Abstract
The COVID-19 pandemic has highlighted structural inequalities and racism promoting health disparities among communities of color. Taking cardiovascular disease as an example, we provide a framework for multidisciplinary efforts leveraging translational and epidemiologic approaches to decode the biological impacts of inequalities and racism and develop targeted interventions that promote health equity.
The COVID-19 pandemic has highlighted structural inequalities and racism promoting health disparities among communities of color. Taking cardiovascular disease as an example, we provide a framework for multidisciplinary efforts leveraging translational and epidemiologic approaches to decode the biological impacts of inequalities and racism and develop targeted interventions that promote health equity.
Main text
Combining epidemiology and translational science to address long-standing health disparities
2020 brought into stark focus the entrenched health disparities burdening Black, Indigenous, Latinx, and Pacific Islander populations and the systemic injustices that underlie these disparities not only in the United States but around the world. In the US, coronavirus disease 2019 (COVID-19) and the psychological trauma associated with excessive brutality at the hands of law enforcement have disproportionately affected communities of color. The economic devastation as a result of the COVID-19 pandemic has fallen on the shoulders of the same populations, just as the Great Recession did more than a decade ago, with women of color disproportionately impacted by the economic effects of the pandemic. Racial discrimination and systemic racism that reduce economic opportunity and subvert access to quality healthcare, adequate education, safe and affordable housing, or a well-resourced and socially connected neighborhood environment have led directly to disproportionate COVID-19 mortality rates among Black, Latinx, Native American, and Pacific Islander communities (Cooper and Williams, 2020).
Unfortunately, while these health disparities may be more apparent during the COVID-19 pandemic, they are not new. Epidemiologic studies of national or international public health surveillance data, as well as data from racially/ethnically diverse population-based cohorts, highlight the existence of race-based health disparities in several healthcare areas. For example, US cohort data demonstrate that mortality rates for coronary artery disease among Black populations have declined more slowly than among white populations since the 1970s despite significant advances in interventional treatment approaches and therapeutics in cardiovascular medicine (Vaughan et al., 2020). In analyses comparing premature cardiovascular disease (CVD) mortality (before age 65) between 2000 to 2015 across racial/ethnic groups in the US, Black populations had the highest premature CVD mortality rates, followed by Native Americans/Alaskan Natives. Native Americans/Alaskan Natives also had significant increases in CVD mortality among those aged 25–49 years over the same time period (Vaughan et al., 2020). Across racial/ethnic groups, structural racism determines the impact of social determinants of health, which are the socioeconomic, social, and environmental factors that dictate where we live, work, and play and can ultimately lead to disparate health outcomes (Cooper and Williams, 2020).
Epidemiologic studies have provided great insights into the existence of race-based health disparities, such as those seen in CVD, but they have the potential to be even more enlightening. Longitudinal epidemiologic studies of diverse population-based cohorts in the US and around the globe can be leveraged to examine the biologic consequences of adversity or the “biology of adversity” (Albert, 2019). These epidemiologic studies can serve as a starting point for conducting translational studies that determine biologic mechanisms of action connecting social inequities and their related stressors to chronic disease development. The racial/ethnic and geographic diversity of population-based epidemiologic cohorts coupled with available phenotypic and genotypic data as well as biobanked samples make these studies excellent resources for more translational inquiries. Now is the time for us, as a scientific community, to address a fundamental question: how do we not only shed light on the existence of racial/ethnic health disparities but identify the wide-ranging biologic effects of structural racism and social inequities or adversity to develop targeted and tailored interventions?
There are numerous ways to combine mechanistic, translational science with epidemiologic studies, but we must also consider potential challenges to be overcome. For instance, the ability to develop interdisciplinary collaborations is critical, particularly when working with populations who have been most impacted by social inequities. Collaborations between academic investigators and community members in populations most impacted by health disparities may be difficult to develop but can engender trust for greater research engagement and sustainability. Moreover, funding opportunities that provide for the breadth of work needed to combine translational science with population-based studies are limited. Here, we propose a general framework for addressing race-based disparities that exist in chronic diseases using illustrative examples from CVD research. Through these examples, we explain how cross-cutting approaches using both epidemiology and translational science can reduce health disparities and lead to targeted interventions, and we illustrate ways of overcoming potential challenges in this type of work.
Discovery of biologic pathways linking psychosocial and environmental stress to chronic disease
Numerous epidemiologic studies have shown the relationships between chronic stress caused by psychosocial and environmental factors, including racism, crime, or limited safety, and a greater likelihood of developing chronic diseases (i.e., obesity, diabetes, hypertension, CVD) or having poor health outcomes. However, only a limited amount of research has focused on understanding the mechanisms by which the chronic stress of oppressive psychosocial and environmental conditions leads to chronic diseases like CVD. For instance, we need further insights into stress-signaling mechanisms, immune system regulation, gene expression, telomere attrition, and epigenetic regulation that are likely impacted by psychosocial and environmental adversities.
Elucidating the impact of chronic adversity on stress-signaling pathways
There are abundant opportunities to uncover the physiological signaling mechanisms that mediate the impacts of chronic stress due to adverse psychosocial and environmental conditions on chronic disease. Since the 1930s and 1940s, it has been known that the human body responds to stress by activating two distinct pathways (Russell and Lightman, 2019): the sympathetic nervous system (SNS), including the sympatho-adrenomedullary (SAM) axis, and the hypothalamic-pituitary-adrenal (HPA) axis with catecholamines and cortisol, respectively, as their biologic signaling molecules. Thus, past work has focused on the relationship between social or environmental exposures and catecholamines or cortisol as biomarkers of stress measured from blood samples in epidemiologic cohorts. As an example, it has been shown that chronic activation of both the SNS and HPA pathways can induce glucocorticoid receptor resistance (Miller et al., 2002), and a dysfunctional immune system accompanied by low-grade chronic inflammation can ultimately worsen CVD outcomes. However, cortisol and catecholamines may not be the only signaling molecules involved. Future studies should examine the relationship between psychosocial or environmental stressors and signaling molecules outside of cortisol or catecholamines, including metabolites or lipid subspecies. Wider investigation of the network of molecules altered and influenced by chronic psychosocial and environmental stress might allow us to determine common signaling pathways.
Examining the impact of psychosocial and environmental stress on immune cell physiology
Data suggest that specific immune cells are functionally impaired by chronic psychosocial and environmental stressors, but mechanistic insight is still lacking. In epidemiologic research examining associations between psychosocial or environmental stressors and immune cell changes, most studies focus on alterations in immune cell distribution and, potentially, characterization of immune cell-surface-receptor expression. For instance, social adversities impair monocytes by promoting a more pro-inflammatory profile, while T cells display shifts in their subsets. Natural killer (NK) cells can display changes in their overall proportions or receptor expression profile and lose cytotoxic function in individuals exposed to chronic stressors, like depression, loneliness, bereavement, childhood trauma, or violence (Baumer et al., 2020). Future translational work in epidemiologic studies could evaluate cellular function more closely as a shift in immune cell subtypes or surface markers does not always translate into a dysfunctional immune system. Additionally, scientists with basic/translational and epidemiologic expertise can work together in large cohort studies to examine signaling networks that connect psychosocial or environmental stressors to immune cell dysfunction and subsequent disease development.
Identifying the role of adversity in gene expression and DNA modification
Investigating the impact of chronic social and environmental inequities on genes and DNA modifications is also crucial. There is an important role for genomics when examining connections between adverse social and environmental conditions and health outcomes. The concept of gene-environment interactions has been investigated for some time (Goldenberg et al., 2013). For example, the conserved transcriptional response to adversity (CTRA) genes appear to be dysregulated in individuals exposed to various chronic psychosocial and environmental stressors (Cole, 2014). Future translational-epidemiologic studies could be designed to examine the impact of structural racism and accompanying psychosocial factors or environmental exposures on gene expression and epigenetic modifications related to CVD risk (e.g., diabetes or obesity) and CVD outcomes including incident myocardial infarction, stroke, or other thromboembolic events (Mancilla et al., 2020). In addition to understanding effects on gene expression and epigenetic modifications, a key translational marker for further investigation is telomere shortening, which has been linked to various diseases including CVD (Rentscher et al., 2020). For example, it has been reported that Black individuals have greater telomere length earlier in life when compared to white individuals; however, over the life-course, the rate by which telomere length decreases is accelerated in Black individuals (Baumer et al., 2020). Psychosocial and environmental factors, including racial discrimination and lower neighborhood socioeconomic status, have also been linked to telomere shortening (Powell-Wiley et al., 2019). Moreover, studies suggest that telomere dynamics might be an important mechanism by which the effects of psychosocial trauma or environmental stressors are passed intergenerationally (Haussmann and Heidinger, 2015). More detailed studies of telomere data in diverse epidemiologic cohorts with well-characterized psychosocial and environmental exposures could uncover exact biomarkers that mediate the relationships between specific stressors and telomere shortening.
Interventions, interdisciplinary collaborations, and reimagined funding to achieve health equity
Unfortunately, the mere identification of pathways altered by chronic psychosocial and environmental stress will not be sufficient in moving toward health equity. The scientific community needs to also identify potential interventions to mitigate the adverse effects on biologic pathways connecting psychosocial and environmental stressors to disease outcomes. For instance, various behavioral interventions (e.g., physical activity, dietary modifications) have been shown to be beneficial in at least partially decreasing systemic inflammation and restoring immune cell function (Seiler et al., 2020). Physical activity may serve as a particularly powerful intervention, as it has been shown to help individuals cope with stress, reduce inflammatory cytokines, like IL-6 and TNFα, and boost immune cell function independent of weight loss or reduction in fat mass. Future studies can also examine how digital health technology or internet-based tools can be used for delivery of behavioral interventions to vulnerable populations with limited access to clinical care (Bakken et al., 2019). Multi-level interventions that reduce individual-level barriers to behavioral change and target improvements to the environment for greater access to a healthy lifestyle can be most effective for reducing chronic disease risk. Such interventions should address social factors such as crime and neighborhood deprivation that limit healthy behaviors (Bakken et al., 2019) and incorporate biomarkers of stress, immune cell function, and genomic or epigenetic markers impacted by adversity. In addition, clinical trials developed to test behavioral interventions targeting chronic disease risk reduction should precisely measure social determinants of health so that translational studies can examine the impact of behavior change on relevant signaling pathways connecting adverse social and environmental conditions to disease risk.
Identifying the impact of social and environmental stressors on cellular phenotype and function is an immense challenge requiring interdisciplinary collaborations across multiple stakeholder groups. Interdisciplinary teams doing this type of work may include behavioral and social scientists, epidemiologists, geneticists, immunologists, physician-scientists, and/or vascular biologists. While it can be challenging to meet researchers outside of one’s area of scientific expertise, large professional organizations working to improve cardiovascular health, such as the American Heart Association or European Society of Cardiology, are examples of places where scientists from different disciplines can come together. One of the most difficult first steps in developing these interdisciplinary collaborations is finding a common language across varying scientific areas. Researchers must remember that despite different terminology within each area, the scientific method is common to us all. Collaborations with vulnerable populations at highest risk of being adversely affected by health disparities are also crucial. Well-established partnerships between academic researchers, community leaders, and community health advocates can engender trust in research study development and implementation, which ultimately improves the sustainability of research efforts with populations at high-risk for chronic diseases. These types of collaborations require specific efforts in engaging communities that may be distrustful of research and the healthcare system (Mensah et al., 2018). For instance, community-based participatory research principles encourage working with established community advisory boards to take the time to listen, learn, and understand a community’s needs prior to study design and implementation. Therefore, insights from collaborators with expertise in community engagement efforts may be critical.
For progress to happen, funding partners must recognize the need for research that combines epidemiologic and translational approaches to address the role of adverse social or environmental conditions in health disparities. Over the years, several funding agencies have started to allow for multi-disciplinary and innovative research projects, such as program project (P01) grants from NIH/NHLBI or the American Heart Association’s Strategically Focused Research Networks. Furthermore, various epidemiologic cohort studies accept proposals to allow further analyses of samples collected during the main data collection and ancillary projects, like the Multi-Ethnic Study of Atherosclerosis (MESA), Jackson Heart Study (JHS), or the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Additionally, the NHLBI offers access to psychosocial, environmental, biomarker, and genomic data from racially and ethnically diverse cohorts through BioData Catalyst (https://biodatacatalyst.nhlbi.nih.gov/), which provides digital workspaces with tools for analyses of large-scale datasets, such as the Trans-Omics for Precision Medicine (TOPMed) Program (https://www.nhlbiwgs.org/). In fact, the BioData Catalyst Fellows Program provides funding to early-career researchers, including graduate students, postdoctoral fellows, and junior faculty, for innovative data science projects that use BioData Catalyst resources and is another potential source of funding for studies that combine epidemiologic and translational data. In addition to existing grant mechanisms, public and private research funders must dismantle the funding silos between epidemiologic work and translational studies to expand funding opportunities for collaborative research. Funders must also acknowledge the importance of and time required to build trust with study participants who make this research possible by creating funding streams specifically for community engagement efforts in interdisciplinary studies addressing racial/ethnic health disparities.
Conclusions
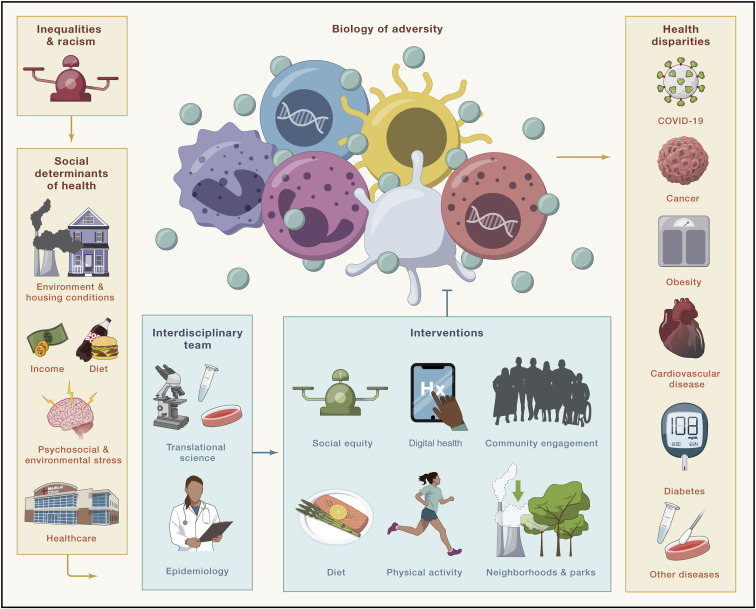
Thus, we put forward a framework to address the biology of adversity (Figure 1 ). We call on the scientific community to shift the research paradigm toward work connecting epidemiologic studies to translational science to identify (1) biologic mechanisms by which structural racism and social inequities lead to CVD and other diseases and (2) targets for effective, multi-level interventions to reduce health disparities. Most importantly, the research community must recognize community engagement as a fundamental process by which to foster and sustain trust in research participation from populations most impacted by disparate health outcomes. If we are going to truly address racial/ethnic health disparities that shorten the lives of far too many, the time is now for scientists to not only advocate for societal change to end structural inequalities in our spheres of influence but to also build the interdisciplinary research approaches that will delineate a path toward health equity.
Figure 1.
A framework to address the biology of adversity
Structural inequalities and racism cause Black, Indigenous, Latinx, and Pacific Islander populations to disproportionately experience adverse social determinants of health, including poor housing conditions, lower individual-level socioeconomic status, decreased access to healthy food (food deserts), higher likelihood of living in polluted and economically deprived neighborhoods, and/or decreased access to health care. The psychosocial and environmental stressors related to these social determinants of health alter biology within the human body, including the immune system. It will be of utmost importance for scientists to use interdisciplinary approaches, particularly in the context of community engagement, to further understand the biology of adversity in relation to diseases like CVD. Additionally, now is the time to investigate the impact of multi-level interventions on the biology of adversity to address health disparities and promote health equity.
Acknowledgments
Y.B. is funded by the Division of Intramural Research of the National Heart, Lung, and Blood Institute of the NIH. T.M.P.-W. is funded by the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the NIH and the Intramural Research Program of the National Institute on Minority Health and Health Disparities (ZIA HL006168, ZIA HL006225, ZIA HL006252, ZIA MD000010).
Web resources
BioData Catalyst, https://biodatacatalyst.nhlbi.nih.gov/
TOPMed, https://www.nhlbiwgs.org/
References
- Albert M.A. Ignored in Plain Sight. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005647. doi: 10.1161/CIRCOUTCOMES.119.005647. [DOI] [PubMed] [Google Scholar]
- Bakken S., Marden S., Arteaga S.S., Grossman L., Keselman A., Le P.T., Creber R.M., Powell-Wiley T.M., Schnall R., Tabor D., et al. Behavioral Interventions Using Consumer Information Technology as Tools to Advance Health Equity. Am. J. Public Health. 2019;109(S1):S79–S85. doi: 10.2105/AJPH.2018.304646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer Y., Farmer N., Premeaux T.A., Wallen G.R., Powell-Wiley T.M. Health Disparities in COVID-19: Addressing the Role of Social Determinants of Health in Immune System Dysfunction to Turn the Tide. Front. Public Health. 2020;8:559312. doi: 10.3389/fpubh.2020.559312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S.W. Human social genomics. PLoS Genet. 2014;10:e1004601. doi: 10.1371/journal.pgen.1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper L.A., Williams D.R. Excess Deaths From COVID-19, Community Bereavement, and Restorative Justice for Communities of Color. JAMA. 2020;324:1491–1492. doi: 10.1001/jama.2020.19567. [DOI] [PubMed] [Google Scholar]
- Goldenberg A.J., Hartmann C.D., Morello L., Brooks S., Colón-Zimmermann K., Marshall P.A. Gene-environment interactions and health inequalities: views of underserved communities. J. Community Genet. 2013;4:425–434. doi: 10.1007/s12687-013-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann M.F., Heidinger B.J. Telomere dynamics may link stress exposure and ageing across generations. Biol. Lett. 2015;11:20150396. doi: 10.1098/rsbl.2015.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla V.J., Peeri N.C., Silzer T., Basha R., Felini M., Jones H.P., Phillips N., Tao M.H., Thyagarajan S., Vishwanatha J.K. Understanding the Interplay Between Health Disparities and Epigenomics. Front. Genet. 2020;11:903. doi: 10.3389/fgene.2020.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah G.A., Cooper R.S., Siega-Riz A.M., Cooper L.A., Smith J.D., Brown C.H., Westfall J.M., Ofili E.O., Price L.N., Arteaga S., et al. Reducing Cardiovascular Disparities Through Community-Engaged Implementation Research: A National Heart, Lung, and Blood Institute Workshop Report. Circ. Res. 2018;122:213–230. doi: 10.1161/CIRCRESAHA.117.312243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Cohen S., Ritchey A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Powell-Wiley T.M., Gebreab S.Y., Claudel S.E., Ayers C., Andrews M.R., Adu-Brimpong J., Berrigan D., Davis S.K. The relationship between neighborhood socioeconomic deprivation and telomere length: The 1999-2002 National Health and Nutrition Examination Survey. SSM Popul. Health. 2019;10:100517. doi: 10.1016/j.ssmph.2019.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentscher K.E., Carroll J.E., Mitchell C. Psychosocial Stressors and Telomere Length: A Current Review of the Science. Annu. Rev. Public Health. 2020;41:223–245. doi: 10.1146/annurev-publhealth-040119-094239. [DOI] [PubMed] [Google Scholar]
- Russell G., Lightman S. The human stress response. Nat. Rev. Endocrinol. 2019;15:525–534. doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- Seiler A., Fagundes C.P., Christian L.M. In: Stress Challenges and Immunity in Space. Choukèr A., editor. Springer International Publishing; Cham: 2020. The Impact of Everyday Stressors on the Immune System and Health; pp. 71–92. [Google Scholar]
- Vaughan A.S., Schieb L., Casper M. Historic and recent trends in county-level coronary heart disease death rates by race, gender, and age group, United States, 1979-2017. PLoS ONE. 2020;15:e0235839. doi: 10.1371/journal.pone.0235839. [DOI] [PMC free article] [PubMed] [Google Scholar]