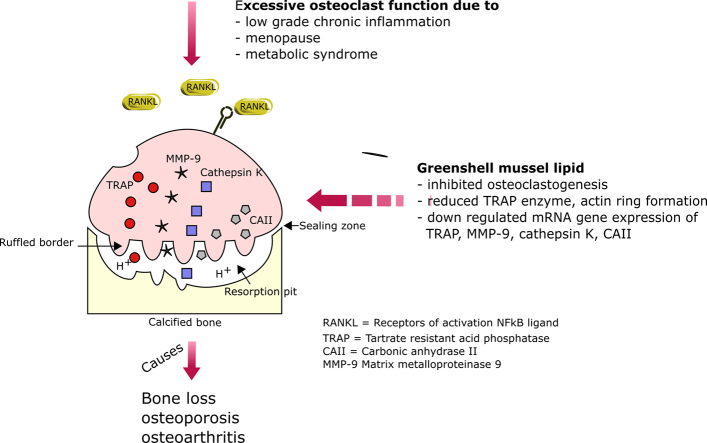
Abstract
The osteoclast-dependent bone resorption process is a crucial part of the bone regulatory system. The excessive function of osteoclasts can cause diseases of bone, joint, and other tissues such as osteoporosis and osteoarthritis. Greenshell mussel oil (GSM), a good source of long chain omega-3 polyunsaturated fatty acids (LCn-3PUFAs), was fractionated into total lipid, polar lipid, and non-polar lipid components and their anti-osteoclastogenic activity tested in RAW 264.7 cell cultures. Osteoclast differentiation process was achieved after 5 days of incubation with RANKL in 24-well culture plates. Introducing the non-polar lipid fraction into the culture caused a lack of cell differentiation, and a reduction in tartrate-resistant acid phosphatase (TRAP) activity and TRAP cell numbers in a dose-dependent manner (50% reduction at the concentration of 20 μg/mL, p < 0.001). Moreover, actin ring formation was significantly diminished by non-polar lipids at 10–20 μg/mL. The bone digestive enzymes released by osteoclasts into the pit formation were also compromised by downregulating gene expression of cathepsin K, carbonic anhydrase II (CA II), matrix metalloproteinase 9 (MMP-9), and nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1). This study revealed that the non-polar lipid fraction of GSM oil contains bioactive substances which possess potent anti-osteoclastogenic activity.
Abbreviations: AA, Arachidonic acid; ALA, Alpha linolenic acid; CAII, Carbonic anhydrase II; DHA, Docosahexaenoic acid; DPA, Docosapentaenoic acid; DMSO, dimethyl sulfoxide; EPA, Eicosapentaenoic acid; FFAR, Free fatty acid receptor; GSM, Greenshell mussel; LA, Linoleic acid; LPS, Lipopolysaccharide; MMP-9, Matrix metalloproteinase 9; MUFA, Monounsaturated fatty acid; NFATc1, Nuclear factor of activated T-cells, cytoplasmic 1; NF-κB, Nuclear factor κB; OA, Osteoarthritis; PA, Palmitic acid; PPAR, Peroxisome proliferator activated receptor; PUFA, Polyunsaturated fatty acid; RANKL, Receptor activator of nuclear factor κB ligand; SFA, Saturated fatty acid; TRAP, Tartrate-resistant acid phosphatase
Keywords: Greenshell mussel, Osteoclasts, Omega 3 fatty acid, Osteoarthritis, Osteoporosis
Graphical abstract
1. Introduction
Greenshell mussel (GSM) or Perna canaliculus, a species of bivalve shellfish that is endemic to New Zealand, has solid demonstrated evidence of providing health benefits. Its biological activity has been reported since 1975 (Whitehouse et al., 1997). GSM lipids, the most well-researched component, contain a high proportion of long-chain polyunsaturated fatty acids (LCPUFAs) including EPA and DHA (Miller et al., 2014; Singh et al., 2008). These are renowned as having potent anti-inflammatory activity, as they both inhibit the production of pro-inflammatory compounds and can be converted into resolvins, protectins and maresins which protect against tissue inflammation and organ injury. Most studies using GSM oil extracted by supercritical fluid CO2 method support its anti-inflammatory property showing inhibition of prostaglandin E2 (PGE2) in LPS-activated mononuclear cells (Whitehouse et al., 1997), which relates to both cyclooxygenase and lipoxygenase pathways (McPhee et al., 2007; Treschow et al., 2007). Both enzymatic pathways are responsible for cleaving arachidonic acid into many species of inflammatory mediators including a family of prostaglandins and leukotrienes (Calder et al., 2013). Later clinical studies in animals or humans were mainly focused on GSM's anti-inflammatory and anti-arthritis property (Brien et al., 2008; Cobb and Ernst, 2006; Eason et al., 2018; Zawadzki et al., 2013). Our study of metabolic osteoarthritis (OA) in rats fed a high-energy diet found that GSM prevented the early occurrence of OA pathological markers (Siriarchavatana et al., 2019). The early pathogenesis of metabolic OA is hypothetically associated with subchondral bone remodelling (Burr and Gallant, 2012; Goldring and Goldring, 2016).
Bone remodelling is a physiological function occurring continuously through human life. It is driven by two processes, osteoblast-mediated bone formation and osteoclast-mediated bone resorption in which the equilibrium is a key factor to maintain bone health. Theoretically, bone-resorbing osteoclasts create an empty space for osteoblasts to reconstruct new microarchitecture in response to the microdamage caused by load-bearing stress on the bones and joints (Li et al., 2013). Excessive osteoclast function leads to skeletal disorders, so osteoclasts currently are considered to be a target for drugs such as bisphosphonates in the treatment of osteoporosis (Mastaglia et al., 2006). Osteoclasts, large multinucleated cells, are derived from hematopoietic stem cells of monocyte lineage (Marino et al., 2014). The osteoclast differentiation of the precursor cells or murine macrophage cell line (RAW 264.7) of this study is established by an osteoblast-secretory factor called RANKL (Collin-Osdoby and Osdoby, 2012).
The increased number of osteoclasts in the subchondral bone plate is evident in early-stage OA in animals (Botter et al., 2011; Pelletier et al., 2004). Suppression of the bone resorption process in a preclinical study improved cartilage health, whereas increased resorption caused more severe cartilage deterioration (Karsdal et al., 2008). Moreover, osteoclasts probably have a direct involvement in cartilage degradation (Knowles et al., 2012) as chondrocytes, similar to osteoblasts, produce a great abundance of RANKL (Tat et al., 2009; Xiong et al., 2011). A study in equines demonstrated that osteoclasts were recruited to the subchondral bone plate of post-traumatic OA and their density was highly correlated with the expression of RANKL in the hyaline cartilage layer (Bertuglia et al., 2016). An in vitro model also proved that osteoclasts are able to resorb cartilage and calcified cartilage by the means of MMP and cathepsin K (Lofvall et al., 2018). In addition, a subset of osteoclasts, arising at the subchondral bone plate or near calcified cartilage, was recognized as chondroclasts (Knowles et al., 2012; Lewinson and Silbermann, 1992). Both cells are morphologically similar and positive by TRAP staining; however, a recent study has showed the distinction of metabolic genes between those cells (Khan et al., 2020). This revealed a pathogenic relationship between bone and cartilage.
Despite the fact that DHA and EPA have consistent evidence of anti-inflammatory and anti-osteoclastogenic effects leading to a therapeutic option for bone and joint diseases (Kim et al., 2017; Rahman et al., 2008), no study has addressed the potential of GSM oils in association with bone or osteoclasts. This current study hypothesized that GMS oil could have an anti-osteoclastogenic effect as it contains omega 3 fatty acid. To investigate this, we conducted experiments using an osteoclast differentiation assay and analysed TRAP cell number, enzymatic activity, actin ring formation and gene expression. This is the first study using GSM oil in osteoclast differentiation. The results reveal a new perspective of using GSM for the treatment or prevention of skeletal disorders.
2. Methodology and research design
2.1. Reagents and materials
Dulbecco's Modified Eagle Medium (DMEM) was purchased from GE Life sciences (Pittsburgh, PA, USA). Heat inactivated fetal bovine serum (FBS) and antibiotic solution was supplied by GIBCO (Invitrogen, Corp., Victoria, Australia). RANKL (#462-TEC) was acquired from Research and Diagnostic Systems (R&D Systems, Minneapolis, MN, USA). Docosahexaenoic acid (DHA), napthol-AS-BI phosphate, 4-nitrophenyl phosphate, pararosaniline, hematoxylin, Phalloidin-Atto® 488, and Hoescht 33342 were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA). RNA extraction kit (Direct-zol™ RNA Miniprep Plus) was purchased from Zymo Research (Tustin, CA, USA). SuperScript™ IV First-Strand Synthesis System and primers were bought from Invitrogen (Life Technologies, New Zealand). SYBR™ Green Master Mix was purchased from Bio-Rad Laboratories (New South Wales, Australia). 24-well culture plates were obtained from Greiner Bio-one (Kremsmünster, Austria).
2.2. Greenshell mussel (GSM) extraction and identification
All the extraction and identification process were carried out by the Food Testing Laboratory of Cawthron Analytical Services (Nelson, NZ). Live GSM, shipped overnight on ice from the Marlborough Sounds NZ, were shucked and meat was homogenized and extracted by a modified Bligh Dyer procedure (Bligh and Dyer, 1959), providing a total lipid fraction. Later, it was separated into 2 different fractions, polar-lipid and non-polar lipid, by silica column running mobile phase with ether and hexane respectively. After evaporating the solvent, all fractions were treated with lipases (Candida antarctica: lipase B (CALB) Novozyme 435, Sigma Aldrich) at 40 °C for 5 h (Kahveci and Xu, 2011), extracted by a modified Bligh dyer, dried under nitrogen then kept at -20 °C for in vitro assay. To prevent lipid oxidation, the sample vials were filled with nitrogen gas before capping. The lipid compositions were identified as follows: 1 mL of analytical samples were made from lipid extracts with an internal injection standard (C19:0 methyl nonadecanoate; NuCheck Elysian, MN, USA) and external standards (Supelco 37 Component FAME Mix, Merck, Auckland, NZ) then analysed by gas chromatography – flame ionised spectroscopy (GC-FID) according to AOAC 963.22 (Association of Official Agricultural Chemists (AOAC)).
2.3. Cell culture maintenance
Murine macrophage cell line RAW 264.7 (#TIB71) was purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were maintained in DMEM with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin and incubated at 37 °C with 5% CO2. Cells in flasks were passaged or harvested for the assay when reaching 90% confluence and a scraping technique was used to prepare a single-cell suspension. All GSM lipid fractions and DHA were dissolved in DMSO at various concentrations and stored at −20 °C prior to introducing into the culture wells. The final concentration of DMSO in culture media was equal to 0.1%.
2.4. MTT assay
The cytotoxicity of the test substances were determined by assessing the presence of metabolically active RAW 264.7 macrophages using MTT assay (Ferrari et al., 1990). The optimisation study comparing 1 day with 6-day-incubation periods found no differences in the interpretation of cytotoxic levels; therefore, the incubation period for the main study was selected to be a shorter time point. Cells were seeded on 96-well culture plates at 10,000 cells per well for 24 h prior to new media replacement together with induction of test substances in duplicate wells. The concentrations of all GSM lipid fractions were tested at 5–80 μg/mL while DHA as the positive control was evaluated at 5–20 μg/mL. After a 48-h incubation period, 10 μL of 5 mg/mL MTT (purchased from Sigma-Aldrich Inc.) was added to each well and incubated further for 3 h at 37 °C. Then the media from each well was replaced with 100 μL of DMSO. After 5 mins, the plates were shaken until the formazan colour was homogeneous and the absorbance measured at 550 nm on a microplate reader (Multiskan FC, Thermo Fisher Scientific, Vantaa, Finland). Data were normalized and reported as percentage compared to the vehicle control (DMSO treatment only). Three independent experiments were conducted, each in duplicate wells, and the data pooled.
2.5. Measurement of tartrate resistant acid phosphatase (TRAP) activity
The pilot study of this assay tested the GSM oil at the concentrations of 1.25–20 μg/mL but concentrations lower than 2.5 μg/mL showed no effect. In subsequent assays the test concentrations were limited to 5–20 μg/mL. The assay was performed according to Boeyens et al. (2014). Briefly, RAW 264.7 cells were routinely prepared as describe above. 1 mL of cell suspension at density of 1.5 × 104 cells/mL was seeded in each well of 24 well plates. The GSM lipid fractions at three concentrations (5, 10, and 20 μg/mL) were introduced into the culture on day 1 simultaneously with RANKL at 15 ng/mL. 10 μg/mL of DHA were used as a reference for the assay. Each treatment was performed in triplicate. At day 4, new media and test substances were replaced. At day 6, culture medium from each well was collected separately and kept at −80 °C for further enzymatic activity measurement. TRAP enzyme from the culture media was measured by enzymatic methods. Briefly, 30 μL of test sample was added into 96 well plates followed by 170 μL of assay reagent (4-nitrophenyl phosphate in tartrate-acetate buffer). The reaction was stopped by adding 50 μL of 1 M NaOH after 1 h of incubation period at 37 °C. Optical density was read at 405 nm. The percentage of the enzymatic activity compared to the control was reported. The osteoclast differentiation assay was done in duplicate with three separate experiments.
2.6. Tartrate resistant acid phosphatase (TRAP)-positive cell staining
The 24-well culture plates from the osteoclast cultures were processed for TRAP cell staining immediately. The method was modified from Boeyens et al. (2014). Briefly, the cells were fixed with fixative buffer then washed with warm deionized water. A 500 μL of 0.125 mg/mL naphthol-AS-BI phosphate in acetate-tartrate buffer was added and incubated at 37 °C for 30 min. Then, pararosaniline dye in acetate-tartrate buffer was added and incubated for 15 min. Hematoxylin was used for counter staining. All TRAP-positive multinucleated cells containing more than 4 nuclei in each well were counted as osteoclasts under 10× objective lens of an inverted microscope (Olympus CK40). Images were taken with an Olympus camedia C-5060 camera.
2.7. Actin ring formation staining
RAW 264.7 cells were seeded at 15,000 cells per well in 24-well culture plates together with 15 ng/mL RANKL and test substances. The process of cell maintenance was similar to the above. After the end of incubation period, cells were washed with PBS and fixed with 3.75% formaldehyde solution in PBS for 15 min. Later, cell permeabilization was done by 0.5% Triton X-100 in PBS for 10 min. Actin ring formation was stained with 0.5 nmol/mL fluorescent phalloidin conjugate solution (Phalloidin-Atto® 488, Sigma-Aldrich) for 40 min and nuclei were stained with 5 μg/mL Hoescht 33,342. Actin ring formation was visualized using an inverted microscope (Olympus IX-71) with dual filters: Hoescht (dichromatic filter 400 nm); Phalloidin (dichromatic filter 505 nm) and images were taken using Olympus camera XC 50.
2.8. Quantification of mRNA expression using RT-PCR
RAW 264.7 cells were grown and assessed similarly as the above osteoclast differentiation studies. At the end of incubation period, cells in osteoclast culture were lysed by Trizol reagent and then RNA was separated using the RNA extraction kit (Direct-zol™ RNA Miniprep Plus, Zymo Research, USA). cDNA was synthesised from the total RNA according to manufacturer's protocol using SuperScript™ IV First-Strand Synthesis System (Invitrogen, USA). Real-time quantitative PCR, using SYBR™ Green Master Mix, was performed on LightCycler® 480 Real-Time PCR instrument (Roche Applied Science). Four genes were quantitatively measured: cathepsin K, NFATc1, MMP-9, and CA II; each was normalized to GAPDH. The relative quantification was calculated using (2−∆∆CT) method. The conditions for the PCR process and primer sequences are presented in Table 1, Table 2 respectively.
Table 1.
Protocol for quantitative RT-PCR.
| Programs | Temperature | Duration | Cycles |
|---|---|---|---|
| Pre-incubation | 95 °C | 10 min | 1 |
| Amplification (3 steps) | 95 °C | 20 s | 35 |
| 60 °C | 20 s | ||
| 72 °C | 20 s | ||
| Melting | 95 °C | 5 s | 1 |
| 65 °C | 1 min |
Table 2.
Primer sequences.
| Gene description | Sequences | Length (bp) | TM(°C) | GC% | |
|---|---|---|---|---|---|
| GAPDH | Forward | 5′-AGGTCGGTGTGAACGGATTTG-3′ | 21 | 60.88 | 52.38 |
| Reverse | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ | 23 | 58.59 | 43.48 | |
| NFATc1 | Forward | 5′-GTGGAGAAGCAGAGCAC-3 | 17 | 54.77 | 58.82 |
| Reverse | 5′- ACGCTGGTACTGGCTTC-3 | 17 | 56.45 | 58.82 | |
| CathepsinK | Forward | 5′-CTGGAGGGCCAACTCAAGA-3′ | 19 | 58.93 | 57.89 |
| Reverse | 5′-CCTCTGCATTTAGCTGCCTT-3′ | 20 | 58.24 | 50.00 | |
| MMP-9 | Forward | 5′-GTCATCCAGTTTGGTGTCGCG-3 | 21 | 62.11 | 57.14 |
| Reverse | 5′-AGGGGAAGACGCACAGCTC-3 | 19 | 61.95 | 63.16 | |
| CAII | Forward | 5′-GAGTTTGATGACTCTCAGGACAA-3 | 23 | 58.11 | 43.48 |
| Reverse | 5′-CATATTTGGTGTTCCAGTGAACCA-3 | 24 | 59.48 | 41.67 | |
2.9. Statistical analysis
Data were analysed by one-way ANOVA and least significant difference test, using IBM statistics software (SPSS) version 25 (Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
3. Results
3.1. GSM lipids and composition
Three lipid fractions were recovered from GSM lipids (Table 3). Fatty acid profiles of all three lipid fractions were almost equivalent in term of total recovery. They were rich in PUFA (37.68–45.07 g/100 g) rather than SFA (22.42-28.80 g/100 g) and also contained a high proportion of omega 3: omega 6 (~9:1). The three fatty acids found in high abundance were palmitic acid (13.50-16.70 g/100 g), EPA (13.60-20.10 g/100 g) and DHA (14.40–15.40 g/100 g). Despite the equivalence of fatty acids contained in those lipid fractions, the lipid classes of each lipid fraction were obviously distinct depending on their polarity. Phospholipids (PL) were highly present in the polar lipid fraction (79.50 g/100 g) but completely absent in the non-polar lipid fraction. Triglycerides on the other hand were highly present in the non-polar lipid fraction (35.40 g/100 g) but absent in the polar lipid fraction. Free fatty acids were also pronounced in the non-polar fraction (34.80 g/100 g) and the total lipid fraction (15.40 g/100 g) but only small amount was detected in the polar lipid fraction (3.90 g/100 g).
Table 3.
GSM lipid composition.
| Fatty acid in GSM oil (g/100 g) |
The amount of fatty acid in free form (g/100 g) |
|||||
|---|---|---|---|---|---|---|
| Total lipid | Polar | Non-polar | Total lipid (15.4%) |
Polar (3.9%) |
Non-polar (34.8%) |
|
| Fatty acid | ||||||
| C14:0 myristic acid | 4.50 | 1.60 | 6.30 | 0.69 | 0.06 | 2.19 |
| C15:0 pentadecanoic acid | 0.71 | 0.31 | 0.83 | 0.11 | 0.01 | 0.29 |
| C16:0 palmitic acid | 16.10 | 13.50 | 16.70 | 2.48 | 0.53 | 5.81 |
| C16:1 palmitoleic acid | 5.60 | 2.20 | 8.80 | 0.86 | 0.09 | 3.06 |
| C18:0 stearic acid | 4.40 | 5.20 | 3.60 | 0.68 | 0.20 | 1.25 |
| C18:1n7 vaccenic acid | 2.20 | 0.91 | 3.00 | 0.34 | 0.04 | 1.04 |
| C18:2n6c linoleic acid | 1.80 | 1.70 | 1.90 | 0.28 | 0.07 | 0.66 |
| C18:3n3 alpha linolenic acid (ALA) | 1.10 | 0.79 | 1.40 | 0.17 | 0.03 | 0.49 |
| C18:3n6 gamma linolenic (GLA) | 0.22 | 0.12 | 0.33 | 0.03 | 0.00 | 0.11 |
| C18:4n3 stearidonic acid (SDA) | 2.10 | 0.80 | 3.40 | 0.32 | 0.03 | 1.18 |
| C20:1 gadoleic acid | 1.80 | 2.30 | 1.40 | 0.28 | 0.09 | 0.49 |
| C20:4n6 arachidonic acid (AA) | 1.90 | 3.00 | 1.00 | 0.29 | 0.12 | 0.35 |
| C20:5n3 eicosapentaenoic acid (EPA) | 16.90 | 13.60 | 20.10 | 2.60 | 0.53 | 6.99 |
| C22:5n3 docosapentaenoic acid (DPA) | 1.30 | 1.60 | 1.00 | 0.20 | 0.06 | 0.35 |
| C22:6n3 docosahexaenoic acid (DHA) | 15.00 | 15.40 | 14.40 | 2.31 | 0.60 | 5.01 |
| ∑SFA | 27.21 | 22.42 | 28.80 | 4.19 | 0.87 | 10.02 |
| ∑MUFA | 10.72 | 6.10 | 14.72 | 1.65 | 0.24 | 5.12 |
| ∑PUFA | 41.40 | 37.68 | 45.07 | 6.38 | 1.47 | 15.68 |
| - ∑n-3 PUFA | 36.71 | 32.31 | 40.83 | 5.65 | 1.26 | 14.21 |
| - ∑n-6 PUFA | 4.14 | 4.97 | 3.49 | 0.64 | 0.19 | 1.21 |
| Lipid class (%) | ||||||
| TAG | 5.10 | n.d. | 35.40 | |||
| FFA | 15.40 | 3.90 | 34.80 | |||
| Sterols | 9.80 | 15.80 | 13.10 | |||
| MAG & DAG | 4.90 | 2.90 | 9.80 | |||
| PL | 62.30 | 79.50 | n.d. | |||
As free fatty acids are well-recognized molecules influencing biological mechanisms, the amount of the fatty acids in free form was retrieved by calculating data in lipid profiles against percentage of free fatty acids in the lipid class; the results are shown in the last three columns of Table 3. The non-polar lipid fraction contained the highest amount of at least three free fatty acids, which were palmitic acid (5.81 g/100 g), EPA (6.99 g/100 g) and DHA (5.01 g/100 g). The total lipid fraction showed around 50% lower abundance of those free fatty acids, and those fatty acids were only minimally present in the polar lipid fraction. Interestingly, although the three fractions were similar in quantities of total SFA, n-3 PUFA and n-6 PUFA as noted above, in free form these differed markedly across the fractions. The non-polar fraction contained over ten-fold more of each of these components in free form compared to the polar fraction, and at least two-fold more compared to the total lipid fraction.
Greenshell mussel was extracted as described in Methods Section 2.2 and three lipid fractions (total, polar, and non-polar lipid) were obtained. The total composition of fatty acid in each individual lipid fraction is presented in g/100 g and shown in the first three data columns. Lipid classes as the percentage of each fraction are indicated at the bottom of the table. The amount of fatty acid in free form was calculated using the fatty acid profile multiple by the percentage of lipid class and the results are shown in the last three data columns. SFA = saturated fatty acid, MUFA = monounsaturated fatty acid, PUFA = polyunsaturated fatty acid, n-3 = omega-3 fatty acid, n-6 = omega-6 fatty acid, TAG = triacylglycerol, DAG = diacylglycerol, MAG = monoacylglycerol, FFA = free fatty acid, PL = phospholipid nd = not detectable.
3.2. Cell viability
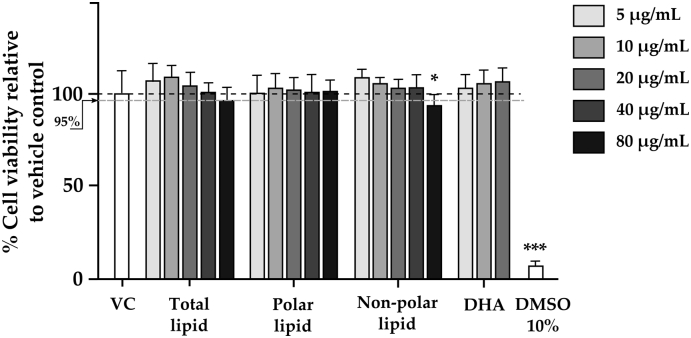
RAW 264.7 cells were incubated for 48 h with individual fractions of GSM lipids at concentrations of 5-80 μg/mL, or with DHA at concentrations of 5-20 μg/mL. The relative numbers of metabolically active cells per well, assumed to equate to viable cells, were determined by MTT assay. The results showed that the vehicle control (VC) containing 0.1% DMSO caused no toxic effects while 10% DMSO as a cytotoxic reference severely reduced cell viability. All GSM fractions and DHA were not cytotoxic at the concentrations of 5–20 μg/mL; however, the percent cell viability when treated with non-polar lipid at 80 μg/mL was lower than 95% indicating mild cytotoxicity (Fig. 1). Therefore, the concentrations of 5, 10, and 20 μg/mL for all lipids fractions were selected for subsequent study in an osteoclast differentiation assay.
Fig. 1.
Cell viability
RAW 264.7 cells were incubated with different fractions of GSM lipids at concentration of 5-80 μg/mL or docosahexaenoic acid (DHA) at concentrations of 5-20 μg/mL in 96-well plates for 48 h. The viability of the cells was measured by MTT assay. Bar chart represents mean ± SD to demonstrate intra-and inter-experimental variability of percent cell viability from three independent experiments each conducted in duplicate. 10% DMSO was used as a cytotoxic reference. Individual fractions were compared with the vehicle control (VC) using one-way ANOVA followed by LSD post hoc test. Asterisk * indicates significantly different when compared to vehicle control at p < 0.05.
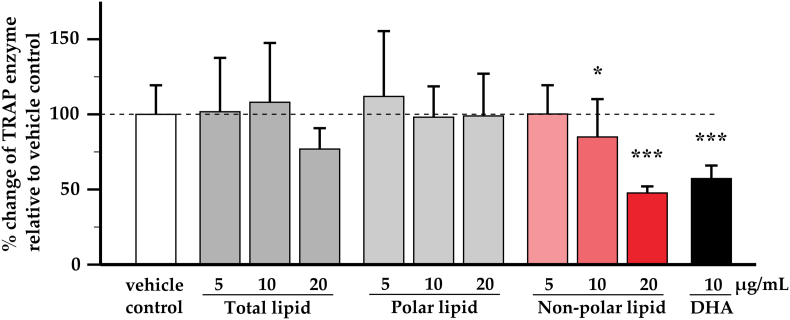
3.3. TRAP enzymatic activity
As can be seen in Fig. 2, only non-polar lipid GSM reduced the TRAP enzyme in a dose dependent manner by which the highest concentration displayed approximately 50% reduction (% change = 47.66 ± 2.20, p < 0.001) when compared to the vehicle control. At the highest concentration the total lipid fraction, which included the non-polar lipids, slightly reduced TRAP. The polar lipids had no effect. The positive control (DHA) significantly reduced percent change of TRAP enzyme.
Fig. 2.
Effect of greenshell mussel lipid on TRAP enzymatic activity
The three fractions of GSM lipids at the concentration range from 5 to 20 μg/mL were tested in osteoclast differentiation assay and 10 μg/mL of docosahexaenoic acid (DHA) was used as a positive control for anti-osteoclastogenesis. The amount of TRAP was measured in supernatants by enzymatic activity. Bar chart shows mean ± SD of percentage of differences between the test substances and the vehicle control (n = 3 per condition). Individual fractions were compared with the vehicle control using one-way ANOVA followed by LSD post hoc test. Asterisk * and *** indicate significant different when compared to vehicle control at p < 0.05 and 0.001 respectively.
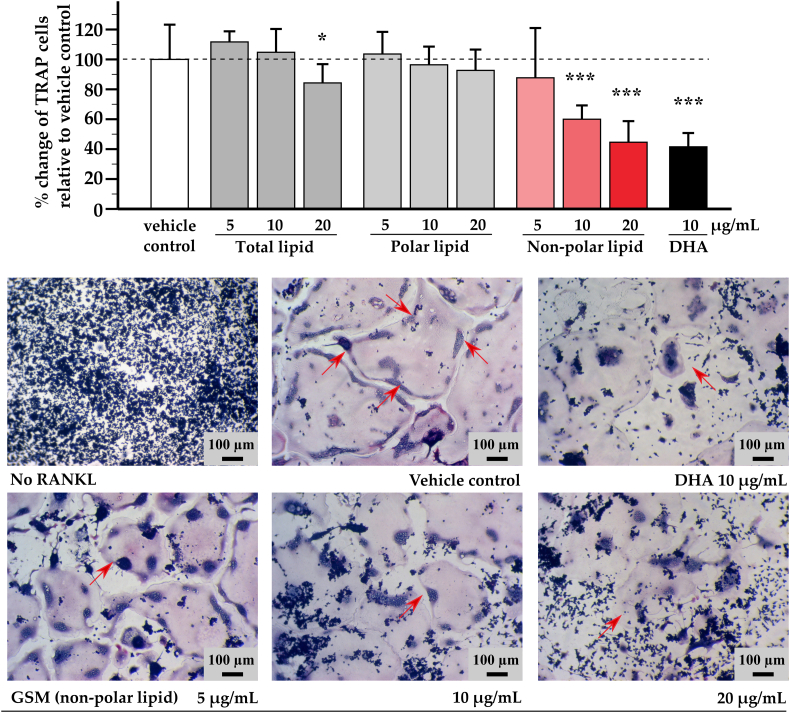
3.4. TRAP-positive cell counting
Excessive osteoclast activity due to increasing of osteoclast numbers is a pathological mechanism of bone associated diseases. As shown in Fig. 3, RAW264.7 cells without RANKL could not transform into osteoclasts, which appear morphologically as large multinucleated cells with red staining in the cytoplasm that demonstrates the presence of the TRAP enzyme. The positive control DHA significantly inhibited the number of RANKL-induced osteoclasts as expected (Boeyens et al., 2014) and reduced the number of red-staining TRAP-positive cells. Non-polar lipid extract at 5-20 μg/mL showed an inhibitory effect in a dose dependent manner and at the highest concentration halved the number of osteoclasts when compared to the vehicle control. The total lipid, in which the non-polar lipids were present, at the highest concentration also significantly reduced the number of TRAP positive cells by approximately 20% (p < 0.05). The polar lipid did not show an inhibitory effect similar to its lack of effect in the TRAP enzymatic assay. As the result of these findings, only the non-polar lipid was investigated in subsequent assays.
Fig. 3.
Effect of greenshell mussel lipid on TRAP-positive cell counting
The three fractions of GSM lipids at the concentration ranging from 5 to 20 μg/mL were tested in osteoclast differentiation assay. All cultures but “No RANKL” were treated with 15 ng/mL RANKL to induce osteoclastogenesis; vehicle control represents the negative control with RANKL only while 10 μg/mL of docosahexaenoic acid (DHA) was used as a positive control for anti-osteoclastogenesis. All treatments, including No RANKL, contained the vehicle (0.1% DMSO). TRAP positive cells with more than four nuclei were visually counted in each well under a microscope and expressed as percentage. Bar chart shows mean ± SD of percentage of differences between the test substances and the vehicle control (n = 3 per condition). Individual fractions were compared with the vehicle control using one-way ANOVA followed by LSD post hoc test. Asterisk * and *** indicate significant differences when compared to vehicle control at p < 0.05 and 0.001 respectively. Representative images show the effect of non-polar lipid GSM on anti-osteoclastogenesis in dose dependent manner. The red arrows identify the border of fusion osteoclasts (Vehicle control) or an osteoclast (the other images).
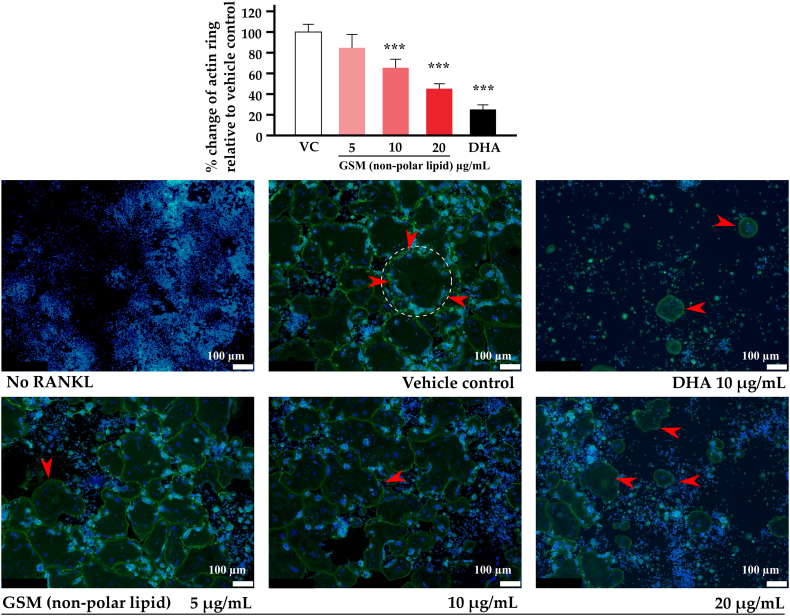
3.5. Actin ring formation
Active osteoclasts create actin rings which are an essential element in the bone resorption process. By incubation with RANKL, RAW 264.7 cells transformed to active osteoclasts showing actin rings, which are large rings of cytoskeletal elements that stained in green due to phalloidin's ability to bind selectively to filamentous actin but not actin monomers (Fig. 4). Inclusion of non-polar lipid reduced the number and size of actin ring formation in a dose-dependent manner. As expected, the positive control (DHA) showed only a few, small sized actin rings.
Fig. 4.
Effect of non-polar lipid GSM on actin ring formation
RAW 264.7 cells were seeded on 24-well culture plates at a density of 1.5 × 104 cells per well with various concentrations of non-polar lipid GSM and 15 ng/mL RANKL. After 5 days of incubation, the cultures were stained with fluorescent dyes phalloidin (green) for actin and Hoechst 3324 (blue) for nuclei and then actin rings were counted in each well. Bar chart shows mean ± SD of percentage of differences between the test substances and the vehicle control (n = 3 per condition). Individual fractions were compared with the vehicle control using one-way ANOVA followed by LSD post hoc test. Asterisk *** indicate significant differences when compared to the vehicle control at p < 0.001. A demonstration of one actin ring is enclosed with a circle in the vehicle control and red arrows indicate actin ring formation. GSM = greenshell mussel, DHA = docosahexaenoic acid. Scale bars = 100 μm.
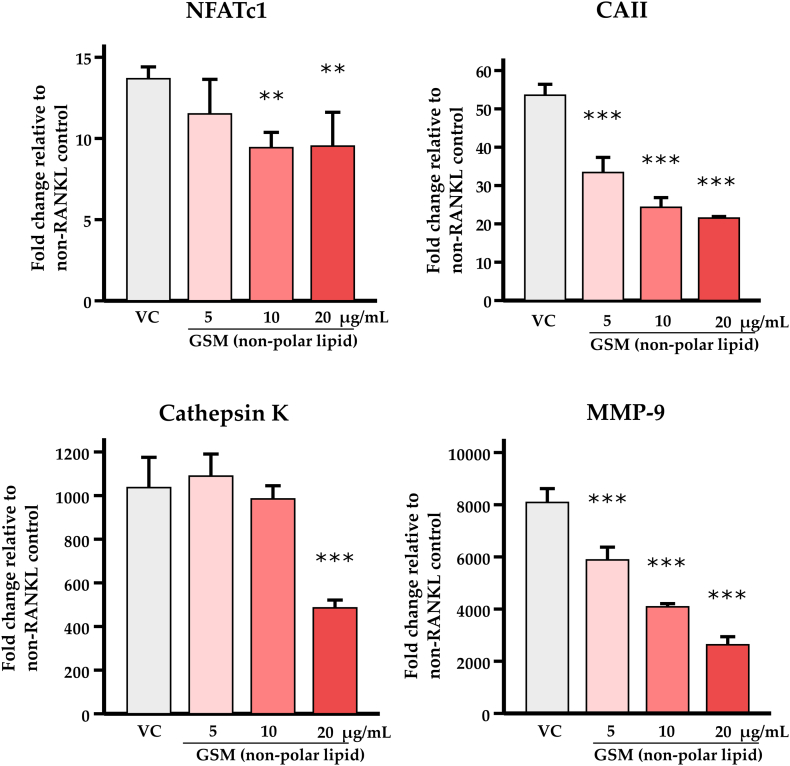
3.6. Gene expression
MMP-9, cathepsin K, carbonic anhydrase II and NFATc1 were expressed at detectable but low levels in undifferentiated cells (data not shown). RANKL-induced osteoclasts however highly upregulated those genes, thus relative quantification using 2−∆∆CT method was compared with non-RANKL control. MMP-9 and carbonic anhydrase II were consistently inhibited by non-polar lipids. Cathepsin K was downregulated at the highest concentration of non-polar lipid (Fig. 5).
Fig. 5.
Effect of non-polar lipid GSM on gene expression
RAW 264.7 cells were seeded on 24-well culture plates at a density of 1.5 × 104 cells per well with various concentrations of non-polar lipid GSM and 15 ng/mL RANKL for 5 days. The specific mRNA expression of gene relevant for osteoclast function was determined by quantitative RT-PCR. Relative quantification to non-RANKL vehicle-only control (0.1% DMSO) was calculated in fold change. The data are shown as mean ± SD (n = 3 per condition). Asterisk * and ** indicate significant differences of each concentration when compared to the vehicle control (VC) using one-way ANOVA followed by LSD post hoc test at p < 0.01 and 0.001 respectively. MMP-9 = Matrix metallopeptidase 9, Cat K = Cathepsin K, CAII = Carbonic anhydrase, NFATc1 = Nuclear Factor of Activated T Cells 1.
4. Discussion
The prevalence of metabolic OA has increased significantly in obese people and postmenopausal women (Woolf and Pfleger, 2003; Litwic et al., 2013; Zhuo et al., 2012). Although the etiology and pathogenesis are inconclusive, many lines of evidence suggest that the initial pathogenesis of this form of OA arises in subchondral bone area rather than from the surface of articular cartilage as occurs in other OA phenotypes (Burr and Gallant, 2012; Goldring and Goldring, 2016; Pelletier et al., 2004; Zhuo et al., 2012). In many circumstances of metabolic-disorder-associated osteoarthritis in obese or postmenopausal subjects, chronic-low-grade inflammation due to a serial activation of inflammatory mediators has a central influence in the disease progression. For instance, elevation of leptin levels in obese people (in which increased body adiposity is more pronounced after menopause) can activate macrophage proliferation, phagocytosis, and natural killer cell function, and subsequently upregulate TNF-α, IL-6, IL-1β, and IL-12 production (Vadacca et al., 2011), resulting in increased RANKL expression on osteoblasts. RANKL directly participates by binding to receptors (RANK) on osteoclasts and initiating the signaling cascade via tumor necrosis factor receptor-associated factor 6 (TRAF6) to activate NFκB and finally stimulate NFATc1, the critical transcription factor for osteoclast differentiation (Boyce et al., 2015). Moreover, the lack of estrogen in postmenopausal women, causing elevation of IL-6 production, can aggravate the bone resorption process (Jilka et al., 1992; Tamura et al., 1993). These relevant circumstances indicate that osteoclasts have a complicated association with many health conditions. Thus, inhibition of osteoclast activity might be a versatile therapeutic approach for osteoporosis and other bone-associated diseases.
There has been an increasing interest of using marine animals as a source of n-3 PUFA to supply the omega3 requirement in health markets since many health authorities have recommended regular daily intake to prevent many chronic diseases (Punia et al., 2019). Therefore, aqua-cultured GSM is a focus as it is sustainable and some health benefits are scientifically evident. This current study has proved another pharmacological activity of GSM which is anti-osteoclastogenesis.
The total lipid in this study was a parent fraction in which the most abundant lipid class was PL (62%). The further separation steps gave rise to the other two daughter fractions, polar and non-polar lipid. By the processes, PL was enriched in more hydrophilic phase, which was the polar lipid fraction, and triglyceride was concentrated in hydrophobic phase, the non-polar lipid. At the end of the oil extraction process, all lipid fractions were enzymatically treated with lipase. This enzymatic process had hydrolysed ester bonds of triglyceride chemical structure and then released fatty acid branches into free form. However, not all units of triglyceride had been digested through this enzymatic reaction as the large amounts of triglycerides still remained in those fractions. The activity of lipase in this study was not able to cleave ester bonds in PL; thus, a small amount of free fatty acid was present in the polar lipid which probably obtained from the reaction with mono-diacylglycerols or naturally occurred by partial hydrolysis (Burri et al., 2012). There has been an increased interest in comparing efficacy between marine n-3 LC-PUFA in triglyceride form versus PL form. It was assumed that PL form may have advantages over triglyceride form in oral bioavailability. However, the advantage of PL on bioavailability or therapeutic efficacy is still not conclusively demonstrated in animal or clinical trials (Schuchardt et al., 2011; Sugasini et al., 2019; Miller et al., 2020).
Our results showed that GSM non-polar lipid fraction contained a higher percentage of free fatty acids (34.8%) than the polar fraction (4%) in which the most abundant of PUFAs was n-3 PUFA, especially EPA (20%) and DHA (14%). Free form of the fatty acids was calculatedly extrapolated in order to estimate the approximate amounts of bioactive molecules in the assay. The data showed that the non-polar lipid contained the highest amount of PUFA especially EPA and DHA and also contained the highest of SFA such as palmitic acid, palmitoleic acid, and myristic acid. At the highest concentration (20 μg/mL) of the tested lipids in the assay, the non-polar lipid would contain approximately 2.8 μg of n-3PUFA (mainly composed of 1 μg of DHA and 1.4 μg of EPA), and 2 μg of SFA (mainly composed of 1.2 μg of palmitic acid and 0.6 μg of palmitoleic acid). These would equate to total DHA at 2.88 μg/mL and EPA at 4 μg/mL, or free-form DHA at 1 μg/mL and free-form EPA at 1.4 μg/mL in the assay conditions using the non-polar fraction at 20 μg/mL. DHA and EPA have been shown to individually significantly decrease TRAP-positive cells in this assay in a linear dose-response fashion in concentrations from 5 to 20 and 10–20 μg/mL respectively (Boeyens et al., 2014) and for DHA to be significantly effective when present as low as 3.2 μg/mL (Rahman et al., 2008). Thus it is reasonable to speculate that the anti-osteoclastic activity observed with the non-polar fraction is due largely to the combination of DHA and EPA. In contrast, the total lipid extract would contain less than half of the free fatty acid amount found in the non-polar lipid, and no individual species of free fatty acid would be available in quantities greater than 0.1 μg in the polar lipid. In the non-polar fraction, approximately a third of the DHA and EPA were present in the free form. This finding leads to the possibility that fatty acid in free form could be at least partially responsible for the biological response with non-polar lipids in this assay.
There is evidence that an anti-inflammatory property of DHA and EPA is exerted by activating one of the free fatty acid receptor (FFAR) family, FFAR4, on cell membranes of macrophages (Itoh et al., 2003; Hirasawa et al., 2005). Similarly, anti-osteoclastogenesis of these free fatty acids was demonstrated via the same mechanism on osteoclasts (Kasonga et al., 2019). In this study, 10 μg/mL of DHA in free form was used as a reference and clearly exhibited the anti-osteoclastogenic effect. It is possible that DHA could activate FFAR4 because FFAR4 is highly expressed in RAW264.7 cells (Tazoe et al., 2008) and DHA possesses the highest binding affinity to this receptor compared to other FAs (Kimura et al., 2020).
Actin ring formation is the essential cytoskeleton structure of active osteoclasts and is also considered as a functional marker (Teitelbaum, 2000). The formation of this structure is the foundation for osteoclasts to attach to the bone surface prior to the excavation of bone matrix. Bioactive compounds capable of disrupting actin ring foundation in osteoclast development could be beneficial in prevention of bone-related diseases. The GSM non-polar lipid extract at a concentration of 5-20 μg/mL effectively inhibited actin ring formation, showing a reduction in both size and number of rings. The positive control treatment DHA at the concentration of 10 μg/mL similarly inhibited the differentiation and actin ring formation of osteoclasts. This evidence is consistent to a previous study using 20 μg/mL of DHA which completely inhibited the actin ring formation (Boeyens et al., 2014).
NFATc1, the master transcription factor of osteoclast differentiation, regulates gene expression of TRAP, cathepsin K, calcitonin receptor, and osteoclast-associated receptor (OSCAR) through cooperation with microphthalmia-associated transcription factor (MITF) and c-Fos (Kim and Kim, 2014). The GSM non-polar lipid suppressed the expression of NFATc1 and the downstream functional enzymes (CAII, cathepsin K and MMP-9) active in bone digestion. All these findings confirm that the non-polar lipid fraction of GSM oil has a potent anti-osteoclastogenic property involving several genes.
Changes in bioavailability due to lipid form may have minimal impact on therapeutic effects as the efficacy and potency of most lipid bioactives are likely to remain similar. On the other hand, the effect of multiple bioactives working in concert can modify the biological response with marked shifts in efficacy or potency. In this experiment, the free form of DHA at the concentration of 10 μg/mL was used as a reference and it produced the strongest biological response in the assay. Treatment with 20 μg/mL of the non-polar lipid exerted a nearly equivalent effect to the reference in the TRAP cell count but was less effective than DHA in the actin ring assay. DHA in the 20 μg/mL non-polar lipid was present at less than a third of the reference for total DHA and one-tenth for free DHA; however, this treatment contained other free fatty acids such as EPA, palmitic acid, palmitoleic acid, and myristic acid. Anti-osteoclastogenic effects have been reported in studies for many lipids such as EPA, GLA, AA (Boeyens et al., 2014), steric acid, palmitic acid (Cornish et al., 2008), palmitoleic acid (van Heerden et al., 2017) and myristic acid (Bao et al., 2020). Despite the obvious evidence of the effects of individual free fatty acid on osteoclasts, it is not clear what the biological response of a fatty acid combination would be as there is no supporting evidence currently available. However, different fatty acids may activate different receptors, pathways or mechanisms and consequently may modulate the biological response in an additive or even synergistic manner. In addition, allosteric modulation of lipid receptors such as GRP40 (FFAR1) has been reported in which a simultaneous activation of the orthosteric and allosteric binding sites by different fatty acids can modulate the response (Audet and Stevens, 2019). There are still many gaps to be filled in order to elucidate which and how fatty acids in the non-polar lipid extract used in this study exerted its activity. Meanwhile we postulate that free fatty acids in the non-polar lipid extract were responsible for some or all of the anti-osteoclastogenic effect, as this extract was found to be high in n-3 LC-PUFAs, DHA and EPA.
Despite the positive evidence of GSM oil on bone health of this study, there are some limitations. Firstly, bone health is dependent on at least two types of cells and functions, namely bone-resorbing osteoclasts and bone-forming osteoblasts. The results of this study only assessed one of these aspects; therefore, further studies in osteoblast assays would be useful to confirm additional bone-protective effects of GSM lipids. Secondly, our results do not disprove biological activity of GSM phospholipids, as the unresponsiveness observed in the test assay may be due to the lack of enzymatic processes which would normally occur in the gastrointestinal system to cleave PL into a metabolically active form. Further, we speculated that the inhibition of osteoclasts was related to free fatty acid composition in GSM oil but we have not yet identified which of these free fatty acids were responsible for the biological response.
Our study concluded that the non-polar lipids relating to free fatty acid of GSM oil inhibited osteoclast differentiation and function in vitro. This bioactivity may reduce the bone resorption process and increase bone formation in humans. While the results of the in vitro studies shed initial light, there is a need for more evidence in animal studies to confirm whether GSM could improve bone mass in cortical or cancellous bone and have health benefits relating to osteoporosis and osteoarthritis prevention.
Funding
This study was funded by the National Science Challenge, High Value Nutrition (HVN) “Musseling up: high-value Greenshell™ mussel foods” (UOAX1421), and a Masssey University College of Health PhD scholarship, as a collaboration between Massey University, Cawthron Institute, and Sanford Ltd.
CRediT authorship contribution statement
Parkpoom Siriarchavatana: Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Marlena C. Kruger: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Funding acquisition. Matthew R. Miller: Conceptualization, Investigation, Writing – review & editing, Funding acquisition. Hong Tian: Conceptualization, Writing – review & editing, Funding acquisition. Frances M. Wolber: Conceptualization, Methodology, Investigation, Resources, Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
H.S.T. has a conflict of interest as she is employed by Sanford Limited which is a mussel producer and exporter.
References
- Audet M., Stevens R.C. Emerging structural biology of lipid G protein-coupled receptors. Protein Sci. 2019;28(2):292–304. doi: 10.1002/pro.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M., Zhang K., Wei Y., Hua W., Gao Y., Li X., Ye L. Therapeutic potentials and modulatory mechanisms of fatty acids in bone. Cell Prolif. 2020;53(2):e12735. doi: 10.1111/cpr.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuglia A., Lacourt M., Girard C., Beauchamp G., Richard H., Laverty S. Osteoclasts are recruited to the subchondral bone in naturally occurring post-traumatic equine carpal osteoarthritis and may contribute to cartilage degradation. Osteoarthr. Cartil. 2016;24(3):555–566. doi: 10.1016/j.joca.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boeyens J.C., Deepak V., Chua W.H., Kruger M.C., Joubert A.M., Coetzee M. Effects of omega3- and omega6-polyunsaturated fatty acids on RANKL-induced osteoclast differentiation of RAW264.7 cells: a comparative in vitro study. Nutrients. 2014;6(7):2584–2601. doi: 10.3390/nu6072584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botter S.M., van Osch G.J.V.M., Clockaerts S., Waarsing J.H., Weinans H., van Leeuwen J.P.T.M. Osteoarthritis induction leads to early and temporal subchondral plate porosity in the tibial plateau of mice an in vivo microfocal computed tomography study. Arthritis Rheum. 2011;63:2690–2699. doi: 10.1002/art.30307. [DOI] [PubMed] [Google Scholar]
- Boyce B.F., Yan X., Jinbo L., Lianping X., Zhenqiang Y. NF-κB-mediated regulation of osteoclastogenesis. Endocrinol. Metab. 2015;30(1):35–44. doi: 10.3803/EnM.2015.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien S., Prescott P., Coghlan B., Bashir N., Lewith G. Systematic review of the nutritional supplement Perna Canaliculus (green-lipped mussel) in the treatment of osteoarthritis. QJM. 2008;101(3):167. doi: 10.1093/qjmed/hcm108. [DOI] [PubMed] [Google Scholar]
- Burr D.B., Gallant M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- Burri L., Hoem N., Banni S., Berge K. Marine omega-3 phospholipids: metabolism and biological activities. Int. J. Mol. Sci. 2012;13(11):15401–15419. doi: 10.3390/ijms131115401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C., Ahluwalia N., Albers R., Bosco N., Bourdet-Sicard R., Haller D., Holgate S.T., Latulippe M.E., Marcos A., Moreines J., Jonsson L.S., M’Rini C., Müller M., Pawelec G., van Neerven R.J.J., Watzl B., Zhao J. A consideration of biomarkers to be used for evaluation of inflammation in human nutrition. Br. J. Nutr. 2013;109(1):34. doi: 10.1017/S0007114512005119. [DOI] [PubMed] [Google Scholar]
- Cobb C.S., Ernst E. Systematic review of a marine nutriceutical supplement in clinical trials for arthritis: the effectiveness of the New Zealand green-lipped mussel perna canaliculus. Clin. Rheumatol. 2006;25(3):275–284. doi: 10.1007/s10067-005-0001-8. [DOI] [PubMed] [Google Scholar]
- Collin-Osdoby P., Osdoby P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods Mol. Biol. 2012;816:187–202. doi: 10.1007/978-1-61779-415-5_13. [DOI] [PubMed] [Google Scholar]
- Cornish J., MacGibbon A., Lin J.-M., Watson M., Callon K.E., Tong P.C., Dunford J.E., van der Does Y., Williams G.A., Grey A.B., Naot D., Reid I.R. Modulation of osteoclastogenesis by fatty acids. Endocrinol. Metab. 2008;149:5688–5695. doi: 10.1210/en.2008-0111. [DOI] [PubMed] [Google Scholar]
- Eason C.T., Adams S.L., Puddick J., Romanazzi D., Miller M.R., King N., Johns S., Forbes-Blom E., Hessian P.A., Stamp L.K., Packer M.A. Greenshell™ mussels: a review of veterinary trials and future research directions. Vet. Sci. 2018;5(2) doi: 10.3390/vetsci5020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M., Fornasiero M.C., Isetta A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods. 1990;131(2):165–172. doi: 10.1016/0022-1759(90)90187-Z. [DOI] [PubMed] [Google Scholar]
- Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016;12:632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogi K., Hosoya M., Tanaka Y., Uejima H., Tanaka H., Maruyama M., Satoh R., Okubo S., Kizawa H., Komatsu H., Matsumura F., Noguchi Y., Shinohara T. Free fatty acids regulate insulin secretion from pancreatic B cells through GPR40. Nature. 2003;422(6928):173. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Jilka R.L., Hangoc G., Girasole G., Passeri G., Williams D.C., Abrams J.S., Boyce B., Broxmeyer H., Manolagas S.C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Kahveci D., Xu X. Repeated hydrolysis process is effective for enrichment of omega 3 polyunsaturated fatty acids in salmon oil by Candida rugosa lipase. Food Chem. 2011;129(4):1552–1558. doi: 10.1016/j.foodchem.2011.05.142. [DOI] [Google Scholar]
- Karsdal M.A., Leeming D.J., Dam E.B., Henriksen K., Alexandersen P., Pastoureau P., Altman R.D., Christiansen C. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthr. Cartil. 2008;16(6):638–646. doi: 10.1016/j.joca.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Kasonga A.E., Kruger M.C., Coetzee M. Free fatty acid receptor 4-β-arrestin 2 pathway mediates the effects of different classes of unsaturated fatty acids in osteoclasts and osteoblasts. Biochim. Biophys. Acta. 2019;1864(3):281–289. doi: 10.1016/j.bbalip.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Khan N.M., Clifton K.B., Lorenzo J., Hansen M.F., Drissi H. Comparative transcriptomic analysis identifies distinct molecular signatures and regulatory networks of chondroclasts and osteoclasts. Arthritis Res. Ther. 2020;22(1) doi: 10.1186/s13075-020-02259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kim N. Regulation of NFATc1 in osteoclast differentiation. J. Bone Metab. 2014;21(4):233–241. doi: 10.11005/jbm.2014.21.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Ohk B., Yoon H.J., Kang W.Y., Seong S.J., Kim S.Y., Yoon Y.R. Docosahexaenoic acid signaling attenuates the proliferation and differentiation of bone marrow-derived osteoclast precursors and promotes apoptosis in mature osteoclasts. Cell. Signal. 2017;29:226–232. doi: 10.1016/j.cellsig.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Kimura I., Ichimura A., Ohue-Kitano R., Igarashi M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020;100(1):171–210. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- Knowles H.J., Moskovsky L., Thompson M.S., Grunhen J., Cheng X., Kashima T.G. Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Arch. 2012;461:205–210. doi: 10.1007/s00428-012-1274-3. [DOI] [PubMed] [Google Scholar]
- Lewinson D., Silbermann M. Chondroclasts and endothelial cells collaborate in the process of cartilage rsorption. Anat. Rec. 1992;233:504–514. doi: 10.1002/ar.1092330403. [DOI] [PubMed] [Google Scholar]
- Li G., Yin J., Gao J., Cheng T.S., Pavlos N.J., Zhang C., Zheng M.H. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013;15:223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwic A., Edwards M.H., Dennison E.M., Cooper C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofvall H., Newbould H., Karsdal M.A., Dziegiel M.H., Richter J., Henriksen K., Thudium C.S. Osteoclasts degrade bone and cartilage knee joint compartments through different resorption processes. Arthritis Res. Ther. 2018;20 doi: 10.1186/s13075-018-1564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S., Logan J.G., Mellis D., Capulli M. Generation and culture of osteoclasts. Bonekey Rep. 2014;3:570. doi: 10.1038/bonekey.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastaglia S.R., Pellegrini G.G., Mandalunis P.M., Chaves M.M.Gonzales, Friedman S.M., Zeni S.N. Vitamin D insufficiency reduces the protective effect of bisphosphonate on ovariectomy-induced bone loss in rats. Bone. 2006;39(4):837–844. doi: 10.1016/j.bone.2006.04.015. [DOI] [PubMed] [Google Scholar]
- McPhee S., Hodges L.D., Wright P.F.A., Wynne P.M., Kalafatis N., Harney D.W., Macrides T.A. Anti-cyclooxygenase effects of lipid extracts from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol. B. 2007;146:346–356. doi: 10.1016/j.cbpb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Miller M.R., Pearce L., Bettjeman B.I. Detailed distribution of lipids in Greenshell™ mussel (Perna canaliculus) Nutrients. 2014;6(4):1454–1474. doi: 10.3390/nu6041454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.R., Kruger M.C., Wynne C., Waaka D., Li W., Frampton C., Wolber F.M., Eason C. Bioavailability of orally administered active lipid compounds from four different Greenshell™ mussel formats. Mar. Drugs. 2020;18(11):524. doi: 10.3390/md18110524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J.P., Boileau C., Brunet J., Boily M., Lajeunesse D., Reboul P., Martel-Pelletier J., Laufer S. The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone. 2004;34(3):527–538. doi: 10.1016/j.bone.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Punia S., Sandhu K.S., Siroha A.K., Dhull S.B. Omega 3-metabolism, absorption, bioavailability and health benefits–a review. PharmaNutrition. 2019;10 doi: 10.1016/j.phanu.2019.100162. [DOI] [Google Scholar]
- Rahman M.M., Bhattacharya A., Fernandes G. Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. J. Cell. Physiol. 2008;214(1):201–209. doi: 10.1002/jcp.21188. [DOI] [PubMed] [Google Scholar]
- Schuchardt J.P., Schneider I., Meyer H., Neubronner J., von Schacky C., Hahn A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations - a comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011;10(1) doi: 10.1186/1476-511X-10-145. 145.10.1186/1476-511X-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Hodges L.D., Wright P.F.A., Cheah D.M.Y., Wynne P.M., Kalafatis N., Macrides T.A. The CO2-SFE crude lipid extract and the free fatty acid extract from perna canaliculus have anti-inflammatory effects on adjuvant-induced arthritis in rats. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2008;149(2):251–258. doi: 10.1016/j.cbpb.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Siriarchavatana P., Kruger M.C., Miller M.R., Tian H.S., Wolber F.M. The preventive effects of greenshell mussel (Perna canaliculus) on early-stage metabolic osteoarthritis in rats with diet-induced obesity. Nutrients. 2019;11(7):1601. doi: 10.3390/nu11071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasini D., Yalagala P.C.R., Goggin A., Tai L.M., Subbaiah P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019;74 doi: 10.1016/j.jnutbio.2019.108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Udagawa N., Takahashi N., Miyaura C., Tanaka S., Yamada Y., Koishihara Y., Ohsugi Y., Kumaki K., Taga T. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc. Natl. Acad. Sci. U. S. A. 1993;90(24):11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tat S.K., Amiable N., Pelletier J.-P., Boileau C., Lajeunesse D., Duval N., Martel-Pelletier J. Modulation of OPG, RANK and RANKL by human chondrocytes and their implication during osteoarthritis. Rheumatology (Oxford) 2009;48:1482–1490. doi: 10.1093/rheumatology/kep300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe H., Otomo Y., Kaji I., Tanaka R., Karaki S.I., Kuwahara A. Roles of short-chain fatty acids receptors, gpr41 and gpr43 on colonic functions. J. Physiol. Pharmacol. 2008;59:251–262. [PubMed] [Google Scholar]
- Teitelbaum S.L. Bone resorption by osteoclasts. Science (New York, N.Y.) 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Treschow A.P., Hodges L.D., Kalafatis N., Macrides T.A., Wright P.F.A., Wynne P.M. Novel anti-inflammatory ω-3 PUFAs from the New Zealand green-lipped mussel Perna canaliculus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;147(4):645–656. doi: 10.1016/j.cbpb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Vadacca M., Margiotta D.P.E., Navarini L., Afeltra A. Leptin in immuno-rheumatological diseases. Cell. Mol. Immunol. 2011;8(3):203–212. doi: 10.1038/cmi.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerden B., Kasonga A., Kruger M.C., Coetzee M. Palmitoleic acid inhibits RANKL-induced osteoclastogenesis and bone resorption by suppressing NF-κB and MAPK signalling pathways. Nutrients. 2017;9(5):441. doi: 10.3390/nu9050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse M.W., Macrides T.A., Kalafatis N., Betts W.H., Haynes D.R., Broadbent J. Anti-inflammatory activity of a lipid fraction (Lyprinol) from the NZ green-lipped mussel. Inflammopharmacology. 1997;5(3):237–246. doi: 10.1007/s10787-997-0002-0. [DOI] [PubMed] [Google Scholar]
- Woolf A.D., Pfleger B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- Xiong J., Onal M., Jilka R.L., Weinstein R.S., Manolagas S.C., O'Brien C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011;17:1235–1262. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzki M., Janosch C., Szechinski J. Perna canaliculus lipid complex PCSO-524 demonstrated pain relief for osteoarthritis patients benchmarked against fish oil, a randomized trial, without placebo control. Mar. Drugs. 2013;11(6):1920–1935. doi: 10.3390/md11061920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Q., Yang W., Chen J., Wang Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012;8(12):729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]