Abstract
The global obesity epidemic is well established, with increases in obesity prevalence for most countries since the 1980s. Obesity contributes directly to incident cardiovascular risk factors, including dyslipidemia, type 2 diabetes, hypertension, and sleep disorders. Obesity also leads to the development of cardiovascular disease and cardiovascular disease mortality independently of other cardiovascular risk factors. More recent data highlight abdominal obesity, as determined by waist circumference, as a cardiovascular disease risk marker that is independent of body mass index. There have also been significant advances in imaging modalities for characterizing body composition, including visceral adiposity. Studies that quantify fat depots, including ectopic fat, support excess visceral adiposity as an independent indicator of poor cardiovascular outcomes. Lifestyle modification and subsequent weight loss improve both metabolic syndrome and associated systemic inflammation and endothelial dysfunction. However, clinical trials of medical weight loss have not demonstrated a reduction in coronary artery disease rates. In contrast, prospective studies comparing patients undergoing bariatric surgery with nonsurgical patients with obesity have shown reduced coronary artery disease risk with surgery. In this statement, we summarize the impact of obesity on the diagnosis, clinical management, and outcomes of atherosclerotic cardiovascular disease, heart failure, and arrhythmias, especially sudden cardiac death and atrial fibrillation. In particular, we examine the influence of obesity on noninvasive and invasive diagnostic procedures for coronary artery disease. Moreover, we review the impact of obesity on cardiac function and outcomes related to heart failure with reduced and preserved ejection fraction. Finally, we describe the effects of lifestyle and surgical weight loss interventions on outcomes related to coronary artery disease, heart failure, and atrial fibrillation.
Keywords: AHA Scientific Statements, atrial fibrillation, cardiovascular diseases, coronary artery disease, death, sudden, heart, heart failure, obesity
Obesity is a multifactorial disease with a complex pathogenesis related to biological,1 psychosocial,2 socioeconomic,3 and environmental4,5 factors and heterogeneity in the pathways and mechanisms by which it leads to adverse health outcomes.6–8 The “2013 AHA [American Heart Association]/ACC [American College of Cardiology]/TOS [The Obesity Society] Guideline for the Management of Overweight and Obesity in Adults”7 uses the World Health Organization criteria9 to define overweight as a body mass index (BMI) ≥25 and <30 kg/m2 and obesity as a BMI ≥30 kg/m2.7 Although BMI is strongly correlated with percent body fat across populations, there are limitations in its predictive ability to estimate body fat for any given individual,10–12 with considerable variation by sex, age, and race/ethnicity.13,14 Country-specific cut points have been developed for Asian subpopulations such as in China, for which cut points of 24 kg/m2 for overweight and 28 kg/m2 for obesity are recommended.15 The GBD (Global Burden of Disease) Obesity Collaborators estimated that a total of 603.7 million adults had obesity, with obesity prevalence doubling between 1980 and 2015 in 73 countries and continuously increasing in most of the other countries.16 It is estimated that 39% to 49% of the world’s population (2.8–3.5 billion people) have overweight or obesity.17 In addition, the GBD investigators found an increase in the burden of elevated BMI, with high BMI accounting for 4.0 million deaths in 2015, more than two-thirds of which were caused by cardiovascular disease (CVD),16 even after accounting for smoking and ill health.18 Furthermore, a large proportion of both BMI-related deaths (41%) and BMI-related disability-adjusted life-years (34%) were caused by CVD among individuals with obesity.16 The most recent nationally representative US estimates for obesity prevalence based on the National Health and Nutrition Examination Survey reported a crude prevalence of 39.8% in 2015 to 2016, which is an increase from the crude prevalence of 37.9% in 2013 to 2014.19 The prevalence of class 3 obesity (BMI ≥40 kg/m2) is relatively high at an unadjusted prevalence of 7.7% in the total sample, with racial/ethnic and sex differences in class 3 obesity prevalence ranging from 5.5% in non-Hispanic White men to 16.9% in non-Hispanic Black women.19 Important contributors to racial/ethnic differences in obesity prevalence in the United States include racial/ethnic discrimination,20,21 weight stigmatization,22 and disproportionate experience of psychosocial stressors,23 as well as structural racism that promotes obesogenic environments and socioeconomic inequalities.24 Disparate exposure to psychosocial and environmental factors that contribute to both obesity and other CVD risk factors directly relates to disparities in CVD outcomes across racial/ethnic groups in the United States.25 Among pediatric populations, adolescent obesity is a global health epidemic; worldwide, marked increases in obesity prevalence among adolescents over the past 35 years ultimately contribute to CVD risk into adulthood.26 Moreover, the trends in obesity prevalence in the United States and around the world highlight the significant impact that obesity will continue to have on CVD incidence and prevalence globally. Therefore, the purpose of this scientific statement is to provide an update to the 2006 American Heart Association scientific statement “Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss.”27 Although obesity is linked to numerous diseases of the cardiovascular system, including stroke, venous thromboembolic disease, and pulmonary hypertension,28,29 this statement focuses on the impact of obesity on the pathophysiology, diagnosis, treatment, and clinical outcomes of atherosclerotic CVD, heart failure (HF), and arrhythmias, especially sudden cardiac death (SCD) and atrial fibrillation (AF). Before focusing on the relationship between obesity and these CVD outcomes, we review recent data linking abdominal obesity and visceral adiposity to CVD risk.
VISCERAL ADIPOSITY, LIVER FAT, AND CVD RISK
There is a strong correlation between overall obesity and abdominal obesity; however, some individuals may be classified as having overall obesity but not abdominal obesity. The converse may occur as well with abdominal obesity in the absence of overall obesity based on the BMI definition of obesity. The presence of cardiometabolic disease and CVD in those with “normal-weight obesity” leads to misclassification and under-diagnosis of CVD risk in clinical practice, particularly among patients who have excess fat but not obesity as classified by BMI.30–32 Thus, high waist circumference (WC) even in individuals with normal weight may unmask higher CVD risk because WC is an indicator of abdominal body fat, which is associated with cardiometabolic disease and CVD and is predictive of mortality.33,34 WC as a measure of abdominal obesity provides an indicator of body composition and adds critical information along with BMI.33 Several organizations and expert panels have recommended that WC measures be assessed along with BMI in clinical evaluations7,14,35,36 because increasing evidence supports visceral adiposity as a marker of cardiovascular risk.37–39
The development of imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) has been a remarkable advance in the study of human body composition and of its relationship with CVD risk.40,41 With these methods, cross-sectional images of the body at any level allow the quantification of areas or volumes of various adipose tissue and ectopic fat depots. An ectopic fat depot is generally considered a lipid deposit that is not physiologically stored in adipose tissues such as in the liver, the pancreas, the heart, and skeletal muscle.42 Cohort imaging studies have shown that all adipose and ectopic fat depots are correlated with one another.43,44 However, at any BMI or total adiposity level, there is considerable individual variation in the amount of subcutaneous versus intra-abdominal or visceral adipose tissue (VAT) in the abdominal cavity.42,45,46 There may be a 2- to 3-fold variation in the amount of VAT at any level of total or subcutaneous adiposity.42,43,47 Within overweight and obese categories individuals with low levels of VAT are characterized by a more favorable CVD risk profile sometimes referred to as metabolically healthy obesity.48,49 Recent data suggest that metabolically healthy obesity may be a transient phenotype for the majority of the population with the duration of metabolically healthy obesity differing by race/ethnicity and sex.49 When those with metabolically healthy obesity are compared with patients with excess VAT those with excess VAT represent a subgroup of individuals at highest CVD risk regardless of BMI.42,46,50 Studies that have examined the relationships between VAT and cardiovascular outcomes have also confirmed that VAT serves as a clear health hazard.51–53 Imaging studies have shown that a frequent partner of visceral obesity is higher liver fat accumulation,54,55 for which nonalcoholic fatty liver disease is a clinical manifestation.56 Overall excess liver fat has generally been associated with the same alterations in cardiovascular risk factors as visceral obesity.56,57 However the question remains as to whether excess liver fat in isolation is associated with higher cardiovascular risk. Mendelian randomization studies that have measured genetic variants predisposing to higher liver fat have not been able to show associations with CVD.58 Excess liver fat is likely to play a major role in the pathogenesis of the dysmetabolic state that can be found in individuals with overweight/obesity.59 From a clinical standpoint, health care practitioners should be aware of the fact that the most prevalent form of non-alcoholic fatty liver disease is found among individuals with excess VAT.60,61 Thus, from a prevention standpoint, reducing visceral obesity by promoting improved lifestyle habits is key to addressing the current epidemic of nonalcoholic fatty liver disease.
ECTOPIC FAT DEPOTS AND CVD RISK
Other ectopic fat depots of interest are pericardial and epicardial adipose tissues. In the literature, the two are often used interchangeably but have distinct anatomic locations and functions that should be clearly defined.62 Pericardial fat can be imaged with CT and consists of the total fat content within the pericardial sac63 below the superior extent of the left64,65 or right66 main coronary artery. This depot has been associated with higher BMI, traditional cardiovascular risk factors, and more atherogenic lipoprotein particles.64 Pericardial fat correlates with CVD after adjustment for age, sex, BMI, and WC but not after adjustment for cardiovascular risk factors.63 In the Multi-Ethnic Study of Atherosclerosis, pericardial fat was associated with a higher risk of allcause CVD, hard atherosclerotic CVD, and HF.65 Adding pericardial fat to clinical parameters and coronary artery calcium (CAC) scores improved risk discrimination for these outcomes. In the Rancho Bernardo Study, allcause mortality risk was higher by 34% per 1-SD increment in pericardial fat after adjustment for age, sex, lifestyle variables, lipids, glucose, and adipocytokines.66 However, this study did not show that pericardial fat was predictive of incident CVD beyond traditional risk factors; additional studies must be done to assess this relationship. Epicardial adipose tissue represents visceral fat between the outer wall of the myocardium and the visceral layer of the pericardium. This adipose tissue originates from embryonic brown adipose tissue and releases cytokines and chemokines into the vasculature.67 It has been associated with overall cardiovascular health score68 and arterial stiffness in patients with CVD and type 2 diabetes.69 Studies have shown that epicardial adipose tissue thickness is significantly correlated with WC, blood pressure, markers of insulin resistance, and dyslipidemia,68,70 suggesting that this adipose tissue depot could be considered highly insulin resistant and may be an indicator of cardiovascular risk. In addition, epicardial fat thickness has been shown to be associated with sleep apnea severity in women independently of BMI,71 and sleep apnea is associated with higher CVD risk.72 This fat depot can be mobilized, with reductions observed after continuous positive airway pressure treatment.71 However, short-term (8–12 weeks) continuous positive airway pressure use in patients with sleep apnea does not appear to affect VAT.73,74 One must question, then, whether thicker epicardial fat is a predictor or a consequence of sleep-disordered breathing.
IMPACT OF LIFESTYLE INTERVENTIONS ON ECTOPIC/PERICARDIAL FAT
Given the associations of ectopic fat with CVD risk, numerous interventions to reduce these adipose tissue depots have been investigated. Although a number of pharmacological agents exist to reduce body fat, lifestyle interventions such as the Diabetes Prevention Program may be as effective as, if not more effective than, medications.75,76 Randomized studies in both men and women across varying ages have found that exercise, usually 3 to 5 sessions per week for 12 to 52 weeks, reduces VAT compared with a nonexercise control group.77–79 Well-controlled studies have demonstrated that exercise can reduce VAT even in the absence of weight loss,77–79 and a meta-analysis reported exercise to result in a 6.1% loss of VAT in the absence of weight loss.80 Loss of VAT in the absence of weight loss may relate to increases in fat-free mass.81 However, not all studies have demonstrated a significant reduction of VAT compared with control.82,83 The most beneficial exercise interventions appear to be aerobic in nature; data on the reductions of VAT by only resistance training are equivocal.84,85 Similarly, reductions in VAT with high-intensity exercise have not been consistently superior to those with moderate-intensity exercise,86,87 and even 3 months of walking resulted in greater VAT reductions compared with control.88 Meeting the current recommendations for physical activity of 150 min/wk may be sufficient to reduce VAT, with no further reductions with additional activity.89 Interventions targeting weight loss through caloric restriction have also demonstrated effectiveness in reducing VAT.90,91 Compared with dietary interventions, exercise interventions have demonstrated greater VAT reductions in most studies92,93 and in a meta-analysis80 but not in all studies.90,91,94 Combined interventions carried out in the Diabetes Prevention Program and the Look AHEAD Trial (Action for Health in Diabetes) have reported greater VAT reductions compared with control groups.75,95 Exercise interventions also appear to be effective at reducing hepatic96,97 and epicardial and pericardial fat.98,99 However, a meta-analysis did not find a significant reduction in epicardial fat with exercise.100 Caloric restriction has been demonstrated to reduce hepatic101 and epicardial and pericardial fat.100,102
OTHER ADIPOSITY AND BODY COMPOSITION MEASURES
Although WC is meaningful on its own, the ratio of WC to height, which takes body size into account, may be a better predictor of CVD and may be considered a measure of adiposity.103,104 Moreover, waist-to-hip ratio (WHR) has been shown to predict cardiovascular mortality independently of BMI. According to data from the National Health and Nutrition Examination Survey, those in the US population with a WHR indicative of central obesity had a higher risk of cardiovascular mortality compared with those with the same BMI but without central adiposity.34,105 Nonanthropometric measures based on CT, MRI, ultrasonography, dual-energy x-ray absorptiometry, air displacement plethysmography, and bioelectric impedance analysis can be used to quantify body composition. Details on how these body composition measures relate to cardiovascular risk have been summarized in the American Heart Association scientific statement “Identification of Obesity and Cardiovascular Risk in Ethnically and Racially Diverse Populations.”14
Thus, visceral adiposity as measured by WC, WHR, or detailed imaging methods has been shown to be a risk factor for CVD independently of BMI. Lifestyle interventions, particularly physical activity interventions or interventions combining dietary changes and physical activity, have been shown to reduce VAT and ectopic fat, in some cases independently of weight loss.
PATHOPHYSIOLOGY OF CORONARY ARTERY DISEASE IN OBESITY
Atherosclerosis and Coronary Artery Disease
The atherosclerotic process is initiated in childhood, with ingestion of cholesterol esters by macrophage foam cells and their deposition in vessel walls resulting in thickening of the arterial intima. Further lipid accumulation leads to the development of fatty streaks,106 which appear to be nearly ubiquitously present in young adults.107 Obesity accelerates these early atherosclerotic changes through several mechanisms, including insulin resistance and inflammation.108 Obesity and several related downstream metabolic cardiovascular risk factors, including elevated blood pressure, dyslipidemia, and hyperglycemia, have been linked to the extent of atherosclerotic disease in autopsy studies of children and young adults.109,110 However, obesity is associated with overt atherosclerotic lesions even after accounting for the impact of these metabolic cardiovascular risk factors. The association of obesity with raised atherosclerotic lesions among men in the Pathobiological Determinants of Atherosclerosis in Youth study was present only for those with a thick abdominal panniculus, indicating the fundamental role of central adiposity in the development of atherosclerotic disease.111 Visceral adiposity promotes systemic and vascular inflammation, which is fundamental to all aspects of the atherosclerotic process, from fatty streak development to atherothrombosis.112,113 Inflammation induced by obesity increases the likelihood of low-density lipoprotein oxidation,114 which in turn promotes atherogenesis. Insulin resistance is associated with dyslipidemia (high triglycerides; low high-density lipoprotein cholesterol; small, dense low-density lipoprotein particles) and metabolic syndrome (multiplex CVD risk factor including abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, insulin resistance with or without glucose intolerance, and proinflammatory and prothrombotic states), which are linked to atherosclerosis.115 Endothelial dysfunction in obesity, principally caused by diminished bioavailability of nitric oxide in the setting of inflammation and oxidative stress,116 is also fundamental to atherosclerosis progression. Carotid intima-media thickness as an early marker of atherosclerosis in young adults is associated with obesity,117 particularly chronically elevated weight from youth through adulthood.118
Incident Coronary Artery Disease Events
Several prospective epidemiological studies demonstrate that obesity is associated with higher risk of incident coronary artery disease (CAD).119–122 A meta-analysis of >300 000 adults with 18 000 CAD events demonstrated that BMI in the overweight and obese ranges was associated with elevated CAD risk.123 Of clinical importance, at each level of BMI, higher measures of central adiposity, including WC and WHR, were associated with a greater risk of CAD and cardiovascular mortality, including among those with normal weight as assessed by BMI.31,34,105,124,125 The degree and duration of obesity, as measured by total cumulative exposure to excess overall and abdominal adiposity and expressed as excess BMI-years and WC-years, have been shown to be stronger predictors of CAD events beyond BMI or WC alone.126 There are conflicting results on the extent to which the association of obesity with CAD is independent of the metabolic cardiovascular risk factors linked to excess weight. Some large prospective analyses have indicated that the link between obesity and CAD is mediated largely by hypertension, dyslipidemia, diabetes, and other comorbidities,127 whereas other prospective studies suggest a significant residual CAD risk in obesity even after accounting for these risk factors.120,128 Similarly, some studies have indicated that obesity without metabolic syndrome is not associated with incident myocardial infarction,129 in contrast to other studies.130,131 A meta-analysis of 21 studies including 1.8 million individuals suggested that approximately half of the associations of overweight and obesity with CAD are explained by levels of blood pressure, cholesterol, and glucose.132 However, this may be an underestimation resulting from residual confounding from cardiovascular risk factors assessed at a single time point or not measured directly in some studies. Production of adipocytokines, oxidative stress, and a prothrombotic state in individuals with metabolic syndrome may contribute to CAD risk beyond that explained by routinely measured cardiovascular risk factors.115 Ectopic fat deposition, including within the pericardial and epicardial spaces, may further contribute to the burden of coronary atherosclerosis.133 A pathological study in humans reported that part of the left anterior descending artery with an intramyocardial course was in perfect condition (ie, without any intimal atherosclerotic lesion), which was in contrast to the epicardial segment of the same artery in which atherosclerosis was documented.134 Likewise, in hypercholesterolemic rabbits, epicardial coronary arteries surrounded by adipose tissue develop atherosclerosis, whereas the intramyocardial segments of the same arteries remain unaltered.135 Thus, local production of adipocytokines by epicardial fat may modulate blood vessel biology through paracrine signaling or through vasa vasorum.
Obesity and Microvascular Disease
In addition to the effects of excess adiposity on epicardial coronary vessels described above, obesity is linked to abnormalities in the coronary microvasculature, a key regulator of coronary blood flow.136,137 Coronary microvascular disease often coexists with and compounds the effects of obstructive or nonobstructive CAD on myocardial ischemia and CAD events.138,139 Coronary microvascular disease is pathophysiologically linked to endothelial dysfunction and possibly to small vessel remodeling; this microvascular disease is independently associated with higher BMI140 and provides independent prognostic information on cardiovascular risk among those with obesity.141 In prospective studies, weight loss via bariatric surgery has been associated with improvements in coronary microvascular function.142
DIAGNOSIS OF CAD IN OBESITY
CAD assessment can be challenging in patients with obesity. The baseline ECG may be influenced by obesity, and patients with obesity have impaired maximal exercise testing capacity (dyspnea, mechanical limitations, left ventricular [LV] diastolic dysfunction [LVDD]).27 Thus, other modalities such as nuclear medicine approaches, stress echocardiography, or pharmacological stress and stress cardiac MRI may be of interest in the evaluation of CAD in this population. CAC screening and CT coronary angiography can be used in diagnosing CAD, but ultimately, coronary angiography remains the gold standard test for identifying the presence and extent of CAD. Here, we review specific considerations for the use of noninvasive and invasive modalities to assess CAD in patients with obesity (summarized in Table 1).
Table 1.
Considerations for Use of Noninvasive and Invasive Diagnostic Tools in Patients With Obesity
| Diagnostic tool | Strengths | Limitations |
|---|---|---|
| Noninvasive diagnostic tools | ||
| ECG | Widely available, cheap | Low sensitivity and specificity |
| Treadmill stress test | Widely available Functional testing | Patients may stop because of symptoms unrelated to CVD |
| SPECT | Available, good precision | Irradiation, technical limitation because of body size Residual uncorrected attenuation |
| PET (rubidium) | Nuclear imaging technique of choice for patients with obesity | Less radiation exposure than SPECT but technical limitations because of body size |
| Stress echocardiography | Widely available, valid technique in patients with obesity Radiation free Has no weight limits Functional testing | Highly operator dependent Can be limited because of poor acoustic windows related to pulmonary disease, breast size, obesity, and respiratory motion |
| Stress cardiac MRI | Accurate assessment of the complex cardiac effect of chronic pressure overload and high cardiac output in patients with obesity | Table weight limit WC may limit access depending on bore diameter Length of examination Claustrophobia |
| CT calcium scan | Inexpensive and reproducible technique to determine the presence and extent of CAC | Obesity may limit the diagnostic accuracy and value of cardiac CT calcium scan Gantry/bore diameter limitations |
| Invasive diagnostic tools | ||
| Cardiac CT coronary angiography | Sensitivity and negative predictive values are high in patients with obesity | Image quality degrades as BMI increases Degradation is related to an increase in background noise, subsequent reduced signal-to-noise ratio, and low vessel opacification |
| Intravascular ultrasound | Allows in vivo assessment of plaque burden, plaque morphology (ie, stages of plaque development, high-risk plaque features) | Invasive technique |
BMI indicates body mass index; CAC, coronary artery calcium; CT, computed tomography; CVD, cardiovascular disease; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single photon emission computed tomography; and WC, waist circumference.
Noninvasive CAD Assessment in Obesity
Electrocardiographic Assessment
Obesity has the potential to affect the ECG in several ways: displacing the heart by elevating the diaphragm in the supine position, increasing the cardiac workload, and increasing the distance between the heart and the recording electrodes.27 Several electrocardiographic changes are associated with obesity (Table 2). More frequent ST-segment depression is seen in patients with overweight and CAD,143 and insulin concentration may be related to the development of the ST-segment depression over time.144 Multiple electrocardiographic criteria for LV hypertrophy (LVH) are present more regularly in patients with severe obesity compared with individuals with normal weight but less frequently than would be expected on the basis of the high prevalence of echocardiographic criteria for LVH.27 Therefore, LVH is probably underdiagnosed according to the usual ECG criteria in individuals with severe obesity. In LVH and in obesity, the heart is oriented more horizontally in the mediastinum, which may explain the usefulness of the R wave in AVL.27 Thus, it has been proposed that for men of all ages, LVH is considered present on the basis of the QRS voltage alone when the amplitudes of the R wave in lead AVL and the S wave in lead V3 are >35 mm. For women, the same criteria were set at >25 mm.27 When electrocardiographic voltage criteria were compared with LV mass estimated by echocardiography, a sensitivity of 49%, specificity of 93%, and overall accuracy of 76% were reported.27 These percentages representing the Cornell score are higher than other widely used criteria such as the Sokolow-Lyon voltage or Romhilt-Estes score.
Table 2.
Electrocardiographic Changes That May Occur in Individuals With Obesity
| Clinically significant |
|---|
| ↑ Heart rate |
| ↑ QRS interval |
| ↑ QTc interval |
| False-positive criteria for inferior myocardial infarction |
| Less clinically significant |
| ↑ PR interval |
| ↑ QRS voltage |
| ↑ QT dispersion |
| ↑ SAECG (late potentials) |
| ↑ ST-T abnormalities |
| ↑ ST-segment depression |
| Left axis deviation |
| Flattening of the T wave (inferolateral leads) |
| Left atrial abnormalities |
SAECG indicates signal-averaged ECG.
Adapted from Poirier et al.27 Copyright © 2006, American Heart Association, Inc.
Treadmill Stress Test
Standard treadmill stress test performance is limited in patients with obesity by several factors. Electrocardiographic abnormalities seen with obesity might limit accurate interpretation, and aerobic capacity can be diminished because of pulmonary dysfunction, orthopedic limitations, and LVDD.145 Many patients with obesity fail to achieve 80% to 85% of the age-predicted heart rate needed for diagnostically valid results.146,147 Chronotropic competence can be reduced in obesity, with a prior study showing that peak heart rate, heart rate recovery, and chronotropic index are lower in patients with obesity, regardless of fitness level.146 Higher systolic and diastolic blood pressures also may be observed during the exercise stress test in patients with obesity.148 However, standard Bruce and modified Ramp protocols achieve valid results in most patients with obesity, with patients terminating the test because of fatigue, leg pain, or dyspnea.149
Single Photon Emission CT
Single photon emission CT can be used with exercise, vasodilator (dipyridamole), and dobutamine stress. Two-day protocols with larger tracer doses, which are weight based, are recommended in patients who weigh 250 to 350 lb (113–160 kg). Attenuation artifacts, most commonly resulting from attenuation by the diaphragm or breast, are common in obesity. Tissue attenuation decreases single photon emission CT image quality and thus diagnostic accuracy. Improved cameras, software, and CT-based attenuation correction algorithms are techniques that enable a reduction of attenuation artifacts. Technetium sestamibi is the marker of choice in patients with obesity because of greater energy emission, which generates better images.150 Disadvantages include the limitations of relative perfusion imaging with reduced ability to detect triple-vessel or left main stem disease and residual uncorrected attenuation. Weight-based limitations might occur at 350 lb (160 kg), which might necessitate planar imaging. Newer and more sensitive cameras might eliminate some of these issues, but their use still leads to table weight and size issues because proper positioning of the patient is required with this system. Thus, single photon emission CT is generally avoided when the patient’s BMI is >35 kg/m2 because of the above limitations, and positron emission tomography (PET) is recommended in those cases when looking for myocardial ischemia and an imaging modality is indicated.
PET Rubidium
PET rubidium has a 91% sensitivity and 89% specificity; is faster than sestamibi single photon emission CT; and produces less radiation exposure, better-quality images, correction for attenuation, a greater degree of diagnostic precision, and a reduced need for invasive examinations. Normal PET myocardial perfusion imaging is associated with very low cardiac death rates in all categories of obesity.151 PET allows the ability to quantify absolute coronary blood flow, adding to the diagnostic and prognostic capabilities beyond relative perfusion imaging, especially in the detection of triplevessel and left main stem disease. Therefore, PET rubidium is the nuclear imaging technique of choice for patients with obesity.
Stress Echocardiography
Despite some limitations, exercise stress echocardiography is a valid technique in patients with obesity.152,153 Stress echocardiography is highly feasible in most cases for patients with obesity through either physiological stress (treadmill exercise) or pharmacological stress (dobutamine). It is widely available, low cost, and radiation free and has no weight limits. However, stress echocardiography is highly operator dependent and can be limited in the presence of poor acoustic windows related to pulmonary disease, breast size, obesity, and respiratory motion.154 Excellent 1-year outcomes have been shown in patients with obesity and normal stress echocardiography.155 Contrast injection can be used to improve the number of heart segments visualized.155 In a prospective study of patients with overweight and obesity who underwent coronary angiography and dobutamine stress echocardiography with and without contrast, contrasted images improved sensitivity and specificity (82% versus 70% and 78% versus 67% with and without contrast, respectively).154 Retrospectively, Lerakis et al156 assessed dobutamine stress echocardiography as a preoperative screen for CAD in a bariatric surgery population. Adequate imaging was obtained in 97% of patients in light of intravenous echocardiographic contrast use in 72% of cases. Indeed, higher rates of contrast use have been reported in patients with severe obesity who undergo transthoracic dobutamine echocardiography.152,153,157,158 If severe limitations exist, transesophageal echocardiography with dobutamine might be useful.159,160
Stress Cardiac MRI
Stress cardiac MRI is a technique that allows the assessment of perfusion defects, regional wall motion abnormalities, and LV ejection fraction and the detection of scar with the use of gadolinium. It allows accurate assessment of the complex cardiac effect of chronic pressure overload and high cardiac output in patients with obesity.153,155 Stress cardiac MRI and PET are likely the diagnostic techniques least affected by obesity. Newer-generation MRIs have larger bore sizes (70 instead of 60 cm) and greater magnet strengths, which have accommodated patients with obesity more easily and led to improved image quality. The usefulness of stress cardiac MRI was studied in 285 participants with an average BMI of 34 kg/m2 who underwent testing and long-term follow-up. Of the patients imaged, 89% had diagnostic image quality.161 The presence of ischemia predicted adverse events at 5 years of follow-up, regardless of whether scar was present. Lack of inducible ischemia is associated with a low annual major adverse coronary events (MACEs) rate of 0.3% at 2 years in patients with obesity.161 Table weight limit, bore diameter, and length can be significant limitations, and some centers might not be able to accommodate patients with more severe obesity despite the benefits of diagnosis. Besides the weight limit of 335 lb (152 kg) that comes with MRI tables, higher WC and claustrophobia might also limit the feasibility of MRI in patients with severe obesity.155
CT Calcium Scan
Obesity is associated with elevated CAC, a marker of coronary atherosclerosis that is predictive of cardiovascular events162,163 and more rapid CAC progression.164 The presence of high CAC score offers an inexpensive and reproducible technique to determine the presence and extent of calcified coronary artery plaque. Despite advances in CT scanners, obesity may limit the diagnostic accuracy and value of cardiac CT calcium scan. CT equipment has table weight limits of 350 to 450 lb (160–204 kg) and is also limited by gantry/bore diameter. Studies suggest that WC and WHR provide more useful prognostic information than BMI on the likelihood of elevated CAC,165,166 again indicating the importance of abdominal obesity in the pathophysiology of atherosclerosis.
Cardiac CT Coronary Angiography
CT coronary angiography is emerging as an alternative approach for the quantification of both coronary calcified and noncalcified plaque. This approach may be particularly useful in specific subsets of symptomatic patients with obesity, unknown CVD, and equivocal or uninterpretable stress tests or in cases when a discrepancy exists between clinical presentation and stress test results. CAC score allows risk stratification and plaque burden assessment, whereas CT coronary angiography allows evaluation of luminal stenosis and plaque characterization and quantification. Registry data showed that those with obesity who were symptomatic were more likely than patients without obesity to have any CVD at CT coronary angiography.167 Imai et al168 studied 553 patients who underwent serial CT coronary angiography and observed that the risk of noncalcified plaques became higher as abdominal visceral adiposity was higher, with the highest quartile conferring the greatest risk, regardless of underlying cardiovascular risk factors. One major challenge with CT coronary angiography is that image quality degrades as BMI increases; this degradation is related to an increase in background noise and subsequent reduced signal-to-noise ratio. In addition, low vessel opacification may occur in patients with overweight or obesity because of differences in the distribution of blood volume in peripheral venous and central circulation when contrast is injected,169 which ultimately leads to a higher rate of nonevaluable segments in patients with overweight or obesity. Nevertheless, sensitivity and negative predictive values are invariably high even in patients with obesity.
Invasive Evaluation of CAD in Obesity
Coronary Angiography
Individuals with obesity have several limitations when undergoing evaluation in the catheterization laboratory. Potential technical difficulties include suboptimal radiographic visualization that may limit detection of angiographic results and may result in a greater likelihood of complications. Vascular access may be laborious; radial access is preferred in this population because of fewer vascular complications, especially bleeding, earlier ambulation, and a shorter hospital stay.170,171 When cardiac catheterization is pursued for diagnostic or therapeutic purposes for those with severe obesity, radial artery access has been associated with a 3 times lower rate of complications than a transfemoral approach.170 The radial approach is particularly useful for patients with limited capability to tolerate supine positions because upright mobilization can be immediate after the procedure. If the femoral approach is used, vascular closure devices should be used to accelerate ambulation in patients with obesity.172 The fluoroscopy needed to achieve adequate x-ray penetration and sufficient image quality may also result in higher radiation exposure to both patients with obesity and staff.173 In addition to issues with vascular access and radiographic imaging, the engineering parameters and physical limitations of the catheterization table and its supporting structures may limit patients’ ability to undergo clinically indicated coronary angiography.
Intravascular Ultrasound
Several intravascular imaging techniques such as intravascular ultrasound, virtual histology intravascular ultrasound, and optical coherence tomography allow in vivo assessment of plaque burden, plaque morphology (ie, stages of plaque development, high-risk plaque features), and response to therapy, particularly for higher-risk patients. In a large retrospective database of 3158 patients designed to evaluate plaque characteristics, 32% of patients with BMI >25 kg/m2 demonstrated evidence of high-risk plaque features (positive remodeling, spotty calcification, and low-attenuation plaque), and BMI itself was an independent predictor of future acute coronary syndrome events.174 Abdominal visceral adiposity independently predicted the presence and extent of noncalcified coronary plaque that also contained multiple features of plaque vulnerability.175 Thus, numerous tests can diagnose atherosclerosis, myocardial ischemia, or both. The appropriate choice of test to assess CVD depends on local expertise, the relative strengths and weaknesses of each modality, and individual patient characteristics that contribute to the pretest likelihood of CVD and the risk/benefit ratio of using a given modality.
CLINICAL MANAGEMENT AND TREATMENT OF CAD IN OBESITY
The Obesity Paradox
Obesity is a strong risk factor for the development of CVD because patients with obesity experience CVD events at an earlier age, live with CVD for a greater proportion of their lifetime, and have a shorter average life span than individuals with normal weight.176 However, in patients with overweight or obesity, particularly among those who develop symptomatic CVD, BMI and other parameters of body composition are not consistent cardiovascular risk factors for adverse short-term CVD outcomes (≤10 years).177–179 This reversal of traditional epidemiology, called the obesity paradox, is now well documented in numerous studies, particularly in diverse populations who have overweight or class 1 obesity. The underlying cause of the obesity paradox is unclear. The paradox may relate to potential lead time bias that occurs when patients with overweight or obesity develop CVD earlier in their lifetime or are tested earlier for CVD than patients with normal weight, resulting in earlier diagnoses and treatment and confounding differences in outcomes. In addition, differences in cardiorespiratory fitness may explain more favorable CVD outcomes regardless of BMI. Finally, some propose that a “lean paradox” may exist in which low body fat percentage and low BMI with less reserve to avoid cardiac cachexia may be the more pertinent predictors of poor CVD outcomes.177–179
Weight Loss and CAD Risk
Lifestyle modification, with associated weight loss, improves both the diagnostic components of metabolic syndrome and associated pathophysiologic abnormalities such as systemic inflammation and endothelial dysfunction.27,180,181 Interventional trials of medical weight loss have not demonstrated a clear reduction in CAD rates.182–184 In contrast, reduced CAD risk has been demonstrated in prospective studies comparing patients undergoing bariatric surgery with nonsurgical patients with obesity, with the Swedish Obesity Study finding significantly lower rates of fatal and nonfatal cardiovascular events in those undergoing bariatric surgery.185 The reason for the disparate results of medical and surgical weight loss studies is likely the degree of weight loss achieved (5–10 kg with medical weight loss versus 10–40 kg with surgery) and the risk factor reduction seen with bariatric surgery.186 Modest short-term weight loss may not be sufficient to fully overcome the deleterious effects of long-term obesity on the vasculature.
Benefits of Weight Loss on CAD
The general goals of weight loss and management are, at a minimum, to prevent further weight gain, to reduce body weight, and to maintain a lower body weight over the long term. Patients should have their BMI and WC measured not only for the initial assessment of the degree of overweight and obesity but also as a guide to the efficacy of weight loss treatment.187 The Mediterranean diet decreases MACEs in patients with high cardiovascular risk and is an interesting option for this population.188 Future studies should determine how much adherence to a Mediterranean dietary pattern is needed or how best to personalize diets on the basis of genetic or other objective factors for CVD risk reduction in obesity.189 Moreover, no studies have shown a clear reduction of CVD or mortality with weight loss through lifestyle modification. Look AHEAD, one of the largest clinical trials of lifestyle modification for obesity treatment in patients with type 2 diabetes, failed to show a significant reduction of MACEs or cardiovascular mortality after 9.6 years, which may be related to the limited differential weight loss between the intervention and control groups by the end of the trial.182 Post hoc analyses of Look AHEAD showed that participants who lost ≥10% of their body weight had significant reductions in cardiovascular events.190 In addition, physical activity, particularly aerobic exercise, is associated with improved insulin sensitivity, endothelial function, and reduction in proinflammatory markers independently of weight loss,189 but more data are needed in populations with CVD. Liraglutide has been shown to reduce MACEs and cardiovascular death in the LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results), but this was in a population with type 2 diabetes who were using the 1.8-mg dosing specified for diabetes treatment.191 Lorcaserin appeared to be safe in terms of CVD, but no benefits in cardiovascular mortality or CVD events were demonstrated (Lorcaserin was removed from the US market by the US Food and Drug Administration in 2020).192 An interim analysis of the LIGHT trial (Cardiovascular Outcomes Study of Naltrexone SR/Bupropion SR in Overweight and Obese Subjects With Cardiovascular Risk Factors) showed that naltrexone-bupropion has cardiovascular safety; however, no solid conclusion can be drawn from this trial given that it was terminated early because of public release of the interim data by the sponsor.193 A retrospective study of 20 235 surgical and nonsurgical patients194 showed that bariatric surgery is associated with a lower incidence of macrovascular disease (first occurrence of CAD or cerebrovascular events) driven mainly by a lower incidence of CAD (acute myocardial infarction, unstable angina, percutaneous coronary intervention [PCI], or coronary artery bypass grafting [CABG]).194 The SOS study (Swedish Obese Subjects), which is a nonrandomized prospective controlled study, also demonstrated a reduction of cardiovascular death in the bariatric surgery group compared with the control group.185 To date, there is no randomized controlled trial on the effects of bariatric surgery on MACE incidence.
PCI and Obesity
Short-Term Outcomes After PCIs
The CathPCI Registry examined in-hospital complications of 83 861 patients with severe obesity, including patients after myocardial infarction.195 After multivariable adjustment, obesity was independently associated with a greater mortality rate and a lower bleeding rate.195 Although obesity affects weight-based dosing protocols for unfractionated heparin, patients with severe obesity are underrepresented or even excluded from major trials.196 A doubling in the time necessary to obtain adequate anticoagulation in patients weighing 110 kg with an initial infusion rate based on a nomogram was reported.196 Another study of 227 042 registry patients, including patients after myocardial infarction, with 37.2% (n=84 479) having obesity and 7.4% (n=16 730) having severe obesity,197 reported that patients with severe obesity had significantly more contrast-induced nephropathy, nephropathy requiring dialysis, and vascular complications (almost exclusively femoral) compared with patients with overweight.197 Gastrointestinal bleeding and MACE incidences were not statistically different. The British Cardiovascular Intervention Society Registry reported adverse in-hospital outcomes and mortality of 345 192 patients undergoing PCI.198 At 30 days after PCI, there was evidence of the obesity paradox, with lower mortality observed in patients with BMI ≥25 kg/m2. Up to 5 years after PCI, BMI >25 kg/m2 was an independent predictor of greater survival compared with normal weight, regardless of the clinical presentation (unstable angina, non–ST-segment–elevation myocardial infarction or ST-segment–elevation myocardial infarction).198 The APPROACH registry (Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease) reported mortality in 30 258 patients who had PCI and showed further evidence of the obesity paradox given that the 6-month mortality was lower in patients who were in the overweight or obese category compared with patients with normal BMI.199
Long-Term Outcomes After PCIs
Patients with low BMI tend to have more events after PCI than patients with obesity.200,201 A study of 23 181 patients from 11 prospective PCI studies used a BMI of 22.5 to 24.9 kg/m2 as the reference category. The risk of major cardiovascular events was higher among patients with a lower BMI (<18.5 kg/m2) and declined among patients with a higher BMI (>30.0 kg/m2).202 A recent meta-analysis of 865 774 patients undergoing PCI or CABG confirmed these findings and demonstrated a U-shaped association across all BMI categories for all-cause mortality and MACEs after PCI or CABG.203 This obesity paradox seems to wane when severe obesity is taken into consideration.199,204 The APPROACH registry demonstrated that the 5- and 10-year mortality rate after PCI in patients with class 3 obesity and high-risk coronary anatomy was higher than that of patients with normal BMI (odds ratio, 1.78 at 5 years and 1.57 at 10 years).199
Antiplatelet Therapy in Obesity
Compared with patients with normal weight, individuals with obesity display higher platelet reactivity in a number of ex vivo assays of platelet function, including platelet aggregation.205,206 Adipose tissue produces multiple bioactive substances and hormones such as leptin, adiponectin, TNF-α (tumor necrosis factor-α), interleukin-6, and resistin, all of which may directly or indirectly affect platelet function.206,207 High levels of platelet aggregation and turnover are also found in patients with insulin resistance and hyperglycemia.208 High on-aspirin platelet reactivity is the laboratory-defined failure of aspirin to appropriately inhibit platelet thromboxane production or to inhibit platelet function. Several studies have linked obesity to an elevated risk of high on-aspirin platelet reactivity.209 In comparisons with individuals without obesity, postaspirin platelet reactivity was higher in the group with obesity at peak 1 hour after aspirin administration and trough 24 hours after aspirin administration time points.210,211 In a pharmacokinetic/pharmacodynamic comparison of aspirin formulations in patients with obesity and type 2 diabetes, high on-aspirin platelet reactivity was highest with enteric-coated aspirin because of the increase in esterase and phase II conjugation enzymes in obesity.212 Obesity-related endothelial dysfunction and persistent, low-grade inflammation can cause higher platelet consumption, leading to higher platelet turnover and acceleration of COX-1 (cyclooxygenase 1) renewal and resulting in a faster recovery of thromboxane-dependent platelet function and the loss of aspirin effect.213,214 Similar correlations between patients’ BMI and residual platelet reactivity under treatment were observed with clopidogrel and prasugrel in patients with obesity. However, patients with obesity but without metabolic syndrome had a better response to thienopyridines compared with patients with obesity and metabolic syndrome and had a response similar to that of patients without obesity, suggesting that metabolic status is a better correlate of platelet inhibition than BMI.206,215 Some data suggest that patients with obesity receiving prasugrel had lower rates of high on-treatment platelet reactivity than those taking clopidogrel. However, a comparison of patients with and without obesity on prasugrel revealed that 28% of patients with obesity had high on-treatment platelet reactivity compared with 4% of patients without obesity (P<0.01). Although it seems that prasugrel is more effective in obesity compared with clopidogrel, it should be noted that additional data suggest that obesity might lead to a variable response effect to prasugrel compared with nonobesity.206,215 Conversely to thienopyridines, no correlation was reported between BMI and high on-treatment platelet reactivity with ticagrelor; patients with obesity do not express significantly higher levels of platelet reactivity, whereas ticagrelor seems to induce significantly higher platelet inhibition than prasugrel in patients with obesity.206,216 Although studies suggest that obesity may promote platelet activation and blunt effects of antiplatelet medications, clinical observations have pointed to an obesity paradox, namely that patients with obesity may have better post–acute coronary syndrome outcomes and may have a lower risk of reinfarction or death. Data involving platelet assays are often conflicting and involve sample sizes too small to draw decisive conclusions about clinical outcomes and to make recommendations about dosing adjustments of antiplatelet therapy in obesity.206
Surgical Revascularization
Obesity has been inconsistently associated with higher in-hospital mortality after CABG. An analysis of the Society of Thoracic Surgeons’ database (559 004 patients who underwent isolated CABG between 1997 and 2000)217 showed a higher risk of in-hospital mortality in patient with moderate obesity (n=42 060; BMI, 35–39.9 kg/m2) and patients with severe obesity (n=18 735; BMI >40 kg/m2) compared with patients with a BMI of 18.5 to 34.9 kg/m2. These results contrasted with previous studies that found no significantly greater postoperative mortality in patients with obesity after CABG.218,219 A meta-analysis showed that the rate of in-hospital mortality after CABG was even less in the population with obesity.220 A potentially protective effect was also shown in a retrospective multicenter cohort study,221 which showed that 30-day operative mortality was highest in extreme BMI groups (BMI <20 and >40 kg/m2) and lowest near a BMI of 30 kg/m2, suggesting a U-shaped relationship.222 Evidence on long-term mortality is still conflicting.171 A meta-analysis found decreased long-term mortality (1–5 years) for the population with overweight and obesity.203,220 In contrast, a recent retrospective study showed that obesity was associated with a higher rate of long-term mortality after CABG.223 Several studies have documented the association between obesity and numerous postoperative CABG complications such as renal failure,224 respiratory failure, arrhythmias, and greater intraoperative transfusion rate.27,225,226 In contrast, postoperative cerebrovascular events, myocardial infarction, and postoperative bleeding do not appear to be higher in patients with obesity.225,226 A greater incidence of postoperative AF was reported in patients with obesity,227 as was a longer length of stay.199 In a large cohort study of patients who underwent isolated CABG, WC was associated with a higher risk of postoperative AF, prolonged mechanical ventilation and reintubation, renal failure and new postoperative renal replacement therapy, bloodstream infection, sternal wound infections, and intensive care unit and hospital length of stay independently of BMI.228 Post-operative deep sternal wound infection is also more common in patients with obesity. CABG surgery using bilateral internal mammary artery instead of single internal mammary artery was associated with a higher risk of postoperative deep sternal wound infection without improving survival for patients with obesity.229 The large, poorly vascularized panniculus, the higher incidence of dysglycemia among those with obesity, and the difficulty in wound surveillance may predispose to wound infections.199,230 Obesity has also been identified as a risk factor for superficial wound infection and saphenous vein harvest site infection.218
PATHOPHYSIOLOGY OF HF AND ARRHYTHMIAS IN OBESITY
Impact of Obesity on Heart Function
Excess adiposity promotes changes in cardiac function both directly through the effects on the myocardium and vasculature and indirectly through obesity-related comorbidities.231 Excess adipose tissue accumulation leads to hemodynamic changes, including higher blood volume and cardiac output and a reduction in systemic vascular resistance.27 Excess adiposity also leads to higher blood pressure as a result of activation of the renin-angiotensin-aldosterone and sympathetic nervous systems.232 Obesity also directly affects the myocardium with myocardial fat accumulation and subsequent fibrosis that can lead to the development of LVDD and HF with preserved ejection fraction (HFpEF). Detailed phenotyping of patients with HFpEF with and without obesity compared with controls depicted that patients with obesity and HFpEF had greater concentric LV remodeling, right ventricular dilatation, and right ventricular dysfunction. There was also evidence of more pericardial restraint and ventricular interdependence for those with obesity and HFpEF in the setting of greater epicardial fat thickness and epicardial fat volume.233,234 Patients with obesity and HFpEF also had significantly lower exercise capacity compared with patients without obesity with HFpEF and control subjects. This was one of the first studies to demonstrate a distinct pathophysiological phenotype of HFpEF in the setting of obesity.233 Atherosclerotic heart disease related to obesity can lead to systolic dysfunction and, ultimately, HF with reduced ejection fraction (HFrEF). Finally, comorbidities associated with obesity such as diabetes, sleep apnea, and hypoventilation syndrome can increase the risk for pulmonary hypertension and right ventricular and LV failure.231
Obesity and HF
Numerous studies have established obesity to be a major risk factor for hypertension, CVD, and LVH, all strong risk factors for the development of HF.177,178 In addition, obesity has potent adverse effects on LV systolic and, particularly, LV diastolic function. Multiple studies have established obesity as a major risk factor for the development of HF. In a study of 5881 Framingham Heart Study participants, HF incidence increased by 5% in men and 7% in women for every 1-unit BMI increase after adjustment for other risk factors, and the risk of HF increased across the entire spectrum of BMI.235 This was subsequently confirmed in several large, prospective epidemiological studies.235–237 Other anthropometric parameters of excess adiposity such as WC, WHR, and waist-to-height ratio have also been independently associated with HF risk, but they generally do not add substantive risk information for HF beyond BMI measurement.236–239 Visceral obesity has a number of local effects on the myocardium, including inducing cardiomyocyte hypertrophy, myocardial fibrosis, and activation of inflammatory pathways relating to macrophage infiltration and cytokine gene expression. Excessive fat accumulation in VAT and ectopic sites such as the pericardium/epicardium and liver results in higher circulating blood volume and local and systemic proatherogenic inflammatory factors, which act to increase stroke volume, cardiac wall stress, and myocardial injury, leading to concentric LVH, LV remodeling, and ultimately diastolic and systolic cardiac failure (Figure 1).240–242 Recent work has also suggested that higher BMI is more strongly associated with the risk of HFpEF than with HFrEF.243 In fact, in a pooled analysis using data from 3 large longitudinal studies, Pandey et al244 demonstrated a greater association between higher BMI and risk of HFpEF, with participants with overweight and class 1 obesity having 38% and 56% higher risk of HFpEF, respectively, independently of other cardiovascular risk factors (Figure 2).244 Low fitness has been associated with a significantly higher risk of HF across all BMI categories and may explain close to 50% of HF risk associated with BMI.245
Figure 1. Pathophysiology of heart failure in obesity.
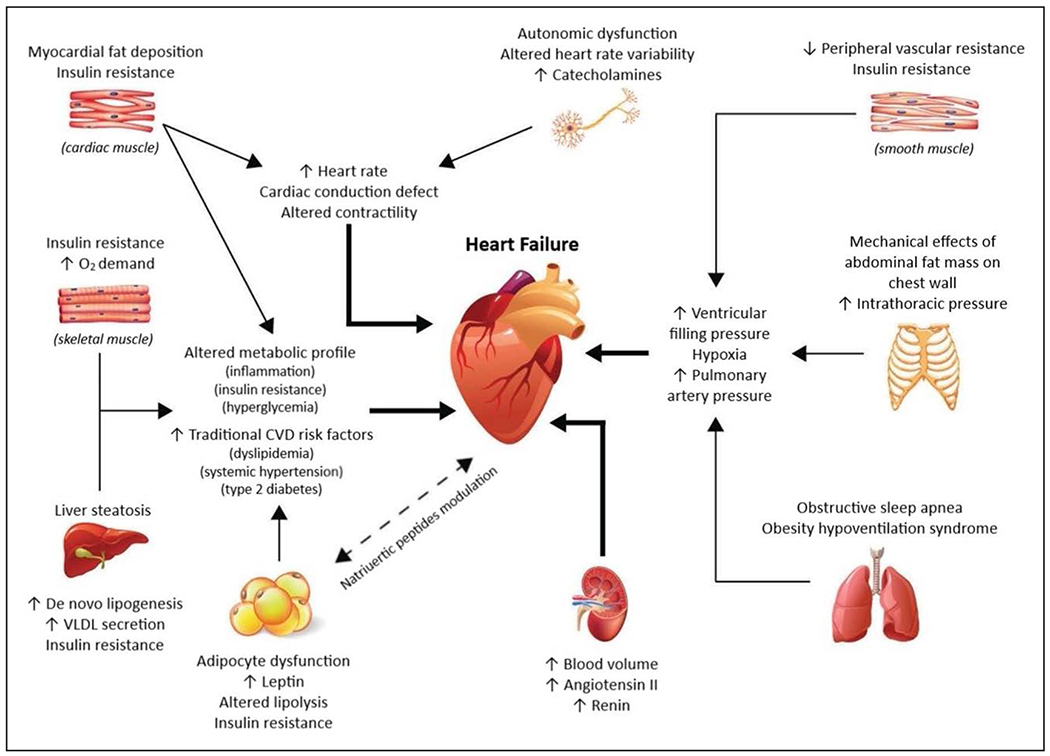
CVD indicates cardiovascular disease; and VLDL, very-low-density lipoprotein. Adapted from Rodriguez Flores et al240 with permission from Taylor & Francis Ltd (https://www.tandfonline.com). Copyright © 2017, Taylor & Francis Ltd.
Figure 2. Association between body mass index and risk of heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF).
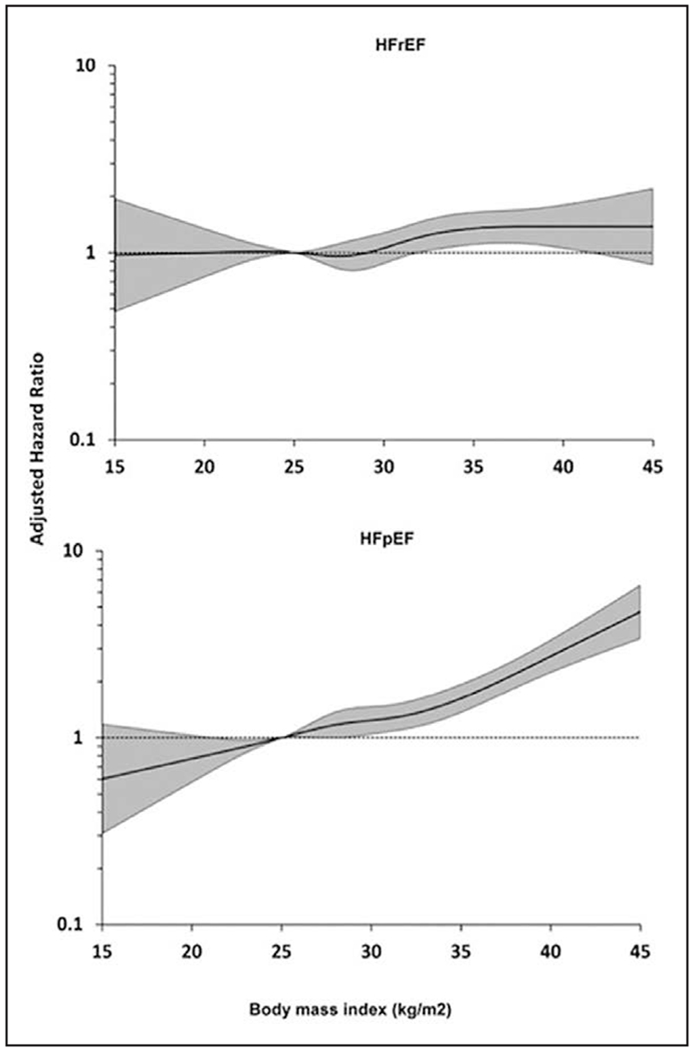
Reprinted from Pandey et al243 with permission from the American College of Cardiology Foundation. Copyright © 2018, American College of Cardiology Foundation.
Obesity and HF Outcomes
Data support the presence of the obesity paradox in HF: Patients with overweight or class 1 obesity have better clinical outcomes than patients with normal weight and similar degrees of HF, and this is seen more for HFrEF than for HFpEF.177–179 In addition, the protective effects of obesity on cardiovascular outcomes have now been noted for HFrEF, HFpEF, and acutely decompensated HF.177–179,237 This obesity paradox has also been noted for BMI, WC, and percent body fat,178,179,237,246,247 although a recent study in HFpEF suggested that higher WC was associated with better outcomes in univariate analysis but worse outcomes in multivariate analysis.248,249 Epicardial adipose tissue has recently been found to be low in patients with HF compared with the general population, and a recent study found that low epicardial adipose tissue in HF was associated with higher HF mortality, another aspect of the obesity paradox.237,250,251 Patients with obesity have lower levels of BNP (brain natriuretic peptide) than patients with normal weight, including in HF.177,178,237 In patients with severe obesity, weight loss after bariatric surgery increases NT-proBNP (N-terminal pro-BNP) levels concomitantly with improved LVDD.252 In advanced HF, extra adipose tissue and higher lean muscle mass may also provide reserves against cardiac cachexia and sarcopenia, which are associated with very poor cardiac prognosis in HF.253–255 However, the exact reasons why those who have overweight and mild obesity with less severe forms of HF are also protected are not entirely clear.177–179
Obesity and Arrhythmias
There is now compelling evidence to support the importance of excess adiposity in determining arrhythmic risk, particularly focused on SCD and AF.256,257
Obesity and SCD
There is an established association between obesity and SCD.258,259 Every 5-unit increment in BMI confers a 16% higher risk of SCD,260 and obesity has been identified as the most common nonischemic cause of SCD.261 Data suggest that there may be an important role for body fat distribution, implicating abdominal adiposity as a marker of SCD.259,262 The potential mechanisms for this association are varied and may include LVH, QT prolongation, premature ventricular complexes, and autonomic imbalance.257,263,264 Both mild obesity and severe obesity are reported to be associated with greater risk of ventricular tachycardia (VT)/ventricular fibrillation (VF)265,266 and late potentials,267 highlighting a role in the formation of arrhythmic substrate. Clinical data reporting the substrate for VT/VF in obesity have come largely from autopsy studies, tissue Doppler, or endomyocardial biopsy. VT/VF in obesity is associated with increased LV diameter and mass,268 concentric LV hypertrophy,269 LVDD,268,270 and repolarization abnormalities. A common finding in obesity and obesity-mediated SCD is QRS fragmentation, a surrogate for heterogeneous conduction.271,272 Both QRS fragmentation273 and fibrosis274 are shown to be independent predictors of SCD, indicative of a potential role in mediating reentrant ventricular arrhythmias in obesity. Mechanistic studies from animal models have identified involvement of fibrosis, ion channel remodeling, and reduction of connexin proteins as likely drivers of lethal ventricular arrhythmias and SCD. In mice, rabbit, and rat models, high-fat diet has demonstrated (1) greater frequency of ventricular arrhythmias, attributed to oxidation RyR2 (ryanodine receptor type 2) and subsequently greater RyR2 calcium release; (2) changes in oxidative stress state, calcium handling, and putative components of the mitochondrial membrane permeability transition pore275; and (3) LVH and repolarization abnormalities.276 However, it remains to be determined whether these changes can be replicated in ventricles of models with established obesity. Epicardial adipose tissue was reported to be associated with higher occurrence of premature ventricular contractions, VT/VF,277 and all-cause long-term mortality277 and mortality from SCD.278 Furthermore, epicardial adipose tissue is significantly correlated with traditional SCD and VT/VF risk factors.63,65,279–281 In a post–myocardial infarction ovine model, intramyocardial adiposity and discontinuous conduction at scar borders were found to be associated with altered electrophysiological properties and higher propensity for VT.282 Perhaps even more important, epicardial adipose tissue infiltrations and subsequent fibrosis (as shown in the atria) may drive reentrant circuits for lethal arrhythmias and SCD (Figure 3). Given that SCD is responsible for approximately half of all deaths resulting from CVD, obesity also represents a modifiable target to reduce the public health burden of SCD in our society. Also of clinical importance is that, in patients with obesity, the efficacy of chest compressions and airway protection may be compromised because of body habitus in case of sudden cardiac arrest, and the problem is likely to worsen as the patient’s weight increases. Higher thoracic impedance associated with an increase in BMI may also reduce defibrillation success.283,284 It was shown that severe obesity is associated with higher mortality after in-hospital cardiac arrest caused by either non-VF or VF arrest if it occurs late during hospitalization, and among survivors, discharge to home is significantly lower.285
Figure 3. Relationships between obesity and cardiac arrhythmias.
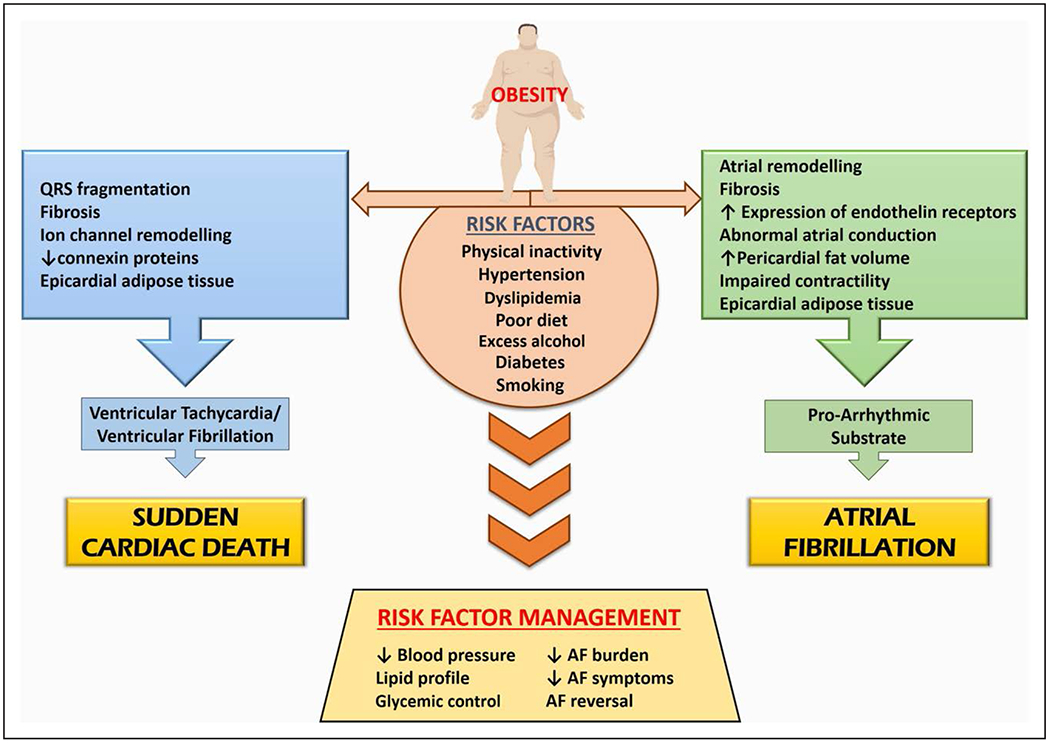
AF indicates atrial fibrillation.
Obesity and AF
Estimates suggest that obesity may account for one-fifth of AF cases and 60% of recently documented population increases.286–288 Weight gain and a higher midlife BMI are strongly correlated with incident AF in later life.289,290 Every 5-unit increment in BMI confers an ≈29% greater risk of incident AF.291 Moreover, these figures may underestimate the impact of adiposity when body fat distribution is considered. In addition, each 5-unit increment in BMI confers a 10% increase in postoperative AF and a 13% increase in postablation AF.291 Progression of the disease has also been demonstrated in the context of obesity, with a BMI in the range of 30 to 34.9 kg/m2 associated with a 54% increase in the likelihood of progression from paroxysmal to permanent AF and class 2 obesity (BMI 35.0 to 39.9 kg/m2) associated with an 87% increase in risk.292 Overweight and obesity elevate the risk of AF through numerous mechanisms, including structural and electric remodeling, which contribute to development of the arrhythmogenic substrate (Figure 3). Experimental studies in the ovine model have demonstrated short-term weight gain results in progressive remodeling of the atria, including a greater deposition of fibrous tissue, a greater expression of endothelin receptors, and abnormalities in atrial conduction, which in turn resulted in greater AF inducibility.293 A subsequent chronic ovine model of obesity extended these findings and described a unique component of the substrate for AF. This study demonstrated a marked increase in pericardial fat volumes. Histological samples of the atrial myocardium from regions adjacent to pericardial fat depots showed epicardial fat infiltrating the myocardium, potentially resulting in voltage abnormalities, conduction block, and higher AF vulnerability.294 Clinical data also demonstrate the role of obesity and epicardial fat in the promotion of AF. Early studies demonstrated that, compared with individuals with normal weight, patients with obesity undergoing electrophysiological studies were significantly more likely to have higher left atrial pressure and volumes.295 In addition, individuals with obesity had significant left atrial remodeling and impaired contractility. These features remained significant after adjustment for common cardiovascular risk factors such as hypertension, sleep apnea, and diabetes. More recently, a larger cohort who were undergoing AF ablation were studied with cardiac MRI and electroanatomic mapping of the left atrium before undergoing ablation.296 This study demonstrated that there was significantly more atrial remodeling, with areas of low voltage, conduction slowing, and increased fractionation of ECGs in patients with obesity. More distinct changes were noted in regions with greater epicardial fat depots, highlighting the role of epicardial fat in the promotion of AF. Epicardial adipose tissue has emerged as an important proarrhythmic substrate that may explain the excess risk of AF in obesity.296–298 The strength of associations of AF with epicardial fat is greater than for measures of abdominal and overall obesity, raising the possibility that adiposity may be more influential than previously suspected when quantified through BMI alone.299 The anatomic proximity of epicardial adipose tissue to the atrial myocardium lends credence to potential paracrine signaling.300 Mechanisms by which adiposity may lead to a susceptible electrophysiological substrate in the atria include fatty infiltration, adipokine-mediated fibrosis, LVDD, and inflammation, among many possibilities.301
TREATMENT OF HF AND ARRHYTHMIAS IN OBESITY
Lifestyle Interventions in HF and Obesity
Currently, there is little evidence that weight reduction in HF leads to better major clinical outcomes or better survival, but weight loss may reduce symptoms and improve quality of life and other medical conditions such as sleep apnea or diabetes.237 In addition, weight loss in advanced HF could improve the candidacy of patients with obesity for aggressive interventions such as LV assist device implantation and heart transplantation.240,302 Clearly, higher levels of physical activity and fitness have major impacts in reducing the development of HF,243–245 and in patients with established HF, high fitness is a major determinant of prognosis.177–179 Among patients with HF with preserved levels of fitness, several studies show a very good prognosis, regardless of BMI.177,178,237,303–305 Therefore, greater physical activity and exercise training, especially with the goal of improving fitness, are highly encouraged for individuals with obesity with HF For elderly populations with obesity who may be at greatest risk for HF, more work is needed to develop effective strategies for maintaining weight and improving functional outcomes as opposed to weight loss interventions.
Medications for Weight Loss in HF
Although numerous medications are currently indicated for weight loss,177,237 only orlistat, a lipase inhibitor, has limited efficacy and safety for the treatment of obesity with HF.306,307 Several new classes of medications originally developed for patients with type 2 diabetes have shown promise for the treatment of both obesity and HF. Glucagon-like peptide agonists (at least liraglutide)191,308,309 and sodium-glucose cotransporter 2 inhibitors310 have demonstrated efficacy for weight loss and reduced hospitalization for HF and cardiovascular death. Trials of these agents are currently ongoing, focusing on both patients with HFrEF and those with HFpEF with and without diabetes, with results forthcoming over the next 5 years. Recently, it was reported in individuals with overweight/obesity and HFrEF that the risk of worsening HF or cardiovascular death was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.311
Obesity Management in Advanced HF
Advanced HF is typically considered a contraindication for bariatric surgery, but small studies of bariatric surgery have indicated improvements in LV function, myocardial mechanics, and functional classification among patients with HF with obesity.237,240,312 A recent retrospective study has also suggested that bariatric surgery reduces hospitalizations for HF in patients with a history of HF.313 Although recent HF guidelines have not emphasized weight reduction, these guidelines recognize the high risk associated with severe obesity.237,314 Clearly, efforts to reduce obesity and, especially, to reduce the progression of obesity to class 2 or 3 levels are needed.177 Class 3 obesity is a relative contraindication for heart transplantation because patients with obesity who undergo heart transplantation have higher acute rejections and higher 5-year mortality than patients with normal weight receiving heart transplantation.302 However, obesity has not universally been considered a contraindication for LV assist device implantation,240 although there are adverse effects of obesity such as higher drive-line infection rates, and the clinical trials generally have excluded individuals with class 3 obesity. Clearly, there are opportunities to improve weight loss efforts in patients with an LV assist device with obesity who are anticipating heart transplantation, including a multidisciplinary approach with caloric restriction, physical activity/exercise training, and even bariatric surgery, that allow greater weight loss and greater ability to perform physical activity/exercise, allowing increases in muscle mass and function, possibly facilitating LV recovery, but certainly allowing the potential for better success with heart transplantation.302
Obesity Management and AF
Convincing data now demonstrate the benefits of weight loss in patients with AF, supporting a strong causative role for adiposity in these patients.315 A randomized controlled trial of 150 individuals demonstrated that an intense weight loss and cardiometabolic risk factor management program resulted in a greater reduction in cumulative time in AF, symptom burden, and severity scores and beneficial cardiac remodeling, as evidenced by a reduction in interventricular septal dimension and left atrial area, after 15 months of follow-up.316 This approach was further validated in a cohort study that resulted in an almost 5-fold higher likelihood of freedom from AF after ablation for those who attended this clinic compared with control subjects.317 Concomitant improvements in numerous other cardiovascular risk factors were observed, including a reduction in blood pressure and improved lipid profiles, commensurate with weight reduction.317 Long-term follow-up at 5 years demonstrated the sustainability of this approach, with individuals able to achieve a ≥10% reduction in body weight and having an almost 6-fold higher likelihood of freedom from AF.315 In addition, a reduced propensity for progression of the disease was observed, with greater degrees of weight loss associated with a reduced likelihood of progression to more permanent forms of the arrhythmia.318 Collectively, these studies prove the dynamic nature of the AF substrate and solidify the role of cardiovascular risk factor management, in addition to rate and rhythm control and appropriate anticoagulation to mitigate stroke risk, as the essential fourth pillar of AF management.319
CONCLUSIONS
Obesity is recognized as a heterogeneous condition in which individuals with similar BMIs may have distinct metabolic and CVD risk profiles. Thus, susceptibility to obesity-related cardiovascular complications is not mediated solely by overall body fat mass but depends largely on individual differences in regional body fat distribution, which negatively affect cardiac structure and function. With increasing prevalence of obesity in populations with a longer life span, there is a need to evaluate mechanisms underlying obesity-related cardiac dysfunction and to improve the management of patients with obesity and CVD through future research (Table 3). In addition, the dramatic increase in the proportion of young patients with severe obesity invokes the need for more upstream interventions for the primary prevention and better treatment of obesity as a chronic disease.
Table 3.
Summary of Recommendations for Future Research
| Evaluation of lifestyle interventions with randomized controlled trials to identify the role of intentional weight loss and decreased visceral adiposity for improving CVD outcomes in obesity |
| Development of dietary interventions with large randomized controlled trials to identify healthful dietary patterns or personalized diets for CVD risk reduction in obesity |
| Development of upstream interventions for primary prevention and better treatment of obesity as a chronic disease among young patients with severe obesity |
| Identification of best practices for use of glucagon-like peptide agonists and sodium-glucose cotransporter 2 inhibitors to reduce hospitalization for HF and cardiovascular death for patients with HFrEF and HFpEF with and without diabetes |
| Development of effective strategies for weight maintenance and improved functional outcomes as opposed to weight loss interventions in elderly populations at risk for HF |
CVD indicates cardiovascular disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; and HFrEF, heart failure with reduced ejection fraction.
ACKNOWLEDGMENTS
The authors would like to acknowledge Ms Audrey Auclair and Ms Sam Neally for their assistance in formatting the scientific statement for publication.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on November 6, 2020, and the American Heart Association Executive Committee on December 16, 2020. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or email Meredith.Edelman@wolterskluwer.com.
The American Heart Association requests that this document be cited as follows: Powell-Wiley TM, Poirier P, Burke LE, Després J-P, Gordon-Larsen P Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge M-P; on behalf of the American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e•••–e•••. doi: 10.1161/CIR.0000000000000973.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
Disclosures
Writing Group Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Tiffany M. Powell-Wiley | National Institutes of Health | NIH (funded intramural investigator at NIH)† | None | None | None | None | None | None |
| Paul Poirier | Universite Laval, Faculte de Pharmacie Institut Universitaire de Cardiologie et de Pneumologie de Quebec (Canada) | None | None | Abbott*; Amgen*; Astra-Zeneca*; Bausch Health*; Bayer*; Boehringer Ingelheim*; Eli Lilly*; Janssen*; Novartis*; Novo Nordisk*; Sanofi*; Servier*; HLS Therapeutics* | None | None | Amgen*; Bausch Health*; Bayer*; Boehringer Ingelheim*; Eli Lilly*; Janssen*; Novartis*; Novo Nordisk*; Servier*; Sanofi* | None |
| Lora E. Burke | University of Pittsburgh | NIH† | None | None | None | None | None | NIH (principal investigator)† |
| Jean-Pierre Després | Université Laval, VITAM – Centre de recherche en santé durable (Canada) | Fondation IUCPQ-UL (coinvestigator)†; CIHR (PI)† | None | None | None | None | None | None |
| Penny Gordon-Larsen | Gillings School of Global Public Health, University of North Carolina at Chapel Hill | NIH (principal investigator)*; NIH (coinvestigator)* | None | None | None | Astra Zeneca (immediate family members)*; Amgen (immediate family members)*; Johnson & Johnson (immediate family members)*; Pfizer (immediate family members)*; Merck (immediate family members)* | NIDDK*; Boston Nutrition Obesity Research Center* | None |
| Carl J. Lavie | Ochsner Medical Center | None | None | AstraZeneca/FARXIGA* | None | None | AstraZeneca/FARXIGA* | None |
| Scott A. Lear | Simon Fraser University, St. Paul’s Hospital (Canada) | Robert Wood Johnson Foundation†; Hamilton Health Sciences (research contract)† | None | None | None | None | None | None |
| Chiadi E. Ndumele | Johns Hopkins University | NIH (grant on mechanisms linking obesity to heart failure)† | None | None | None | None | None | None |
| Ian J. Neeland | University Hospitals Cleveland Medical Center and Case Western Reserve University School of Medicine | NIH/NIDDK (K23 career development grant)† | None | None | None | None | Merck* | None |
| Prashanthan Sanders | University of Adelaide and Royal Adelaide Hospital (Australia) | None | None | None | None | None | None | None |
| Marie-Pierre St-Onge | Columbia University, Irving Medical Center | None | None | None | None | None | None | None |
| Leena P. Bharath | Merrimack College | None | None | None | None | None | None | None |
| Kevin P. Davy | Virginia Polytechnic Institute and State University | None | None | None | None | None | None | None |
| Francisco Lopez-Jimenez | Mayo Clinic | None | None | None | None | None | None | None |
| Eric D. Peterson | Duke Clinical Research Institute | Novo Nordisk*; AstraZeneca†; Janssen† | None | None | None | None | AstraZeneca*; Janssen* | None |
| Stephen Sidney | Kaiser Permanente | NHLBI (1RC2HL101666, relating to surveillance of cardiovascular disease, including stroke, in the Cardiovascular Research Network)†; NINDS (1U54NS081760-01, Stroke Prevention/Intervention Program [SPIRP])† | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
REFERENCES
- 1.Loos RJ. Genetic determinants of common obesity and their value in prediction. Best Pract Res Clin Endocrinol Metab. 2012;26:211–226. doi: 10.1016/j.beem.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Gebreab SZ, Vandeleur CL, Rudaz D, Strippoli MF, Gholam-Rezaee M, Castelao E, Lasserre AM, Glaus J, Pistis G, Kuehner C, et al. Psychosocial stress over the lifespan, psychological factors, and cardiometabolic risk in the community. Psychosom Med. 2018;80:628–639. doi: 10.1097/PSY.0000000000000621 [DOI] [PubMed] [Google Scholar]
- 3.Sommer I, Griebler U, Mahlknecht P, Thaler K, Bouskill K, Gartlehner G, Mendis S. Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC Public Health. 2015;15:914. doi: 10.1186/s12889-015-2227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallis JF, Glanz K. Physical activity and food environments: solutions to the obesity epidemic. Milbank Q. 2009;87:123–154. doi: 10.1111/j.1468-0009.2009.00550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franks PW, McCarthy MI. Exposing the exposures responsible for type 2 diabetes and obesity. Science. 2016;354:69–73. doi: 10.1126/science.aaf5094 [DOI] [PubMed] [Google Scholar]
- 6.Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a disease: The Obesity Society 2018 position statement. Obesity (Silver Spring). 2019;27:7–9. doi: 10.1002/oby.22378 [DOI] [PubMed] [Google Scholar]
- 7.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon-Larsen P, Heymsfield SB. Obesity as a disease, not a behavior. Circulation. 2018;137:1543–1545. doi: 10.1161/CIRCULATIONAHA.118.032780 [DOI] [PubMed] [Google Scholar]
- 9.Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity, Geneva, 3–5 June 1997. World Health Organization, Division of Noncommunicable Disease, Programme of Nutrition Family and Reproductive Health; 1998. [PubMed] [Google Scholar]
- 10.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond). 2008;32:959–966. doi: 10.1038/ijo.2008.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pack QR, Rodriguez-Escudero JP, Thomas RJ, Squires RW, Johnson L, Somers VK, Lopez-Jimenez F. Diagnostic performance of weight loss to predict body fatness improvement in cardiac rehabilitation patients. J Cardiopulm Rehabil Prev. 2013;33:68–76. doi: 10.1097/HCR.0b013e31827fe7e3 [DOI] [PubMed] [Google Scholar]
- 12.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond). 2010;34:791–799. doi: 10.1038/ijo.2010.5 [DOI] [PubMed] [Google Scholar]
- 13.Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17:262–275. doi: 10.1111/obr.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao G, Powell-Wiley TM, Ancheta I, Hairston K, Kirley K, Lear SA, North KE, Palaniappan L, Rosal MC; on behalf of the American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health . Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: a scientific statement from the American Heart Association [published correction appears in Circulation. 2015;132:e130]. Circulation. 2015;132:457–472. doi: 10.1161/CIR.0000000000000223 [DOI] [PubMed] [Google Scholar]
- 15.Zhou BF; Cooperative Meta-Analysis Group of the Working Group on Obesity in China . Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 16.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, et al. ; GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maffetone PB, Rivera-Dominguez I, Laursen PB. Overfat and underfat: new terms and definitions long overdue. Front Public Health. 2017;4:e00279. doi: 10.3389/fpubh.2016.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, Cairns BJ, Huxley R, Jackson CL, Joshy G, et al. ; Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beccia AL, Jesdale WM, Lapane KL. Associations between perceived everyday discrimination, discrimination attributions, and binge eating among Latinas: results from the National Latino and Asian American Study. Ann Epidemiol. 2020;45:32–39. doi: 10.1016/j.annepidem.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cozier YC, Yu J, Coogan PF, Bethea TN, Rosenberg L, Palmer JR. Racism, segregation, and risk of obesity in the Black Women’s Health Study. Am J Epidemiol. 2014;179:875–883. doi: 10.1093/aje/kwu004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YK, Berry DC, Schwartz TA. Weight stigmatization and binge eating in Asian Americans with overweight and obesity. Int J Env Res Public Health. 2020;17:4319. doi: 10.3390/ijerph17124319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuevas AG, Chen R, Slopen N, Thurber KA, Wilson N, Economos C, Williams DR. Assessing the role of health behaviors, socioeconomic status, and cumulative stress for racial/ethnic disparities in obesity. Obesity (Silver Spring). 2020;28:161–170. doi: 10.1002/oby.22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell CN, Kerr J, Young JL. Associations between Obesity, Obesogenic Environments, and Structural Racism Vary by County-Level Racial Composition. Int J Env Res Public Health. 2019;16:861. doi: 10.3390/ijerph16050861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Freedman ND, Albert PS, Huxley RR, Shiels MS, Withrow DR, Spillane S, Powell-Wiley TM, Berrington de González A. Association of cardiovascular disease with premature mortality in the United States. JAMA Cardiol. 2019;4:1230–1238. doi: 10.1001/jamacardio.2019.3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardel MI, Atkinson MA, Taveras EM, Holm JC, Kelly AS. Obesity treatment among adolescents: a review of current evidence and future directions. JAMA Pediatr. 2020;174:609–617. doi: 10.1001/jamapediatrics.2020.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 28.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahmani J, Roudsari AH, Bawadi H, Thompson J, Fard RK, Clark C, Ryan PM, Ajami M, Sakak FR, Salehisahlabadi A, et al. Relationship between body mass index, risk of venous thromboembolism and pulmonary embolism: a systematic review and dose-response meta-analysis of cohort studies among four million participants. Thromb Res. 2020;192:64–72. doi: 10.1016/j.thromres.2020.05.014 [DOI] [PubMed] [Google Scholar]
- 30.Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Millán D, Vila N, Ibañez P, Gil MJ, Valentí V, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond). 2012;36:286–294. doi: 10.1038/ijo.2011.100 [DOI] [PubMed] [Google Scholar]
- 31.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–746. doi: 10.1093/eurheartj/ehp487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F. Normal weight obesity and mortality in United States subjects ≥60 years of age (from the Third National Health and Nutrition Examination Survey). Am J Cardiol. 2013;112:1592–1598. doi: 10.1016/j.amjcard.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 33.Piché ME, Poirier P, Lemieux I, Després JP Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61:103–113. doi: 10.1016/j.pcad.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 34.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. 2015;163:827–835. doi: 10.7326/M14-2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, Daniels SR, Wadden TA, Tsai AG, et al. The science of obesity management: an Endocrine Society scientific statement. Endocr Rev. 2018;39:79–132. doi: 10.1210/er.2017-00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R ; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive clinical practice guidelines For medical care of patients with obesity.Endocr Pract. 2016;22(suppl 3):1–203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 37.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JAC, Ding JZ, Allison MA, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA study. J Am Coll Cardiol Cardiovasc Imag. 2014;7:1222–1235. doi: 10.1016/j.jcmg.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–1647. doi: 10.1161/CIRCULATIONAHA.114.015000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsumi Y, Nakao YM, Masuda I, Higashiyama A, Takegami M, Nishimura K, Watanabe M, Ohkubo T, Okamura T, Miyamoto Y. Risk for metabolic diseases in normal weight individuals with visceral fat accumulation: a cross-sectional study in Japan. BMJ Open. 2017;7:e013831. doi: 10.1136/bmjopen-2016-013831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 41.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring). 2013;21:E439–E447. doi: 10.1002/oby.20135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018; 137:1391–1406. doi: 10.1161/CIRCULATIONAHA.117.029617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nazare JA, Smith J, Borel AL, Aschner P, Barter P, Van Gaal L, Tan CE, Wittchen HU, Matsuzawa Y, Kadowaki T, et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA Study). Am J Cardiol. 2015; 115:307–315. doi: 10.1016/j.amjcard.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 44.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Levy D, Long MT. Visceral and intrahepatic fat are associated with cardiometabolic risk factors above other ectopic fat depots: the Framingham Heart Study. Am J Med. 2018;131:684–692.e12. doi: 10.1016/j.amjmed.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol. 2009;5:319–325. doi: 10.1038/nrendo.2009.78 [DOI] [PubMed] [Google Scholar]
- 46.Després JP Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012; 126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264 [DOI] [PubMed] [Google Scholar]
- 47.Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizaka Y, Oka R, Okada M, Suzuki S, Takaya N, Nakagawa T, Fukui T, et al. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med. 2012;44:82–92. doi: 10.3109/07853890.2010.526138 [DOI] [PubMed] [Google Scholar]
- 48.Després JP. What is “metabolically healthy obesity”? From epidemiology to pathophysiological insights. J Clin Endocrinol Metab. 2012;97:2283–2285. doi: 10.1210/jc.2012-2081 [DOI] [PubMed] [Google Scholar]
- 49.Camhi SM, Must A, Gona PN, Hankinson A, Odegaard A, Reis J, Gunderson EP, Jacobs DR, Carnethon MR. Duration and stability of metabolically healthy obesity over 30 years. Int J Obes (Lond). 2019;43:1803–1810. doi: 10.1038/s41366-018-0197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 51.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and allcause mortality. J Am Coll Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mongraw-Chaffin M, Allison MA, Burke GL, Criqui MH, Matsushita K, Ouyang P, Shah RV, Shay CM, Anderson CAM. CT-derived body fat distribution and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2017; 102:4173–4183. doi: 10.1210/jc.2017-01113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neeland IJ, Turer AT, Ayers CR, Berry JD, Rohatgi A, Das SR, Khera A, Vega GL, McGuire DK, Grundy SM, de Lemos JA. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65:2150–2151. doi: 10.1016/j.jacc.2015.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Alméras N, Després JP Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr. 2012;96:714–726. doi: 10.3945/ajcn.112.035758 [DOI] [PubMed] [Google Scholar]
- 56.Lim S, Taskinen MR, Borén J. Crosstalk between nonalcoholic fatty liver disease and cardiometabolic syndrome. Obes Rev. 2019;20:599–611. doi: 10.1111/obr.12820 [DOI] [PubMed] [Google Scholar]
- 57.Klein S Is visceral fat responsible for the metabolic abnormalities associated with obesity? Implications of omentectomy. Diabetes Care. 2010;33:1693–1694. doi: 10.2337/dc10-0744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauridsen BK, Stender S, Kristensen TS, Kofoed KF, Køber L, Nordestgaard BG, Tybjærg-Hansen A . Liver fat content, non-alcoholic fatty liver disease, and ischaemic heart disease: Mendelian randomization and meta-analysis of 279013 individuals. Eur Heart J. 2018;39:385–393. doi: 10.1093/eurheartj/ehx662 [DOI] [PubMed] [Google Scholar]
- 59.Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192 [DOI] [PubMed] [Google Scholar]
- 60.Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32–42. doi: 10.1038/nrgastro.2016.147 [DOI] [PubMed] [Google Scholar]
- 61.Ndumele CE, Nasir K, Conceiçao RD, Carvalho JA, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1927–1932. doi: 10.1161/ATVBAHA.111.228262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iacobellis G Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring). 2009;17:625; author reply 626; author reply 627. doi: 10.1038/oby.2008.575 [DOI] [PubMed] [Google Scholar]
- 63.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O’Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ong KL, Ding J, McClelland RL, Cheung BM, Criqui MH, Barter PJ, Rye KA, Allison MA. Relationship of pericardial fat with lipoprotein distribution: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;241:664–670. doi: 10.1016/j.atherosclerosis.2015.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah RV, Anderson A, Ding JZ, Budoff M, Rider O, Petersen SE, Jensen MK, Koch M, Allison M, Kawel-Boehm N, et al. Pericardial, but not hepatic, fat by CT Is Associated With cv outcomes and structure: the Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imag. 2017;10:1016–1027. doi: 10.1016/j.jcmg.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Larsen BA, Laughlin GA, Saad SD, Barrett-Connor E, Allison MA, Wassel CL. Pericardial fat is associated with all-cause mortality but not incident CVD: the Rancho Bernardo Study. Atherosclerosis. 2015;239:470–475. doi: 10.1016/j.atherosclerosis.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iacobellis G Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–371. doi: 10.1038/nrendo.2015.58 [DOI] [PubMed] [Google Scholar]
- 68.Hruskova J, Maugeri A, Podrouzkova H, Stipalova T, Jakubik J, Barchitta M, Medina-Inojosa JR, Homolka M, Agodi A, Kunzova S, et al. Association of cardiovascular health with epicardial adipose tissue and intima media thickness: the Kardiovize study. J Clin Med. 2018;7:113. doi: 10.3390/jcm7050113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Talabany S, Mordi I, Graeme Houston J, Colhoun HM, Weir-McCall JR, Matthew SZ, Looker HC, Levin D, Belch JJF, Dove F, et al. Epicardial adipose tissue is related to arterial stiffness and inflammation in patients with cardiovascular disease and type 2 diabetes. BMC Cardiovasc Disord. 2018;18:31. doi: 10.1186/s12872-018-0770-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698 [DOI] [PubMed] [Google Scholar]
- 71.Akilli H, Kayrak M, Bekci TT, Erdogan Hi, Aribas A, Yildirim O, Taner A, Erer M, Unlu A . Gender-related changes of the epicardial fat thickness and leptin in obstructive sleep apnea. Echocardiography. 2014;31:411–419. doi: 10.1111/echo.12392 [DOI] [PubMed] [Google Scholar]
- 72.St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL; on behalf of the American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ng SS, Liu EK, Ma RC, Chan TO, To KW, Chan KK, Ngai J, Yip WH, Ko FW, Wong CK, et al. Effects of CPAP therapy on visceral fat thickness, carotid intima-media thickness and adipokines in patients with obstructive sleep apnoea. Respirology. 2017;22:786–792. doi: 10.1111/resp.12963 [DOI] [PubMed] [Google Scholar]
- 74.Sivam S, Phillips CL, Trenell MI, Yee BJ, Liu PY, Wong KK, Grunstein RR. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. 2012;40:913–918. doi: 10.1183/09031936.00177011 [DOI] [PubMed] [Google Scholar]
- 75.Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett-Connor E, Haffner S, Hanson R, Hill JO, Hubbard V, Stamm E, et al. ; Diabetes Prevention Program Research Group. Body size and shape changes and the risk of diabetes in the Diabetes Prevention Program. Diabetes. 2007;56:1680–1685. doi: 10.2337/db07-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao S, Pandey A, Garg S, Park B, Mayo H, Després JP, Kumbhani D, de Lemos JA, Neeland IJ. Effect of exercise and pharmacological interventions on visceral adiposity: a systematic review and meta-analysis of long-term randomized controlled trials. Mayo Clin Proc. 2019;94:211–224. doi: 10.1016/j.mayocp.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008 [DOI] [PubMed] [Google Scholar]
- 78.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95 [DOI] [PubMed] [Google Scholar]
- 79.Boudou P, de Kerviler E, Erlich D, Vexiau P, Gautier JF. Exercise training-induced triglyceride lowering negatively correlates with DHEA levels in men with type 2 diabetes. Int J Obes Relat Metab Disord. 2001;25:1108–1112. doi: 10.1038/sj.ijo.0801637 [DOI] [PubMed] [Google Scholar]
- 80.Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17:664–690. doi: 10.1111/obr.12406 [DOI] [PubMed] [Google Scholar]
- 81.Cruz P, Johnson BD, Karpinski SC, Limoges KA, Warren BA, Olsen KD, Somers VK, Jensen MD, Clark MM, Lopez-Jimenez F. Validity of weight loss to estimate improvement in body composition in individuals attending a wellness center. Obesity (Silver Spring). 2011;19:2274–2279. doi: 10.1038/oby.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lesser IA, Singer J, Hoogbruin A, Mackey DC, Katzmarzyk PT, Sohal P, Leipsic J, Lear SA. Effectiveness of exercise on visceral adipose tissue in older South Asian women. Med Sci Sports Exerc. 2016;48:1371–1378. doi: 10.1249/MSS.0000000000000906 [DOI] [PubMed] [Google Scholar]
- 83.Cooper JH, Collins BE, Adams DR, Robergs RA, Donges CE. Limited effects of endurance or interval training on visceral adipose tissue and systemic inflammation in sedentary middle-aged men. J Obes. 2016;2016:2479597. doi: 10.1155/2016/2479597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hintze LJ, Messier V, Lavoie MÉ, Brochu M, Lavoie JM, Prud’homme D, Rabasa-Lhoret R, Doucet É. A one-year resistance training program following weight loss has no significant impact on body composition and energy expenditure in postmenopausal women living with overweight and obesity. Physiol Behav. 2018;189:99–106. doi: 10.1016/j.physbeh.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 85.Slentz CA, Bateman LA, Willis LH, Shields AT, Tanner CJ, Piner LW, Hawk VH, Muehlbauer MJ, Samsa GP, Nelson RC, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301 :E1033–E1039. doi: 10.1152/ajpendo.00291.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maillard F, Rousset S, Pereira B, Traore A, de Pradel Del Amaze P, Boirie Y, Duclos M, Boisseau N. High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes Metab. 2016;42:433–441. doi: 10.1016/j.diabet.2016.07.031 [DOI] [PubMed] [Google Scholar]
- 87.Zhang H, Tong TK, Qiu W, Zhang X, Zhou S, Liu Y, He Y. Comparable effects of high-intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J Diabetes Res. 2017;2017:5071740. doi: 10.1155/2017/5071740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herzig KH, Ahola R, Leppäluoto J, Jokelainen J, Jämsä T, Keinänen-Kiukaanniemi S. Light physical activity determined by a motion sensor decreases insulin resistance, improves lipid homeostasis and reduces visceral fat in high-risk subjects: PreDiabEx study RCT. Int J Obes (Lond). 2014;38:1089–1096. doi: 10.1038/ijo.2013.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedenreich CM, Neilson HK, O’Reilly R, Duha A, Yasui Y, Morielli AR, Adams SC, Courneya KS. Effects of a high vs moderate volume of aerobic exercise on adiposity outcomes in postmenopausal women: a randomized clinical trial. JAMA Oncol. 2015;1:766–776. doi: 10.1001/jamaoncol.2015.2239 [DOI] [PubMed] [Google Scholar]
- 90.van Gemert WA, Peeters PH, May AM, Doornbos AJH, Elias SG, van der Palen J, Veldhuis W, Stapper M, Schuit JA, Monninkhof EM. Effect of diet with or without exercise on abdominal fat in postmenopausal women: a randomised trial. BMC Public Health. 2019;19:174. doi: 10.1186/s12889-019-6510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trussardi Fayh AP, Lopes AL, Fernandes PR, Reischak-Oliveira A, Friedman R.Impact of weight loss with or without exercise on abdominal fat and insulin resistance in obese individuals: a randomised clinical trial. Br J Nut. 2013;110:486–492. doi: 10.1017/S0007114512005442 [DOI] [PubMed] [Google Scholar]
- 92.Murphy JC, McDaniel JL, Mora K, Villareal DT, Fontana L, Weiss EP. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. J Appl Physiol (1985). 2012;112:79–85. doi: 10.1152/japplphysiol.00355.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giannopoulou I, Ploutz-Snyder LL, Carhart R, Weinstock RS, Fernhall B, Goulopoulou S, Kanaley JA. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1511–1518. doi: 10.1210/jc.2004-1782 [DOI] [PubMed] [Google Scholar]
- 94.Pedersen LR, Olsen RH, Jürs A, Astrup A, Chabanova E, Simonsen L, Wisøff U, Haugaard SB, Prescott E. A randomised trial comparing weight loss with aerobic exercise in overweight individuals with coronary artery disease: the CUT-IT trial. Eur J Prev Cardiol. 2015;22:1009–1017. doi: 10.1177/2047487314545280 [DOI] [PubMed] [Google Scholar]
- 95.Gallagher D, Heshka S, Kelley DE, Thornton J, Boxt L, Pi-Sunyer FX, Patricio J, Mancino J, Clark JM; MRI Ancillary Study Group of Look AHEAD Research Group. Changes in adipose tissue depots and metabolic markers following a 1-year diet and exercise intervention in overweight and obese patients with type 2 diabetes. Diabetes Care. 2014;37:3325–3332. doi: 10.2337/dc14-1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63:174–182. doi: 10.1016/j.jhep.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 97.Zhang HJ, Pan LL, Ma ZM, Chen Z, Huang ZF, Sun Q, Lu Y, Han CK, Lin MZ, Li XJ, et al. Long-term effect of exercise on improving fatty liver and cardiovascular risk factors in obese adults: a 1-year follow-up study. Diabetes Obes Metab. 2017;19:284–289. doi: 10.1111/dom.12809 [DOI] [PubMed] [Google Scholar]
- 98.. Fernandez-del-Valle M, Gonzales JU, Kloiber S, Mitra S, Klingensmith J, Larumbe-Zabala E. Effects of resistance training on MRI-derived epicardial fat volume and arterial stiffness in women with obesity: a randomized pilot study. Eur J Appl Physiol. 2018;118:1231–1240. doi: 10.1007/s00421-018-3852-9 [DOI] [PubMed] [Google Scholar]
- 99.Honkala SM, Motiani KK, Eskelinen JJ, Savolainen A, Saunavaara V, Virtanen KA, Löyttyniemi E, Kapanen J, Knuuti J, Kalliokoski KK, et al. Exercise training reduces intrathoracic fat regardless of defective glucose tolerance. Med Sci Sports Exerc. 2017;49:1313–1322. doi: 10.1249/MSS.0000000000001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev. 2015;16:406–415. doi: 10.1111/obr.12270 [DOI] [PubMed] [Google Scholar]
- 101.Yoshimura E, Kumahara H, Tobina T, Matsuda T, Ayabe M, Kiyonaga A, Anzai K, Higaki Y, Tanaka H. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J Obes. 2014;2014:197216. doi: 10.1155/2014/197216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brinkley TE, Ding J, Carr JJ, Nicklas BJ. Pericardial fat loss in postmenopausal women under conditions of equal energy deficit. Med Sci Sports Exerc. 2011;43:808–814. doi: 10.1249/MSS.0b013e3181fb512d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsieh SD, Yoshinaga H. Abdominal fat distribution and coronary heart disease risk factors in men: waist/height ratio as a simple and useful predictor. int J Obesity. 1995;19:585–589. [PubMed] [Google Scholar]
- 104.Ashwell M, Gibson S. Waist-to-height ratio as an indicator of “early health risk”: simpler and more predictive than using a “matrix” based on BMI and waist circumference. BMJ Open. 2016;6:e010159. doi: 10.1136/bmjopen-2015-010159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coutinho T, Goel K, de Sa DC, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of “normal weight central obesity”. J Am Coll Cardiol. 2013;61:553–560. doi: 10.1016/j.jacc.2012.10.035 [DOI] [PubMed] [Google Scholar]
- 106.McGill HC Jr. Fatty streaks in the coronary arteries and aorta. Lab invest. 1968;18:560–564. [PubMed] [Google Scholar]
- 107.Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP 3rd, Herderick EE, Cornhill JF. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281:727–735. doi: 10.1001/jama.281.8.727 [DOI] [PubMed] [Google Scholar]
- 108.McGill HC Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP ; for the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce [DOI] [PubMed] [Google Scholar]
- 109.Berenson GS, Srinivasan SR, Bao WH, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302 [DOI] [PubMed] [Google Scholar]
- 110.McGill HC Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP, Wissler RW, Robertson AL, Cornhill JF, Gay S, Gay RE, et al. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Arterioscler Throm Vasc Biol. 1995;15:431–440. doi: 10.1161/01.atv.15.4.431 [DOI] [PubMed] [Google Scholar]
- 111.Zieske AW, Malcom GT, Strong JP. Natural history and risk factors of atherosclerosis in children and youth: the PDAY study. Pediatr Pathol Mol Med. 2002;21:213–237. doi: 10.1080/15227950252852104 [DOI] [PubMed] [Google Scholar]
- 112.Rocha VZ, Libby P Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55 [DOI] [PubMed] [Google Scholar]
- 113.Ross R Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138(pt 2):S419–S420. doi: 10.1016/s0002-8703(99)70266-8 [DOI] [PubMed] [Google Scholar]
- 114.Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, Lamarche B, Bergeron N. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab. 2005;90:6454–6459. doi: 10.1210/jc.2004-2438 [DOI] [PubMed] [Google Scholar]
- 115.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26:364–373. doi: 10.1016/j.tcm.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 116.Engin A Endothelial dysfunction in obesity. Adv Exp Med Biol. 2017;960:345–379. doi: 10.1007/978-3-319-48382-5_15 [DOI] [PubMed] [Google Scholar]
- 117.De Michele M, Panico S, Iannuzzi A, Celentano E, Ciardullo AV, Galasso R, Sacchetti L, Zarrilli F, Bond MG, Rubba P. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002;33:2923–2928. doi: 10.1161/01.str.0000038989.90931.be [DOI] [PubMed] [Google Scholar]
- 118.Freedman DS, Dietz WH, Tang R, Mensah GA, Bond MG, Urbina EM, Srinivasan S, Berenson GS. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:159–166. doi: 10.1038/sj.ijo.0802515 [DOI] [PubMed] [Google Scholar]
- 119.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501 [DOI] [PubMed] [Google Scholar]
- 120.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968 [DOI] [PubMed] [Google Scholar]
- 121.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101 [DOI] [PubMed] [Google Scholar]
- 122.Folsom AR, Stevens J, Schreiner PJ, McGovern PG. Body mass index, waist/hip ratio, and coronary heart disease incidence in African Americans and Whites: Atherosclerosis Risk in Communities Study Investigators. Am J Epidemiol. 1998;148:1187–1194. doi: 10.1093/oxfordjournals.aje.a009608 [DOI] [PubMed] [Google Scholar]
- 123.Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, van Dam RM, Hu FB, Visscher TL, Menotti A, et al. ; BMI-CHD Collaboration Investigators. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch intern Med. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720 [DOI] [PubMed] [Google Scholar]
- 124.Canoy D, Cairns BJ, Balkwill A, Wright FL, Green J, Reeves G, Beral V; Million Women Study Collaborators. Coronary heart disease incidence in women by waist circumference within categories of body mass index. Eur J Prev Cardiol. 2013;20:759–762. doi: 10.1177/2047487313492631 [DOI] [PubMed] [Google Scholar]
- 125.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714 [DOI] [PubMed] [Google Scholar]
- 126.Reis JP, Allen N, Gunderson EP, Lee JM, Lewis CE, Loria CM, Powell-Wiley TM, Rana JS, Sidney S, Wei G, et al. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity (Silver Spring). 2015;23:879–885. doi: 10.1002/oby.21023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E, et al. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc. 2016;5:e003921. doi: 10.1161/JAHA.116.003921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wilson PW, Bozeman SR, Burton TM, Hoaglin DC, Ben-Joseph R, Pashos CL. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. Circulation. 2008;118:124–130. doi: 10.1161/CIRCULATIONAHA.108.772962 [DOI] [PubMed] [Google Scholar]
- 129.Mørkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trøndelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–1078. doi: 10.1016/j.jacc.2013.11.035 [DOI] [PubMed] [Google Scholar]
- 130.Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med. 2014;174:15–22. doi: 10.1001/jamainternmed.2013.10522 [DOI] [PubMed] [Google Scholar]
- 131.Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J. 2018;39:397–406. doi: 10.1093/eurheartj/ehx448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G; Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects). Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–983. doi: 10.1016/S0140-6736(13)61836-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H, Fukuda D, Soeki T, Kitagawa T, Takanashi S, et al. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:1077–1084. doi: 10.1161/ATVBAHA.112.300829 [DOI] [PubMed] [Google Scholar]
- 134.Ishii T, Asuwa N, Masuda S, Ishikawa Y. The effects of a myocardial bridge on coronary atherosclerosis and ischaemia. J Pathol. 1998;185:4–9. doi: [DOI] [PubMed] [Google Scholar]
- 135.Ishikawa Y, Ishii T, Asuwa N, Masuda S. Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol-fed rabbits. Virchows Arch. 1997;430:163–171. doi: 10.1007/BF01008038 [DOI] [PubMed] [Google Scholar]
- 136.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–640. doi: 10.1016/j.jcmg.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 137.Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2625–2641. doi: 10.1016/j.jacc.2018.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATI0NAHA.114.012636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015; 131: 19–27. doi: 10.1161/CIRCULATI0NAHA.114.011939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–1195. doi: 10.1016/j.jacc.2005.10.062 [DOI] [PubMed] [Google Scholar]
- 141.Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol. 2018;72:707–717. doi: 10.1016/j.jacc.2018.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Quercioli A, Montecucco F, Pataky Z, Thomas A, Ambrosio G, Staub C, Di Marzo V, Ratib O, Mach F, Golay A, et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J. 2013;34:2063–2073. doi: 10.1093/eurheartj/eht085 [DOI] [PubMed] [Google Scholar]
- 143.Nomura A, Zareba W, Moss AJ. Obesity does not influence electrocardiographic parameters in coronary patients. Am J Cardiol. 2000;85:106–108, A9. doi: 10.1016/s0002-9149(99)00617-7 [DOI] [PubMed] [Google Scholar]
- 144.Adachi H, Hashimoto R, Tsuruta M, Jacobs DR Jr, Crow RS, Imaizumi T. Hyperinsulinemia and the development of ST-T electrocardiographic abnormalities: an 11-year follow-up study. Diabetes Care. 1997;20:1688–1692. doi: 10.2337/diacare.20.11.1688 [DOI] [PubMed] [Google Scholar]
- 145.Karason K, Lindroos AK, Stenlöf K, Sjöström L. Relief of cardiorespiratory symptoms and increased physical activity after surgically induced weight loss: results from the Swedish Obese Subjects study. Arch Intern Med. 2000;160:1797–1802. doi: 10.1001/archinte.160.12.1797 [DOI] [PubMed] [Google Scholar]
- 146.Gondoni LA, Titon AM, Nibbio F, Augello G, Caetani G, Liuzzi A. Heart rate behavior during an exercise stress test in obese patients. Nutr Metab Cardiovasc Dis. 2009;19:170–176. doi: 10.1016/j.numecd.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 147.Lear SA, Brozic A, Myers JN, Ignaszewski A. Exercise stress testing: an overview of current guidelines. Sports Med. 1999;27:285–312. doi: 10.2165/00007256-199927050-00002 [DOI] [PubMed] [Google Scholar]
- 148.Chrysohoou C, Skoumas J, Georgiopoulos G, Liontou C, Vogiatzi G, Tsioufis K, Lerakis S, Soulis D, Pitsavos C, Tousoulis D. Exercise capacity and haemodynamic response among 12,327 individuals with cardiometabolic risk factors undergoing treadmill exercise. Eur J Prev Cardiol. 2017;24:1627–1636. doi: 10.1177/2047487317726069 [DOI] [PubMed] [Google Scholar]
- 149.Bires AM, Lawson D, Wasser TE, Raber-Baer D. Comparison of Bruce treadmill exercise test protocols: is ramped Bruce equal or superior to standard Bruce in producing clinically valid studies for patients presenting for evaluation of cardiac ischemia or arrhythmia with body mass index equal to or greater than 30? J Nucl Med Technol. 2013;41:274–278. doi: 10.2967/jnmt.113.124727 [DOI] [PubMed] [Google Scholar]
- 150.Korbee RS, Boiten HJ, Ottenhof M, Valkema R, van Domburg RT, Schinkel AF. What is the value of stress (99m)Tc-tetrofosmin myocardial perfusion imaging for the assessment of very long-term outcome in obese patients? J Nucl Cardiol. 2013;20:227–233. doi: 10.1007/s12350-012-9657-z [DOI] [PubMed] [Google Scholar]
- 151.Chow BJ, Dorbala S, Di Carli MF, Merhige ME, Williams BA, Veledar E, Min JK, Pencina MJ, Yam Y, Chen L, et al. Prognostic value of PET myocardial perfusion imaging in obese patients. JACC Cardiovasc Imaging. 2014;7:278–287. doi: 10.1016/j.jcmg.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 152.Supariwala A, Makani H, Kahan J, Pierce M, Bajwa F, Dukkipati SS, Teixeira J, Chaudhry FA. Feasibility and prognostic value of stress echocardiography in obese, morbidly obese, and super obese patients referred for bariatric surgery. Echocardiography. 2014;31:879–885. doi: 10.1111/echo.12481 [DOI] [PubMed] [Google Scholar]
- 153.Argulian E, Halpern DG, Agarwal V, Agarwal SK, Chaudhry FA. Predictors of ischemia in patients referred for evaluation of exertional dyspnea: a stress echocardiography study. J Am Soc Echocardiogr. 2013;26:72–76. doi: 10.1016/j.echo.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 154.Hu SJ, Liu SX, Katus HA, Luedde M. The value of contrast dobutamine stress echocardiography on detecting coronary artery disease in overweight and obese patients. Can J Cardiol. 2007;23:885–889. doi: 10.1016/s0828-282x(07)70844-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shah BN, Senior R. Stress echocardiography in patients with morbid obesity. Echo Res Pract. 2016;3:R13–R18. doi: 10.1530/ERP-16-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lerakis S, Kalogeropoulos AP, El-Chami MF, Georgiopoulou VV, Abraham A, Lynch SA, Lewis AJ, Leach GC, Osier EJ, Veledar E, et al. Transthoracic dobutamine stress echocardiography in patients undergoing bariatric surgery. Obes Surg. 2007;17:1475–1481. doi: 10.1007/s11695-008-9425-y [DOI] [PubMed] [Google Scholar]
- 157.Mulvagh SL, DeMaria AN, Feinstein SB, Burns PN, Kaul S, Miller JG, Monaghan M, Porter TR, Shaw LJ, Villanueva FS. Contrast echocardiography: current and future applications. J Am Soc Echocardiogr. 2000;13:331–342. doi: 10.1067/mje.2000.105462 [DOI] [PubMed] [Google Scholar]
- 158.Medical Advisory Secretariat. Stress echocardiography with contrast for the diagnosis of coronary artery disease: an evidence-based analysis. Ont Health Technol Assess Ser. 2010;10:1–59. [PMC free article] [PubMed] [Google Scholar]
- 159.Madu EC. Transesophageal dobutamine stress echocardiography in the evaluation of myocardial ischemia in morbidly obese subjects. Chest. 2000;117:657–661. doi: 10.1378/chest.117.3.657 [DOI] [PubMed] [Google Scholar]
- 160.Legault S, Sénéchal M, Bergeron S, Arsenault M, Tessier M, Guimond J, Poirier P. Usefulness of an accelerated transoesophageal stress echocardiography in the preoperative evaluation of high risk severely obese subjects awaiting bariatric surgery. Cardiovasc Ultrasound. 2010;8:30. doi: 10.1186/1476-7120-8-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shah RV, Heydari B, Coelho-Filho O, Abbasi SA, Feng JH, Neilan TG, Francis S, Blankstein R, Steigner M, Jerosch-Herold M, et al. Vasodilator stress perfusion CMR imaging is feasible and prognostic in obese patients. JACC Cardiovasc Imaging. 2014;7:462–472. doi: 10.1016/j.jcmg.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation. 2007; 115:402–426. doi: 10.1161/CIRCULATIONAHA.107.181425 [DOI] [PubMed] [Google Scholar]
- 163.Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung HS, Choi Y, Ahn J, et al. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–2686. doi: 10.1016/j.jacc.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 164.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143 [DOI] [PubMed] [Google Scholar]
- 165.See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50:752–759. doi: 10.1016/j.jacc.2007.04.066 [DOI] [PubMed] [Google Scholar]
- 166.Park J, Lee ES, Lee DY, Kim J, Park SE, Park CY, Lee WY, Oh KW, Park SW, Rhee EJ. Waist circumference as a marker of obesity is more predictive of coronary artery calcification than body mass index in apparently healthy Korean adults: the Kangbuk Samsung Health Study. Endocrinol Metab (Seoul). 2016;31:559–566. doi: 10.3803/EnM.2016.31.4.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Labounty TM, Gomez MJ, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, et al. Body mass index and the prevalence, severity, and risk of coronary artery disease: an international multicentre study of 13 874 patients. Eur Heart J Cardiovasc Imaging. 2013;14:456–463. doi: 10.1093/ehjci/jes179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Imai A, Komatsu S, Ohara T, Kamata T, Yoshida J, Miyaji K, Takewa M, Kodama K. Visceral abdominal fat accumulation predicts the progression of noncalcified coronary plaque. Atherosclerosis. 2012;222:524–529. doi: 10.1016/j.atherosclerosis.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 169.Husmann L, Leschka S, Boehm T, Desbiolles L, Schepis T, Koepfli P, Gaemperli O, Marincek B, Kaufmann P, Alkadhi H. Influence of body mass index on coronary artery opacification in 64-slice CT angiography [in German]. Rofo. 2006;178:1007–1013. doi: 10.1055/s-2006-926871 [DOI] [PubMed] [Google Scholar]
- 170.Hibbert B, Simard T, Wilson KR, Hawken S, Wells GA, Ramirez FD, Le May MR, So DY, Glover CA, Froeschl M, et al. Transradial versus transfemoral artery approach for coronary angiography and percutaneous coronary intervention in the extremely obese. JACC Cardiovasc Interv. 2012;5:819–826. doi: 10.1016/j.jcin.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 171.McNulty PH, Ettinger SM, Field JM, Gilchrist IC, Kozak M, Chambers CE, Gascho JA. Cardiac catheterization in morbidly obese patients. Catheter Cardiovasc Interv. 2002;56:174–177. doi: 10.1002/ccd.10186 [DOI] [PubMed] [Google Scholar]
- 172.Wong P, Harding S, Walters D, Hull ML, Jang IK. Vascular complications after hemostatic puncture closure device (Angio-Seal) are not higher in overweight patients. J Invasive Cardiol. 2001;13:623–625. [PubMed] [Google Scholar]
- 173.Plourde G, Pancholy SB, Nolan J, Jolly S, Rao SV, Amhed I, Bangalore S, Patel T, Dahm JB, Bertrand OF. Radiation exposure in relation to the arterial access site used for diagnostic coronary angiography and percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. 2015;386:2192–2203. doi: 10.1016/S0140-6736(15)00305-0 [DOI] [PubMed] [Google Scholar]
- 174.Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015;66:337–346. doi: 10.1016/j.jacc.2015.05.069 [DOI] [PubMed] [Google Scholar]
- 175.Ohashi N, Yamamoto H, Horiguchi J, Kitagawa T, Kunita E, Utsunomiya H, Oka T, Kohno N, Kihara Y. Association between visceral adipose tissue area and coronary plaque morphology assessed by CT angiography. JACC Cardiovasc Imaging. 2010;3:908–917. doi: 10.1016/j.jcmg.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 176.Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, Lloyd-Jones DM. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–287. doi: 10.1001/jamacardio.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Reprint of: healthy weight and obesity prevention: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(Pt B):3027–3052. doi: 10.1016/j.jacc.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 178.Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, Milani RV. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–150. doi: 10.1016/j.pcad.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 179.Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61:151–156. doi: 10.1016/j.pcad.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 180.Bassi N, Karagodin I, Wang S, Vassallo P, Priyanath A, Massaro E, Stone NJ. Lifestyle modification for metabolic syndrome: a systematic review. Am J Med. 2014;127:1242.e1–1242.e10. doi: 10.1016/j.amjmed.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 181.Lien LF, Brown AJ, Ard JD, Loria C, Erlinger TP, Feldstein AC, Lin PH, Champagne CM, King AC, McGuire HL, et al. Effects of PREMIER lifestyle modifications on participants with and without the metabolic syndrome. Hypertension. 2007;50:609–616. doi: 10.1161/HYPERTENSIONAHA.107.089458 [DOI] [PubMed] [Google Scholar]
- 182.Wing R, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, et al. ; Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, Sharma P, Fraser C, MacLennan G. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. doi: 10.1136/bmj.j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Thomas RJ, Squires RW, Allison TG. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15:336–340. doi: 10.1097/HJR.0b013e3282f48348 [DOI] [PubMed] [Google Scholar]
- 185.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914 [DOI] [PubMed] [Google Scholar]
- 186.Batsis JA, Sarr MG, Collazo-Clavell ML, Thomas RJ, Romero-Corral A, Somers VK, Lopez-Jimenez F. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol. 2008;102:930–937. doi: 10.1016/j.amjcard.2008.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, et al. ; on behalf of the American Heart Association Obesity Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing, Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease, and Stroke Council. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a [DOI] [PubMed] [Google Scholar]
- 188.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. ; PREDIMED Study Investigators . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 189.Heffron SP, Parham JS, Pendse J, Alemán JO. Treatment of obesity in mitigating metabolic risk. Circ Res. 2020; 126:1646–1665. doi: 10.1161/CIRCRESAHA.119.315897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gregg EW, Jakicic JM, Lewis CE, Regensteiner JG, Pi-Sunyer X, Wing RR, Curtis JM, Yanovski SZ, Evans M, Lang W, et al. ; Look AHEAD Research Group . Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. doi: 10.1016/S2213-8587(16)30162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. ; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bohula EA, Wiviott SD, Scirica BM. Lorcaserin safety in overweight or obese patients. N Engl J Med. 2019;380:100. doi: 10.1056/NEJMc1813971 [DOI] [PubMed] [Google Scholar]
- 193.Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, Perez A, Smith SR. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315:990–1004. doi: 10.1001/jama.2016.1558 [DOI] [PubMed] [Google Scholar]
- 194.Fisher DP, Johnson E, Haneuse S, Arterburn D, Coleman KJ, O’Connor PJ, O’Brien R, Bogart A, Theis MK, Anau J, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320:1570–1582. doi: 10.1001/jama.2018.14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Payvar S, Kim S, Rao SV, Krone R, Neely M, Paladugu N, Daggubati R. Inhospital outcomes of percutaneous coronary interventions in extremely obese and normal-weight patients: findings from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2013;62:692–696. doi: 10.1016/j.jacc.2013.05.058 [DOI] [PubMed] [Google Scholar]
- 196.Joncas SX, Poirier P, Ardilouze JL, Carrier N, Fayad T, Farand P Delayed efficient anticoagulation with heparin in patients with a weight of 110 kg and more treated for acute coronary syndrome. Obesity (Silver Spring). 2013;21:1753–1758. doi: 10.1002/oby.20029 [DOI] [PubMed] [Google Scholar]
- 197.Buschur ME, Smith D, Share D, Campbell W, Mattichak S, Sharma M, Gurm HS. The burgeoning epidemic of morbid obesity in patients undergoing percutaneous coronary intervention: insight from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. J Am Coll Cardiol. 2013;62:685–691. doi: 10.1016/j.jacc.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 198.Holroyd EW, Sirker A, Kwok CS, Kontopantelis E, Ludman PF, De Belder MA, Butler R, Cotton J, Zaman A, Mamas MA; British Cardiovascular Intervention Society and National Institute of Cardiovascular Outcomes Research. The relationship of body mass index to percutaneous coronary intervention outcomes: does the obesity paradox exist in contemporary percutaneous coronary intervention cohorts? Insights from the British Cardiovascular Intervention Society Registry. JACC Cardiovasc Interv. 2017;10:1283–1292. doi: 10.1016/j.jcin.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 199.Terada T, Forhan M, Norris CM, Qiu WY, Padwal R, Sharma AM, Nagendran J, Johnson JA. Differences in short- and long-term mortality associated with BMI following coronary revascularization. J Am Heart Assoc. 2017;6:e005335. doi: 10.1161/JAHA.116.005335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lancefield T, Clark DJ, Andrianopoulos N, Brennan AL, Reid CM, Johns J, Freeman M, Charter K, Duffy SJ, Ajani AE, et al. ; MIG (Melbourne Interventional Group) Registry. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. 2010;3:660–668. doi: 10.1016/j.jcin.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 201.Mehta L, Devlin W, McCullough PA, O’Neill WW, Skelding KA, Stone GW, Boura JA, Grines CL. Impact of body mass index on outcomes after percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol. 2007;99:906–910. doi: 10.1016/j.amjcard.2006.11.038 [DOI] [PubMed] [Google Scholar]
- 202.Park DW, Kim YH, Yun SC, Ahn JM, Lee JY, Kim WJ, Kang SJ, Lee SW, Lee CW, Park SW, et al. Association of body mass index with major cardiovascular events and with mortality after percutaneous coronary intervention. Circ Cardiovasc Interv 2013;6:146–153. doi: 10.1161/CIRCINTERVENTIONS.112.000062 [DOI] [PubMed] [Google Scholar]
- 203.Ma WQ, Sun XJ, Wang Y, Han XQ, Zhu Y, Liu NF. Does body mass index truly affect mortality and cardiovascular outcomes in patients after coronary revascularization with percutaneous coronary intervention or coronary artery bypass graft? A systematic review and network meta-analysis. Obes Rev. 2018;19:1236–1247. doi: 10.1111/obr.12713 [DOI] [PubMed] [Google Scholar]
- 204.Li YH, Lin GM, Lin CL, Wang JH, Han CL. Relation of body mass index to mortality among patients with percutaneous coronary intervention longer than 5 years follow-up: a meta-analysis. Int J Cardiol. 2013;168:4315–4318. doi: 10.1016/j.ijcard.2013.04.174 [DOI] [PubMed] [Google Scholar]
- 205.Unek IT, Bayraktar F, Solmaz D, Ellidokuz H, Sisman AR, Yuksel F, Yesil S. The levels of soluble CD40 ligand and C-reactive protein in normal weight, overweight and obese people. Clin Med Res. 2010;8:89–95. doi: 10.3121/cmr.2010.889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Beavers CJ, Heron P, Smyth SS, Bain JA, Macaulay TE. Obesity and antiplatelets: does one size fit all? Thromb Res. 2015;136:712–716. doi: 10.1016/j.thromres.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 207.Farb MG, Bigornia S, Mott M, Tanriverdi K, Morin KM, Freedman JE, Joseph L, Hess DT, Apovian CM, Vita JA, et al. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–237. doi: 10.1016/j.jacc.2011.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Neergaard-Petersen S, Hvas AM, Kristensen SD, Grove EL. Platelets and antiplatelet therapy in patients with coronary artery disease and diabetes. Semin Thromb Hemost. 2016;42:234–241. doi: 10.1055/s-0036-1571308 [DOI] [PubMed] [Google Scholar]
- 209.Norgard NB. Obesity and altered aspirin pharmacology. Clin Pharmacokinet. 2018;57:663–672. doi: 10.1007/s40262-017-0611-8 [DOI] [PubMed] [Google Scholar]
- 210.Bordeaux BC, Qayyum R, Yanek LR, Vaidya D, Becker LC, Faraday N, Becker DM. Effect of obesity on platelet reactivity and response to low-dose aspirin. Prev Cardiol. 2010;13:56–62. doi: 10.1111/j.1751-7141.2009.00058.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tamminen M, Lassila R, Westerbacka J, Vehkavaara S, Yki-Jarvinen H. Obesity is associated with impaired platelet-inhibitory effect of acetylsalicylic acid in nondiabetic subjects. Int J Obes Relat Metab Disord. 2003;27:907–911. doi: 10.1038/sj.ijo.0802312 [DOI] [PubMed] [Google Scholar]
- 212.Bhatt DL, Grosser T, Dong JF, Logan D, Jeske W, Angiolillo DJ, Frelinger AL 3rd, Lei L, Liang J, Moore JE, et al. Enteric coating and aspirin non-responsiveness in patients with type 2 diabetes mellitus. J Am Coll Cardiol. 2017;69:603–612. doi: 10.1016/j.jacc.2016.11.050 [DOI] [PubMed] [Google Scholar]
- 213.Stohlawetz P, Folman CC, von dem Borne AE, Pernerstorfer T, Eichler HG, Panzer S, Jilma B. Effects of endotoxemia on thrombopoiesis in men. Thromb Haemost. 1999;81:613–617. [PubMed] [Google Scholar]
- 214.Guthikonda S, Alviar CL, Vaduganathan M, Arikan M, Tellez A, DeLao T, Granada JF, Dong JF, Kleiman NS, Lev EI. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52:743–749. doi: 10.1016/j.jacc.2008.05.031 [DOI] [PubMed] [Google Scholar]
- 215.Pankert M, Quilici J, Loundou AD, Verdier V, Lambert M, Deharo P, Bonnet G, Gaborit B, Morange PE, Valero R, et al. Impact of obesity and the metabolic syndrome on response to clopidogrel or prasugrel and bleeding risk in patients treated after coronary stenting. Am J Cardiol. 2014;113:54–59. doi: 10.1016/j.amjcard.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 216.Deharo P, Pankert M, Bonnet G, Quilici J, Bassez C, Morange P, Alessi MC, Bonnet JL, Cuisset T. Body mass index has no impact on platelet inhibition induced by ticagrelor after acute coronary syndrome, conversely to prasugrel. Int J Cardiol. 2014;176:1200–1202. doi: 10.1016/j.ijcard.2014.07.228 [DOI] [PubMed] [Google Scholar]
- 217.Prabhakar G, Haan CK, Peterson ED, Coombs LP, Cruzzavala JL, Murray GF. The risks of moderate and extreme obesity for coronary artery bypass grafting outcomes: a study from the Society of Thoracic Surgeons’ database. Ann Thorac Surg. 2002;74:1 125–1130. doi: 10.1016/s0003-4975(02)03899-7 [DOI] [PubMed] [Google Scholar]
- 218.Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation. 1996;94(suppl):II87–II92. [PubMed] [Google Scholar]
- 219.Birkmeyer NJ, Charlesworth DC, Hernandez F, Leavitt BJ, Marrin CA, Morton JR, Olmstead EM, O’Connor GT. Obesity and risk of adverse outcomes associated with coronary artery bypass surgery: Northern New England Cardiovascular Disease Study Group. Circulation. 1998;97:1689–1694. doi: 10.1161/01.cir.97.17.1689 [DOI] [PubMed] [Google Scholar]
- 220.Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity (Silver Spring). 2008;16:442–450. doi: 10.1038/oby.2007.36 [DOI] [PubMed] [Google Scholar]
- 221.Prapas SN, Panagiotopoulos IA, Salama Ayyad MA, Protogeros DA, Linardakis IN, Kotsis VN, Katinioti AA, Michalopoulos AS. Impact of obesity on outcome of patients undergoing off-pump coronary artery bypass grafting using aorta no-touch technique. Interact Cardiovasc Thorac Surg. 2010;1 1:234–237. doi: 10.1510/icvts.2010.234443 [DOI] [PubMed] [Google Scholar]
- 222.Wagner BD, Grunwald GK, Rumsfeld JS, Hill JO, Ho PM, Wyatt HR, Shroyer AL. Relationship of body mass index with outcomes after coronary artery bypass graft surgery. Ann Thorac Surg. 2007;84:10–16. doi: 10.1016/j.athoracsur.2007.03.017 [DOI] [PubMed] [Google Scholar]
- 223.Benedetto U, Danese C, Codispoti M. Obesity paradox in coronary artery bypass grafting: myth or reality? J Thorac Cardiovasc Surg. 2014;147:1517–1523. doi: 10.1016/j.jtcvs.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 224.Virani SS, Nambi V, Lee VV, Elayda MA, Pan W, Petersen LA, Wilson JM, Willerson JT, Ballantyne CM. Obesity an independent predictor of in-hospital postoperative renal insufficiency among patients undergoing cardiac surgery? Tex Heart I J. 2009;36:540–545. [PMC free article] [PubMed] [Google Scholar]
- 225.Nolan HR, Davenport DL, Ramaiah C. BMI is an independent preoperative predictor of intraoperative transfusion and postoperative chest-tube output. Int J Angiol. 2013;22:31–36. doi: 10.1055/s-0033-1333865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Totaro P Obesity and coronary surgery: new concepts for an old problem. Expert Rev Cardiovasc Ther. 2008;6:897–903. doi: 10.1586/14779072.6.6.897 [DOI] [PubMed] [Google Scholar]
- 227.Hernandez AV, Kaw R, Pasupuleti V, Bina P, loannidis JP, Bueno H, Boersma E, Gillinov M; Cardiovascular Meta-Analyses Research Group. Association between obesity and postoperative atrial fibrillation in patients undergoing cardiac operations: a systematic review and meta-analysis. Ann Thorac Surg. 2013;96:1104–1116. doi: 10.1016/j.athoracsur.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 228.Chassé M, Mathieu P, Voisine P, Després JP, Pibarot P, Baillot R, Lellouche F, Poirier P The underestimated belly factor: waist circumference is linked to significant morbidity following isolated coronary artery bypass grafting. Can J Cardiol. 2016;32:327–335. doi: 10.1016/j.cjca.2015.06.031 [DOI] [PubMed] [Google Scholar]
- 229.Ruka E, Dagenais F, Mohammadi S, Chauvette V, Poirier P, Voisine P Bilateral mammary artery grafting increases postoperative mediastinitis without survival benefit in obese patients. Eur J Cardiothorac Surg. 2016;50:1188–1195. doi: 10.1093/ejcts/ezw164 [DOI] [PubMed] [Google Scholar]
- 230.Parisian Mediastinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: a prospective, multicenter study. J Thorac Cardiovasc Surg. 1996;111:1200–1207. [DOI] [PubMed] [Google Scholar]
- 231.Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res. 2014;164:345–356. doi: 10.1016/j.trsl.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 232.Csige I, Ujvárosy D, Szabó Z, Lőrincz I, Paragh G, Harangi M, Somodi S. The impact of obesity on the cardiovascular system. J Diabetes Res 2018;2018:3407306. doi: 10.1155/2018/3407306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kitzman DW, Lam CSP Obese heart failure with preserved ejection fraction phenotype: from pariah to central player. Circulation. 2017;136:20–23. doi: 10.1161/CIRCULATIONAHA.117.028365 [DOI] [PubMed] [Google Scholar]
- 235.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245 [DOI] [PubMed] [Google Scholar]
- 236.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237–244. doi: 10.1161/CIRCULATIONAHA.109.887893 [DOI] [PubMed] [Google Scholar]
- 237.Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, Jessup M, Kosiborod M, Pritchett AM, Ramasubbu K, et al. ; on behalf of the American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular and Stroke Nursing; Council on Hypertension; and Council on Quality and Outcomes Research. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e535–e578. doi: 10.1161/CIR.0000000000000450 [DOI] [PubMed] [Google Scholar]
- 238.Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Folsom AR, Chambless LE, Heiss G. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities Study. Circ Heart Fail. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Levitan EB, Yang AZ, Wolk A, Mittleman MA. Adiposity and incidence of heart failure hospitalization and mortality: a population-based prospective study. Circ Heart Fail. 2009;2:202–208. doi: 10.1161/CIRCHEARTFAILURE.108.794099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rodriguez Flores M, Aguilar Salinas C, Piché ME, Auclair A, Poirier P. Effect of bariatric surgery on heart failure. Expert Rev Cardiovasc Ther. 2017;15:567–579. doi: 10.1080/14779072.2017.1352471 [DOI] [PubMed] [Google Scholar]
- 241.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, Berry JD, Khera A, McGuire DK, Vega GL, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6:800–807. doi: 10.1161/CIRCIMAGING.113.000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Murase T, Hattori T, Ohtake M, Abe M, Amakusa Y, Takatsu M, Murohara T, Nagata K. Cardiac remodeling and diastolic dysfunction in DahlS.ZLepr(fa)/Lepr(fa) rats: a new animal model of metabolic syndrome. Hypertens Res. 2012;35:186–193. doi: 10.1038/hr.2011.157 [DOI] [PubMed] [Google Scholar]
- 243.Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD, Lavie CJ. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:975–982. doi: 10.1016/j.jchf.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 244.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–1142. doi: 10.1016/j.jacc.2016.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pandey A, Cornwell WK 3rd, Willis B, Neeland IJ, Gao A, Leonard D, DeFina L, Berry JD. Body mass index and cardiorespiratory fitness in midlife and risk of heart failure hospitalization in older age: findings from the Cooper Center Longitudinal Study. JACC Heart Fail. 2017;5:367–374. doi: 10.1016/j.jchf.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 246.Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. 2012;110:77–82. doi: 10.1016/j.amjcard.2012.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17:374–380. doi: 10.1016/j.cardfail.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 248.Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70:2739–2749. doi: 10.1016/j.jacc.2017.09.1111 [DOI] [PubMed] [Google Scholar]
- 249.Lavie CJ, Milani RV, Ventura HO. Adipose composition and heart failure prognosis: paradox or not? J Am Coll Cardiol. 2017;70:2750–2751. doi: 10.1016/j.jacc.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 250.Futter JE, Cleland JG, Clark AL. Body mass indices and outcome in patients with chronic heart failure. Eur J Heart Fail. 2011;13:207–213. doi: 10.1093/eurjhf/hfq218 [DOI] [PubMed] [Google Scholar]
- 251.Doesch C, Suselbeck T, Leweling H, Fluechter S, Haghi D, Schoenberg SO, Borggrefe M, Papavassiliu T. Bioimpedance analysis parameters and epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure. Obesity (Silver Spring). 2010;18:2326–2332. doi: 10.1038/oby.2010.65 [DOI] [PubMed] [Google Scholar]
- 252.Martin J, Bergeron S, Pibarot P, Bastien M, Biertho L, Lescelleur O, Bertrand F, Simard S, Poirier P. Impact of bariatric surgery on N-terminal fragment of the prohormone brain natriuretic peptide and left ventricular diastolic function. Can J Cardiol. 2013;29:969–975. doi: 10.1016/j.cjca.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 253.Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, Loncar G, Springer J, Doehner W, Lainscak M, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-Morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2018;20:1580–1587. doi: 10.1002/ejhf.1304 [DOI] [PubMed] [Google Scholar]
- 254.Ventura HO, Carbone S, Lavie CJ. Muscling up to improve heart failure prognosis. Eur J Heart Fail. 2018;20:1588–1590. doi: 10.1002/ejhf.1314 [DOI] [PubMed] [Google Scholar]
- 255.Carbone S, Billingsley HE, Rodriguez-Miguelez P, Kirkman DL, Garten R, Franco RL, Lee DC, Lavie CJ. Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol. 2020;45:100417. doi: 10.1016/j.cpcardiol.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pathak RK, Mahajan R, Lau DH, Sanders P. The implications of obesity for cardiac arrhythmia mechanisms and management. Can J Cardiol. 2015;31:203–210. doi: 10.1016/j.cjca.2014.10.027 [DOI] [PubMed] [Google Scholar]
- 257.Plourde B, Sarrazin JF, Nault I, Poirier P Sudden cardiac death and obesity. Expert Rev Cardiovasc Ther. 2014;12:1099–1110. doi: 10.1586/14779072.2014.952283 [DOI] [PubMed] [Google Scholar]
- 258.Chiuve SE, Sun Q, Sandhu RK, Tedrow U, Cook NR, Manson JE, Albert CM. Adiposity throughout adulthood and risk of sudden cardiac death in women. JACC Clin Electrophysiol. 2015;1:520–528. doi: 10.1016/j.jacep.2015.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Adabag S, Huxley RR, Lopez FL, Chen LY, Sotoodehnia N, Siscovick D, Deo R, Konety S, Alonso A, Folsom AR. Obesity related risk of sudden cardiac death in the Atherosclerosis Risk in Communities study. Heart. 2015;101:215–221. doi: 10.1136/heartjnl-2014-306238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Aune D, Schlesinger S, Norat T, Riboli E. Body mass index, abdominal fatness, and the risk of sudden cardiac death: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2018;33:711–722. doi: 10.1007/s10654-017-0353-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hookana E, Junttila MJ, Puurunen VP, Tikkanen JT, Kaikkonen KS, Kortelainen ML, Myerburg RJ, Huikuri HV. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011;8:1570–1 575. doi: 10.1016/j.hrthm.2011.06.031 [DOI] [PubMed] [Google Scholar]
- 262.Empana JP, Ducimetiere P, Charles MA, Jouven X. Sagittal abdominal diameter and risk of sudden death in asymptomatic middle-aged men: the Paris Prospective Study I. Circulation. 2004;110:2781–2785. doi: 10.1161/01.CIR.0000146395.64065.BA [DOI] [PubMed] [Google Scholar]
- 263.Messerli FH, Nunez BD, Ventura HO, Snyder DW. Overweight and sudden death: increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med. 1987;147:1725–1728. doi: 10.1001/archinte.147.10.1725 [DOI] [PubMed] [Google Scholar]
- 264.Fraley MA, Birchem JA, Senkottaiyan N, Alpert MA. Obesity and the electrocardiogram. Obes Rev. 2005;6:275–281. doi: 10.1111/j.1467-789X.2005.00199.x [DOI] [PubMed] [Google Scholar]
- 265.Pietrasik G, Goldenberg I, McNitt S, Moss AJ, Zareba W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol. 2007;18:181–184. doi: 10.1111/j.1540-8167.2006.00680.x [DOI] [PubMed] [Google Scholar]
- 266.Sabbag A, Goldenberg I, Moss AJ, McNitt S, Glikson M, Biton Y, Jackson L, Polonsky B, Zareba W, Kutyifa V. Predictors and risk of ventricular tachyarrhythmias or death in Black and White cardiac patients: a MADIT-CRT Trial substudy. JACC Clin Electrophysiol. 2016;2:448–455. doi: 10.1016/j.jacep.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 267.Lalani AP, Kanna B, John J, Ferrick KJ, Huber MS, Shapiro LE. Abnormal signal-averaged electrocardiogram (SAECG) in obesity. Obes Res. 2000;8:20–28. doi: 10.1038/oby.2000.4 [DOI] [PubMed] [Google Scholar]
- 268.Kasper EK, Hruban RH, Baughman KL. Cardiomyopathy of obesity: a clinicopathologic evaluation of 43 obese patients with heart failure. Am J Cardiol. 1992;70:921–924. doi: 10.1016/0002-9149(92)90739-l [DOI] [PubMed] [Google Scholar]
- 269.Duflou J, Virmani R, Rabin I, Burke A, Farb A, Smialek J. Sudden death as a result of heart disease in morbid obesity. Am Heart J. 1995;130:306–313. doi: 10.1016/0002-8703(95)90445-x [DOI] [PubMed] [Google Scholar]
- 270.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Konno T, Hayashi K, Fujino N, Oka R, Nomura A, Nagata Y, Hodatsu A, Sakata K, Furusho H, Takamura M, et al. Electrocardiographic QRS fragmentation as a marker for myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26:1081–1087. doi: 10.1111/jce.12742 [DOI] [PubMed] [Google Scholar]
- 272.Narayanan K, Zhang L, Kim C, Uy-Evanado A, Teodorescu C, Reinier K, Zheng ZJ, Gunson K, Jui J, Chugh SS. QRS fragmentation and sudden cardiac death in the obese and overweight. J Am Heart Assoc. 2015;4:e001654. doi: 10.1161/JAHA.114.001654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Brenyo A, Pietrasik G, Barsheshet A, Huang DT, Polonsky B, McNitt S, Moss AJ, Zareba W. QRS fragmentation and the risk of sudden cardiac death in MADIT II. J Cardiovasc Electrophysiol. 2012;23:1343–1348. doi: 10.1111/j.1540-8167.2012.02390.x [DOI] [PubMed] [Google Scholar]
- 274.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363 [DOI] [PubMed] [Google Scholar]
- 275.Littlejohns B, Pasdois P, Duggan S, Bond AR, Heesom K, Jackson CL, Angelini GD, Halestrap AP, Suleiman MS. Hearts from mice fed a nonobesogenic high-fat diet exhibit changes in their oxidative state, calcium and mitochondria in parallel with increased susceptibility to reperfusion injury. PLoS One. 2014;9:e100579. doi: 10.1371/journal.pone.0100579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zarzoso M, Mironov S, Guerrero-Serna G, Willis BC, Pandit SV. Ventricular remodelling in rabbits with sustained high-fat diet. Acta Physiol (Oxf). 2014;211:36–47. doi: 10.1111/apha.12185 [DOI] [PubMed] [Google Scholar]
- 277.Wu CK, Tsai HY, Su MY, Wu YF, Hwang JJ, Tseng WY, Lin JL, Lin LY. Pericardial fat is associated with ventricular tachyarrhythmia and mortality in patients with systolic heart failure. Atherosclerosis. 2015;241:607–614. doi: 10.1016/j.atherosclerosis.2015.05.025 [DOI] [PubMed] [Google Scholar]
- 278.Fuller B, Garland J, Anne S, Beh R, McNevin D, Tse R. Increased epicardial fat thickness in sudden death from stable coronary artery atherosclerosis. Am J Forensic Med Pathol. 2017;38:162–166. doi: 10.1097/PAF.0000000000000310 [DOI] [PubMed] [Google Scholar]
- 279.Chi PC, Chang SC, Yun CH, Kuo JY, Hung CL, Hou CJ, Liu CY, Yang FS, Wu TH, Bezerra HG, et al. The associations between various ectopic visceral adiposity and body surface electrocardiographic alterations: potential differences between local and remote systemic effects. PLoS One. 2016;11:e0158300. doi: 10.1371/journal.pone.0158300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Al-Mosawi AA, Nafakhi H, Hassan MB, Alareedh M, Al-Nafakh HA. ECG markers of arrythmogenic risk relationships with pericardial fat volume and BMI in patients with coronary atherosclerosis. J Electrocardiol. 2018;51:569–572. doi: 10.1016/j.jelectrocard.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 282.Pouliopoulos J, Chik WW, Kanthan A, Sivagangabalan G, Barry MA, Fahmy PN, Midekin C, Lu J, Kizana E, Thomas SP, et al. Intramyocardial adiposity after myocardial infarction: new implications of a substrate for ventricular tachycardia. Circulation. 2013; 128:2296–2308. doi: 10.1161/CIRCULATIONAHA.113.002238 [DOI] [PubMed] [Google Scholar]
- 283.Fumagalli S, Boni N, Padeletti M, Gori F, Boncinelli L, Valoti P, Baldasseroni S, Di Bari M, Masotti G, Padeletti L, et al. Determinants of thoracic electrical impedance in external electrical cardioversion of atrial fibrillation. Am J Cardiol. 2006;98:82–87. doi: 10.1016/j.amjcard.2006.01.065 [DOI] [PubMed] [Google Scholar]
- 284.Jain R, Nallamothu BK, Chan PS; for the American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Body mass index and survival after in-hospital cardiac arrest. Circ Cardiovasc Qual Outcomes. 2010;3:490–497. doi: 10.1161/CIRCOUTCOMES.109.912501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Shahreyar M, Dang G, Waqas Bashir M, Kumar G, Hussain J, Ahmad S, Pandey B, Thakur A, Bhandari S, Thandra K, et al. Outcomes of inhospital cardiopulmonary resuscitation in morbidly obese patients. JACC Clin Electrophysiol. 2017;3:174–183. doi: 10.1016/j.jacep.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 286.Wong CX, Brooks AG, Lau DH, Leong DP, Sun MT, Sullivan T, Roberts-Thomson KC, Sanders P. Factors associated with the epidemic of hospitalizations due to atrial fibrillation. Am J Cardiol. 2012;110:1496–1499. doi: 10.1016/j.amjcard.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 287.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 Year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. Eur Heart J. 2009;30:1113–1120. doi: 10.1093/eurheartj/ehp076 [DOI] [PubMed] [Google Scholar]
- 290.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation: the WHS (Women’s Health Study). J Am Coll Cardiol. 2010;55:2319–2327. doi: 10.1016/j.jacc.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp M, Twomey D, Ganesan AN, Rangnekar G, Roberts-Thomson KC, et al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol. 2015;1:139–152. doi: 10.1016/j.jacep.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 292.Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–2233. doi: 10.1093/eurheartj/ehn324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M, Mahajan R, Kuklik P, Zhang Y, Brooks AG, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. doi: 10.1016/j.hrthm.2012.08.043 [DOI] [PubMed] [Google Scholar]
- 294.Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP, Finnie JW, Samuel CS, Royce SG, Twomey DJ, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66:1–11. doi: 10.1016/j.jacc.2015.04.058 [DOI] [PubMed] [Google Scholar]
- 295.Munger TM, Dong YX, Masaki M, Oh JK, Mankad SV, Borlaug BA, Asirvatham SJ, Shen WK, Lee HC, Bielinski SJ, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol. 2012;60:851–860. doi: 10.1016/j.jacc.2012.03.042 [DOI] [PubMed] [Google Scholar]
- 296.Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, Carbone A, Teo K, Agbaedeng T, Linz D, et al. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol. 2018;4:1529–1540. doi: 10.1016/j.jacep.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 297.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010;56:784–788. doi: 10.1016/j.jacc.2010.03.071 [DOI] [PubMed] [Google Scholar]
- 298.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts-Thomson KC, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745–1751. doi: 10.1016/j.jacc.2010.11.045 [DOI] [PubMed] [Google Scholar]
- 299.Wong CX, Sun MT, Odutayo A, Emdin CA, Mahajan R, Lau DH, Pathak RK, Wong DT, Selvanayagam JB, Sanders P, et al. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9:e004378. doi: 10.1161/CIRCEP116.004378 [DOI] [PubMed] [Google Scholar]
- 300.Hatem SN, Sanders P Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res. 2014;102:205–213. doi: 10.1093/cvr/cvu045 [DOI] [PubMed] [Google Scholar]
- 301.Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–2035. doi: 10.1016/j.jacc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 302.Lavie CJ, Mehra MR, Ventura HO. Body composition and advanced heart failure therapy: weighing the options and outcomes. JACC Heart Fail. 2016;4:769–771. doi: 10.1016/j.jchf.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 303.Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Guazzi M, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251–258. doi: 10.1016/j.mayocp.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.McAuley PA, Keteyian SJ, Brawner CA, Dardari ZA, Al Rifai M, Ehrman JK, Al-Mallah MH, Whelton SP, Blaha MJ. Exercise capacity and the obesity paradox in heart failure: the FIT (Henry Ford Exercise Testing) Project. Mayo Clin Proc. 2018;93:701–708. doi: 10.1016/j.mayocp.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 305.Pandey A, Patel KV, Lavie CJ. Obesity, central adiposity, and fitness: understanding the obesity paradox in the context of other cardiometabolic parameters. Mayo Clin Proc. 2018;93:676–678. doi: 10.1016/j.mayocp.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 306.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, et al. ; HF-ACTION Investigators. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Beck-da-Silva L, Higginson L, Fraser M, Williams K, Haddad H. Effect of orlistat in obese patients with heart failure: a pilot study. Congest Heart Fail. 2005;11:118–123. doi: 10.1111/j.1527-5299.2005.03827.x [DOI] [PubMed] [Google Scholar]
- 308.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, et al. ; NHLBI Heart Failure Clinical Research Network. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–508. doi: 10.1001/jama.2016.10260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, Nilsson B, Møller JE, Hjort J, Rasmussen J, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE): a multi-centre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657 [DOI] [PubMed] [Google Scholar]
- 310.Ghosh RK, Ghosh GC, Gupta M, Bandyopadhyay D, Akhtar T, Deedwania P, Lavie CJ, Fonarow GC, Aneja A. Sodium glucose co-transporter 2 inhibitors and heart failure. Am J Cardiol. 2019;124:1790–1796. doi: 10.1016/j.amjcard.2019.08.038 [DOI] [PubMed] [Google Scholar]
- 311.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 312.Koshino Y, Villarraga HR, Somers VK, Miranda WR, Garza CA, Hsiao JF, Yu Y, Saleh HK, Lopez-Jimenez F. Changes in myocardial mechanics in patients with obesity following major weight loss after bariatric surgery. Obesity (Silver Spring). 2013;21:1111–1118. doi: 10.1002/oby.20168 [DOI] [PubMed] [Google Scholar]
- 313.Shimada YJ, Tsugawa Y, Brown DFM, Hasegawa K. Bariatric surgery and emergency department visits and hospitalizations for heart failure exacerbation: population-based, self-controlled series. J Am Coll Cardiol. 2016;67:895–903. doi: 10.1016/j.jacc.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 314.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 315.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, et al. Longterm effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 316.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. doi: 10.1001/jama.2013.280521 [DOI] [PubMed] [Google Scholar]
- 317.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 318.Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JML, Linz D, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018;20:1929–1935. doi: 10.1093/europace/euy117 [DOI] [PubMed] [Google Scholar]
- 319.Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017; 136:583–596. doi: 10.1161/CIRCULATIONAHA.116.023163 [DOI] [PubMed] [Google Scholar]


