Abstract
Since the beginning of the COVID-19 pandemic in 2020 caused by SARS-CoV-2, the question of the origin of this virus has been a highly debated issue. Debates have been, and are still, very disputed and often violent between the two main hypotheses: a natural origin through the “spillover” model or a laboratory-leak origin. Tenants of these two options are building arguments often based on the discrepancies of the other theory. The main problem is that it is the initial question of the origin itself which is biased. Charles Darwin demonstrated in 1859 that all species are appearing through a process of evolution, adaptation and selection. There is no determined origin to any animal or plant species, simply an evolutionary and selective process in which chance and environment play a key role. The very same is true for viruses. There is no determined origin to viruses, simply also an evolutionary and selective process in which chance and environment play a key role. However, in the case of viruses the process is slightly more complex because the “environment” is another living organism. Pandemic viruses already circulate in humans prior to the emergence of a disease. They are simply not capable of triggering an epidemic yet. They must evolve in-host, i.e. in-humans, for that. The evolutionary process which gave rise to SARS-CoV-2 is still ongoing with regular emergence of novel variants more adapted than the previous ones. The real relevant question is how these viruses can emerge as pandemic viruses and what the society can do to prevent the future emergence of pandemic viruses.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, Emerging diseases, Origin, Evolution
1. Introduction
In January 2020 the whole world heard for the first time of a novel disease, COVID-19, caused by a novel betacoronavirus related to SARS-CoV, SARS-CoV-2 (Zhu et al., 2019; Guan et al., 2020; Huang et al., 2020; WHO, 2020). Since the very beginning, the origin of SARS-CoV-2 has been a strongly debated issue. The controversy is still undergoing. This debate can sometimes be very violent mostly because it has left the sole field of science to enter also that of journalism and politics. Furthermore, internet and social networks are worsening the situation with a permanent flow of opinions, fake-news and violent attacks. There are now a lot of hidden agendas and manipulations attached to this issue. Although perhaps difficult to achieve because of its current status of public debate with all sorts of rational and irrational arguments, the debate on the origin of SARS-CoV-2 and other pandemic viruses should come back to the scientific framework and stay within. There are several reasons for that and beyond the obvious need for scientific rationality, there is a major societal issue. The society must be protected against future emerging pandemics. Pushing toward wrong issues only for opinions, political, corporatist or ideological reasons will result in leaving the society unprotected in face of the next pandemic (Frutos et al., 2021a). It is the duty of scientists to work for the good of the society and to ensure that rational analyses based on scientific evidences are brought forward to lead to rational and efficient decisions and protection measures. It is a major public health and security issue where scientific evidence rather than unsubstantiated opinions should be considered.
The COVID-19 pandemic officially started on December 8, 2019 at the Huanan Seafood Wholesale Market (HSWM) in Wuhan (Huang et al., 2020; WHO, 2020). However, this corresponds only to the official date and place and does not even correspond to the actual index case. Earlier cases have been reported which were not linked HSWM and phylogenetic studies showed that the virus was certainly already circulating in autumn 2019 (Frutos et al., 2020a; Li et al., 2020a, 2020b, 2020c; Lai, 2020). Unravelling the dynamic of disease emergence is essential not only to understand how COVID-19 emerged as a disease but also to organize ourselves as a society to prevent the future emergence of other infectious diseases (Frutos et al., 2021a).
Several hypotheses have been brought forward to explain the emergence of SARS-CoV-2, sometimes with violence and aggressiveness when politics, geostrategic conflicts and personal interests are taking over the scientific analysis. It is therefore essential to review the process of emergence of SARS-CoV-2, and other pandemic viruses, as well as the human societal context in which this emergence of viral pandemics is taking place.
1.1. To be or not to be … a pandemic virus
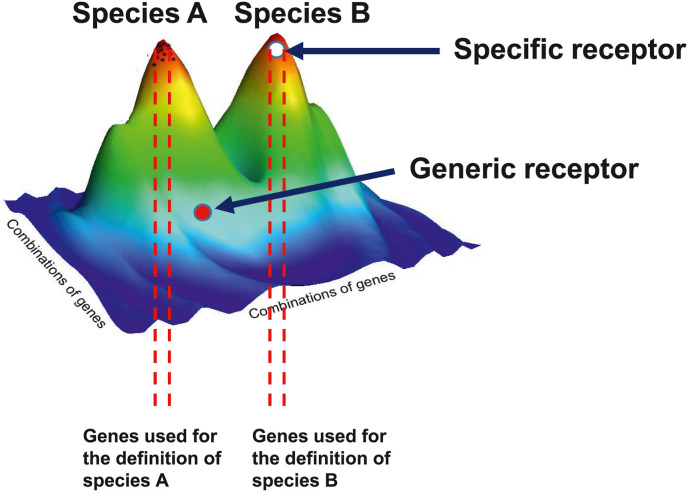
SARS-CoV-2 is a pandemic virus but what means being a pandemic virus? Being a pandemic virus simply means being able to spread over the whole human population, worldwide. This is not strictly a phenotypic trait. It is the consequence of a life cycle and of a specific evolutionary process. It is also the consequence of the host populational context into which these take place. Talking about human diseases, this context corresponds by definition to the organization of the human society and to human behavior. There is hardly anything more societal than a pandemic. Not all viruses can be pandemic. Only few can be and, indeed, pandemics are rare due to both biological and societal reasons. Emerging pandemic viruses are, by definition, multi-host viruses. This is the result of an evolutionary process. These viruses have evolved to recognize ubiquitous proteins as receptors. They do not recognize specific receptors, i.e. present in only one species. If so, they would be specifically attached to only one host species, or a limited number of related species, and if this host species disappears, the virus will disappear as well. Furthermore, the diffusion of the virus would be limited to that species. Recognizing instead a generic, ubiquitous receptor or at least a receptor present in various species, allows the virus to survive even if one or more of its host species disappear. Furthermore, receptors are proteins essential for the deep cell physiology of the host who cannot evolve to suppress them. These are very favorable evolutionary traits for the virus. The receptor recognized by SARS-CoV-2 is ACE2 or Angiotensin I Converting Enzyme 2 (Cao et al., 2020; Hamming et al., 2004). This protein is a cell surface protein primarily involved in the modulation of the Angiotensin-Converting Enzyme (ACE) (Oudit et al., 2003; Burrell et al., 2004). ACE cleaves angiotensin I into a vasoconstricting form named angiotensin II, whereasACE2 is converting angiotensin II into a vasodilatating form named angiotensin-(1–7) (Donoghue et al., 2000; Keidar et al., 2007; Wang et al., 2016; Devaux et al., 2020). ACE2 is also involved in the cleavage of other proteins such as bradykinin, apelin, neurotensin, dynorphin A or ghrelin (Ferrão et al., 2014). ACE2 is vital to the host and cannot be lost. It is a perfect receptor for a multi-host virus. Receptors are not proteins meant to allow viruses to dock and invade cells. They are proteins vital for the physiology of the host that viruses have evolved to recognize. This receptor binding process also demonstrates that the so-called “species barrier” concept often referred to for explaining that SARS-CoV-2 is not a naturally occurring virus does simply not make sense. At this stage it is important to come back to the concept of species. A species is an artificial, human-made concept developed for the purpose of classification based on reproductive isolation, i.e. two different species cannot reproduce or give a sterile offspring, and on recognizable morphological traits, and for molecular classification on the genetic similarity of few genes. As a matter of fact, a species is defined based on a very small number of genes encoding highly “specific” traits (Fig. 1 ). These “specific” traits are by definition only found in one given species. However, any species comprises several thousand or tens of thousand genes which are not encoding any “specific” trait. These “core” genes are common to several species and genera, and, in some cases to larger taxa like vertebrates (Fig. 1). This is typically the case for ACE2 which is shared by all vertebrates (Devaux et al., 2021a). It is not a “specific” receptor. The “species barrier” is simply an illusion based on the misconception that a species is an isolated island with only specific genes, and proteins, which force a virus to adapt to species B in order to be able “jump” from species A to species B. For a virus there are only two kinds of hosts; susceptible hosts and resistant hosts, regardless of the species status given by humans in a classification system. Either the virus can recognize a receptor, and the host is then susceptible, or it cannot recognize a receptor making this host resistant. The receptor recognition can be either at low or high affinity but this is not a major limitation since the virus will evolve later on to optimize its adaptation to the host. Only a limited affinity is needed for the primary infection. The host susceptibility is also modulated by a second factor which is the capacity for a virus to avoid host defenses. A virus can thus infect a host if it can both recognize a receptor and avoid the host defenses. If the receptor is a ubiquitous protein encoded by a “core” gene, such as ACE2 recognized by SARS-CoV-2, the concept of species becomes irrelevant for the virus and that of “species barrier” inexistent.
Fig. 1.
Schematic representation of the position of specific and generic receptors in the genome of species.
Being a pandemic virus is more than just being capable of binding to a ubiquitous receptor. Other traits characterize viruses with a potential for pandemics. They must display a low virulence, i.e. low pathogenicity, with a significant period of incubation. Indeed, for a virus to be able to circulate widely and trigger a pandemic, it is a mandatory requirement not to kill the host or to display a limited fatality rate. This is exactly what is seen with SARS-CoV-2. COVID-19 is a mild to moderate disease with a fatality rate currently estimated at 2.13% (Johns Hopkins University, 2021) but which will most likely be lower since asymptomatic cases are not considered in the calculation. The high number of deaths is a consequence of the size of the human population (Coelho et al., 2020; Iacus et al., 2020; Sigler et al., 2021) and to the transmissibility of the virus, which is itself a consequence of both the human population density and the virus capacity to easily infect a new host. The mortality is in most cases not directly linked to a pathogenic effect of the virus but instead to the weakness of deceased patients who are most frequently aged and/or affected by major comorbidities. Doing so, the virus can easily and efficiently spread. A comparison with a virus displaying no pandemic potential, like the Zaire strain of Ebola virus, can help understanding the specific traits of pandemic viruses. The Zaire strain of Ebola virus displays a very high fatality rate around 70% (Kucharski and Edmunds, 2014). This virus kills very fast and generates very strong and disabling symptoms. The Zaire strain of Ebola virus cannot spread into a pandemic. It kills too much and too fast and is immediately detected. Ebola outbreaks are limited to a given area with sporadic cases occurring over a given period of time. The only exception is the 2014–2015 outbreak in West Africa, but this is due to bad management and active transportation of the virus by humans. The Zaire strain of Ebola virus is an accidental multi-host virus not adapted to humans to whom it quickly causes enormous and lethal damages, impeding its propagation in a pandemic, or even an epidemic, way. Adaptation is a consequence of the host-virus coevolution and is characterized by a low virulence. The Zaire strain of the Ebola virus is compatible with humans in whom it can replicate and can generate lethal damage, but is not adapted owing to the lack of coevolution. However, if given the opportunity, the Zaire strain of the Ebola virus can, like other viruses, adapt to humans and its virulence starts declining. This happened during the 2014–2015 West-African outbreak (Urbanowicz et al., 2016). This leads to another major concept, the distinction between the primary case and the index case. The primary case is the first person to be infected by a given virus. It corresponds to the primo-infection in the human population. The index case is the first person to have been diagnosed with the novel disease. These are two very different concepts. For a pandemic virus like SARS-CoV-2, there may be a long time, and large geographic distance, between the occurrence of the primary case and that of the index case during which the virus spread unnoticed in the human population. The index case can sometimes be identified but the primary case is never found. In the case of COVID-19, there is an official index case who was declared as such by an administrative decision for a provisional dating of the beginning of a viral outbreak but which is not the real one. The primary case is not known and will never be identified. In the case of Ebola, it is impossible to ignore both the primary case and the index case who are often the same person.
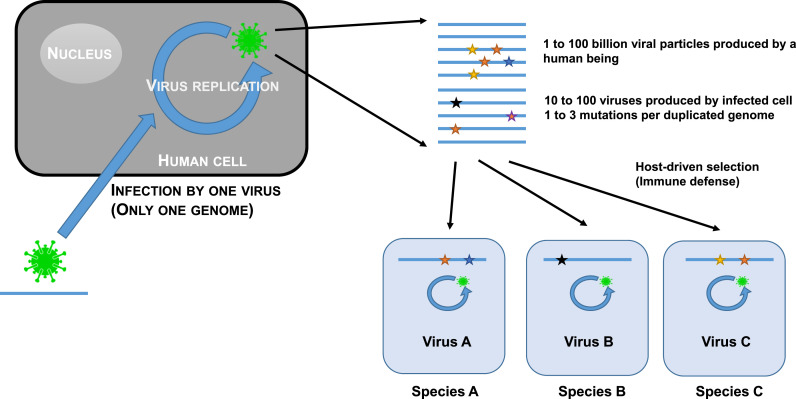
Another trait displayed by pandemic viruses is their ability to evolve in-host through a quasispecies process (Fig. 2 ). The quasispecies model of evolution is a specific trait of RNA virus (Andino and Domingo, 2015; Song et al., 2005; Karamitros et al., 2020; Chaudhry et al., 2020). The vast majority of zoonotic viruses are RNA viruses (Woolhouse et al., 2013). This is not a coincidence. The quasispecies mode of evolution corresponds to the generation of a multitude of variants covering all possibilities of mutations in the viral genome. This is referred to as the sequence space. Almost all of these variants are not viable and are eliminated. However, some of them carry mutations which allow avoiding the immune defenses of the host and can replicate. Their sequence will be the starting point of a new generation of variants exploring the sequence space. Progressively, the virus will better adapt to humans and under the host pressure, a favorable sequence, the matrix sequence, will be selected. This evolution will sometimes lead to mutations making the virus very highly transmissible. This represents of course a clear selective advantage and viruses bearing these mutations will quickly be selected. This is for instance what most likely happened with the D614G mutation in the Receptor Binding Domain (RBD) of SARS-CoV-2 or the furin cleavage sites between the two subunits of the spike protein (Andersen, 2020; Coutard et al., 2020). These mutations conferred a considerable evolutionary advantage to the variants bearing them which quickly became dominants. This process of in-host evolution and adaptation through a quasispecies process is still going on. The host can adapt and its immune system can control the virus and the latter adapts automatically by quasipecies evolution yielding a new variant capable of evading the immune defenses. This is exactly what we see today in the case of COVID-19 with the regular emergence and spread of new variants replacing the previous ones like the current Delta variant. New variants will continue to appear, a process also observed with other viruses (Carrillo-Valenzo et al., 2010; Zhang et al., 2005; Suzuki et al., 2019; Duong et al., 2013a, 2013b; Anggraeni et al., 2021). Coming back to SARS-CoV-2, it is simply a disease-triggering, highly human-transmissible variant having evolved from a previous form of the same virus already circulating in humans but unable to trigger a disease which we could then call SARS-CoV-2-AD, AD standing for “Ante Disease”.
Fig. 2.
Schematic representation of the quasispecies evolution process.
1.2. Confusing the primary case and the index case
An emerging infectious disease is of course a disease and as a consequence is considered as a medical issue. However, the problem is that it is considered exclusively a medical issue. Regarding a pandemic such as COVID-19 only as a disease is the origin of many confusions and misconceptions. In particular, confusion is often made between the disease and the infection. A disease is a medical concept defined by the presence of a specific set of symptoms that physicians can recognize and to which a name is given. The art of the physician is to identify the set of symptoms, name the disease and then prescribe the relevant treatment. COVID-19 is the disease which has been identified in Wuhan. Conversely, an infection is a biological concept corresponding to the multiplication of pathogen within a host but which does not presuppose the occurrence of symptoms, thus of a disease. Asymptomatic cases are major actors in the dynamic of expansion of pathogen, and of course in the dynamic of a pandemic, but are not visible to physicians who focus only on symptoms. Epidemiologists also focus only on symptoms and their role is to identify the first patient having declared the disease, the index case, to unravel the pattern of diffusion of the disease within the human population and propose actions to block or, at least, to slow down the expansion of the disease. This is exactly what has been done worldwide since the identification of COVID-19 in January 2020. However, this is only the very end of the process leading the emergence of the disease. This process started long before with the infection of the first human being, the primo-infection. As already mentioned, a disease is a physiological disorder characterized by a specific pattern of symptoms. However, an emerging infectious disease is by definition a disease with no associated specific pattern of symptoms and name. This is the origin of the problem. Physicians only recognize that there is an unknown disease outbreak when confronted to a flow of patients with the same atypical pattern of symptoms. This is the main drawback of this approach. The detection of the disease outbreak is delayed and occurs only after the virus has already spread in the population. All nations worldwide as well as WHO are basing their strategy of emerging disease control on the same medical approach. All actions are taken only after the disease has been recognized and the outbreak alert has been given. This is exactly what happened with COVID-19. No actions were taken outside China when WHO released the information about a new emerging disease in Wuhan in late December 2020. National actions were considered only after WHO has declared COVID-19 a pandemic in March 2020. It was too late for prevention and the only option left was to try to control the disease. The current worldwide situation with COVID-19 clearly demonstrates that we should not focus on the origin of the disease (the index case) but instead on the origin of the primo-infection (the primary case).
1.3. Hunting down the virus in the wild
COVID-19 being considered a “zoonotic disease”, i.e. a disease coming from animals, the search for the origin of SARS-CoV-2 was immediately oriented towards wildlife. This search for the wild ancestors of SARS-CoV-2 is driven today by the “spillover” theory. This theory, as formulated by Power and Mitchell (2004), states that there must be an “animal intermediate species” also often referred to as “reservoir” bearing the same virus as the one causing the epidemic. This spillover theory is the reference driving strategies for preventing and controlling emerging infectious diseases at the early stage. It is at the origin of the search for intermediate species and screening projects such as the Global Virome or PREDICT (Carroll et al., 2018; Jonas and Seifman, 2019) which objectives are to identify potential zoonotic viruses circulating in the wild. In the COVID-19 context, this intermediate species is supposed to make the link between bats, the putative original virus reservoir (Burki, 2020; Boni et al., 2020) and humans, the final recipient host. However, none of the predictions from the spillover model have been confirmed. The pangolin was for a while identified as the intermediate species between the reservoir, i.e. bats, and the recipients, i.e. humans. However, the pangolin virus sequences considered came from metagenomic analyses performed on smuggled Malayan pangolins confiscated by Chinese customs before the COVID-19 crisis (Zhang et al., 2020; Liu et al., 2020; Liu et al., 2019). Furthermore, a screening conducted in Malaysia on pangolins seized by customs led to no detection of coronavirus (Lee et al., 2020). The status of the pangolin as formal intermediary was only built through successive deformation of the initial reports even though articles showed that this hypothesis was not valid (Zhang et al., 2020; Frutos et al., 2020b; Li et al., 2020d; Liu et al., 2020; Lee et al., 2020; Wenzel, 2020; Tang et al., 2020). No intermediate or reservoir species have ever been found, despite numerous investigations. No related epizootic was described in pangolins or other animals in China or elsewhere. Furthermore, no SARS-CoV-2 has been reported until now in wild animals. Viruses, like other living organisms, are organized in metapopulations, i.e. a population of populations (Levins, 1969). Viruses causing pandemics in humans only represent the human evolution through quasispecies of a group of viruses which in other hosts would evolve differently. SARS-CoV-2 is merely one human form of the SARS metapopulation of viruses among others having emerged. SARS-CoV, the causative agent of SARS which emerged in 2002 (Stockman et al., 2006), is another one coming from a previous emergence from the same virus metapopulation. There is nothing surprising at seeing multiple emergence events from the same metapopulation of virus. This is exactly what happened with the dengue viruses (Moncayo et al., 2004; Vasilakis et al., 2011). Four different dengue viruses are known today: DENV1, DENV2, DENV3 and DENV4. These four viruses, being at the same time highly similar but significantly different, are coming from four independent events of emergence of four different populations from the same dengue virus metapopulation (Moncayo et al., 2004; Vasilakis et al., 2011). To date there is no experimental data to support a spillover of SARS-CoV-2 from any animal species. Only related viruses have been found in the wild but never the same virus as in the human population. The exception is animals in rearing or captivity which are contaminated by humans and can contaminate humans back. This is exactly what happened with minks in the case of COVID-19 (Munnink et al., 2020; Frutos and Devaux, 2020; Devaux et al., 2021b) and civets in the case of SARS (Frutos et al., 2021b; Tu et al., 2004; Han, 2020).
The closest sequences to SARS-CoV-2 found in the wild were obtained from bats. The first one to have been identified is RaTG13, which was obtained from a Rhinolophus affinis bat from an abandoned copper-mine in Tong Guan (Mojiang, Yunnan, China) (Ge et al., 2016; Zhou et al., 2020a). RaTG13 display 96% of sequence identity with SARS-CoV-2. Another batCoV sequence, RmYN02, was identified also in Yunnan in the horseshoe bat Rhinolophus malayanus (Zhou et al., 2020b). RmYN02 shared 93.3% similarity with SARS-CoV-2, a percentage rising to 97.2% in ORF 1 ab. However, the Receptor Binding Domain (RBD) displayed only 61.3% similarity with that of SARS-CoV-2 (Zhou et al., 2020b). Two other related sequences, RshSTT182 and RshSTT200, have been described in Rhinolophus shameli bats caught in 2010 in Cambodia (Hul et al., 2021) which displayed 92.6% of overall similarity with SARS-CoV-2. Another Sarbecovirus sequence has been described in a Rhinolophus acuminatus bat from Thailand (Wacharapluesadee et al., 2021). This sequence, named RacCS203, displayed 95.86% similarity with SARS-CoV-2 (Wacharapluesadee et al., 2021). However, all these sequences obtained from bats share the same characteristics. First of all, none of them are SARS-CoV-2 sequences. They are merely related sequences. Furthermore, they have all been obtained by metagenomics. None of them corresponds to an actual virus having been isolated and grown in cell culture. They have all been assembled in silico from a multitude of small random reads using SARS-CoV-2 as a template. Biases and assembly mistakes may have occurred. There is therefore no certainty as whether these sequences correspond to an actual virus or to a viable virus. It is not known either whether each sequence corresponds to only one virus or to an in silico chimera made with fragments coming from several viruses. These are serious limitations mentioned as a warning by the GISAID database whenever a metagenomic sequence is deposited. Therefore, all analyses conducted with these sequences should be considered with caution.
1.4. The laboratory leak narrative
The impossibility to find any reservoir, intermediate species or SARS-CoV-2 virus in the wild prompted the development of what isknown as the laboratory leak narrative (Domingo, 2021a, 2021b; Rahalkar and Bahulikar, 2020; Segreto and Deigin, 2021; Sirotkin and Sirotkin, 2020; Kaina, 2021; Relman, 2020). The initial rationale behind this narrative is that since both SARS-CoV-2, reservoirs and intermediaries are not found in the wild, the virus must come from a laboratory from which it has accidently escaped or has been voluntarily released. This narrative also builds on the fact that Wuhan, where COVID-19 was first described, is also home to the Wuhan Institute of Virology (WIV) which runs a P4 safety laboratory where previous works have been conducted on SARS and other coronaviruses (Menachery et al., 2015; Cohen, 2020). Furthermore, a team from WIV has collected samples from bats in the Mojiang mine in Yunnan from which they obtained the RaTG13 sequence (Ge et al., 2016; Zhou et al., 2020a). However, this laboratory leak narrative is composite and comprises several self-excluding theories: i) SARS-CoV-2 is originating from the Mojiang mine where WIV members were contaminated, ii) SARS-CoV-2 has accidently escaped from the WIV laboratory, and iii) SARS-CoV-2 has been engineered at WIV and accidently escaped or was voluntarily released. These hypotheses are often brought together. However, they are excluding each other. If SARS-CoV-2 is a naturally occurring virus from the Mojiang mine, it cannot come from genetic engineering and vice versa. If SARS-CoV-2 was spread by WIV staff members infected in the Mojiang mine, it cannot come from a laboratory accident and vice versa. A detailed analysis brings a better understanding of these narratives.
Infection of WIV staff members by SARS-CoV-2 in the Mojiang mine. The origin of this narrative is an accident which occurred in 2012. Six miners sent to clean up an abandoned copper-mine in Mojiang, Yunnan, were all hospitalized for pneumonia. Three of them died whereas the other three recovered. This narrative is based on the analysis by Rahalkar and Bahulikar (2020) who diagnosed a COVID-19-like infection in the case of the six miners and made a direct link between the Mojiang mine incident and COVID-19. However, the authors are not physicians but specialists in agriculture and bioenergy. Nevertheless, this analysis has been used to claim that the Mojiang mine might be the origin of COVID-19 through the involvement of WIV who investigated this mine following the incident (Rahalkar and Bahulikar, 2020). The clinical files of the six miners were analyzed by specialists, a clinician and a radiologist, working in a hospital specializing in infectious diseases and having handled thousands of patients with COVID-19 (Frutos et al., 2021c). Their diagnosis is that the miners did not have COVID-19 or SARS. Neither the clinical features nor the radiologic images corresponded to these diseases. The symptoms reported by Rahalkar and Bahulikar (2020) are generic symptoms associated with any kind of pneumonia regardless of the etiology and are not representative of COVID-19. Interestingly, several clinical and radiologic symptoms incompatible with COVID-19 or any coronavirus infection have been omitted by Rahalkar and Bahulikar (Frutos et al., 2021c). Four out of six miners, including the three who died, have been tested for both COVID-19 and SARS and the result was negative for both diseases (Zhou et al., 2021). The Mojiang miners did not die of COVID-19 which was not present in the Mojiang mine. Furthermore, SARS-CoV-2 was never found in the Mojiang mine by the teams who investigated it in 2012 and 2013 (Ge et al., 2016; Wu et al., 2014). It is thus not possible for WIV staff members to have been infected by SARS-CoV-2 in this mine. Furthermore, all WIV staff members were tested for COVID-19 and were negative (Cohen, 2020). This event occurred in 2012 and one can also wonder how a highly transmissible virus which killed 4.3 million people and infected more than 200 million persons in 18 months (Johnson et al., 2021) could have stayed silent for seven years. This narrative on the origin of SARS-CoV-2 can be dismissed. Not only there is no evidence to support it but there is evidence against it.
The laboratory leak of the virus from the WIV laboratory. This second narrative is saying that SARS-CoV-2 is a naturally occurring virus which accidently escaped during the handling of a sample from the Mojiang mine. Laboratory accidents occurred in the past (Heymann et al., 2004; Watts, 2004; Webster, 2004; WHO, 2004) and will without any doubt occur in the future. However, in the case of SARS-CoV-2, there are several evidences against that hypothesis in addition to the conclusion of the official WHO mission who concluded that it was “extremely unlikely” (Dyer, 2021). SARS-CoV-2 was not present in the Mojiang mine making it not possible to be present in any sample. SARS-CoV-2 was never found in samples from the Mojiang mine (Ge et al., 2016; Zhou et al., 2020a; Wu et al., 2014). Only other virus sequences were found. RaTG13 which is the closest sequence to that of SARS-CoV-2 and which was detected in samples from the Mojiang mine is not a virus. It was never isolated and grown in cell culture. It is only a sequence obtained by metagenomics. It has no physical existence. Furthermore, having been obtained by metagenomics, there is no certainty on whether it is viable, if it corresponds to a real virus and even if it corresponds to a single virus. As mentioned above, it could be an in silico chimeric construction. Nevertheless, it is simply not possible for a virus which does not physically exist, for a virtual sequence in computer, to escape from a laboratory and trigger an epidemic.
Genetic engineering of SARS-CoV-2 at WIV. The last narrative about the laboratory origin of SARS-CoV-2 is the engineering of the virus through gain-of-function experiments followed by an accidental release of the virus (Segreto and Deigin, 2021; Segreto et al., 2021; Sirotkin and Sirotkin, 2020; Kaina, 2021; Relman, 2020). The main argument in support of this narrative is the presence of a furin cleavage site between the two subunits of the RBD and the presence of the less-frequent CGG codon at this level (Segreto and Deigin, 2021; Segreto et al., 2021; Sirotkin and Sirotkin, 2020). Furin cleavage sites are naturally very frequent in many viruses including coronaviruses (Andersen, 2020; Huang et al., 2006; Yamada and Liu, 2009; Hao, 2020; Dimitrov, 2004; Coutard et al., 2020; Frutos et al., 2021d). The CGG arginine codon is indeed less frequent than other arginine codon in coronaviruses. However, being less frequent does not mean it is never present. This CGG codon is naturally present in SARS-CoV-2 and is even present at a higher rate in MERS-CoV (Chen et al., 2017; Hou, 2020). Its presence is by no means a proof of genetic engineering. The lower presence of CpG (intrachain Cytosine-Guanosine dinucleotide linked by a phosphate bond) in human pathogens has been shown to be a selective process. CpGs trigger direct B-Cell activation and therefore these dinucleotides provide a selective disadvantage (Krieg et al., 1995). However, they nevertheless exist in human pathogens. When considering the huge selective advantage in transmissibility brought by the furin-cleavage site over the disadvantage brought by the B-cell activation, the net result in largely in favor of the furin-cleavage site leading to the fixation of these mutations in the human populations even if it involves the rare CGG codons. This is a simple and straightforward selective and evolutionary process. Furthermore, RmYN02 also carries an indel at the same place as the furin site (PRRA) of SARS-CoV-2 with the insertion of a PAA sequence and the deletion of the immediate upstream QTQT sequence (Zhou et al., 2020b). The naturally occurring PAAR sequence displayed by RmYN02 is not active as a furin-cleavage site but is only one mutation away from the active RNNR furin cleavage site. One additional mutation turning the proline (P) into an arginine (R) will generate a RAAR active furin-cleavage site. This suggests that viruses other than SARS-CoV-2 are under similar selective pressure. The SARS-CoV-2 furin activation sites was also shown to be highly adaptable (Whittaker, 2021). Scientists at the Wuhan Institute of Virology have indeed carried out gain of function experiments, but only on SARS-CoV in published and openly displayed international collaborations and not on SARS-CoV-2 (Cohen, 2020; Menachery et al., 2015; Frutos et al., 2021c). There is currently no evidence to support the claim for genetic engineering of SARS-CoV-2. These are only unsubstantiated accusations and a narrative based on a virtual scenario.
1.5. Humanization and socialization
Before moving to the explanation on how SARS-CoV-2 emerged, it is important to address the issue of the in-host virus evolution and adaptation. All viruses are evolving and adapting and RNA viruses are particularly efficient in evolution and adaptation due to their quasispecies process of evolution (Andino and Domingo, 2015). Multi-host viruses evolve in their hosts to continuously adapt. In humans, this leads to the process of humanization. The virus is permanently adapting to its human host by overcoming the host defenses while keeping the lethality low. This leads to the generation of novel lineages replacing the previous ones. This process is for instance very well known on the dengue virus which is characterized by the continuous replacement of lineages (Carrillo-Valenzo et al., 2010; Zhang et al., 2005; Suzuki et al., 2019; Duong et al., 2013a, 2013b). This was also shown on the chikungunya virus (Anggraeni et al., 2021) or on influenza viruses (Fitch et al., 1991; Hay et al., 2001; Huang et al., 2012). The same process occurs with SARS-CoV-2 for which these novel, more adapted lineages are called variants. It is an ongoing process which will not end just as for dengue, chikungunya or influenza. As mentioned above, SARS-CoV-2 must simply be seen as a highly-transmissible variant of a previous virus, SARS-CoV-2-AD, already circulating in humans which was not capable of triggering a disease because its transmissibility was not high enough. The gain through evolution of mutations like the furin cleavage site or the D614G substitution were essential for this increase in transmissibility (Xia et al., 2020; Johnson et al., 2021; Zhou et al., 2021; Zhang et al., 2021; Plante et al., 2021). Viruses are also using their hosts for dissemination. In particular, they use the ecology and behavior of their hosts to be disseminated through intraspecies or interspecies contacts. The same occurs with SARS-CoV-2 in humans. However, one must consider the specific nature of human ecology and behavior. Humans have created their own ecosystem, the human society, which is driven by societal rules. The human society also impacts wild areas through different ways such as deforestation, land conversion or recreational activities. The drivers for virus dissemination within or outside the human population are societal. It is essential to understand that because of this specific nature of the human ecology, SARS-CoV-2 became socialized at the same time it became humanized and its expansion is governed by societal rules. This is true also for other pandemic viruses like influenza, dengue, chikungunya, Zika or HIV. Their expansion is always driven by human societal traits and territorial factors.
On the origin of SARS-CoV-2 by means of natural selection or the preservation of the favoured variants in the struggle for life.
The process of emergence of a pandemic can be divided into three phases: a circulation phase, a societal phase and a medical phase (Fig. 3 ). The circulation phase corresponds to an environmental stage whereas the societal and the medical phases together make an anthropogenic stage. The circulation phase is characterized by a permanent multidirectional circulation of multi-host viruses upon contact. Humans are part of this broad circulation process, acquiring viruses from other species and transmitting viruses to other species. The most likely places for humans to acquire multi-host viruses are rural anthropized environments (Afelt et al., 2017, 2018), especially pioneer, i.e. settlement, fronts. These rural environments are close to wildlife. They are characterized by a mosaic of landscapes where wild animals can find favorable living conditions. Human settlements can provide food and shelter to many animals and provide opportunities for contacts and virus transmission which would not occur in the wild (Afelt et al., 2017, 2018). The diversity and population density of viruses was shown to be greatly higher close to human settlements than in the wild (Afelt et al., 2017; Plowright et al., 2015). Anthropized rural environments are thus the most likely areas for the primo-infection to occur. These are the places where primary cases should be sought for instead of urban areas. Once the primo-infection occurred, the virus is entering the societal phase. Both evolution and transmission are now taking place within the human population. The virus will evolve within the human population following the quasispecies process. However, this phase is a stochastic one during which many things may happen due to evolutionary and environmental factors. The virus can be eliminated, stay in a low-transmissibility form or mutate into a high-transmissibility form (Fig. 4 ). The latter is what happened with SARS-CoV-2. It is not possible to determine how long this process can last but on the case of SARS-CoV-2, it left traces of positive selection, i.e. host-driven selection, in the genome (MacLean et al., 2021). The evolution into a highly transmissible form corresponds to a first accident, a genetic one. A second accident, a societal one, must then occur to ensure the amplification of the virus population up to the level of the epidemic threshold. An epidemic never starts with only one infected individual. It is a probabilistic process, i.e. the probability to transmit the virus by contact, and there must be a minimal number of infected individuals to initiate an epidemic. This is referred to as the epidemic threshold, or in the case of an emerging disease as the outbreak threshold (Hartfield and Alizon, 2013). This requires a high human density in a limited area for a limited time. This corresponds to what is called an amplification loop, a process allowing the virus population to be quickly amplified to the level of the epidemic threshold. This also corresponds to what is described as “clusters” of cases. Simply, a “cluster” is the static representation while an “amplification loop” is the dynamic representation of the same process. This corresponds to the definition of societal events and explains why pandemic diseases emerge in cities. The probability for a high-population density societal event to occur is indeed significantly higher in urban areas than in rural areas. There is thus a geographic discrimination between the primary case and the index case. Societal events leading to the amplification of the viral population are diverse and may depend on the society. Societies themselves are diverse and even though there is today a broad harmonization due to globalization, internet and social networks, social events in each society depend for a large part on traditions, history and culture. However, some common features can easily be identified such as sportive events, fairs, concerts, religious meetings, political meetings, etc. Once the epidemic threshold is reached, the virus enters a deterministic phase characterized by an exponential growth of the viral population, or in other words of the number of persons contaminated. This is due to the combination of a highly transmissible virus with an initial number of contaminated persons high enough to statistically generate a high number of contacts with naive persons. At this stage, the process enters the last phase, the medical phase. The disease is not identified when the viral population reaches the epidemic threshold. There is a delay due to the fact that an emerging disease is by definition unknown and that the specific pattern of symptoms is not characterized. Physicians detect the occurrence of an unknown disease when facing a flow of patients displaying the same unknown pattern of symptoms. The index case can be identified at that stage. The causative agent is also identified allowing thus the development of detection tests and screening in the population. However, during the time needed to go over all these steps, the virus has already widely spread in the population triggering an epidemic, and can, owing to the high international mobility which characterizes our society, translate into a pandemic (Steverding, 2020; Sheller and Urry, 2006). This whole process corresponds to what has been described as the “circulation model” (Frutos et al., 2020b, 2021d, 2021e). The circulation model was recently confirmed on Ebola (Keita et al., 2021). The virus responsible for the 2020–2021 Ebola outbreak has been circulating within the human population for several year and no spillover from the wild occurred (Keita et al., 2021).
Fig. 3.
Schematic representation of the process of circulation and emergence of pandemic viruses in the human population.
Fig. 4.
Different ways of evolution of RNA viruses within the human population.
On November 24, 1859 Charles Darwin published a major work titled “On the origin of species by means of natural selection or the preservation of the favoured races in the struggle for life”. In this founding work, Darwin demonstrated that there is no determined “origin” to cats, blue whales, elephant, humans, kangaroos or pine trees. There is a permanent ongoing process of evolution, adaptation and selection in which chance and environment play a major role and which leads to the development of adapted species. Similarly, there is no determined “origin” to SARS-CoV-2 and other viruses but a permanent process of evolution, adaptation and selection shaped by chance and environment which gives rise to novel lineages, i.e. variants. However, there is something specific to viruses which is that their environment is another living organism and this “environment” has its own specific ecology and behavior which influence the expansion and transmission of the viruses. In human viruses, this specific ecology is the human society. There is also something specific to humans beside human behavior, it is the exponential growth of the human population. This growth generates many contacts facilitating both primo-infection and dissemination after emergence of the disease.
1.6. Perception, deception and perversion
Charles Darwin gave us the answer 162 years ago, but it is still extremely difficult for people to accept that the emergence of a disease is only the result of evolution, stochastic events, human society and human behavior. People need to find answers to the question on the “origin” of any infectious disease and specifically “when”, “who”, “where from” and “why”. It is a common human behavior to search for a culprit to blame, whether it is human or animal. It is very difficult for people to accept a natural situation on which they have little control. This human need to find a source, and possibly a reason, to a pandemic triggers the development of scenarios, narratives and intellectual constructions to answer these questions which in fact have no answer because they have no reality. The only question that exists and to which we can bring an answer is “how”. What is the process leading to the emergence of an infectious disease? This process is simply evolution, adaptation and selection as for any other living organism on Earth. These are the words of wisdom Darwin brought to us in 1859. Why are they not understood today? The answer is multiple. A first factor is ignorance. Many people, even in the medical and scientific communities, are not familiar with the concepts of evolution. Therefore, they imagine a straightforward, linear process of transmission with an origin, “THE origin”, eventually an intermediate and a final recipient. This linear model is very simple and attractive owing to the fact that our society responds to infectious diseases through a medical approach which is a straightforward, linear approach. In medicine and epidemiology, there is an origin of contamination and a recipient who becomes sick. However, if this is true within the human population for an already declared disease, it is not true for the emergence of a disease which is the result of evolution and occurrence of stochastic events. Another key factor to consider is the human behavior and the will to take benefit from any situation. In face of a threat, corporatist attitudes will often prevail and groups will lobby toward the implementation of their own field of activity as a source of income and power even though it does not bring any solution. A last factor to consider in this need to blame something or someone is the manipulation for political or commercial reasons. We are living today in a time of intense geopolitical conflicts between superpowers during which manipulations, false accusations and hidden agendas are common. Therefore, it is essential to take all narratives with caution, in particular when they are initiated by governments. They might be forgeries driven by national or commercial interests with the aim to destabilize an opponent. Other people, also forward unsubstantiated accusations simply because they need to accuse someone, they need to find a culprit, or because they hate other people, nations, countries, etc. All these issues are strongly worsened by internet, social networks and permanent news channels where everyone can broadcast and advertise his/her opinion even without any expertise and knowledge. Internet has erased the concept of specialist and everyone is claiming a right to be heard not on the basis of knowledge, expertise and scientific evidence but on the right to express their opinion regardless of reality. Therefore, it is essential to analyze things in a rational way, to base conclusions only on scientific evidence and to step away from opinions and opinion-driven narratives (Vosoughi et al., 2018).
1.7. A plea for rationality
Other pandemics will occur in the future. It is only a matter of time. Since the probability of occurrence is directly linked to the number of contacts and opportunities of amplification, the permanent growth of the human population makes this probability higher every year. The main question is therefore: how to prevent another pandemic? The first step is to understand the multi-host nature of pandemic viruses, the whole process of virus circulation and evolution and the dynamic of emergence of diseases. The current situation with COVID-19 is also giving information on what cannot be done. Medicine cannot prevent the emergence of a pandemic. Being symptom-based by essence, medicine comes too late. Screening for viruses in the wild and surveillance cannot bring relevant information and cannot prevent an epidemic either. This necessitates to know the target. One can detect and monitor only what is already known and it is thus impossible to detect something that is not known yet. Multi-host pandemic viruses are evolving within humans and the genotype of virus responsible for the pandemic does not exist in the wild. Screenings will only yield related viruses, members of the same metapopulation, but not the pandemic one. Screenings cannot provide any clue on which virus out of the multitude circulating may one day infect humans and, further, which one out of the multitude infecting humans may turn into a pandemic virus. The solution to prevent pandemics is not to target the disease or putative causative agents but instead to target the process of disease emergence itself. This process of disease emergence being socially-driven, the only reliable approach which can be implemented ahead of disease emergence is the societal approach. Society-driven amplification loops are mandatory to reach the epidemic threshold and we must prevent these amplification loops from taking place. Interactions, processes and events involved in these amplification loops are man-made and thus can be modeled and analyzed. All we need to do is to organize these interactions and processes to introduce dilution factors and prevent the virus to reach the epidemic threshold. An additional positive point of this approach is that we do not need to know the virus. Whatever the virus, it will have to go through these amplification loops. What is needed is a global effort of modeling human activities. Efficient tools like multi-agent modeling exist and are already in use for this same purpose of optimizing activities and management of the society through adapted regulation. However, the human society is diverse and amplification loops might be triggered by different processes depending on the specific society. A global and coordinated effort is needed to model the different type of human activities in order to isolate the components leading to amplification loops and to determine how to block the chain of events leading to the amplification of the viral population without negatively impacting the society. The problem is global but the solution is local. There is no single universal solution. The solution is society-driven and each society must implement its own regulations aiming at eliminating amplification loops and designed according to the modeling of its own traits. Unlike what has been done in an empirical way since the beginning of the COVID-19 crisis with dramatic impacts, i.e. lockdowns, mental impact, destruction of the economy, job losses, blockades, this optimization of the organization can be realized with no economic, human, and societal impact if properly modeled and translated into national and international regulations. This is the endeavor mankind must engage in. Our society has been able to organize itself to control other major threats like nuclear power or climate change. Why should the society not be able to do the same for pandemics?
Authors contributions
All authors contributed to the manuscript. All authors participated in the writing and correction of the manuscript. All authors read the manuscript and agreed with its content.
Ethics declarations
No human samples or clinical data were used.
Funding
The work was supported by institutional funds from Institut de Recherche pour le Développement and Aix-Marseille University. Other funding sources involved are the institutions of affiliation of each author: CIRAD (Centre de Coopération Internationale en Recherche Agronomique pour le Développement) for RF, University of Montpellier for LG, and CNRS (Centre National de la Recherche Scientifique) for OP and CAD and were limited to the salaries of the authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Afelt A., Lacroix A., Zawadzka-Pawlewska U., Pokojski W., Buchy P., Frutos R. Distribution of bat-borne viruses and environment patterns. Infect. Genet. Evol. 2017;58:181–191. doi: 10.1016/j.meegid.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afelt A., Frutos R., Devaux C. Bats, coronaviruses and deforestation: towards the emergence of novel infectious diseases? Front. Microbiol. 2018;9:702. doi: 10.3389/fmicb.2018.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino R., Domingo E. Viral quasispecies. Virology. 2015;479:46–51. doi: 10.1016/j.virol.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggraeni Y.M., Garjito T.A., Prihatin M.T., Handayani S.W., Negari K.S., Yanti A.O., Hidajat M.C., Prastowo D., Satoto T.B.T., Manguin S., Gavotte L., Frutos R. Fast expansion of the Asian-Pacific genotype of the Chikungunya virus in Indonesia. Frontiers in Cellular and Infection Microbiology. 2021;11:631508. doi: 10.3389/fcimb.2021.631508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni M.F., Lemey P., Jiang X., Lam T.T.Y., Perry B.W., Castoe T.A., et al. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nature microbiology. 2020;5:1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- Burki T. The origin of SARS-CoV-2. Lancet Infect. Dis. 2020;20:1018–1019. doi: 10.1016/S1473-3099(20)30641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell L.M., Johnston C.I., Tikellis C., Cooper M.E. ACE2, a new regulator of the renin–angiotensin system. Trends Endocrinol. Metabol. 2004;15:166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discovery. 2020;6:1–4. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Valenzo E., Danis-Lozano R., Velasco-Hernández J.X., Sánchez-Burgos G., Alpuche C., López I., et al. Evolution of dengue virus in Mexico is characterized by frequent lineage replacement. Arch. Virol. 2010;155:1401–1412. doi: 10.1007/s00705-010-0721-1. [DOI] [PubMed] [Google Scholar]
- Carroll D., Daszak P., Wolfe N.D., Gao G.F., Morel C.M., Morzaria S., et al. The global virome project. Science. 2018;359:872–874. doi: 10.1126/science.aap7463. [DOI] [PubMed] [Google Scholar]
- Chaudhry M.Z., Eschke K., Grashoff M., Abassi L., Kim Y., Brunotte L., et al. SARS-CoV-2 quasispecies mediate rapid virus evolution and adaptation. bioRxiv. 2020 2020.08.10.241414. [Google Scholar]
- Chen Y., Xu Q., Yuan X., Li X., Zhu T., Ma Y., et al. Analysis of the codon usage pattern in Middle East respiratory syndrome coronavirus. Oncotarget. 2017;8:110337. doi: 10.18632/oncotarget.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho M.T.P., Rodrigues J.F.M., Medina A.M., Scalco P., Terribile L.C., Vilela B., et al. Global expansion of COVID-19 pandemic is driven by population size and airport connections. PeerJ. 2020;8 [Google Scholar]
- Cohen J. Wuhan coronavirus hunter Shi Zhengli speaks out. Science. 2020;369:487–488. doi: 10.1126/science.369.6503.487. [DOI] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Pinault L., Osman I.O., Raoult D. Can ACE2 receptor polymorphism predict species susceptibility to SARS-CoV-2? Front. Public Health. 2021;8:608765. doi: 10.3389/fpubh.2020.608765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Pinault L., Delerce J., Raoult D., Levasseur A., Frutos R. Spread of mink SARS-CoV-2 variants in humans: a model of sarbecovirus interspecies evolution. Front. Microbiol. 2021;12:675528. doi: 10.3389/fmicb.2021.675528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D.S. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004;2:109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L. SARS-CoV-2/COVID-19: natural or laboratory origin? Environ. Res. 2021;201:111542. doi: 10.1016/j.envres.2021.111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L. Environmental Research; 2021. What we know and what we need to know about the origin of SARS-CoV-2; p. 111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., et al. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87:e1–e9. doi: 10.1161/01.res.87.5.e1. (REF) [DOI] [PubMed] [Google Scholar]
- Duong V., Henn M.R., Simmons C., Ngan C.Y.B., Gavotte L., Viari A., et al. Complex dynamic of dengue virus serotypes 2 and 3 in Cambodia following series of climate disasters. Infect. Genet. Evol. 2013;15:77–86. doi: 10.1016/j.meegid.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Duong V., Simmons C., Gavotte L., Viari A., Ong S., Chantha N., et al. Genetic diversity and lineage dynamic of dengue virus serotype 1 (DENV-1) in Cambodia. Infect. Genet. Evol. 2013;15:59–68. doi: 10.1016/j.meegid.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Dyer O. Covid-19: WHO says laboratory escape theory is “extremely unlikely” after mission to China. BMJ Br. Med. J. (Clin. Res. Ed.) 2021;372 doi: 10.1136/bmj.n428. [DOI] [PubMed] [Google Scholar]
- Ferrão F.M., Lara L.S., Lowe J. Renin-angiotensin system in the kidney: what is new? World J. Nephrol. 2014;3:64. doi: 10.5527/wjn.v3.i3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch W.M., Leiter J.M., Li X.Q., Palese P. Positive Darwinian evolution in human influenza A viruses. Proc. Natl. Acad. Sci. Unit. States Am. 1991;88:4270–4274. doi: 10.1073/pnas.88.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Devaux C.A. Mass culling of minks to protect the COVID-19 vaccines: is it rational? New Microbes and New Infections. 2020;38:100816. doi: 10.1016/j.nmni.2020.100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Lopez-Roig M., Serra-Cobo J., Devaux C.A. COVID-19: the conjunction of events leading to the pandemic and lessons to learn for future threats. Front. Med. 2020;7:223. doi: 10.3389/fmed.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Serra-Cobo J., Chen T., Devaux C.A. COVID-19: time to exonerate the pangolin from the transmission of SARS-CoV-2 to humans. Infect. Genet. Evol. 2020;84:104493. doi: 10.1016/j.meegid.2020.104493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Gavotte L., Serra-Cobo J., Chen T., Devaux C.A. COVID-19 and emerging infectious diseases: the society is still unprepared for the next pandemic. Environ. Res. 2021;202:111676. doi: 10.1016/j.envres.2021.111676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Serra-Cobo J., Pinault L., Lopez Roig M., Devaux C.A. Emergence of bat-related betacoronaviruses: hazard and risks. Front. Microbiol. 2021;12:591535. doi: 10.3389/fmicb.2021.591535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Javelle E., Barberot C., Gavotte L., Tissot-Dupont H., Devaux C.A. Origin of COVID-19: dismissing the Mojiang mine theory and the laboratory accident narrative. Environ. Res. 2021 doi: 10.1016/j.envres.2021.112141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Gavotte L., Devaux C.A. Understanding the origin of COVID-19 requires to change the paradigm on zoonotic emergence from the spillover to the circulation model. Infect. Genet. Evol. 2021:104812. doi: 10.1016/j.meegid.2021.104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Gavotte L., Devaux C.A. Unravelling the origin of SARS-CoV-2: is the model good? New Microbes and New Infections. 2021;43:100918. doi: 10.1016/j.nmni.2021.100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.-Y., Wang N., Zhang W., Hu B., Li B., Zhang Y.Z., et al. Coexistence of multiple coronaviruses in several bat colonies in an abandoned mineshaft. Virol. Sin. 2016;31:31–40. doi: 10.1007/s12250-016-3713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.V., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol.: A Journal of the Pathological Society of Great Britain and Ireland. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G.Z. Pangolins harbor SARS-CoV-2-related coronaviruses. Trends Microbiol. 2020;28:515–517. doi: 10.1016/j.tim.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao P. Is SARS-CoV-2 originated from laboratory? A rebuttal to the claim of formation via laboratory recombination. Emerg. Microb. Infect. 2020;9:545–547. doi: 10.1080/22221751.2020.1738279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartfield M., Alizon S. Introducing the outbreak threshold in epidemiology. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A.J., Gregory V., Douglas A.R., Lin Y.P. The evolution of human influenza viruses. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 2001;356:1861–1870. doi: 10.1098/rstb.2001.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D.L., Aylward R.B., Wolff C. Dangerous pathogens in the laboratory: from smallpox to today's SARS setbacks and tomorrow's polio-free world. Lancet. 2004;363:1566–1568. doi: 10.1016/S0140-6736(04)16234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins University Johns. 2021. https://coronavirus.jhu.edu/map.html
- Hou W. Characterization of codon usage pattern in SARS-CoV-2. Virol. J. 2020;17:1–10. doi: 10.1186/s12985-020-01395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I.C., Bosch B.J., Li F., Li W., Lee K.H., Ghiran S., et al. SARS coronavirus, but not human coronavirus NL63, utilizes cathepsin L to infect ACE2-expressing cells. J. Biol. Chem. 2006;281:3198–3203. doi: 10.1074/jbc.M508381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K., Zhu H., Fan X., Wang J., Cheung C.L., Duan L., et al. Establishment and lineage replacement of H6 influenza viruses in domestic ducks in southern China. J. Virol. 2012;86:6075–6083. doi: 10.1128/JVI.06389-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hul V., Delaune D., Karlsson E.A., Hassanin A., Tey P.O., Baidaliuk A., et al. A novel SARS-CoV-2 related coronavirus in bats from Cambodia. BioRxiv. 2021 doi: 10.1101/2021.01.26.428212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacus S.M., Santamaria C., Sermi F., Spyratos S., Tarchi D., Vespe M. Human mobility and COVID-19 initial dynamics. Nonlinear Dynam. 2020;101:1901–1919. doi: 10.1007/s11071-020-05854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B.A., Xie X., Bailey A.L., Kalveram B., Lokugamage K.G., Muruato A., et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature. 2021;591:293–299. doi: 10.1038/s41586-021-03237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas O., Seifman R. Do we need a global virome project? The Lancet Global Health. 2019;7:e1314–e1316. doi: 10.1016/S2214-109X(19)30335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaina B. On the origin of SARS-CoV-2: did cell culture experiments lead to increased virulence of the progenitor virus for humans? Vivo. 2021;35:1313–1326. doi: 10.21873/invivo.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamitros T., Papadopoulou G., Bousali M., Mexias A., Tsiodras S., Mentis A. SARS-CoV-2 exhibits intra-host genomic plasticity and low-frequency polymorphic quasispecies. J. Clin. Virol. 2020;131:104585. doi: 10.1016/j.jcv.2020.104585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidar S., Kaplan M., Gamliel-Lazarovich A. ACE2 of the heart: from angiotensin I to angiotensin (1–7) Cardiovasc. Res. 2007;73:463–469. doi: 10.1016/j.cardiores.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Keita A.K., Koundouno F.R., Faye M., Düx A., Hinzmann J., Diallo H., et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature. 2021;597:539–543. doi: 10.1038/s41586-021-03901-9. [DOI] [PubMed] [Google Scholar]
- Krieg A.M., Yi A.K., Matson S., Waldschmidt T.J., Bishop G.A., Teasdale R., et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Kucharski A.J., Edmunds W.J. Case fatality rate for Ebola virus disease in west Africa. Lancet. 2014;384:1260. doi: 10.1016/S0140-6736(14)61706-2. [DOI] [PubMed] [Google Scholar]
- Lai A. Early phylogenetic estimate of the effective reproduction number of SARS-CoV-2. J. Med. Virol. 2020;92:675–679. doi: 10.1002/jmv.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Hughes T., Lee M.H., Field H., Rovie-Ryan J.J., Sitam F.T., et al. No evidence of coronaviruses or other potentially zoonotic viruses in Sunda pangolins (Manis javanica) entering the wildlife trade via Malaysia. EcoHealth. 2020;17:406–418. doi: 10.1007/s10393-020-01503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull. Entomol. Soc. Am. 1969;15:237–240. [Google Scholar]
- Li X., Wang W., Zhao X., Zai J., Zhao Q., Chaillon A. Transmission dynamics and evolutionary history of 2019-nCoV. J. Med. Virol. 2020;92:501–511. doi: 10.1002/jmv.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zai J., Wang X., Li Y. Potential of large “first generation” human-to-human transmission of 2019‐nCoV. J. Med. Virol. 2020;92:448–454. doi: 10.1002/jmv.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zai J., Zhao Q., Nie Q., Li Y., Foley B.T., Chaillon A. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J. Med. Virol. 2020;92:602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Chen W., Chen J.P. Viral metagenomics revealed Sendai virus and coronavirus 703 infection of Malayan pangolins (Manis javanica) Viruses. 2019;11:979. doi: 10.3390/v11110979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jiang J.Z., Wan X.F., Hua Y., Li L., Zhou J., et al. Are pangolins the intermediate host of the 2019 novel coronavirus (SARS-CoV-2)? PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean O.A., Lytras S., Weaver S., Singer J.B., Boni M.F., et al. Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery V.D., Yount B.L., Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncayo A.C., Fernandez Z., Ortiz D., Diallo M., Sall A., Hartman S., et al. Dengue emergence and adaptation to peridomestic mosquitoes. Emerg. Infect. Dis. 2004;10:1790. doi: 10.3201/eid1010.030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnink B.B.O., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., et al. Jumping back and forth: anthropozoonotic and zoonotic transmission of SARS-CoV-2 on mink farms. BioRxiv. 2020 doi: 10.1101/2020.09.01.277152. [DOI] [Google Scholar]
- Oudit G.Y., Crackower M.A., Backx P.H., Penninger J.M. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc. Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- Plante J.A., Liu Y., Liu J., Xia H., Johnson B.A., Lokugamage K.G., et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592:116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright R.K., Eby P., Hudson P.J., Smith I.L., Westcott D., Bryden W.L., et al. Ecological dynamics of emerging bat virus spillover. Proc. Biol. Sci. 2015;282:20142124. doi: 10.1098/rspb.2014.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power A.G., Mitchell C.E. Pathogen spillover in disease epidemics. Am. Nat. 2004;164:S79–S89. doi: 10.1086/424610. [DOI] [PubMed] [Google Scholar]
- Rahalkar M.C., Bahulikar R.A. Lethal pneumonia cases in Mojiang miners (2012) and the mineshaft could provide important clues to the origin of SARS-CoV-2. Frontiers in Public Health. 2020;8:638. doi: 10.3389/fpubh.2020.581569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D.A. Opinion: to stop the next pandemic, we need to unravel the origins of COVID-19. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:29246–29248. doi: 10.1073/pnas.2021133117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segreto R., Deigin Y. The genetic structure of SARS‐CoV‐2 does not rule out a laboratory origin: SARS‐COV‐2 chimeric structure and furin cleavage site might be the result of genetic manipulation. Bioessays. 2021;43:2000240. doi: 10.1002/bies.202000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segreto R., Deigin Y., McCairn K., Sousa A., Sirotkin D., Sirotkin K., et al. Should we discount the laboratory origin of COVID-19? Environ. Chem. Lett. 2021;19:2743–2757. doi: 10.1007/s10311-021-01211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheller M., Urry J. ‘The new mobilities paradigm’, environment and planning A: economy and space. 2006;38:207–226. [Google Scholar]
- Sigler T., Mahmuda S., Kimpton A., Loginova J., Wohland P., Charles-Edwards E., Corcoran J. The socio-spatial determinants of COVID-19 diffusion: the impact of globalisation, settlement characteristics and population. vGlobalization and health. 2021;17:1–14. doi: 10.1186/s12992-021-00707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin K., Sirotkin D. Might SARS‐CoV‐2 have arisen via serial passage through an animal host or cell culture? A potential explanation for much of the novel coronavirus' distinctive genome. Bioessays. 2020;42:2000091. doi: 10.1002/bies.202000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.D., Tu C.C., Zhang G.W., Wang S.Y., Zheng K., Lei L.C., et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2430–2435. doi: 10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steverding D. The spreading of parasites by human migratory activities. Virulence. 2020;11:1177–1191. doi: 10.1080/21505594.2020.1809963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Phadungsombat J., Nakayama E.E., Saito A., Egawa A., Sato T., et al. Genotype replacement of dengue virus type 3 and clade replacement of dengue virus type 2 genotype Cosmopolitan in Dhaka, Bangladesh in 2017. Infect. Genet. Evol. 2019;75:103977. doi: 10.1016/j.meegid.2019.103977. [DOI] [PubMed] [Google Scholar]
- Tang X., Wu C., Li X., Song Y., Yao X., Wu X., et al. On the origin and continuing evolution of SARS-CoV-2. National Science Review. 2020;7:1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Crameri G., Kong X., Chen J., Sun Y., Yu M., et al. Antibodies to SARS coronavirus in civets. Emerg. Infect. Dis. 2004;10:2244. doi: 10.3201/eid1012.040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowicz R.A., McClure C.P., Sakuntabhai A., Sall A.A., Kobinger G., Müller M.A., et al. Human adaptation of Ebola virus during the west african outbreak. Cell. 2016;167:1079–1087. doi: 10.1016/j.cell.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis N., Cardosa J., Hanley K.A., Holmes E.C., Weaver S.C. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat. Rev. Microbiol. 2011;9:532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosoughi S., Roy D., Aral S. The spread of true and false news online. Science. 2018;359:1146–1151. doi: 10.1126/science.aap9559. [DOI] [PubMed] [Google Scholar]
- Wacharapluesadee S., Tan C.W., Maneeorn P., Duengkae P., Zhu F., Joyjinda Y., et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021;12:1–9. doi: 10.1038/s41467-021-21240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., McKinnie S.M., Farhan M., Paul M., McDonald T., McLean B., et al. Angiotensin-converting enzyme 2 metabolizes and partially inactivates pyr-apelin-13 and apelin-17: physiological effects in the cardiovascular system. Hypertension. 2016;68:365–377. doi: 10.1161/HYPERTENSIONAHA.115.06892. [DOI] [PubMed] [Google Scholar]
- Watts J. SARS under control, but lab-safety questions remain. Lancet. 2004;363:1780. doi: 10.1016/S0140-6736(04)16344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R.G. Wet markets-a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363:234–236. doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J. Origins of SARS-CoV-1 and SARS-CoV-2 are often poorly explored in leading publications. Cladistics. 2020;36:374–379. doi: 10.1111/cla.12425. [DOI] [PubMed] [Google Scholar]
- Whittaker G.R. SARS-CoV-2 spike and its adaptable furin cleavage site. The Lancet. Microbe. 2021 doi: 10.1016/S2666-5247(21)00174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO China confirms SARS infection in another previously reported case. 2004. https://www.who.int/csr/don/2004_04_30/en/
- WHO Novel coronavirus (2019-nCoV) situation report-1. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf
- Woolhouse M.E., Adair K., Brierley L. RNA viruses: a case study of the biology of emerging infectious diseases. Microbiol. Spectr. 2013;1:1. doi: 10.1128/microbiolspec.OH-0001-2012. 1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Yang L., Yang F., Ren X., Jiang J., Dong J., et al. Novel henipa-like virus, Mojiang paramyxovirus, in rats, China, 2012. Emerg. Infect. Dis. 2014;20:1064. doi: 10.3201/eid2006.131022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Lan Q., Su S., Wang X., Xu W., Liu Z., et al. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Transduction and Targeted Therapy. 2020;5:1–3. doi: 10.1038/s41392-020-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Liu D.X. Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells. J. Virol. 2009;83:8744–8758. doi: 10.1128/JVI.00613-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Mammen M.P., Jr., Chinnawirotpisan P., Klungthong C., Rodpradit P., Monkongdee P., et al. Clade replacements in dengue virus serotypes 1 and 3 are associated with changing serotype prevalence. J. Virol. 2005;79:15123–15130. doi: 10.1128/JVI.79.24.15123-15130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cai Y., Xiao T., Lu J., Peng H., Sterling S.M., et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science. 2021;372:525–530. doi: 10.1126/science.abf2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Chen X., Hu T., Li J., Song H., Liu Y., et al. A novel bat coronavirus closely related to SARS-CoV-2 contains natural insertions at the S1/S2 cleavage site of the spike protein. Curr. Biol. 2020;30:2196–2203. doi: 10.1016/j.cub.2020.05.023. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Thao T.T.N., Hoffmann D., Taddeo A., Ebert N., Labroussaa F., et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature. 2021;592:122–127. doi: 10.1038/s41586-021-03361-1. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2019;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]