Abstract
Introduction
The use of flavors in electronic cigarettes appeals to adults and never-smoking youth. Consumption has rapidly increased over the last decade, and in the U.S. market alone, there are over 8000 unique flavors. The U.S. Food and Drug Administration (FDA) has begun to regulate e-liquids, but many have not been tested, and their impact, both at the cellular level, and on human health remains unclear.
Methods
We tested e-liquids on the human cell line HEK293T and measured toxicity, mitochondrial membrane potential (ΔΨ m), reactive oxygen species production (ROS), and cellular membrane potential (Vm) using high-throughput screening (HTS) approaches. Our HTS efforts included single-dose and 16-point dose–response curves, which allowed testing of ≥90 commercially available e-liquids in parallel to provide a rapid assessment of cellular effects as a proof of concept for a fast, preliminary toxicity method. We also investigated the chemical composition of the flavors via gas chromatography–mass spectrometry.
Results
We found that e-liquids caused a decrease in ΔΨ m and Vm and an increase in ROS production and toxicity in a dose-dependent fashion. In addition, the presence of five specific chemical components: vanillin, benzyl alcohol, acetoin, cinnamaldehyde, and methyl-cyclopentenolone, but not nicotine, were linked with the changes observed in the cellular traits studied.
Conclusion
Our data suggest that ΔΨ m, ROS, Vm, and toxicity may be indicative of the extent of cell death upon e-liquid exposure. Further research on the effect of flavors should be prioritized to help policy makers such as the FDA to regulate e-liquid composition.
Implications
E-liquid cellular toxicity can be predicted using parameters amenable to HTS. Our data suggest that ΔΨ m, ROS, Vm, and toxicity may be indicative of the extent of cell death upon e-liquid exposure, and this toxicity is linked to the chemical composition, that is, flavoring components. Further research on the effect of flavors should be prioritized to help policy makers such as the FDA to regulate e-liquid composition.
Introduction
Electronic cigarettes (e-cigarettes) were introduced onto the market in the early 2000s. Since then, their use has drastically increased worldwide both among adults and youth.1 E-cigarettes comprise an electric heater that aerosolizes a liquid (e-liquid), which usually contains different concentrations of nicotine; variable ratios of propylene glycol (PG) and vegetable glycerin (VG), used as vehicle; and diverse chemicals for flavoring.2 In the United States alone, there are over 1200 different vendors and over 8000 flavors.3 In January 2020, after many years without proper regulatory measures, the FDA issued an enforcement policy on unauthorized flavored cartridge-based e-cigarette products, including fruit and mint flavors.4
We have previously shown that human embryonic kidney 293 (HEK293T) cells elicit similar cell responses to cigarette smoke as airway epithelia, including those involved in cell signaling, protein trafficking, and similar membrane stiffening when exposed to PG/VG.5,6 Moreover, we recently demonstrated that HEK293T cells are suitable to compare the cytotoxic effects of many e-liquids in a quick and reliable way that is representative of the toxicity induced in pulmonary cell types, including air–liquid interface epithelia.7 In this approach, we found that 123 flavors, out of 138 e-liquids tested, decreased viability over a range of concentrations from 0 to 60%.7 Notably, high-throughput screening (HTS) approaches opens up a convenient and fast way to test market-available e-cigarettes flavors and their chemical components.8
Some flavored e-cigarettes contain variable amounts of chemicals that are approved for food use/ingestion, but associated with respiratory diseases when inhaled, such as the flavoring agents: benzaldehyde (fruit, bitter almond)9,10 diacetyl (butter, pastry),11 2,3-pentanedione (butter and fermented diary),9,12 and acetoin (butter, green pepper).12–14 Chemicals that raise concerns for human health were also identified, such as coumarin, nitrosamides, aldehydes (forming toxic adducts with DNA), and other toxic carbonyl compounds found in cigarette smoke.15 Here, we used a more diverse set of e-liquids, from different online vendors, and tested the hypothesis that the effects seen with cell death could be replicated with other germane end points, including reactive oxygen species (ROS), mitochondrial membrane potential (ΔΨ m), and cellular membrane potential (Vm). The overall goal of this work was to screen neat e-liquids and identify potential flavors and/or chemical constituents that show other detrimental cellular effects beyond the basic live/dead assay that we previously performed.
Materials and Methods
Refer to the Supplementary Methods section for a detailed description.
E-cigarette Products
A total of 98 e-liquids were purchased from 74 different vendors (Supplementary Table S1).
Chemicals and Reagents
All reagents were ACS grade and purchased from Sigma–Aldrich unless stated otherwise.
Cell Culture
HEK293T cells were cultured as previously described.7
Cytotoxicity Assays
Cell Survival
The effects of the e-liquids on cell survival were evaluated as previously reported.7
Mitochondrial Membrane Potential (ΔΨ m)
Briefly, HEK239T cells were exposed to e-liquids and after 22–20 hours, cells were stained using Mitotracker Red and DAPI for 30 minutes. Fluorescent images were acquired and corrected using DAPI.
ROS Production
HEK293T cells were exposed to e-liquids and later stained with 2′,7′-dichlorofluorescin diacetate for 30 minutes. Fluorescent images were acquired and corrected for cell number using bright-field images DAPI.
Membrane Potential (Vm)
HEK293T cells were exposed to e-liquids and later stained using the FLIPR Membrane Potential Blue Assay Kit according to the manufacturer’s recommendations. Vm was measured as fluorescence emission before (Vm-pre) and after (Vm-post) adding KCl to induce membrane depolarization.
Gas Chromatography–Mass Spectrometry Analysis of E-liquids and Data Analysis
See Supplementary Methods section.
Results
E-Liquids Decrease Cell Viability in a Dose-Dependent Fashion
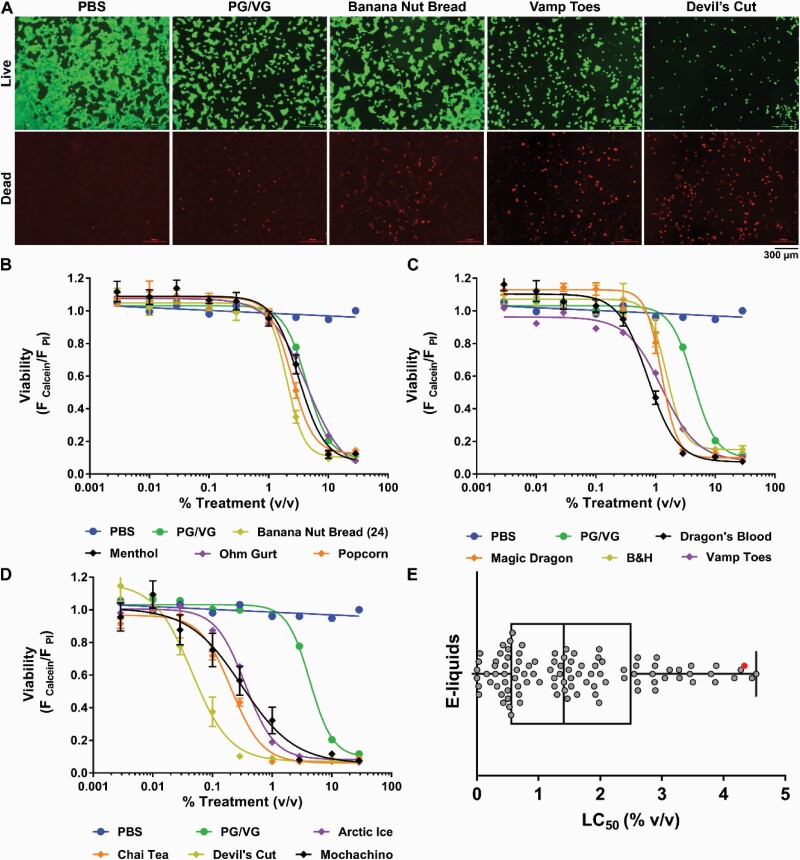
We have previously shown that e-liquids directly affect cell growth and viability.7 However, this study was predominantly performed on locally sourced e-liquids from North Carolina. Here, we extended our study to include national vendors (see Supplementary Table S1). To perform a comparison with our previous study, we first performed HTS in HEK293T cells using the same protocol, that is, calcein-AM and propidium iodide as markers of live and dead cells respectively (Figure 1A). The HTS assay was validated according to the coefficient of variation for each 384 plate as previously described.16 After 22–24 hours, all e-liquids caused a dose-dependent decrease in the number of live cells, an increase in dead cell number and the concomitant decrease in live/dead cell number ratio (Supplementary Table S1). The dose–response curves were then used to calculate the LC50 values of the e-liquids (Figure 1B–D; Supplementary Table S1). As expected, the phosphate buffer (PBS) control did not have a toxic effect, whereas PG/VG decreased cell viability in a dose-dependent manner (Figure 1B–D). PG/VG had an LC50 of 4.34% vol/vol, showing values within range of our previous studies.7 The LC50s of the e-liquids tested ranged from 4.53 to 0.0025% vol/vol (Figure 1E; Supplementary Table S1). Consistent with our previous study, all e-liquids caused toxicity in a dose-dependent fashion.
Figure 1.
E-liquids affect HEK293T cell toxicity. (A) Cells were stained with calcein-AM (live, green) and propidium iodide (dead, red). PBS, PG/VG, and representative e-liquids are shown. (B–D) Dose–response curves for PBS, PG/VG, and representative e-liquids. (E) LC50 distribution (reported as concentration, % vol/vol) of the 98 e-liquids tested. ● = PG/VG (e-liquid vehicle). n = 9.
E-Liquids Affect ΔΨ m
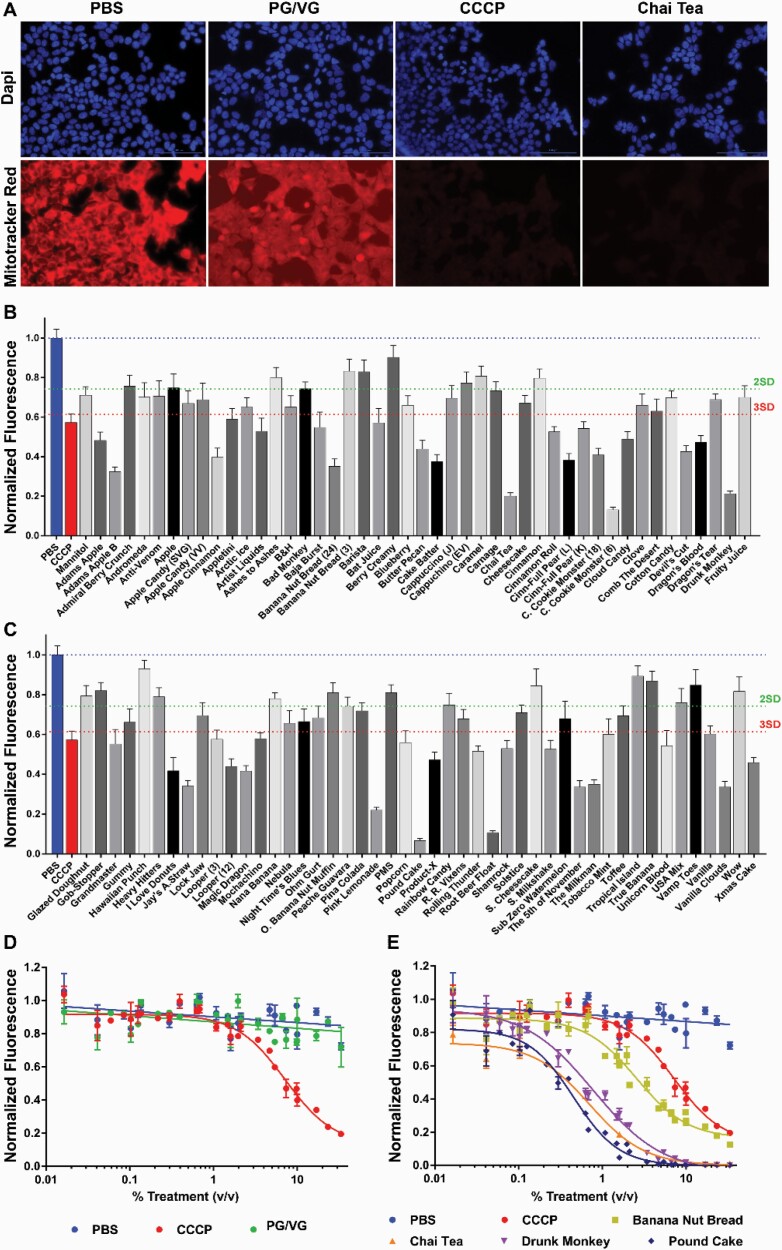
Because we consistently saw effects of e-liquids on cell viability, we tested other commercially available dyes that are suitable for HTS approaches and relevant to cell death. We investigated changes in ΔΨ m using the Mitotracker Red stain. We also added DAPI to identify cell nuclei to normalize the signal to cell number (Figure 2A). HEK293T cells were exposed to e-liquids at 1% vol/vol for 24 hours. Out 92 e-liquids, 57 fell beyond the average ± 3 SD cutoff, including the positive control, CCCP (Figure 2B and C). These were selected to perform dose-responses. The positive control CCCP and all tested e-liquids showed a dose-dependent inhibition of ΔΨ m, whereas PG/VG and PBS did not (Figure 2D and E; Supplementary Table S1).
Figure 2.
E-liquids impair the mitochondrial membrane potential (ΔΨ m). (A) Images showing Mitotracker red and DAPI (blue) staining for PBS, PG/VG, and representative flavors. (B and C) Cells were exposed to e-liquids (1% vol/vol) and then stained. CCCP was used as a positive control (3 µM). Values were normalized to PBS response (blue dotted line). Green and red dotted lines show average response – 2 SD or 3 SD, respectively. N = 6. (D, E) E-liquids were tested at several concentrations. Dose-dependent responses are shown. Values were normalized to the PBS response (blue line). All n = 9 per group.
E-Liquid Effects on Reactive Oxygen Production
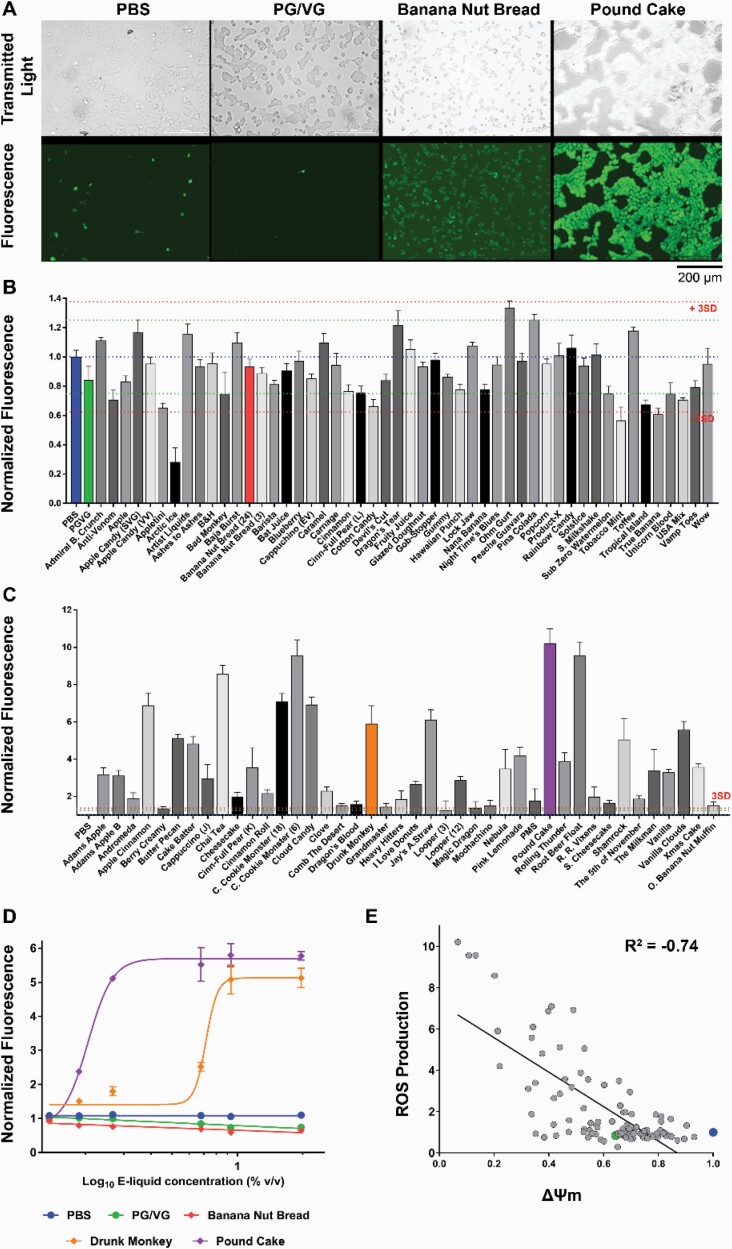
Oxidative stress was evaluated using the fluorescent marker 2′,7′-dichlorofluorescin diacetate (Figure 3A). At 1% vol/vol, 34 e-liquids showed values below and above ± 3 SD of the control’s ROS production. PG/VG did not show any significant effect when compared with the PBS control (Figure 3B and C). E-liquids that responses above or below the average PBS signal ± 3 SD response were tested in a dose-dependent manner. Figure 3D shows representative curves for PG/VG, Banana Nut Bread, Drunk Monkey, and Pound Cake. We then analyzed the relationship between ROS production versus ΔΨ m, and we found that these are negatively correlated (R = −.74; p < .0001; Figure 3E).
Figure 3.
E-liquids effect on ROS production in HEK293T. (A) Transmitted light and fluorescent images (DCFDA stain) for PBS (control), PG/VG (e-liquid base), and representative flavors. (B and C) Primary screen of 98 e-liquids (1% vol/vol). Values normalized to PBS response (blue dotted line). PG/VG is shown in green. Green and red dotted lines show average response ± 2 SD or 3 SD, respectively. N = 8. (D) Dose–response curves for PBS (blue), PG/VG (green), and representative e-liquids. (E) ROS production versus ΔΨ m showed a negative correlation (R = −.74; p < .0001). PG/VG (●) and PBS (●) values are highlighted. All n = 9 per group.
E-Liquids Depolarize Vm
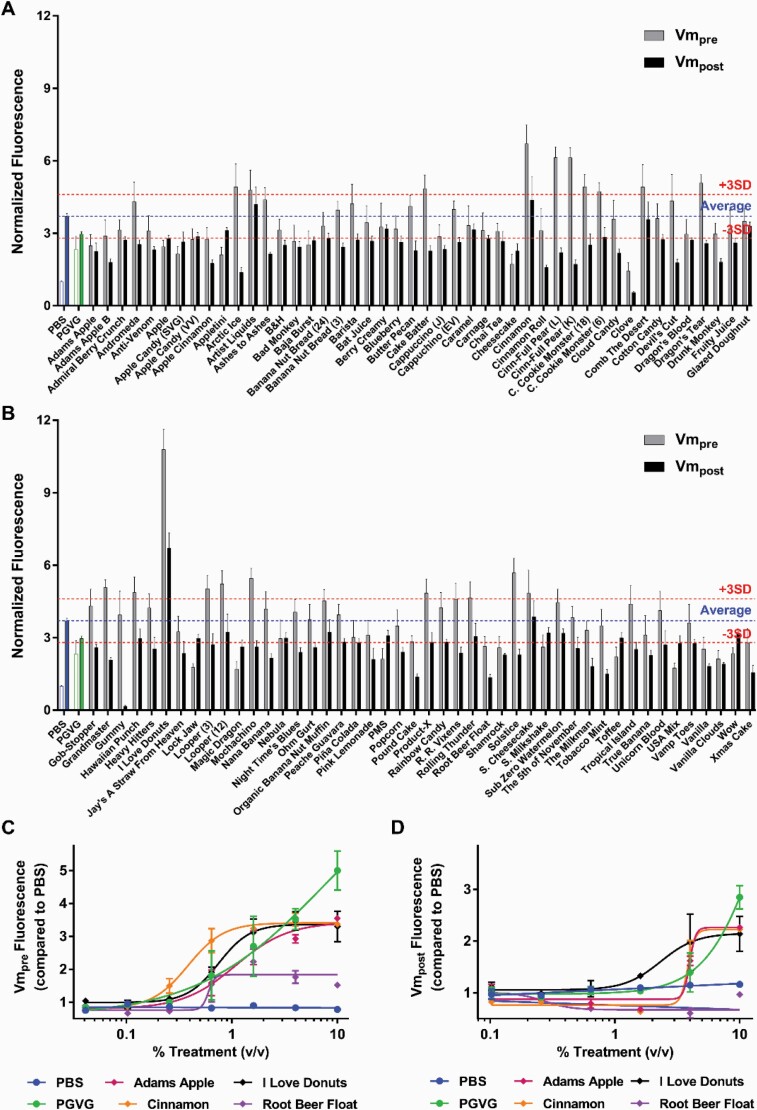
Several reports have shown a relationship between ROS production and Vm depolarization, so we followed our ROS production findings with Vm assessments.17,18 An appropriate resting Vm is critical for cell survival. Vm is typically approximately −70 mV, and persistent depolarizations are an early indicator of apoptosis.19 We therefore investigated the effects of e-liquids on Vm by measuring changes in fluorescence. After incubating HEK293T cells with e-liquids, Vm was measured before (Vm-pre) and after (Vm-post) adding KCl, which induced membrane depolarization. Before adding KCl (Vm-pre), several e-liquids (n = 39) caused significant increase in fluorescence (above 3SD compared with the control), indicating spontaneous depolarization compared with the PBS control (Figure 4A and B). After KCl addition (Vm-post), PBS-treated cells showed depolarization, as expected, whereas 22 e-liquids showed a decrease in Vm-post, suggesting that these e-liquids induced repolarization of Vm or, at a minimum, those e-liquids prevented the cell membrane to depolarize after KCl exposure (Figure 4A and B). E-liquids that showed an effect in the primary assay were used to exposed cell to a range of doses (Figure 4A and B). PG/VG induced a dose-dependent increase in both Vm-pre and Vm-post (Figure 4C and D). To assess the effect after spontaneous depolarization by e-liquids, we plotted Vm-pre and ΔVm (Vm-post − Vm-pre) (Supplementary Figure S1). By comparison to PBS (normal Vm), e-liquids aligned into three distint groups: (1) depolarization after KCl exposure (normal Vm, like PBS control); (2) spontaneous depolarization and no polarization after KCl exposure (compared with PBS control), and (3) spontaneous depolarization and hyperpolarization due to KCl addition (Supplementary Figure S1, red, blue, and green, respectively).
Figure 4.
E-liquids effect on Vm. (A–B) Primary screen of 92 e-liquids (1% vol/vol). Values normalized to Vm-pre PBS response. Blue dotted lined represents average PBS Vm-post. PG/VG is shown in green. Red dotted lines show average Vm-post response ± 3 SD. N = 8. (C) Vm-pre dose–response curves for PBS (●), PG/VG (●), and representative e-liquids. (D) Vm-post dose–response curves for PBS (●), PG/VG (●), and the same representative e-liquids. N = 4 per group.
E-liquid Chemical Composition Is Varied
Gas chromatography–mass spectrometry was used to identify e-liquids chemical constituents, as described.7 Chromatograms obtained using this approach were compared with the NIST mass spectral database for compound identification. As shown in Supplementary Figure S2, distinct peaks, corresponding to unique chemicals, were readily identifiable. Indeed, a total of 128 different chemicals were identified in the 92 e-liquids (Supplementary Table S3). However, only 30 chemicals were present in more than 10 e-liquids.
Analysis of Chemical Composition in relation with E-Liquids Toxicity
We analyzed the relationship between chemical composition and cellular effects using PCA. The first dimension PC1 included the original variables: ΔΨ m, ROS production, live/dead ratio, and LC50 values. The correlation or redundancy value was .75, and the contribution of the variables was 23%. This dimension showed that ΔΨ m and ROS were inversely associated with toxicity (live/dead ratio), whereas live/dead ratio were directly associated to LC50 values (Supplementary Figure S3). The analysis also separated e-liquids according to their toxicity within these traits, with the most toxic e-liquids being on the left side of the axis (more negative axis values) and the least toxic on the right side (more positive axis values) (Supplementary Figure S3). The second dimension, or PC2, represented e-liquid toxicity according to the cellular membrane potential change after adding KCl (Vm-post) or ΔVm (Vm-post − Vm-pre). These variables had a correlation of .73, and their contribution to variability was 50%. This dimension showed a direct correlation between these two traits; it ordered e-liquids from most toxic at the bottom of the axis to least toxic toward the top. The two first PCA dimensions accounted for 54% and 21%, respectively, of the total variance in e-liquids toxicity in all cellular traits (Supplementary Figure S3).
The non-metric multidimensional scaling ordination of the e-liquids showed an acceptable stress level of 0.20 (>0.3 is a poor representation of the data). The ordination of PC1 indicated a clear separation between two groups of chemicals that show below or above toxicity score when compared with the average number of chemical components present in each e-liquid (Supplementary Figure S3). To compare e-liquids grouped according to traits, we used PERMANOVA to define the space between the groups’ centroids and dispersion. The centroid is defined as the mean location of all data points and dispersion accounts for the variability in measured space from the centroid.20 Analysis of PC1 showed a significant difference between the two groups regarding their location (p = .0066) and dispersion (p = .026), thus revealing that the least toxic group is more variable than the other. On the other hand, the same analysis of PC2 did not show significant differences (p = .60 and .61, respectively). These results could tentatively explain that the number of chemical components in an e-liquid could be directly related to its toxicity.
Finally, a linear model was used to assess toxicity ranking scores according to the presence and/or absence of certain chemical components in a e-liquid formulation. The model used 6 degrees of freedom (independent variables) in the analysis and had an adjusted R2 value (goodness of fit for this model) of .401 and a p-value lower than .001. With this analysis, we got a final model that best explained the variability in toxicity among e-liquids. We identified five chemicals that significantly increased the toxicity score (PC1) when present in e-liquids (Supplementary Table S2). These chemicals and their % presence in e-liquids were: ethyl-vanillin and vanillin, 33% incidence; benzyl alcohol, 31%; acetoin, 19%; cinnamaldehyde, 10%; and methyl-cyclopentenolone, 8%. Supplementary Table S2 shows a comprehensive list of chemical compounds that showed increased toxicity in e-liquids. For each compound, we have included its flavor category.
Discussion
Increasing evidence shows that exposure to e-cigarette vapor in human models affects pulmonary and vascular functions,21 which correlate with e-liquids altering a variety of human cells in vitro.1,6,22 Several studies have shown that e-cigarette vapors trigger apoptosis and necrosis in human endothelial cells, epithelial cells, and alveolar macrophages.23–25 Human airway epithelia showed decreased ciliary beating, immune defense, and ion channel conductance; increased levels of oxidative stress via amino acids and ADP levels; and apoptosis when exposed to e-cigarette vapors.26–28
The transmembrane potential of the mitochondria plays an important role in cell homeostasis as it drives the production of ATP in this organelle. We found that 35 of the e-liquids tested induced a significant decrease in ΔΨ m compared with the PBS control (p < .05). Moreover, eight e-liquids showed a greater inhibitory effect than the respiratory chain uncoupling agent, CCCP, suggesting that particular flavors, contaminants, or additives may contribute to cellular toxicity by impairing mitochondrial function. Independent of flavors, some authors have reported that e-cigarette vapors disturb the mitochondrial respiration complexes III and IV in human myofibroblasts and primary lung fibroblasts, respectively.29,30 A recent study showed that e-cigarette aerosols induce mitochondrial depolarization in vascular endothelial cells in a similar way as cigarette smoke.31 Mitochondrial dysfunction is increasingly recognized as a key component in acute and chronic cellular stress and we propose that screening for e-liquid effects on ΔΨ m become standard due to the importance of this measure.
Mitochondrial respiration chain inhibition leads to the formation of ROS.32 We have reported that 64% of the e-liquids tested, induced a significant change in cellular ROS levels in a concentration dependent manner. PG/VG (55:45) did not induce ROS production suggesting that it is not responsible for this effect. Conceivably, it is a combination of nicotine, flavoring chemicals, and/or nonflavor components of e-liquids that cause changes in ROS production. Some studies showed that unvaporized e-liquids were oxidative, depending on the presence of PG and glycerin33 and that some flavored e-cigarette aerosols increased ROS independently of nicotine.34 Altered ROS in the lung can lead to the increased expression of proteins that regulate ΔΨ m. In turn, these changes can cause fibrosis, inflammation, structural damage, promoted airway remodeling, and bronchial hyperresponsiveness, which are involved in the development of asthma, chronic obstructive pulmonary disease, and lung cancer.35–37 Lipid-soluble components in cigarette smoke induce mitochondrial production of ROS in lung epithelial cells.32
Regarding the effects of e-liquids on Vm, 42% of tested e-liquids induced a decrease in Vm followed by a lack of response to KCl, suggesting that they had caused spontaneous depolarization. In most cell types, the plasma membrane Na+/K+-ATPase establishes the ionic gradients, and then K+ channels are responsible for determining the resting Vm, due to K+’s Nernst potential across the plasma membrane.38 It is currently unclear whether the observed effects on Vm were due to alterations in the Na+/K+-ATPase or K+ channels. However, given that we observed a depolarization with extracellular K+ addition that was diminished after e-liquid addition; these data suggest that K+ channel activity has been altered. E-liquids have previously been shown to affect ion channels. For example, the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel was affected by both nicotine and flavors.39 Moreover, transient receptor potential channels, which are nonselective cation channels, can be directly stimulated by certain flavors like menthol40 and more recently have been shown to be activated by nicotine.41 An appropriate Vm is important for multiple aspects of cell homeostasis. For example, if the cell is chronically depolarized, this would disrupt influx of the key second messenger Ca2+. Moreover, dysregulation of ionic gradients and Vm could alter intracellular ion concentrations, which could have ramifications for everything from cell growth to lysosomal functions.42,43 However, more testing is needed to validate these results and to elucidate the underlying mechanism.
The e-liquids tested in this study also caused cell death at higher concentrations. However, both their LC50 and their chemical constituents greatly varied, suggesting that e-liquid composition has a direct impact on toxicity. In agreement with these results, other studies revealed that chemical composition had an influence on the extent of human endothelial cells apoptosis.44 Here, we identified flavoring compounds that were associated with ΔΨ m, ROS production, and cell death. As an example, ethyl-vanillin has been identified as one of the most common flavoring compounds used in e-liquid formulation with concentrations ranging from 0.5 to 335 mg/mL.45 Ethyl-vanillin is known as a potent agonist of several transient receptor potential channels in rat neurons, leading to inflammation.46 Moreover, methyl-vanillin, which is widely found in e-liquids, increases ROS production, promoting inflammation and cell death in human monocytes and endothelial cells.47,48 As a whole, there is sufficient evidence that the “vanillin” family of flavoring compounds is involved in cell apoptosis; consequently, these class of chemicals should be banned from e-liquid formulations.
Acetoin, also related with toxicity according to our study, was identified in vapor mixtures associated with “Popcorn workers’ lung,” 13,49 an obstructive pulmonary disease, and it has been classified as a “High Priority Substance” in the Federal Emergency Management Agency (FEMA) report on respiratory health and safety in the food manufacturing workplace.50 In addition to its use in the food industry, this compound is one of the most prevalent flavors in e-cigarettes, being detected in e-liquids at up to 529 µg per e-liquid vial.14 In agreement with our results, it has been recently found that acetoin increases ROS levels in human monocytic cell lines leading to inflammatory responses and that this effect is independent of nicotine presence.48 Similarly, we did not find an effect due to nicotine presence in our model.
We found benzyl alcohol was present in 31% of our samples. In support of our results, other studies have reported that this chemical reduces ΔΨ m and induces cell death when applied in high doses to mice and human hepatocytes, respectively.51 Last, methylcyclopentenolone (coffee flavor) has been found in e-liquids although to our knowledge this is the only study that has evaluated its cytotoxicity in e-liquids.52 Benzyl alcohol is known to cause respiratory failure, vasodilation, hypotension, and convulsions in adults53 and gasping syndrome in neonates,11,54 and methyl-cyclopentenolone was deemed toxic by the European Union.55 Chemical diversity in e-liquid formulation is a key component of e-liquid safety.
We recognize that our study presents a limitation as the e-liquids were tested neat and not vaped. Several researchers have now found evidence of chemical transformation (ie, pyrolysis) after vaping.56,57 In agreement with our previous studies,7,58 we did not find that vaping e-liquids changed their relative toxicity. Indeed, we have found that vaping is correlated with direct e-liquid addition in several pulmonary cell types,7 suggesting that this phenomenon may be flavor dependent. In our experience, vaping is more variable and less amenable to HTS. We have also reported that e-liquids have a LC50s of ~6% or less; this would suggest that e-liquids may reach biologically relevant levels in the lung. Indeed, it has recently been reported that vaping significantly alters the secreted human airway proteome, suggesting that this may be the case.59 In addition, we recognize that our approach uses HEK293T cells and not air–liquid interface cultures of pulmonary epithelial primary cells or cell lines. With over 8000 different flavors on the market, we believe that it is not be possible to study all e-liquids under vaped/“physiological conditions.” We have previously demonstrated that HEK293T cells are suitable to compare the cytotoxic effects of many e-liquids in a quick and reliable way that is representative of the toxicity induced in pulmonary cell types, including air–liquid interface epithelia.7 Other groups have reported cytotoxicity of e-liquids, some using air–liquid interface cultures, but their screening capacity was 40 e-liquids or less.8,39,60 Our study provides a first step to identify toxic effects of e-liquids that would require further investigation.
A founding principle of toxicology is “the dose makes the poison.” This study was FDA funded, and knowing the upper limit of toxicity will be useful to the FDA even if it is not achieved during normal vaping. Moreover, although every e-liquid is toxic at high doses, individual e-liquids are differentiated based on the LC50 and whether or not the curves are left-shifted relative to PG/VG. Indeed, as per Figure 1, “Devils’ Cut” has an LC50 of ~0.1%, whereas PG/VG has an LC50 of ~10%.
Diversity in flavor composition is one of the main marketing strategies practiced by e-liquid vendors. The appeal of new flavors creates collateral consequences, including nicotine addiction.61 The US FDA has been taking steps to regulate e-cigarette sales: first by deeming electronic nicotine delivery systems to be tobacco products and thus including them in 2009 Family Smoking Prevention and Tobacco Control Act, and subsequently by issuing an “Advanced Notice of Proposed Rulemaking” that gives importance to the regulation of flavors in tobacco products.62 Our study provides new cytotoxic information of a varied and representative sample of flavored e-liquids, and importantly, we have incorporated this information into our publicly available database (https://eliquidinfo.org/). This website allows searching the LC50 value, chemical composition, PG/VG ratio, and vendor information for 320+ e-liquids, and thus, it can inform users, academic researchers, and government researchers. Remarkably, we have revealed that the presence of specific flavors is associated with higher levels of toxicity regarding oxidative stress, ΔΨ m, and cell death. Thus, given their heterogeneous nature, the overall goal of this project was to screen a greater number of neat e-liquids to identify flavors and/or chemical constituents that are more toxic and prompt new research on the potential effects of flavor chemical components on human health in order to limit their use on e-liquid formulation.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
This work was funded by NIH/FDA Grant P50 HL120100 and by NIH/NHLBI HL135642. Research reported in this publication was supported by NIH and the Family Smoking Prevention and Tobacco Control Act. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Declaration of Interests
None declared.
References
- 1. Bals R, Boyd J, Esposito S, et al. . Electronic cigarettes: a task force report from the European Respiratory Society. Eur Respir J. 2019;53(2):1801151. [DOI] [PubMed] [Google Scholar]
- 2. Hahn J, Monakhova YB, Hengen J, et al. . Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Services USDoHaH. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. In: Services USDoHaH, ed. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016. [Google Scholar]
- 4. FDA. Enforcement Priorities for Electronic Nicotine Delivery System (ENDS) and Other Deemed Products on the Market Without Premarket Authorization. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-priorities-electronic-nicotine-delivery-system-ends-and-other-deemed-products-market.
- 5. Marklew AJ, Patel W, Moore PJ, et al. . Cigarette smoke exposure induces retrograde trafficking of CFTR to the endoplasmic reticulum. Sci Rep. 2019;9(1):13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghosh A, Coakley RC, Mascenik T, et al. . Chronic E-cigarette exposure alters the human bronchial epithelial proteome. Am J Respir Crit Care Med. 2018;198(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sassano MF, Davis ES, Keating JE, et al. . Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018;16(3):e2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iskandar AR, Gonzalez-Suarez I, Majeed S, et al. . A framework for in vitro systems toxicology assessment of e-liquids. Toxicol Mech Methods. 2016;26(6):389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FEMA. Recent Progress in the Consideration of Flavoring Ingredients Under the Food Additives. Amendment. 2020. https://www.femaflavor.org/flavor-library/benzaldehyde. [Google Scholar]
- 10. Kosmider L, Sobczak A, Prokopowicz A, et al. . Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71(4):376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baum CR. Examples of mass exposures involving the pediatric population. In: Paloucek JBLFP, ed. Poisoning and Toxicology Handbook. 4th ed. Cambridge, MA: Informa; 2008:726. [Google Scholar]
- 12. Allen JG, Flanigan SS, LeBlanc M, et al. . Flavoring chemicals in E-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect. 2016;124(6):733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Center OSLT. Acetoin and/or Diacetyl. OSHA; 2008. https://www.osha.gov/dts/sltc/methods/validated/1013/1013.html. [Google Scholar]
- 14. Vas CA, Porter A, McAdam K. Acetoin is a precursor to diacetyl in e-cigarette liquids. Food Chem Toxicol. 2019;133:110727. [DOI] [PubMed] [Google Scholar]
- 15. Khlystov A, Samburova V. Flavoring compounds dominate toxic aldehyde production during E-cigarette vaping. Environ Sci Technol. 2016;50(23):13080–13085. [DOI] [PubMed] [Google Scholar]
- 16. Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4(2):67–73. [DOI] [PubMed] [Google Scholar]
- 17. Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR. Relation between mitochondrial membrane potential and ROS formation. Methods Mol Biol. 2012;810:183–205. [DOI] [PubMed] [Google Scholar]
- 18. Sharaf MS, Stevens D, Kamunde C. Mitochondrial transition ROS spike (mTRS) results from coordinated activities of complex I and nicotinamide nucleotide transhydrogenase. Biochim Biophys Acta Bioenerg. 2017;1858(12):955–965. [DOI] [PubMed] [Google Scholar]
- 19. Smith GS, Voyer-Grant JA, Harauz G. Monitoring cleaved caspase-3 activity and apoptosis of immortalized oligodendroglial cells using live-cell imaging and cleaveable fluorogenic-dye substrates following potassium-induced membrane depolarization. J Vis Exp. 2012;( 59). doi: 10.3791/3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Protter MMCB. College Calculus With Analytic Geometry. 2nd ed. Boston, MA: Addison-Wesley; 1970. [Google Scholar]
- 21. Bozier J, Chivers EK, Chapman DG, et al. . The evolving landscape of E-cigarettes: a systematic review of recent evidence. Chest. 2020;157(5):1362–1390. [DOI] [PubMed] [Google Scholar]
- 22. Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L193–L206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson C, Majeste A, Hanus J, Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol Sci. 2016;154(2):332–340. [DOI] [PubMed] [Google Scholar]
- 24. Scott A, Lugg ST, Aldridge K, et al. . Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018;73(12):1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rowell TR, Reeber SL, Lee SL, et al. . Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2017;313(1):L52–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aug A, Altraja S, Kilk K, Porosk R, Soomets U, Altraja A. E-cigarette affects the metabolome of primary normal human bronchial epithelial cells. PLoS One. 2015;10(11):e0142053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moses E, Wang T, Corbett S, et al. . Molecular impact of electronic cigarette aerosol exposure in human bronchial Epithelium. Toxicol Sci. 2017;155(1):248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scheffler S, Dieken H, Krischenowski O, Aufderheide M. Cytotoxic evaluation of e-liquid aerosol using different lung-derived cell models. Int J Environ Res Public Health. 2015;12(10):12466–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lei W, Lerner C, Sundar IK, Rahman I. Myofibroblast differentiation and its functional properties are inhibited by nicotine and e-cigarette via mitochondrial OXPHOS complex III. Sci Rep. 2017;7:43213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lerner CA, Rutagarama P, Ahmad T, Sundar IK, Elder A, Rahman I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem Biophys Res Commun. 2016;477(4):620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaisar MA, Sivandzade F, Bhalerao A, Cucullo L. Conventional and electronic cigarettes dysregulate the expression of iron transporters and detoxifying enzymes at the brain vascular endothelium: In vivo evidence of a gender-specific cellular response to chronic cigarette smoke exposure. Neurosci Lett. 2018;682:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Toorn M, Rezayat D, Kauffman HF, et al. . Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297(1):L109–L114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lerner CA, Sundar IK, Yao H, et al. . Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ganapathy V, Manyanga J, Brame L, et al. . Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One. 2017;12(5):e0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gonzalez-Gonzalez FJ, Chandel NS, Jain M, Budinger GRS. Reactive oxygen species as signaling molecules in the development of lung fibrosis. Transl Res. 2017;190:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boukhenouna S, Wilson MA, Bahmed K, Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid Med Cell Longev. 2018;2018:5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang L, Xu J, Liu H, Li J, Hao H. PM2.5 inhibits SOD1 expression by up-regulating microRNA-206 and promotes ROS accumulation and disease progression in asthmatic mice. Int Immunopharmacol. 2019;76:105871. [DOI] [PubMed] [Google Scholar]
- 38. Armstrong CM, Hollingworth S. A perspective on Na and K channel inactivation. J Gen Physiol. 2018;150(1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res. 2016;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oz M, El Nebrisi EG, Yang KS, Howarth FC, Al Kury LT. Cellular and molecular targets of menthol actions. Front Pharmacol. 2017;8:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kichko TI, Neuhuber W, Kobal G, Reeh PW. The roles of TRPV1, TRPA1 and TRPM8 channels in chemical and thermal sensitivity of the mouse oral mucosa. Eur J Neurosci. 2018;47(3):201–210. [DOI] [PubMed] [Google Scholar]
- 42. Reyes-García J, Flores-Soto E, Carbajal-García A, Sommer B, Montaño LM. Maintenance of intracellular Ca2+ basal concentration in airway smooth muscle (Review). Int J Mol Med. 2018;42(6):2998–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiong J, Zhu MX. Regulation of lysosomal ion homeostasis by channels and transporters. Sci China Life Sci. 2016;59(8):777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee WH, Ong SG, Zhou Y, et al. . Modeling cardiovascular risks of E-cigarettes with human-induced pluripotent stem cell-derived endothelial cells. J Am Coll Cardiol. 2019;73(21):2722–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25(e1):e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meents JE, Fischer MJ, McNaughton PA. Agonist-induced sensitisation of the irritant receptor ion channel TRPA1. J Physiol. 2016;594(22):6643–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fetterman JL, Weisbrod RM, Feng B, et al. . Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol. 2018;38(7):1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol. 2017;8:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Health NIfOSa. Comments of the National Institute for Occupational Safety and Health to the Food and Drug Administration (FDA) in response to Establishment of a Public Docket; Electronic Cigarettes and the Public Health Workshop. NIOSH Comments to FDA. Docket No. FDA-2014-N-1936. Cincinnati, OH: Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health Cincinnati; 2015. [Google Scholar]
- 50. FEMA. Flavor and Extract Manufacturers Association of the United States Letter. 2008. https://www.femaflavor.org/flavor-library/acetoin. Accessed July 15, 2020.
- 51. Du K, McGill MR, Xie Y, Jaeschke H. Benzyl alcohol protects against acetaminophen hepatotoxicity by inhibiting cytochrome P450 enzymes but causes mitochondrial dysfunction and cell death at higher doses. Food Chem Toxicol. 2015;86:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, et al. . Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J Anal Toxicol. 2015;39(4):262–269. [DOI] [PubMed] [Google Scholar]
- 53. Nair B. Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol. 2001;20(Suppl. 3):23–50. [DOI] [PubMed] [Google Scholar]
- 54. Gershanik J, Boecler B, Ensley H, McCloskey S, George W. The gasping syndrome and benzyl alcohol poisoning. N Engl J Med. 1982;307(22):1384–1388. [DOI] [PubMed] [Google Scholar]
- 55. Farsalinos K, Lagoumintzis G. Toxicity classification of e-cigarette flavouring compounds based on European Union regulation: Analysis of findings from a recent study. Harm Reduct J. 2019;16(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health. 2014;11(11):11192–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ogunwale MA, Li M, Ramakrishnam Raju MV, et al. . Aldehyde detection in electronic cigarette aerosols. ACS Omega. 2017;2(3):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rowell TR, Reeber SL, Lee SL, et al. . Flavored E-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2017;313(1):L52–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reidel B, Radicioni G, Clapp PW, et al. . E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am J Respir Crit Care Med. 2018;197(4):492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Behar RZ, Luo W, Lin SC, et al. . Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control. 2016;25(Suppl. 2):ii94–ii102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Garrison KA, O’Malley SS, Gueorguieva R, Krishnan-Sarin S. A fMRI study on the impact of advertising for flavored e-cigarettes on susceptible young adults. Drug Alcohol Depend. 2018;186:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. FDA. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. In: SERVICES DOHAH, ed. 21 CFR Parts 1100, 1140, and 1143 Vol RIN 0910–AG38 2016 and 2018. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.