Abstract
Mechanisms explaining the link between psoriasis, a pro-inflammatory condition, and cardiovascular disease (CVD) are not fully known. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is predominantly expressed in hepatocytes as a critical regulator of lipid metabolism and clinical trials targeting PCSK9 reduce CVD. Independent of its role in lipid metabolism, PCSK9 levels associate with endothelial dysfunction and predict CV events. We used two separate human psoriasis cohorts and the K14-Rac1V12−/+ murine model of psoriasis to investigate PCSK9 and CV risk in psoriasis. In both psoriasis cohorts, (n=88 and n=20), PCSK9 levels were 20% and 13% higher than age, sex, and cholesterol matched controls respectively (p<0.05 for each comparison), and correlated with psoriasis area severity index (r=0.43, p<0.05). Despite no difference in hepatocyte expression, K14-Rac1V12−/+ mice demonstrated skin-specific PCSK9 staining which was confirmed in human psoriatic lesional skin. In psoriasis patients, PCSK9 levels correlated with impaired endothelial vascular health (e.g. early atherosclerosis, β=4.5, p<0.01) and coronary artery calcium score (β=0.30, p=0.01) which remained significant after adjustment for Framingham risk, body mass index and active biologic use. Taken together, these findings suggest independent of cholesterol, an association between circulating PCSK9, and early and advanced stages of atherosclerosis in psoriasis.
INTRODUCTION
Patients with chronic inflammatory diseases such as psoriasis exhibit an increased risk of cardiovascular disease (CVD) independent of traditional CV risk factors.(Garshick M. et al., 2019, Gelfand et al., 2006) The inflammatory response in psoriasis includes systemic immune activation and overexpression of cytokines including IL17A, IL6 and TNFα which are also implicated in the development of atherosclerosis.(Greb et al., 2016) Despite this well-established association, specific mechanisms linking psoriasis and CVD are needed to identify and develop therapeutic targets.
The discovery of proprotein convertase subtilisin kexin 9 (PCSK9) as a critical regulator of cholesterol homeostasis revolutionized management of both hypercholesterolemia and CVD.(Seidah et al., 2014, Shapiro et al., 2018) PCSK9 expression is highest in the liver and regulates low-density lipoprotein (LDL) levels by diverting LDL receptor lysosomal degradation and inhibiting LDL receptor recycling.(Shapiro et al., 2018) Therapeutic agents which block PCSK9 promote enhanced LDL receptor expression, dramatically lowering LDL cholesterol and reduce CV events.(Shapiro et al., 2018)
Beyond hepatocytes, PCSK9 is also expressed in multiple organs including psoriatic lesional skin and in murine models, implicated in the pathogenesis of psoriasis lesion development.(Luan et al., 2019) PCSK9 is also secreted and circulates freely and attached to LDL particles.(Shapiro et al., 2018) Over the past 5 years, commercially available assays have been developed to quantify blood levels of PCSK9 which are shown to correlate with future CV events independent of traditional CV risk factors including LDL cholesterol.(Leander et al., 2016, Shapiro et al., 2018) In other inflammatory conditions including human immunodeficiency virus (HIV), PCSK9 levels are elevated compared to matched controls and associated with coronary endothelial dysfunction, (Leucker et al., 2018) suggesting that PCSK9 has relevance in the development of early atherosclerosis in inflammatory conditions including psoriasis.
Endothelial vascular pro-inflammatory activation is the first step in atherosclerosis development, (Gimbrone and Garcia-Cardena, 2016) and elevated in patients with psoriasis when compared to matched controls.(Garshick M. S. et al., 2019) More advanced atherosclerosis is also seen in patients with psoriasis including a higher prevalence of coronary artery calcium (CAC).(Mansouri et al., 2016) Therefore, utilizing two ongoing human studies of psoriasis and CVD in addition to a murine model of psoriasis associated with cardiometabolic disease, (Baumer et al., 2018) we investigated the association between psoriasis, PCSK9, early and advanced stages of atherosclerosis.
RESULTS
PCSK9 levels are increased in Psoriasis Patients
Demographic and clinical characteristics of psoriasis and age, sex, and cholesterol-matched healthy controls are listed in Table 1. The National Institutes of Health (NIH) cohort (Supplementary Figure S1) consisted of 88 middle-aged individuals (46.8 ± 13 years), predominantly male (57%), low CV risk by 10-year Framingham risk score (2% [1–4]), with a median psoriasis area severity index (PASI) score of 7.1 (3.6–12.3) and not on statin or biologic therapy. Despite matching by age, sex, and total cholesterol, the psoriasis cohort had higher body mass index (BMI, 28.4 ± 5.6 kg/m2 vs. 25.7 ± 4.1 kg/m2, p=0.02) and high-sensitivity C-reactive protein (hsCRP, 2.0 mg/L [0.8–4.5] vs. 1.0 mg/L [0.7–1.3], p<0.01) compared to healthy controls.
Table 1.
Characteristics of psoriasis and healthy control patients recruited from the National Institutes of Health
| Variable | Psoriasis (N=88) | Healthy controls (N = 21) | P-value |
|---|---|---|---|
| Demographic and Clinical Characteristics | |||
|
| |||
| Age, years | 46.9 ± 12.8 | 42.1 ± 10.4 | 0.06 |
| Males | 50 (57%) | 15 (71%) | 0.22 |
| Hyperlipidemia | 22 (25%) | 3 (15%) | 0.29 |
| Type-2 diabetes mellitus | 4 (5%) | 0 (0%) | 0.32 |
| Body mass index, kg/m2 | 28.6 ± 5.8 | 25.7 ± 4.1 | 0.02 |
| Hypertension | 12 (14%) | 1 (5%) | 0.26 |
| Current smoker | 12 (14%) | 0 (0%) | 0.07 |
| Lipid treatment | 0 (0%) | 0 (0%) | - |
|
| |||
| Clinical and Lab Values | |||
|
| |||
| Total cholesterol, mg/dl | 191.8 ± 37.1 | 195.8 ± 29.6 | 0.33 |
| HDL cholesterol, mg/dl | 57.2 ± 18.1 | 62.1 ± 22.5 | 0.15 |
| LDL cholesterol, mg/dl | 112.5 ± 28.7 | 108.4 ± 25.0 | 0.27 |
| Triglycerides, mg/dl | 110.5 ± 46.0 | 126.0 ± 80.6 | 0.12 |
| Framingham risk score, median, (IQR) | 2.0 (1.0–4.0) | 1.0 (0.6–2.1) | 0.02 |
| hsCRP, mg/L, median, (IQR) | 2.1 (0.8–4.5) | 1.0 (0.7–1.3) | 0.006 |
|
| |||
| Psoriasis Severity | |||
|
| |||
| Psoriasis area severity index score, median, (IQR) | 7.1 (3.6–12.3) | - | - |
| Systemic or Biologic treatment | 0 (0%) | - | - |
|
| |||
| PCSK9 Levels | |||
|
| |||
| PCSK9, ng/mL | 253 ± 94 | 209 ± 119 | 0.03 |
|
| |||
| Coronary Artery Calcium Score | |||
|
| |||
| CAC score,* median, range | 0 (0–653) | 0 (0–13) | 0.03 |
Data are mean ± SD or N (%) unless otherwise stated. CAC, coronary artery calcium. HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; IQR, interquartile range; PCSK9, Proprotein convertase subtilisin/kexin type 9.
CAC in Agatston units log-converted and analyzed as (lnCAC +1) to allow for normalizing the high percentage of zero values.
While psoriasis disease duration did not associate with PCSK9 (r=0.03, p=0.80), we noted PCSK9 was significantly higher in the psoriasis (vs. control) cohort (253 ± 94 ng/mL vs. 209 ± 119 ng/mL, p=0.03).
Murine Psoriasis, Circulating and Skin Expression of PCSK9
PCSK9 is expressed in multiple organs throughout the body and others have shown PCSK9 present in psoriatic skin plaque using the imiquimod model of psoriasis.(Luan et al., 2019) To further validate this in another murine psoriasis model, (Baumer et al., 2018, Winge et al., 2016) we leveraged the K14-Rac1V12−/+psoriasis murine model which is shown to have chronic psoriasis-like skin inflammation concomitant with a pro-atherosclerotic phenotype.(Baumer et al., 2018)
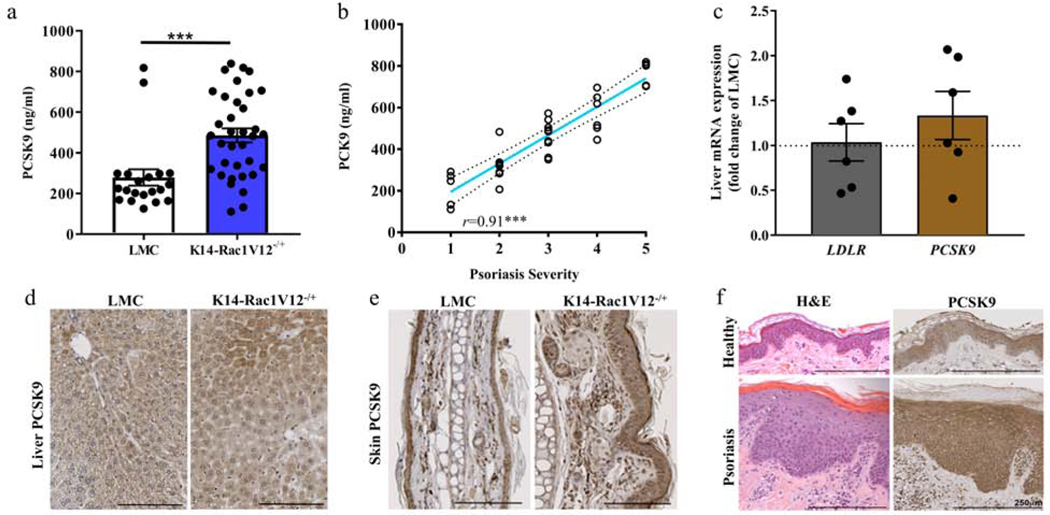
Consistent with our human psoriasis sample, plasma PCSK9 levels were elevated in the K14-Rac1V12−/+ mice vs. littermate controls (LMCs, Figure 1a, mean ± SEM; 485.9 ± 40 ng/mL vs. 278.8 ± 35 ng/mL, p<0.001). These levels positively correlated with murine skin disease severity (r=0.92, p<0.001, Figure 1b). Given PCSK9 is expressed in the liver as a critical regulator of hepatic LDL receptor expression, (Seidah et al., 2014) we evaluated hepatocyte PCSK9 and LDL receptor mRNA and PCSK9 protein expression in K14-Rac1V12−/+ and in LMC mice (Figure 1c and d, Supplementary Figure S3a and b). There was no change in liver-specific PCSK9 and LDL receptor mRNA and PCSK9 protein expression. In contrast, skin-specific PCSK9 expression was elevated in the skin of K14-Rac1V12−/+ mice (Figure 1e), a finding confirmed in lesional biopsies from human psoriasis (Figure 1f).
Figure 1: PCSK9 levels are increased in K14-Rac1V12−/+ psoriatic mice and human skin.
(a) PCSK9 levels in plasma of K14-Rac1V12−/+ mice and their LMC were detected using a commercially available ELISA (n LMC/n K14-Rac1V12−/+=20/35). (b) Severity of mouse skin phenotype correlated with PCSK9 in K14-Rac1V12−/+ mice (n=32). (c), Relative mRNA expression of genes involved in lipid homeostasis including PCSK9 and LDLR detected in mouse liver by qPCR (≥5 each). PCSK9 protein expression in murine liver (d) and skin (e) determined in K14-Rac1V12−/+ mice and their LMC by histology. (f) Human biopsy samples subjected to PCSK9 histological staining. Increased expression of PCSK9 in psoriatic lesional skin can be detected as compared to healthy volunteer skin (spotty uneven staining). LMC, litter mate controls; PCSK9, Proprotein convertase subtilisin/kexin type 9. Mean ± SEM, ***p<0.001. Commercially available ELISA assesses the Pro-Pro PCSK9, SV-Splice variant of PCSK9.
Association between PCSK9 and Circulating Inflammatory Profiles in Psoriasis
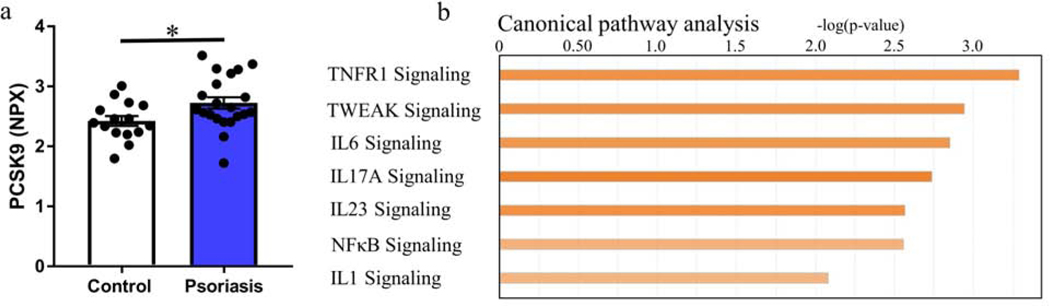
To characterize the relationship between PCSK9 and vascular dysfunction, we studied endothelial cell inflammation in psoriasis using a second validation cohort of deeply phenotyped psoriasis patients (NYU cohort, Table 2, Supplementary Figure S2). PCSK9 levels were higher than age-, sex- matched healthy controls (Figure 2a). Differences in PCSK9 levels between psoriasis and control remained significant in multivariable linear regression after adjustment for Framingham risk score and BMI (β=0.37, p=0.01), and in additional models incorporating the individual CV risk factors (age, gender, LDL-cholesterol, triglycerides, and BMI [β=0.31, p=0.03]). Finally, while there was no association between PCSK9 and psoriasis disease duration (r=0.002, p=0.99), PCSK9 associated with total cholesterol (r=0.34, p<0.05), LDL cholesterol (r=0.32, p=0.06), and PASI (r=0.43, p<0.05) with the association between PASI and PCSK9 still significant after adjusting for Framingham risk score, BMI and active biologic use (β=0.02, p<0.05).
Table 2.
Characteristics of psoriasis and healthy control patients recruited from NYU Langone Health
| Variable | Psoriasis (N=20) | Healthy controls (N = 15) | P-value |
|---|---|---|---|
| Demographic and Clinical Characteristics | |||
|
| |||
| Age, years | 42.7 ± 15 | 40.6 ± 14 | 0.68 |
| Male sex | 9 (45) | 8 (53) | 0.63 |
| Hyperlipidemia | 1 (5%) | 0 (0%) | 0.38 |
| Type-2 diabetes mellitus | 0 (0%) | 0 (0%) | - |
| Body mass index, kg/m2 | 27 (23 – 32) | 25 (23 – 30) | 0.66 |
| Hypertension | 0 (0%) | 0 (0%) | - |
| Current smoker | 0 (0%) | 0 (0%) | - |
| Lipid treatment | 0 (0%) | 0 (0%) | - |
|
| |||
| Clinical and Lab Values | |||
|
| |||
| Total cholesterol, mg/dl | 180 ± 42 | 156 ± 26 | 0.06 |
| HDL cholesterol, mg/dl | 49 ± 15 | 51 ± 12 | 0.69 |
| LDL cholesterol, mg/dl | 110 (81 – 121) | 84 (68 – 115) | 0.25 |
| Triglycerides, mg/dl | 86 (62–148) | 65 (56 – 103) | 0.09 |
| Framingham risk score, %, median (IQR) | 2.9 (1.7 – 5.0) | 1.7 (1.0 – 3.0) | 0.16 |
| hsCRP, mg/L, median (IQR) | 2.0 (0.6 – 4.5) | 1.4 (0.5 – 1.5) | 0.15 |
|
| |||
| Psoriasis Severity | |||
|
| |||
| Psoriasis area severity index score, median (IQR) | 5.1 (3.6 – 12.3) | - | - |
| Systemic or Biologic treatment | 10 (50) | - | - |
|
| |||
| PCSK9 Levels | |||
|
| |||
| PCSK9, NPX | 2.7 ± 0.4 | 2.4 ± 0.3 | 0.03 |
Data are mean ± SD or N (%) unless otherwise stated. HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; IQR, interquartile range; NPX, normalized eXpression units on a Log2 scale; PASI, psoriasis area severity index; PCSK9, Proprotein convertase subtilisin/kexin type 9.
Figure 2.
Inflammation in psoriasis associates with PCSK9 levels. (a) PCSK9 levels in patients with psoriasis compared with those in age- and sex-matched controls assessed through the Olink proteomic platform (n = 20 psoriasis; n = 15 controls). (b) Upregulated canonical pathways using Ingenuity Pathway Analysis of the association between PCSK9 levels and blood transcripts assessed in a subset of recruited participants with the most severe psoriasis (n = 10 psoriasis). Mean ± SEM, ∗P < 0.05. hsCRP, high-sensitivity C-reactive protein; NPX, normalized protein eXpression on a log2 scale.
To assess the relationship between PCSK9 levels and systemically altered pathways in an unbiased manner, we investigated the association between the whole blood transcriptome, pro-inflammatory proteins, and PCSK9.(Garshick M. S. et al., 2019) Whole blood RNA sequencing was performed in a subset of 10 moderate-to-severe psoriasis patients and 10 age, sex-matched controls (Supplementary Figure S2). In total, 322 blood transcripts correlated with PCSK9 (nominal p-value <0.05). Canonical pathway analysis (and confirmed at the protein expression level, Table S1) identified upregulated pro-inflammatory signaling pathways including IL17A, IL23, NFκB and inflammasome related pathways IL1 and IL6, also implicated in the underlying pathogenesis of psoriasis (Figure 2b and Supplementary Figure S4).(Greb et al., 2016)
Association between PCSK9 and Vascular Endothelial Inflammation in Psoriasis
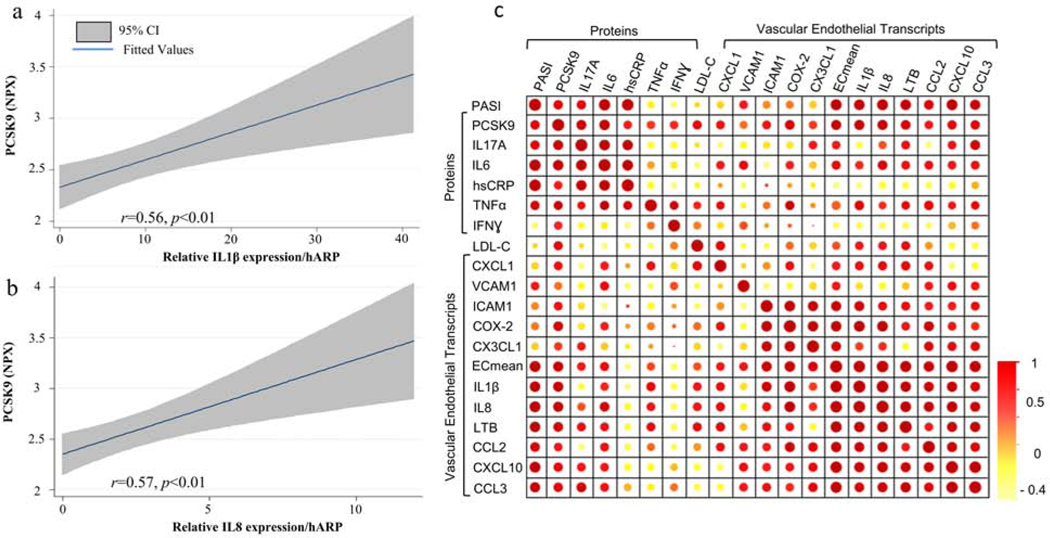
Endothelial pro-inflammatory activation is essential in the initiation of atherosclerosis. Prior data from our group demonstrated endothelial pro-inflammatory activation in patients with psoriasis.(Garshick M. S. et al., 2019) We therefore investigated the relationship between circulating PCSK9 and endothelial pro-inflammatory activation to highlight potential pathways through which psoriasis patients develop CVD. PCSK9 levels positively correlated with brachial vein endothelial expression of the mean composite endothelial pro-inflammatory transcriptome (β=4.5, p<0.01, Figure 3 a/b/c) which remained statistically significant after adjustment for Framingham risk, BMI and active biologic use (β=3.6, p<0.05). PCSK9 levels also correlated with individual endothelial vascular expression of IL1β and IL8 (Figure 3a/b/c). The point estimates in the observed correlations between PCSK9 and endothelial pro-inflammatory transcript expression were higher than between endothelial inflammation and circulating levels of IL6, IL17A. TNFα, and LDL cholesterol (correlation matrix - Figure 3c).
Figure 3. PCSK9 correlates with measures of vascular endothelial inflammation.
(a) Regression plot and correlation coefficient between PCSK9 levels and brachial vein endothelial expression of IL1β and (b) IL8. (c) Correlation matrices assessing the association between markers of psoriatic activity, lipids and brachial vein endothelial pro-inflammatory transcript expression (size and color indicate degree of correlation). hARP, human acidic ribosomal protein; human hsCRP, high-sensitivity C-reactive protein; IL, interleukin; IFN, interferon; LDL, low-density lipoprotein; PASI, psoriasis area and severity index; PCSK9, Proprotein convertase subtilisin/kexin type 9. TNF, tumor necrosis factor; Ecmean is the mean composite of all assessed vascular endothelial transcripts. Mean ± SEM.
Psoriasis, PCSK9, Advanced Atherosclerosis and CV Risk
Finally, to investigate whether PSCK9 associated with the burden of atherosclerosis in psoriasis, we analyzed whether PCSK9 levels were associated with log converted CAC score, a reliable surrogate marker of advanced atherosclerosis burden and CV risk which is shown to be elevated in psoriasis (Table 1). As expected, CAC correlated with age (r=0.54, p<0.001), hypertension (r=0.27, p=0.03) and Framingham risk score (r=0.38,p<0.01). (Khera and Greenland, 2018, Mansouri et al., 2016) PCSK9 was associated with CAC in both unadjusted (β=0.30, p=0.01), and adjusted (Framingham risk and BMI) analyses (β=0.28, p=0.02). Taken together, these data highlight the pro-inflammatory nature of PCSK9 in psoriasis and a relationship with both early and advanced stages of atherosclerosis.
DISCUSSION
Despite epidemiologic evidence linking psoriasis to elevated CV risk, the underlying mechanisms are not fully known. Beyond its role in lipid metabolism, PCSK9 is independently linked to CV events.(Leander et al., 2016) In this study we show in two separate observational psoriasis cohorts and the K14-Rac1V12−/+ murine model of psoriasis, that PCSK9 is elevated in psoriasis compared to control and preferentially expressed in lesional skin. PCSK9 levels were associated with psoriatic severity and pro-inflammatory signaling pathways. Finally, PCSK9 levels independent of traditional CV risk factors, associated with both endothelial inflammation and CAC thus suggesting a link between PCSK9, the development of atherosclerosis, and enhanced CV risk in psoriasis.
In our study, we observed positive PCSK9 staining in both human and the K14-Rac1V12−/+ murine model of psoriasis. These finding are similar to a 2019 study describing preferential skin staining in human and murine imiquimod treated psoriatic lesional skin.(Luan et al., 2019) In this study, PCSK9 was overexpressed in both the epidermal and dermal layers. Inhibition of PCSK9 reduced skin lesion severity including suppressing keratinocyte proliferation and inflammation via reduced NFκB expression. Our study adds to the literature by validating expression of PCSK9 in skin lesions using a robust murine model of psoriasis, and correlating systemic PCSK9 levels to skin lesion severity and atherosclerosis burden. These data suggest psoriatic skin as a source of PCSK9 elevation in psoriasis and PCSK9 as a potential therapeutic target, both to reduce atherosclerosis and skin lesion severity.
When evaluating the impact of inflammation on PCSK9 levels, others have observed no relationship between stimuli such as lipopolysaccharide injection and PCSK9, suggesting that drivers of PCSK9 may be unique to specific pro-inflammatory conditions.(Heinzl et al., 2020) Therefore, to understand the connection between the skin, systemic pro-inflammatory pathways, and PCSK9 elevation in psoriasis, we investigated the blood transcriptome in psoriasis and matched controls.(Figgett et al., 2019, Garshick M. S. et al., 2019) In our analysis, we identify specific pathways strongly associated with PCSK9 which are also causal in psoriasis pathophysiology including TNF, IL6, IL17A and IL23.(Nestle et al., 2009) These findings inform future investigations into mechanisms of PCSK9 elevation in psoriasis and highlight PCSK9 as a promising candidate for further investigation in psoriasis.
In HIV, another pro-inflammatory condition linked to enhanced CV risk and similar to our findings in psoriasis, PCSK9 is elevated and correlates with coronary artery endothelial dysfunction and circulating markers of endothelial inflammation independent of lipid levels.(Leucker et al., 2018) How PCSK9 induces atherosclerosis independent of serum lipids is not yet known.(Shapiro et al., 2018) Intracellularly, PCSK9 interacts with the LDL receptor promoting degradation.(Horton et al., 2007) Inhibition of PCSK9 allows for enhanced LDL receptor expression thus reducing LDL cholesterol by over 60% and dramatically reducing CV events.(Rosenson et al., 2018) PCSK9 is also secreted and circulates with, and independent of LDL cholesterol.(Shapiro et al., 2018) Blocking of PCSK9 inhibits an NFκB response in macrophages (Ricci et al., 2018, Tang et al., 2017) and in HepG2 cells, TNFα induces PCSK9 expression.(Ruscica et al., 2016) Finally, in patients with a recent myocardial infarction and hyperlipidemia, treatment with an IL6 inhibitor reduces PCSK9 levels.(Ueland et al., 2018) These observations suggest PCSK9 may be a pro-inflammatory mediator to induce atherosclerosis in psoriasis, either independently, or through lipid-mediated inflammatory pathways, augmented via circulating PCSK9.
Finally, to identify if elevated PCSK9 may promote atherosclerosis, we investigated the association between both early stages of atherosclerosis, endothelial vascular inflammation and late stages such as CAC. We show that the point estimates in the correlation between PCSK9 levels and endothelial vascular inflammation are higher than other known mediators of psoriatic pathophysiology such as IL6, IL17A and TNFα, and higher than LDL cholesterol.(Greb et al., 2016) We also show in a separate, larger cohort of psoriasis patients that not only is PCSK9 elevated, but in adjusted models correlated with CAC. CAC is a strong predictor of CV risk and elevated in psoriasis.(Khera and Greenland, 2018, Mansouri et al., 2016) In summary, we link PCSK9 to CV risk independent of LDL, to suggest a possible pathway of how psoriasis may lead to CVD.
Limitations
In this study we aimed to identify if PCSK9 is elevated in psoriasis and related to impaired endothelial vascular health and atherosclerosis development. To do this, we utilized two different psoriasis cohorts with relatively moderate skin lesion severity and a murine model to identify PCSK9 elevation in psoriasis. Despite this validation, our study is an observational one and the results could be influenced by small sample size, unmeasured confounders leading to potential section bias and deserves to be validated in larger, prospective studies.
Additionally, we used venous as opposed to arterial cells to evaluate early markers of atherosclerosis burden. However, direct analysis of venous endothelial cells is now a well-validated technique and shown to have a similar expression to arterial endothelial cells.(Onat et al., 2007) We also connect PCSK9, venous endothelial inflammation, and CAC as a way to further enhance the clinical implications of our findings of PCSK9 elevation in psoriasis. Finally, despite other cellular sources of PCSK9 production, the majority of circulating PCSK9 is produced by the liver. (Shapiro et al., 2018) Therefore, given the cross-sectional study design, we can only infer and hypothesize, but not prove that elevations in PCSK9 we observe, directly come from psoriatic skin lesions and induces atherosclerosis. To do this and assess the clinical utility of our findings, requires future investigations.
Conclusions
In conclusion, in psoriasis patients at low risk for CVD traditionally, we identified increased levels of PCSK9 and enhanced expression of PCSK9 in the psoriatic skin plaque. Further, in a murine model of psoriasis, we show that despite no differences in hepatocyte expression of PCSK9, PCSK9 levels may be elevated systemically. Finally, PCSK9 levels are associated with endothelial cell inflammation independent of traditional CV risk factors and also correlated with CAC, a marker of both advanced atherosclerotic burden and CV risk. These findings suggest that PCSK9 may be associated with the development of atherosclerosis and enhanced CV risk seen in psoriasis. However, our study was observational in nature and thus study findings should be interpreted as such. Further studies are required to validate and investigate the clinical significance of these observations.
MATERIAL AND METHODS
Study/Ethical Approval
A full description of the study methods, including participant inclusion/exclusion criteria, is available in the supplemental methods. Study approval was obtained from the Institutional Review Board at New York University (NYU) Langone Health and the National Heart, Lung and Blood Institute (NHLBI), NIH in accordance with the principles of Declaration of Helsinki. All guidelines for good clinical practice and those set forth by the NIH Radiation Safety Commission and in the Belmont Report (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research) were followed. All study participants in the cohort provided written informed consent. All the participants and healthy volunteers were adequately compensated. Data for all psoriasis patients were obtained under a protocol titled Psoriasis, Atherosclerosis and Cardiometabolic Disease Initiative (13-H-0065), while data from healthy volunteers recruited through community advertising, were obtained under a separate study protocol (13-H-0194), both through the NHLBI. The NYU cohort was recruited as part of an ongoing study (NCT03228017) investigating endothelial vascular health in psoriasis. The patient recruitment scheme is displayed in Supplementary Figures S1 and S2 with more detail in the online supplemental methods.
Human Sample Collection
In both the NIH (Supplementary Figure S1) and NYU (Supplementary Figure S2) cohorts, blood of study participants was collected, processed and stored as per previously documented protocols.(Garshick M. S. et al., 2019, Joshi et al., 2016) Human 3mm skin biopsies were fixed using 10% Formalin (Azer Science, PA, USA) overnight with subsequent paraffin embedding. To directly assess endothelial vascular health, participants underwent an endovascular endothelial harvesting procedure. After brachial vein angiocatheter placement, a sterile endovascular J – shaped wire was inserted, and endothelial cells scraped from the vein. Brachial vein endothelial cells were isolated using magnetic beads coated with CD146 antibody and assessed for transcripts of interest using real-time quantitative polymerase chain reaction as described previously.(Garshick M. S. et al., 2019) Brachial vein endothelial cell transcript expression is presented as a relative value standardized to the housekeeping gene human acidic ribosomal protein (hARP). Further details are provided in the supplemental methods.
Blood RNA Sequencing Analysis
A subset of psoriasis patients (Figure S2) underwent blood RNA sequencing (10 psoriasis/10 controls, GEO accession number GSE147339). As previously described, peripheral blood samples were collected in PAXgene Blood RNA tubes (PreAnalytiX, Qiagen/BD), underwent RNA extraction, and sequencing libraries generated on an Illumina TruSeq (Sand Diego, CA) platform.(Garshick M. S. et al., 2019) Completed libraries were quantitated, normalized, and pooled. Pooled libraries were run on 2 lanes of single read 50 on the Illumina Hiseq 4000 sequencer. Sequencing reads were mapped to the human reference genome (GRCh37/hg19) using the STAR aligner (v2.5.0c).(Dobin et al., 2013) Read count tables were generated using HTSeq (v0.6.0),(Anders et al., 2015) normalized based on their library size factors using DESeq (v3.7),(Anders and Huber, 2010) and differential expression analysis was performed. The correlation between normalized count values (of the genes) and protein levels of PCSK9 were assessed in patients with psoriasis (n=10). All downstream statistical analyses and generating plots were performed in R environment (v3.1.1) (http://www.r-project.org/). An exploratory P value <0.05 was used to determine statistical significance. Genes significantly associated with protein levels of PCSK9 were uploaded into Ingenuity Pathway Analysis (Qiagen Bioinformatics, Redwood City, CA) which was used to discover differentially expressed pathways between circuiting PCSK9 and blood transcripts.
Mice
K14-Rac1V12−/+ mice and their age and gender matched LMC mice were used for experiments at 12–16 weeks of age. Mice were handled, housed, scored and sacrificed as described previously (Baumer et al., 2018). Skin lesion severity was recorded on a scale from 0 (genotype but no phenotype) to 5 (substantial lesions with scaling and exhibiting fatigue, pain or lethargy [details in supplemental methods]).(Baumer et al., 2018) Blood was collected and plasma stored at −80°C until use. Organs were collected after perfusion with PBS. Liver and skin samples were either flash frozen for future western blot or real-time quantitative polymerase chain reaction analysis or fixed using 10% Buffered Formalin (Azer Science, PA, USA) overnight for subsequent paraffin embedding.
PCSK9 Measurement
For the NIH cohort, human PCSK9 was measured using the Quantikine ELISA kit from R&D (USA, catalogue number DPC900) according to the manufacturer’s recommendation after a 1:20 dilution in the supplied calibrator diluent RD5P. For the NYU cohort, OLINK Proseek multiplex assay panels were used to measure proteins including PCSK9.(Kim et al., 2018) Briefly, oligonucleotide-labeled antibody probes with proximity extension assay technology bind to their designed target. These antibody pairs attach to their designed target to create a new DNA amplicon. The amplicons were quantified using a Fluidigm BioMark HD real-time PCR platform. Data is reported as Normalized Protein eXpression (NPX), a unit of measurement based on a Log2 scale. Mouse plasma samples were diluted 1:200 in Calibrator Diluent RD5–26 and subjected to the mouse proprotein convertase 9 (PCSK9) ELISA from R&D (both Cat# MPC900) according to manufacturer’s recommendation. When possible, human blood samples were obtained in the morning under fasting conditions.
Histological Staining
10μm paraffin sections were prepared from mouse liver and skin samples as well as human skin biopsies of healthy study volunteers and untreated psoriasis patients. For staining of paraffin sections, sections were de-paraffinized in ethanol dilutions of various concentrations. Subsequently a citrate buffer (pH 6) antigen retrieval was performed for 10min. Samples were then incubated with 0.3%H2O2 in methanol for 30min, washed 3 times with PBS, blocked using 10% normal goat serum (Sigma, USA) and incubated with the according primary antibody over night at 4°C (PCSK: Novus Bio NBP2–31364 at 5μg/ml, LDLR: R&D AF2255 at 15μg/ml). The following day sections were washed using PBS 3 times and incubated with a biotinylated secondary antibody (1:200 dilution) for 1hour, washed afterwards 3 times and incubated with Elite ABC reagent in PBS for 30min at RT. Finally, sections were washed twice with PBS and DAB stained with subsequent hematoxylin counter staining, followed by a dehydrations step before being mounted with a cover slip. Images were take using the NanoZoomer (Hamamatsu, USA).
Coronary Computed Tomography Angiography Image Acquisition
All patients underwent coronary computed tomography angiography on the same day as blood draw, using the same CT scanner (320-detector row Aquilion ONE ViSION, Toshiba, Japan). CAC was evaluated as a part of normal workflow by an experienced cardiologist using semiautomatic software (SmartScore, GE Healthcare). CAC (mean total Agatston scores) was measured using electron beam tomography from 40 continuous 3-mm thick computed tomograms (Imatron, San Francisco, CA). A single, experienced radiological technologist performed scoring, blinded to clinical and laboratory data, using customized software (Imatron). Natural log-transformation of CAC scores, (ln[CAC+1]), were performed to account for the high percentage of CAC scores of 0 in all groups (details in supplemental methods).
Statistics
Summary statistics were generated and expressed as mean with standard deviation for normally distributed and median with interquartile range for non-normally distributed variables. Categorical variables were recorded as frequencies and percentages. Normality was evaluated using skewness, kurtosis, and histogram plots and Shapiro-Wilk test with a p-value <0.05 indicating a non-normally distributed population. Intergroup differences were assessed using t-tests for normally distributed and Mann-Whitney U tests for non-normally distributed but continuous data. Dichotomous variable comparisons were conducted using Pearson’s chi-square test. Correlation coefficients and regression modeling with multivariable adjustment and tests for interaction were performed as indicated, including adjustment for Framingham risk (incorporates age, gender, and cholesterol) and BMI. STATA 12 (StataCorp, College Station, TX), PRISM7.0 (GraphPad) or JMP® 13.2 (SAS Institute Inc, Cary, NC) was utilized for all analyses. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
SOURCES OF SUPPORT
This study was funded by a National Institutes of Health (NIH, Bethesda, MD) training grant T32HL098129, NIH CTSA at NYU Awards (NY, NY) - UL1TR001445, KL2TR001446 and TL1TR001447, American Heart Association Career Development Grant (Dallas, TX) 18CDA34080540 and National Psoriasis Foundation Grant, all awarded to Michael S. Garshick. American Heart Association Career Development Grant (Dallas, TX) 18CDA34110203AHA, and American Society of Hematology (18-A0–00-1001884) to Tessa Barrett. Dr. Mehta is funded by NIH HL-06193–06, a grant to the Intramural Program. Jeffrey S. Berger was supported, in part, by NIH (Bethesda, MD) grants R01HL139909, R01HL114978 and R35HL144993.
ABBREVIATIONS
- BMI
Body mass index
- CAC
Coronary artery calcium
- CVD
Cardiovascular disease
- hARP
human acidic ribosomal protein
- HIV
Human immunodeficiency virus
- HsCRP
high-sensitivity C-reactive protein
- LDL
Low-density lipoprotein
- LMC
littermate controls
- NHLBI
National Heart Lung and Blood Institute
- NIH
National Institutes of Health
- NPX
Normalized protein eXpression
- PASI
psoriasis area severity index
- PCSK9
proprotein convertase subtilisin kexin 9
Footnotes
DATA AVAILABILITY
All data that supports the finding from the manuscript are available by the corresponding authors upon reasonable request.
CRediT Statement
Conceptualization: MG, JG, JB, NM; Data curation: MG, TB, YB; Formal analysis: MG, AD, MT, RG, TB, NM; Funding acquisition: MG, JB, NM; Investigation: MG, YB, AD, QN, HT, MT, TB, NM; Methodology: MG, YB, RB, YZ, MC, TB, TPW, MP; Resources: EF, JK, JB, NM; Supervision: EF, JK, TPW, MP, NM. Writing: MG, AD, JB, NM; Writing – review and editing; JU.
CONFLICTS OF INTEREST
Dr. Mehta is a full-time US government employee and has served as a consultant for Amgen, Eli Lilly, and Leo Pharma receiving grants/other payments; as a principal investigator and/or investigator for AbbVie, Celgene, Janssen Pharmaceuticals, Inc, and Novartis receiving grants and/or research funding; and as a principal investigator for the National Institute of Health receiving grants and/or research funding. Dr. Underberg has received Speaker Bureau Honoraria from Amgen, Sanofi, Regeneron, Amarin, Alexion, Esperion; has consulted for Amarin, Amgen; has served on advisory boards for Amgen, Regeneron, Sanofi, Alexion, Akcea,, esperion, Invitae, Ambry, Medicines Company; performed contracted research for Aegerion, Pfizer; and is a steering committee member for the Aegerion LOWER Registry Trial. Dr. Krueger has received grants from: Novartis, Pfizer, Amgen, Lilly, Boehringer, Innovaderm, BMS, Janssen, Abbvie, Paraxel, Kineta, Leo Pharma, Vitae, Akros, Regeneron, Allergan, Novan, Biogen MA, Sienna, UCB, Celgene, Botanix, Incyte, Avillion, Exicure. He has received personal fees from: Novartis, Pfizer, Amgen, Lilly, Janssen, Boehringer, BiogenIdec, Abbvie, Leo Pharma, Escalier, Acros, Valeant, Aurigne, Allergan, Asana, UCB, Sienna, Celgene, Nimbus, Menlo, Aristea, Sanofi, Sun Pharma, Almirall, Arena, BMS. Dr. Berger serves on the Amgen Advisory Board.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 2010;11(10):R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015;31(2):166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer Y, Ng Q, Sanda GE, Dey AK, Teague HL, Sorokin AV, et al. Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis. JCI Insight 2018;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figgett WA, Monaghan K, Ng M, Alhamdoosh M, Maraskovsky E, Wilson NJ, et al. Machine learning applied to whole-blood RNA-sequencing data uncovers distinct subsets of patients with systemic lupus erythematosus. Clin Transl Immunology 2019;8(12):e01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garshick M, Vaidean G, Nikain C, Chen Y, Smilowitz NR, Berger JS. Sex Differences in the Prevalence of Vascular Disease and Risk Factors in Young Hospitalized Patients with Psoriasis. International Journal of Women’s Dermatology 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garshick MS, Barrett T, Wechter T, Azarchi S, Scher J, Neimann A, et al. Inflammasome Signaling and Impaired Vascular Health in Psoriasis. Arterioscler Thromb Vasc Biol 2019:ATVBAHA118312246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296(14):1735–41. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA Jr., Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 2016;118(4):620–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers 2016;2:16082. [DOI] [PubMed] [Google Scholar]
- Heinzl MW, Resl M, Klammer C, Egger M, Dieplinger B, Clodi M. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Is Not Induced in Artificial Human Inflammation and Is Not Correlated with Inflammatory Response. Infect Immun 2020;88(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci 2007;32(2):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res 2016;119(11):1242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera A, Greenland P. Coronary Artery Calcium: If Measuring Once Is Good, Is Twice Better? Circulation 2018;137(7):680–3. [DOI] [PubMed] [Google Scholar]
- Kim J, Tomalin L, Lee J, Fitz LJ, Berstein G, Correa-da Rosa J, et al. Reduction of Inflammatory and Cardiovascular Proteins in the Blood of Patients with Psoriasis: Differential Responses between Tofacitinib and Etanercept after 4 Weeks of Treatment. J Invest Dermatol 2018;138(2):273–81. [DOI] [PubMed] [Google Scholar]
- Leander K, Malarstig A, Van’t Hooft FM, Hyde C, Hellenius ML, Troutt JS, et al. Circulating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. Circulation 2016;133(13):1230–9. [DOI] [PubMed] [Google Scholar]
- Leucker TM, Weiss RG, Schar M, Bonanno G, Mathews L, Jones SR, et al. Coronary Endothelial Dysfunction Is Associated With Elevated Serum PCSK9 Levels in People With HIV Independent of Low-Density Lipoprotein Cholesterol. J Am Heart Assoc 2018;7(19):e009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan C, Chen X, Zhu Y, Osland JM, Gerber SD, Dodds M, et al. Potentiation of Psoriasis-Like Inflammation by PCSK9. J Invest Dermatol 2019;139(4):859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri B, Kivelevitch D, Natarajan B, Joshi AA, Ryan C, Benjegerdes K, et al. Comparison of Coronary Artery Calcium Scores Between Patients With Psoriasis and Type 2 Diabetes. JAMA Dermatol 2016;152(11):1244–53. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009;361(5):496–509. [DOI] [PubMed] [Google Scholar]
- Onat D, Jelic S, Schmidt AM, Pile-Spellman J, Homma S, Padeletti M, et al. Vascular endothelial sampling and analysis of gene transcripts: a new quantitative approach to monitor vascular inflammation. J Appl Physiol (1985) 2007;103(5):1873–8. [DOI] [PubMed] [Google Scholar]
- Ricci C, Ruscica M, Camera M, Rossetti L, Macchi C, Colciago A, et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci Rep 2018;8(1):2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS, Hegele RA, Fazio S, Cannon CP. The Evolving Future of PCSK9 Inhibitors. J Am Coll Cardiol 2018;72(3):314–29. [DOI] [PubMed] [Google Scholar]
- Ruscica M, Ricci C, Macchi C, Magni P, Cristofani R, Liu J, et al. Suppressor of Cytokine Signaling-3 (SOCS-3) Induces Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Expression in Hepatic HepG2 Cell Line. J Biol Chem 2016;291(7):3508–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res 2014;114(6):1022–36. [DOI] [PubMed] [Google Scholar]
- Shapiro MD, Tavori H, Fazio S. PCSK9: From Basic Science Discoveries to Clinical Trials. Circ Res 2018;122(10):1420–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZH, Peng J, Ren Z, Yang J, Li TT, Li TH, et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-kappaB pathway. Atherosclerosis 2017;262:113–22. [DOI] [PubMed] [Google Scholar]
- Ueland T, Kleveland O, Michelsen AE, Wiseth R, Damas JK, Aukrust P, et al. Serum PCSK9 is modified by interleukin-6 receptor antagonism in patients with hypercholesterolaemia following non-ST-elevation myocardial infarction. Open Heart 2018;5(2):e000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winge MC, Ohyama B, Dey CN, Boxer LM, Li W, Ehsani-Chimeh N, et al. RAC1 activation drives pathologic interactions between the epidermis and immune cells. J Clin Invest 2016;126(7):2661–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.