Abstract
Cystic hepatobiliary neoplasms with mucin-producing epithelium—mucinous cystic neoplasm of the liver (MCN) and intraductal papillary neoplasm of the bile duct (IPNB)—are rare and distinct entities that have unique clinical, pathologic, and imaging features. They are differentiated pathologically by the presence of subepithelial ovarian-like hypercellular stroma (OLS), which is the defining histopathologic feature of MCN. MCN is commonly a benign, large, solitary, symptomatic, multiloculated cystic mass without biliary communication that occurs in middle-aged women. On the other hand, IPNBs are a heterogeneous spectrum of tumors, which are commonly associated with invasive carcinoma, occur in older patients, and can be differentiated from MCN by communication with the biliary tree, intraductal masses, associated biliary ductal dilatation, and absent OLS. Understanding of these rare neoplasms has grown and evolved over time and continues to today, but uncertainty and controversy persist, related to the rarity of these tumors, relatively recent designation as separate entities, inherent clinicopathologic heterogeneity, overlapping imaging features, and the fact that many prior studies likely included MCN and cystic IPNB together as a single entity. Confusion regarding these neoplasms is evident by historical inconsistencies and nonstandardized nomenclature through the years. Awareness of these entities is important for the interpreting radiologist to suggest a particular diagnosis or generate a meaningful differential diagnosis in the appropriate setting, and is of particular significance as MCN and cystic IPNB have overlapping imaging features with other more common hepatobiliary cystic masses but have different management and prognosis.
Online supplemental material is available for this article.
Work of the U.S. Government published under an exclusive license with the RSNA.
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Identify the distinguishing clinical and pathologic features of MCN and IPNB.
■ Describe the characteristic imaging features of MCN and IPNB and how to differentiate these entities from other hepatobiliary cystic masses.
■ Recognize differences in workup, management, and prognosis of MCN and IPNB.
Introduction
Cystic hepatobiliary neoplasms with mucin-producing epithelium—mucinous cystic neoplasm of the liver (MCN) and intraductal papillary neoplasm of the bile duct (IPNB)—are rare and distinct tumors that have unique clinical, pathologic, and imaging features, management strategies, and prognosis (Table 1). MCN and IPNB can be differentiated pathologically by the presence of subepithelial ovarian-like stroma (OLS), which is the key distinguishing feature of MCN that was originally established by the World Health Organization (WHO) in 2010 (1–3). Before this, “biliary cystic tumors” such as MCN and cystic IPNB were subsumed more generically under the term biliary cystadenoma or biliary cystadenocarcinoma despite variability and heterogeneity in clinicopathologic and imaging features, biologic activity, and prognosis (eg, neoplasms with OLS tended to be more indolent with better prognosis) (4–6). Controversy surrounding MCN and an emerging concept of IPNB persisted until the presence of OLS was established as a required diagnostic feature of MCN (3,6).
Table 1:
MCN versus IPNB
MCN and IPNB are examples of “biliary diseases with pancreatic counterparts,” which is a concept that provides a valuable framework for our continued and evolving understanding of these entities (7). Although our understanding of MCN and IPNB has progressed, confusion persists related to the rarity of these tumors, relatively recent classification into distinct entities, inherent pathologic heterogeneity, overlapping imaging features, inconsistent application of pathologic diagnostic criteria in practice, and the fact that prior studies likely included MCN and cystic IPNB together as a single entity (3–5,8–14).
The purpose of this review is to address and clarify salient issues regarding MCN and IPNB pertinent to radiologists. We present updated nomenclature and characteristic clinical, pathologic, and imaging features of MCN and IPNB with attention to management and prognosis and discuss features differentiating them from other cystic hepatobiliary entities (Fig E1), so that interpreting radiologists can gain comfort and familiarity with these rare neoplasms.
Mucinous Cystic Neoplasm
Background and Nomenclature
MCN is rare, accounting for less than 5% of cystic liver masses (11,18). According to the fifth edition of the WHO classification, MCN is a cyst-forming epithelial neoplasm lined by cuboidal, columnar, or flattened mucin-producing epithelium overlying OLS (Fig 1), typically without communication with the bile ducts (1). The presence of OLS is the key distinguishing histopathologic feature required to diagnose MCN. Rarely, MCN gives rise to invasive carcinoma and is considered a premalignant hepatobiliary neoplasm (19).
Figure 1.
MCN. (A) High-power photomicrograph shows mucinous-type columnar epithelium lining the cyst wall with underlying spindled OLS (arrows). The columnar epithelium is of the gastric/foveolar subtype. (Hematoxylin-eosin [H-E] stain; original magnification, ×400.) (B) High-power photomicrograph highlights immunopositive spindled mesenchymal-type cells (arrows) located underneath the mucinous epithelium, confirming the presence of OLS and the diagnosis of MCN. (Immunohistochemistry stain for progesterone receptor; original magnification, ×400.)
MCN was formerly referred to as “biliary cystadenoma,” “biliary cystadenocarcinoma,” or “biliary cystic neoplasm or tumor,” but “mucinous cystic neoplasm” is now the preferred nomenclature. Inconsistencies in nomenclature are attributable in part to controversy in distinguishing between MCN and IPNB until OLS was identified as a required feature for MCN, although this criterion and updated nomenclature have not been uniformly and consistently followed (3,20,21).
Clinical Features
MCN occurs almost exclusively in middle-aged women (87%–100% of cases; median age, 45–54 years), although it may rarely occur in men (9:1 female-to-male predominance) (11,13). The rare malignant MCN tends to occur in older patients (3,11,18). Patients with MCN are typically younger than patients with IPNB, and MCN is more common in Western countries than IPNB (3,13). Although previous series reported a higher proportion of male patients and malignant MCN (“biliary cystadenocarcinoma”), it is now thought that those cases would be classified as cystic IPNB (3,14).
MCN is commonly symptomatic, but the clinical manifestation is often nonspecific. Patients may present with abdominal pain (57%), fullness (20%), or early satiety due to large size and mass effect (11). A minority of patients are asymptomatic (14%) (11). MCN may enlarge with oral contraceptive use and during pregnancy, suggesting a role for hormonal influence (22).
Liver function tests do not allow reliable distinction between benign and malignant MCNs (10). Although levels of serum tumor markers such as carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) may be elevated, particularly in cases with associated invasive carcinoma, normal levels do not exclude invasive carcinoma (10). CEA and CA 19-9 levels in cyst fluid are also limited for differentiating MCN from nonneoplastic simple hepatic cysts (SHCs) (23).
Pathogenesis and Pathologic Features
Although the precise etiology of MCN is unknown, several potential causes have been proposed. One hypothesis suggests aberrant descent or implantation of primordial germ cells during development related to close proximity of the embryonic gonads and the dorsal and ventral mesenteries—from which the pancreatic body and tail and the liver are derived—and close temporal development of the pancreas and liver during embryogenesis (24). MCN may also develop from primordial germ cells within hepatic periductal fetal mesenchyme, which transdifferentiate into ovarian stromal cells (24). The strong predilection of MCN for the left hepatic lobe supports a developmental origin, as other developmental entities (eg, ciliated hepatic foregut cyst, hamartoma) commonly develop in segment IV (3,15).
Morphologically, MCN is a well-delineated, multiloculated/multiseptated, or less commonly unilocular cystic mass (Fig 2) (3). MCN tends to be large (range, 5–29 cm; mean, 11 cm) and is typically larger than cystic IPNB (12,20,25). Stromal inflammation and degenerative changes such as hemorrhage, calcification, or necrosis can be seen (12,20). MCN is most commonly separate from the biliary tree but rarely may have intraluminal erosion (26). It may have associated upstream ductal dilatation related to mass effect, but this is not a dominant feature. Papillary projections may be grossly identified within the cyst, particularly with invasive carcinoma (3). Rarely, MCN occurs in the extrahepatic bile ducts or gallbladder.
Figure 2.
MCN in a 41-year-old woman. (A) Intraoperative color Doppler US image shows a multiloculated cystic mass with thick septa (arrows) and internal echogenic debris. (B) Intraoperative photograph shows the partially exophytic cystic hepatic mass (arrows).
MCN follows a multistep progression from low- to high-grade dysplasia to invasive carcinoma (27). Per the fifth edition of the WHO classification, MCNs are defined as MCN with low-grade dysplasia, MCN with high-grade dysplasia, or MCN with associated invasive carcinoma. OLS cells are estrogen receptor (ER), progesterone receptor (PR) (Fig 1), and α-inhibin immunoreactive (3) and show hormonal responsiveness and production, suggesting a role for estrogen in biliary neoplasia (24).
The vast majority of MCNs (>90%) are benign. Associated invasive carcinoma is rare, occurring in approximately 3%–6% of cases (3,13,20). Historical discrepancies with a reported malignancy rate of up to 26% may be attributable to inclusion of cystic IPNBs (4,14).
The pancreatic counterpart to MCN is MCN of the pancreas (MCN-P) (3,7); like with MCN, OLS is the characteristic defining histologic feature of MCN-P. MCN and MCN-P occur almost exclusively in women and are commonly left sided, involving the left hepatic lobe and the body and tail of the pancreas, respectively (3). Although both are indolent, MCN is less frequently associated with malignancy (3%–6%) compared with MCN-P (12%) (3,13,20,27).
Imaging Features
MCN is often a solitary, large, multiloculated (Figs 2, 3) or infrequently unilocular (6%–10%) (Fig 4) cystic mass with associated septa, mural calcifications, and sometimes mural nodules (5,10,16,28). Sixty-nine percent to 76% occur in the left hepatic lobe with a predilection for segment IV (3,4,13).
Figure 3.
US, CT, and MRI features of MCN. (A) Doppler US image of the liver shows a large multiloculated cystic mass with echogenic septa (arrows). The septa are of varying thickness and centrally located and arise from the cyst wall without external indentation or macrolobulation. (B) Axial contrast-enhanced CT image shows a solitary cystic mass (white arrow) with thin central internal septa (black arrow) in hepatic segment IV. (C) Axial T2-weighted image shows a circumscribed fluid-hyperintense mass with hypointense internal septa (arrow). The septa are centrally located and arise from the cyst wall without associated external indentation or macrolobulation.
Figure 4.
Unilocular MCN in a 52-year-old woman. Gray-scale US image of the liver shows a large simple anechoic unilocular cystic mass (arrow) with no septa, solid component, or associated vascularity.
At CT, in the absence of invasive carcinoma, MCN is a large, circumscribed, multiloculated cystic mass with a well-defined fibrotic capsule (Figs 3, 5). Internal septa vary in thickness (16,29) and may be central or peripheral in location (16,30). The presence of septa and septa arising from the cyst wall without associated wall indentation (Figs 3, 5) are sensitive CT features of MCN (94% and 100%, respectively) (16). Mural calcifications (47%–63%) are also specific for MCN (90%) (16,30). Mural irregularity or nodules (20%–27%) and intracystic debris (21%–40%) may be present (16,30). In addition, the capsule, septa, and nodules may enhance (Fig 5) (29).
Figure 5.
MCN in a 49-year-old woman. (A) Axial contrast-enhanced CT image shows a complex cystic mass in the left hepatic lobe with septa arising from the wall without external indentation or macrolobulation (solid arrows), as well as a septum arising from a macrolobulation (dashed arrow). (B) Axial T2-weighted image shows a large intermediate-signal-intensity mass with septa (arrows). (C) Axial T1-weighted image shows a hyperintense multiseptated mass. (D) Axial contrast-enhanced portal venous phase fat-suppressed T1-weighted image with subtraction shows mild septal enhancement (arrow).
At MRI, MCN is typically T2 hyperintense and shows variable T1 signal intensity owing to proteinaceous or hemorrhagic cyst content (31). A low-signal-intensity rim may be observed on T2-weighted images related to blood products or calcification (5). MRI is superior to CT for demonstrating capsular, septal, or nodular enhancement, particularly with subtraction images (Fig 5) (31). Septal enhancement is a highly sensitive feature of MCN at MRI (16). MR cholangiopancreatography (MRCP) depicts the relationship of an MCN to the bile ducts and can help assess for upstream biliary obstruction, conferring an advantage over endoscopic retrograde cholangiopancreatography (ERCP) (32).
At US, MCN is a complex multiloculated cystic mass with echogenic septa (Figs 2, 3). Cyst fluid is typically anechoic but can be variable, depending on the content (5). Nodules or a solid component larger than 1.0 cm can be associated with malignancy (Fig 6) (33). Contrast-enhanced US (CEUS) is of limited utility in differentiating benign from malignant MCN, as the walls, septa, or nodules may show a similar pattern of enhancement in both benign and malignant MCN (33). At PET/CT, fluorodeoxyglucose (FDG) uptake has been reported in malignant cases and may be helpful when malignancy is suspected, but its use remains unsubstantiated (18).
Figure 6.
Malignant MCN in a 53-year-old woman. (A) Axial contrast-enhanced CT image shows a complex cystic mass in the left hepatic lobe, with a dominant large partially calcified solid component (solid arrow) and smaller mural nodules (dashed arrow). (B) Gray-scale US image shows the complex cystic mass with a partially calcified solid component (arrow).
In a systematic review assessing the effect of imaging findings on surgical management of “biliary cystadenomas/cystadenocarcinomas,” mural nodules, wall enhancement, and calcifications were significantly associated with malignancy (Fig 6) (10). Mural nodules, a solid component, calcifications, and hypervascularity also have high negative predictive value (NPV) (91%) but low positive predictive value (PPV) (11%) in distinguishing benign from malignant MCN (11).
Imaging Differential Diagnosis
The imaging differential diagnosis for MCN includes cystic IPNB, SHC (Figs E2, E3), cystic metastasis, cystic hepatocellular carcinoma (HCC), choledochal cyst (Fig E4), and infections, including pyogenic (Fig E5) or amebic abscesses and hydatid disease (Figs E1, E6) (15). MCN can be distinguished from cystic IPNB by absence of biliary communication, intraductal masses, or bile duct dilatation as a dominant feature (17,25). Patient demographics and large mass size may also help distinguish MCN from IPNB. Choledochal cyst (Fig E4) can be distinguished from MCN, as MCN should not originate from the biliary tree.
In many instances, differential considerations can be narrowed on the basis of history and clinical parameters. Known extrahepatic malignancy (eg, mucinous adenocarcinomas like colorectal or ovarian carcinoma, neuroendocrine tumors, or gastrointestinal stromal tumor) may help distinguish cystic metastases from MCN (15). A history of viral hepatitis or cirrhosis and characteristic mass enhancement characteristics (ie, arterial phase enhancement with washout in the portal venous phase) can help distinguish cystic HCC from MCN (15). Clinical features of infection, recent travel to endemic areas, and predilection of abscesses for the right hepatic lobe can help distinguish pyogenic (Fig E5) and amebic abscesses and hydatid disease (Fig E6) from MCN (15).
Management and Prognosis
Although the vast majority of MCNs are benign, distinguishing benign from malignant MCN is difficult. Furthermore, biopsy does not allow reliable distinction of benign from malignant MCN, as a nondirected biopsy specimen is unlikely to be representative of the lesion as a whole (10). Complete surgical excision (eg, enucleation or hepatic resection) is the treatment of choice for MCN and for definitive diagnosis (21).
Studies reporting higher rates of malignancy among MCNs (17%–19%) show that malignancy typically occurs in older patients, suggesting the importance of early detection and treatment to mitigate the risk of malignant transformation (3). However, poor preoperative imaging specificity to distinguish between MCN and “atypical” SHC may result in overtreatment and operative morbidity (10,28).
The prognosis for MCN is excellent with complete resection, but recurrence is common with incomplete excision. Percutaneous aspiration, sclerotherapy, surgical fenestration, and marsupialization are all associated with a high recurrence rate (21). The recurrence rate after enucleation or resection is low (3%–5%), versus 82% after fenestration or marsupialization (10).
Although data are limited, long-term outcomes after complete resection are good (4,11). Disease-related mortality for benign and malignant MCN is 0% and 24%, respectively (10). MCN with invasive carcinoma has worse prognosis than benign MCN but better long-term prognosis than conventional cholangiocarcinoma (10,11).
Intraductal Papillary Neoplasm of Bile Duct
Background and Nomenclature
IPNBs are premalignant biliary epithelial tumors that are estimated to represent 7%–38% of bile duct tumors (9,34–36). According to the WHO classification, IPNB is a grossly visible premalignant neoplasm demonstrating intraductal papillary or villous growth of biliary-type epithelium (Fig 7) (2). IPNBs originate from and communicate with the biliary tree and can occur anywhere along the biliary tree (Figs 7–11). They comprise a heterogeneous spectrum of tumors that vary in morphology, location, clinicopathologic features, biologic activity, and prognosis (8). They are variably mucin secreting, and 30%–40% exhibit mucin hypersecretion (37,38).
Figure 7.
Typical pattern of intrahepatic IPNB—an intraductal mass and both upstream and downstream ductal dilatation—in a 62-year-old man. (A) Axial T2-weighted image through the liver shows a lobulated intraductal mass (arrow), which is hypointense to adjacent bile and mucin with associated bile duct dilatation. (B) Axial contrast-enhanced fat-suppressed T1-weighted image shows the lobulated mass (arrow) within dilated hypointense bile ducts. (C) Maximum intensity projection MRCP image shows intrahepatic bile duct dilatation predominating in the left hepatic lobe, as well as extrahepatic bile duct dilatation with linear and curved hypointense filling defects (arrows), resulting in the thread sign. (D) Photograph of a section through the gross specimen after resection shows the intraductal mass (arrow) within the left hepatic lobe, which corresponds to the associated invasive carcinoma. (E) Low-power photomicrograph shows a papillary lesion protruding into the bile duct lumen. (Hematoxylin-eosin [H-E] stain; original magnification, ×20.) (F) On a higher-magnification photomicrograph, the biliary epithelium that approaches the papillary lesion (black arrows) appears more hyperchromatic owing to the presence of high-grade dysplasia. At the base of the papillary lesion is invasive carcinoma (green arrow). (Hematoxylin-eosin [H-E] stain; original magnification, ×40.) (G) Intermediate-power photomicrograph of the IPNB shows extensive high-grade dysplasia with rounded nuclei, architectural complexity, and abundant mitoses. (Hematoxylin-eosin [H-E] stain; original magnification, ×200.)
Figure 11.
Extrahepatic IPNB in a 68-year-old man with an extrahepatic intraductal mass and upstream ductal dilatation. (A) Coronal contrast-enhanced CT image shows an intraductal mass (arrow) filling and expanding the mid and distal CBD. (B) Maximum intensity projection MRCP image shows a filling defect (arrow) in the mid and distal CBD, which corresponds to the intraductal mass with mild upstream ductal dilatation. (C) Cholangioscopic photograph shows a papillary mass (arrows) in the CBD, which corresponds to the mass identified at CT and MRI.
Many different terms have been applied to IPNBs, including biliary papilloma, biliary papillomatosis, mucin-hypersecreting bile duct tumor, mucin-producing cholangiocarcinoma, intraductal papillary mucinous neoplasm of the bile duct (IPMN-B), and biliary tract IPMN (39,40). Additional distinctions based on the presence or absence of mucin hypersecretion have also been made (eg, IPNB-M for IPNB with mucin secretion and IPNB-NM for IPNB without mucin secretion) (41,42).
Clinical Features
Although it occurs worldwide, IPNB is more common in East Asia, where it accounts for approximately 10%–38% of bile duct tumors, compared with approximately 7%–11% of bile duct tumors in North America and Europe (34,35). IPNB shows a slight male predominance, and the age at diagnosis is 50–70 years (9,34,35,42,43). IPNB is more common than MCN in East Asia (13).
A significant number of patients with IPNB are asymptomatic (44). When symptomatic, patients present with recurrent abdominal pain, cholangitis, and jaundice (40). Sloughed friable intraductal masses and mucin cause recurrent biliary obstruction and cholangitis (45), and patients may have abnormal liver function test results (23%–100%), most commonly elevated alkaline phosphatase level (44). Cancer antigen 19-9 (CA 19-9) level may be elevated in up to 42% (44).
Risk factors for IPNB include primary sclerosing cholangitis (Fig 9), hepatolithiasis, parasitic infections or liver flukes (eg, clonorchiasis and opisthorchiasis), biliary malformation (eg, choledochal cysts), familial adenomatous polyposis, and Gardner syndrome (19). A common feature of many of these conditions is chronic biliary inflammation.
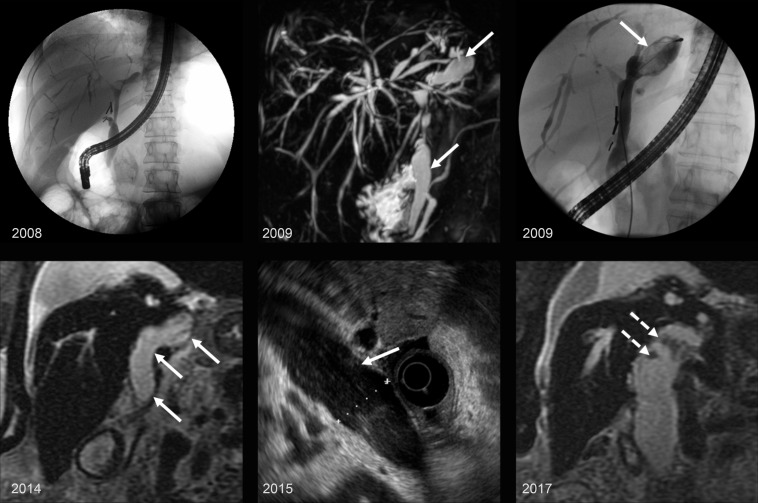
Figure 9.
IPNB in a 66-year-old woman with a history of primary sclerosing cholangitis who developed an IPNB over a decade. Top left: Endoscopic retrograde cholangiopancreatography (ERCP) image from 2008 shows multifocal strictures and irregular beaded appearance of the biliary tree, with mild dilatation of the common bile duct (CBD) and left hepatic duct. Top center: Maximum intensity projection image from MRCP in 2009 shows persistent irregular beaded appearance of the biliary tree and progressive dilatation of the CBD and left hepatic duct (arrows). Top right: ERCP image from 2009 shows ductal dilatation with amorphous and cordlike filling defects in the left hepatic duct (arrow). Bottom: Coronal T2-weighted image from 2014 (bottom left), endoscopic US (EUS) image from 2015 (bottom center), and coronal T2-weighted image from 2017 (bottom right) show progressive enlargement of the CBD and common hepatic duct (solid arrows) with small polypoid intraductal masses (dashed arrows).
Pathogenesis and Pathologic Features
IPNB develops from normal biliary epithelium, although some cystic forms of IPNB showing aneurysmal or diverticular dilatation are thought to arise from peribiliary glands (38,40,46).
IPNB follows an adenoma-carcinoma sequence, with sequential progression from low- to high-grade dysplasia to invasive adenocarcinoma (Fig E7) (40,47). Progression to invasive carcinoma involves stepwise acquisition of genetic alterations in common oncogenic pathways (eg, KRAS mutation, TP53 mutation, and loss of p16) (47). Invasive carcinoma is commonly associated with IPNB, and 40%–80% of cases have invasive carcinoma at presentation (35,47). Increased expression in IMP3, DNMT1, and EZH2 has also been reported in invasive IPNB (48). Carcinoma derived from IPNB can be evident at resection originating from a papillary intraductal mass but cannot be readily distinguished from conventional cholangiocarcinoma histologically. Atypia is often underestimated at biopsy, and submission of the entire cystic lesion is required to establish a diagnosis and determine the presence or absence of invasive carcinoma (40,49).
The exact etiology and pathogenesis of IPNB remain unclear. Hepatolithiasis and liver flukes are implicated in development of IPNB in East Asia, where they are endemic, although these associations are not observed in Western countries (19,35,45), suggesting an interplay between genetic and environmental risk factors (45). There is a 6–8-year latent period between development of hepatolithiasis and IPNB, during which inflammation, cell repair, dysplasia, and malignant transformation occur (36). Evolution of carcinoma in situ to invasive carcinoma is shorter, occurring over 1–2 years (36).
Macroscopically, IPNB manifests as a solitary or multiple polypoid intraductal masses (Figs 7, 11) ranging in size from 0.5 to 2.0 cm, with associated cystic, cylindrical, or fusiform bile duct dilatation (2). Unilocular or multilocular cystic ductal dilatation is more common with intrahepatic IPNBs, whereas extrahepatic IPNBs are associated with cylindrical or fusiform ductal dilatation (6,49). Intrahepatic IPNBs have greater morphologic diversity (eg, cystic and duct-ectatic subtypes), and cystic IPNB can appear grapelike with papillary and mural nodules (3).
Histologically, IPNB shows papillary structures with delicate fine fibrovascular cores and biliary epithelium with or without tubular or glandular components. The epithelium shows pancreaticobiliary, intestinal, gastric, and oncocytic-type differentiation on the basis of the appearance and immunoprofile. The epithelium subtype appears to be an important factor in grade of dysplasia and associated invasive carcinoma (6,9,49), which is significantly more common in the pancreaticobiliary subtype (44). The pancreaticobiliary subtype may also be more common in Western countries (13).
The pancreatic counterpart to IPNB is IPMN. They are both preinvasive neoplasms that can be classified into the same four histologic epithelium subtypes and demonstrate mucin hypersecretion (35,42,43). Intrahepatic IPNBs and IPNBs arising from peribiliary glands—which commonly show cystic dilatation—may correspond to branch-type IPMNs, whereas extrahepatic IPNBs—which typically show cylindrical or fusiform dilatation—may correspond to main duct and combined branch and main duct IPMNs (38,46).
Despite these similarities, there are important differences between IPNB and IPMN. Mucin hypersecretion, which is highly characteristic and seen in nearly all cases of IPMN, is present in only one-third of IPNBs (40). Additionally, mucin-secreting variants are pathologically more similar to IPMN than non-mucin-secreting IPNBs, and mucin hypersecretion and cystic changes predominate in intrahepatic IPNBs, suggesting that intrahepatic IPNBs may be truer counterparts to IPMN than extrahepatic IPNBs (42). Although both IPNB and IPMN have malignant potential, IPNB is more commonly associated with high-grade dysplasia, invasive carcinoma, and more-advanced stage and has a worse prognosis than IPMN (13,49).
Heterogeneity among IPNBs and similarities to and differences from IPMN led expert pathologists from the Japan Biliary Association (JBA) and Korean Association of Hepatobiliary-Pancreatic Surgery (KAHBPS) to propose that IPNB is not a singular disease but at least two different tumors (9,49). They subsequently proposed subclassification of IPNB into two types, type 1 and type 2 (Table 2), which is now included in the fifth edition of the WHO classification (9).
Table 2:
Type 1 IPNB versus Type 2 IPNB
Type 1 or classic IPNB has features similar to those of IPMN (8). It is typically intrahepatic, associated with mucin hypersecretion (~80%), and more likely to be cystic (50,51). Twenty-seven percent to 50% of type 1 IPNBs have associated invasive carcinoma (8,9,38). In contrast, type 2 IPNB has more complex histology and is more commonly associated with invasive carcinoma (49). It is often extrahepatic and infrequently mucin hypersecreting (~10%); 71%–94% of type 2 IPNBs have associated invasive carcinoma (9).
Importantly, some IPNBs have combined features of type 1 and type 2, making classification challenging. Patients may also have synchronous type 1 and type 2 IPNBs involving the intra- and extrahepatic biliary tree, suggesting that IPNBs occur along a spectrum and that location may play a role in tumor type (49).
Location and Morphology
IPNB can occur anywhere along the biliary tree, although there is variability regarding predominant location. Some studies report that nearly 80% of IPNBs occur in intrahepatic locations, while others report that more than 70% occur in extrahepatic locations (4,35–37,41,42,47,52). There are also geographic differences, with intrahepatic IPNBs being more common in East Asia and extrahepatic IPNBs being more common in Western countries. When intrahepatic, IPNB tends to occur in the left hepatic lobe (3,35,40,44). Ultimately, localization of the primary tumor is of paramount importance because the extent and type of surgery depend on tumor location (50).
Jang and Kim’s modified anatomic classification is an example of a simple and intuitive system based on primary tumor location that is used for surgical planning to optimize resection and is similar in principle to classification for conventional cholangiocarcinoma (39). Primary tumor location (ie, intraductal mass, tumor-related stricture, or wall thickening) is categorized as intrahepatic, extrahepatic, or diffuse (Fig 10) (39).
Figure 10.
Jang and Kim’s modified anatomic classification (39). This classification system categorizes the location of the primary tumor as (a) intrahepatic, defined as tumor located in the periphery, beyond the first confluence of the intrahepatic ducts; (b) extrahepatic, defined as tumor confined to the CBD or common hepatic duct; or (c) diffuse, defined as tumor involving both intra- and extrahepatic ducts.
Although different morphologic classification systems have been devised for IPNB based on presence or absence of tumor, tumor morphology, location, and pattern of ductal dilatation (33,41,53,54), morphologic classifications have not been shown to be predictive of prognosis (4,39,43). Tumor morphologies at imaging include polypoid (pedunculated or sessile masses); faintly visible mucosal spreading tumor with a serrated or granular appearance; castlike tumor longitudinally filling the duct; cystic tumors and cystic ductal dilatation with or without associated solid tumor; plaquelike tumor and associated stricture; and infiltrative mass (52–54).
Imaging Features
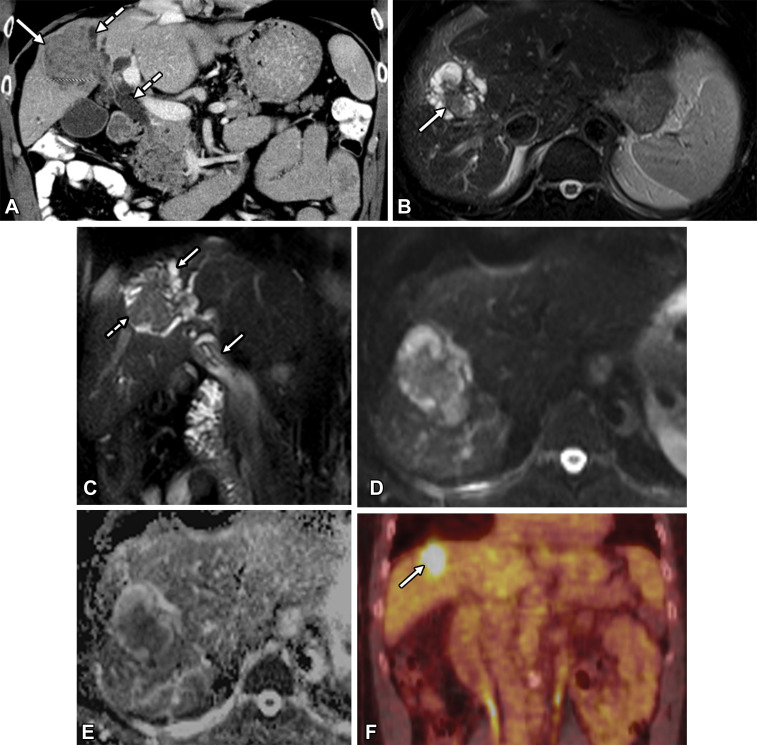
Imaging plays an essential role in diagnosis and management of IPNB, and a multimodality multidisciplinary approach is required (44). The characteristic imaging features of IPNB are an intraductal mass (Figs 7, 11, 12) or masses (Fig 8), bile duct dilatation (Fig 13), or a combination of a mass and ductal dilatation (Figs 7, 12, 14). Strictures can also be observed. Imaging manifestations are related to tumor size, multiplicity, and location and the interplay between epithelial tumor proliferation and mucin production (33,55).
Figure 12.
Moderately differentiated invasive adenocarcinoma arising from an intrahepatic IPNB in a 62-year-old man. (A) Coronal contrast-enhanced CT image shows a large intraductal mass (solid arrow) with associated upstream and downstream ductal dilatation (dashed arrows). (B) Axial T2-weighted image shows a lobulated intraductal mass (arrow) with adjacent bile duct dilatation. (C) Coronal T2-weighted image shows an intraductal mass (dashed arrow) with both upstream and downstream ductal dilatation (solid arrows). (D) Axial diffusion-weighted image shows hyperintense signal within the mass. (E) Axial apparent diffusion coefficient (ADC) map shows hypointense signal within the mass, indicative of restricted diffusion and corresponding to invasive tumor. (F) Coronal PET/CT image shows increased metabolic activity in the mass (arrow).
Figure 8.
IPNB in a 72-year-old man. Axial contrast-enhanced CT (A) and axial T2-weighted MR (B) images show intraductal masses (arrows) filling the intrahepatic biliary tree.
Figure 13.
Intrahepatic IPNB with predominant ductal dilatation but no discernible mass in a 70-year-old man. (A) Axial T2-weighted image shows ductal dilatation (solid arrow) throughout the left hepatic lobe with associated parenchymal atrophy (dashed arrow). (B) Axial contrast-enhanced fat-suppressed T1-weighted image shows hypointense dilated bile ducts (solid arrow) in the left hepatic lobe with associated parenchymal atrophy (dashed arrow) and transient hepatic intensity difference.
Figure 14.
Cystic intrahepatic IPNB in a 43-year-old man. (A) Axial contrast-enhanced arterial phase CT image shows a cystic mass (solid arrow) with an eccentric solid mass component (dashed arrow). (B) Coronal T2-weighted image shows the dominant cystic mass with associated bile duct dilatation (arrows). (C) Axial gadoxetate disodium–enhanced hepatobiliary phase fat-suppressed T1-weighted image shows the lobulated hypointense cystic mass (solid arrow). Note the gadoxetate (dashed arrow) within normal-caliber bile ducts in the right hepatic lobe. (D) Maximum intensity projection MRCP image shows the dominant lobulated cystic mass with adjacent upstream and mild downstream ductal dilatation. (E) Endoscopic retrograde cholangiopancreatography (ERCP) image shows cordlike filling defects (arrows) in the common hepatic duct and CBD, which correspond to mucin. (F) Endoscopic photograph shows a bulging papilla with mucin.
An intraductal mass with upstream and downstream ductal dilatation (Figs 7, 12, 14, 15) is the most commonly reported imaging pattern and is considered to be specific to IPNB, rarely seen with other diseases (33). Tumor, when evident, may be associated with disproportionately dilated bile ducts (46). When IPNB manifests as ductal dilatation without a visible mass (Fig 13), it is imperative to assess the segment or lobe with the most dilated ducts and to assess for associated parenchymal atrophy, which can be severe (33,53,55,56).
Figure 15.
US, CT, and MRI features of an intrahepatic IPNB in a 59-year-old woman. (A) Gray-scale (left) and color Doppler (right) US images show a possible lobulated intraductal mass (arrow) within a dilated duct in hepatic segment II. The mass stands out against the anechoic bile or mucin within the duct. (B) Axial contrast-enhanced portal venous phase CT image shows left intrahepatic biliary dilatation and an enhancing lobulated mass (arrow) within the dilated ducts. (C) Axial T2-weighted image shows a hypointense intraductal mass (arrow) with adjacent dilated ducts, which corresponds to the mass at CT. (D) Axial contrast-enhanced hepatobiliary phase fat-suppressed T1-weighted image shows hypointense dilated bile ducts (arrow) in segment II. (E) Maximum intensity projection MRCP image shows bile duct dilatation predominantly in segments II and III with linear and curved hypointense striae (arrow), the so-called thread sign. (F) Intraoperative Doppler US image shows a lobulated intraductal mass (arrow) within a dilated bile duct and adjacent dilated ducts.
When IPNB manifests as a complex cystic mass (Figs 14, E8), segmental and lobar ducts become part of the tumor and the degree of ductal dilatation is dependent on mucin production and intraluminal pressure (46). Cystic IPNBs are lobulated unilocular or multiloculated cystic masses, which may have enhancing solid masses or papillary excrescences. Associated biliary communication and bile duct dilatation may be apparent (46). Branch aneurysmal dilatation is a characteristic feature of intrahepatic IPNBs (56). Associated calcification is uncommon (30).
At CT, intraductal masses are iso- to hyperattenuating relative to adjacent liver. Mucin within dilated ducts has similar attenuation to that of bile (56). The tumor enhancement pattern at CT relative to background liver is iso- to hyperenhancement in the arterial phase, which diminishes in the portal venous and delayed phases (57). This is in contradistinction to the typical pattern of enhancement for conventional cholangiocarcinoma, which is peripheral rim arterial phase enhancement with progressive and incomplete centripetal enhancement.
MRI with MRCP is the most sensitive imaging modality for mass detection, followed by CT and US (17,45,58). MRI offers excellent contrast between hyperintense bile and mucin and relatively hypointense tumor. Intraductal masses are T1 iso- to hypointense and mildly T2 hyperintense to liver and T2 hypointense to bile (Figs 7–9, 12, 15) (52,59). Tumor enhancement patterns at MRI can be variable, with enhancement in the arterial and portal venous phases (52,59) or arterial phase enhancement that diminishes in the portal venous phase (41). Periductal inflammation and edema can be seen. A visible intraductal mass, tumor size greater than 2.5 cm, tumor multiplicity, ductal wall thickening, and adjacent hepatic invasion are significant features associated with invasive carcinoma at MRI (60).
MRCP depicts bile duct dilatation and can show linear hypointense striae in mucin-secreting IPNBs with a resultant thread sign (Figs 7, 15), which is highly specific for IPNB (99%–100%) (61). Gadoxetic acid (Gd-EOB-DTPA)–enhanced MRI may be helpful for detection of mucin, determination of tumor extent in cases of invasive IPNB, and distinguishing tumor from adjacent tissue inflammation (62). In the hepatobiliary phase, one may observe filling defects and abnormal or absent excretion related to mucin within affected bile ducts (Figs 14, 15) (62). Intraductal masses may be more conspicuous at diffusion-weighted imaging (DWI) (Fig 12) (59). Restricted diffusion and corresponding low signal intensity on an apparent diffusion coefficient (ADC) map within a mass or cyst wall may also be helpful for identifying invasive tumor, but not the extent of tumor invasion (59).
US shows a hypo- or hyperechoic intraductal mass within dilated ducts in up to 41% of IPNBs (58). Contrast-enhanced US (CEUS) can show mass enhancement in a similar pattern to that of CT (63). CEUS also allows distinction between masses and biliary sludge, nonshadowing stones, or hematoma (63). Intraoperative US (IOUS) can help delineate sites of involvement for resection (Figs 7, 15).
PET/CT may be useful for detecting IPNB, and increased fluorodeoxyglucose (FDG) uptake can be seen across the spectrum of IPNBs from low-grade dysplasia to invasive carcinoma (Fig 12) (64,65). Although the utility of PET/CT in differentiating between benign and malignant IPNB remains unclear, some advocate for PET/CT in invasive IPNBs with infiltrating tumor (41). PET/CT may also help assess distant metastases (45).
Endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography show ductal dilatation and mucobilia, with amorphous filling defects corresponding to mucin in bile ducts (Fig 14) (40,45). However, cholangiography is of limited value for mass detection when there is coexisting mucin (66).
Cholangioscopy, endoscopic US, and intraductal US are helpful adjuncts for detecting intraductal tumors in the setting of mucobilia (66). Cholangioscopy is an essential component of the preoperative workup for IPNB to confirm histology and assess extent of disease (Fig 11) (40,45,58). Cholangioscopy is particularly useful for superficial spreading tumor and assessing tumor extent (40), potentially allowing identification of additional tumors occult at imaging in 30% of cases (58). As extent of tumor may be underestimated at preoperative imaging, some advocate routine cholangioscopy and intraoperative frozen sections to determine extent of disease (67), although biopsy has low sensitivity for detecting invasive disease (40).
Imaging Differential Diagnosis
The imaging differential diagnosis for IPNB depends on the imaging pattern. Differential considerations for cystic IPNB include MCN, localized Caroli disease, and SHC. IPNB can be distinguished from MCN and SHC by biliary ductal communication, intraductal masses, and a characteristic pattern of upstream and downstream ductal dilatation (17). Caroli disease, a rare autosomal recessive disorder that results in abnormal intrahepatic duct development and saccular dilatation of intrahepatic ducts, may mimic cystic IPNB (Fig E9). The central dot sign (Fig E9), which corresponds to a central enhancing portal radicle surrounded by a dilated intrahepatic duct and can be seen with Caroli disease, may help distinguish Caroli disease from IPNB (15).
When IPNB manifests with a dominant intraductal mass, the differential diagnosis includes conventional cholangiocarcinoma with intraductal growth, intraductal tubulopapillary neoplasm of the bile duct (ITPN) (Fig E10), hepatocellular carcinoma (HCC) with bile duct invasion, intrabiliary metastases (Figs E11, E12), and hepatolithiasis (Fig E9) (17,49). ITPN is an intraductal biliary neoplasm that differs from IPNB pathologically by characteristic tubular architecture and lack of mucin production (68). Intraductal masses in ITPN are typically larger than in IPNB without associated downstream ductal dilatation, given absent mucin secretion (Fig E10) (69).
A history of viral hepatitis or cirrhosis and an intraductal mass contiguous with a hepatic parenchymal mass showing characteristic arterial phase enhancement and washout at portal venous imaging distinguish HCC from IPNB (17). Known extrabiliary malignancy—particularly colorectal cancer—an intraductal mass with contiguous parenchymal metastasis, and an expansile intraductal growth pattern are useful findings for distinguishing intrabiliary metastases from IPNB (70). Hepatolithiasis can be distinguished from IPNB as nonenhancing intraductal stones that are hyperattenuating at CT and hypointense on T2-weighted images (Fig E9). Stones may be hyper- or hypointense on T1-weighted images depending on their composition (17,41).
Differential considerations for a duct-ectatic pattern of IPNB without a mass include choledochal cyst, primary sclerosing cholangitis (Fig 9), and sequelae of recurrent cholangitis (17). Severe parenchymal atrophy in the segment with predominant ductal dilatation but no discernible mass and compensatory hypertrophy of the unaffected liver can help distinguish IPNB from recurrent cholangitis (55). The thread sign at MRCP—which corresponds to mucin within disproportionately dilated ducts—can also help distinguish IPNB from cholechodal cyst and recurrent cholangitis.
Management and Prognosis
Early surgical intervention is the cornerstone of management for IPNB, and all patients with IPNB should be considered for treatment, given the high potential for malignancy and for recurrent cholangitis and obstructive jaundice in nonmalignant cases (40). R0 resection, indicating tumor-free margins, is the treatment of choice and can be achieved in 46%–90% of cases. Lower-range percentages may be attributable to workup without cholangioscopy to assess tumor extent before resection (44,71).
Tumors involving the extrahepatic bile ducts typically undergo bile duct resection or pancreaticoduodenectomy, whereas tumors confined to intrahepatic bile ducts undergo hepatic resection, the extent of which depends on the degree of biliary involvement (39,44). Diffuse disease requires expanded hepatobiliary resection to achieve negative margins (39).
IPNB has better long-term prognosis compared with conventional cholangiocarcinoma (37,50,51). Extrahepatic and diffuse IPNB have more-advanced tumors, incomplete resection, lymph node metastases, and worse survival compared with intrahepatic IPNB (50,71). Diffuse IPNB has a higher rate of incomplete resection and worse prognosis compared with extrahepatic and intrahepatic IPNB (50). Positive resection margins, lymph node metastases, and tumor multiplicity are associated with worse outcomes (35,72,73). Significantly higher survival in type 1 IPNB compared with type 2 IPNB suggests that subtyping may be predictive of outcome (Table 2) (9).
Distinguishing Neoplastic from Nonneoplastic Cysts
Although cystic hepatobiliary neoplasms with mucin-producing epithelium are rare, cystic hepatic masses are common, occurring in up to 20% of the general population and diagnosed with increased frequency (11,28). Once infection and other malignancy have been ruled out, differentiating neoplasms from nonneoplastic SHCs is important because they have different management. SHCs (Figs 16, E3) are benign and require no specific intervention unless symptomatic (28). Unfortunately, distinguishing cystic hepatobiliary neoplasms from SHCs and particularly atypical SHCs such as hemorrhagic cysts (Fig E2) and other complex cystic hepatic masses can be difficult (Fig E1). Although US, CT, and MRI are sensitive (88%–100%) for diagnosing hepatobiliary neoplasms, they are not specific (43%–53%), with high false-positive rates (37%–45%) (28).
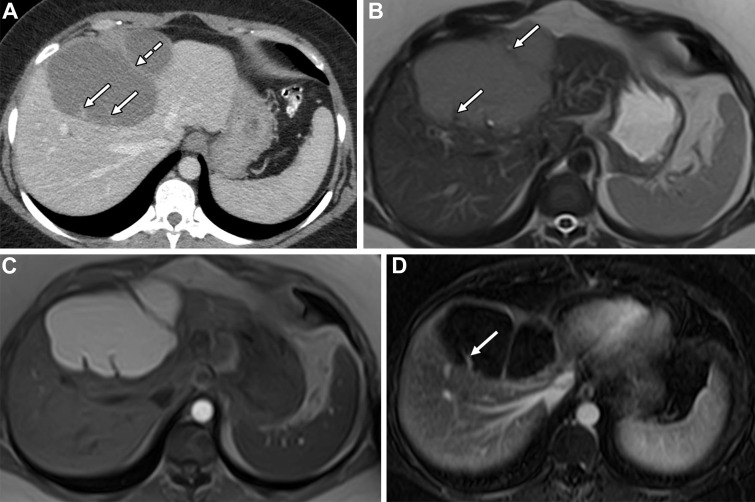
Figure 16.
Symptomatic SHC in a 60-year-old woman. Coronal contrast-enhanced CT (left) and coronal T2-weighted (right) images show an SHC with a thin septum arising from a macrolobulation (arrows).
Comparing features of mucin-producing neoplasms and SHCs at CT, Kim et al (30) found that septa, central pattern or location of septa, mural nodules, upstream ductal dilatation, and downstream ductal dilatation were significant distinguishing imaging features. In combination, cystic masses with more than three of these features statistically favored neoplasm and made the diagnosis of nonneoplastic cyst unlikely. Upstream bile duct dilatation, fewer than three additional cysts, perilesion transient hepatic arterial difference, left hepatic lobe location, and cyst wall calcification have also been described as significant CT features for distinguishing neoplasms from SHC (16,29).
Recently, the presence or absence of septa and the relationship of septa to the cyst wall have been studied and may be more predictive for differentiating MCN from SHC than previously reported features (eg, septum thickness). A unilocular cystic mass is predictive of SHC, with high specificity (94%) and high positive predictive value (PPV) (95%) (16), although the rare unilocular MCN (Fig 4) may be indistinguishable from an SHC (Fig E3). It is important to assess the relationship of septa to the cyst wall, as septa arising from the wall without external indentation or macrolobulation are sensitive (91%–100%) and specific (56%–93%) for MCN, whereas septa arising only from macrolobulations (Fig 16) are highly specific for SHC (91%–100%) (16,74). Additionally, mural calcifications and septa at CT and septal enhancement at MRI are significant features differentiating MCN from SHC (16).
Conclusion
Awareness of MCN and IPNB, their key distinguishing features, and how to differentiate them from each other and from more common cystic hepatic masses is important because they have different malignant potential, management, and prognosis. The majority of MCNs are benign and manifest as large, solitary, symptomatic multiloculated cystic masses without biliary communication in middle-aged women. The presence of characteristic OLS is determined after surgical resection and is not evident at imaging.
On the other hand, IPNBs are a heterogeneous spectrum of tumors that are commonly associated with invasive carcinoma. They have varied imaging appearances related to tumor proliferation and mucin production. Biliary ductal communication, intraductal masses, and associated bile duct dilatation are essential to differentiate IPNB from MCN and other hepatic cystic masses.
Although confusion and controversy surrounding these tumors persist, we illuminate pertinent issues from the past and provide up-to-date nomenclature and current perspectives on these tumors in the present, with the goal of equipping interpreting radiologists with greater understanding for the future.
Acknowledgments
Acknowledgment
We thank Derrick Kempf for the illustrations.
Recipient of a Magna Cum Laude award for an education exhibit at the 2020 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors V.S.K., M.G.L., K.A.M., and P.J.P. have provided disclosures (see end of article); all other authors, the editor, and the reviewers have disclosed no relevant relationships.
M.H.L was a military service member. This work was prepared as part of official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” The views expressed in this presentation are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or U.S. Government.
Disclosures of Conflicts of Interest.— : V.S.K. Activities related to the present article: editorial board member of RadioGraphics (not involved in the handling of this article). Activities not related to the present article: disclosed no relevant relationships. Other activities: disclosed no relevant relationships. M.G.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: grants from Philips and Ethicon. Other activities: disclosed no relevant relationships. K.A.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: honoraria from National Comprehensive Cancer Network; consultant for Elephas Bio; expert testimony for McDowell & Morrissette and for Gutglass, Erickson, Bonville, & Larson. Other activities: disclosed no relevant relationships. P.J.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultant for Bracco and Zebra Medical Vision; stockholder in SHINE Medical Technologies and Elucent. Other activities: disclosed no relevant relationships.
Abbreviations:
- CBD
- common bile duct
- IPMN
- intraductal papillary mucinous neoplasm
- IPNB
- intraductal papillary neoplasm of the bile duct
- MCN
- mucinous cystic neoplasm of the liver
- MRCP
- MR cholangiopancreatography
- OLS
- ovarian-like stroma
- SHC
- simple hepatic cyst
- WHO
- World Health Organization
References
- 1. Nakanuma Y , Klimstra DS , Zen Y , Komuta M . Mucinous cystic neoplasm of the liver and biliary system . In: WHO Classification of Tumours . 5th ed. Lyon, France: : IARC; , 2020. . https://tumourclassification.iarc.who.int/chaptercontent/31/88. Accessed December 21, 2020 . [Google Scholar]
- 2. World Health Organization . Intraductal papillary neoplasm of the bile ducts . In: WHO Classification of Tumours . 5th ed. Lyon, France: : IARC; , 2020. . https://tumourclassification.iarc.who.int/chaptercontent/31/275. Accessed December 21, 2020 . [Google Scholar]
- 3. Zen Y , Pedica F , Patcha VR , et al . Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct . Mod Pathol 2011. ; 24 ( 8 ): 1079 – 1089 . [DOI] [PubMed] [Google Scholar]
- 4. Kubota K , Nakanuma Y , Kondo F , et al . Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association . J Hepatobiliary Pancreat Sci 2014. ; 21 ( 3 ): 176 – 185 . [DOI] [PubMed] [Google Scholar]
- 5. Buetow PC , Buck JL , Pantongrag-Brown L , et al . Biliary cystadenoma and cystadenocarcinoma: clinical-imaging-pathologic correlations with emphasis on the importance of ovarian stroma . Radiology 1995. ; 196 ( 3 ): 805 – 810 . [DOI] [PubMed] [Google Scholar]
- 6. Zen Y , Fujii T , Itatsu K , et al . Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct . Mod Pathol 2006. ; 19 ( 9 ): 1243 – 1254 . [DOI] [PubMed] [Google Scholar]
- 7. Katabathina VS , Flaherty EM , Dasyam AK , et al . “Biliary Diseases with Pancreatic Counterparts”: Cross-sectional Imaging Findings . RadioGraphics 2016. ; 36 ( 2 ): 374 – 392 . [DOI] [PubMed] [Google Scholar]
- 8. Nakanuma Y , Jang KT , Fukushima N , et al . A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage . J Hepatobiliary Pancreat Sci 2018. ; 25 ( 3 ): 181 – 187 . [DOI] [PubMed] [Google Scholar]
- 9. Kubota K , Jang JY , Nakanuma Y , et al . Clinicopathological characteristics of intraductal papillary neoplasm of the bile duct: a Japan-Korea collaborative study . J Hepatobiliary Pancreat Sci 2020. ; 27 ( 9 ): 581 – 597 . [DOI] [PubMed] [Google Scholar]
- 10. Klompenhouwer AJ , Ten Cate DWG , Willemssen FEJA , et al . The impact of imaging on the surgical management of biliary cystadenomas and cystadenocarcinomas: a systematic review . HPB (Oxford) 2019. ; 21 ( 10 ): 1257 – 1267 . [DOI] [PubMed] [Google Scholar]
- 11. Arnaoutakis DJ , Kim Y , Pulitano C , et al . Management of biliary cystic tumors: a multi-institutional analysis of a rare liver tumor . Ann Surg 2015. ; 261 ( 2 ): 361 – 367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albores-Saavedra J , Córdova-Ramón JC , Chablé-Montero F , Dorantes-Heredia R , Henson DE . Cystadenomas of the liver and extrahepatic bile ducts: morphologic and immunohistochemical characterization of the biliary and intestinal variants . Ann Diagn Pathol 2015. ; 19 ( 3 ): 124 – 129 . [DOI] [PubMed] [Google Scholar]
- 13. Zen Y , Jang KT , Ahn S , et al . Intraductal papillary neoplasms and mucinous cystic neoplasms of the hepatobiliary system: demographic differences between Asian and Western populations, and comparison with pancreatic counterparts . Histopathology 2014. ; 65 ( 2 ): 164 – 173 . [DOI] [PubMed] [Google Scholar]
- 14. Devaney K , Goodman ZD , Ishak KG . Hepatobiliary cystadenoma and cystadenocarcinoma: a light microscopic and immunohistochemical study of 70 patients . Am J Surg Pathol 1994. ; 18 ( 11 ): 1078 – 1091 . [PubMed] [Google Scholar]
- 15. Borhani AA , Wiant A , Heller MT . Cystic hepatic lesions: a review and an algorithmic approach . AJR Am J Roentgenol 2014. ; 203 ( 6 ): 1192 – 1204 . [DOI] [PubMed] [Google Scholar]
- 16. Boyum JH , Sheedy SP , Graham RP , et al . Hepatic Mucinous Cystic Neoplasm Versus Simple Biliary Cyst: Assessment of Distinguishing Imaging Features Using CT and MRI . AJR Am J Roentgenol 2021. ; 216 ( 2 ): 403 – 411 . [DOI] [PubMed] [Google Scholar]
- 17. Park HJ , Kim SY , Kim HJ , et al . Intraductal Papillary Neoplasm of the Bile Duct: Clinical, Imaging, and Pathologic Features . AJR Am J Roentgenol 2018. ; 211 ( 1 ): 67 – 75 . [DOI] [PubMed] [Google Scholar]
- 18. Soares KC , Arnaoutakis DJ , Kamel I , et al . Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma . J Am Coll Surg 2014. ; 218 ( 1 ): 119 – 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klöppel G , Adsay V , Konukiewitz B , Kleeff J , Schlitter AM , Esposito I . Precancerous lesions of the biliary tree . Best Pract Res Clin Gastroenterol 2013. ; 27 ( 2 ): 285 – 297 . [DOI] [PubMed] [Google Scholar]
- 20. Quigley B , Reid MD , Pehlivanoglu B , et al . Hepatobiliary Mucinous Cystic Neoplasms With Ovarian Type Stroma (So-Called “Hepatobiliary Cystadenoma/Cystadenocarcinoma”): Clinicopathologic Analysis of 36 Cases Illustrates Rarity of Carcinomatous Change . Am J Surg Pathol 2018. ; 42 ( 1 ): 95 – 102 . [DOI] [PubMed] [Google Scholar]
- 21. Lee CW , Tsai HI , Lin YS , Wu TH , Yu MC , Chen MF . Intrahepatic biliary mucinous cystic neoplasms: clinicoradiological characteristics and surgical results . BMC Gastroenterol 2015. ; 15 ( 1 ): 67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erdogan D , Kloek J , Lamers WH , et al . Mucinous cystadenomas in liver: management and origin . Dig Surg 2010. ; 27 ( 1 ): 19 – 23 . [DOI] [PubMed] [Google Scholar]
- 23. Choi HK , Lee JK , Lee KH , et al . Differential diagnosis for intrahepatic biliary cystadenoma and hepatic simple cyst: significance of cystic fluid analysis and radiologic findings . J Clin Gastroenterol 2010. ; 44 ( 4 ): 289 – 293 . [DOI] [PubMed] [Google Scholar]
- 24. Van Treeck BJ , Lotfalla M , Czeczok TW , et al . Molecular and Immunohistochemical Analysis of Mucinous Cystic Neoplasm of the Liver . Am J Clin Pathol 2020. ; 154 ( 6 ): 837 – 847 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serra S . Precursor neoplastic lesions of the biliary tract . J Clin Pathol 2014. ; 67 ( 10 ): 875 – 882 . [DOI] [PubMed] [Google Scholar]
- 26. Anand S , Chandrasekar S , Raja K , Pottakkat B . Mucinous cystic neoplasm of the liver with biliary communication: an exception to the current classification . BMJ Case Rep 2019. ; 12 ( 1 ): bcr-2018 – 227063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujikura K , Akita M , Abe-Suzuki S , Itoh T , Zen Y . Mucinous cystic neoplasms of the liver and pancreas: relationship between KRAS driver mutations and disease progression . Histopathology 2017. ; 71 ( 4 ): 591 – 600 . [DOI] [PubMed] [Google Scholar]
- 28. Doussot A , Gluskin J , Groot-Koerkamp B , et al . The accuracy of pre-operative imaging in the management of hepatic cysts . HPB (Oxford) 2015. ; 17 ( 10 ): 889 – 895 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim JY , Kim SH , Eun HW , et al . Differentiation between biliary cystic neoplasms and simple cysts of the liver: accuracy of CT . AJR Am J Roentgenol 2010. ; 195 ( 5 ): 1142 – 1148 . [DOI] [PubMed] [Google Scholar]
- 30. Kim HJ , Yu ES , Byun JH , et al . CT differentiation of mucin-producing cystic neoplasms of the liver from solitary bile duct cysts . AJR Am J Roentgenol 2014. ; 202 ( 1 ): 83 – 91 . [DOI] [PubMed] [Google Scholar]
- 31. Cogley JR , Miller FH . MR imaging of benign focal liver lesions . Radiol Clin North Am 2014. ; 52 ( 4 ): 657 – 682 . [DOI] [PubMed] [Google Scholar]
- 32. Lewin M , Mourra N , Honigman I , et al . Assessment of MRI and MRCP in diagnosis of biliary cystadenoma and cystadenocarcinoma . Eur Radiol 2006. ; 16 ( 2 ): 407 – 413 . [DOI] [PubMed] [Google Scholar]
- 33. Xu HX , Lu MD , Liu LN , et al . Imaging features of intrahepatic biliary cystadenoma and cystadenocarcinoma on B-mode and contrast-enhanced ultrasound . Ultraschall Med 2012. ; 33 ( 7 ): E241 – E249 . [DOI] [PubMed] [Google Scholar]
- 34. Barton JG , Barrett DA , Maricevich MA , et al . Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford) 2009. ; 11 ( 8 ): 684 – 691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rocha FG , Lee H , Katabi N , et al . Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 2012. ; 56 ( 4 ): 1352 – 1360 . [DOI] [PubMed] [Google Scholar]
- 36. Yeh TS , Tseng JH , Chen TC , et al . Characterization of intrahepatic cholangiocarcinoma of the intraductal growth-type and its precursor lesions . Hepatology 2005. ; 42 ( 3 ): 657 – 664 . [DOI] [PubMed] [Google Scholar]
- 37. Zen Y , Fujii T , Itatsu K , et al . Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas . Hepatology 2006. ; 44 ( 5 ): 1333 – 1343 . [DOI] [PubMed] [Google Scholar]
- 38. Nakanuma Y , Uesaka K , Kakuda Y , et al . Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations . J Clin Med 2020. ; 9 ( 12 ): E3991 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim JR , Lee KB , Kwon W , Kim E , Kim SW , Jang JY . Comparison of the Clinicopathologic Characteristics of Intraductal Papillary Neoplasm of the Bile Duct according to Morphological and Anatomical Classifications . J Korean Med Sci 2018. ; 33 ( 42 ): e266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohtsuka M , Shimizu H , Kato A , et al . Intraductal papillary neoplasms of the bile duct . Int J Hepatol 2014. ; 2014 459091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ying S , Ying M , Liang W , et al . Morphological classification of intraductal papillary neoplasm of the bile duct . Eur Radiol 2018. ; 28 ( 4 ): 1568 – 1578 . [DOI] [PubMed] [Google Scholar]
- 42. Ohtsuka M , Kimura F , Shimizu H , et al . Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion . Am J Surg Pathol 2011. ; 35 ( 4 ): 512 – 521 . [DOI] [PubMed] [Google Scholar]
- 43. Kim KM , Lee JK , Shin JU , et al . Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype . Am J Gastroenterol 2012. ; 107 ( 1 ): 118 – 125 . [DOI] [PubMed] [Google Scholar]
- 44. Gordon-Weeks AN , Jones K , Harriss E , Smith A , Silva M . Systematic Review and Meta-analysis of Current Experience in Treating IPNB: Clinical and Pathological Correlates . Ann Surg 2016. ; 263 ( 4 ): 656 – 663 . [DOI] [PubMed] [Google Scholar]
- 45. Wan XS , Xu YY , Qian JY , et al . Intraductal papillary neoplasm of the bile duct . World J Gastroenterol 2013. ; 19 ( 46 ): 8595 – 8604 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lim JH , Zen Y , Jang KT , Kim YK , Nakanuma Y . Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects . AJR Am J Roentgenol 2011. ; 197 ( 5 ): 1111 – 1120 . [DOI] [PubMed] [Google Scholar]
- 47. Schlitter AM , Born D , Bettstetter M , et al . Intraductal papillary neoplasms of the bile duct: stepwise progression to carcinoma involves common molecular pathways . Mod Pathol 2014. ; 27 ( 1 ): 73 – 86 . [DOI] [PubMed] [Google Scholar]
- 48. Sasaki M , Sato Y . Insulin-like growth factor II mRNA-binding protein 3 (IMP3) is a marker that predicts presence of invasion in papillary biliary tumors . Hum Pathol 2017. ; 62 ( 152 ): 159 . [DOI] [PubMed] [Google Scholar]
- 49. Nakanuma Y , Kakuda Y , Uesaka K . Characterization of Intraductal Papillary Neoplasm of the Bile Duct with Respect to the Histopathologic Similarities to Pancreatic Intraductal Papillary Mucinous Neoplasm . Gut Liver 2019. ; 13 ( 6 ): 617 – 627 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim JR , Jang KT , Jang JY , et al . Clinicopathologic analysis of intraductal papillary neoplasm of bile duct: Korean multicenter cohort study . HPB (Oxford) 2020. ; 22 ( 8 ): 1139 – 1148 . [DOI] [PubMed] [Google Scholar]
- 51. Harada F , Matsuyama R , Mori R , et al . Outcomes of surgery for 2010 WHO classification–based intraductal papillary neoplasm of the bile duct: case-control study of a single Japanese institution’s experience with special attention to mucin expression patterns . Eur J Surg Oncol 2019. ; 45 ( 5 ): 761 – 768 . [DOI] [PubMed] [Google Scholar]
- 52. Aslam A , Wasnik AP , Shi J , Sahai V , Mendiratta-Lala M . Intraductal papillary neoplasm of the bile duct (IPNB): CT and MRI appearance with radiology-pathology correlation . Clin Imaging 2020. ; 66 ( 10 ): 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim H , Lim JH , Jang KT , et al . Morphology of intraductal papillary neoplasm of the bile ducts: radiologic-pathologic correlation . Abdom Imaging 2011. ; 36 ( 4 ): 438 – 446 . [DOI] [PubMed] [Google Scholar]
- 54. Lim JH , Jang KT . Mucin-producing bile duct tumors: radiological-pathological correlation and diagnostic strategy . J Hepatobiliary Pancreat Sci 2010. ; 17 ( 3 ): 223 – 229 . [DOI] [PubMed] [Google Scholar]
- 55. Lim JH , Jang KT , Choi D . Biliary intraductal papillary-mucinous neoplasm manifesting only as dilatation of the hepatic lobar or segmental bile ducts: imaging features in six patients . AJR Am J Roentgenol 2008. ; 191 ( 3 ): 778 – 782 . [DOI] [PubMed] [Google Scholar]
- 56. Lim JH , Yoon KH , Kim SH , et al . Intraductal papillary mucinous tumor of the bile ducts . RadioGraphics 2004. ; 24 ( 1 ): 53 – 66 ; discussion 66–67 . [DOI] [PubMed] [Google Scholar]
- 57. Ogawa H , Itoh S , Nagasaka T , Suzuki K , Ota T , Naganawa S . CT findings of intraductal papillary neoplasm of the bile duct: assessment with multiphase contrast-enhanced examination using multi-detector CT . Clin Radiol 2012. ; 67 ( 3 ): 224 – 231 . [DOI] [PubMed] [Google Scholar]
- 58. Lee SS , Kim MH , Lee SK , et al . Clinicopathologic review of 58 patients with biliary papillomatosis . Cancer 2004. ; 100 ( 4 ): 783 – 793 . [DOI] [PubMed] [Google Scholar]
- 59. Yoon HJ , Kim YK , Jang KT , et al . Intraductal papillary neoplasm of the bile ducts: description of MRI and added value of diffusion-weighted MRI . Abdom Imaging 2013. ; 38 ( 5 ): 1082 – 1090 . [DOI] [PubMed] [Google Scholar]
- 60. Lee S , Kim MJ , Kim S , Choi D , Jang KT , Park YN . Intraductal papillary neoplasm of the bile duct: assessment of invasive carcinoma and long-term outcomes using MRI . J Hepatol 2019. ; 70 ( 4 ): 692 – 699 . [DOI] [PubMed] [Google Scholar]
- 61. Hong GS , Byun JH , Kim JH , et al . Thread sign in biliary intraductal papillary mucinous neoplasm: a novel specific finding for MRI . Eur Radiol 2016. ; 26 ( 9 ): 3112 – 3120 . [DOI] [PubMed] [Google Scholar]
- 62. Ying SH , Teng XD , Wang ZM , et al . Gd-EOB-DTPA–enhanced magnetic resonance imaging for bile duct intraductal papillary mucinous neoplasms . World J Gastroenterol 2015. ; 21 ( 25 ): 7824 – 7833 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu LN , Xu HX , Zheng SG , et al . Ultrasound Findings of Intraductal Papillary Neoplasm in Bile Duct and the Added Value of Contrast-Enhanced Ultrasound . Ultraschall Med 2015. ; 36 ( 6 ): 594 – 602 . [DOI] [PubMed] [Google Scholar]
- 64. Takanami K , Hiraide T , Kaneta T , et al . FDG PET/CT findings in malignant intraductal papillary mucinous neoplasm of the bile ducts . Clin Nucl Med 2010. ; 35 ( 2 ): 83 – 85 . [DOI] [PubMed] [Google Scholar]
- 65. Dong A , Dong H , Zhang L , Zuo C . F-18 FDG uptake in borderline intraductal papillary neoplasms of the bile duct . Ann Nucl Med 2012. ; 26 ( 7 ): 594 – 598 . [DOI] [PubMed] [Google Scholar]
- 66. Tsuyuguchi T , Sakai Y , Sugiyama H , et al . Endoscopic diagnosis of intraductal papillary mucinous neoplasm of the bile duct . J Hepatobiliary Pancreat Sci 2010. ; 17 ( 3 ): 230 – 235 . [DOI] [PubMed] [Google Scholar]
- 67. Luvira V , Pugkhem A , Bhudhisawasdi V , et al . Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct . J Gastroenterol Hepatol 2017. ; 32 ( 2 ): 527 – 533 . [DOI] [PubMed] [Google Scholar]
- 68. Schlitter AM , Jang KT , Klöppel G , et al . Intraductal tubulopapillary neoplasms of the bile ducts: clinicopathologic, immunohistochemical, and molecular analysis of 20 cases . Mod Pathol 2015. ; 28 ( 9 ): 1249 – 1264 . [Published correction appears in Mod Pathol 2016;29(1):93.] [DOI] [PubMed] [Google Scholar]
- 69. Wu CH , Yeh YC , Tsuei YC , et al . Comparative radiological pathological study of biliary intraductal tubulopapillary neoplasm and biliary intraductal papillary mucinous neoplasm . Abdom Radiol (NY) 2017. ; 42 ( 10 ): 2460 – 2469 . [DOI] [PubMed] [Google Scholar]
- 70. Lee YJ , Kim SH , Lee JY , et al . Differential CT features of intraductal biliary metastasis and double primary intraductal polypoid cholangiocarcinoma in patients with a history of extrabiliary malignancy . AJR Am J Roentgenol 2009. ; 193 ( 4 ): 1061 – 1069 . [DOI] [PubMed] [Google Scholar]
- 71. Matsumoto T , Kubota K , Hachiya H , et al . Impact of Tumor Location on Postoperative Outcome of Intraductal Papillary Neoplasm of the Bile Duct . World J Surg 2019. ; 43 ( 5 ): 1313 – 1322 . [DOI] [PubMed] [Google Scholar]
- 72. Jung G , Park KM , Lee SS , Yu E , Hong SM , Kim J . Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct . J Hepatol 2012. ; 57 ( 4 ): 787 – 793 . [DOI] [PubMed] [Google Scholar]
- 73. Kang MJ , Jang JY , Lee KB , Han IW , Kim SW . Impact of macroscopic morphology, multifocality, and mucin secretion on survival outcome of intraductal papillary neoplasm of the bile duct . J Gastrointest Surg 2013. ; 17 ( 5 ): 931 – 938 . [DOI] [PubMed] [Google Scholar]
- 74. Kovacs MD , Sheafor DH , Burchett PF , Picard MM , Hardie AD . Differentiating biliary cystadenomas from benign hepatic cysts: preliminary analysis of new predictive imaging features . Clin Imaging 2018. ; 49 ( 44 ): 47 . [DOI] [PubMed] [Google Scholar]





![MCN. (A) High-power photomicrograph shows mucinous-type columnar epithelium lining the cyst wall with underlying spindled OLS (arrows). The columnar epithelium is of the gastric/foveolar subtype. (Hematoxylin-eosin [H-E] stain; original magnification, ×400.) (B) High-power photomicrograph highlights immunopositive spindled mesenchymal-type cells (arrows) located underneath the mucinous epithelium, confirming the presence of OLS and the diagnosis of MCN. (Immunohistochemistry stain for progesterone receptor; original magnification, ×400.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/2126/8493652/f8105243b49d/rg.2021210011.fig1.jpg)




![Typical pattern of intrahepatic IPNB—an intraductal mass and both upstream and downstream ductal dilatation—in a 62-year-old man. (A) Axial T2-weighted image through the liver shows a lobulated intraductal mass (arrow), which is hypointense to adjacent bile and mucin with associated bile duct dilatation. (B) Axial contrast-enhanced fat-suppressed T1-weighted image shows the lobulated mass (arrow) within dilated hypointense bile ducts. (C) Maximum intensity projection MRCP image shows intrahepatic bile duct dilatation predominating in the left hepatic lobe, as well as extrahepatic bile duct dilatation with linear and curved hypointense filling defects (arrows), resulting in the thread sign. (D) Photograph of a section through the gross specimen after resection shows the intraductal mass (arrow) within the left hepatic lobe, which corresponds to the associated invasive carcinoma. (E) Low-power photomicrograph shows a papillary lesion protruding into the bile duct lumen. (Hematoxylin-eosin [H-E] stain; original magnification, ×20.) (F) On a higher-magnification photomicrograph, the biliary epithelium that approaches the papillary lesion (black arrows) appears more hyperchromatic owing to the presence of high-grade dysplasia. At the base of the papillary lesion is invasive carcinoma (green arrow). (Hematoxylin-eosin [H-E] stain; original magnification, ×40.) (G) Intermediate-power photomicrograph of the IPNB shows extensive high-grade dysplasia with rounded nuclei, architectural complexity, and abundant mitoses. (Hematoxylin-eosin [H-E] stain; original magnification, ×200.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/2126/8493652/31b57339e30b/rg.2021210011.fig7.jpg)