Abstract
Osteoarthritis (OA), the most common form of arthritis, is characterized by inflammation of joints and cartilage degradation leading to disability, discomfort, severe pain, inflammation, and stiffness of the joint. It has been shown that adenosine, a purine nucleoside composed of adenine attached to ribofuranose, is enzymatically produced by the human synovium. However, the functional significance of adenosine signaling in homeostasis and pathology of synovial joints remains unclear. Adenosine acts through four cell surface receptors, i.e., A1, A2A, A2B, and A3, and here, we have systematically analyzed mice with a deficiency for A3 receptor as well as pharmacological modulations of this receptor with specific analogs. The data show that adenosine receptor signaling plays an essential role in downregulating catabolic mechanisms resulting in prevention of cartilage degeneration. Ablation of A3 resulted in development of OA in aged mice. Mechanistically, A3 signaling inhibited cellular catabolic processes in chondrocytes including downregulation of Ca2+/calmodulin-dependent protein kinase (CaMKII), an enzyme that promotes matrix degradation and inflammation, as well as Runt-related transcription factor 2 (RUNX2). Additionally, selective A3 agonists protected chondrocytes from cell apoptosis caused by pro-inflammatory cytokines or hypo-osmotic stress. These novel data illuminate the protective role of A3, which is mediated via inhibition of intracellular CaMKII kinase and RUNX2 transcription factor, the two major pro-catabolic regulators in articular cartilage.
Keywords: Articular cartilage, Adenosinereceptor3, Osteoarthritis, RUNX2, CaMKII
Introduction
Osteoarthritis (OA) is one of the most common disorders of musculoskeletal system particularly common in adults 60 years of age or older [1]. OA is characterized by accelerated degeneration and loss of articular cartilage. Even though OA is often age-related, excessive mechanical stimulation and compression force on the joints can also result in degenerative changes in cartilage. In OA, changes seen in articular cartilage are the result of interaction between the components of the cartilage matrix (proteoglycans and collagens) and the chondrocytes which produce this matrix [2]. The pathologic characteristic of OA is directly perceptible by the initial degradative changes in the cartilage matrix. Understanding of how to prevent this escalated destruction is medically and socially significant.
Numerous genes which are active during normal development that drive hypertrophy and catabolism are transcriptionally silenced in healthy adult articular chondrocytes. Upregulation of this pro-hypertrophic program, which results in activation of catabolic enzymes which destroy the matrix, is detrimental in mature adult joints, and there are multiple mechanisms and signaling pathways that drive this degeneration. Some of the most notorious and prominent players in cartilage degeneration are Ca(2+)/calmodulin-dependent protein kinase (CaMKII) and Runt-related transcription factor 2 (RUNX2) [3]. By upregulating catabolic enzymes, both proteins promote terminal differentiation of chondrocytes, hypertrophy, and extracellularmatrix (ECM) destruction [3, 4]. While CaMKII and RUNX2 are naturally expressed in the developing joint [3], evidence suggests that their ectopic activation in adult OA is associated with activation of catabolic enzymes and cartilage degeneration [3–6]. Upstream pathways that can activate CaMKII and RUNX2 are relatively well characterized [3, 7], but signaling pathways that can inhibit this program are not as well known. Our study identifies adenosine receptor signaling as one potential signaling pathway that can regulate hypertrophy and catabolism in articular cartilage.
Adenosine is a natural molecule produced through degradation of adenine nucleotides and acts as a building block for nucleic acids. In human synovial tissue, a subpopulation of human mesenchymal stem cells expressing the ectonucleotidases CD39 and CD73, which control extracellular adenosine synthesis, have been shown to play a role in adenosine production in the joint [8, 9]. Furthermore, endogenous adenosine levels were reported to regulate homeostasis in cartilage matrix [10]. It was also shown that exogenous adenosine injection in the joint prevents OA development in a rat model of post-traumatic OA [11]. Anti-apoptotic and anti-inflammatory functions of adenosine have been previously reported in different tissues, and abnormal adenosine signaling has been implicated as a common mechanism in inflammatory damage [12]. Adenosine functions through the activation of four adenosine receptors A1, A2A, A2B, and A3 [12]. Adenosine receptors have been implicated in several key physiological processes, ranging from neuromodulation to immune regulation. A1 is expressed in all tissues with the highest levels found in the brain, where it functions by blocking neurotransmitter release and reducing the firing rate [13]. A2A plays a role in the modulation of inflammation, myocardial oxygen consumption, coronary blood flow, angiogenesis, and the control of cancer pathogenesis [12]. In the brain, A2A interacts with neurotransmitters to regulate motor activity, psychiatric behaviors, and neuronal cell death [12]. In addition, it was previously shown that blocking receptor activation of A2A with antagonists leads to cartilage matrix degradation in horse cartilage explants [10], and it was recently shown that A2A is important for the maintenance of articular cartilage [11]. A2B signaling plays a role in tissue adaptation to hypoxia or attenuation of acute inflammation; however, it also stimulates inflammatory pathways involving mitogen-activated protein kinases. A3 mediates a cardioprotective function during cardiac ischemia, and it has been implicated in both neuroprotective and neurodegenerative effects [12]. It also has been shown that A3 exerts anti-inflammatory effects in experimental animal models of inflammatory bowel disease, pulmonary inflammation, rheumatoid arthritis, OA, and liver inflammation [14]. Upregulation of A3 signaling causes downregulation of the NF-κB signaling pathway, inhibiting TNF-α, IL-6, IL-12, MIP-1α, MIP-2, and RANKL, which results in apoptosis of inflammatory cells [14].
The role of adenosine signaling in cartilage diseases has been investigated previously as the emergence of adenosine agonists that have allowed for the interrogation of specific adenosine receptors and downstream signaling. Pharmacological utilization of A3 agonists makes them a promising small molecule drug candidate. Various orally administered A3 agonists were efficacious in combating growth of solid tumors, including melanoma, prostate, colon, and hepatocellular carcinoma, which not only have shown anticancer effects but also immunological. In tumor lesions of A3 agonist-treated animals, the expression levels of PKB/Akt, IKK, and c-Myc and proteins were downregulated [14–16]. A3 agonists administered in combination with chemotherapeutic agents to tumor-bearing mice prevented the myelotoxic effects of chemotherapy [17]. In addition, based on the pre-clinical pharmacology data and encouraging data in clinical studies, the data obtained from orally administered A3 agonist IB MECA showed excellent safety profile and efficacy in patients with rheumatoid arthritis, psoriasis, and dry eye syndrome [14, 18, 19]. Prior studies have shown that IB MECA induces apoptosis of inflammatory cells and prevents articular cartilage damage in induced OA in rat models [20]. However, higher concentrations of IB MECA have a potential ability to activate other adenosine receptors such as A1 and A2A [21, 22], and these non-selective effects in vivo could not be completely ruled out for previously published studies. Further complicating matters, the bioavailability of this compound is difficult to control in vivo. Recent study showed that mice lacking A2A develop OA, which was characterized by loss of cartilage and chondrocyte hypertrophy [11]. Taking both studies into account, it remains unclear to what extent of IB MECA’s protective effects are actually driven by A3 signaling or if the effect results in simultaneous consequences of activation of A2A. Given this ambiguity and the lack of mechanistic insight into the role of adenosine signaling pathway in chondrocytes, we chose to implement a systematic genetic approach to simultaneously study A3 via receptor ablation in mouse models over an extended period of time.
Even though studies have shown that adenosine is produced by synovial cells in the joint, the exact role of A3 in chondrocytes is not very clear. Based off of preliminary adenosine agonist studies, we hypothesized that A3 plays an important role in cartilage homeostasis and disease.
Materials and methods
General methods
For all experiments, biological replicates were employed to generate data. For experiments expected to yield large differences, standard practice of using four to eight replicates was followed. All statistical methods are described in corresponding figure legends.
Cell culture, isolation of chondrocytes, and treatments
Only early passages of chondrocytes (passage 0–2) were used for experimentation to avoid de-differentiation and loss of cartilage phenotype. Normal and OA adult articular cartilage (three males and two females, 50 to 70 years old, 2–3 on the Kellgren–Lawrence grading scale for OA) were obtained from the National Disease Research Interchange (NDRI). All donated material was anonymous and carried no personal identity. Yucatan minipig cartilage tissue was obtained from S&S Farm (Ramona, CA) and chondrocytes isolated. Briefly, the tissue was minced and digested with 1 mg/mL of Collagenase II (Worthington, Lakewood, NJ) and 1 mg/mL of dispase (Invitrogen, Carlsbad, CA) in DMEM/F12 containing 10% FBS (Sigma-Aldrich, St. Louis, MO) at 37 °C for more than 16 h, and pellets were re-suspended in PBS. Adult and pig chondrocytes as well as explants of pig articular cartilage were cultured in DMEM/F12 medium containing 10% (v/v) fetal bovine serum and 1% penicillin-streptomycin (v/v) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Media was replenished with DMEM/F12 medium containing charcoal stripped 1% (v/v) fetal bovine serum and 1% penicillin-streptomycin (v/v) once treatments were added. Cell culture reagents were purchased from Life Technologies, Inc. (Grand Island, NY). TNF-alpha was purchased from Life Technologies, Inc. (Grand Island, NY). CCPA (Cat. No C7938) and IB-MECA (Cat. No 1066) were purchased from Sigma-Aldrich (St. Louis, MO). MRS 5698 (Cat. No 5428) was purchased from Tocris Bioscience (Minneapolis, MN).
Generation of adenosine receptor KO mice
The experiments were approved by the institutional review board (the Stockholm Ethical Committee for Animal Experiments; Protocols: N139/15 & N314/12) and were conducted in accordance with the National Institutes of Health guidelines for the conduct of experiments in animals. The generation, breeding, and genotyping of the different adenosine receptor KO have been described previously [23–26].
Aged 64–72-week-old male (n = 4) and female (n = 4) A3 KO and corresponding wild-type mice (n = 8) (equal sex distribution and age-matched) from homozygous breeding pairs were used. Twelve-week-old male (n = 4) and female (n = 4) A3 KO and corresponding wild-type mice (n = 8) (equal sex distribution and age-matched) from homozygous breeding pairs were used. All genotypes were generated from C57BL/6J backcrossed on C57 background. Mice were housed under temperature and humidity controlled conditions with 12 h light/dark cycle. Details about the primers used for genotyping have been described previously [26]. At termination, the mice were anesthetized by lethal dose of isoflurane and hind legs were dissected, processed and stored for later analyses.
Histology and immunohistochemistry
For histology, mouse and human tissues were fixed in 10% formalin for 48 h, dehydrated with ethanol, and embedded in paraffin using routine procedures. A microtome (Leica, Germany) was used to cut 5-μm sections. Slides were then deparaffinized and stained for sulfated glycosaminoglycans (GAGs) with Safranin O/Fast Green. For OARSI scoring, two observers performing the analysis were blinded to mouse genotype.
For immunohistochemistry, paraffin sections were processed using routine procedures. Rabbit pCaMKII, RUNX2, COLX, MMP13, and ADAMTS4 antibodies were used on sections followed by incubation with HRP-conjugated secondary antibodies against rabbit IgG. Antibodies were then visualized by peroxidase substrate Kit DAB (Vector Laboratories, Burlingame, CA). Slides were viewed using a Zeiss Imager A.2 Microscope and images were taken using an Axiocam 105 color camera with Zen 2 program. Standard microscope camera settings were used. Auto-exposure was used to normalize background light levels across all images. Protein expression was quantified using ImageJ by measuring number of positively labeled cells in each section within articular cartilage layer normalized to total number of analyzed cells and expressed as a percentage of positive cells.
All antibodies are listed in Supplementary Table 1.
Survival assay
To induce osmotic shock, adult articular chondrocytes were incubated with different shock solutions of NaCl (Thermo Scientific Rockford, IL) for 16 h. Dead cells were visualized by DAPI staining (diluted 1:1000; Molecular Probes, Cat. No. D1306) followed by FACS analysis.
Flow cytometry-based analysis of cell death
Pig chondrocytes were exposed to hypo-osmotic (0.18% w/v of sodium chloride) conditions with and without A3 receptor agonist, IB MECA (1 nM) for 24 h. Briefly, cells were blocked in 1% FBS in PBS, and cells were then washed twice in 1% FBS. Next, cells were collected and washed in Annexin V binding buffer and then incubated with PE-conjugated Annexin V (BD Biosciences, Cat. No. 556421). Labeled cells were analyzed using a BD LSRII flow cytometer (BD Biosciences). Data files were exported and analyzed with FACSDiva software (BD Biosciences). DAPI (diluted 1:1000; Molecular Probes, Cat. No. D1306) was added 15 min prior the analysis to exclude the dead cells.
Pig biopsy punch and explant culture
Biopsy punches (1-mm biopsy punches (Miltex, Inc., York, PA)) of adult pig articular cartilage were processed by removing 1 mm full-thickness explants. Explants were cultured with the 12-well plates with DMEM/F12 medium treated with or without IB MECA (1 nM) and/or TNF-alpha (10 ng/mL) and/or osmotic shock (0.18% w/v NaCl).
Western blot
For primary cells, healthy or OA human adult articular chondrocytes were maintained in DMEM (10% FBS, 1% PSA) and were either left untreated or were stimulated either IB MECA (1 nM), MRS 5698 (3 nM), TNF-alpha (10 ng/mL), or osmotic shock (0.18% w/v NaCl) for 24 h before cell harvest and protein extraction. Treated and non-treated articular chondrocytes were dissolved in RIPA Lysis and Extraction Buffer (Pierce, Rockford, IL) containing protease inhibitors (Pierce) followed by sonication with a 15-s pulse at a power output of 2 using the VirSonic 100 (SP Industries Company, Warminster, PA). Protein concentrations were determined by BCA protein assay (Pierce) and boiled for 5 min with Laemmli Sample Buffer (Bio-Rad, Hercules, CA). Proteins were separated on acrylamide gel and analyzed by Western blot using pCaMKII and RUNX2 primary antibodies. Histone 3 antibody was used as loading control. Proteins were resolved with SDS-PAGE utilizing 4–15% Mini-PROTEAN TGX Precast Gels and transferred to Trans-Blot Turbo Transfer Packs with a 0.2-μm pore-size nitrocellulose membrane. The SDS-PAGE running buffer, 4–15% Mini-PROTEAN TGX Precast Gels, and Trans-Blot Turbo Transfer Packs with a 0.2-μm pore-size nitrocellulose membrane were purchased from Bio-Rad (Hercules, CA). Nitrocellulose membranes were blocked in 5% nonfat milk in 0.05% (v/v) Tween 20 (PBST) (Corning, Manassas, VA). Membranes were then incubated with primary antibodies overnight. After washing in PBS containing 0.05% (v/v) Tween 20 (PBST), membranes were incubated with secondary antibodies (Thermo Scientific, Rockford, IL). After washing, development was performed with the Clarity Western ECL Blotting Substrate (Bio-Rad, Hercules, CA), and images were quantified by ImageJ software.
For cartilage explants, pig articular cartilage explants were made using 1-mm biopsy punch and wet weight of each explant determined prior to experimentation. Explants were cultured for 7 days in conditioned DMEM/F12 medium treated with or without IB MECA (1 nM) and/or TNF-alpha (10 ng/mL) and/or osmotic shock (0.18% w/v NaCl). The conditioned medium of pig articular cartilage explant from an equivalent wet weight of cartilage was digested for 2 h at 37 °C with 0.01 U chondroitinase ABC (Sigma-Aldrich, St. Louis, MO). Samples were then dialyzed with ultrapure water for 24 h at 4 °C, freeze dried, and dissolved in RIPA Lysis and Extraction Buffer (Pierce, IL) containing protease inhibitors (Pierce) followed by sonication with a 15-s pulse at a power output of 2 using the VirSonic 100 (SP Industries Company, Warminster, PA). Proteins were separated and analyzed by Western blot as mentioned above using aggrecan neo-epitope (Novus Biologicals, Littleton, CO) and collagen neo-epitope primary antibodies (IBEX Pharmaceuticals Inc. Mount Royal, QC). After washing in PBS containing 0.05% (v/v) Tween 20 (PBST), membranes were incubated with secondary antibodies (Thermo Scientific, Rockford, IL). After washing, membranes were developed with the Clarity Western ECL Blotting Substrate (Bio-Rad, Hercules, CA). Wet weight of explants was used as loading control.
Statistics
Numbers of repeats for each experiment are indicated in figure legends. Statistical analysis was performed using one-way analysis of variance test (ANOVA) followed by Tukey HSD post hoc Test (Prism 7 Software) to compare more than two groups, or two-tailed Student’s t test to compare two groups. P values < 0.05 were considered to be significant.
Results
A3 receptor knockout results in progressive loss of articular cartilage in mice
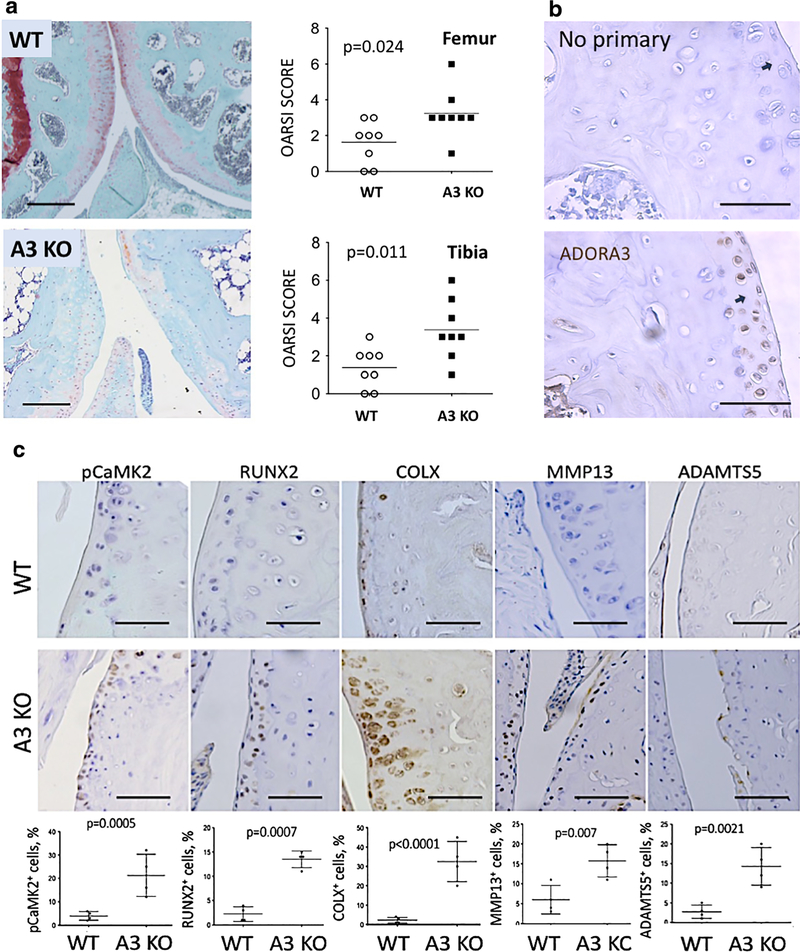
We hypothesized that genetic ablation of adenosine receptor 3 (A3) in mice will predispose these animals to OA and that this condition will further progress with age. To reveal if A3 is involved in homeostasis of articular cartilage in mice, we analyzed Safranin O/Fast Green stained knee joint sections from individual A3 knockouts (KOs). A3 KO aged mice showed significant changes with marked degeneration of the articular cartilage tissue confirmed by OARSI scoring (Fig. 1a) with no significant differences observed between males (n = 4) or females (n = 4) (data not shown). No significant phenotype was observed in 12-week-old A3 KO mice (Fig. 1S), indicating that OA development upon A3 deficiency is age-related. Next, we performed immunohistochemical (IHC) assessment of A3 expression in healthy joints in wild-type (WT) mice. As shown (Fig. 1b), positive signal was primarily detected in the superficial areas of articular cartilage of knee joints. As expected, no protein expression for A3 was detected in articular chondrocytes of A3 KO mice (not shown).
Fig. 1.
A3 receptor knockout results in progressive loss of articular cartilage in mice. a Representative images of Safranin O/Fast Green stained sections of adult mouse knee joint from wild type (WT) and genetic knockouts (KO) of adenosine receptor A3. A3 KO sections showed articular cartilage degradation and loss of glycosaminoglycans (GAGs). Arrows point to articular cartilage. Both males (n = 4) and females (n = 4) were analyzed. Scale bar = 100 mM. Quantitative assessment of cartilage degradation in femur and tibia was performed by two blinded observers using OARSI scoring system. Lower OA scores indicate better outcomes. Data represent eight biological replicates and are presented as mean ± SD; P values were calculated with one-way ANOVA followed by Tukey honest significant difference post hoc test. P values < 0.05 were considered to be significant. NS not significant. b A3 protein is expressed in the superficial layer of articular cartilage in healthy mice. Scale bar = 20 μM. c Sections of wild-type (WT) or A3 KO mouse knee joints were stained for various markers of osteoarthritis: pCaMKII, Runx2, Collagen type X, MMP13, and ADAMTS5. Representative images are shown. Quantitative assessment of positive cells for each marker is shown below each section. Data represent four biological replicates with equal sex distribution (two males, two females) and are presented as mean ± SD. P values were calculated using Student’s t test (two-tailed). P values < 0.05 were considered to be significant. Scale bar = 20 μM
One of the hallmarks of OA progression is the damage inflicted upon the superficial layer of cartilage matrix. This degradative process is driven by expression of catabolic factors such as matrix metalloproteinase 13 (MMP13) [27] and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) [28] that are responsible for the degradation of collagen and aggrecan, respectively, two major structural components of articular cartilage. Aged A3-deficient mice had a significantly higher increase in expression of these enzymes relative to WT as seen in the IHC analysis of the knee joints (Fig. 1c).
To further dissect the molecular profile of A3 KO joints, we performed IHC staining for other markers of articular cartilage degeneration and hypertrophy including CaMKII, RUNX2, and Collagen Type X (COLX). It was previously reported that CaMKII and RUNX2 regulate the MMP13 gene expression [29, 30], and both are upregulated in human cartilage from OA patients and in animal models of OA [31]. Histological staining of A3-deficient mice showed a significant upregulation of active phosphorylated CaMKII (pCaMKII) and Runx2 (Fig. 1c). Histological staining also revealed strong upregulation of COLX, further confirming loss of articular cartilage and increased hypertrophy (Fig. 1c). Thus, loss of A3 causes activation of catabolic program in articular cartilage as well as chondrocyte hypertrophy.
Adenosine receptor A3 is expressed in normal and osteoarthritic articular cartilage
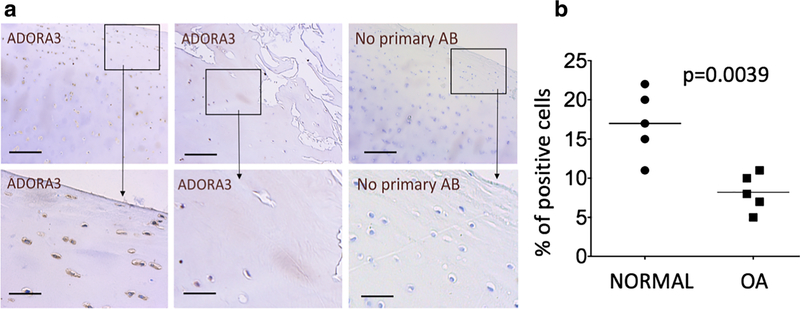
We next analyzed if A3 is endogenously expressed in human chondrocytes by performing an analysis of healthy and osteoarthritic (OA) articular cartilage from the knee joint, which showed that the receptor was present in superficial cells in human articular cartilage (Fig. 2a, b). Interestingly, expression of A3 was consistently lower in osteoarthritic human cartilage relative to the healthy human adult (Fig. 2a, b), which correlates well with progressive loss of superficial chondrocytes in OA and potentially suggests that A3 might be mechanistically involved in pathogenesis of OA in humans.
Fig. 2.
Adenosine receptor A3 is expressed in normal and osteoarthritic articular cartilage. a Protein expression of A3 was detected in the superficial and transitional layer of healthy human articular cartilage. Control sections were stained with secondary antibody only. Images in the lower panels represent a higher magnification view of areas marked in the corresponding upper panels. b Expression of A3 reduced in OA adult cartilage is characterized by progressive loss of the superficial chondrocytes. N = 5 independent donors from both healthy and OA articular cartilage from the knee joint. P values were calculated using Student’s t test (two-tailed). P values < 0.05 were considered to be significant. Scale bar = 20 μM. Representative images are shown. Detailed information about donors can be found in the “Materials and methods” section
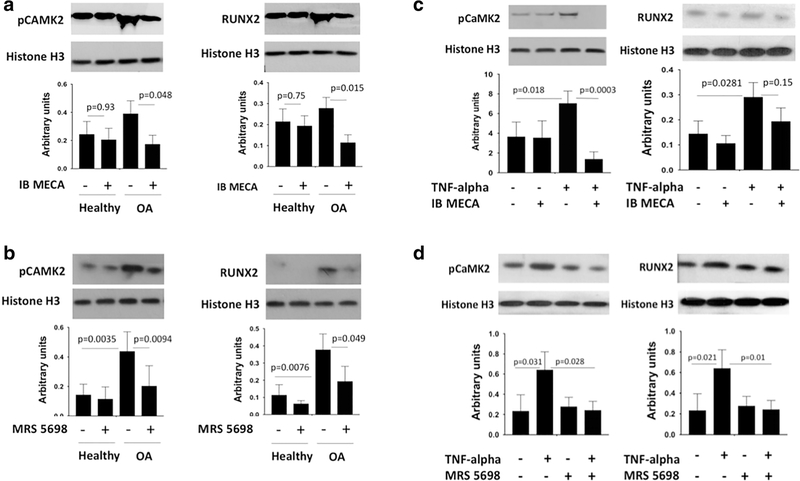
A3 receptor agonists downregulate RUNX2 levels and CaMKII phosphorylation in osteoarthritic human articular chondrocytes
CaMKII and RUNX2 are upregulated in OA [6, 32]. In our study, chondrocytes isolated from osteoarthritic (OA) cartilage showed elevated expression of active pCaMKII and RUNX2 (Fig. 3a), as expected. In line with our observations of KO mice, the A3 agonist, IB MECA, caused significant downregulation of both pCaMKII and RUNX2 (Fig. 3a). As previously mentioned, IB MECA can also act as an agonist for other adenosine receptors. In order to confirm that the effect was specifically mediated through A3, we used another highly selective A3 agonist, MRS 5698 (Fig. 3b), which has been shown to reverse mechanoallodynia in chronic neuropathic pain models [33]. MRS 5698 had the same pattern of downregulating RUNX2 and pCaMKII in OA samples as IB MECA verifying that the effect is regulated by A3.
Fig. 3.
A3 receptor agonists downregulate RUNX2 levels and CaMKII phosphorylation in osteoarthritic (OA) human articular chondrocytes. a Addition of A3 agonist, IB MECA (1 nM), to human adult healthy and OA chondrocytes in culture decreased the protein expression of pCaMKII and RUNX2 as detected by Western blot analysis. b Addition of A3 agonist, MRS 5698 (3 nM), to human adult healthy and OA chondrocytes in culture decreased the protein expression of pCaMKII and RUNX2 as detected by Western blot analysis. c In human adult healthy chondrocytes, IB MECA (1 nM) counteracts TNF-alpha (10 ng/mL)-induced elevation of pCAMKII levels as detected by Western blot, but no statistically significant effect was seen on RUNX2. d In human adult healthy chondrocytes, MRS 5698 (3 nM) counteracts TNF-alpha (10 ng/mL)-induced elevation of pCAMKII and RUNX2 levels as detected by Western blot. In panels a, b, c, and d, healthy and OA articular chondrocytes were treated for 24 h in indicated conditions and expression levels were normalized to Histone 3. Data represent four biological replicates and are presented as mean ± SD; P values were calculated with one-way ANOVA followed by Tukey honest significant difference post hoc test. P values < 0.05 were considered to be significant. NS not significant
We next explored how A3 signaling would function in a pro-catabolic environment stimulated by pro-inflammatory cytokines. It is well known that pro-inflammatory cytokines are one of the factors that can trigger cartilage degradation. Tumor necrosis factor alpha (TNF-alpha) is one of the most potent pro-catabolic cytokines, and numerous studies report increased levels of TNF-alpha in OA [34]. To determine anticatabolic potency of A3, we treated adult articular chondrocytes with IB MECA in combination with TNF-alpha. IB MECA fully abrogated TNF-alpha-induced pCaMKII activation (Fig. 3c). However, TNF-alpha had no statistically significant effect on RUNX2 expression alone or in combination with IB MECA. Upon treatment with TNF-alpha, MRS 5698 showed a similar trend as IB MECA in downregulating pCAMKII, but MRS 5698 was also able to significantly downregulate RUNX2 (Fig. 3d). Thus, in both knockout experiments in mice and pharmacological manipulations in human chondrocytes, A3 plays a role in regulation of the catabolic and hypertrophic pathways in cartilage.
A3 receptor agonist protects primary pig articular chondrocytes from hypo-osmotic shock
Osmotic fluctuation is a natural physiological stress that chondrocytes experience when the joint is mechanically loaded causing change in the water content and matrix osmolarity [35]. It has been previously shown that in chondrocytes subjected to a 50% dilution, [Ca2+] rapidly increases by approximately 250% [36].
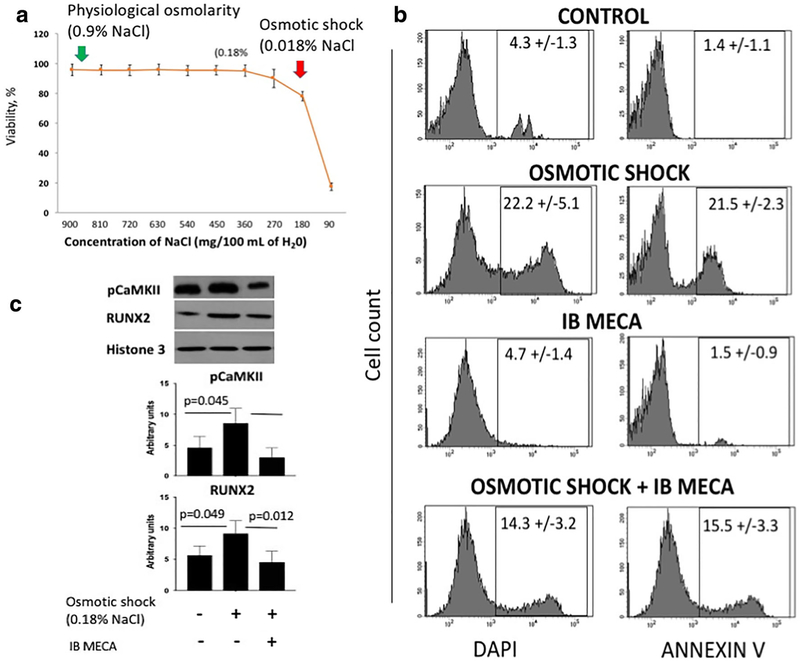
Excessive mechanical stimulation of the joint is a well-documented cause of OA [34]. During OA, a breakdown of collagen fibers results in an increase of water uptake. In the initial stages of OA, cartilage swelling is the first detectable macroscopic event in the initial stages of OA. Naturally, chondrocytes activate specific membrane transporters, such as calcium channels, which release or accumulate solutes in response to cell swelling or shrinking [37]. Calcium homeostasis is lost when extracellular fluid osmolarity is greater than that of the intracellular fluid, inducing hypo-osmotic stress and death in cells. ECM synthesis is also sensitive to calcium concentration, and chondrocyte calcium concentration level is determined by the balance of influx and efflux across the cell membrane and uptake and release from intracellular stores [38]. Imbalance of calcium and lack of osmotic homeostasis are damaging to cells. In physiological saline, natural human blood osmolarity consists of 0.9% w/v of sodium chloride or 9.0 g per liter of water [39]. To determine at what concentration of salt and water content does chondrocytes experience death, we have performed a survival assay as described previously [36]. We find that during hypotonic shock, pig articular chondrocytes undergo cell death when sodium chloride concentration is decreased to 0.18% w/v in the culture medium (Fig. 4a).
Fig. 4.
A3 receptor agonist protects primary pig articular chondrocytes from hypo-osmotic shock. a Survival assay line graph showing percentage of viable primary pig articular chondrocytes at different osmolarity. Viability of cells was measured by DAPI staining. Physiological osmolarity consists of 0.9% w/v of sodium chloride (green arrow). As shown, chondrocytes undergo death during hypo-osmotic shock at 0.18% w/v of sodium chloride (red arrow). b Fluorescence-activated cell sorting (FACS) was performed to show the percentage of dead cells (DAPI+) and apoptotic cells (Annexin V+) in primary pig articular chondrocytes exposed to control and hypo-osmotic shock with and without A3 receptor agonist, IB MECA (1 nM). Addition of IB MECA protected the chondrocytes from cell death and apoptosis during hypo-osmotic shock. The plots represent mean ± SD of dead and apoptotic cells. Data represent four biological replicates; P values were calculated with one-way ANOVA followed by Tukey honest significant difference post hoc test. P values < 0.05 were considered to be significant. c Western blot showing the protein levels of pCaMKII and RUNX2 in primary pig articular chondrocytes treated with the indicated conditions. Addition of A3 receptor agonist, IB MECA (1 nM), to hypo-osmotic shock-treated (0.18% w/v of sodium chloride) chondrocytes downregulated pCaMKII and RUNX2. Cells were treated for 24 h. Data shown are normalized to Histone 3. Data represent four biological replicates and are presented as mean ± SD; P values were calculated with one-way ANOVA followed by Tukey honest significant difference post hoc test. P values < 0.05 were considered to be significant. NS not significant
Given the relationship between intracellular calcium levels and activation of CaMKII combined with the direct effects of A3 signaling on CaMKII that we have previously observed, we reasoned that A3 signaling may play a chondro-protective role during osmotic shock. To assess the protective role of A3 selective agonist, IB MECA, in osmotic resistance in culture, we performed flow cytometry on adult articular chondrocytes subjected to osmotic shock in the presence or absence of IB MECA. The results revealed that relative to control, IB MECCA was able to decrease cell death and apoptosis as shown by DAPI staining and Annexin V positivity (Fig. 4b). This demonstrates the protective role of A3 receptor signaling in homeostatic balance and its function in chondrocyte survival during osmotic shock.
As previously mentioned, CaMKII activation is directly correlated to the amount of calcium present in the cell, and calcium concentration in chondrocytes arises due to hypotonic shock [36]. We wanted to investigate if both CaMKII and RUNX2 were upregulated due to osmotic shock, resulting in chondrocyte death and if simultaneous treatment with A3 agonist can reverse this effect. To determine the expression of CaMKII and RUNX2 under hypotonic conditions in adult articular chondrocytes, we induced osmotic shock and treated the cells with or without IB MECA. Western blot analysis was performed using specific pCaMKII and RUNX2 antibodies. As expected, with an influx of calcium stress conditions, phosphorylation of CaMKII was significantly upregulated. Interestingly, osmotic shock also increased RUNX2 protein expression. Upon addition of IB MECA, both CaMKII and RUNX2 were downregulated (Fig. 4c). This confirmed that A3 agonist inhibits CaMKII and RUNX2 activation in response to hypotonic shock. Our results demonstrated that functional adenosine A3 receptor signaling is essential in protecting chondrocytes from osmotic imbalances.
A3 receptor agonist prevents degradation of cartilage matrix induced by either TNF-alpha or hypo-osmotic shock
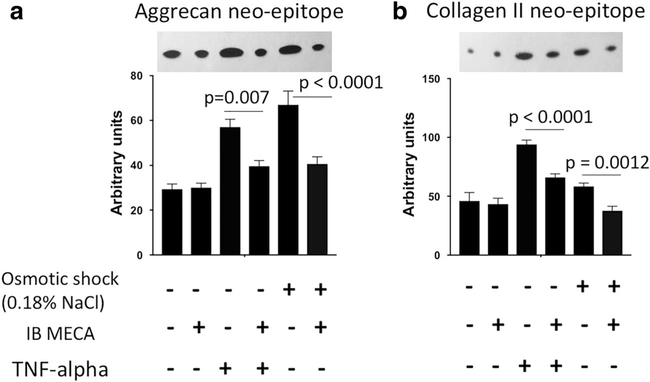
We have demonstrated that A3 signaling inhibits expression of master regulators of cartilage degeneration and hypertrophy, i.e., RUNX2 and CaMKII. To further investigate whether A3 signaling can prevent proteolytic activity in cartilage, we measured levels of aggrecan and collagen type II released in the media as described previously [40] after treating pig articular cartilage explants with either TNF-alpha or induced osmotic shock.. The amount of aggrecan and collagen type II released in the explant media directly correlates to aggrecanase and collagenase activity in the cartilage [40]. As expected, TNF-alpha treated and osmotic shock-induced explants had significantly higher concentrations of aggrecan and collagen type II neo-epitopes in the media implying high aggrecanase and collagenase activity (Fig. 5). This effect was ameliorated in both conditions by simultaneous treatment with the A3 agonist IB MECA (Fig. 5). This finding demonstrates that A3 signaling prevents cartilage catabolism by downregulating aggrecanase and collagenase activity in vitro.
Fig. 5.
A3 receptor agonist prevents articular cartilage matrix degradation induced by TNF-alpha and hypo-osmotic shock. Collagenase-generated collagen neo-epitope (a) and aggrecanase-generated aggrecan neo-epitope (b) in the media of pig articular cartilage explants as visualized by Western blot. Explants were cultured for 24 h in the indicated conditions. A3 receptor agonist, IB MECA (1 nM), was able to decrease collagenase and aggrecanase activity in culture as shown by the downregulation of aggrecan neo-epitope and collagen type II neoepitope expression. Data shown are normalized to explant wet weight. Data represent four biological replicates and are presented as mean ± SD; P values were calculated with one-way ANOVA followed by Tukey honest significant difference post hoc test. P values < 0.05 were considered to be significant
Discussion
Currently, there are no clinical drugs which can prevent cartilage catabolism during OA and, accordingly, deeper understanding of underlying mechanisms is required. The novel data presented here demonstrates that the cartilage degenerative activity can be prevented by adenosine via A3 signaling and subsequent inhibition of CaMKII and RUNX2. We provide in vivo evidence for A3’s role in cartilage homeostasis in mouse KO models, and further substantiate our findings by implementing A3 agonists, IB-MECA and MRS5698, to characterize the role of adenosine signaling in the prevention of catabolic mechanisms related to OA. Previous reports demonstrated that IB MECA prevented manifestation of collagen and adjuvant induced arthritis (AIA) in animal models including decreased cartilage and bone destruction [41]. A3 has also been reported to reduce OA development in rats due to the anti-inflammatory effects of IB-MECA in a monosodium iodoacetate-induced model of OA and played a protective role in cartilage degeneration [20]. However, to date, there was no substantial evidence addressing the underlying mechanism of action. Our experiments in healthy and OA human and pig articular chondrocytes show that IB MECA acts via inhibition of CaMKII and RUNX2, two catabolic factors pathophysiologically upregulated in OA chondrocytes.
A significant body of literature implicates A3 in regulating immune responses and inflammation. It was previously noted that IB MECA administration resulted in downregulation of TNF-alpha in AIA rats [41] and reduced pro-inflammatory NF-κB upregulation in cancer-associated osteolytic lesions in MRMT-rat mammary gland carcinoma cells [42]. In addition, treatment with IB MECA in a chemically induced OA resulted in deregulation of the NF-κB signaling pathway causing a downregulation of TNF-alpha [20]. We recently showed that A3 deficiency is associated with “boosted” immune system, e.g., display increased antigen presenting cells (APC) [26]. However, these immune changes were not associated with any phenotype in young mice [26]. Our present study confirms the anti-inflammatory effect of A3 signaling in OA chondrocytes. In culture, IB MECA and MRS 5698 were able to decrease TNF-alpha activity as demonstrated by downregulation of TNF-alpha-induced pCaMKII protein expression. At the same time, IB MECA showed no downregulation of RUNX2 in OA chondrocytes, while MRS 5698 did, indicating that upregulation of this transcription factor in OA is multifactorial.
Previous literature has suggested that IB-MECA induces survival of murine bone marrow cells during chemotherapy [43]. In ischemia-reperfusion (IR) injury, it was noted that IB-MECA administered before or during IR decreased apoptosis in lung tissue and injured-alveoli [44]. However, the role of IB MECA in chondrocyte survival was not well established. Here, we show that addition of IB MECA to hypo-osmotic pig chondrocytes significantly decreased cell death and apoptosis. In addition, osmotic stressed-induced upregulation of pCaMKII and RUNX2 was blocked by IB MECA treatment, while aggrecanase and collagenase activity were attenuated in pig articular cartilage explants. Together, these results convey a potential chondroprotective role of IB MECA. These observations, which link IB MECA and its effect on catabolic mechanisms, provide mechanistic evidence for the potential involvement of A3 signaling in OA.
In summary, our studies show for the first time that A3 signaling plays an important role in regulating age-related OA. Deletion of the A3 is linked to cartilage degeneration in mouse knee joint during aging mediated by upregulation of catabolic factors pCaMKII and RUNX2. Moreover, the A3 agonists decrease the levels of key catabolic pathways in human OA cartilage and prevent matrix degradation in pig articular cartilage explants exposed to osmotic shock or pro-inflammatory cytokines. This study adds important knowledge to the recent body of literature further highlighting the role of adenosine signaling for joint homeostasis and disease. This study also offers novel insight into the complex network by establishing a relationship between adenosine receptor signaling and its function on regulatory mechanisms that initiate OA.
Supplementary Material
Key messages.
Adenosine receptor A3 (A3) knockout results in progressive loss of articular cartilage in vivo.
Ablation of A3 results in activation of matrix degradation and cartilage hypertrophy.
A3 agonists downregulate RUNX2 and CaMKII expression in osteoarthritic human articular chondrocytes.
A3 prevents articular cartilage matrix degradation induced by inflammation and osmotic fluctuations.
A3 agonist inhibits proteolytic activity of cartilage-degrading enzymes.
Acknowledgments
Funding information This work is supported by NIH grant R01AR071734, DOD grant W81XWH-13-1-0465, and CIRM grant TRAN1-09288, all to DE. This work was also supported by grants from the Swedish Heart and Lung Foundation (Dnr: 20170124 & 20140448, M.C.), the Swedish Research Council (Dnr: 2016-01381 to M.C. and Dnr. 2016-02835 to ASC), and by KID-funding from the Karolinska Institutet (Dnr 2415/2012-225 and Dnr 2-3707/2013, M.C.).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
The experiments were approved by the institutional review board (the Stockholm Ethical Committee for Animal Experiments; Protocols: N139/15 & N314/12) and were conducted in accordance with the National Institutes of Health guidelines for the conduct of experiments in animals.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00109-018-1680-3) contains supplementary material, which is available to authorized users.
References
- 1.Yelin E, Weinstein S, King T (2016) The burden of musculoskeletal diseases in the United States. Semin Arthritis Rheum 46(3):259–260 [DOI] [PubMed] [Google Scholar]
- 2.Muller-Fassbender H, Bach GL, Haase W, Rovati LC, Setnikar I (1994) Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthr Cartil 2(1):61–69 [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Ahrens MJ, Wu A, Liu J, Dudley AT (2011) Calcium/calmodulin-dependent protein kinase II activity regulates the proliferative potential of growth plate chondrocytes. Development 138(2):359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W, Li X, Gao B, Gan H, Lin X, Liao L et al. (2016) Observing the development of the temporomandibular joint in embryonic and post-natal mice using various staining methods. Exp Ther Med 11(2):481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreier R (2010) Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Res Ther 12(5):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao L, Zhang S, Gu J, Takarada T, Yoneda Y, Huang J, Zhao L, Oh CD, Li J, Wang B, Wang M, Chen D (2017) Deletion of Runx2 in articular chondrocytes decelerates the progression of DMM-induced osteoarthritis in adult mice. Sci Rep 7:2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Solal KA, Boregowda RK, Lasfar A (2015) RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression. Mol Cancer 14(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández P, Perez-Aso M, Smith G, Wilder T, Trzaska S, Chiriboga L, Franks A Jr, Robson SC, Cronstein BN, Chan ESL (2013) Extracellular generation of adenosine by the ectonucleotidases CD39 and CD73 promotes dermal fibrosis. Am J Pathol 183(6):1740–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gullo F, De Bari C (2013) Prospective purification of a subpopulation of human synovial mesenchymal stem cells with enhanced chondro-osteogenic potency. Rheumatology (Oxford) 52(10): 1758–1768 [DOI] [PubMed] [Google Scholar]
- 10.Tesch AM, MacDonald MH, Kollias-Baker C, Benton HP (2004) Endogenously produced adenosine regulates articular cartilage matrix homeostasis: enzymatic depletion of adenosine stimulates matrix degradation. Osteoarthr Cartil 12(5):349–359 [DOI] [PubMed] [Google Scholar]
- 11.Corciulo C, Lendhey M, Wilder T, Schoen H, Cornelissen AS, Chang G, Kennedy OD, Cronstein BN (2017) Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat Commun 8:15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JF, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12(4):265–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daly JW, Padgett WL (1992) Agonist activity of 2- and 5′-substituted adenosine analogs and their N6-cycloalkyl derivatives at A1- and A2-adenosine receptors coupled to adenylate cyclase. Biochem Pharmacol 43(5):1089–1093 [DOI] [PubMed] [Google Scholar]
- 14.Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA (2012) Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today 17(7–8):359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, del Valle L, Fishman P (2008) The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappaB signal transduction pathways. Int J Oncol 33(2):287–295 [PubMed] [Google Scholar]
- 16.Fishman P, Bar-Yehuda S, Ardon E, Rath-Wolfson L, Barrer F, Ochaion A, Madi L (2003) Targeting the A3 adenosine receptor for cancer therapy: inhibition of prostate carcinoma cell growth by A3AR agonist. Anticancer Res 23(3A):2077–2083 [PubMed] [Google Scholar]
- 17.Bar-Yehuda S, Madi L, Barak D, Mittelman M, Ardon E, Ochaion A, Cohn S, Fishman P (2002) Agonists to the A3 adenosine receptor induce G-CSF production via NF-kappaB activation: a new class of myeloprotective agents. Exp Hematol 30(12):1390–1398 [DOI] [PubMed] [Google Scholar]
- 18.Avni I, Garzozi HJ, Barequet IS, Segev F, Varssano D, Sartani G, Chetrit N, Bakshi E, Zadok D, Tomkins O, Litvin G, Jacobson KA, Fishman S, Harpaz Z, Farbstein M, Yehuda SB, Silverman MH, Kerns WD, Bristol DR, Cohn I, Fishman P (2010) Treatment of dry eye syndrome with orally administered CF101: data from a phase 2 clinical trial. Ophthalmology 117(7):1287–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman MH, Strand V, Markovits D, Nahir M, Reitblat T, Molad Y, Rosner I, Rozenbaum M, Mader R, Adawi M, Caspi D, Tishler M, Langevitz P, Rubinow A, Friedman J, Green L, Tanay A, Ochaion A, Cohen S, Kerns WD, Cohn I, Fishman-Furman S, Farbstein M, Yehuda SB, Fishman P (2008) Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol 35(1): 41–48 [PubMed] [Google Scholar]
- 20.Bar-Yehuda S, Rath-Wolfson L, Del Valle L, Ochaion A, Cohen S, Patoka R et al. (2009) Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum 60(10):3061–3071 [DOI] [PubMed] [Google Scholar]
- 21.Gallo-Rodriguez C, Ji XD, Melman N, Siegman BD, Sanders LH, Orlina J, Fischer B, Pu Q, Olah ME, van Galen P (1994) Structure-activity relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J Med Chem 37(5):636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klotz KN (2000) Adenosine receptors and their ligands. Naunyn Schmiedeberg’s Arch Pharmacol 362(4–5):382–391 [DOI] [PubMed] [Google Scholar]
- 23.Halldner L, Ådén U, Dahlberg V, Johansson B, Ledent C, Fredholm BB (2004) The adenosine A1 receptor contributes to the stimulatory, but not the inhibitory effect of caffeine on locomotion: a study in mice lacking adenosine A1 and/or A2A receptors. Neuropharmacology 46(7):1008–1017 [DOI] [PubMed] [Google Scholar]
- 24.Peleli M, Hezel M, Zollbrecht C, Persson AEG, Lundberg JO, Weitzberg E et al. (2015) In adenosine A(2B) knockouts acute treatment with inorganic nitrate improves glucose disposal, oxidative stress, and AMPK signaling in the liver. Front Physiol 6:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang T, Gao X, Sandberg M, Zollbrecht C, Zhang X-M, Hezel M, Liu M, Peleli M, Lai EY, Harris RA, Persson AEG, Fredholm BB, Jansson L, Carlström M (2015) Abrogation of adenosine A1 receptor signalling improves metabolic regulation in mice by modulating oxidative stress and inflammatory responses. Diabetologia 58(7): 1610–1620 [DOI] [PubMed] [Google Scholar]
- 26.Yang T, Zollbrecht C, Winerdal ME, Zhuge Z, Zhang XM, Terrando N, Checa A, Sällström J, Wheelock CE, Winqvist O, Harris RA, Larsson E, Persson AEG, Fredholm BB, Carlström M (2016) Genetic abrogation of adenosine A(3) receptor prevents uninephrectomy and high salt–induced hypertension. J Am Heart Assoc: Cardiovascular and Cerebrovascular Disease 5(7):e003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Sampson ER, Jin H, Li J, Ke QH, Im H-J et al. (2013) MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther 15(1):R5–R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tortorella MD, Malfait AM, Deccico C, Arner E (2001) The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthr Cartil 9(6):539–552 [DOI] [PubMed] [Google Scholar]
- 29.Quinn C Parathyroid hormone induces rat interstitial collagenase mRNA through Ets-1 facilitated by cyclic AMP response element-binding protein and Ca(2+)/calmodulin-dependent protein kinase II in osteoblastic cells 2000. 73–84 [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH (2004) Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr Cartil 12(12):963–973 [DOI] [PubMed] [Google Scholar]
- 31.Wojdasiewicz P, Poniatowski LA, Szukiewicz D (2014) The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm 2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugita S, Hosaka Y, Okada K, Mori D, Yano F, Kobayashi H, Taniguchi Y, Mori Y, Okuma T, Chang SH, Kawata M, Taketomi S, Chikuda H, Akiyama H, Kageyama R, Chung UI, Tanaka S, Kawaguchi H, Ohba S, Saito T (2015) Transcription factor Hes1 modulates osteoarthritis development in cooperation with calcium/calmodulin-dependent protein kinase 2. Proc Natl Acad Sci U S A 112(10):3080–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tosh DK, Padia J, Salvemini D, Jacobson KA (2015) Efficient, large-scale synthesis and preclinical studies of MRS5698, a highly selective A3 adenosine receptor agonist that protects against chronic neuropathic pain. Purinergic Signal 11(3):371–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunke M, Schulze-Koops H (2006) Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann Rheum Dis 65(4):555–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urban JPG, Hall AC, Gehl KA (1993) Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol 154(2):262–270 [DOI] [PubMed] [Google Scholar]
- 36.Sanchez JC, Danks TA, Wilkins RJ (2003) Mechanisms involved in the increase in intracellular calcium following hypotonic shock in bovine articular chondrocytes. Gen Physiol Biophys 22(4):487–500 [PubMed] [Google Scholar]
- 37.Brocker C, Thompson DC, Vasiliou V (2012) The role of hyperosmotic stress in inflammation and disease. Biomol Concepts 3(4):345–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busa WB (1996) Regulation of intracellular free calcium. In: Schultz SG, Andreoli TE, Brown AM, Fambrough DM, Hoffman JF, Welsh MJ (eds) Molecular biology of membrane transport disorders. Springer US, Boston, MA, pp 427–446 [Google Scholar]
- 39.Martini WZ, Cortez DS, Dubick MA (2013) Comparisons of normal saline and lactated Ringer’s resuscitation on hemodynamics, metabolic responses, and coagulation in pigs after severe hemorrhagic shock. Scand J Trauma Resusc Emerg Med 21:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P, Zucman-Rossi J (2009) Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 457(7226):200–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rath-Wolfson L, Bar-Yehuda S, Madi L, Ochaion A, Cohen S, Zabutti A, Fishman P (2006) IB-MECA, an A3 adenosine receptor agonist prevents bone resorption in rats with adjuvant induced arthritis. Clin Exp Rheumatol 24(4):400–406 [PubMed] [Google Scholar]
- 42.Strazzulla LC, Cronstein BN (2016) Regulation of bone and cartilage by adenosine signaling. Purinergic Signalling 12(4):583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panjehpour M, Karami-Tehrani F (2007) Adenosine modulates cell growth in the human breast cancer cells via adenosine receptors. Oncol Res 16(12):575–585 [DOI] [PubMed] [Google Scholar]
- 44.Rivo J, Zeira E, Galun E, Matot I (2004) Activation of A3 adenosine receptor provides lung protection against ischemia-reperfusion injury associated with reduction in apoptosis. Am J Transplant 4(12):1941–1948 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.