Long before recorded history, fatty acids must have been hard for any organism bent on survival to ignore. Fatty acid combustion yields more energy than almost any other resource— 9 kcal per g, more than double the yield from glucose. Moreover, these aliphatic, anhydrous carboxylic acids are easily stored, lowering the amount of water required for deposition as compared to other energy combustibles. Thus, as a portable energy resource, fatty acids are lighter and require less space, fostering mobility.
The complexity of fatty acids may reflect their importance. Fatty acids vary in chain length and saturation, with an elaborate handling system in place for uptake into the gastrointestinal tract, packaging into distinct lipoproteins for transit through the circulation, release through diverse lipases, cellular uptake by receptors and chaper-oning both inside and outside the cell by fatty acid–binding proteins (FABPs)1 (Fig. 1).
Figure 1.
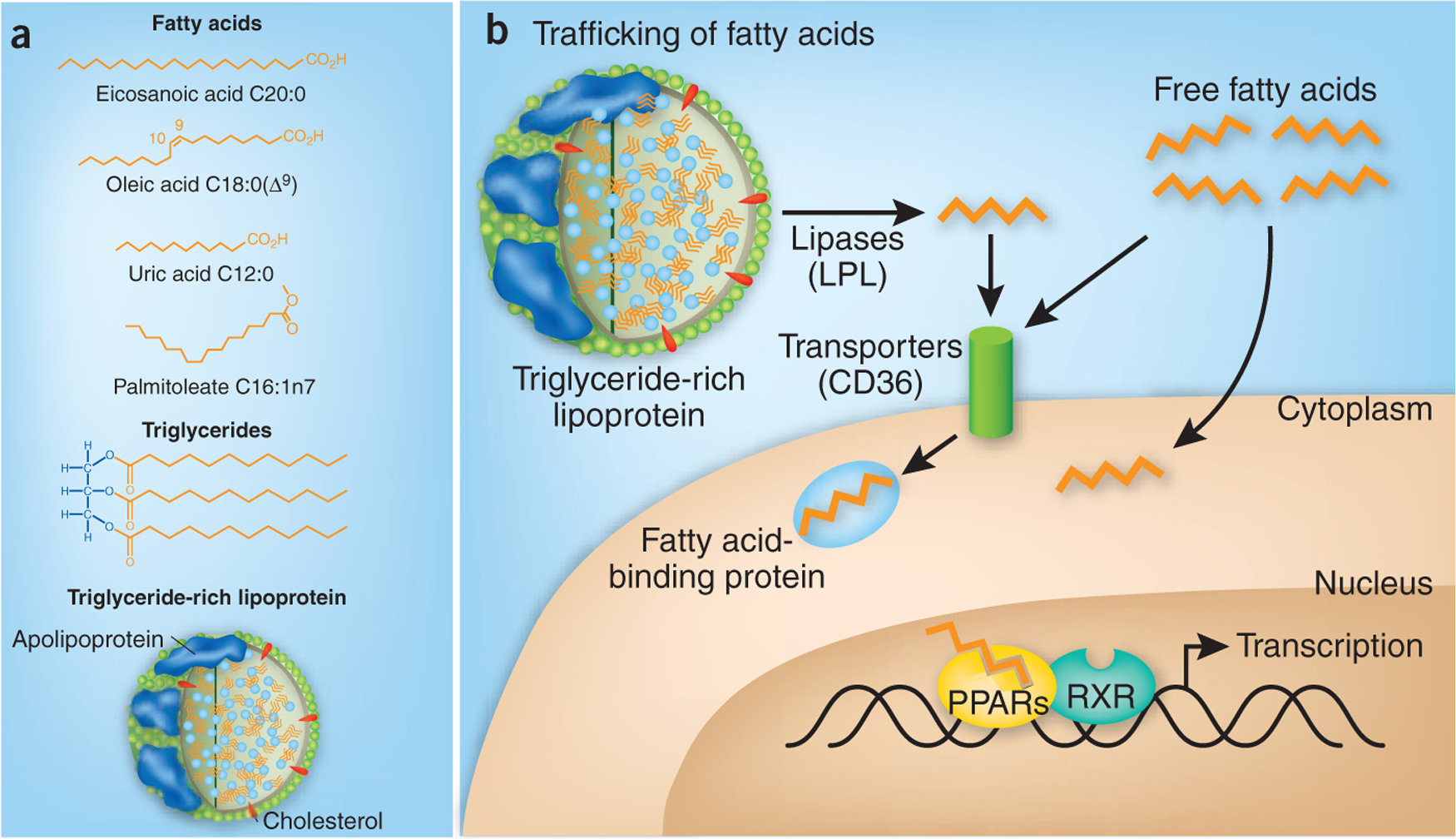
Complexities in fatty acids and their handling. (a) Fatty acids, which vary in chain length, saturation and configuration, can be assembled into triglycerides, which circulate within certain lipoproteins. Specific fatty acids can have different functional effects, as also associated with the metabolism of triglycerides and lipoproteins. (b) Various lipases, for example, lipoprotein lipase (LPL), release fatty acids from circulating lipoproteins, for example, triglyceride-rich VLDL. Fatty acids can enter cells in various ways, including through fatty acid transporters such as CD36. Within cells, fatty acids interact with FABPs that help determine their destination and fate, such as serving as ligands for nuclear receptors (for example, PPARs) that regulate gene expression, including ones controlling fatty acid oxidation and triglyceride metabolism. Elevated levels of FFAs are associated with various cardiometabolic disorders.
If nutrients were in limited supply, the advantage of carefully managing and storing fatty acids must have been clear. Likewise, putting in place transcription factors activated by fatty acids that could initiate programmed control of gene expression, such as peroxisome prolif-erator–activated receptors (PPARs), must have made good engineering sense2.
Fast forward a few million years: dietary calories are no longer a limited commodity for much of industrialized society, promoting the obesity and type 2 diabetes epidemics that dominate many aspects of current medicine3. Despite this shift in available energy, fatty acids remain key players, although now as possible drivers of excess adiposity and its complications.
Elevated concentrations of free fatty acids (FFAs) in the circulation—in other words, fatty acids not secured to triglycerides and tucked into lipoproteins such as very low density lipoprotein (VLDL)—are common in type 2 diabetes, even predicting its arrival4. Elevated FFA concentrations increase oxidant stress, promote endothelial dysfunction, induce inflammatory cytokine release and provoke insulin resistance. Increased FFA levels are also closely associated with hypertriglyceridemia and abnormal accumulation of triglyceride in skeletal muscle and liver, conditions strongly linked to insulin resistance. Moreover, therapies that improve insulin sensitivity typically lower circulating FFA levels5.
It remains under discussion how circulating FFAs and triglycerides reflect what is occurring within cells, where the real toxicity from fatty acids may be occurring, for example through increased formation of cell death- inducing ceramide and insulin resistance- promoting diacylglycerol. Regardless, these connections between FFAs, type 2 diabetes and its complications raise the possibility that increased FFA concentrations are not merely a consequence of obesity and type 2 diabetes but also contributors.
Does the inability to adequately and appropriately ‘put away’ excess fatty acids contribute to cardiometabolic disorders? Several recent studies point in this direction, highlighting along the way how specific complexities of the fatty acid system may be in dictating specific systemic responses.
In the Journal of Clinical Investigation, Kim et al.6 provide evidence that, indeed, at least part of the issue in obesity and insulin resistance may be excess fatty acids. These investigators were able to manipulate the elevated FFA levels in ob/ob mice, a classic mouse model that lacks leptin and is characterized by hyperglycemia, hyperinsulinemia, increased FFA and low levels of adiponectin, a fat-derived anti-inflammatory protein7. Ob/ob mice have increased triglyceride deposition in nonadipose tissues such as the liver, suggestive of an inability to further accommodate fatty acid and triglyceride in fat.
In these ob/ob mice, Kim et al.6 transgenically overexpressed adiponectin—a polymeric protein made in fat, associated with anti-inflammatory, insulin-sensitizing and fat-promoting effects. As a result, these obese mice became even fatter. But with this expansion of fat, glucose and triglycerides were normalized, and FFA levels fell6. Treating ob/ob mice with a PPAR-γ–activating agent (thiazolidinedione), which promotes adipogenesis, had similar effects, increasing fat mass while lowering triglyceride and FFA concentrations. The findings are reminiscent of observations of the effects of thiazolidinediones in humans. Thiazolidinediones lower FFA concentrations, lower glucose levels and expand subcutaneous fat, which may be less pathogenic than visceral adiposity8.
An increased capacity to ‘put away’ fatty acids in an appropriate location may have some bearing on so-called ‘fit but fat’ individuals9. Despite the extensive connections between obesity and risk for diabetes and cardiovascular disease, some overweight individuals seem to maintain insulin sensitivity and avoid these cardiometabolic complications.
The study of Kim et al.6 leaves unresolved the question of whether metabolic improvements in more obese adiponectin-transgenic ob/ob mice are derived from increased fatty acid storage, decreased adverse FFA effects, such as inflammatory mediator release or decreased inflammatory cells in fat, or a direct action of adiponectin. Perhaps the ability of adipocytes to accommodate these FFAs reflects improved adipocyte survival, as adipocyte death can promote adipose tissue inflammation10, or a broader capacity of adipose tissue to expand11.
If increased fatty acid storage protects against diabetes, then does loss of fatty acid storage promote diabetes? In a clinical paradox that informs this area, individuals with lipodystrophy, or a loss of fat stores in specific areas, often have diabetes12. One such lipodystrophy involves loss-of-function variants in PPAR-γ. Thus, although weight loss usually improves insulin sensitivity and obesity worsens it, in the absence of fat, glucose and fatty acid homeostasis is abnormal. In mice, interference with fat or its ablation also causes diabetes13–15. Thus, as humans and other obligate aerobic organisms seem to have known since prehistoric times, fatty acids are a valuable and essential commodity. But, along the way, researchers are also learning new aspects of the impact of specific components of fatty acid signaling and handling.
A spate of recent studies reveals how discrete, specific steps in triglyceride metabolism and fatty acid handling help determine systemic patterns of metabolism, inflammation and atherosclerosis. Ketharisan et al.16, using genome-wide association studies, reported some 30 loci associated with polygenic dyslipidemia, including 11 previously undescribed targets. Genes associated with lower triglyceride levels included those encoding phospholipid transfer protein and fatty acid desaturases 1 and 3, proteins that convert polyunsaturated fatty acids to other signaling molecules, as well as cetain fatty acid–releasing molecules.
Once released from triglyceride-rich lipoproteins at the endothelial surface, fatty acids interact with various proteins that seem designed to guide them appropriately. At the cell surface, fatty acids can be channeled through receptors such as CD36 and on to specific FABPs (Fig. 1). Our own work suggests that the endothelium participates in fatty acid handling: high fat- fed mice lacking endothelial PPAR-γ show decreased adiposity and improved insulin sensitivity despite their considerable dyslipidemia and elevated FFA abundance17. In essence, by leaving fatty acids and triglycerides in the circulation and not metabolizing and storing them in fat, these mice were leaner and more insulin sensitive.
FABPs can direct metabolic phenotypes, with mice genetically deficient for FABPs being protected against atherosclerosis, obesity and diabetes. More recently, Furuhashi et al.18 found that FABP-4 (aP2 in mice) and FABP-5 (mal1 in mice) in hematopoietic cells (macrophages) and adipocytes work together to govern these metabolic effects. Further studies in mice deficient in both FABP-4 and FABP-5 mice suggested that a specific fatty acid released from adipocytes— palmitoleate (C16:1n7-palmitoleate)—could improve systemic metabolism. Cao et al.19 suggest that release of palmitoleate from de novo lipogenesis in fat, stimulated by the lack of FABP in adipose tissue, sends a signal to liver, decreasing lipogenesis, and to muscle, improving insulin action.
All of these lines of investigation reveal fatty acids as a valuable resource that requires careful handling; when they are not handled carefully, pathological consequences ensue. Circulating FFAs and elevated concentrations of triglyceride-rich lipoproteins may literally bathe the endothelium and the circulatory system in a proatherogenic mix, stimulating oxidative stress, inflammation and atherosclerosis. Indeed, fatty meals, high triglycerides concentrations, elevated FFA concentrations and even just a strong family history of diabetes have been associated with impaired endothelial-dependent relaxation of the arteries. Left in the circulation, FFAs can end up being stored in abnormal locations such as in the liver, causing hepatosteatosis, or in muscle, promoting insulin resistance. Perhaps simply lowering FFA levels will improve this scenario, especially if studies such as the ones noted here help lead back to the sources of these fatty acids or specific therapeutic targets involved in their handling. Certainly the benefits of one intervention on these metabolic disorders is clear: even a modest amount of exercise, consistent with an increased combustion of fatty acids, improves insulin sensitivity to a seemingly disproportionate extent.
The pages of journals such as this one are replete with important molecules with diverse, important effects. But when it comes to survival—both long ago during caloric scarcity and now, when calories abound but activity is scarce—the impact of fatty acids and specific components of their handling is impressive. It’s increasingly clear that fatty acids need to be put away appropriately, through combustion or storage.
Physicists, electrical companies and nations would be among those quick to note that energy is power. Investigators studying energy balance can do the same, pointing to the power of fatty acids in both survival and pathogenesis. Perhaps additional work might yet yield interventions that improve adiposity, diabetes and their cardiovascular complications. It seems likely any such advances will involve, either directly or indirectly, specific changes in fatty acid handling.
Figure 2.
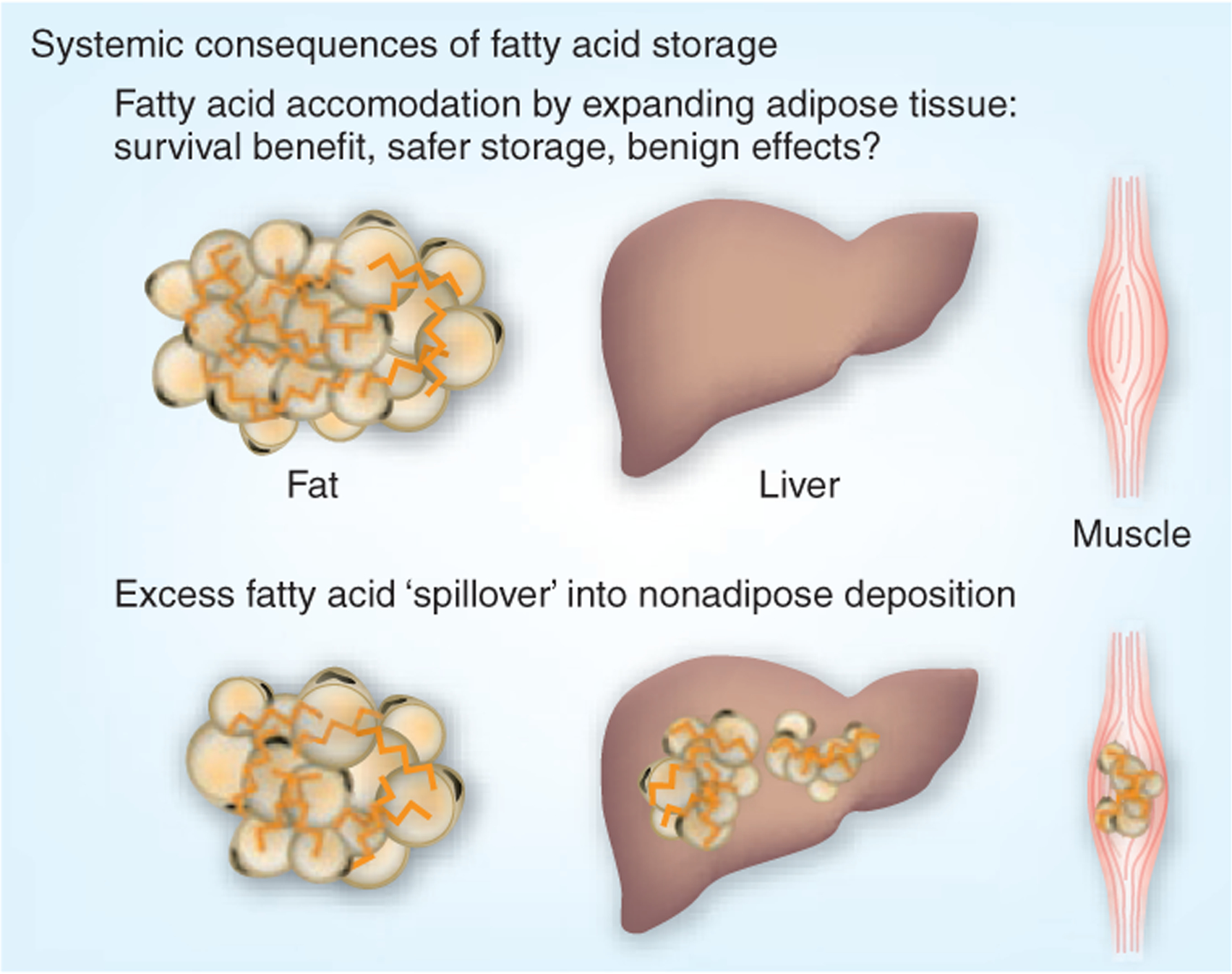
Systemic consequences of fatty acid and triglyceride storage. The direct relationship between elevated FFA levels and cardiometabolic disorders such as hepatic steatosis, diabetes and atherosclerosis implicates fatty acid handling in the pathogenesis of these problems. Recent work suggests that decreasing FFAs by driving them into safer storage, that is, subcutaneous fat, may improve metabolic states, whereas an inability to accommodate fatty acids may promote fat (triglyceride and fatty acid) deposition in abnormal locations, such as liver and muscle, initiating problems with hepatic steatosis and insulin resistance.
References
- 1.Wang S et al. Mol. Genet. Metab 95, 117–126 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Bensinger SJ & Tontonoz P Nature 454, 470–477 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Van Gaal LF, Mertens IL & De Block CE Nature 444, 875–880 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Savage DB, Petersen KF & Shulman GI Physiol. Rev 87, 507–520 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eleftheriadou I, Grigoropoulou P, Katsilambros N & Tentolouris N Curr. Diabetes Rev 4, 340–356 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Kim JY et al. J. Clin. Invest 117, 2621–2637 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speakman J, Hambly C, Mitchell S & Krol E Lab. Anim 42, 413–432 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Yki-Järvinen HN Engl. J. Med 351, 1106–1118 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Wildman RP et al. Arch. Intern. Med 168, 1617–1624 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Strissel KJ et al. Diabetes 56, 2910–2918 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Slawik M & Vidal-Puig AJ Genes Nutr. 2, 41–45 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg A & Agarwal AK Biochim. Biophys. Acta published online, doi: 10.1016/j.bbalip.2008.12.014 (7 January 2009). [DOI] [PMC free article] [PubMed]
- 13.Shimomura I et al. Genes Dev. 12, 3182–3194 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moitra J et al. Genes Dev. 12, 3168–3181 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolonin MG, Saha PK, Chan L, Pasqualini R & Arap W Nat. Med 10, 625–632 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Kathiresan S et al. Nat. Genet 41, 56–65 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanda T et al. J. Clin. Invest 119, 110–124 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuhashi M et al. J. Clin. Invest 118, 2640–2650 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao H et al. Cell 134, 933–944 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]


