Abstract
Brain growth and development occur at peak rates in early childhood through adolescence, and for some children this must happen in conjunction with chronic kidney disease (CKD), associated medical conditions, and their treatment(s). This review provides an overview of key findings to date on the topic of the brain in pediatric CKD. Here, we specifically address the topics of neuroimaging and cognition in pediatric CKD with consideration to biomarkers of disease progression that may impact cognition. Current cognitive data suggest that most children with mild to moderate CKD do not exhibit significant cognitive impairments; but, rather, the presence of somewhat lower intellectual abilities and subtle deficits in selected executive functions. Although promising, modern neuroimaging data remain inconclusive in linking cognitive findings to neuroimaging correlates in the pediatric CKD population. Certainly, it is important to note that even subtle cognitive concerns can present barriers to learning, social functioning, and overall quality of life if not appropriately recognized or addressed. Further longitudinal research utilizing concurrent and targeted cognitive and neuroimaging evaluations is warranted to better understand the impact of CKD progression on brain development and associated neurocognitive outcomes.
Keywords: chronic kidney disease, pediatric, cognition, neuroimaging, biomarkers
Introduction –
For the pediatric patient, chronic kidney disease (CKD) represents a life-long process. There may be a transition from early-stage disease to dialysis and then to subsequent transplant; however, the CKD life-cycle begins again within the transplanted kidney, in nearly every instance as a “new normal.” The progression of CKD is accompanied by a multitude of multisystem effects, with an impact on cognition observed across the disease spectrum.
Cognition and neurodevelopment exist in tandem. Normal neurodevelopment in pediatric patients represents a purposeful, sequential process of gray and white matter deposition and synaptic pruning in elegant juxtaposition – beginning in utero and extending into early adulthood before arrival at the mature adult brain. Our understanding of brain development and cognition is generally well-characterized within the context of the healthy child. As we see increased survival into adulthood among children with advanced chronic kidney disease, it behooves us to consider the long-term impact of CKD progression on cognition and the developing brain and identify disease-related risk factors, particularly those that are modifiable, that can be harnessed to mitigate cognitive risk. As such, this review provides an overview of key, contemporary research findings examining three basic questions: (1) What cognitive deficits are observed in pediatric CKD? (2) Are there biomarkers of disease progression that impact cognition? (3) What are the neuroimaging findings in pediatric CKD? Does neuroimaging data yield developmental correlates of cognitive functioning? Available evidence addressing each of these questions should provide guidance to both the clinician and researcher working with children with CKD and, hopefully, lay the foundation for future work in this population. In particular, we provide a call to action for the scientific community to examine clinical biomarkers, brain structure, brain function, and neurocognition simultaneously in order to improve our understanding of interaction between the kidney and the brain in CKD.
What cognitive deficits are observed in pediatric CKD?
Historically, advanced CKD in infancy was associated with high rates of developmental delay, microcephaly, and chronic seizures. Data from the early 1990s evaluating infants and toddlers on dialysis documented the risk of pronounced developmental delay, compared to children with mild or no renal disease (1). Estimates from these early-era findings reported approximately 20-25% of young children with advanced kidney disease had significant developmental delay (1, 2) and that those with a diagnosis of end-stage kidney disease (ESKD) from infancy were more likely to have lower intelligence (IQ) (3). Nutritional advances, anemia control, and the avoidance of aluminum-containing compounds/binders were likely substantial contributors to the reported improvement in cognitive outcomes within the pediatric CKD population. These changes in care have enhanced pediatric CKD/ESKD survival; thus, creating a unique opportunity for researchers to better understand how CKD progression and associated comorbidities accelerate the emergence of cognitive deficits.
Little attention has been given to the cognitive assessment of preschool age children with CKD. Duquette et al. (4) reported the presence of cognitive and developmental differences between a group of preschool CKD patients – including those with advanced CKD requiring dialysis and transplant - and healthy controls. In this sample, lower cognitive scores were directly related to disease duration. A more recent study on cognitive outcomes in a large (N = 126) preschool sample of patients with mild to moderate CKD (estimated glomerular filtration rate [eGFR] 30-90 ml/min/1.73m2) evaluated developmental level/IQ, attention regulation, and parent ratings of executive functions, social-behavior, and adaptive behaviors (5). In general, the sample demonstrated intact cognitive and developmental functioning. The percentage of preschoolers who met an at-risk developmental designation was determined within the sample based on an at-risk definition of performance at least one standard deviation (S.D.) below the normative mean for the tasks and rating scales administered. When examining those considered at-risk, rates of performance lower than 1 S.D. below the mean were uniformly higher than would be expected from normative standards (approximately 16%). Specifically, within the developmental level/IQ domain, 27% of the children were deemed at risk. Within the attention regulation domain, 23% of the children were at least one SD below the mean on errors of commission, suggesting a heightened rate of impulsive responding in this sample. Parent ratings of executive functioning placed approximately 30% of the students at risk for their overall executive capabilities. Notably, approximately 40% of the sample was found to be “at-risk” on two or more measures.
The impact of CKD on cognition is better described among school-age children and adolescents. Intelligence (IQ) is generally within the average range for children and adolescents with mild to moderate CKD (6). Hooper et al., (2011) found that a majority of children in the Chronic Kidney Disease in Children (CKiD) (7) sample had average range IQ; however, 25% of the sample had scores greater than 1 standard deviation below the mean, indicating below average intelligence (6).
Children and adolescents across the CKD spectrum have shown neurocognitive difficulties, notably, in the domains of attention regulation and executive function. The term executive function (EF) represents an interrelated and overlapping set of neuropsychological constructs that include a variety of higher-order cognitive capacities such as judgment, planning, decision-making, response monitoring, attention, working memory, insight, and self-regulation. Much of our understanding of executive function in pediatric CKD comes from the CKiD Study (7). Hooper et al. (2011) sought to identify the presence of cognitive difficulties in children with mild to moderate CKD in relationship to disease-related variables (6). This large-scale sample provided data for 368 children ages 6-16 years at the time of analysis. Data analyzed included parental reports of EF and attention – in addition to a measure of IQ. Within the sample, 40% (95% CI) of children were found to be at-risk (i.e., scores at least one standard deviation below the mean) for lower overall EF as per parental report from the Behavior Rating Inventory of Executive Function (BRIEF) (8), which was more than double the normative expectation.
Further analysis of EF data has detailed the association between duration of CKD and level of CKD severity on EF performance (9). Data from children having mild to moderate CKD demonstrated that a longer duration of CKD was associated with increased odds for poor performance on the Conners Continuous Performance Test-II, a neurocognitive task utilized to assess attention regulation as a component of EF. Participants evidenced the most deficit in the domain “errors of commission” – a measure used to assess the inhibitory control component of EF. Other single-center studies have also shown the presence of poorer attention regulation in children with advanced CKD on dialysis compared to healthy, age-matched controls (10, 11).
It is a logical extension, then, that the presence of subtle cognitive difficulties, and perhaps deficits, may drive academic difficulties. Academic underachievement is present in slightly over 30% of children within the CKiD sample (12), and single-center data also reveal an increased rate of grade retention among those with CKD (13). The risk for academic underachievement is likely multifactorial and impacted by subtle cognitive deficits, the higher rates of chronic school absenteeism with greater medical complexity (14), and perhaps other key factors such as family stress and lack of school supports.
Summary.
In general, the preponderance of contemporary cognitive findings for children with CKD demonstrate that most children with mild to moderate CKD do not exhibit significant global cognitive impairments; but, rather, average to slightly low-average intelligence (IQ) and related deficits in various executive functions, specifically attention regulation (15). While significant cognitive impairment may not be present in most children with CKD, it is important to note that even subtle cognitive concerns can provide barriers to learning, social functioning, and overall quality of life if not appropriately recognized and addressed. Concern for the presence of cognitive deficits should prompt early engagement with developmental and educational specialists and a steady practice of developmental surveillance on the part of all care providers.
Are there biomarkers of disease progression that impact cognition?
A clinical biomarker is a feature that can be objectively measured and evaluated as an indicator of normal (or pathological) biological processes as well as the accompanying biological responses to a therapeutic intervention (16). Research in the field of pediatric nephrology has sought to identify clinical biomarkers of pediatric CKD progression that may impact cognition. Table 1 summarizes currently known relationships between clinical biomarkers and cognitive outcomes in pediatric CKD. Specific examples of clinical biomarkers include eGFR, proteinuria, and hypertension.
Table 1 -.
Relationships between clinical biomarkers and cognitive outcomes in pediatric CKD.
| Biomarker | Development | Intelligence (IQ) | Executive Functions |
|---|---|---|---|
| Hypertension | Elevated BP associated with lower full scale and performance IQ [20] | Heightened blood pressure variability is associated with set shifting errors [21] | |
| Anemia | Anemia in preschoolers with CKD predicts risk of lower developmental level [5] | ||
| Proteinuria | Proteinuria (UPC > 2) may be associated with lower verbal and full scale IQ [6] | ||
| Duration of CKD | Poorer performance on measures of attention (executive function) notably in those with longer duration of CKD [9] | ||
| Renal function (level of eGFR) | Lower eGFR is independently associated with at risk status for lower developmental level in preschoolers with CKD [5] | Better renal function predicts lesser risk of poor performance on measures of executive function [6] | |
| Genomic variants | Those with copy number variants associated with renal disease also have significantly lower IQ than those without genomic variants [24] | Those with copy number variants associated with renal disease also have significantly worse scores on measure of executive function those without genomic variants [24] |
Existing data support a relationship between renal function (eGFR) and cognition. For example, data from preschool patients with CKD indicate that higher eGFR is associated with more intact developmental level/IQ (5). Similarly, older children with mild to moderate CKD who have higher eGFR are at lower risk for executive dysfunction (6).
Proteinuria has been identified as a clinical renal biomarker for poorer or at-risk cognitive outcomes. Participants in the CKiD study with proteinuria were approximately 2.5 times more likely to be at-risk for both low IQ and lower scores on measures of attention variability than those without proteinuria (6).
The impact of hypertension (HTN) on cerebrovascular disease in adulthood also makes this comorbidity a plausible biomarker of disease progression that can impact cognition. In particular, hypertension may serve as one of the primary mechanisms underlying the cognitive dysfunction in children with CKD (17-20) and as a potential modifiable risk factor with positive treatment. Lande et al. (2011) evaluated data from children mild to moderate CKD having elevated blood pressure (i.e., systolic or diastolic blood pressure > 90th percentile for age) (20). Children with elevated blood pressure were more likely to have lower nonverbal IQ than normotensive children (20). Within the analysis, it was noted that the blood pressure index (i.e., the subject’s blood pressure divided by the 95th percentile blood pressure for that subject’s gender, age, and height) correlated inversely with nonverbal IQ, and this relationship was maintained even after controlling for demographic and disease related variables (20).
Additional data analysis from the CKiD Study has investigated the relationship between visit-to-visit blood pressure variability (BPV) and cognition. Visit-to-visit BPV was assessed by two methods: 1) the standard deviation of visit BPs (BPV-SD), and 2) average real variability (ARV), with all BPs indexed to the 95th percentile for age, gender, and height for that subject. Lande et al. (2016) assessed blood pressure and blood pressure variability in relationship to cognitive performance on measures of intelligence, attention, behavior, and executive function (21). There was no effect of BPV noted on intelligence, attention, or behavior; however, heightened visit-to-visit BPV placed hypertensive children with mild to moderate CKD at-risk for lower performance on a measure of EF called category switching (21). Category switching represents the ability to adjust thinking or attention in response to changing expectations, goals, or environmental stimuli. This finding was particularly noteworthy given previous data demonstrating that both CKD and primary hypertension in adults are linked to performance deficits on neurocognitive measures of cognitive efficiency and processing speed (22, 23).
Finally, do causative genomic variants that lead to development of CKD also predispose children to higher risk of neurocognitive impairment? To answer this, genetic data were analyzed to determine whether the subtle neurocognitive differences, as discussed herein, could be attributed to genomic differences in addition to, or perhaps rather than, renal impairment. Verbitsky et al. (2017) found that children with genomic disorders scored significantly poorer on measures of IQ, EF, and ratings of anxiety/depressive symptoms compared to noncarriers (differences of 0.6–0.7 SD; P=1.2×10−3–2.4×10−4) (24). Interestingly, the effect of genomic disorders on cognition was attenuated by level of maternal education, which may suggest the potential for a strong positive (or negative) inducible environmental modifier on cognition in CKD patients with genomic disorders.
Summary.
Decreased renal function, proteinuria, and hypertension are identifiable clinical biomarkers that are associated with poorer cognitive outcomes in pediatric CKD. Additionally, causative genomic variants for CKD are linked to worse performance on measures of IQ, EF and behavior. In particular, hypertension appears to be a key clinical biomarker by which individual care can be optimized to improve cognitive outcomes in pediatric CKD.
What are the neuroimaging findings in pediatric CKD? Are there neuroimaging correlates to cognitive functioning?
Neuroimaging provides a noninvasive opportunity to examine brain structure and function. Given the observed neurocognitive findings in the pediatric CKD population, research has more recently moved to defining the neural mechanisms for cognitive dysfunction. In seeking a deeper understanding of these cognitive problems, it is critical to ask: 1) is there a structural or functional brain basis for the observed neurocognitive deficits in this population; and 2) are there specific regions of the brain that are most associated with these deficits?
Published work in the domain of neuroimaging in CKD has largely been hypothesis generating in nature; that is, seeking to describe and define differences in the pediatric CKD brain compared to healthy children. Nineteen neuroimaging studies that include pediatric CKD patients have been published in the literature between 1977 and the present (25). Of these studies, 13 utilized CT-based neuroimaging and 6 utilized MRI. The majority of early studies on the topic of neuroimaging in pediatric CKD used computerized tomography (CT) scans. Within the more modern pediatric MRI subset, five have evaluated structural MRI and only one has examined brain function through use of regional cerebral blood flow. Study populations in the published literature vary widely in sample size, age, and primary disease etiology.
Computerized tomography (CT).
Contemporary neuroimaging data available from CT are available from 1997-2006. Nine of the thirteen published studies in the pediatric CKD literature using CT as a neuroimaging modality were case-control or prospective in nature. The majority of these early CT-based studies focused on pediatric populations with ESKD or post-transplantation without inclusion of less severe disease CKD phenotypes. In general, CT data from these studies demonstrate higher risk for global cerebral atrophy, silent white matter infarcts, and ventriculomegaly (26-28). Cerebral atrophy is well-described in pediatric nephrology literature dating prior to 1990, with reports of up to 60% of patients having atrophy that was not associated with type of renal disease, hypertension severity, or corticosteroid therapy (29). Cerebral atrophy in ESRD, however, has been correlated with age of onset of renal disease (26) and dialytic modality (30, 31). Qualitative imaging also shows that lower cerebral density (30, 32) and ventriculomegaly secondary to brain atrophy (33) are more often associated with requirement for and duration of pediatric hemodialysis compared with receipt of peritoneal dialysis (31).
Magnetic resonance imaging (MRI).
The use of MRI can provide both qualitative and quantitative opportunity to assess the brain. Quantitative MRI – commonly referred to as structural MRI (sMRI) – provides information related to volumes within and between regions of the brain. Additionally, if the MRI is performed in a research-based scan sequence, it is often possible to obtain sequences that evaluate white and gray matter as well as blood flow within the brain – the former representing sMRI and latter representing functional MRI (fMRI).
Data from the Neurocognitive Assessment and Magnetic Resonance Imaging Analysis of Children and Young Adults with Chronic Kidney Disease (NiCK) Study performed volumetric brain assessment using sMRI in youth with CKD compared to normal controls (34). Although the sample size was adequately powered to detect a statistical difference between populations (N = 90), the CKD sample was very heterogenous with regard to chronological age, stage of disease, disease etiology, and inclusion of dialysis/transplant patients. Statistical analyses, including corrections for multiple comparisons and adjustments for age and sex, did not support any specific brain regional differences between CKD patients and controls. Furthermore, there were no CKD-related clinical predictors to link differences in brain regions of interest to neurocognitive performance.
sMRI also serves to inform the microstructural white matter integrity of the brain via diffusion tensor imaging (DTI). DTI is an MRI modality that allows for examination of white matter integrity – i.e., axonal or white matter microstructural changes that disrupt the diffusive property of water in the axon. These microstructural changes have been shown in diseases such as hypertension, diabetes mellitus, and atherosclerosis (35, 36). The result of this microstructural disruption is a change in a physical diffusive property of the axon called decreased fractional anisotropy. Matsuda-Abedini et al. (37) conducted a quantitative white matter analysis utilizing a sample of patients with CKD (including patients on peritoneal dialysis and post-transplant) and control patients. This demonstrated the presence of decreased white matter integrity, specifically decreased fractional anisotropy, within the anterior limb of the internal capsule. It is possible that the entirety of the white matter integrity difference within the sample was not captured given the multi-site nature of the study; specifically, MRI sequences are highly scanner dependent and statistically significant differences can emerge (or be lost) due to differences in magnet strength or brand of scanner utilized. Lastly, the sample did not have parallel neurocognitive data to further inform the significance of white matter changes within the anterior limb of the internal capsule finding. Despite this, the data underscore a critical need to understand the potential impact of white matter injury on cognition in the pediatric CKD population. This, again, emphasizes the vascular, multisystem impact of CKD, with need for early recognition of non-traditional disease manifestations, such as white matter injury.
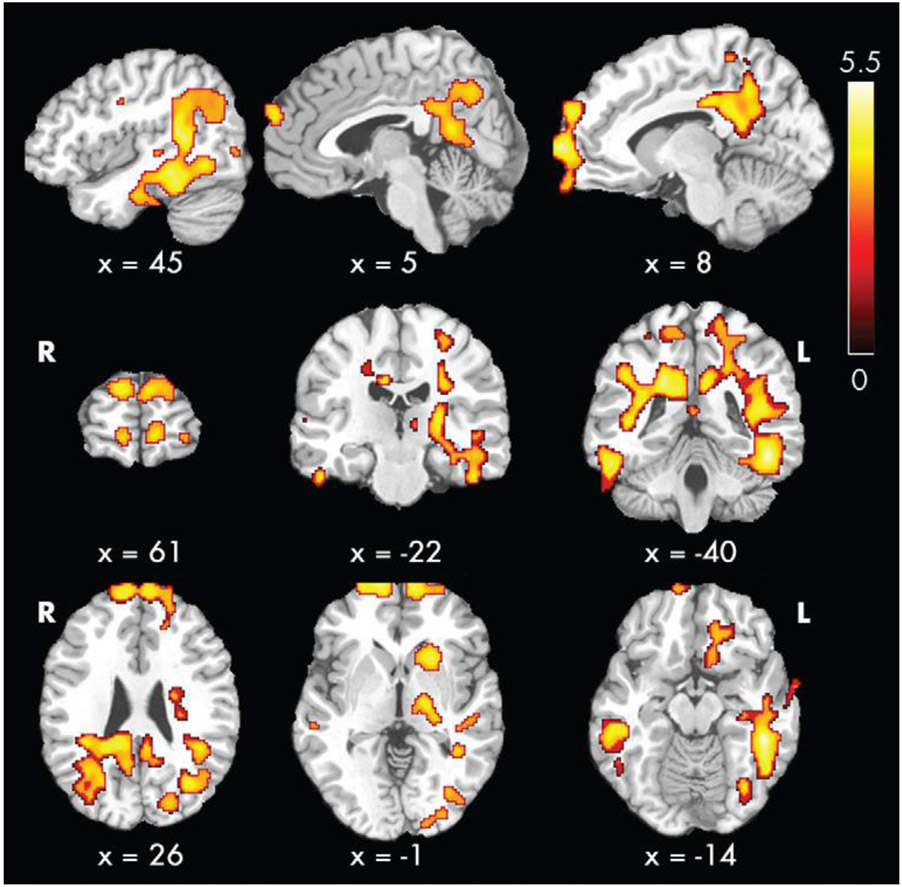
fMRI studies examine the use of oxygen within the brain or rate of arterial cerebral blood flow with specialized scan sequences called ‘resting state’ or ‘arterial spin labeling,’ respectively. Additional analysis of data from the NiCK Study lends evidence for regional cerebral blood flow abnormalities that may underlie cognitive changes in pediatric CKD (38). In Liu et al. (2018) patients with CKD, including dialysis and transplant, showed higher global cerebral blood flow compared to control subjects. Perhaps of most interest, one particular area showing regional cerebral blood flow differences between patients and control subjects included a region called the “default mode” network (Figure 1). Neuroscience literature supports that the brain is organized into functional networks, and the default mode network is a critical loop for attention regulation and, perhaps, EF processes (39-42). Hematocrit-related effects explained most of the observed group differences in cerebral blood flow. Thus, Liu et al. hypothesized that chronic anemia experienced in pediatric CKD could be a potential cause of vascular endothelial damage due to increased compensatory blood flow to meet demands for deficits in tissue oxygen delivery.
Figure 1 -.
From Liu 2018 [38], reprinted with permission: demonstration of voxel-wise group comparison of cerebral blood flow after removal of effects of hematocrit level, age, and sex. Contrast shown here demonstrates the regions where those with CKD have greater cerebral blood flow than controls. Of note, there were no regions in this analysis where controls had greater regions than those with CKD. Color bar indicates t scores. x, y, z = coordinates in Montreal Neurological Institute (MNI) space.
Summary.
Although promising, modern neuroimaging data remain somewhat inconclusive in linking specific cognitive processes in pediatric CKD to specific brain regions and/or networks. It may be that current findings are limited by the heterogeneity of our patient samples within a small sample size – both by etiology and stage of disease. For example, neuroimaging research in homogenous populations such as sickle cell disease clearly demonstrate a vascular (white matter) effect of disease that is associated with sickle cell disease type (e.g., SS vs SC disease) (43). Certainly, this supports the notion that a disease with a vascular disease phenotype, such as hypertension in CKD, can manifest robust findings on neuroimaging in a pediatric population.
A scientific call to action –
Adult data support the concept of the “kidney-brain axis” whereby interactions between renal impairment and brain function occur that may accentuate the risk for future cognitive impairment (44, 45). Our understanding of the brain in parallel to pediatric CKD progression is limited by numerous factors – sample heterogeneity within published data, small sample sizes, and notably no studies fully integrating cognition, biomarkers, and neuroimaging into a multifaceted assessment. There are no longitudinal (specifically, lifespan) studies in pediatric CKD that fully characterize cognitive and neurodevelopmental outcomes over time in parallel with CKD progression and clinical biomarkers. It is critical, then, that our field address the concept of the pediatric kidney-brain axis through future research in an accelerated longitudinal model. This model should prioritize systematic collection of clinical biomarker data across the disease course, comprehensive structural and functional neuroimaging at multiple time points, consideration to task-based functional neuroimaging, and tandem neurocognitive assessment that focuses on measures of executive function. Such research should utilize a healthy control model in parallel to inclusion of children with CKD and also strongly consider ways to ally with experts across pediatric specialties to include a focused chronic disease control group. Inclusion of a chronic disease control group may allow our field to more clearly attribute cognitive and neuroimaging changes to the presence of CKD, as opposed to the presence of a chronic disease in general.
Conclusion –
The cognitive deficits observed in pediatric CKD patients represent a potentially under-recognized component of the CKD disease process in day-to-day clinical practice. This review of key findings to date demonstrates that our pediatric CKD patients are most at risk for cognitive dysfunction in the domain of executive functions – specifically, attention and working memory. These cognitive difficulties emerge in early childhood and can be detected during early CKD. This signals a need for greater attention to the developing brain in the midst of a life-long, chronic disease process. Current data represent a limited understanding of the medical determinants of cognitive dysfunction in CKD and an even more minimal understanding of the genetic and epigenetic drivers of cognition in CKD. Certainly, research efforts to understand cognition in CKD may be better served through parallel inclusion of a chronic disease control model alongside healthy comparisons.
In parallel to this, it is critical to understand the influence of clinical biomarkers of disease that may drive cognitive functioning in children with CKD (e.g., hypertension, proteinuria, genomic variants, renal function). The potential for complex interactions between clinical disease biomarkers and CKD progression creates challenges for both clinicians and scientists working with this population. There also is significant heterogeneity within the pediatric CKD population, and attention to the various subgroups – specifically examining the effect of disease etiology - will require further distinction in future studies. For example, although having the common problem of kidney disease and progression, children with Autosomal Recessive Polycystic Kidney Disease (ARPKD) and those with Lupus Nephritis show little in the way of cognitive impairments when compared to children having CKD only (46, 47). This heterogeneity should be considered when examining this literature. Further, with respect to clinical biomarkers that may drive cognitive dysfunction, there is some sense from the available pediatric and adult literature that the cerebrovascular system, and potential for a hypertension as a modifiable risk factor, may be of keen interest as one of the primary mechanisms underlying the cognitive dysfunction in children with CKD (17-20). This will require ongoing scientific scrutiny with need for longitudinal assessment in parallel to therapeutic intervention in those with hypertension (48).
Our field continues to push for improved CKD treatment – certainly it is easy to argue that the neurocognitive characteristics observed now are much less pervasive than those observed 30 years ago in pediatric CKD-ESKD; however, this does not negate the need to pursue early identification and early intervention for potential cognitive and associated learning difficulties that may be present in a milder form. As a field, we must seek to push boundaries in order to understand the neural mechanisms of cognition across the spectrum of pediatric CKD, ESKD, and transplant to improve adherence to therapies, enhance quality of life, and bolster successful transitions to adult care.
Summary Points –
1. Children and adolescents with CKD are at-risk for cognitive dysfunction.
2. The cognitive dysfunction seems to be manifest in the form of somewhat lower intellectual abilities, with associated problems in short-term memory, attention regulation, and selected aspects of executive functions. Academic achievement skills and adaptive behavior also may be of concern. These findings appear to be present in both preschool and school-age children.
3. Underlying neurological mechanisms remain unknown, but the available neuroimaging findings to date point to both structural and functional differences in the brain, with keen interest in the neurovascular system.
4. Hypertension may be a modifiable CKD biomarker that directly impacts cognition. Increased blood pressure index and heightened blood pressure variability both have been associated with lower cognitive functioning within pediatric CKD populations.
5. There is a need to examine neuroimaging and neurocognitive functions concurrently in the pediatric CKD population, and the field has reached a level of maturity that a longitudinal examination of these functions is needed. This is particularly important given the progressive processes inherent in both neurodevelopment and CKD.
Multiple Choice Questions:
In describing the impact of pediatric CKD on cognition, which of the following statements is false?
Nutritional advances, anemia control, and the avoidance of aluminum-containing compounds/binders are thought to have been substantial contributors to the reported improvement in cognitive outcomes within the pediatric CKD population.
Early data from the 1990s suggested a risk for pronounced developmental delay for young patients on dialysis.
Current data support significant differences in IQ between pediatric CKD patients and healthy children.
Evaluation of preschool patients with CKD suggests that these children may be more at risk for executive function and adaptive behavior concerns than healthy children.
Which of the following statements regarding executive function are true?
Executive function is a neurocognitive construct that include the abilities to purposefully plan, attend to complex stimuli, attention regulation, inhibitory control, set-shifting, working memory, and the simultaneous consideration of both conscious and subconscious information pertinent to decision-making.
Longer duration of CKD may be associated with worsened attention regulation
Heightened blood pressure variability is associated with worsened performance on category switching measures of executive function
All of the above are true statements
Current data from neuroimaging in pediatric CKD suggests:
The possibility of increased white matter integrity in major white matter tracts, specifically the anterior limb of the internal capsule.
Alterations in blood oxygen utilization on function brain sequences that suggest cerebral blood flow differences in brain networks engaged in executive function.
Significant differences in brain frontal lobe volume in pediatric CKD
Increased cortical thickness in the pediatric CKD brain
Biomarkers associated with pediatric CKD that may impact cognitive outcomes include:
Proteinuria
Hypertension
Copy number variants (genomic disorders)
All of the above
References
- 1.Ledermann SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS. Long-term outcome of peritoneal dialysis in infants. J Pediatr. 2000;136(1):24–9. [DOI] [PubMed] [Google Scholar]
- 2.Hulstijn-Dirkmaat GM, Damhuis IH, Jetten ML, Koster AM, Schroder CH. The cognitive development of pre-school children treated for chronic renal failure. Pediatr Nephrol. 1995;9(4):464–9. [DOI] [PubMed] [Google Scholar]
- 3.Warady BA, Belden B, Kohaut E. Neurodevelopmental outcome of children initiating peritoneal dialysis in early infancy. Pediatr Nephrol. 1999;13(9):759–65. [DOI] [PubMed] [Google Scholar]
- 4.Duquette PJ, Hooper SR, Icard PF, Hower SJ, Mamak EG, Wetherington CE, et al. Neurodevelopmental status and adaptive behaviors in preschool children with chronic kidney disease. J Sp Ed. 2009;43(1):45–51. [Google Scholar]
- 5.Hooper SR, Gerson AC, Johnson RJ, Mendley SR, Shinnar S, Lande MB, et al. Neurocognitive, Social-Behavioral, and Adaptive Functioning in Preschool Children with Mild to Moderate Kidney Disease. J Dev Behav Pediatr. 2016;37(3):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper SR, Gerson AC, Butler RW, Gipson DS, Mendley SR, Lande MB, et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(8):1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Odessa, FL: PAR, Inc; 2000. [Google Scholar]
- 9.Mendley SR, Matheson MB, Shinnar S, Lande MB, Gerson AC, Butler RW, et al. Duration of chronic kidney disease reduces attention and executive function in pediatric patients. Kidney Int. 2015;87(4):800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gipson DS, Hooper SR, Duquette PJ, Wetherington CE, Stellwagen KK, Jenkins TL, et al. Memory and executive functions in pediatric chronic kidney disease. Child Neuropsychol. 2006;12(6):391–405. [DOI] [PubMed] [Google Scholar]
- 11.Fennell EB, Fennell RS, Mings E, Morris MK. The effects of various modes of therapy for end stage renal disease on cognitive performance in a pediatric population--a preliminary report. Int J Pediatr Nephrol. 1986;7(2):107–12. [PubMed] [Google Scholar]
- 12.Harshman LA, Johnson RJ, Matheson MB, Kogon AJ, Shinnar S, Gerson AC, et al. Academic achievement in children with chronic kidney disease: a report from the CKiD cohort. Pediatr Nephrol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duquette PJ, Hooper SR, Wetherington CE, Icard PF, Gipson DS. Brief report: intellectual and academic functioning in pediatric chronic kidney disease. J Pediatr Psychol. 2007;32(8):1011–7. [DOI] [PubMed] [Google Scholar]
- 14.Richardson KL, Weiss NS, Halbach S. Chronic School Absenteeism of Children with Chronic Kidney Disease. J Pediatr. 2018;199:267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Didsbury M, van Zwieten A, Howell M, Kim S, Tong A, et al. Neurocognitive and Educational Outcomes in Children and Adolescents with CKD: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol. 2018;13(3):387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amur S. From our perspective: Clinical biomarker qualification: U.S. Food & Drug Administration; Accessed Aug 20 2019. [Available from: https://www.fda.gov/drugs/news-events-human-drugs/our-perspective-clinical-biomarker-qualification. [Google Scholar]
- 17.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke. 2002;33(1):26–30. [DOI] [PubMed] [Google Scholar]
- 18.Crichton GE, Elias MF, Dore GA, Torres RV, Robbins MA. Measurement-to-measurement blood pressure variability is related to cognitive performance: the Maine Syracuse study. Hypertension. 2014;64(5):1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, et al. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64(5):983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lande MB, Gerson AC, Hooper SR, Cox C, Matheson M, Mendley SR, et al. Casual blood pressure and neurocognitive function in children with chronic kidney disease: a report of the children with chronic kidney disease cohort study. Clin J Am Soc Nephrol. 2011;6(8):1831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lande MB, Mendley SR, Matheson MB, Shinnar S, Gerson AC, Samuels JA, et al. Association of blood pressure variability and neurocognition in children with chronic kidney disease. Pediatr Nephrol. 2016;31(11):2137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jassal SV, Roscoe J, LeBlanc D, Devins GM, Rourke S. Differential impairment of psychomotor efficiency and processing speed in patients with chronic kidney disease. Int Urol Nephrol. 2008;40(3):849–54. [DOI] [PubMed] [Google Scholar]
- 23.Waldstein SR. Hypertension and neuropsychological function: a lifespan perspective. Exp Aging Res. 1995;21(4):321–52. [DOI] [PubMed] [Google Scholar]
- 24.Verbitsky M, Kogon AJ, Matheson M, Hooper SR, Wong CS, Warady BA, et al. Genomic Disorders and Neurocognitive Impairment in Pediatric CKD. J Am Soc Nephrol. 2017;28(8):2303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moodalbail DG, Reiser KA, Detre JA, Schultz RT, Herrington JD, Davatzikos C, et al. Systematic review of structural and functional neuroimaging findings in children and adults with CKD. Clin J Am Soc Nephrol. 2013;8(8):1429–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passer JA. Cerebral atrophy in end-stage uremia. Proc Clin Dial Transplant Forum. 1977;7:91–4. [PubMed] [Google Scholar]
- 27.Elzouki A, Carroll J, Butinar D, Moosa A. Improved neurological outcome in children with chronic renal disease from infancy. Pediatr Nephrol. 1994;8(2):205–10. [DOI] [PubMed] [Google Scholar]
- 28.Papageorgiou C, Ziroyannis P, Vathylakis J, Grigoriadis A, Hatzikonstantinou V, Capsalakis Z. A comparative study of brain atrophy by computerized tomography in chronic renal failure and chronic hemodialysis. Acta Neurol Scand. 1982;66(3):378–85. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg A, Efrat R, Pomeranz A, Drukker A. Computerized tomography of the brain in children with chronic renal failure. The International journal of pediatric nephrology. 1985;6(2):121–6. [PubMed] [Google Scholar]
- 30.Schnaper HW, Cole BR, Hodges FJ, Robson AM. Cerebral cortical atrophy in pediatric patients with end-stage renal disease. Am J Kidney Dis. 1983;2(6):645–50. [DOI] [PubMed] [Google Scholar]
- 31.La Greca G, Biasioli S, Chiaramonte S, Dettori P, Fabris A, Feriani M, et al. Studies on brain density in hemodialysis and peritoneal dialysis. Nephron. 1982;31(2):146–50. [DOI] [PubMed] [Google Scholar]
- 32.Dettori P, La Greca G, Biasioli S, Chiaramonte S, Fabris A, Feriani M, et al. Changes of cerebral density in dialyzed patients. Neuroradiology. 1982;23(2):95–9. [DOI] [PubMed] [Google Scholar]
- 33.Kretzschmar K, Nix W, Zschiedrich H, Philipp T. Morphologic cerebral changes in patients undergoing dialysis for renal failure. AJNR Am J Neuroradiol. 1983;4(3):439–41. [PMC free article] [PubMed] [Google Scholar]
- 34.Hartung EA, Erus G, Jawad AF, Laney N, Doshi JJ, Hooper SR, et al. Brain Magnetic Resonance Imaging Findings in Children and Young Adults With CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2018;72(3):349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57(11):3083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozera GM, Dubaniewicz M, Zdrojewski T, Madej-Dmochowska A, Mielczarek M, Wojczal J, et al. Cerebral vasomotor reactivity and extent of white matter lesions in middle-aged men with arterial hypertension: a pilot study. Am J Hypertens. 2010;23(11):1198–203. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda-Abedini M, Fitzpatrick K, Harrell WR, Gipson DS, Hooper SR, Belger A, et al. Brain abnormalities in children and adolescents with chronic kidney disease. Pediatr Res. 2018;84(3):387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu HS, Hartung EA, Jawad AF, Ware JB, Laney N, Port AM, et al. Regional Cerebral Blood Flow in Children and Young Adults with Chronic Kidney Disease. Radiology. 2018;288(3):849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson JR Jr., Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A. 2001;98(2):683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raichle ME. The brain's default mode network. Annu Rev Neurosci. 2015;38:433–47. [DOI] [PubMed] [Google Scholar]
- 43.Steen RG, Emudianughe T, Hankins GM, Wynn LW, Wang WC, Xiong X, et al. Brain imaging findings in pediatric patients with sickle cell disease. Radiology. 2003;228(1):216–25. [DOI] [PubMed] [Google Scholar]
- 44.Miranda AS, Cordeiro TM, Dos Santos Lacerda Soares TM, Ferreira RN, Simoes ESAC. Kidney-brain axis inflammatory cross-talk: from bench to bedside. Clin Sci (Lond). 2017;131(11):1093–105. [DOI] [PubMed] [Google Scholar]
- 45.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol. 2013;24(3):353–63. [DOI] [PubMed] [Google Scholar]
- 46.Knight A, Kogon AJ, Matheson MB, Warady BA, Furth SL, Hooper SR. Cognitive Function in Children with Lupus Nephritis: A Cross-Sectional Comparison with Children with Other Glomerular Chronic Kidney Diseases. J Pediatr. 2017;189:181–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hooper SR. Risk Factors for Neurocognitive Functioning in Children with Autosomal Recessive Polycystic Kidney Disease. Front Pediatr. 2017;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lande MB, Batisky DL, Kupferman JC, Samuels J, Hooper SR, Falkner B, et al. Neurocognitive Function in Children with Primary Hypertension after Initiation of Antihypertensive Therapy. J Pediatr. 2018;195:85–94 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]