weighing 30 to less than 40 kg, and 18 mg/d for those weighing 20 to less than 30 kg.
Efficacy Assessments
The primary efficacy end point was change in YGTSS-TTS from baseline to week 12. The YGTSS rating scale is a clinician-administered rating instrument in which patients and/or caregivers report the number, frequency, intensity, complexity, and degree of interference for motor and phonic tics in the prior week on a scale from 0 (none) to 5 (severe).15,16 The total Motor Tic Severity Score (MTSS; 0-25) is added to the total Vocal Tic Severity Score (VTSS; 0-25) to determine the TTS (0-50). Key secondary efficacy end points included change from baseline to week 12 in the (1) TS–Clinical Global Impression (TS-CGI)17; (2) TS–Patient Global Impression of Impact (TS-PGII), which is the question, “How much do your current tics disrupt things in your life?” on a scale from 1 (not at all) to 5 (very much); and (3) Child and Adolescent Gilles de la TS–Quality of Life Activities of Daily Living (CA-GTS-QOL ADL) subscale score.18 Exploratory analyses included assessment of the changes from baseline in YGTSS-TTS, TS-CGI, TS-PGII, the proportion of responders (defined as patients with ≥25% reduction from baseline in YGTSS-TTS), along with changes from baseline in YGTSS-MTSS and YGTSS-VTSS, at all postbaseline time points.
Safety Assessments
The incidence of adverse events (AEs) was assessed from signing of informed consent to the end of the follow-up period. Preferred AE terms were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1. Treatment-emergent AEs (TEAEs) were defined as AEs that began or worsened after the first administration of study drug. TEAEs were considered to be treatment related if the investigator assessed the event as having a reasonable possibility of being caused by study drug. The severity of each AE was recorded by the investigator as either mild (no limitations on usual activities), moderate (some limitations on usual activities), or severe (inability to carry out usual activities). Standardized MedDRA queries (SMQs) for TEAEs of depression and suicide and/or self-injury were summarized for the overall study period.
The Children’s Depression Inventory–2 (CDI-2) parent-report and self-report versions were used to assess depressive symptoms and depression-related behaviors.19 The children’s Columbia Suicide Severity Rating Scale (C-SSRS) was used to assess past and current suicidal ideation and behaviors to determine suicide risk.20 Observed values and changes from baseline were assessed for vital signs, electrocardiogram measurements, and clinical laboratory parameters.
Statistical Analysis
It was estimated that approximately 58 patients per group would enable a 90% or greater power to detect a beneficial effect (ie, a difference of 6.0 in change from baseline to week 12 in YGTSS-TTS, assuming an SD of 9.5 in each group), with a 2-sided type I error rate of 5%, after accounting for potential dropouts. The intent-to-treat (ITT) population included all randomized patients. Efficacy analyses were performed for the modified ITT (mITT) population, defined as all patients in the ITT population who received at least 1 dose of the study drug and had a baseline and at least 1 postbaseline YGTSS assessment. The safety population included all patients who received at least 1 dose of study drug.
The primary end point analysis was a mixed-model, repeated measures, with the change in YGTSS-TTS as the dependent variable and treatment group, week, and treatment group × week interaction as fixed effects. Baseline YGTSS-TTS, region, and age group at baseline were included as covariates. Least squares (LS) mean change from baseline to week 12 in YGTSS-TTS was compared for deutetrabenazine vs placebo using a 2-sided test at the α = .05 significance level.
Change from baseline to week 12 in TS-CGI, CA-GTS-QOL ADL subscale, YGTSS-MTSS, and YGTSS-VTSS scores were analyzed in the same manner as the primary analysis, except the baseline value of the given end point was included as the covariate. Change from baseline to week 12 in TS-PGII score was analyzed using a Cochran-Mantel-Haenszel (CMH) row mean score test with modified ridit scoring, controlling for baseline TS-PGII. YGTSS responder rates were compared between treatment groups using a CMH test stratified by baseline age group. A hierarchical (fixed-sequence) testing approach was used for the analysis of the primary and key secondary end points to maintain the experimentwise type I error rate of 5%. Statistical analysis was conducted with SAS version 9.4 (SAS Institute).
Results
Participants
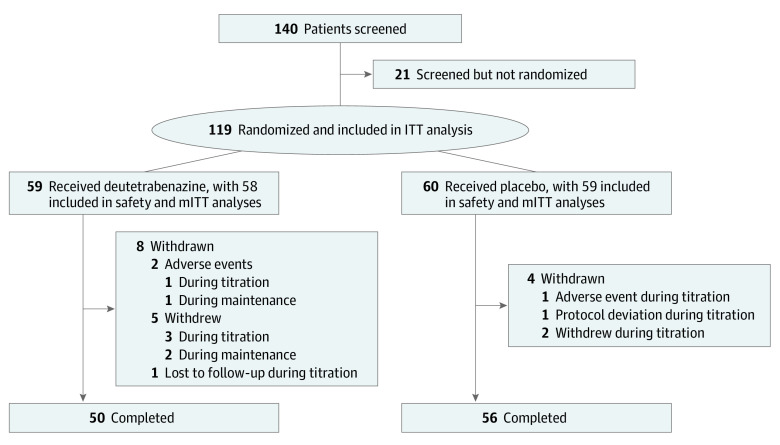
Of 140 patients screened, 119 were randomized to deutetrabenazine (59 participants; mean [SD] age, 11.5 [2.5] years; 53 [90%] boys; 49 [83%] White; 3 [5%] Black) or placebo (60 participants; mean [SD] age, 11.5 [2.6] years; 51 [85%] boys; 53 [88%] White; 3 [5%] Black) (Figure 1; Table 1). The mITT population included 117 patients evaluated for efficacy. Baseline characteristics were similar between groups (Table 1). Overall, 61 patients (51%) were aged 6 to 11 years, and 58 (49%) were aged 12 to 16 years. A total of 104 patients (87%) were boys, and 102 (86%) were White; the mean (SD) time since TS diagnosis was 3.0 (2.9) years, and the mean (SD) baseline YGTSS-TTS was 32.3 (5.9). Medical history was comparable between the deutetrabenazine and placebo groups, except for the proportion of patients with psychiatric disorders (48 [81%] vs 40 [67%]), including ADHD (37 [63%] vs 31 [52%]).
Figure 1. Study Flowchart.
ITT indicates intention to treat; mITT, modified intention to treat.
Table 1. Baseline Demographic and Clinical Characteristics in the Intention-to-Treat Population.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Deutetrabenazine (n = 59) | Placebo (n = 60) | Total (N = 119) | |
| Age, y | |||
| Mean (SD) | 11.5 (2.5) | 11.5 (2.6) | 11.5 (2.5) |
| 6-11 | 30 (51) | 31 (52) | 61 (51) |
| 12-16 | 29 (49) | 29 (48) | 58 (49) |
| Sex | |||
| Male | 53 (90) | 51 (85) | 104 (87) |
| Female | 6 (10) | 9 (15) | 15 (13) |
| Racea | |||
| White | 49 (83) | 53 (88) | 102 (86) |
| Black | 3 (5) | 3 (5) | 6 (5) |
| Asian | 1 (2) | 1 (2) | 2 (2) |
| Native American | 1 (2) | 0 | 1 (1) |
| Multiple | 3 (5) | 1 (2) | 4 (3) |
| Otherb | 2 (3) | 2 (3) | 4 (3) |
| Region | |||
| North America | 44 (75) | 43 (72) | 87 (73) |
| Eurasia | 15 (25) | 17 (28) | 32 (27) |
| Ethnicityc | |||
| Hispanic or Latino | 5 (8) | 8 (13) | 13 (11) |
| Not Hispanic or Latino | 51 (86) | 50 (83) | 101 (85) |
| Unknown | 3 (5) | 2 (3) | 5 (4) |
| Weight, kgc | |||
| Mean (SD) | 50.9 (21.8) | 53.5 (24.2) | 52.1 (23.0) |
| 20 to <30 | 10 (17) | 9 (15) | 19 (16) |
| 30 to <40 | 10 (17) | 13 (22) | 23 (19) |
| ≥40 | 38 (64) | 37 (62) | 75 (63) |
| BMI categoryd | |||
| Underweight | 2 (3) | 3 (5) | 5 (4) |
| Normal | 30 (51) | 30 (50) | 60 (50) |
| Overweight | 9 (15) | 6 (10) | 15 (13) |
| Obese | 17 (29) | 21 (35) | 38 (32) |
| Time since TS diagnosis, mean (SD), y | 3.1 (3.1) | 3.0 (2.8) | 3.0 (2.9) |
| YGTSS-TTS score, mean (SD) | 31.7 (5.8) | 33.0 (6.0) | 32.3 (5.9) |
| CYP2D6 status | |||
| Not impaired | 50 (85) | 49 (82) | 99 (83) |
| Impaired | 9 (15) | 11 (18) | 20 (17) |
| Psychiatric comorbiditiesc | |||
| ADHD | 37 (63) | 31 (52) | 68 (57) |
| OCD | 10 (17) | 9 (15) | 19 (16) |
| Anxiety | 8 (14) | 11 (18) | 19 (16) |
| Depression | 5 (8) | 3 (5) | 8 (7) |
| Concomitant medicationse | |||
| ADHDf | 31 (53) | 25 (42) | 56 (48) |
| Antidepressants | 14 (24) | 10 (17) | 24 (21) |
| Previous TS treatment | 31 (53) | 26 (43) | 57 (48) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index; CYP2D6, cytochrome P450 2D6; OCD, obsessive compulsive disorder; TS, Tourette syndrome; YGTSS-TTS, Yale Global Tic Severity Scale–Total Tic Score.
Race information was collected using predefined options in the case report form.
One patient reported unknown; 1 patient reported Canadian First Nations; 1 patient reported Métis; 1 patient reported “mother is Chinese and father is Danish.”
Percentages may not total to 100% due to rounding.
Age- and sex-based BMI categories were defined as follows: underweight, less than 5th percentile; normal, 5th to less than 85th percentile; overweight, 85th to less than 95th percentile; and obese, 95th percentile or greater.
Percentages are calculated based on the number of patients in the safety population (deutetrabenazine, 58; placebo, 59; total, 117).
Includes stimulant and nonstimulant medications.
Prior to the study, psychostimulants (14 [12%]) and antipsychotics (11 [9%]) were most frequently used. Approximately half of patients received prior treatment for TS (deutetrabenazine group: 31 [53%]; placebo group: 26 [43%]). In the deutetrabenazine and placebo groups, psychostimulants (31 [53%] vs 25 [42%]), antidepressants (14 [24%] vs 10 [17%]), systemic antihistamines (6 [10%] vs 6 [10%]), and hypnotics or sedatives (7 [12%] vs 5 [8%]) were the most common concomitant medications.
Treatment Exposure
Mean (SD) duration of exposure was 11.3 (2.4) weeks in the deutetrabenazine group and 11.6 (2.0) weeks in the placebo group; median exposure was 12.1 (range, 2.4-13.7) weeks in both groups. Adherence to deutetrabenazine was high (111 participants [95%] were between 80% and 105% adherent). Daily dose information by body weight and CYP2D6 impairment categories at the end of titration (week 7) and the end of maintenance (week 12) is shown in eTable 1 in Supplement 2. Overall, 31 patients (63%) titrated to their maximum allowable dose.
Efficacy
At week 12, the difference in YGTSS-TTS between the deutetrabenazine and placebo groups was not significant, and the primary end point was not met (Figure 2A). LS mean (SE) change from baseline to week 12 in YGTSS-TTS was –9.1 (1.28) in the deutetrabenazine group and –8.4 (1.25) in the placebo group (LS mean difference, –0.7; 95% CI, –4.1 to 2.8; P = .69; Cohen d, –0.07).
Figure 2. Change From Baseline Through Week 12 in Primary and Key Secondary Efficacy End Points.
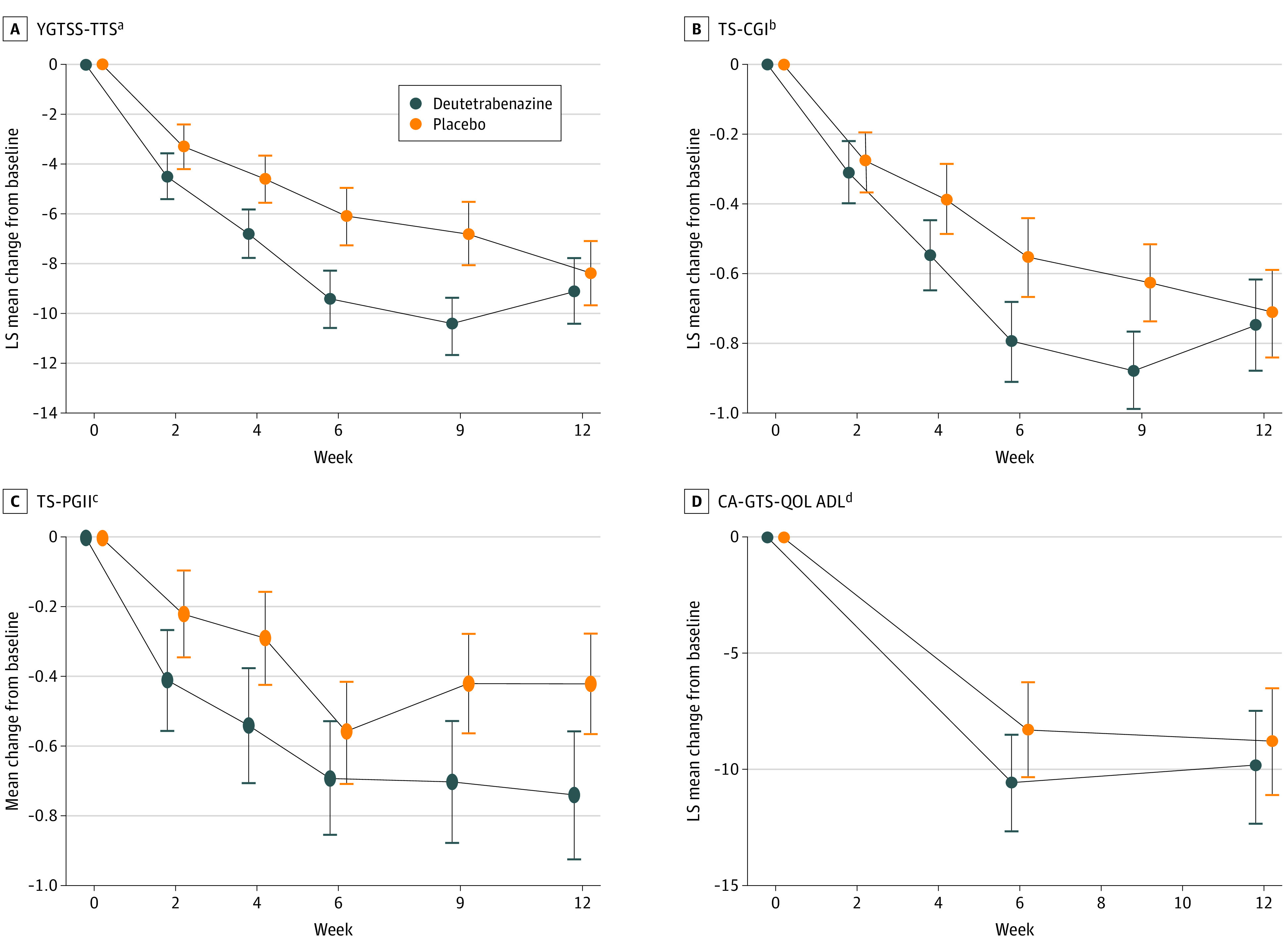
Whiskers indicate SEs; CA-GTS-QOL ADL, Child and Adolescent Gilles de la Tourette Syndrome–Quality of Life Activities of Daily Living; LS, least squares; TS-CGI, Tourette Syndrome–Clinical Global Impression; TS-PGII, Tourette Syndrome–Patient Global Impression of Impact; and YGTSS-TTS, Yale Global Tic Severity Scale–Total Tic Score.
aThe YGTSS rating scale is a semistructured clinician-administered rating instrument in which the number, frequency, intensity, complexity, and degree of interference of tics are each evaluated on a scale from 0 (none) to 5 (severe) for both motor and phonic tics. The total motor tic score (0-25) is added to the total phonic tic score (0-25) to determine the TTS (0-50). The primary end point was LS mean change from baseline in YGTSS-TTS score.
bThe TS-CGI is the clinician-reported impact of tics on the patient’s quality of life and is assessed using a 7-point Likert scale in which 1 indicates normal and 7 indicates extreme. The key secondary end point was LS mean change from baseline in TS-CGI score.
cThe TS-PGII is a single-item questionnaire that asks the patient, “How much do your current tics disrupt things in your life?” on a scale from 1 (not at all) to 5 (very much). The key secondary end point was LS mean change from baseline in TS-PGII score.
dThe CA-GTS-QOL ADL subscale is a 3-question assessment in which patients rate the extent to which their quality of life related to performance of daily activities is impacted by their Tourette syndrome symptoms. Scores range from 0 to 100, with higher scores indicating worse quality of life related to ADL. The key secondary end point was LS mean change from baseline in CA-GTS-QOL ADL score.
Similarly, there were no nominally significant differences between groups for key secondary end points at week 12 (Figure 2B, 2C, and 2D). LS mean (SE) change from baseline to week 12 in TS-CGI was –0.7 (0.13) in the deutetrabenazine group and –0.7 (0.12) in the placebo group (LS mean difference, 0.0; 95% CI, –0.4 to 0.3; nominal P = .85; Cohen d, –0.04). Mean (SE) change from baseline to week 12 in TS-PGII was –0.7 (0.18) in the deutetrabenazine group and –0.4 (0.14) in the placebo group (nominal P = .05). LS mean (SE) change from baseline to week 12 in CA-GTS-QOL ADL was –9.9 (2.37) in the deutetrabenazine group and –8.8 (2.27) in the placebo group (LS mean difference, –1.1; 95% CI, –7.3 to 5.1; nominal P = .73; Cohen d, –0.06).
The proportion of patients with a 25% or greater reduction from baseline in YGTSS-TTS was numerically higher with deutetrabenazine compared with placebo at all postbaseline time points (eFigure 2 in Supplement 2). Over 12 weeks, YGTSS-MTSS improved in both groups, with a favorable numeric difference for deutetrabenazine vs placebo at each visit through week 12. Change from baseline to week 12 in YGTSS-VTSS was similar for the deutetrabenazine and placebo groups, but a favorable numeric difference was observed at earlier points (eFigure 3 in Supplement 2).
Safety
During the overall study period, TEAEs were reported for 38 of 58 patients (65.5%) in the deutetrabenazine group and 33 of 59 patients (55.9%) in the placebo group (Table 2). There were no deaths or serious TEAEs. TEAEs were generally mild or moderate in severity. No safety signals beyond those described in the current deutetrabenazine labeling were observed. The most common TEAEs that occurred more often in patients receiving deutetrabenazine vs placebo were fatigue, increased weight, and headache (Table 2).
Table 2. Summary of TEAEs in Safety Group.
| TEAE | Patients, No. (%) | |
|---|---|---|
| Deutetrabenazine (n = 58) | Placebo (n = 59) | |
| Any TEAE | 38 (66) | 33 (56) |
| Treatment-related TEAE | 29 (50) | 12 (20) |
| Serious TEAE | 0 | 0 |
| Serious treatment-related TEAE | 0 | 0 |
| TEAE | ||
| Leading to death | 0 | 0 |
| Leading to study drug discontinuation | 1 (2) | 1 (2) |
| Leading to dose interruption | 4 (7) | 5 (8) |
| Leading to dose reduction | 7 (12) | 1 (2) |
| Most common (ie, >4%) TEAEs in either treatment group | ||
| Fatigue | 7 (12) | 3 (5) |
| Weight increase | 7 (12) | 1 (2) |
| Headache | 6 (10) | 6 (10) |
| Somnolence | 5 (9) | 1 (2) |
| Nausea | 4 (7) | 5 (8) |
| Diarrhea | 4 (7) | 1 (2) |
| Enuresis | 4 (7) | 0 |
| Vomiting | 3 (5) | 3 (5) |
| Pyrexia | 3 (5) | 2 (3) |
| Increased appetite | 3 (5) | 1 (2) |
| Anxiety | 2 (3) | 3 (5) |
| Depressed mood | 2 (3) | 3 (5) |
| Suicidal ideation | 1 (2) | 3 (5) |
| Abdominal pain | 1 (2) | 3 (5) |
| Upper respiratory tract infection | 0 | 7 (12) |
Abbreviation: TEAE, treatment-emergent adverse event.
During the titration period, TEAEs were reported for 33 patients (57%) in the deutetrabenazine group and 25 patients (42%) in the placebo group. During the maintenance period, TEAEs were reported for 14 patients (26%) and 16 patients (29%) in the deutetrabenazine and placebo groups, respectively.
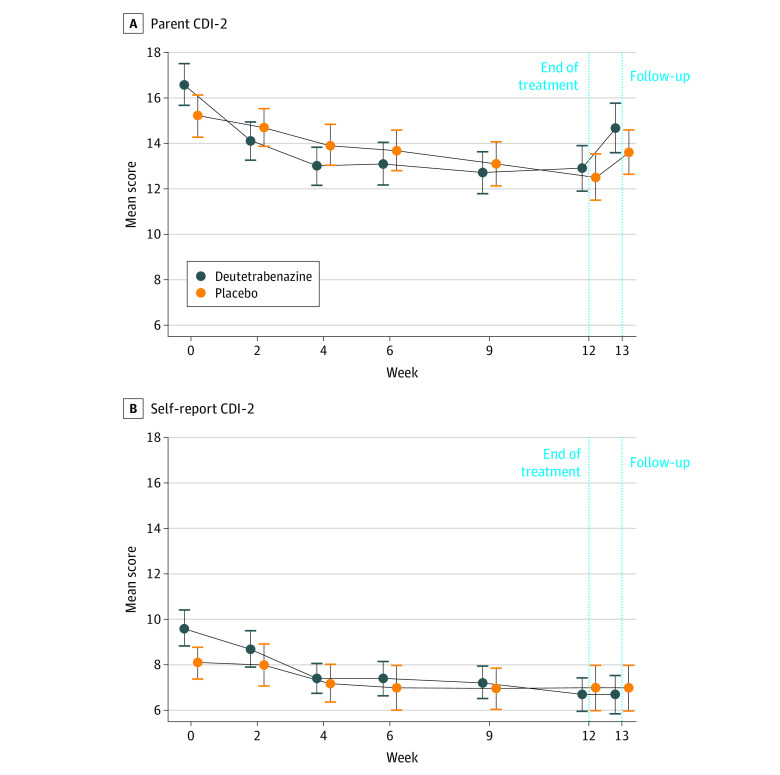
Rates of TEAEs within the depression and suicide/self-injury SMQs were low; most were considered mild in severity (eTable 2 in Supplement 2). Six patients receiving deutetrabenazine and 3 patients receiving placebo had TEAEs within the depression SMQ, with 1 event in the placebo group considered moderate in severity. One patient receiving deutetrabenazine and 3 patients receiving placebo had TEAEs within the suicide and self-injury SMQ. Results from the CDI-2 parent and self-report versions indicate no worsening of depression in either group (Figure 3). Mean (SE) change from baseline to week 13 in CDI-2 parent-reported total score was −2.7 (0.95) for the deutetrabenazine group and −1.7 (0.82) for the placebo group. Mean (SE) changes from baseline to week 13 in CDI-2 self-reported total score were −2.8 (0.95) and −1.2 (0.69) for the deutetrabenazine and placebo groups, respectively. Overall, 3 patients (3%) displayed a suicidal ideation score of 1 (wish to be dead) at baseline, and no patients showed suicidal behavior at baseline according to the children’s C-SSRS. One patient in the placebo group with suicidal ideation at baseline and week 12 had 1 recorded suicide attempt at week 13. No other patients with postbaseline suicidal ideation and/or behavior exhibited suicidal ideation and/or behavior at baseline.
Figure 3. Parent and Self-report Results From the Children’s Depression Inventory 2 (CDI-2) During Study Period.
Whiskers indicate SEs.
There were no significant changes from baseline in metabolic parameters, serum chemistry, hematology, urinalysis, vital signs, or electrocardiogram measures. Mean (SD) body weight increased from baseline to week 12 for both groups, but to a greater extent for the deutetrabenazine group (by a mean [SE] of 3.23 [0.39] kg from a baseline of 50.91 [21.8] kg) than the placebo group (by a mean [SE] of 0.84 [0.33] kg from a baseline of 53.47 [24.2] kg). Of note, the number of patients in the deutetrabenazine group who were in the body mass index category of obesity (defined as body mass index ≥95th percentile) increased from baseline (15 [30%]) to week 12 (21 [42%]). AEs of weight increased were reported in 7 patients (12%) and 1 patient (2%) in the deutetrabenazine and placebo groups, respectively; all were considered mild in severity.
Discussion
TS is a childhood-onset chronic disease characterized by motor and phonic tics, often accompanied by behavioral-emotional comorbid disorders, including ADHD and OCD. Tic severity is associated with reduced quality of life in children and adolescents with TS, and frequent severe tics can negatively affect mobility and performance of everyday activities.2,21,22 Currently, FDA-approved treatments for tics in TS are limited to antipsychotics with known risks for intolerable AEs that can outweigh potential benefits for many patients with TS.10,12
Results of the current study support the generally favorable safety profile of deutetrabenazine in children and adolescents with TS. TEAEs were mild or moderate in severity, with few differences between the deutetrabenazine and placebo groups. Incidence of TEAEs was higher during titration vs maintenance in both treatment groups. TEAEs that were more common with deutetrabenazine than placebo (eg, fatigue, increased weight, and somnolence) were generally mild and consistent with the known safety profile of deutetrabenazine in adult patients with chorea associated with Huntington disease or TD. Careful monitoring of TEAEs related to SMQs for depression and suicidality did not identify any noteworthy safety signals for deutetrabenazine.
Deutetrabenazine did not demonstrate efficacy in tic reduction compared with placebo at week 12, despite initial favorable numeric differences. The reason for the diminished improvement with deutetrabenazine from week 9 to 12 while the placebo effect increased is unknown. This is consistent with findings from the phase 3 ARTISTS 2 study,23 which found that fixed doses of deutetrabenazine demonstrated favorable numeric differences but did not significantly reduce tics in TS compared with placebo at the end of the trial. Future examination of pharmacokinetic data collected during these studies may provide insights into whether responses to deutetrabenazine differ based on drug and active drug metabolite exposure.
In contrast to favorable open-label experiences in patients with TS,14,24,25 a double-masked, placebo-controlled study of another VMAT2 inhibitor, valbenazine, also failed to meet its primary end point in a 12-week study in pediatric patients with moderate to severe TS. Changes from baseline to week 12 in YGTSS-TTS were not significantly different with once-daily valbenazine compared with placebo.26 However, full results have not been published, so explanations for this outcome are unknown.
Limitations
Several limitations of this study are shared with other tic intervention studies that failed to confirm efficacy in patients with TS. Natural fluctuations in TS tic frequency and intensity over time complicate the ability to accurately capture responses to treatment interventions over short study periods with presently available scales and measures. The time frame for experiencing beneficial therapeutic effects of VMAT2 inhibition may differ in TS compared with other indications that have different pathophysiology (ie, Huntington disease and TD), such that a longer time interval may be necessary to assess efficacy. In addition, limited information on the effects of deutetrabenazine in patients with comorbidities, including ADHD and OCD, was available, as these conditions were relatively mild at baseline compared with patients’ tics.
Although the response-driven titration design of this study is likely a reasonable model for how deutetrabenazine would be used in clinical practice, this design can promote treatment bias. For example, poor initial responders may have repeatedly titrated to higher doses, while favorable initial responders may have continued to take lower doses throughout the study.27,28 Furthermore, it may have been challenging for investigators to consistently identify an optimal dose that maximized efficacy without compromising safety for each patient. This is supported by the finding that less than two-thirds of patients reached their maximum allowable dose despite relative lack of troublesome adverse effects. Therefore, it is possible that improvements in tic control may have been achieved if investigators had continued to increase the dose, a strategy typically employed with VMAT2 inhibitors in real-world practice.25 Another potential consideration is the magnitude of the placebo effect observed, which has been similarly reported in other trials and may have contributed to negative results.23,29
Conclusions
In conclusion, this short-term, double-masked, placebo-controlled study of efficacy, safety, and tolerability of flexible doses of deutetrabenazine in patients aged 6 to 16 years with TS did not meet the primary efficacy end point despite initial favorable numeric differences. There were no new safety signals compared with the known safety profile of deutetrabenazine.
Trial Protocol
eTable 1. Daily Dose of Deutetrabenazine by Baseline Weight Category and CYP2D6 Impairment Status
eTable 2. TEAEs Within the Depression and Suicide and Self-injury SMQs
eFigure 1. Study Design
eFigure 2. Proportion of Patients With 25% or Greater Reduction From Baseline in YGTSS-TTS
eFigure 3. Change From Baseline Through Week 12 in YGTSS-MTSS and YGTSS-VTSS
eAppendix. Full Study Inclusion and Exclusion Criteria
Data Sharing Statement
References
- 1.Efron D, Dale RC. Tics and Tourette syndrome. J Paediatr Child Health. 2018;54(10):1148-1153. doi: 10.1111/jpc.14165 [DOI] [PubMed] [Google Scholar]
- 2.Robertson MM, Eapen V, Singer HS, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primers. 2017;3:16097. doi: 10.1038/nrdp.2016.97 [DOI] [PubMed] [Google Scholar]
- 3.Black KJ, Jankovic J, Hershey T, McNaught KS, Mink JW, Walkup J. Progress in research on Tourette syndrome. J Obsessive Compuls Relat Disord. 2014;3(4):359-362. doi: 10.1016/j.jocrd.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuire JF, Nyirabahizi E, Kircanski K, et al. A cluster analysis of tic symptoms in children and adults with Tourette syndrome: clinical correlates and treatment outcome. Psychiatry Res. 2013;210(3):1198-1204. doi: 10.1016/j.psychres.2013.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pringsheim T, Okun MS, Müller-Vahl K, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019;92(19):896-906. doi: 10.1212/WNL.0000000000007466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jankovic J. Treatment of tics associated with Tourette syndrome. J Neural Transm (Vienna). 2020;127(5):843-850. doi: 10.1007/s00702-019-02105-w [DOI] [PubMed] [Google Scholar]
- 7.Billnitzer A, Jankovic J. Current management of tics and tourette syndrome: behavioral, pharmacologic, and surgical treatments. Neurotherapeutics. 2020;17(4):1681-1693. doi: 10.1007/s13311-020-00914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey S, Dash D. Progress in pharmacological and surgical management of Tourette syndrome and other chronic tic disorders. Neurologist. 2019;24(3):93-108. doi: 10.1097/NRL.0000000000000218 [DOI] [PubMed] [Google Scholar]
- 9.Pringsheim T, Holler-Managan Y, Okun MS, et al. Comprehensive systematic review summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019;92(19):907-915. doi: 10.1212/WNL.0000000000007467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quezada J, Coffman KA. Current approaches and new developments in the pharmacological management of Tourette syndrome. CNS Drugs. 2018;32(1):33-45. doi: 10.1007/s40263-017-0486-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jankovic J. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. 2016;17(18):2461-2470. doi: 10.1080/14656566.2016.1258063 [DOI] [PubMed] [Google Scholar]
- 12.Novak L, Svab V. Antipsychotics side effects’ influence on stigma of mental illness: focus group study results. Psychiatr Danub. 2009;21(1):99-102. [PubMed] [Google Scholar]
- 13.Bashir HH, Jankovic J. Treatment of tardive dyskinesia. Neurol Clin. 2020;38(2):379-396. doi: 10.1016/j.ncl.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 14.Jankovic J, Jimenez-Shahed J, Budman C, et al. Deutetrabenazine in tics associated with Tourette syndrome. Tremor Other Hyperkinet Mov (N Y). 2016;6:422. doi: 10.5334/tohm.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566-573. doi: 10.1097/00004583-198907000-00015 [DOI] [PubMed] [Google Scholar]
- 16.Martino D, Pringsheim TM, Cavanna AE, et al. ; Members of the MDS Committee on Rating Scales Development . Systematic review of severity scales and screening instruments for tics: critique and recommendations. Mov Disord. 2017;32(3):467-473. doi: 10.1002/mds.26891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leckman JF, Towbin KE, Ort SI, Cohen DJ. Clinical assessment of tic disorder severity. In: Cohen DJ, Braun RD, Leckman JF. Tourette’s Syndrome and Tic Disorders. John Wiley & Sons; 1988:550-578. [Google Scholar]
- 18.Su MT, McFarlane F, Cavanna AE, et al. The English version of the Gilles de la Tourette Syndrome–Quality of Life Scale for Children and Adolescents (C&A-GTS-QOL). J Child Neurol. 2017;32(1):76-83. doi: 10.1177/0883073816670083 [DOI] [PubMed] [Google Scholar]
- 19.Sun S, Wang S.. The Children’s Depression Inventory in worldwide child development research: a reliability generalization study. J Child Fam Stud. 2015;24(8):2352-2363. doi: 10.1007/s10826-014-0038-x [DOI] [Google Scholar]
- 20.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storch EA, Merlo LJ, Lack C, et al. Quality of life in youth with Tourette’s syndrome and chronic tic disorder. J Clin Child Adolesc Psychol. 2007;36(2):217-227. doi: 10.1080/15374410701279545 [DOI] [PubMed] [Google Scholar]
- 22.Murphy TK, Lewin AB, Storch EA, Stock S; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI) . Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1341-1359. doi: 10.1016/j.jaac.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 23.Coffey B, Jankovic J, Claassen DO, et al. Efficacy and safety of deutetrabenazine treatment for Tourette syndrome in children and adolescents: results from the ARTISTS 1 and ARTISTS 2 studies. Presented at: Tourette Association of America’s Inaugural Virtual Conference; May 15, 2020. [Google Scholar]
- 24.Kenney C, Hunter C, Mejia N, Jankovic J. Tetrabenazine in the treatment of Tourette syndrome. J Ped Neurol. 2007;5:9-13. doi: 10.1055/s-0035-1557350 [DOI] [Google Scholar]
- 25.Niemann N, Jankovic J. Real-world experience with VMAT2 inhibitors. Clin Neuropharmacol. 2019;42(2):37-41. doi: 10.1097/WNF.0000000000000326 [DOI] [PubMed] [Google Scholar]
- 26.Neurocrine Biosciences . Neurocrine Biosciences announces topline data from phase IIb T-Force GOLD study demonstrating valbenazine did not meet primary endpoint in pediatric patients with Tourette syndrome. December 12, 2018. Accessed June 7, 2020. https://neurocrine.gcs-web.com/news-releases/news-release-details/neurocrine-biosciences-announces-topline-data-phase-iib-t-force
- 27.Lipkovich I, Adams DH, Mallinckrodt C, Faries D, Baron D, Houston JP. Evaluating dose response from flexible dose clinical trials. BMC Psychiatry. 2008;8:3. doi: 10.1186/1471-244X-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu XS, Yuan M, Nandy P. Analysis of dose-response in flexible dose titration clinical studies. Pharm Stat. 2012;11(4):280-286. doi: 10.1002/pst.1498 [DOI] [PubMed] [Google Scholar]
- 29.Sallee F, Kohegyi E, Zhao J, et al. Randomized, double-blind, placebo-controlled trial demonstrates the efficacy and safety of oral aripiprazole for the treatment of Tourette’s disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27(9):771-781. doi: 10.1089/cap.2016.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Daily Dose of Deutetrabenazine by Baseline Weight Category and CYP2D6 Impairment Status
eTable 2. TEAEs Within the Depression and Suicide and Self-injury SMQs
eFigure 1. Study Design
eFigure 2. Proportion of Patients With 25% or Greater Reduction From Baseline in YGTSS-TTS
eFigure 3. Change From Baseline Through Week 12 in YGTSS-MTSS and YGTSS-VTSS
eAppendix. Full Study Inclusion and Exclusion Criteria
Data Sharing Statement