Highlights
-
•
Apple by-products are a source of phenolic compounds associated with bioactivities.
-
•
Apple processing industries generate by-products that could be better used.
-
•
This work provides an up-to-date literature overview on extraction techniques.
-
•
Gaps and future trends related to apple by-products are critically presented.
Keyword: Apple pomace, Malus sp., Phenolics compounds, Bioactive compounds, Conventional extraction, Non-conventional extraction
Abstract
Apple is one of the most consumed fruits worldwide and has recognized nutritional properties. Besides being consumed fresh, it is the raw material for several food products, whose production chain generates a considerable amount of by-products that currently have an underestimated use. These by-products are a rich source of chemical compounds with several potential applications. Therefore, new ambitious platforms focused on reusing are needed, targeting a process chain that achieves well-defined products and mitigates waste generation. This review covers an essential part of the apple by-products reuse chain. The apple composition regarding phenolic compounds subclasses is addressed and related to biological activities. The extraction processes to recover apple biocompounds have been revised, and an up-to-date overview of the scientific literature on conventional and emerging extraction techniques adopted over the past decade is reported. Finally, gaps and future trends related to the management of apple by-products are critically presented.
1. Introduction
Apples (Malus sp.) are among the most popular fruits consumed worldwide and are a rich source of valuable chemical compounds (e.g., polyphenols, pectin, and fibers) in the human diet. Bioactive compounds such as polyphenols are naturally produced by a plant or induced by physical or chemical stresses. Generally, polyphenols act as regulators of growth factors and secondary antioxidant defense in different vegetable tissues. These compounds act as antioxidants and anti-inflammatory agents for human health, playing an important role in preventing (or treat) non-communicable chronic diseases (Kumar and Pandey, 2013, Ponte et al., 2021).
Due to the wide variety of phenolic compounds and potential biological properties, the investigation of apple and apple by-products is a boundless field, notably focusing on a better understanding of the main bioactive compounds, the most appropriate extraction methods, purification techniques, and refinement of the final product and its biological applications and analysis methods. Thus, this review aims to fill part of this gap by gathering studies from the past two decades dealing with phenolic extraction from apple and apple by-products through conventional and non-conventional techniques. The primary fruit compounds are comprehensively summarized, and the future trends and perspectives for apple by-product extraction are provided.
2. Apple and its main bioactive compounds
>60 phenolic compounds are currently identified in apple fruit. They are part of plants’ secondary metabolism, performing essential roles, such as growth, defense mechanisms against pathogens, coloring, and aroma properties. Moreover, they are vital to growth and reproduction and are synthesized mainly when the plant is submitted to stressful conditions, such as infections, wounds, and ultra-violet radiation (Haminiuk et al., 2012, Hyson, 2011).
Phenolic compounds have one or more aromatic rings in their molecular structures with one or more hydroxyl groups, which are related to the human body’s antioxidant properties, i.e., they react with free radicals forming stable radicals (Fu et al., 2011). The antioxidant properties promote biological benefits such as anti-cancer, antimicrobial, and cardiovascular protection (Carocho & Ferreira, 2013), among others.
Table 1 presents an overview of the main classes, subclasses, and health benefits of phenolic compounds from the apple, which can be classified into non-flavonoids or flavonoids. Among the non-flavonoids, phenolic acids are subdivided into hydroxycinnamic and hydroxybenzoic. The quinic and caffeic acids have been the most identified compounds in different apple cultivars, reaching between 4 and 18% of total polyphenols in the fruit. In addition, 5́-caffeoylquinic or chlorogenic acid, p-coumaroylquinic, and p-coumaric acids have also been reported. Both hydroxycinnamic and hydroxybenzoic acids can be found in higher amounts conjugated to components of cell walls, such as cellulose or lignin, or even forming protein complexes that can be bounded to sugar or organic acids (Barros et al., 2009).
Table 1.
An overview of the main classes, subclasses, and health benefits of the major phenolic compounds identified in apples.
| Classes | Subclasses | Compounds | Benefits associated | References |
|---|---|---|---|---|
| Acids | Hydroxycinnamic | Chlorogenic; Cryptochlorogenic | Antiobesity, antihypertensive and neuroprotective | Naveed et al. (2018) |
| Caffeic; 4-Caffeoylquinic; 5-Caffeoylquinic | Aid against injury to ischemia–reperfusion | Sato et al. (2011) | ||
| p-coumaric; 5-p-Coumaroylquinic; p-coumaric acid-O-hexoside; 4-O-p-coumaroylquinic; p-coumaroylquinic | Lung anticancer, analgesic and mitigating effects in diabetes | Pei et al. (2016) | ||
| Ferulic | Vasodilator, antidiabetic and anticarcinogenic action in gastrointestinal tumors | Kumar & Pruthi (2014) | ||
| Sinapic acid-O-glucoside | Antidiabetic, aid against neurodegeneration and anxiety | Chen (2016) | ||
| Hydroxybenzoic | Syringic | Cardiovascular diseases, cerebral ischemia and liver anticancer | Srinivasulu et al. (2018) | |
| Gentisic | Antioxidant and anti-inflammatory | Zhou et al. (2017) | ||
| Protocatechuic | Antiobesity and antihyperglycemic | D’Archivio et al. (2014) | ||
| Salicylic | Anti-inflammatory and chemoprotective properties | Dachineni et al. (2016) | ||
| Ascorbic | Antitumor, antiviral, antioxidant | Macan et al. (2019) | ||
| Vanillic | Antioxidant, anti-inflammatory, and neuroprotective effects | Ullah et al. (2020) | ||
| Flavonoids | Flavonols | Quercetin; Quercetin-3-O-diglucoside; Quercetin-3-O-galactoside; Quercetin-3-O-glucoside; Quercetin-3-O-rhamnoside; Quercetin-3-O-rutinoside; Quercetin-3-O-xylanoside; Quercetin-O-xylosyl-pentoside | Anti-inflammatory and antioxidant | Lesjak et al. (2018) |
| Rhamnetin; Rhamnetin-3-O-glucoside | Antiviral | Ferenczyova et al. (2020) | ||
| Isorhamnetin-3-O-galactoside, isorhamnetin-3-O-glucoside, isorhamnetin-3-O-rutinoside, isorhamnetin-3-O-rhamnoside | Antihypertensive, aid against cardiovascular diseases | Eisvand et al. (2020) | ||
| Kaempferol-O-glucoside | Breast, prostate and colon anticancer properties | Wang et al. (2019) | ||
| Rutin | Antitumor, antimicrobial, anti-inflammatory, and neuroprotector | Budzynska et al. (2017) | ||
| Reynoutrin | Antithrombotic, anticancer, and antidiabetic | Li et al. (2016) | ||
| Avicularin | Anti-allergic and aid against gastric cancer development | Guo et al. (2018) | ||
| Quercitrin | Neuropharmacological actions, anti-viral and anticancer | Zhi et al. (2016) | ||
| Flavones | Apigenin | Antidiabetic, aid against amnesia, Alzheimer's disease, depression and insomnia | Salehi et al. (2019) | |
| Chrysoeriol | Inhibit the activity of pancreatic lipase | Ramirez et al. (2016) | ||
| Luteolin; Luteolin-7-O-galactoside; Luteolin-7-O-glucoside | Anti-inflammation, anti-allergy, and pancreatic anticancer | Imran et al. (2019) | ||
| Flavanones | Hesperidin-O-pentoside | Neuroprotection | Hajialyani et al. (2019) | |
| Eriodictyol; Eriodictyol-hexoside | Aid to Insulin secretion | Hameed et al. (2018) | ||
| Naringenin-7-O-glucoside; Naringenin-7-O-neohesperidoside; Naringenin-7-O-rutinoside; Naringenin-O-glucuronide. | Immunomodulatory agent, neuroprotective and anti-atherosclerotic properties | Hartogh & Tsiani (2019) | ||
| Flavanols | (−)-Epicatechin; (−)-Epigallocatechin 3-gallate; (−)-Epigallocatechin; (−)-Epigallocatechin 3-gallate | Potential in the treatment and diabetes and cardiac pathology | Shay et al. (2015) | |
| (+) Catechin | Prevention of hypertension and high blood cholesterol, Stomach anticancer | Matsui (2015) | ||
| Procyanidin B1; Procyanidin B2; Procyanidin B3; Procyanidin B5 Procyanidin C1 | Esophagus anticancer | Connor et al. (2014) | ||
| Anthocyanins | Cyanidin 3-O-galactoside; Cyanidin 3-O-arabinoside; Cyanidin-7-arabinoside; Cyanidin 3-O-xyloside | Anti-inflammation and aid to decrease cholesterol levels | Ding et al. (2006) | |
| Peonidin | Antioxidant, anticancer and antidiabetic | Rajan et al. (2018) | ||
| Dihydrochalcones | Phloretin; 3-Hydroxyphloretin 2′-O-glucoside; Phloretin 2′-O-xylosyl-glucoside | Antimicrobial | Barreca et al. (2014) | |
| Phloridzin; 3-hydroxyphloridzin | Antihyperglycemic |
Makarova et al. (2014) |
||
Flavonoid groups in apples can be subdivided into flavonols (71–90%), flavan-3-ols (1–11%), dihydrochalcones (2–6%), and anthocyanins (1–3%). Catechins, epicatechins, and procyanidin B2 are the main flavan-3-ols in apples’ skin and pulp. The subgroup of flavanols is usually found in different quercetin glycosides, with quercetin 3-glycoside as the leading representative (Bondonno et al., 2017, Hyson, 2011). From the anthocyanins group, cyanidin 3-galactoside is the most representative in the red apples’ skin since it is responsible for the red color of the apples. Dihydrochalcones, such as phloridzin and phloretin, are associated with the fruit sugar content, such as glucose and xyloglucan. Dihydrochalcones are found predominantly in apple seeds and stems, typical compounds of the industrial by-product of the fruit (Da Silva et al., 2020, Jakobek and Barron, 2016).
Different apple species, cultivar characteristics, maturation degree, storage conditions, among others, are the main factors that determine the phenolic concentration in the fruit (Jakobek & Barron, 2016). Exposure to ultraviolet radiation, predators, and soil diversities, for example, make the fruit skin more concentrated in phenolics than other parts, such as pulp and seeds.
In general, the dry basis concentration of phenolics may be higher in fresh fruit than in pomace as the latter comes from industrial processing (press, thermal processes for drying, exposure to light, among others). In addition, pomace results from the mixing of all parts of the fruit and, therefore, its chemical composition is a consequence of this mixture and the treatment that the by-product has undergone. For example, the flavonoids are predominant in the skin, while higher phenolic acids amount is found in the pulp and dihydrochalcones, hydroxycinnamic acids, flavan-3-ols, proanthocyanin B2 and flavonols are present in the seeds. The pomace can, but not necessarily must consist of these phenolic compounds. Fruit by-products are complex matrices that present trends and not rules about their composition as they may have undergone different industrial processes that impact their final state.
Nevertheless, the most significant compounds in the apple pomaces are chlorogenic acid, caffeic acid, (+)-catechin, (−)-epicatechin, rutin, quercetin glycosides, and phlorizin. As shown in Table 1, such compounds display several biological activities, allowing opportunities to design new products, especially from their extracts. Therefore, extraction configures an essential step in the reuse chain of by-products.
It is worth mentioning that apple seeds may contain other compounds such as amygdalin and cyanogenic diglucoside. Cyanide toxicity in humans occurs at doses between 0.5 and 3.5 mg/kg body weight. However, this compound is present in apple seeds between 0.06 and 0.2 mg cyanide equivalent/g of apple seeds (Bolarinwa et al., 2015, Lu and Foo, 1998), depending on the cultivation. (Xu et al., 2016). Even so, attention should be paid to extraction products from the pomace containing seeds to monitor these compounds’ concentration.
3. Extraction of bioactive compounds
Several techniques have already been employed to extract bioactive compounds from apples-based raw materials in laboratory scales. Whole apple and apple by-products are similar matrices regarding the composition of the phenolic compounds and, therefore, the selection of the method for the extraction of them depends more on the characteristics of the methods than on the matrix. The extraction processes are commonly classified as conventional and modern extraction methods. Both have been used to recover compounds from apple-based matrices, which are the focus of this section, providing practical and theoretical characteristics and giving perspectives on the direction of this topic in future years.
The classical or conventional extraction methods have been used for at least ten years to obtain compounds from apple-based matrices; maceration, Soxhlet, mild homogenization, and magnetic stirring are the main examples. Maceration and Soxhlet have been less employed than mild homogenization and magnetic stirring, and two first have mainly been used by published papers for comparison or validation of alternative extraction techniques, or even to evaluate some initial extraction parameters like the mass ratio between solvent and sample (Azmir et al., 2013, Ferrentino et al., 2018, Moreira et al., 2017). Conventional methods are of easy operation at lab scale; however, they present some drawbacks like the lack of fine temperature control, light exposure, and longer extraction time, which may reduce the extraction yields and the extract concentration of target compounds (Azmir et al., 2013, Mustafa and Turner, 2011). Additionally, for a long time, they employed extraction solvents that nowadays are avoided due to environmental and safety issues. Thanks to concerns and warnings released by the scientific community, such dangerous solvents have been avoided and replaced by solvents Generally Recognized as Safe (GRAS).
Some of the mentioned conventional extraction techniques, such as Soxhlet and magnetic stirrer, have been applied to apple-based raw materials mainly as preparative methods for lab-scale analytical purposes. Additionally, conventional extractions for process engineering purposes often stumble on scaling up limitations, which corroborates the search for innovative and scalable extraction technologies.
Moreover, scalability is a critical point that needs to be evaluated in new proposals. Unfortunately, most of the published works dealing with laboratory scales do not address discussions about how feasible the method is at large scales. Furthermore, to the best of our knowledge, the possibility of scaling-up is a pivotal point to cross de boundary between research laboratories and industry.
Modern or non-conventional methods have emerged in the last years to overcome the mentioned downsides. In addition, to receive the seal of green extraction or green techniques, they present some advantages over the conventional processes due to the lower energy and solvent consumption, shorter extraction time, and well-defined parameters that result in better extraction performance (higher yields and extract concentration). The ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), pressurized-liquid extraction (PLE), and supercritical fluid extraction (SFE) are examples of non-conventional techniques to recover phenolic compounds from vegetable matrices. These methods have been employed to extract apple bioactive compounds in the past few years. Usually, the studies propose to find the best operational conditions (e.g., type of solvent, temperature, time, pressure, power) to obtain phenolics from this fruit (Armenta et al., 2019, Azmir et al., 2013, Pingret et al., 2012, Santos et al., 2019, Da Silva et al., 2020, Souza et al., 2020, Sumere et al., 2018, Xu et al., 2017). Nevertheless, except for SFE that is already well established in industrial scales, the other methods are starting to gain ground in the industry and therefore still need adjustments for larger scales.
3.1. Conventional extraction techniques
Most of the studies found in the past decade literature dealing with apple compounds obtention through conventional extraction are based on the extraction power of different solvents, temperature, extraction time, and solid to liquid ratio (SLR: sample mass (g)/solvent volume (mL)) (Fromm et al., 2013, Hernández-Carranza et al., 2016, Reis et al., 2012). Table 2 presents works that applied conventional techniques to extract phenolics from apple and apple by-products, including the operational parameters and analyzed most important compounds. Ferrentino et al. (2018) used the Soxhlet apparatus to obtain phenolics from apple pomace using ethanol as the solvent for six hours. Rana et al. (2015) used homogenization to study the effects of different solvents on the extraction of phenolic compounds from apple pomace. The authors observed that acetone was the best solvent to recover phenolic compounds. Mild homogenization was also used to extract phenolics from apple tree wood (Moreira et al., 2017), applying ethanol as the solvent. Some important compounds of the fruit are mainly nonpolar. Thus, organic solvents or their mixtures give the highest yield in conventional extractions.
Table 2.
Summary of publications using conventional extraction techniques for recovery phenolic compounds from apple, and their respective operational extraction conditions (solvent, temperature, time, and SLR), the most important compounds analyzed, and the best conditions reported.
| Technique | Sample | Solvent (%, v/v) | Temperature (°C) | Time (min) | SLR (g/mL) | Most important compounds analyzed | Yield of extraction (TPC) | Best conditions reported | References |
|---|---|---|---|---|---|---|---|---|---|
| HMG | Whole Apples | ACT 50–80 MEOH 70–99.9 |
10–40 | 10–20 | 0.017 | Chlorogenic Acid and Phloridzin | 5.90 mg/g | 84.5% MEOH, 15 min, 28 °C or 65% ACT, 20 min, 10 °C | Alberti et al. (2014) |
| HMG | Apple pomace | ACT 50 ETOH 50 MEOH 50 |
30 | 60 | 0.05 | Quercetin, Phloridzin and Phloretin | 3.31 mg/g | 50% ACT, 60 min, 30 °C | Rana et al. (2015) |
| HMG | Apple pomace | Water MEOH 20–100 ACT 20–100 |
RT | 90 | 0.075 | Hydroxycinnamic Acids, Flavonols, Flavanols, Dihydrochalcones, and Flavones | n.i. | Water, 40% MEOH, 40%, ACT, RT, 90 min | Reis et al. (2012) |
| HMG | Apple tree wood | ETOH 20–80 | 20–55 | 180–1140 | 0.025 | Phenolics Acids, and Phloridzin | 43.2 mg/g | 50% ETOH, 55 °C, 120 min | Moreira et al. (2017) |
| Mag- str | Apple seeds | ACT 60–70 | 0–42 | 60–1440 | 1.25 | Hydroxybenzoic Acid, Flavan-3-ols, and Dihydrochalcone | 2.99 mg/g | 60–70% ACT, 25 °C, 60 min | Fromm et al. (2013) |
| Mag-str | Apple pomace | Water ETOH 50–100 MEOH 50–100 |
60 | 30 | 0.04 | Gallic Acid/Protocatechuic Acid Glucoside, Chlorogenic Acid, Epicatechin, Rutin, Hyperoside, Quercetin Derivatives, Quercetin Rhamnoside, Phloretin Xylosyl Glucoside, Phlorizin | – | 80% MEOH, 50% ETOH, 60 °C, 30 min | Da Silva et al. (2020) |
| Mag-str | Apple pomace | Water | 20–60 | 30–720 | 0.004 | Quercetin, Epicatechin, Chlorogenic Acid, Procyanidin B2, and Phloretin | 6.89 mg/g | Water, 60 °C, 720 min | Hernández-Carranza et al. (2016) |
| MCT | Apple pomace | ETOH | RT | 60 | 0.05 | Gallic Acid, Chlorogenic Acid, Catechin, Rutin and Phloridzin | n.i. | – | Grigoras et al. (2013) |
| MCT | Apple pomace | ETOH MEOH |
RT | 1440 | 0.1 | n.i. | 148 mg/g | MEOH, 1440 min, RT, 0.1 SLR | Rezaei et al. (2013) |
| MCT | Apple pomace | Water | 100 | 37 | 0.016 | Phlorizin, Epicatechin, Quercetin, and Phloretin | 2.41 mg/g | – | Ferrentino et al. (2018) |
| SX | Apple pomace | ETOH MEOH |
RT | 60–240 | n.i. | n.i. | 221 mg/g | MEOH, 180 min, RT | Rezaei et al. (2013) |
| SX | Appel pomace | ETOH | RT | 360 | 0.033 | Phlorizin, Epicatechin, Quercetin, and Phloretin | 4.13 mg/g | – | Ferrentino et al. (2018) |
ACT: acetone; ETOH: ethanol; HMG: homogenization; MEOH: methanol; Mag-str: magnetic stirring; MCT: maceration; n.i: not informed parameter; SLR: solid to liquid ratio; RT: room temperature; SX: Soxhlet; TPC: calculated in terms of total phenolic content depending on the study cited.
It is important to highlight those other parameters, such as temperature and extraction cycles, that also affect the target compounds yield. Pure water appears as a green and low-cost alternative solvent; however, it is necessary to increase the temperature or the number of extraction cycles to assure higher yields (Fromm et al., 2013, Hernández-Carranza et al., 2016). Reis et al. (2012), for example, used pure water, acetone (20–100%, v/v), and methanol (20–100%, v/v) to extract phenolics from apple pomace. Three extraction cycles were used for pure water, while only one extraction cycle was enough for acetone and methanol to achieve equivalent yields. The authors noticed that all solvents could extract phenolics, but pure water extracted less of the target compounds due to the polarity and compatibility. Water also led to a lower selectivity, promoting the extraction of other components and reducing the relative purity of the extracts. Methanol (70–99.9%, v/v) and acetone (50–80%, v/v) were compared in a study conducted by Alberti et al. (2014) to extract chlorogenic acid and phloridzin from apple by mild homogenization. The authors observed that the best operational condition for methanol was 84.5% (v/v) for 15 min at 28 °C, and acetone at 65% (v/v) for 20 min at 10 °C. Corroborating this finding, Rezaei et al. (2013) showed that extractions performed with methanol recovered higher yields of phenolics than those performed with ethanol, using both the Soxhlet (221 mg tannic acid equivalent (TAE)/g dry matter (d.m.)) and maceration (148 mg TAE/g d.m.) techniques. The methanol and acetone’s ability to extract phenolic compounds from plant sources is well established. Although both solvents are acceptable for analytical purposes, substitutes (GRAS) are very welcome, especially when applying the extract for human consumption, besides all the environmental impact inherent in producing these solvents. Moreover, safer substitutes should be applied to replace these organic solvents, such as non-volatile alternative solvents, namely, ionic liquids (ILs), eutectic solvents, and surfactants.
Besides the most appropriate solvent, the particle size is another determinant physical parameter that must be evaluated to guarantee a successful extraction, especially on conventional extraction techniques. The smaller particle size seems for high-performance extraction of bioactive compounds, which promotes better mass transfer, and consequently, the higher release of the bioactive compounds in the extraction solution in a shorter extraction time. The sample preparation steps, e.g., drying or packaging, also may affect the extraction yield and the extract concentration since factors like oxidation of the components induced by light or oxygen can occur during the raw material pretreatment. The drying procedures’ influence on the extraction yield was studied Rana et al. (2015), using quercetin, phloridzin, and phloretin from apple pomace as target compounds. The authors showed that higher phenolics content was found in freeze-dried samples, followed by those dried in an oven and dried under the sun. Although, in general, drying at high temperatures and air circulation is harmful to the maintenance of phenolic compounds, attention must be paid to raw materials with a high content of polyphenol oxidase, such as apple-based products (Illera et al., 2019), since drying above the polyphenol oxidase’ inactivation temperature may be useful to preserve the raw material polyphenols.
Several works used magnetic stirrer as a conventional extraction technique to obtain phenolics from apples. Among the advantages of this method are the mild temperatures, which are favorable to extract thermolabile compounds. Another alternative to overcome temperature downsides is increasing extraction time.
Fromm et al. (2013) evaluated different temperatures (0–42 °C) and extraction time (60–1440 min) to extract phenolics from apple seeds, and the authors observed a higher yield of phenolics at 25 °C for 60 min. Da Silva et al., 2020, Hernández-Carranza et al., 2016 used magnetic stirrer to extract phenolics from apple pomace. In both cases, 60 °C was the optimum temperature, but the use of methanol (80%, v/v) and ethanol (50%, v/v) by Da Silva et al. (2020) resulted in a shorter extraction time (30 min) than that obtained by Hernández-Carranza et al. (2016) (720 min), who used water as the solvent. On this subject, it is worth mentioning that the magnetic stirrer is an alternative to lab-scale extraction that must be replaced by the mechanical stirrer to higher processing scales. In addition, such extraction methods need later processes to separate the solids from the extracts, which impacts the cost and time to carry out a batch. This issue is not problematic on laboratory scales; however, it can generate difficulties and the need for high-capacity filtering equipment on industrial scales.
During an extraction procedure, the SLR plays a fundamental role. An adequate proportion is needed to release the bioactive compounds in the solvent efficiently and, consequently, achieve an adequate extraction (Rostagno et al., 2010). It is important to highlight that the ideal ratio used in the extraction process depends on the interaction between matrix (biomass) and solvent; the biomass particle and their interaction with the solvent directly influence the viscosity of the solution, which affects the mass-transfer coefficient and the extraction efficiency.
Additionally, the extraction yield is limited by the target compounds’ solubility in the solvent; therefore, the amount of solvent must ensure that it is enough to solubilize all the target compounds in the raw material. However, excess solvent diminishes the extract concentration and raises the procedure cost due to the solvent cost and the higher solvent mass to be evaporated from the extract. Hence, the economic evaluations are very welcome to define based on production cost the best SLR. The published paper generally defines the SLR based on the higher yield or extract concentration without considering cost issues. Moreover, especially for dried apple-based raw materials, high SLR is not advised since the pectin extraction may increase the solution viscosity and diminish the recovery of the compounds. Examples of SLR are reported by Grigoras et al. (2013) that used maceration to recover phenolics from apple pomace using an SLR of 0.1 and Hernández-Carranza et al. (2016) that used SLR of 0.004.
The increased concern about the environmental issues due to the waste generated after the extraction processes and the costs of operation and the safety of the workers have stimulated the chase for alternative methods that are cleaner, cheaper, and eco-friendly. Conventional methods can generate satisfactory results, and indeed, some of them are used at industrial scales for different raw materials; however, based on the drawbacks mentioned above and the need to change classical manufacture production, non-conventional techniques come out as feasible options.
3.2. Non-Conventional extraction techniques
The use of non-conventional extraction techniques to recover bioactive compounds has significantly increased in the past decade. Classified by some authors as green techniques, they have many advantages compared to the conventional ones, such as shorter extraction time, better temperature control, lower sample and extract light exposition, scalability, selectivity that impacts the extract concentration, and higher extract yields. Moreover, non-conventional techniques allow the use of a lower amount of solvent, and in some cases, besides the solvent is defined as GRAS, it is cyclically recycled (Belwal et al., 2018, Chemat et al., 2019). The most common methods employed to obtain bioactive compounds from natural matrices are UAE, MAE, PLE, and SFE (Da Silva et al., 2020, Ferrentino et al., 2018, Moreira et al., 2017, Wang et al., 2019).
3.2.1. Ultrasound-assisted extraction (UAE)
The ultrasound-assisted extraction (UAE) has been used to obtain a broad spectrum of natural compounds through ultrasonic bath and/or ultrasonic probe. The ultrasound (US) allows continuous compression/decompression cycles of the bubbles inside the extraction solvent that cause the cavitation phenomena (He et al., 2016). In addition, the US may act in the extraction by single or combined mechanisms, including fragmentation, erosion, capillarity, detexturation, and sonoporation (Chemat et al., 2017). The sum of US effects in the extraction medium facilitates the disruption of the physical structure of the raw material, diminishes the sample particle size, enhances diffusional and convective mass transfer, and, therefore, increases the extraction efficiency by a better solute–solvent contact.
Table 3 summarizes the UAE’s main works to obtain phenolic compounds from apple by-products, including their operational parameters, type of US device, and most important compounds analyzed. Ajila et al. (2011) tested different solvents to extract polyphenols from apple pomace and concluded that 80% acetone (v/v) promoted the highest target compounds yield, resulting in three-fold higher extraction efficiency than the same process performed using pure water as the solvent.
Table 3.
UAE and MAE applied to obtain phenolics from apple including the operational parameters, the most important compounds analyzed, and the best conditions reported.
| Technique | Sample | Solvent (%, v/v) | Temperature (°C) | Time (min) | Frequency (kHz) | Power (W) | SLR (g/mL) | Most important compounds analyzed | Yield of extraction (TPC) | Best conditions tested | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| US bath | Apple pomace | Water ACT 60–80 ETOH 60–80 MEOH 60–80 |
40 | 30 | n.i | n.i | 0.05 | n.i | n.i. | 80% ACT, 30 min, 40 °C | Ajila et al. (2011) |
| US bath | Apple pomace | Water | 9.9–40 | 5–55 | 25 | 0.335–0.764 (W/cm2)** | – | (−)Epicatechin, Phloridzin, Chlorogenic Acid | 5.55 mg/g | Water, 40 °C, 40 min, 0.764 W/cm2 | Pingret et al. (2012) |
| US bath | Unripe Apple | ETOH 50–70 | 50–70 | 20–30 | n.i | 420–560 | 0.04 | (-)-Epicatechin, Procyanidin B2, Chlorogenic Acid, Procyanidin B1 | 13.26 mg/g | 50% ETOH, 519.39 W, 30 min, 50 °C | Yue et al. (2012) |
| US bath | Apple pomace | ETOH | RT | 30 | n.i | n.i | 0.05 | Gallic Acid, Chlorogenic Acid, Catechin, Rutin, Ursolic Acid, Phloridzin | n.i. | – | Grigoras et al. (2013) |
| US bath | Fresh, old and apple peel | MEOH 40–100 MEOH (0.1% HCl) |
RT | 5–15 | n.i. | n.i. | 0.04–0.1 | Flavonols, Anthocyanins, Dihydrochalcones, Flavan-3-Ols | 0.181 to 4.992 mg/g | 80% MEOH, 15 min | Jakobek et al. (2015) |
| US bath | Apple | Water | 20 | 0–30 | 21 or 40 | 180 | 0.25 | n.i | 5.43 and 10.46 mg/g | Water, 20 °C, 21 kHz, 30 min and 180 W or Water, 20 °C, 40 kHz, 5 min | Wiktor et al. (2016) |
| US bath | Apple pomace | Water ETOH Water (1% Rokanol) |
20 | 30 | n.i. | n.i. | 0.05 | Catechin, Quercetin, Phloretin derivates, p‐coumaryl‐quinic, Cryptochlorogenic, Chlorogenic acid | 0.88 mg/g | ETOH, 20 °C, 30 min, SLR 0.05 | Malinowska et al. (2018) |
| US bath | Apple peel and pulp | MEOH 20–100 | 20–80 | 20–40 | 35 | n.i. | 0.06–0.02 | Chlorogenic Acid, Epicatechin, Phloridzin, Catechin, Hyperoside, Quercitrin | n.i. | MEOH 100%, 33 min, 65 °C (peel) or 20% MEOH, 40 min, 80 °C (pulp) | Mihailović et al. (2018) |
| US bath | Apple wood | Water ETOH 20–100 MEOH 20–100 ACT 20–100 |
60 | 30 | n.i. | 800 | 0.01 | Epicatechin gallate, Kaempferol-3-glucoside, Naringin, Naringenin, Rutin, Phloridzin, Phloretin, Procyanidin B1, Procyanidin B2, Vanillic acid, Gallic acid, Ferulic acid, p-Coumaric acid, Caffeic acid | 29 mg/g | Ethanol 40 – 80%, 60 °C, 30 min, 800 W | Withouck et al. (2019) |
| US bath | Apple pomace | Water ETOH 50–100 MEOH 50–100 |
60 | 30 | 37 | n.i. | Gallic Acid/Protocatechuic Acid Glucoside (Pcag), Chlorogenic Acid, Epicatechin, Rutin, Hyperoside, Quercetin Derivatives, Quercetin Rhamnoside, Phloretin Xylosyl Glucoside, Phlorizin | – | MEOH 80, 30 min, 60 °C, 37 kHz | Da Silva et al. (2020) | |
| US probe | Flesh and apple peel | Water | 50 | 180 | 24 | 0–118 | 0.1 | Catechin | – | – | Wang et al. (2018) |
| US probe | Apple peel | ETOH 0–96 | 25–40 | 30 | 24 | 0–400 | 0.1 | n.i | – | 50% ETOH, 50 W | Wang et al. (2019) |
| UAMME | Apple pomace | Water ETOH Water (1% Rokanol) |
20 | 30 | n.i. | n.i. | 0.05 | Catechin, Quercetin, Phloretin derivates, p‐coumarylo‐ quinic, Cryptochlorogenic, Chlorogenic acid | 6.99 mg/g | Water (1% Rokanol), 20 °C, 30 min, SLR 0.05 | Malinowska et al. (2018) |
| MAE | Apple pomace | Water ACT 60–80 ETOH 60–80 MEOH 60–80 |
30–80 | 5–15 | – | 400 | 0.05 | n.i | 16.12 mg/g | 80% ACT, 10 min, 40–60 °C | Ajila et al. (2011) |
| MAE | Apple pomace | ETOH Ethyl Acetate Water:MEOH |
RT | 1.5 | – | 1000 | 0.05 | Gallic Acid, Chlorogenic Acid, Catechin, Rutin, Ursolic Acid, Phloridzin | n.i. | – | Grigoras et al. (2013) |
| MAE | Apple pomace | Water ETOH 35–100 |
RT | 5–20 | – | 90 – 360 | 0.1 – 0.3 | n.i. | 127 mg/g | 90 W, 15 min, | Rezaei et al. (2013) |
| MAE | Apple tree wood | ACT 70 ETOH 60 |
RT | 0.5–3 | – | 100–900 | 0.083–0.25 | Phloridzin, Caffeic Acid, Chlorogenic Acid, Quercetrin | 15.8 mg/g | SLR 0.09, 60% ETOH, 735 W, 2.48 min | Chandrasekar et al. (2015) |
| MAE | Apple dust | ETOH 40–80 | RT | 15–35 | – | 400–800 | 0.1 | n.i. | 30.79 mg/g | 40% ETOH, 25 min, 400 W | Pavlić et al. (2017) |
| MAE | Apple tree wood residues | Water ETOH 20–100 MEOH 20–100 |
66–134 | 3–37 | – | n.i | 0.004 | Phenolics Acids, Phloridzin, Myricetin, Kaempferol-3-O-Glucoside, Naringin and Quercetin-3-O-Glucopyranoside | 47.7 mg/g | 60% ETOH, 20 min, 100 °C | Moreira et al. (2017) |
| MAE | Apple skin | ETOH 20–100 | 90–150 | 30–90 | – | n.i. | 0.1 | Epigallocatechin gallate, Rutin Quercetin, Gallic acid, Catechin and Protocatechuic acid | 50.4 mg/g | Casazza et al. (2020) |
ACT: acetone; ETOH: ethanol; MEOH: methanol; n.i: not informed parameter; RT: room temperature; TPC: calculated in terms of total phenolic content depending on the study cited; UAMME: ultrasound‐assisted micelle‐mediated extraction. *Ultrasonic power expressed as power (W) to mass of sample (g) ratio. **Ultrasonic intensity expressed as power (W) per area of the internal diameter of the ultrasound reactor (cm2).
Da Silva et al. (2020) evaluated the effects of different solvents (pure water, ethanol (50–100%, v/v), and methanol (50–100%, v/v/)) on the extraction of phenolics from apple pomace, being 80% methanol (v/v) the best solvent for recovering phenolic compounds.. Organic solvents, such as ethanol or its mixtures, are the most indicated to extract phenolics (e.g., flavonoids) from apples (Grigoras et al., 2013, Yue et al., 2012).
Withouck et al. (2019) showed no significant differences when extracting phenolics from apple wood with organic solvent mixtures between 40 and 80% (v/v solvent/water), at 60 °C, for 30 min using UAE. However, ethanol was significantly better than others (methanol and acetone) in extracting these compounds. In contrast, pure water is the most suitable to extract polar compounds such as phenolic acids (Pingret et al., 2012, Wang et al., 2018, Wiktor et al., 2016).
Different properties of each solvent are responsible for enhancing the extraction of a specific class of compounds. Water acts as an emollient of the samples eluting the highest polar compounds, while organic solvents easily penetrate in the samples extracting compounds with less polarity than those extracted by water (Azmir et al., 2013, Da Silva et al., 2020, Wang et al., 2019, Wang et al., 2019, Yue et al., 2012). Thus, extractions with a gradient solvent (more polar to less polar, or vice versa) could be an excellent alternative for recovery sequentially different compounds in specific fractions, optimizing the full potential of the biomass. Besides, attention must be paid to using solvents with a low boiling point because, depending on the ultrasound power and exposure time, the solvent temperature may reach the boiling temperature, which causes solvent loss by volatilization.
Temperature is another parameter that needs to be carefully controlled in UAE since the mechanical process of the ultrasonic waves may be dissipated as heat, resulting in overheating the solvent and degradation of the aimed compounds (Chemat et al., 2011). Also, the heat transferred to the system can generate an additional cost to the process (in costs and environmental terms).
For example, Grigoras et al. (2013) extracted phenolics from apple pomace using ethanol as the solvent at room temperature. Otherwise, Pingret et al. (2012) tested different temperatures (10 to 40 °C) using ethanol as the extraction solvent for recovery phenolic compounds from apple pomace. They noticed that the highest extraction yields were obtained at 40 °C, using 50% ethanol, 0.142 W/g, 25 kHz, in 45 min, and water at 40 min and 0.764 W/cm2, respectively.
Yue et al. (2012) studied the phenolic extraction from unripe apple varying temperature and extraction time from 50 to 70 °C, and from 20 to 30 min, respectively. The authors observed that the lowest temperature and the longest time generated the highest total phenolic yield (13.26 ± 0.56 mg GAE/g). Mihailović et al. (2018) evaluated different temperatures (20–80 °C) and extraction time (20–40 min) to obtain phenolics from apple skin and pulp. In this case, 65 °C and 33 min, with pure methanol, were considered the optimum conditions for apple skin, while 80 °C and 40 min with 20% methanol was the best condition for the pulp; therefore, different matrixes and solvents composition slight interfered with the results about time and temperatures.
Still, regarding the temperature, according to a review work recently provided by Kumar et al. (2021), the higher temperature UAE operation may affect the extraction yield by three different hypotheses. One describes that high temperature increases solvent vapor in the cavitation bubble reducing the pressure gradient inside and outside the bubble. Therefore, even though the number of cavitation bubbles is significant, they implode with less intensity at a high temperature, causing lesser damage to the cell and decreasing the yield.
The second hypothesis includes that the increased shear stress causes the degradation of the desired component due to the large number of cavitation bubbles formed at higher temperatures and subsequent collapse. Moreover, the third hypothesis regards reducing the solvent surface tension at higher temperatures, reducing the cavitation bubble’s intensity. Thus, the temperature must be carefully evaluated to determine the specific range that potentializes the extraction performance and prevents the target compounds’ degradation.
In addition to temperature, frequency and US power are the most studied parameters in UAE. Table 3 shows that the frequency interval applied to recover phenolic compounds from different apple samples varied from 21 to 40 kHz. Low-frequency US (<120 kHz) has been reported to be preferable in extracting bioactive compounds from natural raw materials; the low-frequency US allows forming a smaller number of cavitation bubbles with a large diameter than the high-frequency US (>120 kHz). Higher bubbles enhance the cavitation effect by damaging the cell structure and releasing the target compounds, increasing the extraction yield (Kumar et al., 2021, Chemat et al., 2017).
Interestingly, Wiktor et al. (2016) evaluated the effects of frequency (21 or 40 kHz) and extraction time (0–30 min) on the phenolics from apple tissue after being treated by US, concluding that 21 kHz for 30 min or 40 kHz for 5 min displayed the highest yield of total phenolics compounds of extraction namely, 543.4 ± 21.3 and 1046.5 ± 18.9 mg chlorogenic acid/100 g d.m.. The results reported by the authors suggest that the increase in the frequency requires less extraction time and result in higher yield, and therefore there could be a frequency inflection point to maximize the extraction yield. However, frequency is still a fertile field for research since few studies are available focusing on the effect of this parameter. Besides, very-high frequencies (beyond 500 kHz) may be applied for reversible and irreversible sonoporation similar that occurs in the biological application (molecules cell uptake and cell destruction, respectively) (Chemat et al., 2017); however, this issue also deserves further research to be validated for extraction of natural materials.
US power is also a crucial parameter to optimize UAE since it affects the aforementioned US mechanisms that impact the extraction performance. Pingret et al. (2012) reported a direct relation of the US power with the extraction yield of phenolics from apple pomace. On the other hand, Yue et al. (2012) evaluated US power (420–560 W) to extract phenolics from unripe apples. They observed that the highest power diminished the compounds’ recovery, where 50% ethanol (v/v) at 519.39 W, 30 min, and 50 °C, the optimum between the tested conditions.
Wang et al. (2019) studied different temperatures (25–40 °C) and ultrasonic power (0–400 W) to extract polyphenols from apple skin using ultrasonic probes. Counterintuitive, the authors noticed that the optimum condition was achieved at lower power (50 W) in a shorter extraction time (30 min). Thus, along with the temperature and due to the same reasons, the US power must be carefully evaluated. Works have reported that the increase in the US power favor the extraction yield up to a certain point, and above of it, the US mechanisms are affected by the bubbled formed; a high concentration of high bubbles leads to an inter-bubble collision, deformation, and nonspherical collapse resulting in the less impact between bubbles and raw material, which negatively impact the yield (Kumar et al., 2021). Moreover, the very high power may affect the extraction yield of target compounds due to molecular degradation, especially when high powers are combined with water as solvent. The US can dissociate the water molecules in free radicals that may trigger the oxidation of compounds and breakage of the bonds (Dias et al., 2021).
The type of ultrasonic device is another determinant parameter that influences the extraction yield. Baths and probes are the ultrasonic devices usually used. Ultrasonic baths are the most widely available and cheap. In this configuration, the energy may be spread out in the vessel to be transferred to the sample. Besides, in baths, the ultrasonic waves may have difficulties penetrating smaller particles, taking longer extraction times and diminishing the extraction efficiency (Chemat et al., 2017).
In contrast, ultrasonic probes induce the transfer of ultrasonic energy directly to the medium. Consequently, cavitation phenomena are more pronounced, improving the extraction yields (Dias et al., 2017). On the other hand, direct contact of the probe with the medium can contaminate the extract with metals due to erosion. Additionally, ultrasonic probe processes only one sample at a time, while ultrasonic baths allow processing several samples simultaneously (Dias et al., 2021).
Ultrasound technology has proven effective in extracting various raw materials, mainly due to increased extraction yield and reduced processing time. However, the technology still has several challenges to overcome, especially for industrial scales; evidence indicates that it is necessary to consider the cost of implementation and energy consumption (greener processes must demand less energy). In this sense, the application of ultrasonic waves at specific moments of the process should be experimentally and economically validated. For instance, application at the beginning of the extraction process can accelerate solvent saturation. Similarly, application at the end of the extraction process can increase the diffusional mass transfer rate (Dias et al., 2021). In both cases, time and energy can be saved. Another critical aspect to consider is the scale-up; it is advisable to keep the energy density constant (J/m3) in the scale transposition, implying in the power increase proportionally to the new volume, or else the extraction time will need to be extended. Accordingly, ultrasonic probes are restricted for scales of small volume; alternatively, continuous systems or ultrasonic baths with a larger radiating surface and an agitation system could be used (Chemat et al., 2017, Dias et al., 2021).
3.2.2. Microwave-assisted extraction (MAE)
The combination of solid–liquid extraction with microwave radiation is defined as microwave-assisted extraction (MAE). Briefly, MAE is applied to extract soluble products in a fluid using microwave energy to heat the solvent-sample mixture, accelerating the vegetable matrices’ cell walls’ crack (or rupture). Moreover, microwave energy modifies the biological tissues’ physical properties, improving access through the porosity and the extraction yields (Kubra et al., 2016). Generally, the solvent’s choice to extract phenolics compounds from apples is based on solvents with water. The water’s high dielectric constant is a crucial point in MAE since the microwave absorption depends on the solvents’ higher polarity. The higher absorption of the waves promotes an increment in the mixture’s temperature, cell disruption, and consequently a better extraction of the compounds from the matrix (Bouras et al., 2015).
Open and closed systems can be used in MAE. The extraction in open systems is performed at atmospheric pressure. Consequently, the maximum temperature is defined by the solvent boiling point, and losses of vapors can be prevented by cooling systems on the top of the extraction vessel that promotes the condensation of vapors. Closed systems avoid this problem by pressure increase and allow temperatures above the critical point. However, the temperature rises rapidly in closed systems, difficulting the temperature control, damaging thermolabile compounds. Therefore, the temperature should be sufficient to enhance the extraction yield, however not high enough to degrade the target compounds (Rostagno & Prado, 2013).
Table 3 also summarizes the studies that extracted phenolic compounds from apples by MAE. The main operational parameters, the most important compounds analyzed, and the best conditions reported are shown.
Ajila et al. (2011) studied the effects of different solvents, temperature, and time on the MAE of phenolics from apple pomace, concluding that 80% methanol (v/v) at 40–60 °C for 10 min promoted the best extraction yield (16.12 mg/g). In a similar approach, Chandrasekar et al. (2015) tested the influence of different solvents, time, and power on the extraction performance of phenolics from apple tree wood. In this case, the best condition was achieved for 60% ethanol (v/v) at 735 W for 2.5 min yielding 15.8 mg/g. The authors showed that the interaction between solvent and power was significant and inverse, which means that the extraction yield rises when one parameter increased and the other decreased. In another study, Rezaei et al. (2013) concluded that increasing microwave power from 90 W to 360 W decreases extraction yields (127 to 104 mg TAE/g d.m.). They also observed 65% ethanol with SLR at 0.2 for 15 min, and power of 90 W was better to extract phenolic compounds from apple pomace using MAE. It is worth mentioning that the power choice needs to consider a combination with other operational parameters (e.g., temperature, solvent concentration, time) to achieve a complete optimized process.
According to Table 3, the temperature used in the studies to recover phenolics from apples by MAE ranged from 30 to 134 °C. Moreira et al. (2017) evaluated the effects of different solvents (pure water, ethanol (20–100%, v/v), and methanol (20–100%, v/v)), temperature (66–134 °C), and time (3–37 min) to extract phenolics from apple tree wood residues. The use of 60% ethanol (v/v) at 100 °C for 20 min was considered the best condition, recovering 47.7 ± 0.9 mg GAE/g d.m. that included phenolic acids, phloridzin, myricetin, kaempferol-3-o-glucoside, naringin, and quercetin-3-o-glucopyranoside. On the other hand, Pavlić et al. (2017) showed that the lowest yield of phenolics from apple dust was found in MAE extraction processes where the investigated parameters were higher (60% ethanol, 35 min at 800 W). Moreover, they showed that only factors such as time and ethanol concentration and their interaction were significantly relevant (p < 0.05) in the recovery of total phenolics. That is, the longer time may promote the degradation of sensitive compounds during the extraction process.
Casazza et al. (2020) identified that the higher the proportion of organic solvent (ethanol), temperature, and time, the higher the recovery of total flavonoids (13.9 mg catechin equivalents/g d.m.) for extracting flavonoids sub-class (such as epigallocatechin gallate, rutin, quercetin, gallic acid, catechin, and protocatechuic acid) from apple skin using MAE. However, further experiments are necessary to understand the relationship between temperature and other operational parameters when extracting phenolics from apples by MAE, especially the stability of compounds.
To sum up, MAE has been developed over several years at laboratory scales to overcome scale-up limitations. Nowadays, the technology becomes a reality and, although a limited few studies underline the potential of MEA at industrial scales, some industrial or pilot installations can offer the possibility to extract around 100 kg of fresh material.
3.2.3. Pressurized liquid extraction (PLE)
Another alternative and potentially greener technique used to recover bioactive compounds from vegetable matrices is the PLE, also called accelerated solvent extraction (ASE), among others. PLE has some advantages, such as faster extraction time, reduced solvent consumption, and precise adjustment of the operational parameters (Machado et al., 2015). Moreover, GRAS solvents (e.g., water and/or ethanol) make the process safer for the operators and less pollutant than conventional techniques. PLE can operate at higher temperatures (above the boiling point of the solvent) since the solvent is pressurized, with allows the solvent kept in the liquid state; such a feature enhances the solvent properties and increases the desorption and solubility of the aimed compounds (Mustafa & Turner, 2011).
Table 4 shows the reports of PLE to recover phenolic compounds from apple and apple by-products, including the most important compounds analyzed and the best conditions reported.
Table 4.
Summary of studies that applied PLE and SFE to obtain phenolic compounds from apple including the operational parameters, the most important compounds analyzed, and the best conditions reported.
| Technique | Sample | Solvent (%, v/v) | Temperature (°C) | Time (min) | SLR (g/mL) | Pressure (MPa) | Most important compounds analyzed | Yield of extraction (TPC) | Best conditions reported | References |
|---|---|---|---|---|---|---|---|---|---|---|
| PLE | Apple peel and pulp | Water MEOH 100 |
40–100 | 5–15 | 0.09 or 0.13 | 6.89–10.34 | (+)- Catechin, Procyanidin B2, (−)-Epicatechin, Procyanidin, Phloretin-2′-Xyloglucoside, Phloridzin, Hyperoside, Isoquercitrin, Quercetin Glycosides + Rutin, Avicularin, Quercitrin, Chlorogenic Acid, P-Coumaric Acid derivative | n.i. | 40 °C, static extraction time (5 min), 6.89 MPa, 2 extraction cycles | Alonso-Salces et al. (2001) |
| Apple pomace | ETOH 14–85 | 64–135 or 153–200 | 5 | 0.04 | 10.3 | Chlorogenic Acid, Caffeic Acid, P-Coumaric Acid, Quercetin Glycoside, Rutin, Quercetin Glycoside, Quercetin Glycoside, Phloretin Glycoside | 14.42 mg/g | 60% ETOH, 102 °C | Wijngaard & Brunton (2009) | |
| Apple pomace | ETOH | 40 | 15 | 0.08 | 10 | Gallic Acid, Chlorogenic Acid, Catechin, Rutin, Ursolic Acid, Phloridzin | n.i. | – | Grigoras et al. (2013) | |
| Apple | MEOH ACT 70 |
RT | 1–15 | 0.007 – 0.07 | 1 | Flavan-3-Ol Monomers ((+)-Catechin, (−)-Epicatechin), Phloridzin, Chlorogenic Acid, Hyperoside, Isoquercitrin, Quercitrin | 4.113 mg/g | MEOH, 15 min, 3 extraction cycles | Franquin-Trinquier et al. (2014) | |
| Apple pomace | Water ETOH 50–100 MEOH 50–100 |
60 | 30 | 0.04 | 10 | Gallic Acid/Protocatechuic Acid Glucoside (Pcag), Chlorogenic Acid, Epicatechin, Rutin, Hyperoside, Quercetin Derivatives, Quercetin Rhamnoside, Phloretin Xylosyl Glucoside, Phlorizin | – | ETOH 50–80%, 30 min, 60 °C, 10 MPa | Da Silva et al. (2020) | |
| SFE | Apple pomace | CO2 + ETOH 75 | 50 | 180 | 0.27 | 25 | Chlorogenic Acid, Catechin, Epicatechin, Phloridzin, Quercetin-3-Glucoside, Quercetin-3-Galactoside, Quercetin-3-Arabinoside, Quercetin-3-Xyloside, Quercetin-3-Rhamnoside | – | CO2 + 25 mol% cosolvent (96% ETOH), 25 MPa, 50 °C | Massias et al. (2015) |
| Fresh apple pomace | CO2 + ETOH 5 | 45–55 | 120 | n.i. | 20 and 30 | P-OH Benzoic Acid, Phlorizin, Epicatechin, Quercetin, Phloretin | 8.87 mg/g | 5% ETOH (co-solvent), 30 MPa, 45 °C, 120 min | Ferrentino et al. (2018) | |
| Apple seeds | CO2 | 35–60 | 0–120 | 0.008 – 0.002 | 10–30 | n.i. | 8.21 mg/g | 25 MPa, 60 min, 45 °C, 2.5 mL/min |
Panadare et al. (2021) | |
ACT: acetone; ETOH: ethanol; MEOH: methanol; n.i: not informed parameter; RT: room temperature; TPC: calculated in terms of total phenolic content depending on the study cited.
Alonso-Salces et al. (2001) studied the effects of different solvents (pure water and methanol (100%, v/v)), temperature (40–100 °C), extraction time (5–15 min), and pressure (6.89–10.34 MPa) to obtain phenolics from apple skin and pulp, concluding that 40 °C, 6.89 MPa, and 5 min was the optimum operating condition to recover phenolic compounds. Temperatures above that diminished the yield by hydrolysis or polymerization reactions.
Wijngaard and Brunton (2009) used PLE to extract phenolics from apple pomace in different ethanol concentrations (14–85%, v/v) and temperatures (64–135 °C or 153–200 °C) at 10.3 MPa. Milder temperatures (75–125 °C) promoted a better extraction yield (1442 ± 58 mg GAE/100 g d.m.) of total phenolics at 102 °C. Further increase in temperature (up to 200 °C) was concluded to form undesirable products, such as hydroxymethylfurfural.
Divergences regarding the optimal temperature for obtaining bioactive compounds are recurrent in the literature. Therefore, it is essential for the scientific community to critically analyze the results when the temperature is evaluated. For example, it is understandable that the total phenolic yield increases even at high temperatures (>100 °C), as there may is, in addition to extraction, the hydrolysis of the lignocellulosic material into phenolic acids and other compounds, contributing to confusion when comparing results and the phenomena governing the process, and thus, the yield. This effect is particularly relevant when evaluating results based on spectrophotometric analysis, such as total phenolics, which can be affected by many compounds, leading to conflicting observations. Therefore, a critical analysis of each target compound individually is necessary, and with that, a better and deeper understanding of the effect of temperature can be achieved.
Franquin-Trinquier et al. (2014) obtained phenolics by PLE from apple using pure methanol and acetone (70%, v/v) at room temperature and 1 MPa, during extraction intervals of 1 to 15 min, and extraction cycles from 1 to 3. The authors noticed that pure methanol was the most suitable solvent to extract chlorogenic acid, hyperoside, quercitrin, and ideain, for 15 min and three extraction cycles.
Da Silva et al. (2020) recovered phenolics from apple pomace by PLE and other techniques (UAE, shaker, and magnetic stirring). The authors employed different solvents (pure water, ethanol (50–100%, v/v) and methanol (50–100%, v/v)) at 60 °C, 10 MPa for 30 min, being methanol (50–80%, v/v) the best solvent to extract phenolics acids and flavonoids. They found higher amounts of those compounds in PLE than in the other techniques (at least the double yield compared to conventional processes).
Although PLE is very useful to reach higher yields in shorter extraction times, it is not a selective technique. Thus, the authors discuss that for better extraction performance, it is desirable the association/coupling of some different techniques (as SPE) in order to concentrate the extract in the target compounds, improving not only the yield of extraction but also the selectivity, which could assist in the further application of the extract. PLE coupled with solid-phase extraction (SPE) was the one that best recovered phenolics from apple pomace, with recoveries of phenolic acids equal to 2.85 ± 0.19 mg/g and flavonoids equal to 0.97 ± 0.11 mg/g. The chase for alternative solvents also can be a strategy to improve the selectivity of the compound of PLE. As evidenced, mostly organic solvents and water have been used and, therefore, with the formulation of new solvents as eutectic solvents and ILs, other options are available and could be used as the main solvent or as a cosolvent in ethanol or water.
PLE has been widely employed for lab-scale applications, and studies report it in general as the most efficient (high extraction yields and less extraction time) to obtain polar compounds being economically feasible at large scales (Viganó et al., 2017). Besides that, PLE is easily scalable and automated. Nonetheless, the literature lacks studies performing scale-up to ensure that the extraction yields and extract composition have reproducibility at large scales. Along with scale-up, coupling PLE with other extraction methods is also a perspective, mainly due to integrating processes to accomplish the biorefinery concept.
3.2.4. Supercritical fluid extraction (SFE)
Although it has many advantages, among the non-conventional techniques, the SFE is the less used to process apple-based raw materials, probably because SFE is an excellent candidate for extracting nonpolar compounds that are in low concentrations matrices. Moreover, SFE appears as a clean and eco-friendly alternative to recover bioactive compounds. The main advantages of SFE compared to conventional techniques are the use of milder temperatures, reduced solvent amount, higher purity of the extracts (i.e., selectivity), and reduced energy costs. The most used solvent in SFE is carbon dioxide (CO2), which has improved mass transfer properties due to its density and viscosity. In addition, supercritical CO2 is a GRAS solvent, nontoxic, relatively inert, and totally recovered at the end of the process (Dias et al., 2016). Besides, in SFE with CO2, the solvent-free extract is obtained by the fluid depressurization, which impacts energy savings to evaporate solvents; besides not leaving residual organic solvent in the final extract, contrarily than those techniques dependent on liquid solvents.
A recent study by Panadare et al. (2021) used SFE to extract volatile compounds from apple seeds and obtained a yield of 8.21 mg GAE/g. The process parameters were 25 MPa, 60 min, 45 °C, flow 2.5 mL/min. However, SFE can be performed by adding cosolvents (ethanol, water) to overcome low yields; consequently, the desorption of polar compounds from the vegetable matrices is favored (Xu et al., 2017).
In Table 4, one can note the use of ethanol as the cosolvent added to CO2, enhancing the extraction yield of phenolic compounds from apple pomace. Massias et al. (2015) extracted phenolics from apple pomace through SFE at a fixed temperature (50 °C), extraction time (180 min), and pressure (25 MPa). Moreover, the authors used a higher ethanol concentration (75%, v/v) added to CO2 since the presence of quercetin glucoside derivatives associated with polar compounds makes these components less extractable in nonpolar solvents.
Regarding cosolvent applications, it is relevant for the scientific community to critically consider the amount used since it can have critical implications (literally). The cosolvent addition changes the phase equilibrium and can lead the system to conditions outside the critical region depending on the proportion used. For example, Ferrentino et al. (2018) compared SFE to recover phenolics from fresh apple pomace with and without cosolvent (ethanol (5%, v/v) at pressures of 20 and 30 MPa, temperature intervals from 45 to 55 °C for 120 min. The results showed that an optimum total phenolic compounds extraction yield was obtained when the cosolvent was employed, as well as the use of the lowest temperature and the highest pressure. In a supercritical process, pressure and temperature variation change the CO2 density and its solvation power. Therefore, higher densities induce smaller spaces and higher interactions between the molecules (Dias et al., 2021). From this point, the authors could infer that pressure and temperature reduction resulted in a higher solvation power.
As with other techniques, the temperature also needs to be carefully evaluated in SFE. Depending on the target compounds, the solubility can have an inverse effect on SFE. Compounds better extracted at high solvent densities will be favored when temperatures closer to the critical limit are used. On the other hand, for raw materials whose extraction depends on the vapor pressure of the solutes, the increase in temperature favors the extraction. Generally, temperatures higher than 80 °C is not usual in SFE, and therefore, thermal degradation is not presented as a problem for most phenolic compounds.
Analogous to PLE, SFE is easily scaled and automated, and indeed, it is an extraction method with large-scale industrial applications. However, as aforementioned, SFE mainly uses CO2 as the solvent, making it less efficient to extract phenolic compounds. Considering that, the application of CO2 + new cosolvents or even the investigation of other alternative solvents to CO2 could be a sensible approach to overcome its limitations in obtaining compounds of higher polarity.
3.2.5. Sequential extraction processes
The several challenges involved in extracting and purifying compounds from natural products lead to innovative approaches to overcome them. For example, combining different techniques has been widely used to recover phenolics from vegetable matrices and enhance the aimed compounds’ extraction (Viganó et al., 2016). The main advantages of the combined processes are higher extraction yields, less solvent amount, shorter time, and higher purification and/or separation of the final products (Rostagno and Prado, 2013, Santos et al., 2019, Souza et al., 2020, Sumere et al., 2018, Wang et al., 2019). Da Silva et al. (2020) proposed an online extraction/fractionation technique employing PLE coupled to SPE to increase the purification of phenolics from apple pomace. PLE-SPE was used at different temperatures (60–80 °C), solvent concentrations (pure water, methanol (0–100%, v/v), and ethanol (0–100%, v/v)) at 10 MPa during 70 min, and the method was compared to PLE and other conventional techniques, such as magnetic-stirrer, shaker, and UAE. The authors obtained two different fractions of compounds, one of the phenolic acids (yield: 2.85 ± 0.19 mg/g) and another of flavonoids (yield: 0.97 ± 0.11 mg/g); therefore, PLE-SPE presented higher extraction yields than the other techniques (3.69- to 1.45-fold higher than produced by PLE, UAE, shaker, and magnetic stirring).
Besides, some techniques have been used to pre-treatment the apple fruits to extract the target bioactive compounds, such as enzyme-assisted extraction (EAE) and pulse electric field extraction (PEF). EAE may enhance the mass transfer in extraction processes by breaking the cell walls of the vegetable matrices (Krakowska et al., 2018). PEF consists of a technique in which samples are inserted between two electrodes creating pulses that raise the extraction of the desired components. PEF has also been employed as a pre-treatment to extract carotenoids and phenolics from vegetable matrices (Bot et al., 2018). Lohani & Muthukumarappan (2016) noticed that PEF as pre-treatment in apple pomace enhanced the phenolics release up to 37.4% compared to the control. EAE can be used to remove non-phenolic compounds (e.g., pectin) from apples or assist the elution of the phenolics compounds (Wikiera et al., 2015), which could be a suitable biomass pre-treatment to improve the phenolics’ extraction selectivity.
To sum up, sequential extraction processes to integrate techniques and intensify the recovery of target compounds or enhance their concentration in the extract are still incipient in the literature. They are expected to be worthy of investigation remarkably due to the approaches on biorefinery. Due to the concerns in changing the linear to circular economies, it is expected that the integration of processes could be more explored shortly, including technical, economic, social, and environmental aspects, resulting in factual greener platforms to use the food by-products fully, including apple pomace.
3.2.6. Sustainability and the use of non-volatile alternative solvents
Developing new sustainable downstream processes to recover bioactive extracts is a clear tendency in the scientific community. However, much more is needed to create a green process than reduce the processing time and cost. It is necessary to guarantee higher extract yields than those conventionally obtained, besides better extract purity and quality (Chemat et al., 2019, Souza Mesquita et al., 2020). Also, it is desired that the newly developed process promotes low energy consumption, economic costs, and environmental impacts.
From ancient times, many extraction platforms are mediated by petroleum-based organic solvents for recovering compounds to be applied in many industrial sectors, which are not desired considering their real toxicity potential, besides severe implications on the environment (Chemat et al., 2019). Thus, a strategy carried forward is using water as the extraction solvent, which seems to be the best (and safer!) alternative for recovering bioactive compounds from biomass. Nevertheless, due to the high-water polarity, water is not suitable for solubilizing a very broad compound. Considering this, some compounds added in water could modulate the solubility of solutes, such as ILs and eutectic solvents, enhancing the extraction of certain compounds that are usually not well recovered.
The ILs are salts with low melting points that can be used as solvents for selective extraction of a large plethora of biomolecules, from hydrophilic (phenolic compounds) (Silva et al., 2017, Lima et al., 2017) to hydrophobic (carotenoids, chlorophylls, curcuminoids, essential oils) (Souza Mesquita et al., 2020, Souza Mesquita et al., 2019). Also, ILs have been considered design solvents due to their unique tunable properties and eutectic solvents.
However, eutectic solvents are low-transition-temperature mixtures (of two or more compounds), which cover a large variety of anionic and/or cationic molecules, for which the eutectic temperature is under that of an ideal liquid mixture and are used for a myriad of applications (Hansen et al., 2020). Furthermore, both ILs and eutectic mixtures present negligible volatility at atmospheric conditions, besides being considered alternatives for replacing organic solvents in extraction platforms, which are usually labeled as hazards and flammable (Ventura et al., 2017).
Therefore, some authors classify these solvents as an environmentally compatible (or green) alternative. Some reports have already proven the possibility of recycling them without loss of efficiency, besides their low toxicity compared to those organic solvents used in the same extraction process. Another alternative is using an aqueous solution of surfactants (nonionic or ionic), which are also considered alternatives for replacing volatile organic solvents in extraction processes since their amphiphilic structure can extract both hydrophilic and hydrophobic compounds.
In the context of the available reports using apple by-products as a source of bioactive compounds, IL and eutectic solvents have been scarcely explored (Table 5). Also, three reports highlighted the use of aqueous solutions of nonionic surfactants. A conventional approach by stirring rotation was recently optimized by Skrypnik & Novikova (2020) for obtaining phenolics from apple pomace. An aqueous polysorbate 80 solution (1.14 %) at room temperature, SLR 0.009 for 64.6 min extracted 7.75 mg/g of phenolic compounds, representing two-fold the extraction yield obtained by pure water and ethanol (70%, v/v). Also, the authors noticed that the antioxidant activity of the surfactant extract was higher than those extracted by ethanol.
Table 5.
Summary of studies that applied IL, and eutectic solvents to obtain phenolic compounds from apple including the operational parameters and target compounds.
| Sample | Optimum operating conditions |
Extracted compounds | Yield | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | Co-solvent | Concentration | Technique | Temperature (°C) | Time (min) | SLR (g/mL) | ||||
| Apple peels | 1-tetradecyl-3-methylimidazolium chloride - [C14mim]Cl | Water | 500 mM | Rotatory elliptical shaking | 80 | 60 | 0.1 | Ursolic, oleanolic, and betulinic acids | 2.6% | Faria et al. (2017) |
| Apple pomace | 1-Butyl-3-methylimidazolium bromide - [C4mim]Br | Water | 600 mM | MAE | 73 | 15 | 0.03 | TPC | 0.3% | Du et al. (2013) |
| Apple flowers | 1-Butyl-3-methylimidazolium bromide - [C4mim]Br | MEOH | 520 mM | UAE | n.d. | 60 | 0.01 | Phlorizin, Astragalin, and Afzelin | 181.03 mg/g | Li et al. (2018) |
| Whole apple | Choline chloride/glucose-ethyl acetate - ChCl/Glu-EAC- molar ratio: 1:1:2 | Water | 75 % | High-speed countercurrent chromatography based on eutectic solvent | 77.5 | 30–90 | 0.04 | 6 ´ ´-O-coumaroyl-2 ´-O-glucopyranosylphloretin, 3 ´ ´ ´-methoxy-6 ´ ´-O-feruloy- 2 ´-O-glucopyranosylphloretin, vicularin, phloridzin, and sieboldin | 15.% | Cai et al. (2021) |
| Apple pomace | Polysorbate 80 - Tween 80® – Surfactant) | Water | 1.14 % | Stirring rotation | RT | 64.6 | 0.009 | TPC | 7.75 mg/g | Skrypnik & Novikova (2020) |
| Apple juice | Brij-58® - surfactant | Water | 7 mM | UAE (bath) | RT | 10 | n.r. | TPC | 35.4 mg/g | Sharma et al. (2015) |
| Whole apple | Brij-58® - surfactant + 2 % potassium chloride (W/V), pH = 3.7 | Water | 7 mM | UAE (bath) | 25 | n.i. | n.d. | Gallic acid, Catechin, Epi-catechin, Chlorogenic acid, Coumaric acid, Fluoridizin, and Quercetin | 180 mg/g | Hosseinzadeh et al. (2013) |
MAE: microwave assisted extraction; MEOH: methanol n.d.: not described; n.r.: not required step; TPC: calculated in terms of total phenolic content depending on the study cited; SLR: solid–liquid ratio; RT: room temperature; UAE: ultrasound assisted extraction.
Hosseinzadeh et al. (2013) highlighted that surfactant-water solution was selected as the best solvent to recover phenolic compounds from whole apple samples, even compared to those where the surfactant was dissolved in ethanol or methanol. The UAE (bath) was optimized using Brij-58 (7 mM) at 25 °C, recovering between 97 and 104% of the main target compounds from apple. Also, using Brij-58 solution at 7 mM, Sharma et al. (2015) enhanced the extraction performance of total phenolics from apple juice by applying surfactant solution instead of acetone, methanol, and ethanol. Using UAE (bath) at room temperature for 10 min, the authors recovered 180 mg/g of total phenolic compounds, besides 90.4% of antioxidant activity.
Imidazolium-based ILs were the most well-investigated for extracting natural compounds from different biomasses, including apple biomass (Table 5). However, given the significant possibilities of new ILs, such as those made with naturally derived ions (more benign, non-toxic, and low-cost), additional studies are necessary to improve the safety of the obtained extracts since imidazolium-based ILs were not considered as the best choice, especially in the food sector due to their high toxicity (Flieger & Flieger, 2020).
By a conventional approach technique (rotatory elliptical shaking), de Faria et al. (2017) recovered triterpene acids from apple skin using an aqueous solution of the tensioactive IL 1-tetradecyl-3-methylimidazolium chloride ([C14mim]Cl) at 500 mM, 80 °C, SLR 0.1 for 60 min, representing a promising alternative over the replacement of acetone and chloroform, the most organic solvents used for this purpose.
A microwave-assisted extraction using 1-Butyl-3-methylimidazolium bromide ([C4min]Br) at 600 mM, 73 °C, SLR 0.03 for 15 min was applied by (Du et al., 2013). The authors recovered only 0.3% of the total phenolic content from apple pomace, which indeed does not represent a successful extraction method. However, 181 mg/g of phenolic compounds were extracted from apple flowers by using a methanolic solution of [C4min]Br (operational conditions: 520 mM, SLR 0.01, 60 min), which promoted an increased by 25.4% of the extraction yield compared to the same process using organic solvents (Li et al., 2012).
Phenolic compounds, like those present in apple biomass, have been widely extracted by eutectic mixtures from different matrices, namely grapes (Jeong et al., 2015), olive pomace (Chanioti & Tzia, 2018), and wood (Alvarez-Vasco et al., 2016), just to mention a few. A high-speed countercurrent chromatography approach was performed by using Choline chloride/glucose-ethyl acetate eutectic solvent (75%, molar ratio: 1:1:2) for extraction of phenolics from Malus hupehensis. Water was used as a co-solvent under the operational conditions optimized at 77.5 °C, SLR 0.04 for 30–90 min. The methodology recovered 15.3% of the target compounds, which was superior to that obtained by methanol-mediated extraction (Cai et al., 2021).
Non-volatile solvents allow new combinations in different extraction techniques to investigate each case’s best scenario. Thus, considering the countless possibility of alternative solvents that can be formed (∼108 ternary ILs and 106 binary ILs are potentially formed), compared to 600 different organic solvents commonly used in the industrial field, the design solvents are the future (and the present!) since it is possible to modulate a particular solvent for a specific purpose.
However, until now, no sustainable extraction processes have been developed using apple as a source of bioactive compounds, which is worrying, considering the large number of tons yearly discarded of this raw material. Therefore, more studies are needed in order to optimize not only the extraction performance (yield of extraction), but also the sustainability of the developed process (especially the recyclability of solvents) (Souza Mesquita et al., 2021), putting into practice the circular economy concept.
Besides, it is worth mentioning that the alternative solvents are a tool to be used in an extraction method and, in this sense, the application of the new solvent and the method process parameters must be optimized and studied from lab to large scales. Additionally, new solvents generally are costly, which leads to two options. First, the new solvent could give technical or biological roles to the extract that justifies the solvent's presence in the product. Second and contrarily, the solvent must be separated and recycled.
4. Concluding remarks and future perspectives
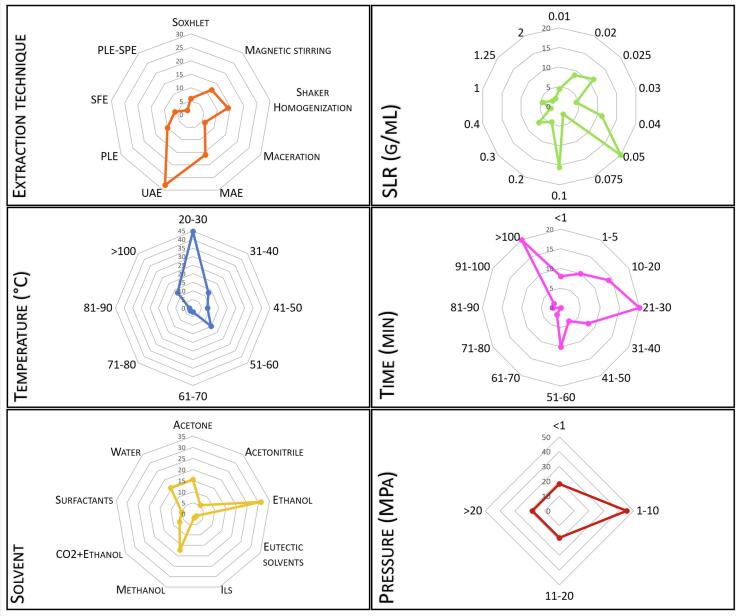
The available information indicates that many strategies were explored to overcome the challenges of recovering phenolic compounds from apples and by-products. Fig. 1 summarizes the data raised by this work, which was detailed presented in Table 2, Table 3, Table 4, Table 5, and allows an overview of the extraction methods and their extraction variables that have been used. According to Fig. 1, magnetic stirring and shaker homogenization was the preferred conventional techniques employed in the last two decades. It is worth mentioning that this work identified majority extraction methods at lab scales; therefore, although these methods are not considered the most efficient in terms of yield and selectivity, they are very ready to hand at lab scales, making them the most applied. Apart from the conventional methods, the UAE is gaining prominence and is the leading emergent technique, possibly due to its advantages like short extraction times with comparable or higher yield of other techniques. Additionally, coupled techniques, such as PLE-SPE, are yet poorly explored to extract/separate bioactive compounds from apple raw materials, having only one work published so far (Da Silva et al., 2020).
Fig. 1.
Distribution of the works dealing with different extraction technique and their respective conditions (solid–liquid ratio (SLR), temperature, time, solvent, and pressure).
Still, it is possible to note in Fig. 1 that the SLR used more times by the authors ranged between 0.04 and 0.05 g/mL, which can be considered a good choice for following works aiming to devolop of new extraction platforms. Mild temperatures (20–30 °C) were the most used in the works, especially in conventional methods. However, temperatures between 51 and 60 °C, and higher than 100 °C were already studied with satisfactory results, especially in pressurized systems like PLE (primarily performed using pressures ranging from 1 to 10 MPa).
The extraction time was the operational condition with more different ranges, reflecting its dependence on other extraction variables. Regardless of the technique used, most extractions are performed using times between 10 and 30 min. Here we must make a very important statement; the behavior of an extraction run shows a typical evolution with time in which the yield increases with the time until the extractable fraction is exhausted from the raw material. The optimal time to stop the run varies with the purpose of the process. For example, in an analytical application, an extraction time is expected to allow the sample depletion and achieve the quantitative extraction.
On the other hand, for industrial purposes, it is well documented that it is not convenient to extract until such time to exhaust the raw material. The extraction time impacts the number of batches produced annually and, consequently, impacts the cost of manufacturing. Therefore, for industrial applications, it is also advisable to verify the impact of the extraction time on the cost of manufacturing.
Fig. 1 allows concluding that organic solvents are yet the most used to extract phenolic compounds from apple-based raw materials (unfortunately!). However, despite the large number of works developed with methanol, acetonitrile, and acetone, the ethanolic solutions with different concentrations (20–99% v/v) were the most employed so far, which is a positive point considering that the ethanol has a low toxicity potential compared to other organic solvents and is not petroleum derivate. Supercritical CO2, ILs, eutectic solvents, and aqueous solutions of surfactants have already been used but just in few cases still in lab scales, and more research is needed to discover new possibilities towards the sustainability of new solvents. Considering the main trends in the industrial sector are going toward the development of sustainable strategies for extraction of biobased molecules from wastes, by-products, and pomaces, shortly we hope to see the scientific community changing the paradigm from the conventional to the modern, with more studies focusing on the development of integrated downstream processes with recycling of the raw materials and using extraction techniques that meet the green chemistry principles.
Due to the large amount of waste produced annually from the apple industries, adequate handling and disposal of the residues are needed to reduce the environmental impacts. In addition to environmental issues, there are the economic aspects, where the reuse of waste (and by-products) allows reducing the waste treatment cost. Thus, the extraction processes using apple-based raw materials are a feasible alternative to create new products with high added value, creating new market opportunities in many industrial fields. In this sense, the concept of transition from linear to circular production systems can be applied to the chain of apple products. The extraction processes we cover in this work consist of only one step in this chain and can be integrated with other processes giving rise to a biorefinery. Specifically, the chemical composition of apple pomace enables it to be used in extraction processes to obtain phenolic compounds, whose by-products can still follow in the transformation chain because they have pectin and lignocellulosic compounds that are interesting for the production of new materials and energy. Indeed, apple by-products as the pomace have already been industrially used to produce pectin, ethanol, citric acid, lactic acid, and enzymes. To the best of our knowledge, such and other processes could be integrated into a biorefinery concept.
Been more restricted to the extraction processes, as presented and discussed in the previous sections, apple pomace has been widely used on a laboratory scale to obtain phenolic compounds, mainly phenolic acids and flavonoids. Nevertheless, there is a lack of reports dealing with coupled extraction techniques. Many published works approach the use of SFE-UAE to recover bioactive compounds from by-products from food industries (Dias et al., 2016, Santos et al., 2015). Some other studies used PLE-UAE to obtain bioactive from food waste, such as pomegranate peels (Sumere et al., 2018, Santos et al., 2019), defatted passion fruit bagasse (Viganó et al., 2020).
Moreover, Santos et al. (2019) used the coupled expanded N2 associated with UAE to enhance phenolics recovery from pomegranate peels. The combination of these techniques, whether in a raw material pre-treatment or by assisting the entire extraction process, or in pulses, aims to enhance the extraction process, i.e., increase the yield and extract concentration, provide less heat to the system, and decrease consumption solvent and extraction time always with the least environmental impact in mind. Therefore, these mentioned coupled extraction techniques arise as promising possibilities to enhance the apple by-products processing.
Apart from the extraction technique, the choice of solvent is a critical step in the process design. Ethanol, water, and mixture have been widely employed to obtain phenolic compounds. Water is compatible with the most phenolic extract application; therefore, it does not always need to be evaporated, unlike ethanol. Consequently, the option for a solvent that is both efficient to extract and may have functionalities in the product in which the extract will be applied is very welcome. For example, Strieder et al. (2020) used milk as a solvent to obtain a blue colorant from genipap, considering the application in foods.
We want illustrate that the solvent’s choice can go beyond simply desorbing and solubilizing the extract; the solvent can, for instance, play as an emulsifier and stabilizer in food or as an emollient in drugs and cosmetics. In this context, ILs and eutectic solvents are good candidates since they have favorable physicochemical properties. Ionic liquids and natural deeps eutectic solvents have been reported as solvents for emerging extraction techniques like UAE and MAE. Accordingly, they are handled as a perspective to be employed as solvents in emerging extraction techniques and modern and coupled ones, such as those where the extraction coincides with the analysis.
Regardless of the extraction technique, to better understand the bioactive compounds’ mass transfer behavior, mathematical modeling is recommended in which the solutes’ behavior in sub- and supercritical media during the extraction procedures can be predicted. Moreover, the economic analysis and life cycle assessment of the new processes are helpful and very welcome, especially if the scale-up of the methods is aimed. Similarly, with the transposition between laboratory and industrial scales in mind, developing methods to concentrate the extract on the target compounds is also a fertile field for research.
Finally, given what was presented in this review, the potential of apple-based raw materials for obtaining bioactive compounds is reaffirmed. In addition to market trends and needs, the apple is grown in all continents, which provides a good supply chain worldwide showing and ensures the promising industrial application for producing phenolic compounds into a biorefinery concept.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to thanks to São Paulo Research Foundation (FAPESP), the National Council for Scientific and Technological Development (CNPq), and the Coordination of Superior Level Staff Improvement ‐ Brazil (CAPES) for supporting this research.
Funding sources
This work was supported by São Paulo Research Foundation (FAPESP), São Paulo, SP [grant number 2019/13496-0; 2018/14582‐5; 2019/24537-0; 2020/15774-5; 2020/08421-9; 2018/17089-8; 2019/18772-6; 2020/04067-6 and 2020/03623-2]; National Council for Scientific and Technological Development (CNPq) [grant number 151005/2019-2] and Coordination of Superior Level Staff Improvement ‐ Brazil (CAPES) [grant number 88887.310558/2018‐00 and Finance Code 001].
References
- Ajila C.M., Brar S.K., Verma M., Tyagi R.D., Valéro J.R. Solid-state fermentation of apple pomace using Phanerocheate chrysosporium - Liberation and extraction of phenolic antioxidants. Food Chemistry. 2011;126(3):1071–1080. doi: 10.1016/j.foodchem.2010.11.129. [DOI] [Google Scholar]
- Alberti A., Zielinski A.A.F., Zardo D.M., Demiate I.M., Nogueira A., Mafra L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chemistry. 2014;149:151–158. doi: 10.1016/j.foodchem.2013.10.086. [DOI] [PubMed] [Google Scholar]
- Alonso-Salces R.M., Korta E., Barranco A., Berrueta L.A., Gallo B., Vicente F. Pressurized liquid extraction for the determination of polyphenols in apple. Journal of Chromatography A. 2001;933(1–2):37–43. doi: 10.1016/S0021-9673(01)01212-2. [DOI] [PubMed] [Google Scholar]
- Alvarez-Vasco C., Ma R., Quintero M., Guo M., Geleynse S., Ramasamy K.K.…Zhang X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chemistry. 2016;18(19):5133–5141. doi: 10.1039/C6GC01007E. [DOI] [Google Scholar]
- Armenta S., Garrigues S., Esteve-Turrillas F.A., de la Guardia M. Green extraction techniques in green analytical chemistry. TrAC - Trends in Analytical Chemistry. 2019;116:248–253. doi: 10.1016/j.trac.2019.03.016. [DOI] [Google Scholar]
- Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F.…Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering. 2013;117(4):426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Barreca D., Bellocco E., Laganà G., Ginestra G., Bisignano C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chemistry. 2014 doi: 10.1016/j.foodchem.2014.03.118. [DOI] [PubMed] [Google Scholar]
- Barros L., Dueñas M., Ferreira I.C.F.R., Baptista P., Santos-Buelga C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food and Chemical Toxicology. 2009;47(6):1076–1079. doi: 10.1016/j.fct.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Belwal T., Ezzat S.M., Rastrelli L., Bhatt I.D., Daglia M., Baldi A.…Atanasov A.G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC – Trends in Analytical Chemistry. 2018;100:82–102. doi: 10.1016/j.trac.2017.12.018. [DOI] [Google Scholar]
- Bolarinwa I.F., Orfila C., Morgan M.R. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food chemistry. 2015;170:437–442. doi: 10.1016/j.foodchem.2014.08.083. [DOI] [PubMed] [Google Scholar]
- Bondonno N.P., Bondonno C.P., Ward N.C., Hodgson J.M., Croft K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends in Food Science & Technology. 2017;69:243–256. doi: 10.1016/j.tifs.2017.04.012. [DOI] [Google Scholar]
- Bot F., Verkerk R., Mastwijk H., Anese M., Fogliano V., Capuano E. The effect of pulsed electric fields on carotenoids bioaccessibility: The role of tomato matrix. Food Chemistry. 2018;240:415–421. doi: 10.1016/j.foodchem.2017.07.102. [DOI] [PubMed] [Google Scholar]
- Bouras M., Chadni M., Barba F.J., Grimi N., Bals O., Vorobiev E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Industrial Crops and Products. 2015;77:590–601. doi: 10.1016/j.indcrop.2015.09.018. [DOI] [Google Scholar]
- Budzynska B., Faggio C., Kruk-Slomka M., Samec D., Nabavi S.F., Sureda A.…Nabavi S.M. Rutin as neuroprotective agent: From bench to bedside. Current Medicinal Chemistry. 2017;26(27):5152–5164. doi: 10.2174/0929867324666171003114154. [DOI] [PubMed] [Google Scholar]
- Cai X., Xiao M., Zou X., Tang J., Huang B., Xue H. Extraction and separation of flavonoids from Malus hupehensis using high-speed countercurrent chromatography based on deep eutectic solvent. Journal of Chromatography A. 2021;1641 doi: 10.1016/j.chroma.2021.461998. [DOI] [PubMed] [Google Scholar]
- Carocho M., Ferreira I. The role of phenolic compounds in the fight against cancer – A review. Anti-Cancer Agents in Medicinal Chemistry. 2013;13(8):1236–1258. doi: 10.2174/18715206113139990301. [DOI] [PubMed] [Google Scholar]
- Casazza A.A., Pettinato M., Perego P. Polyphenols from apple skins: A study on microwave-assisted extraction optimization and exhausted solid characterization. Separation and Purification Technology. 2020;240 doi: 10.1016/j.seppur.2020.116640. [DOI] [Google Scholar]
- Chandrasekar V., Martín-González M.F.S., Hirst P., Ballard T.S. Optimizing microwave-assisted extraction of phenolic antioxidants from red delicious and jonathan apple pomace. Journal of Food Process Engineering. 2015;38(6):571–582. doi: 10.1111/jfpe.12187. [DOI] [Google Scholar]
- Chanioti S., Tzia C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innovative Food Science & Emerging Technologies. 2018;48:228–239. doi: 10.1016/j.ifset.2018.07.001. [DOI] [Google Scholar]
- Chemat F., Abert-Vian M., Fabiano-Tixier A.S., Strube J., Uhlenbrock L., Gunjevic V., Cravotto G. Green extraction of natural products. Origins, current status, and future challenges. TrAC - Trends in Analytical Chemistry. 2019;118:248–263. doi: 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- Zhi K., Li M., Bai J., Wu Y., Zhou S., Zhang X., Qu L. Quercitrin treatment protects endothelial progenitor cells from oxidative damage via inducing autophagy through extracellular signal-regulated kinase. Angiogenesis. 2016 doi: 10.1007/s10456-016-9504-y. [DOI] [PubMed] [Google Scholar]
- Zhou L., Xiong Z., Liu W., Zou L. Different inhibition mechanisms of gentisic acid and cyaniding-3-O-glucoside on polyphenoloxidase. Food Chemistry. 2017;19(3):311–324. doi: 10.1016/j.foodchem.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Chemat, F., Zill-e-Huma, & Khan, M. K. (2011). Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonics Sonochemistry, 18(4), 813–835. https://doi.org/10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed]
- Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications A review. Ultrasonics Sonochemistry. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Chen C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxidative Medicine and Cellular Longevity. 2016;2016:1–10. doi: 10.1155/2016/3571614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor S., Adriaens M., Pierini R., Johnson I.T., Belshaw N.J. Procyanidin induces apoptosis of esophageal adenocarcinoma cells via JNK activation of c-Jun. Nutrition and Cancer. 2014;66(2):335–341. doi: 10.1080/01635581.2014.868914. [DOI] [PubMed] [Google Scholar]
- Da Silva L.C., Souza M.C., Sumere B.R., Silva L.G.S., da Cunha D.T., Barbero G.F.…Rostagno M.A. Simultaneous extraction and separation of bioactive compounds from apple pomace using pressurized liquids coupled on-line with solid-phase extraction. Food Chemistry. 2020;318 doi: 10.1016/j.foodchem.2020.126450. [DOI] [PubMed] [Google Scholar]
- Dachineni R., Ai G., Kumar D.R., Sadhu S.S., Tummala H., Bhat G.J. Cyclin A2 and CDK2 as novel targets of aspirin and salicylic acid: A potential role in cancer prevention. Molecular Cancer Research. 2016;14(3):241–252. doi: 10.1158/1541-7786.MCR-15-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Archivio M., Scazzocchio B., Giovannini C., Masella R. Polyphenols in Human Health and Disease. Academic Press; 2014. Role of protocatechuic acid in obesity-related pathologies. [DOI] [Google Scholar]
- Dias A.L.B., Aguiar A.C., Rostagno M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrasonics Sonochemistry. 2021;74 doi: 10.1016/j.ultsonch.2021.105584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias A.L.B., Arroio Sergio C.S., Santos P., Barbero G.F., Rezende C.A., Martínez J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. var. pendulum) Ultrasonics Sonochemistry. 2016;31:284–294. doi: 10.1016/j.ultsonch.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Dias, A. L. B., Arroio Sergio, C. S., Santos, P., Barbero, G. F., Rezende, C. A., & Martínez, J. (2017). Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. Journal of Food Engineering, 198, 36–44. https://doi.org/10.1016/j.jfoodeng.2016.11.020 Chemistry, 281(25), 17359-17368. https://doi.org/10.1074/jbc.M600861200.
- Ding M., Feng R., Wang S.Y., Bowman L., Lu Y., Qian Y.…Shi X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. Journal of Biological Chemistry. 2006;281(25):17359–17368. doi: 10.1074/jbc.M600861200. [DOI] [PubMed] [Google Scholar]
- Du F., Deng B., Gao L., Xiang Y., Zhang J. Optimization of microwave-assisted extraction technology of total flavonoids from Malus micromalus Makino using Ionic liquids and response surface methodology. Science and Technology of Food Industry. 2013;2013:17. [Google Scholar]
- Eisvand F., Razavi B.M., Hosseinzadeh H. The effects of Ginkgo biloba on metabolic syndrome: A review. Phytotherapy Research. 2020;34(8) doi: 10.1002/ptr.6646. [DOI] [PubMed] [Google Scholar]
- Faria E.L.P., Shabudin S.V., Claúdio A.F.M., Válega M., Domingues F.M.J., Freire C.S.R.…Freire M.G. Aqueous solutions of surface-active ionic liquids: Remarkable alternative solvents to improve the solubility of triterpenic acids and their extraction from biomass. ACS Sustainable Chemistry & Engineering. 2017;5(8):7344–7351. doi: 10.1021/acssuschemeng.7b01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczyova K., Kalocayova B., Bartekova M. Potential implications of quercetin and its derivatives in cardioprotection. International Journal of Molecular Sciences. 2020;34(8):1798–1811. doi: 10.3390/ijms21051585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrentino G., Morozova K., Mosibo O.K., Ramezani M., Scampicchio M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. Journal of Cleaner Production. 2018;186:253–261. doi: 10.1016/j.jclepro.2018.03.165. [DOI] [Google Scholar]
- Flieger J., Flieger M. Ionic liquids toxicity—Benefits and threats. International Journal of Molecular Sciences. 2020;21(17):6267. doi: 10.3390/ijms21176267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franquin-Trinquier S., Maury C., Baron A., Le Meurlay D., Mehinagic E. Optimization of the extraction of apple monomeric phenolics based on response surface methodology: Comparison of pressurized liquid-solid extraction and manual-liquid extraction. Journal of Food Composition and Analysis. 2014;34(1):56–67. doi: 10.1016/j.jfca.2014.01.005. [DOI] [Google Scholar]
- Fromm M., Loos H.M., Bayha S., Carle R., Kammerer D.R. Recovery and characterisation of coloured phenolic preparations from apple seeds. Food Chemistry. 2013;136(3–4):1277–1287. doi: 10.1016/j.foodchem.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Fu L., Xu B.-T., Xu X.-R., Gan R.-Y., Zhang Y., Xia E.-Q., Li H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chemistry. 2011;129(2):345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- Grigoras C.G., Destandau E., Fougère L., Elfakir C. Evaluation of apple pomace extracts as a source of bioactive compounds. Industrial Crops and Products. 2013;49:794–804. doi: 10.1016/j.indcrop.2013.06.026. [DOI] [Google Scholar]
- Guo X.F., Liu J.P., Ma S.Q., Zhang P., Sun W. De. Avicularin reversed multidrug-resistance in human gastric cancer through enhancing Bax and BOK expressions. Biomedicine and Pharmacotherapy. 2018;103:67–74. doi: 10.1016/j.biopha.2018.03.110. [DOI] [PubMed] [Google Scholar]
- Hajialyani M., Farzaei M.H., Echeverría J., Nabavi S.M., Uriarte E., Eduardo S.S. Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules. 2019;24(3):648. doi: 10.3390/molecules24030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A., Hafizur R.M., Hussain N., Raza S.A., Rehman M., Ashraf S.…Choudhary M.I. Eriodictyol stimulates insulin secretion through cAMP/PKA signaling pathway in mice islets. European Journal of Pharmacology. 2018;820:245–255. doi: 10.1016/j.ejphar.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Haminiuk C.W.I., Maciel G.M., Plata-Oviedo M.S.V., Peralta R.M. Phenolic compounds in fruits - an overview. International Journal of Food Science & Technology. 2012;47(10):2023–2044. doi: 10.1111/j.1365-2621.2012.03067.x. [DOI] [Google Scholar]
- Hansen B.B., Spittle S., Chen B., Poe D., Zhang Y., Klein J.M.…Doherty B.W. Deep eutectic solvents: A review of fundamentals and applications. Chemical Reviews. 2020;121:1232–1285. doi: 10.1021/acs.chemrev.0c00385. [DOI] [PubMed] [Google Scholar]
- Hartogh D.J.D., Tsiani E. Antidiabetic properties of naringenin: A citrus fruit Polyphenol. Biomolecules. 2019;9(3):99. doi: 10.3390/biom9030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Zhang L.L., Yue X.Y., Liang J., Jiang J., Gao X.L., Yue P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chemistry. 2016;204:70–76. doi: 10.1016/j.foodchem.2016.02.094. [DOI] [PubMed] [Google Scholar]
- Hernández-Carranza P., Ávila-Sosa R., Guerrero-Beltrán J.A., Navarro-Cruz A.R., Corona-Jiménez E., Ochoa-Velasco C.E. Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. Journal of Food Processing and Preservation. 2016;40(1):103–115. doi: 10.1111/jfpp.12588. [DOI] [Google Scholar]
- Hosseinzadeh R., Khorsandi K., Hemmaty S. Study of the effect of surfactants on extraction and determination of polyphenolic compounds and antioxidant capacity of fruits extracts. PloS One. 2013;8(3) doi: 10.1371/journal.pone.0057353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyson D.A. A comprehensive review of apples and apple components and their relationship to human health. Advances in Nutrition. 2011;2(5):408–420. doi: 10.3945/an.111.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illera A.E., Chaple S., Sanz M.T., Ng S., Lu P., Jones J.…Bourke P. Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food chemistry: X. 2019;3 doi: 10.1016/j.fochx.2019.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M., Rauf A., Abu-Izneid T., Nadeem M., Shariati M.A., Khan I.A.…Mubarak M.S. Luteolin, a flavonoid, as an anticancer agent: A review. Biomedicine and Pharmacotherapy. 2019;12 doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- Jakobek L., Barron A.R. Ancient apple varieties from Croatia as a source of bioactive polyphenolic compounds. Journal of Food Composition and Analysis. 2016;45:9–15. doi: 10.1016/j.jfca.2015.09.007. [DOI] [Google Scholar]
- Jakobek L., Boc M., Barron A.R. Optimization of ultrasonic-assisted extraction of phenolic compounds from apples. Food Analytical Methods. 2015;8(10):2612–2625. doi: 10.1007/s12161-015-0161-3. [DOI] [Google Scholar]
- Jeong K.M., Zhao J., Jin Y., Heo S.R., Han S.Y., Yoo D.E., Lee J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Archives of Pharmacal Research. 2015;38(12):2143–2152. doi: 10.1007/s12272-015-0678-4. [DOI] [PubMed] [Google Scholar]
- Krakowska A., Rafińska K., Walczak J., Buszewski B. Enzyme-assisted optimized supercritical fluid extraction to improve Medicago sativa polyphenolics isolation. Industrial Crops and Products. 2018;124:931–940. doi: 10.1016/j.indcrop.2018.08.004. [DOI] [Google Scholar]
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal. 2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnology Reports. 2014;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K., Srivastav S., Sharanagat V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrasonics Sonochemistry. 2021;70 doi: 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesjak M., Beara I., Simin N., Pintać D., Majkić T., Bekvalac K.…Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. Journal of Functional Foods. 2018;40:68–75. doi: 10.1016/j.jff.2017.10.047. [DOI] [Google Scholar]
- Li H., Deng Z., Wu T., Liu R., Loewen S., Tsao R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chemistry. 2012;130(4):928–936. doi: 10.1016/j.foodchem.2011.08.019. [DOI] [Google Scholar]
- Li W., Shi M., Wang P., Guo X., Li C., Kang W. Efficient determination of three flavonoids in Malus pumila flowers by ionic liquid-HPLC. Journal of Molecular Liquids. 2018;263:139–146. doi: 10.1016/j.molliq.2018.04.135. [DOI] [Google Scholar]
- Li Z., Meng F., Zhang Y., Sun L., Yu L., Zhang Z.…Guo J. Simultaneous quantification of hyperin, reynoutrin and guaijaverin in mice plasma by LC-MS/MS: Application to a pharmacokinetic study. Biomedical Chromatography. 2016;30(7):1124–1130. doi: 10.1002/bmc.3660. [DOI] [PubMed] [Google Scholar]
- Lima Á.S., Soares C.M.F., Paltram R., Halbwirth H., Bica K. Extraction and consecutive purification of anthocyanins from grape pomace using ionic liquid solutions. Fluid Phase Equilibria. 2017;451:68–78. doi: 10.1016/j.fluid.2017.08.006. [DOI] [Google Scholar]
- Lohani U.C., Muthukumarappan K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innovative Food Science & Emerging Technologies. 2016;35:29–35. doi: 10.1016/j.ifset.2016.03.012. [DOI] [Google Scholar]
- Lu Y., Foo L.Y. Constitution of some chemical components of apple seed. Food chemistry. 1998;61(1–2):29–33. doi: 10.1016/S0308-8146(97)00123-4. [DOI] [Google Scholar]
- Macan A.M., Kraljević T.G., Raić-malić S. Therapeutic perspective of vitamin C and its derivatives. Antioxidants. 2019;8(8):247. doi: 10.3390/antiox8080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A.P.D.F., Pasquel-Reátegui J.L., Barbero G.F., Martínez J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Research International. 2015;77:675–683. doi: 10.1016/j.foodres.2014.12.042. [DOI] [Google Scholar]
- Makarova E., Górnaś P., Konrade I., Tirzite D., Cirule H., Gulbe A.…Dambrova M. Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. Journal of the Science of Food and Agriculture. 2014;95(3):560–568. doi: 10.1002/jsfa.6779. [DOI] [PubMed] [Google Scholar]
- Malinowska M., Śliwa K., Sikora E., Ogonowski J., Oszmiański J., Kolniak-Ostek J. Ultrasound-assisted and micelle-mediated extraction as a method to isolate valuable active compounds from apple pomace. Journal of Food Processing and Preservation. 2018;42(10) doi: 10.1111/jfpp.13720. [DOI] [Google Scholar]
- Martins Strieder M., Neves M.I.L., Silva E.K., Meireles M.A.A. Low-frequency and high-power ultrasound-assisted production of natural blue colorant from the milk and unripe Genipa americana L. Ultrasonics Sonochemistry. 2020;66 doi: 10.1016/j.ultsonch.2020.105068. [DOI] [PubMed] [Google Scholar]
- Massias A., Boisard S., Baccaunaud M., Leal Calderon F., Subra-Paternault P. Recovery of phenolics from apple peels using CO2+ethanol extraction: Kinetics and antioxidant activity of extracts. The Journal of Supercritical Fluids. 2015;98:172–182. doi: 10.1016/j.supflu.2014.12.007. [DOI] [Google Scholar]
- Matsui T. Condensed catechins and their potential health-benefits. European Journal of Pharmacology. 2015 doi: 10.1016/j.ejphar.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Mihailović, N. R., Mihailović, V. B., Kreft, S., Ćirić, A. R., Joksović, L. G., & Đurđević, P. T. (2018). Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.), 67, 1-9. Journal of Food Composition and Analysis. https://doi.org/10.1016/j.jfca.2017.11.007.
- Moreira M.M., Barroso M.F., Boeykens A., Withouck H., Morais S., Delerue-Matos C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Industrial Crops and Products. 2017;104:210–220. doi: 10.1016/j.indcrop.2017.04.038. [DOI] [Google Scholar]
- Mustafa A., Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Analytica Chimica Acta. 2011;703(1):8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M.…XiaoHui Z. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomedicine and Pharmacotherapy. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- Panadare D., Dialani G., Rathod V. Extraction of volatile and non-volatile components from custard apple seed powder using supercritical CO2 extraction system and its inventory analysis. Process Biochemistry. 2021;100:224–230. doi: 10.1016/j.procbio.2020.09.030. [DOI] [Google Scholar]
- Pavlić B., Naffati A., Hojan T., Vladić J., Zeković Z., Vidović S. Microwave-assisted extraction of wild apple fruit dust-production of polyphenol-rich extracts from filter tea factory by-products. Journal of Food Process Engineering. 2017;40(4) doi: 10.1111/jfpe.12508. [DOI] [Google Scholar]
- Pei K., Ou J., Huang J., Ou S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. Journal of the Science of Food and Agriculture. 2016;96(9):2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- Pingret D., Fabiano-Tixier A.S., Bourvellec C. Le, Renard C.M.G.C., Chemat F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. Journal of Food Engineering. 2012;111(1):73–81. doi: 10.1016/j.jfoodeng.2012.01.026. [DOI] [Google Scholar]
- Ponte L.G.S., Pavan I.C.B., Mancini M.C.S., da Silva L.G.S., Morelli A.P., Severino M.B.…Simabuco F.M. The hallmarks of flavonoids in cancer. Molecules. 2021;26(7):2029. doi: 10.3390/molecules26072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahath Kubra, I., Kumar, D., & Jagan Mohan Rao, L. (2016). Emerging trends in microwave processing of spices and herbs. Critical Reviews in Food Science and Nutrition, 56(13), 2160-2173. https://doi.org/10.1080/10408398.2013.818933. [DOI] [PubMed]
- Rajan V.K., Shameera S.A., Hasna C.K., Muraleedharan K. A non toxic natural food colorant and antioxidant ‘Peonidin’ as a pH indicator: A TDDFT analysis. Computational Biology and Chemistry. 2018;76:202–209. doi: 10.1016/j.compbiolchem.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Ramirez G., Zamilpa A., Zavala M., Perez J., Morales D., Tortoriello J. Chrysoeriol and other polyphenols from Tecoma stans with lipase inhibitory activity. Journal of Ethnopharmacology. 2016;185:1–8. doi: 10.1016/j.jep.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Rana S., Gupta S., Rana A., Bhushan S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Science and Human Wellness. 2015;4(4):180–187. doi: 10.1016/j.fshw.2015.10.001. [DOI] [Google Scholar]
- Reis S.F., Rai D.K., Abu-Ghannam N. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chemistry. 2012;135(3):1991–1998. doi: 10.1016/j.foodchem.2012.06.068. [DOI] [PubMed] [Google Scholar]
- Rezaei S., Rezaei K., Haghighi M., Labbafi M. Solvent and solvent to sample ratio as main parameters in the microwave-assisted extraction of polyphenolic compounds from apple pomace. Food Science and Biotechnology. 2013;22(5):1–6. doi: 10.1007/s10068-013-0212-8. [DOI] [Google Scholar]
- Rostagno M.A., D’Arrigo M., Martínez J.A., Martínez J.A. Combinatory and hyphenated sample preparation for the determination of bioactive compounds in foods. TrAC - Trends in Analytical Chemistry. 2010;29(6):553–561. doi: 10.1016/j.trac.2010.02.015. [DOI] [Google Scholar]
- Rostagno M.A., Prado J.M. Royal Society of Chemistry; 2013. Natural product extraction: principles and applications. [Google Scholar]
- Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A.…Martins N. The therapeutic potential of Apigenin. International Journal of Molecular Sciences. 2019;20(6):1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, P., Aguiar, A. C., Barbero, G. F., Rezende, C. a, & Martínez, J. (2015). Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrasonics Sonochemistry, 22, 78–88. https://doi.org/10.1016/j.ultsonch.2014.05.001. [DOI] [PubMed]
- Santos M.P., Souza M.C., Sumere B.R., da Silva L.C., Cunha D.T., Bezerra R.M.N., Rostagno M.A. Extraction of bioactive compounds from pomegranate peel (Punica granatum L.) with pressurized liquids assisted by ultrasound combined with an expansion gas. Ultrasonics Sonochemistry. 2019;54:11–17. doi: 10.1016/j.ultsonch.2019.02.021. [DOI] [PubMed] [Google Scholar]
- Sato Y., Itagaki S., Kurokawa T., Ogura J., Kobayashi M., Hirano T.…Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. International Journal of Pharmaceutics. 2011;403(1–2):136–138. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Sharma S., Kori S., Parmar A. Surfactant mediated extraction of total phenolic contents (TPC) and antioxidants from fruits juices. Food Chemistry. 2015;185:284–288. doi: 10.1016/j.foodchem.2015.03.106. [DOI] [PubMed] [Google Scholar]
- Shay J., Elbaz H.A., Lee I., Zielske S.P., Malek M.H., Hüttemann M. Molecular mechanisms and therapeutic effects of (-)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxidative Medicine and Cellular Longevity. 2015;2015:1–13. doi: 10.1155/2015/181260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva F.A., Carmo R.M.C., Fernandes A.P.M., Kholany M., Coutinho J.A.P., Ventura S.P.M. Using ionic liquids to tune the performance of aqueous biphasic systems based on pluronic L-35 for the purification of naringin and rutin. ACS Sustainable Chemistry & Engineering. 2017;5(8):6409–6419. doi: 10.1021/acssuschemeng.7b00178. [DOI] [Google Scholar]
- Skrypnik L., Novikova A. Response surface modeling and optimization of polyphenols extraction from apple pomace based on nonionic emulsifiers. Agronomy. 2020;10(1):92. doi: 10.3390/agronomy10010092. [DOI] [Google Scholar]
- Souza Mesquita, L. M., Martins, M., Pisani, L. P., Ventura, S. P. M., & de Rosso, V. V. (2021). Insights on the use of alternative solvents and technologies to recover bio-based food pigments. Comprehensive Reviews in Food Science and Food Safety, 20, 787-818. https://doi.org/https://doi.org/10.1111/1541-4337.12685. [DOI] [PubMed]
- Souza Mesquita L.M., Martins M., Maricato É., Nunes C., Quinteiro P.S.G.N., Dias A.C.R.V.…Ventura S.P.M. Ionic liquid-mediated recovery of carotenoids from the Bactris gasipaes fruit waste and their application in food-packaging chitosan films. ACS Sustainable Chemistry & Engineering. 2020;8(10):4085–4095. doi: 10.1021/acssuschemeng.9b06424. [DOI] [Google Scholar]
- Souza Mesquita L.M., Ventura S.P.M., Braga A.R.C., Pisani L.P., Dias A., de Rosso V.V. Ionic liquid-high performance extractive approach to recover carotenoids from Bactris gasipaes fruits. Green Chemistry. 2019;21:2380–2391. doi: 10.1039/C8GC03283A. [DOI] [Google Scholar]
- Souza M.C., Santos M.P., Sumere B.R., Silva L.C., Cunha D.T., Martínez J.…Rostagno M.A. Isolation of gallic acid, caffeine and flavonols from black tea by on-line coupling of pressurized liquid extraction with an adsorbent for the production of functional bakery products. Lwt. 2020;117 doi: 10.1016/j.lwt.2019.108661. [DOI] [Google Scholar]
- Srinivasulu C., Ramgopal M., Ramanjaneyulu G., Anuradha C.M., Suresh Kumar C. Syringic acid (SA) – A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomedicine and Pharmacotherapy. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Sumere B.R., de Souza M.C., dos Santos M.P., Bezerra R.M.N., da Cunha D.T., Martinez J., Rostagno M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.) Ultrasonics Sonochemistry. 2018;48:151–162. doi: 10.1016/j.ultsonch.2018.05.028. [DOI] [PubMed] [Google Scholar]
- Ullah R., Ikram M., Park T.J., Ahmad R., Saeed K., Alam S.I.…Kim M.O. Vanillic acid, a bioactive phenolic compound, counteracts LPS-induced neurotoxicity by regulating c-Jun N-Terminal kinase in mouse brain. International Journal of Molecular Sciences. 2020;22(1):361. doi: 10.3390/ijms22010361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura, S. P. M., E Silva, F. A., Quental, M. V., Mondal, D., Freire, M. G., & Coutinho, J. A. P. (2017). Ionic-liquid-mediated extraction and separation processes for bioactive compounds: past, present, and future trends. Chemical Reviews, 117(10), 6984–7052. https://doi.org/10.1021/acs.chemrev.6b00550. [DOI] [PMC free article] [PubMed]
- Viganó J., Aguiar A.C., Moraes D.R., Jara J.L.P., Eberlin M.N., Cazarin C.B.B.…Martínez J. Sequential high pressure extractions applied to recover piceatannol and scirpusin B from passion fruit bagasse. Food Research International. 2016;85:51–58. doi: 10.1016/j.foodres.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Viganó, J., Assis, B. F. de P., Náthia-Neves, G., dos Santos, P., Meireles, M. A. A., Veggi, P. C., & Martínez, J. (2020). Extraction of bioactive compounds from defatted passion fruit bagasse (Passiflora edulis sp.) applying pressurized liquids assisted by ultrasound. Ultrasonics Sonochemistry, 64, 104999. https://doi.org/10.1016/j.ultsonch.2020.104999. [DOI] [PubMed]
- Viganó J., Zabot G.L., Martínez J. Supercritical fluid and pressurized liquid extractions of phytonutrients from passion fruit by-products: Economic evaluation of sequential multi-stage and single-stage processes. The Journal of Supercritical Fluids. 2017;122:88–98. doi: 10.1016/j.supflu.2016.12.006. [DOI] [Google Scholar]
- Wang L., Boussetta N., Lebovka N., Vorobiev E. Selectivity of ultrasound-assisted aqueous extraction of valuable compounds from flesh and peel of apple tissues. LWT. 2018;93:511–516. doi: 10.1016/j.lwt.2018.04.007. [DOI] [Google Scholar]
- Wang L., Boussetta N., Lebovka N., Vorobiev E. Ultrasound assisted purification of polyphenols of apple skins by adsorption/desorption procedure. Ultrasonics Sonochemistry. 2019;55:18–24. doi: 10.1016/j.ultsonch.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang Y., An Y., Fang G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomedicine and Pharmacotherapy. 2019;117 doi: 10.1016/j.biopha.2019.109086. [DOI] [PubMed] [Google Scholar]
- Wijngaard H., Brunton N. The optimization of extraction of antioxidants from apple pomace by pressurized liquids. Journal of Agricultural and Food Chemistry. 2009;57(22):10625–10631. doi: 10.1021/jf902498y. [DOI] [PubMed] [Google Scholar]
- Wikiera A., Mika M., Grabacka M. Multicatalytic enzyme preparations as effective alternative to acid in pectin extraction. Food Hydrocolloids. 2015;44:156–161. doi: 10.1016/j.foodhyd.2014.09.018. [DOI] [Google Scholar]
- Wiktor A., Sledz M., Nowacka M., Rybak K., Witrowa-Rajchert D. The influence of immersion and contact ultrasound treatment on selected properties of the apple tissue. Applied Acoustics. 2016;103:136–142. doi: 10.1016/j.apacoust.2015.05.001. [DOI] [Google Scholar]
- Withouck H., Boeykens A., Vanden Broucke M., Moreira M.M., Delerue-Matos C., De Cooman L. Evaluation of the impact of pre-treatment and extraction conditions on the polyphenolic profile and antioxidant activity of Belgium apple wood. European Food Research and Technology. 2019;245(11):2565–2578. doi: 10.1007/s00217-019-03373-2. [DOI] [Google Scholar]
- Xu Y., Fan M., Ran J., Zhang T., Sun H., Dong M.…Zheng H. Variation in phenolic compounds and antioxidant activity in apple seeds of seven cultivars. Saudi Journal of Biological Sciences. 2016;23(3):379–388. doi: 10.1016/j.sjbs.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.P., Li Y., Meng X., Zhou T., Zhou Y., Zheng J.…Li H. Bin. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. International Journal of Molecular Sciences. 2017;18(1):96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T., Shao D., Yuan Y., Wang Z., Qiang C. Ultrasound-assisted extraction, HPLC analysis, and antioxidant activity of polyphenols from unripe apple. Journal of Separation Science. 2012;35(16):2138–2145. doi: 10.1002/jssc.201200295. [DOI] [PubMed] [Google Scholar]