Abstract
This paper presents attempts to enrich hens eggs with ions of copper, manganese, and zinc through the use of new feed additives (19 mg Cu2+; 124 mg Mn2+ and 85 mg Zn2+) such as biomass of alfalfa and goldenrod after extraction with supercritical carbon dioxide enriched with microelements via biosorption. Mechanical parameters of eggs (shell thickness and strength, Haugh unite), hen's laying performance, microelements content in albumen and yolk were examined and the transfer factor from feed to eggs was determined. The highest transfer of microelements content in albumen occurred in the group of hens fed with enriched goldenrod in a 100% dose (daily dose of microelements from biomass; Cu2+ 106%; Mn2+ 104%; Zn2+ 104% more in comparison to the inorganic salt group), while the highest yolk enrichment with microelements manifested itself for hens fed with enriched goldenrod in a 50% dose (daily dose of microelements from biomass; Cu2+ 32%; Zn2+ 22% more in comparison to the inorganic salt group). These groups also had the highest total microelements concentration. Mechanical properties of eggs varied insignificantly during the trial. Production parameters did not differ statistically among all experimental group. Eggs produced with need additives had better organoleptic parameters than fed with conventional premixes, which is why they were preferred by the respondents. The presented technology allows obtaining low-cost feed materials characterized by high bioavailability of components. The produced feed additives can serve as potential material for biofortification of eggs with nutrients.
Key words: feed additive, food biofortification, functional egg, micronutrient
INTRODUCTION
An optimized and balanced diet is the basis for animal production and has a real impact on the profitability of this sector. A typical dietary strategy relies on nutrients – such as plant or animal materials – that are rich in micro- and macroelements. The compound feed features additives to supplement individual components – including microelements – essential to animals. Although administered in small quantities, they are crucial to the proper growth and functioning of organisms.
Due to their properties, microelements are classified as a group of nutrients responsible for regulating life processes. They are substrates in the synthesis of many cell structures, actively participate in metabolic pathways and act as signal molecules responsible for initiating catalytic cascades in immunological reactions. They regulate osmotic pressure and the pH balance in physiological fluids (Georgievskii et al., 2013).
Copper, manganese and zinc are essential in poultry nutrition. Feeds low in these nutrients can contribute to homeostasis disorders (Michalak et al., 2011). Weak immunity (Cu deficiency; Berwanger et al., 2018), muscle deformation (Zn deficiency; Naz et al., 2016) or even growth inhibition (Mn deficiency; Jankowski et al., 2019) may occur in poultry. Microelement feed additives delivered to farm animals in the right form and dose can prevent all these phenomena. Unfortunately, the mineral compounds or organic chelates used so far as feed additives are characterized by low bioavailability or high prices.
Inorganic salts, mostly due to their low price, appear to be the most popular form of micronutrients in animal nutrition. Unfortunately, this formulation is not fully bioavailable. Large amounts of unconsumed microelements are excreted. An additional disadvantage of this type of supplementation is the problem of storage and transport of such feed mixes, mainly associated with the separation of fractions, which reduces the homogeneity of the material. Also, such forms may form free radicals, as in the case of sulphates, which affect animal health (Abd El-Hack et al., 2017). Alternative forms of micronutrient supplementation are organic forms, including chelates, in which the microelement ion is locked into a complex ligand structure (Stanaćev et al., 2014). Popular are amino acids, carbohydrates or lipids chelates, which additionally provide other nutritional components (Świątkiewicz et al., 2014). This is a more expensive option than the mentioned inorganic forms, but it is characterized by high bioavailability.
An adequately composed diet provides animals with doses of nutrients adapted to age or breeding conditions. The supply of microelements among farm animals significantly affects the condition and quality of breeding, thus new solutions are being sought to obtain low-cost feed materials with high bioavailability of nutrients. Plants and algae are extracted to provide bioactive substances in the food, pharmaceutical, or cosmetic industries, but the postextraction waste is not managed and can be used to produce feed additives. The management of the by-product stream from extraction processes will reduce the cost of feed materials technology, and additionally, this approach is in line with the circular economy with zero waste production (Skrzypczak et al., 2020). The plant material is rich in several functional groups (e.g., carboxyl, carbonyl, or hydroxyl), which can bind microelement ions. The production of feed additives with this method is simple, and the resultant preparations are highly bioavailable. The micronutrient feed additives based on biomass of microalgae (Saeid et al., 2016), macroalgae (Michalak et al., 2011) and soybean meal (Witkowska et al., 2014, Witkowska et al., 2019) have been tested on laying hens with positive results on egg quality parameters.
Due to the increased transfer of microelements in the edible parts of eggs, a biofortification effect can be achieved (White et al., 2009). The nutrients present in the food are in easily assimilable forms and in proper proportions. Consumption of such improved food is aimed at reducing the incidence of diseases caused by deficiencies in trace elements or vitamins (Ruel and Alderman, 2013).
The aim of this study was to explore the potential direction of management of alfalfa and goldenrod as postextraction waste toward the production of feed materials enriched in Zn2+, Cu2+, and Mn2+ ions. The effect of biomass residues as a feed additive with micronutrients on laying hen performance and egg quality properties (weight, eggshell strength and thickness, albumen height) was determined. The possibility of obtaining food biofortification by feed additives usage was investigated, and the level of biofortification was established by determining the microelement transfer to individual egg fractions. The organoleptic properties of eggs were also tested.”
MATERIALS AND METHODS
Materials
Zinc sulphate (ZnSO4·7H2O), manganese sulphate (Mn SO4·2H2O), copper sulphate (CuSO4·5H2O), hydrogen chloride (HCl) and sodium hydroxide (NaOH) were purchased from POCH (Gliwice, Poland). Nitric acid (supra-pure grade, 69%), used for sample mineralization, was purchased from Merc (Whitehouse Station, NJ). Biomass (alfalfa and goldenrod) after supercritical CO2 extraction were provided by Azoty Group (Pulawy, Poland).
Preparation of Microelemental Feed Additives
An appropriate weight of the goldenrod (10 g L−1) was weighed and transferred to a reactor containing 10 L aqueous solution with a concentration of microelements 250 mg L−1 (pH 5). Sorption was carried out at room temperature, with continuous stirring (agitator speed 100 rotation per min), for 4 h. After this time, the enriched biomass was dried at 60°C for 24 h. The process was carried out analogically for the second biomass. Prepared feed additives were used for subsequent research.
Animal Diets and Experimental Design
The research on live animals met the guidelines approved by the institutional animal care and use committee (IACUC). The tests were carried out on 126 laying hens of the Lohmann Brown line. The birds were randomly distributed into 42 cages and divided into 7 groups (6 replications per group; Table 1). The initial age of hens was 21 wk. The investigation took place in a room with controlled microclimatic conditions. The light regime was 16L:8D. The cage dimension was 50 cm × 120 cm × 50 cm. For each hen, the daily feed ratio was 100 g.
Table 1.
Type of feed additives in each experimental group.
| Group | Source of microelements |
|---|---|
| Alfalfa 0% | alfalfa + P100% |
| Alfalfa 50% | mineral compounds mix (50% - P50%) and mix of enriched alfalfa biomass (50%) |
| Alfalfa 100% | mix of enriched alfalfa biomass + P0% |
| Goldenrod 0% | goldenrod + P100% |
| Goldenrod 50% | mineral compounds mix (50% - P50%) and mix of enriched goldenrod biomass (50%) |
| Goldenrod 100% | mix of enriched goldenrod biomass + P0% |
| Control | mineral compounds mix (manganese oxide, zinc oxide and copper (II) sulphate) – P100% |
P0%, P50%, P100% – experimental premixes (Table 3).
The specific amount of each compound was calculated according to data from Nutrient Requirements of Poultry (National Research Council, 1994). The amount of each microelement was equal in all types of additives.
To ensure the nutrient requirements, various feed compositions were chosen (Tables 2 and 3). The composition of the basal diet, which was formulated according to nutrient recommendations for laying hens, is given in Table 2.
Table 2.
Ingredients and chemical composition of the basal diet.
| Ingredient | Unit | Mass |
|---|---|---|
| Basal diet | ||
| Wheat | % | 40.20 |
| Maize | % | 20.00 |
| Soybean meal (46% protein) | % | 17.88 |
| Fodder chalk (37% Ca) | % | 8.25 |
| Sunflower meal | % | 8.00 |
| Experimental premix | % | 3.00 |
| Soybean oil | % | 2.67 |
| Chemical composition, calculated, per kg | ||
| Dry mass | % | 88.7 |
| Crude protein | % | 17.5 |
| Crude fiber | % | 0,4 |
| Crude fat | % | 4.5 |
| Crude ash | % | 12.5 |
| Lysine | % | 0.8 |
| Lysine for poultry | % | 0.7 |
| Methionine | % | 0.4 |
| Methionine for poultry | % | 0.3 |
| Methionine + Cysteine | % | 0.7 |
| Tryptophan | % | 0.2 |
| Threonine | % | 0.5 |
| Linoleic acid | % | 2.1 |
| Na | g | 1.52 |
| K | g | 7.59 |
| Mg | g | 1.54 |
| Ca | g | 35 |
| P | g | 6.63 |
| Cu | mg | 19 |
| Mn | mg | 124 |
| Zn | mg | 85 |
| Cl | mg | 1.91 |
| Fe | mg | 162 |
| Vitamin A (retinyl acetate) | IU | 11,999 |
| Vitamin D | IU | 2,000 |
| Vitamin B12 | μg | 20 |
| Vitamin E (dl-α-tocopheryl acetate) | IU | 10 |
| Net energy for poultry | kcal | 2,647 |
| Net energy for laying hens | kcal | 2,700 |
Table 3.
Composition of experimental premixes.
| Ingredient | Premix 0% selected mineral (P0%) | Premix 50% selected mineral (P50%) | Premix 100% selected mineral (P100%) |
|---|---|---|---|
| Monocalcium phosphate (CaH4P2O8) | 40 | 40 | 40 |
| Polfamix A-Z1 | 33.3 | 33.3 | 33.3 |
| Fodder chalk 37% (CaCO3) | 14.87 | 14.4 | 14.4 |
| Sodium chloride (NaCl) | 8.2 | 8.2 | 8.2 |
| DL-methionine (C5H11NO2S)2 | 3.1 | 3.1 | 3.1 |
| Iron oxide (FeO) | 0.5 | 0.5 | 0.54 |
| Manganese oxide (MnO) | - | 0.27 | 0.5 |
| Zinc oxide (ZnO) | - | 0.12 | 0.24 |
| Copper sulfate (CuSO4•5H2O) | - | 0.09 | 0.17 |
Mixture of supplements for laying hens (Polfamix): Vitamin A1 200,000 IU (retinyl acetate), vitamin D3 (cholecalciferol) 200,000 IU, vitamin E 1,000 IU (dl-α-tocopheryl acetate), vitamin K3 1,000 mg, vitamin B1 100 mg, vitamin B2 3,000 mg, vitamin B6 200 mg, vitamin B12 2,000 mg, vitamin C 5,000 mg, biotin 20,000 mg, folic acid 80 mg, niacin 12,000 mg, calcium pantothenate 4,000 mg.
Source of amino acids (MetAmino, EVONIK), feed grade 99%.
The composition of experimental premixes is shown in Table 3. The experimental design (7 groups in total) necessitated the preparation of 3 separate premixes (P0%, P50%, P100%).
The experiment was conducted for 60 d and was divided into 2 series (30 d each). In that time, all groups were monitored for egg production. Eggs were collected and weighed daily. Eggs production was determined by dividing the number of eggs laid over the course of the experiment by the number of hens in the same period (expressed as the percentage of egg production). Feed intake was recorded once per week. Feed conversion ratio was calculated by dividing the feed intake by the mass of eggs.
The analysis was performed after 30 and 60 d of the experiment in all the examined groups for 5 randomly collected eggs from each replicate.
Sampling Regime
At each stage of the research, 3 eggs were taken from each cage. Eggs were separated into fractions (albumen, yolk, and shell) and weighed. Egg shells were washed and then dried at 60°C for 24 h. After this time, the shells were ground in a titanium mill (Retch, Germany). Individual fractions from one cage were mixed, mineralized (Microwave oven, StartD, Milestone, Italy) and subjected to multielement analysis an Inductively Coupled Plasma Emission Spectrometer.
Egg Quality
Before manual egg breaking in each experimental group, the crush strength of the shell was measured, using materials testing machine Z010 with testXpert II software (Zwick GmbH & Co. KG; Ulm, Germany), equipped with a 100 N load cell (BTC-LC 100N). The eggs were tested at a constant head speed of 10 mm/min (tangential force 0.1 N). The maximum crush strength of the eggshell was determined at the time of its cracking.
The eggs were analyzed with the use of semiautomated egg quality equipment – QCM+ with Eggware 3.0.16 software (Technical Services and Supplies, York, UK). Haugh Unit was calculated using the software based on the height of the albumen and egg weight. Eggshell thickness was measured near the equator at 2 points with an electronic micrometer screw IP 54 (Fowler High Precision, Newton, MA).
Organoleptic Testing
Organoleptic trial ensured the consuming quality of eggs. The 2-staged trail was based on consumers' responses to smell, taste, yolk color, and texture. 20 experts were chosen from group of 60 people in the first trial. The second trial consisted of the survey in which experts subjectively evaluated parameters mentioned earlier, in a scale from 1 (dislike very much) to 5 (like very much) by answering questions: “How do you like taste/smell/texture/yolk color of given samples?”. In each trial, every test participant received 21 samples in 7 rows. In each row, parts of eggs form control, experimental (one from 1–6 groups), and another entrepreneur popular in Poland (industrial) slice of egg was present. Participants were not informed about the origin of each sample.
Calculation
Equation (1) determined the transfer factor (TF), which describes the transfer of microelements to the egg fraction (Witkowska et al., 2019).
| (1) |
where, mef – egg fraction weight (kg), Cmf – microelement concentration in specific fraction (mg kg−1), Cmfeed – microelement concentration in feed (mg kg−1), mf – feed intake (kg).
Multielemental Analysis
Mineralization of Samples
An appropriate amount of sample (0.1 g) was weighed in Teflon vessels and flooded with 69% nitric acid (5 mL). Mineralization was carried out in a microwave oven (Microwave oven, StartD, Milestone, Italy) at 200°C, for 35 min. Delivered power was 100 to 1,000 W.
Analysis
The analysis of elemental content in all samples was carried out using an Inductively Coupled Plasma Emission Spectrometer with Ultrasonic Nebulizer (Varian VISTA-MPX ICP-OES, Victoria, Australia). All the analyses were performed in the Chemical Laboratory of Multielemental Analysis of Wroclaw University of Science and Technology, accredited by ILAC-MRA and Polish Center for Accreditation according to PN-EN ISO/IEC 17025.
Statistical Analysis
Statistical analysis of numerical data (such as micronutrient content, technical properties) was performed in the following order. Initially, the normality of the dependent variables was checked using the Shapiro-Wilk test. For distributions other than normal, the significance of differences was evaluated using the Mann-Whitney test. For the normal distribution, homogeneity of variance was verified. The significance of differences for homogeneous variances was assessed by t test and for heterogeneous variances by Welch test. Statistical differences of ordinal data (organoleptic tests) were evaluated with the Mann-Whitney test. The analysis was performed using Statistica 13.1 TIBCO/StatSoft software at a significance level of P < 0.05.”
RESULTS AND DISCUSSION
Biosorption Experiment
The previous application of biosorption was mainly related to the removal of metal ions from contaminated water. It is because of the mineral richness contained in the biomass and the possibility of its enrichment, due to the available functional groups present on its surface, that the process can be used to prepare fertilizers and feed additives (Michalak et al., 2015). Our previous research showed that there is the possibility of using Chlorella vulgaris biomass for animal supplementation. The biosorption of Mn2+, Cu2+, Zn2+, Co2+, and Cr3+ ions yielded sorption capacities of 20.98, 55.91, 57.51, 51.56, and 30.11 mg g−1 of biomass, respectively (Chojnacka, 2007). In another case, soybean meal enriched with microelements produced levels of 15.7 mg g−1 Cu2+, 16.3 mg g−1 Fe2+, 14.1 mg g−1 Zn2+, and 20.6 mg g−1 Cr3+ (Witkowska et al., 2013). Michalak and Chojnacka (2008) presented the feasibility of using Pithophora varia as a biological supplement for animals. The process was conducted in a single metal system, which resulted in a high sorption capacity (61.1 mg g−1 Zn2+, 55.7 mg g−1 Cu2+, 52.3 mg g−1 Co2+, and 38.3 mg g−1 Mn2+). The process was also carried out in a multimetal system; however, the sorption capacity decreased twice. This is due to the competition of metal ions for active sites. The use of the first method yields higher results, but introduces an additional stage related to the mixing of enriched biomass, which entails higher costs.
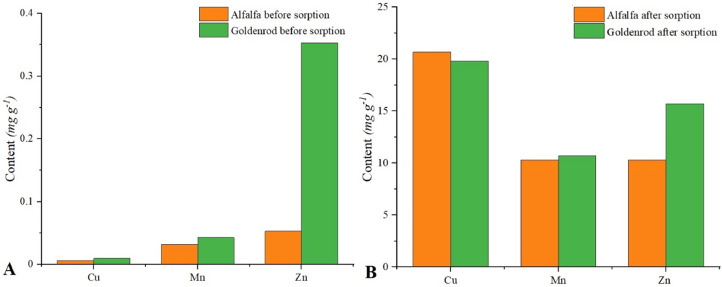
In this research, biosorption was carried out in a multimetal system. The content of microelements in alfalfa and goldenrod before and after enrichment is shown on Figures 1A and 1B. The content of selected microelements in raw biomaterial was low (Figure 1A). A relatively high content of zinc in the biomass before sorption was found in goldenrod (0.353 mg g−1). In both cases the highest level of enrichment was obtained for Cu2+ ions. This could be due to the high affinity of Cu2+ ions to the sorbent (Skrzypczak et al., 2019). In the alfalfa, the zinc and manganese content increased to the same amount (10.3 mg g−1). A significant difference was observed for enrichment of alfalfa with Zn2+ and Mn2+ ions. The final content of Zn2+ ions was higher by 30%, compared to Mn2+ (Figure 1B). Different amounts of sorbed ions were caused by different functional groups present on the surface (Ligas et al., 2018), but also by different properties of sorbed ions (atomic radius, electromagnetism, or ionizing potential; Can and Jianlong, 2007).
Figure 1.
Biomass (goldenrod and alfalfa) before (A) and after microelement sorption (B) (sorbent dosage 10 g L−1; contact time: 4 h; pH:5; C0: 250 mg L−1).
Performance of Laying Hens
The data concerning the egg laying capacity are presented in Table 4. Different sources of micronutrients did not significantly affect either the final values of egg production or the feed conversion ratio. The feed conversion ratio was the lowest in the control group at the begging of the experiment and in goldenrod 100% at the end of the trial. The highest conversions were achieved in alfalfa 50% at the beginning and at the end of the experiment. Hen-day egg production is a proportion/ratio of the number of eggs collected weekly to the maximal hens' production output. At the beginning of the experiment, hens in the control group had the highest egg production rate. At the end of the experiment, hens from the experimental groups had a higher egg production than those from the control group (except alfalfa 50%). It should be noted, however, that differences were small. Similar trends were reported in other application experiments. In the case of iodine yeast biofortification, feed conversion ratio and hen-day egg production did not differ among all types of diets and yielded comparable values at the end of the experiment (95.3–94.6% range of egg laying performance) (Opaliński et al., 2012). With Souporus androgenus as a potential feed additive the feed conversion ratio amounted to 2,57-2,49 (Santoso and Fenita, 2016). The plant also did not enhance the overall laying performance. During the application trial with enriched Spirulina maxima, feed additives also did not make significant differences with the control group (Saeid et al., 2016). Average feed intake was twice higher because of different species of laying hens and their age.
Table 4.
Effect of different microelements supplementation to diet on laying hens performance.
| Egg production [%] |
||||||
|---|---|---|---|---|---|---|
| Group | 1st-7th d | SEM | 24th–30th d | SEM | 54th–60th d | SEM |
| Alfalfa 0% | 40.5 | 0.3 | 73.8 | 0.3 | 88.7 | 0.2 |
| Alfalfa 50% | 32.1 | 0.3 | 51.2 | 0.1 | 86.9 | 0.3 |
| Alfalfa 100% | 40.5 | 0.4 | 82.1 | 0.3 | 90.1 | 0.3 |
| Goldenrod 0% | 46.4 | 0.5 | 73.8 | 0.3 | 90.5 | 0.4 |
| Goldenrod 50% | 36.9 | 0.4 | 72.6 | 0.3 | 88.7 | 0.4 |
| Goldenrod 100% | 44.0 | 0.4 | 92.9 | 0.1 | 90.6 | 0.3 |
| Control | 51.2 | 0.5 | 63.1 | 0.2 | 87.7 | 0.2 |
| Feed conversion ratio [g of feed/egg] |
||||||
|---|---|---|---|---|---|---|
| Group | 1st-7th d | SEM | 24th–30th d | SEM | 54th–60th d | SEM |
| Alfalfa 0% | 320.5 | 16.8 | 177.9 | 12.6 | 186.9 | 14.1 |
| Alfalfa 50% | 433.3 | 20.3 | 326.0 | 17.6 | 208.1 | 18.4 |
| Alfalfa 100% | 326.5 | 17.1 | 166.1 | 12.4 | 156.4 | 13.2 |
| Goldenrod 0% | 289.2 | 16.3 | 185.4 | 13.1 | 160.3 | 13.4 |
| Goldenrod 50% | 367.7 | 18.4 | 189.8 | 13.3 | 194.4 | 14.8 |
| Goldenrod 100% | 296.8 | 16.2 | 142.0 | 11.3 | 125.3 | 11.6 |
| Control | 250.6 | 14.8 | 205.8 | 13.5 | 175.0 | 13.6 |
Quality Parameters of Eggs
The results of the technical properties of eggs are summarized in Table 5. The parameters – Haugh Unit, thickness and strength of eggshell were measured 3 times – at the beginning of the study, in the middle (d 30) and at the end (d 60). The Haugh unit (HU) index, which indicates egg freshness, reached the highest value at the beginning of the study, for the alfalfa 50 group (50% of microelements related to the alfalfa biomass surface) – 91.33 ± 1.58 (statistically significant). All other research groups, regardless of the day of measurement, had a value greater than 72, which assigned eggs into class AA. These results, however, are inconsistent with the literature data. In studies testing soybean enriched in microelements, the HU index increased with the study time, reaching the highest value on the day of their completion (Witkowska et al., 2019). Many studies indicate that the addition of micronutrients in hen diets positively affects protein structure, which increases the freshness of the protein served in Haugh Units (Arpášová et al., 2009; Brodacki et al., 2018).
Table 5.
Technical properties of eggs after 0, 30, and 60 day.
| Group | HU | SEM | Thickness [mm] | SEM | Eggshell strength [N] | SEM | Time period |
|---|---|---|---|---|---|---|---|
| Alfalfa 0% | 72.33abcd | 1.40 | 0.45abcdef | 0.01 | 18.89a | 0.33 | D 0 |
| Alfalfa 50% | 91.33ae | 1.58 | 0.36eg | 0.01 | 20.71 | 0.58 | |
| Alfalfa 100% | 85,00b | 1.32 | 0.39b | 0.01 | 21.42 | 0.46 | |
| Goldenrod 0% | 88.33c | 0.59 | 0.40cgh | 0.01 | 24.20a | 0.98 | |
| Goldenrod 50% | 79.33 | 1.22 | 0.41di | 0.00 | 20.64 | 0.42 | |
| Goldenrod 100% | 77.67ef | 0.74 | 0.39ej | 0.01 | 20.52 | 0.79 | |
| Control | 90,67df | 0.92 | 0.34fhij | 0.00 | 20.72 | 0.99 | |
| Alfalfa 0% | 83,33a | 0.69 | 0.41a | 0.08 | 19.67a | 0.65 | D 30 |
| Alfalfa 50% | 85.33b | 0.69 | 0.40b | 0.05 | 18.61b | 0.75 | |
| Alfalfa 100% | 75.67abcd | 0.56 | 0.38c | 0.03 | 19.49c | 1.03 | |
| Goldenrod 0% | 83.00c | 0.79 | 0.40d | 0.03 | 22.40 | 0.59 | |
| Goldenrod 50% | 86.33d | 1.11 | 0.43 e | 0.03 | 26.06abcd | 1.12 | |
| Goldenrod 100% | 81.00 | 0.89 | 0.42 f | 0.03 | 23.93 | 0.68 | |
| Control | 79.00 | 0.85 | 0.35abcdef | 0.04 | 21.42d | 0.91 | |
| Alfalfa 0% | 83.67 | 1.19 | 0.40 | 0.01 | 21.18 | 0.44 | D 60 |
| Alfalfa 50% | 90.33a | 1.55 | 0.40 | 0.00 | 21.04 | 1.31 | |
| Alfalfa 100% | 87.00 | 1.56 | 0.41 | 0.00 | 22 | 0.57 | |
| Goldenrod 0% | 90.33b | 1.40 | 0.41 | 0.01 | 22.72 | 0.71 | |
| Goldenrod 50% | 88.67 | 0.76 | 0.41 | 0.00 | 23.4 | 0.89 | |
| Goldenrod 100% | 77.00ab | 0.84 | 0.42 | 0.01 | 22.66 | 0.71 | |
| Control | 85.00 | 0.55 | 0.42 | 0.01 | 23.57 | 0.74 |
The averages in the column marked with the same letter differ significantly for P < 0.05, significant statistical differences were determined for each day of egg collection separately.
The thickness of the shell changed during the research. On d 0, it reached its highest value for the group fed on unenriched alfalfa, with the thickness decreasing for this group at further stages of the study. For groups fed with microelement - enriched biomass, the shell thickness increased slightly between the start and end of the study, but these results were not statistically significant. The largest shell thickness of 0.43 ± 0.03 mm and strength were obtained with the application of the feeding enriched with 50% thread for 30 d. These parameters may limit the number of broken eggs. The shell strength for this study group on the 30th d – 26.06 ± 1.12 N – was statistically significant for most of the groups, for which the strength varied between 19 and 23 N. The data from this study are consistent with the literature data. The use of enriched algae biomass results in a thickness increase of 14.3% (Michalak et al., 2011) and 3.27% and a strength increase of 7.47% (Saeid et al., 2016). In another study, after the application of feed additives obtained by biosorption, the shell thickness decreased during the study, but the differences were not statistically significant (Witkowska et al., 2019). The addition of trace elements increases the density of the crust, which translates into increased thickness and strength. Manganese and zinc play a particularly important role, as demonstrated in a study of Świątkiewicz and Koreleski (2007).
Microelements Concentrations in Egg Fractions
The assessment of the bioavailability of the new feed additive was contingent on the examination of the content of microelements (Cu, Mn, Zn) in albumen and yolk in eggs of laying hens (Table 6).
Table 6.
Content of microelements (Cu, Mn, Zn) in albumen (a) and yolk (b) in eggs of laying hens.
| a) | |||||||
|---|---|---|---|---|---|---|---|
| Micronutrient content [mg/kg] in albumen |
|||||||
| Cu2+ |
Mn2+ |
Zn2+ |
|||||
| Group | Content | SEM | Content | SEM | Content | SEM | |
| Alfalfa 0% | 0.725abcdef | 0.063 | 0.039ag | 0.003 | 0.924abcd | 0.067 | D 0 |
| Alfalfa 50% | 0.353a | 0.031 | 0.038bh | 0.003 | 0.259aefg | 0.027 | |
| Alfalfa 100% | 0.252bg | 0.022 | 0.016cghi | 0.001 | 0.247bhij | 0.063 | |
| Goldenrod 0% | 0.485cg | 0.042 | 0.028d | 0.003 | 0.690ehkl | 0.042 | |
| Goldenrod 50% | 0.433d | 0.037 | 0.034 ei | 0.003 | 0.356ckm | 0.046 | |
| Goldenrod 100% | 0.336 e | 0.029 | 0.026f | 0.002 | 1.11filmn | 0.089 | |
| Control | 0.316 f | 0.027 | 0.067abcdef | 0.006 | 0.607dgjn | 0.031 | |
| Alfalfa 0% | 0.324abcdef | 0.061 | 0.018 | 0.006 | 0.368 | 0.127 | D 30 |
| Alfalfa 50% | 0.148a | 0.065 | 0.003 | 0.006 | 0.011a | 0.110 | |
| Alfalfa 100% | 0.203b | 0.008 | 0.022 | 0.001 | 0.737a | 0.008 | |
| Goldenrod 0% | 0.181 c | 0.041 | 0.007 | 0.004 | 0.307 | 0.102 | |
| Goldenrod 50% | 0.169d | 0.020 | 0.006 | 0.004 | 0.246 | 0.204 | |
| Goldenrod 100% | 0.119 e | 0.012 | 0.005 | 0.001 | 0.131 | 0.102 | |
| Control | 0.147f | 0.004 | 0.007 | 0.001 | 0.113 | 0.012 | |
| Alfalfa 0% | 0.197a | 0.061 | 0.019a | 0.004 | 0.292a | 0.127 | D 60 |
| Alfalfa 50% | 0.171b | 0.012 | 0.014b | 0.008 | 0.149b | 0.171 | |
| Alfalfa 100% | 0.166c | 0.012 | 0.013 c | 0.004 | 0.344c | 0.102 | |
| Goldenrod 0% | 0.22d | 0.016 | 0.025d | 0.008 | 0.818d | 0.151 | |
| Goldenrod 50% | 0.274 e | 0.037 | 0.038e | 0.012 | 1.17 e | 0.086 | |
| Goldenrod 100% | 0.466abcdef | 0.049 | 0.117abcdef | 0.016 | 3.61abcdef | 0.204 | |
| Control | 0.244f | 0.118 | 0.019f | 0.049 | 0.495f | 0.037 | |
| b) | |||||||
|---|---|---|---|---|---|---|---|
| Micronutrient content [mg/kg] in albumen |
|||||||
| Cu2+ |
Mn2+ |
Zn2+ |
|||||
| Group | Content | SEM | Content | SEM | Content | SEM | |
| Alfalfa 0% | 2.11 | 0.183 | 0.787 | 0.069 | 30.6 | 2.654 | D 0 |
| Alfalfa 50% | 2.07 | 0.179 | 1.100 | 0.096 | 34.6 | 3.001 | |
| Alfalfa 100% | 2.24 | 0.194 | 0.818 | 0.071 | 29.0 | 2.408 | |
| Goldenrod 0% | 1.96 | 0.170 | 0.988 | 0.086 | 30.2 | 2.579 | |
| Goldenrod 50% | 2.20 | 0.191 | 0.763 a | 0.067 | 34.2 | 2.809 | |
| Goldenrod 100% | 1.63 | 0.142 | 0.894 | 0.078 | 26.2 | 2.480 | |
| Control | 2.21 | 0.191 | 1.16a | 0.101 | 30.3 | 1.524 | |
| Alfalfa 0% | 1.42 a | 0.167 | 0.824abc | 0.033 | 33.5abc | 1.155 | D 30 |
| Alfalfa 50% | 1.15abc | 0.033 | 0.898def | 0.037 | 33.8def | 0.771 | |
| Alfalfa 100% | 1.26 | 0.082 | 0.937ghi | 0.041 | 34.4ghi | 0.976 | |
| Goldenrod 0% | 1.47b | 0.029 | 0.609adgj | 0.033 | 25.3adgjk | 1.461 | |
| Goldenrod 50% | 1.44 c | 0.086 | 0.505behkl | 0.033 | 26.1beh | 1.576 | |
| Goldenrod 100% | 1.21 | 0.049 | 0.785jk | 0.020 | 32.6jkl | 1.710 | |
| Control | 1.37 | 0.037 | 0.653cfil | 0.065 | 24.9 cfil | 1.302 | |
| Alfalfa 0% | 1.42a | 0.057 | 0.643a | 0.057 | 27.9a | 2.347 | D 60 |
| Alfalfa 50% | 1.41b | 0.041 | 0.525b | 0.020 | 23.5abc | 0.380 | |
| Alfalfa 100% | 1.24c | 0.053 | 0.539c | 0.029 | 26.9ef | 1.686 | |
| Goldenrod 0% | 1.29d | 0.057 | 0.758d | 0.037 | 33.3bg | 0.653 | |
| Goldenrod 50% | 2.07abcdef | 0.196 | 0.958 | 0.127 | 43.5 acegh | 4.710 | |
| Goldenrod 100% | 1.26 e | 0.061 | 0.851e | 0.049 | 31.7hi | 1.608 | |
| Control | 1.58f | 0.180 | 1.33abcde | 0.331 | 36.1dif | 3.433 | |
(a) The averages in the column marked with the same letter differ significantly for P < 0.05; significant statistical differences were determined for each day of egg collection separately.
(b) The averages in the column marked with the same letter differ significantly for P < 0.05; significant statistical differences were determined for each day of egg collection separately.
Copper ion content in egg proteins decreased for alfalfa feed additives during supplementation (Table 7). After 30 d, the highest Cu2+ content was recorded in the group fed with unenriched alfalfa (0.324 mg/kg). In contrast, the highest content of manganese and zinc ions (0.022 mg/kg and 0.737 mg/kg, respectively) was obtained by feeding laying hens with a mix of enriched alfalfa biomass (alfalfa 100%). After another 30 d of nutrition, the mix of enriched goldenrod biomass (goldenrod 100%) proved to be the best-available feed additive because the content of microelements Cu, Mn, and Zn in albumin was the highest and amounted to 0.466 mg/kg, 0.117 mg/kg and 3.61mg/kg, respectively. The results were statistically significant. The content of microelements also changed in egg yolks (Table 7). After 60 d of supplementation, the best results were obtained for laying hens fed with enriched goldenrod with a 50% dose of microelements. Copper content in the yolk for 50% goldenrod was 2.07 mg/kg and it was about 30% higher than in the control. Also the highest levels of manganese and zinc ions in egg yolk (0.958 mg/kg and 43.5 mg/kg, respectively) were achieved for the same research group. Goldenrod turned out to be effective than alfalfa and positively influenced the enrichment of eggs with valuable microelements. Witkowska et al. (2019) studied a micronutrient dietary supplement for laying hens, applying soy flour enriched with copper, chromium, iron and zinc ions. Animals were fed for 12 wk. Microelements accumulated mainly in egg albumen. There was a significant iron transfer to eggs (increase by 243%). There was also 47.3% more copper and 32.3% more zinc in biofortified eggs. Michalak et al. (2011) tested bio-based feed additives in the feeding of laying hens. Enriched macroalgae (Enteromorpha prolifera and Cladophora sp.) were the source of microelements (Cu, Zn, Co, Mn, and Cr). Eggs treated in this way had more manganese (by 14%) and copper (by 42%) and 2.5 times more chromium than those in the control group (Michalak et al., 2011). Saeid et al. (2016) in their research showed the possibility of using biosorption to enrich Spirulina maxima algae and use such a preparation for feeding hens. The Fe, Mn, and Zn contents in egg proteins were higher than in controls (by 860, 113, and 195% more Fe, Mn, and Zn, respectively). The yolk had much more Mn and Zn (concentrations increased by 195 and 110%, respectively; Saeid et al., 2016). Opaliński et al. (2012) observed that the use of iodine yeast significantly increased the content of this element in egg yolk. In 2 groups of experimental laying hens there were about 80 and 90% more iodine than in the control (Opaliński et al., 2012).
Table 7.
Micronutrient transfer in albumen.
| Micronutrient transfer [mg/kg] |
|||||||
|---|---|---|---|---|---|---|---|
| Cu2+ |
Mn2+ |
Zn2+ |
|||||
| Group | Content | ±SD | Content | ±SD | Content | ±SD | |
| Alfalfa 0% | 0.042abcdef | 0.006 | 0.043abcdef | 0.006 | 0.039abcdef | 0.006 | D 30 |
| Alfalfa 50% | 0.018a | 0.003 | 0.018a | 0.003 | 0.017a | 0.002 | |
| Alfalfa 100% | 0.024b | 0.004 | 0.024b | 0.004 | 0.022b | 0.003 | |
| Goldenrod 0% | 0.022c | 0.003 | 0.023c | 0.003 | 0.021c | 0.003 | |
| Goldenrod 50% | 0.020d | 0.003 | 0.020d | 0.003 | 0.018d | 0.003 | |
| Goldenrod 100% | 0.016e | 0.002 | 0.016 e | 0.002 | 0.014 e | 0.002 | |
| Control | 0.019f | 0.003 | 0.020f | 0.003 | 0.018f | 0.003 | |
| Alfalfa 0% | 0.046abc | 0.007 | 0.047ab | 0.007 | 0.042a | 0.006 | D 60 |
| Alfalfa 50% | 0.033adefg | 0.010 | 0.033cd | 0.005 | 0.030b | 0.005 | |
| Alfalfa 100% | 0.042dhi | 0.006 | 0.043ef | 0.007 | 0.039c | 0.006 | |
| Goldenrod 0% | 0.046 ejk | 0.007 | 0.047gh | 0.007 | 0.043d | 0.006 | |
| Goldenrod 50% | 0.062 bfhjlm | 0.009 | 0.063acegi | 0.009 | 0.057 e | 0.009 | |
| Goldenrod 100% | 0.103cikln | 0.015 | 0.106bdfhj | 0.016 | 0.096abcdef | 0.014 | |
| Control | 0.050gmn | 0.008 | 0.052ij | 0.008 | 0.047f | 0.007 | |
Values in the column marked with the same letter differ significantly for P < 0.05.
Supplementation of the micronutrient with mineral substances did not affect the content of manganese and copper in egg yolk (Inal et al., 2001). With the application of chelates, it was possible to increase the concentration of copper by 19% in albumen and by 2% in yolk in comparison to the negative control group (Brodacki et al., 2018). Therefore, the use of appropriate feed additives based on biosorption can be more effective for biofortification purposes. It is estimated that the increase in micronutrient density is due to the ability of functional groups located in egg albumen and yolk to bind micronutrients. The condition is a sufficiently increased amount of micronutrients in the feed (Réhault-Godbert et al., 2019).
Microelements Transfer in Egg Fractions
Biomass enriched as a carrier of valuable microelements can be used as food for farm animals. Such an application can also have a positive effect on the quality of food products (eggs, milk, and cheese). Witkowska et al. (2015) also conducted research on feed additives produced by biosorption. Soybeans enriched with Cu, Fe, Mn, Zn constituted food for goats. Feed biopreparations increased the content of microelements in milk in comparison to the control group (more by 8.2% Cu, 29.2% Mn and 29.2% Zn, respectively; Witkowska et al., 2015). Tables 7 and 8 show the calculated amounts of Cu, Mn, and Zn that were transferred to egg yolk and egg albumen during supplementation. Transfer of copper microelements is slightly higher for egg yolk than in the case of manganese, but a clear difference was observed for zinc (e.g., over 60 times more Zn in yolk than in 50% goldenrod albumen). The best results were obtained for a mix of enriched goldenrod (100% goldenrod). For this group, micronutrient transfer was 106% for Cu, 103% for Mn, and 104% for Zn, compared to the controls.
Table 8.
Micronutrient transfer in yolk.
| Micronutrient transfer [mg/kg] |
|||||||
|---|---|---|---|---|---|---|---|
| Cu2+ |
Mn2+ |
Zn2+ |
|||||
| Group | Content | ±SD | Content | ±SD | Content | ±SD | |
| Alfalfa 0% | 0.069a | 0.011 | 0.041abc | 0.007 | 1.523abc | 0.244 | D 30 |
| Alfalfa 50% | 0.047abcd | 0.008 | 0.038def | 0.006 | 1.285def | 0.206 | |
| Alfalfa 100% | 0.056 | 0.009 | 0.043ghi | 0.007 | 1.431ghi | 0.229 | |
| Goldenrod 0% | 0.070b | 0.011 | 0.030adg | 0.005 | 1.121adgj | 0.179 | |
| Goldenrod 50% | 0.062c | 0.010 | 0.022behjk | 0.004 | 1.051behk | 0.168 | |
| Goldenrod 100% | 0.060 | 0.010 | 0.040jk | 0.006 | 1.504jkl | 0.241 | |
| Control | 0.070d | 0.011 | 0.034cfi | 0.005 | 1.186cfil | 0.190 | |
| Alfalfa 0% | 0.116a | 0.018 | 0.054a | 0.009 | 2.113a | 0.338 | D 60 |
| Alfalfa 50% | 0.099b | 0.016 | 0.038b | 0.006 | 1.541bcd | 0.247 | |
| Alfalfa 100% | 0.132c | 0.021 | 0.059c | 0.009 | 2.657ef | 0.425 | |
| Goldenrod 0% | 0.103d | 0.017 | 0.062d | 0.010 | 2.480bg | 0.397 | |
| Goldenrod 50% | 0.182abcdef | 0.029 | 0.086 | 0.014 | 3.561aceghi | 0.570 | |
| Goldenrod 100% | 0.115e | 0.018 | 0.080 e | 0.013 | 2.690 i | 0.430 | |
| Control | 0.137f | 0.022 | 0.118abcde | 0.019 | 2.904dfh | 0.465 | |
Values in the column marked with the same letter differ significantly for P < 0.05.
Organoleptic Trial
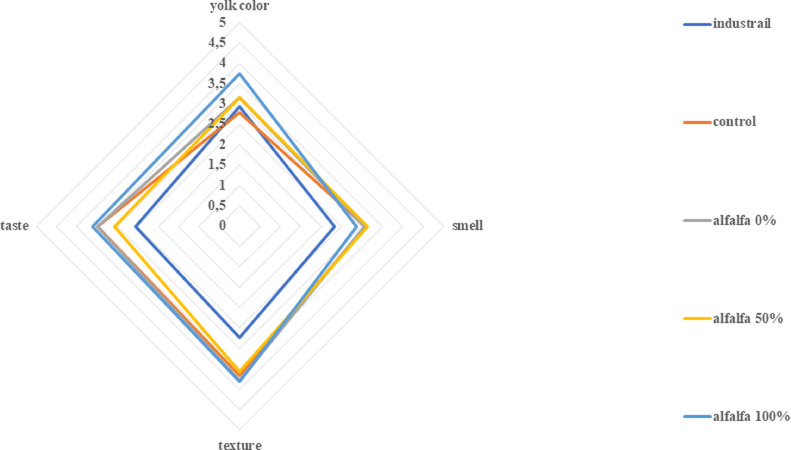
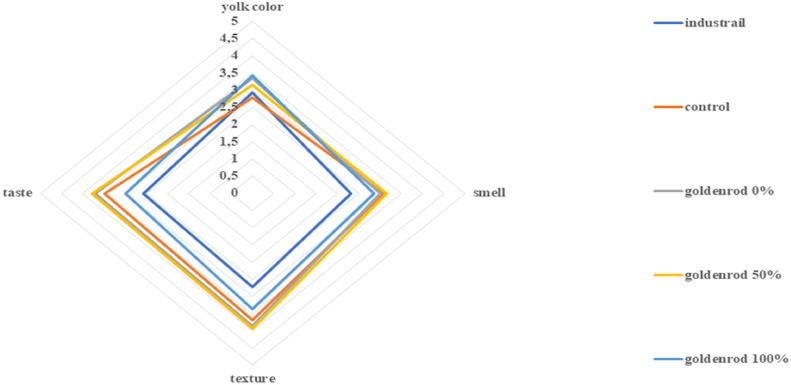
The results of consumer research that focused on the evaluation of the organoleptic properties of eggs are presented in Figures 2 and 3 for alfalfa and goldenrod, respectively. Egg texture, taste, smell, and yolk color were evaluated on a 5-stage scale. Yolk color was rated highest for eggs from hens fed with new feed additives, where microelements were given in 100% biomass-bound form – 3.75 (±1.00 SD) for 100% goldenrod and 3.43 (±1.45 SD) for 100% alfalfa. A lower dose of micronutrients (50%) bound to the biomass significantly affected the smell of eggs, giving the highest level of satisfaction of respondents for goldenrod 50% 3.07 (±1.20 SD) and alfalfa 50% 3.00 (±1.17 SD). In the respondents’ opinion, the taste of eggs and their texture differed between types of biomass. In the case of hens fed with new additives based on alfalfa, these parameters obtained the highest rating when the microelements were completely bound to the biomass (alfalfa 100%). In the case of hens fed with micronutrients enriched with goldenrod, the taste and texture of eggs was in the opinion of the respondents the highest when micronutrients were administered half-way between the biomass-bounded and the salt of micronutrients.
Figure 2.
Organoleptic trial for alfalfa groups.
Figure 3.
Organoleptic trial for goldenrod groups.
Overall data analysis indicated that the addition of biomass into feed has an influence on potential customer response and varies as for types of additives (P < 0.05). The results presented in the radar graphs show that the lowest rating was given to industrial eggs and it corresponds most closely to those from the control group. Similar responses were reported during soybean trial (Witkowska et al., 2014). Eggs from hens fed with micronutrient feed obtained by biosorption received a higher rating from the respondents. Therefore, it can be concluded that the feed additives obtained via biosorption improve the organoleptic parameters of eggs. Better taste, smell, texture, and yolk color are important in the opinion of the respondents. This may constitute a new trend in increasing food quality, using natural products. Thus, it can be concluded that the use of natural feed additives significantly affects egg quality. Biomass not only increases the bioavailability of trace elements, but its compounds can also act as antioxidants in the egg yolk. This results in reduced rancidity of sensitive substances (vitamins), protecting cells from oxidative stress. Such active compounds can reduce unwanted effects (changes in color, taste, and odor) that are caused by oxidation (Gumus et al., 2018).
SUMMARY
The presence of biosorption-based feed additives in the diet did not affect the bird's laying production. Measured values varied between 87.7% (control) and 90.6 (goldenrod 100%) after 60 d of application and did not differ statistically. Similarly, in the case of the feed conversion rate (g of feed per egg), no significant differences were observed (minimum 160.3 for goldenrod 0% and maximum 208.1 alfalfa 100%).
The value of the HU was almost invariable for all groups throughout the application experiment, yet always above 72. This yields a AA qualitative class of eggs. The addition of enriched biomass increased the thickness of the eggshells, compared to the control group on the 30th and 60th d of the experiment. However, long-term feeding is less effective (8%) for eggshell strength vs. mineral compounds.
The content of microelements in edible egg fractions was correlated with the type of feed additive provided into the feed. The highest concentrations in albumen were obtained for goldenrod 100%. It had 90% more Cu2+ ions, 615% Mn2+, and 729% Zn2+ compared to mineral salts. In yolk, the highest Cu2+ and Zn2+ concentrations were obtained for goldenrod 50, 31, and 20% more, respectively, compared to the control. The use of goldenrod-based additives was more effective than alfalfa one.
The transfer of microelements to the edible parts of eggs was as follows; Zn>Cu>>Mn. When enriched biomass was being applied, better results were found in comparison with the rest of the research groups. The highest transfer for albumen was obtained for Zn2+ (goldenrod 50%) – 23% more in comparison to the control group. In the case of yolk, the highest transfer was calculated for goldenrod 50% mixture. The transfer was over 100% higher for all microelements determined vs. the control group. The total content of micronutrients in the albumen and yolk, if enriched with goldenrod, provides a functional food status of eggs.
The presence of plant-based feed additives improves the organoleptic quality for consumers compared to the control group and the products available on the market.
Due to a better transfer of microelements to the egg content for new feed additives, 2 nutritional strategies are possible. The first one assumes the reduction of the total amount of enriched biomass in the feed to obtain the level of micronutrients from the control group (about 20% less total micronutrients in the feed). The second is based on increasing the amount of the feed additive in order to achieve the functional food status of eggs.
CONCLUSIONS
The microelements play a crucial role in the physiological development of laying hens and their productivity. The manipulation of the trace elements supplements is feasible to enhance the economical parameters of breeding and increase the bioavailability of them in edible parts of eggs. The use of feed additives obtained based on biosorption does not affect mechanical or production parameters in the egg-laying process. Enriched biomass had a better effect on the transfer of microelements than unenriched biomass in yolk and albumen. Goldenrod based feed additives has been proven to be a more efficient feed additive than alfalfa in the case of biofortification of edible parts of eggs. Biomass feed strategies have some advantages over the classical approaches, but research is needed for understanding the mechanism of trace elements transfer in eggs to maximize the potential for achieving functional food status.
ACKNOWLEDGMENTS
This project was financed in the framework of grant entitled:“ Cultivated plants and natural products as a source of biologically active sub-stances assign to the production of cosmetic and pharmaceutical products as well as diet supplements” (no. BIOSTRATEG2/298205/9/NCBR/2016) attributed by the National Centre for Research and Development.
The publication is co-financed under the Leading Research Groups support project from the subsidy increased for the period 2020–2025 in the amount of 2% of the subsidy referred to Art. 387 (3) of the Law of 20 July 2018 on Higher Education and Science, obtained in 2019.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Abd El-Hack M.E., Alagawany M., Arif M., Chaudhry M.T., Emam M., Patra A. Organic or inorganic zinc in poultry nutrition: a review. Worlds Poult. Sci. J. 2017;73:904–915. [Google Scholar]
- Arpášová H., Petrovič V., Mellen M., Kačániová M., Čobanová K., Leng L. The effects of supplementing sodium selenite andselenized yeast to the diet for laying hens on thequality and mineral content of eggs. J. Anim. Feed Sci. 2009;18:90–100. [Google Scholar]
- Berwanger E., Vieira S.L., Angel C.R., Kindlein L., Mayer A.N., Ebbing M.A., Lopes M. Copper requirements of broiler breeder hens. Poult. Sci. 2018;97:2785–2797. doi: 10.3382/ps/pex437. [DOI] [PubMed] [Google Scholar]
- Brodacki A., Batkowska J., Stepniowska A., Blicharska E., Drabik K. Quality and mineral composition of eggs from hens supplemented with copper-lysine chelate. Arch. Anim. Breed. 2018;61:109–113. [Google Scholar]
- Can C., Jianlong W. Correlating metal ionic characteristics with biosorption capacity using QSAR model. Chemosphere. 2007;69:1610–1616. doi: 10.1016/j.chemosphere.2007.05.043. [DOI] [PubMed] [Google Scholar]
- Chojnacka K. Using biosorption to enrich the biomass of Chlorella vulgaris with microelements to be used as mineral feed supplement. World J. Microbiol. Biotechnol. 2007;23:1139–1147. [Google Scholar]
- Georgievskii V., Annenkov B., Samokhin V. Mineral nutrition of animals: studies in the agricultural and food sciences. Pages 391-395 in Mineral Feeding of Poultry. Butterworth and Co.; Moscow: 2013. [Google Scholar]
- Gumus H., Oguz M.N., Bugdayci K.E., Oguz F.K. Effects of sumac and turmeric as feed additives on performance, egg quality traits, and blood parameters of laying hens. Rev. Bras. Zootec. 2018;47 [Google Scholar]
- Inal F., Coşkun B., Gülşen N., Kurtoǧlu V. The effects of withdrawal of vitamin and trace mineral supplements from layer diets on egg yield and trace mineral composition. Br. Poult. Sci. 2001;42:77–80. doi: 10.1080/713655024. [DOI] [PubMed] [Google Scholar]
- Jankowski J., Ognik K., Stȩpniowska A., Zduńczyk Z., Kozłowski K. The effect of the source and dose of manganese on the performance, digestibility and distribution of selected minerals, redox, and immune status of turkeys. Poult. Sci. 2019;98:1379–1389. doi: 10.3382/ps/pey467. [DOI] [PubMed] [Google Scholar]
- Ligas B., Izydorczyk G., Mironiuk M., Kowalczyk P., Witek-Krowiak A., Chojnacka K. New feed additives based on alfalfa and goldenrod biomass enriched with microelements by biosorption. Przem. Chem. 2018;97:2141–2144. [Google Scholar]
- Michalak I., Chojnacka K. The application of macroalgaPithophora varia Wille enriched with microelements by biosorption as biological feed supplement for livestock. J. Sci. Food Agric. 2008;88:1178–1186. [Google Scholar]
- Michalak I., Chojnacka K., Dobrzański Z., Górecki H., Zielińska A., Korczyński M., Opaliński S. Effect of macroalgae enriched with microelements on egg quality parameters and mineral content of eggs, eggshell, blood, feathers and droppings. J. Anim. Physiol. Anim. Nutr. (Berl). 2011;95:374–387. doi: 10.1111/j.1439-0396.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- Michalak I., Witek-Krowiak A., Chojnacka K., Bhatnagar A. Advances in biosorption of microelements – the starting point for the production of new agrochemicals. Rev. Inorg. Chem. 2015;35:115–133. [Google Scholar]
- National Research Council . 9th ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Naz S., Idris M., Khalique M.A., Rahman Z.-U., Alhidary I.A., Abdelrahman M.M., Khan R.U., Chand N., Farooq U., Ahmad S. The activity and use of zinc in poultry diets. Worlds. Poult. Sci. J. 2016;72:159–167. [Google Scholar]
- Opaliński S., Dolińska B., Korczyński M., Chojnacka K., Dobrzański Z., Ryszka F. Effect of iodine-enriched yeast supplementation of diet on performance of laying hens, egg traits, and egg iodine content. Poult. Sci. 2012;91:1627–1632. doi: 10.3382/ps.2011-02031. [DOI] [PubMed] [Google Scholar]
- Réhault-Godbert S., Guyot N., Nys Y. The golden egg: nutritional value, bioactivities, and emerging benefits for human health. Nutrients. 2019;11 doi: 10.3390/nu11030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel M.T., Alderman H. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382:536–551. doi: 10.1016/S0140-6736(13)60843-0. [DOI] [PubMed] [Google Scholar]
- Saeid A., Chojnacka K., Opaliński S., Korczyński M. Biomass of Spirulina maxima enriched by biosorption process as a new feed supplement for laying hens. Algal Res. 2016;19:342–347. [Google Scholar]
- Santoso U., Fenita Y. The effect of sauropus androgynus leaf extract on performance, egg quality and chemical composition of eggs. J. Indones. Trop. Anim. Agric. 2016;41:125–134. [Google Scholar]
- Skrzypczak D., Ligas B., Mikula K., Witek-Krowiak A., Samoraj M., Moustakas K., Chojnacka K. Valorization of post-extraction biomass residues as carriers of bioavailable micronutrients for plants and livestock. Biomass Convers. Biorefin. 2020:1–16. [Google Scholar]
- Skrzypczak D., Witek-Krowiak A., Dawiec-Liśniewska A., Podstawczyk D., Mikula K., Chojnacka K. Immobilization of biosorbent in hydrogel as a new environmentally friendly fertilizer for micronutrients delivery. J. Clean. Prod. 2019;241 [Google Scholar]
- Stanaćev V.S., Milošević N., Stanaćev V.Ž., Puvača N., Milić D., Pavlovski Z. Chelating forms of microelements in poultry nutrition. Worlds. Poult. Sci. J. 2014;70:105–112. [Google Scholar]
- Świątkiewicz S., Arczewska-Włosek A., Józefiak D. The efficacy of organic minerals in poultry nutrition: review and implications of recent studies. Worlds Poult. Sci. Assoc. 2014;70:475–485. [Google Scholar]
- Świątkiewicz S., Koreleski J. Eggshell quality in laying hens fed diets supplemented with different levels of zinc and manganese. Polish J. Food Nutr. Sci. 2007;57:551–554. [Google Scholar]
- White P.J., White P.J., Broadley M.R. Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- Witkowska Z., Chojnacka K., Korczyński M., Świniarska M., Saeid A., Opaliński S., Dobrzański Z. Soybean meal enriched with microelements by biosorption - a new biological feed supplement for laying hens. Part I. Performance and egg traits. Food Chem. 2014;151:86–92. doi: 10.1016/j.foodchem.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Witkowska Z., Chojnacka K., Michalak I. Application of biosorption in the production of innovative feed supplements: a novel method. Adsorpt. Sci. Technol. 2013;31:421–431. [Google Scholar]
- Witkowska Z., Michalak I., Korczyński M., Szołtysik M., Świniarska M., Dobrzański Z., Tuhy Ł., Samoraj M., Chojnacka K. Biofortification of milk and cheese with microelements by dietary feed bio-preparations. J. Food Sci. Technol. 2015;52:6484–6492. doi: 10.1007/s13197-014-1696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowska Z., Świniarska M., Korczyński M., Opaliński S., Konkol D., Michalak I., Saeid A., Mironiuk M., Chojnacka K. Biofortification of hens’ eggs with microelements by innovative bio-based dietary supplement. J. Anim. Physiol. Anim. Nutr. (Berl). 2019;103:485–492. doi: 10.1111/jpn.13027. [DOI] [PubMed] [Google Scholar]