Abstract
The data on visceral mycoses that had been reported in the Annual of the Pathological Autopsy Cases in Japan from 1969 to 1994 by the Japanese Society of Pathology were analyzed epidemiologically. The frequency of visceral mycoses among the annual total number of pathological autopsy cases increased noticeably from 1.60% in 1969 to a peak of 4.66% in 1990. Among them, the incidences of candidiasis and aspergillosis increased the most. After 1990, however, the frequency of visceral mycoses decreased gradually. Until 1989, the predominant causative agent was Candida, followed in order by Aspergillus and Cryptococcus. Although the rate of candidiasis decreased by degrees from 1990, the rate of aspergillosis increased up to and then surpassed that of candidiasis in 1991. Leukemia was the major disease underlying the visceral mycoses, followed by solid cancers and other blood and hematopoietic system diseases. Severe mycotic infection has increased over the reported 25-year period, from 6.6% of the total visceral mycosis cases in 1969 to 71% in 1994. The reasons for this decrease of candidiasis combined with an increase of aspergillosis or of severe mycotic infection might be that (i) nonsevere (not disseminated) infections were excluded from the case totals, since they have become controllable by antifungal drugs such as fluconazole, but (ii) the available antifungal drugs were not efficacious against severe infections such as pulmonary aspergillosis, and (iii) the number of patients living longer in an immunocompromised state had increased because of developments in chemotherapy and progress in medical care.
Recently many reports have described an increase of systemic fungal infections, which included aspergillosis (7, 14), zygomycosis (10), fusariosis (48), and candidiasis due to non-Candida albicans Candida spp. (9). Cutaneous or superficial candidiasis has seemed to be controllable by using effective azoles; however, azole-resistant C. albicans strains and pathogenic non-C. albicans Candida species have been emerging (5, 9, 49). Furthermore, severe systemic aspergillosis has been increasing in bone marrow-transplanted patients (4) and in those with other immunocompromised conditions (3).
Over the past 30 years, medical mycologists and pathologists have published several papers on the trends of mycoses (15, 16, 38, 45–47). Groll et al. (14, 15) reported the trends of invasive fungal infections from autopsy findings at the university hospital of Frankfurt, Germany, and Kappe et al. (39) presented analyzed data for invasive aspergillosis cases culled from autopsy records in Heidelberg, Germany. In Japan, several studies analyzing data on mycoses from autopsies had been reported previously; however, few reports on recent trends existed. A study by Miyake and Okudaira covered the 13 years between 1948 and 1961 (45), and a study by Hotchi et al. covered the 10 years between 1966 and 1975 (16). Okudaira et al. then reported the analyzed data from 1972 to 1981 (47), and now this report covers the period after that. As we also wanted to know the impact of new antifungal drugs such as fluconazole or itraconazole, we concentrated our study on the period after 1989. To discern trends in visceral mycoses, we have epidemiologically analyzed the data on visceral mycoses that had been reported in the Annual of the Pathological Autopsy Cases in Japan (18–37). As we reported previously, the total numbers of mycosis cases had been increasing until 1990, whereas the number of candidiasis cases had stopped increasing and had begun to decrease after 1989 (42, 43). Aspergillosis cases were not decreasing but maintained the highest rate of mycosis among the total autopsies. In this report, we review the recent trends and also analyze the effect of antifungal agents introduced in the clinical setting.
(This study has been reported in part at the 13th Congress of the International Society for Human and Animal Mycology, Parma, Italy, 1997.)
MATERIALS AND METHODS
Diagnostic criteria.
The criteria for the pathological diagnosis of each class of mycosis to be described in the Annual of the Pathological Autopsy Cases in Japan are not defined definitively by the Japanese Society of Pathology. Basically, the description of each case is the responsibility of the reporting pathologist and depends on his or her ability to make a diagnostic determination. Most of the autopsies included both gross and histopathological examinations; however, all of the pathologists could not be expected to be equally rigorous in their examination. There might be some differences among the pathologists deriving from their individual experiences with mycoses. Concerning fungemia or candidemia, reporting pathologists might have been given some clinical information on fungemia from the patient’s medical records.
Definitions.
Mycoses were defined as infections caused by eumycotic organisms such as Candida, Aspergillus, Cryptococcus, Zygomycetes, and other fungal species. Infections caused by filamentous bacteria such as Actinomycetes (Actinomadura, Nocardia, and Streptomyces spp.) and pneumonia caused by Pneumocystis carinii were excluded from the criteria for mycoses. Superficial infections such as dermatophytoses were excluded from the category of visceral mycoses. The term complicated infection means a mixed infection with more than two species of fungi. Cultures from specimens might be identified as containing more than two kinds of fungi.
The severe mycotic infections from autopsy records could be defined as (i) the direct cause of death; (ii) severe pulmonary infection involving both lobes of the lung; (iii) severe visceral infections of two or more organ systems, including those involving the central nervous system; (iv) multiorgan systemic infection of three or more organ systems; or (v) fungemia.
Data collection.
Data on visceral mycoses occurring in Japan from 1969 to 1994 were collected in the Annual of the Pathological Autopsy Cases in Japan, which was published from 1970 to 1995 by the Japanese Society of Pathology (18–37). Those data were extracted and compiled to make a database for analysis; however, data from the years 1982 to 1988, except 1985, were not used in this study.
Cases of stillborn babies were excluded from each annual total number of autopsy cases. The data for annual total deaths and the number of certified deaths from mycoses were from Vital Statistics of Japan, edited from 1969 to 1996 by the Minister’s Secretariat, Ministry of Health and Welfare (50). The data were compiled into a database by using Filemaker Pro version 3.0, supplied by Claris Co., with a Power Macintosh 7100 (Apple Computer Co.).
RESULTS
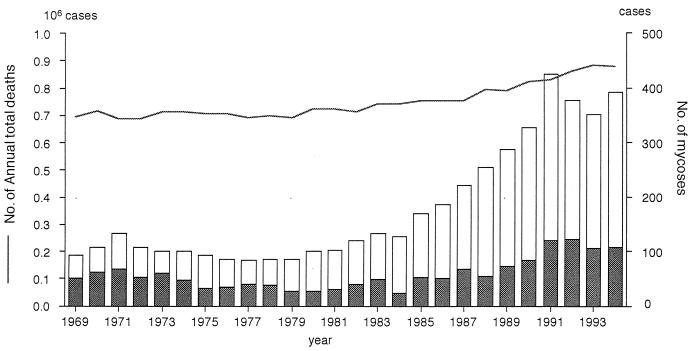
In recent years, the annual total number of deaths in Japan has been about 900,000. Among these, about 3% of bodies are examined by pathological autopsy. Figure 1 shows that among the total deaths, the frequency of mycotic infection or candidiasis as the certified direct cause of death had increased noticeably from 1979 until 1991 (50).
FIG. 1.
Annual trends of total deaths and of deaths certified as resulting from mycoses in Japan. Data were extracted from Vital Statistics of Japan, edited by the Minister’s Secretariat, Ministry of Health and Welfare (50) for the years 1969 through 1994. ——, number of annual total deaths; ▩, candidiasis cases; □, other mycosis cases.
The occurrence of mycoses among total autopsy cases from 1969 to 1994 in Japan is shown in Table 1. The frequency of visceral mycoses among the annual total number of autopsy cases increased significantly, from 1.60% in 1969 to a peak of 4.66% in 1990. After 1990, however, this frequency decreased gradually, from 3.79% in 1991 to 3.17% in 1994. Candidiasis also increased, from 0.41% in 1969 to a peak of 1.89% in 1989, and then decreased to 1.12% by degrees after 1991. In contrast, the aspergillosis rate rose from 0.39% in 1969 to a peak of 1.55% in 1990 and maintained a constant level of about 1.3% after 1991. The rate of zygomycosis increased slowly (from 0.01 to 0.16%), whereas that of cryptococcosis was not remarkably changed (from 0.14 to 0.26%) during that time. Until 1989, the predominant causative agent was Candida, followed by Aspergillus and Cryptococcus in that order. Although the rate of candidiasis decreased by degrees from 1990, the rate of aspergillosis increased up to and then surpassed that of candidiasis in 1991.
TABLE 1.
Changes in rates of mycoses among total autopsy cases and of causative agents of mycoses from 1969 to 1994 in Japan
| Yr | Total no. of autopsies | Total no. of mycoses | % of mycoses among total autopsies | % of cases among total
mycoses (% of cases among total autopsies)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Candida | Aspergillus | Cryptococcus | Zygomycetes | Other | Unknowna | Complicatedb | ||||
| 1969 | 24,715 | 396 | 1.60 | 25.8 (0.41) | 24.5 (0.39) | 8.8 (0.14) | 0.8 (0.01) | 0.3 (0.00) | 37.6 (0.60) | 2.3 (0.04) |
| 1970 | 23,599 | 407 | 1.72 | 27.0 (0.47) | 21.1 (0.36) | 9.6 (0.17) | 4.7 (0.08) | 0.5 (0.01) | 34.6 (0.60) | 2.5 (0.04) |
| 1971 | 23,245 | 433 | 1.86 | 28.9 (0.54) | 24.7 (0.46) | 9.9 (0.18) | 3.7 (0.07) | 0.2 (0.00) | 28.9 (0.54) | 3.7 (0.07) |
| 1972 | 22,769 | 379 | 1.66 | 38.8 (0.65) | 31.7 (0.53) | 14.0 (0.23) | 2.9 (0.05) | 0.0 (0.00) | 7.7 (0.13) | 5.0 (0.08) |
| 1973 | 23,274 | 466 | 2.00 | 37.8 (0.76) | 23.4 (0.47) | 12.7 (0.25) | 1.3 (0.03) | 0.0 (0.00) | 21.7 (0.43) | 3.2 (0.06) |
| 1974 | 23,111 | 531 | 2.30 | 35.4 (0.81) | 22.6 (0.52) | 8.5 (0.19) | 1.9 (0.04) | 0.2 (0.00) | 27.9 (0.64) | 3.6 (0.08) |
| 1975 | 23,048 | 620 | 2.69 | 34.5 (0.93) | 17.3 (0.46) | 8.1 (0.22) | 2.9 (0.08) | 0.0 (0.00) | 34.5 (0.93) | 2.7 (0.07) |
| 1976 | 24,093 | 621 | 2.58 | 43.0 (1.11) | 21.4 (0.55) | 9.5 (0.24) | 2.3 (0.06) | 1.1 (0.03) | 18.4 (0.47) | 4.3 (0.11) |
| 1977 | 25,897 | 664 | 2.56 | 37.5 (0.96) | 22.6 (0.58) | 6.9 (0.18) | 3.5 (0.09) | 0.2 (0.00) | 25.3 (0.65) | 4.1 (0.10) |
| 1978 | 30,742 | 813 | 2.64 | 43.1 (1.14) | 23.2 (0.61) | 8.2 (0.22) | 3.3 (0.09) | 1.8 (0.05) | 16.9 (0.45) | 3.4 (0.09) |
| 1979 | 32,844 | 861 | 2.62 | 35.4 (0.93) | 21.7 (0.57) | 9.1 (0.24) | 3.0 (0.08) | 0.0 (0.00) | 25.9 (0.68) | 4.9 (0.13) |
| 1980 | 35,943 | 970 | 2.70 | 49.2 (1.33) | 20.7 (0.56) | 7.3 (0.20) | 3.2 (0.09) | 0.0 (0.00) | 15.1 (0.41) | 4.5 (0.12) |
| 1981 | 38,841 | 1,096 | 2.82 | 42.0 (1.18) | 26.0 (0.73) | 7.4 (0.21) | 3.8 (0.11) | 0.1 (0.00) | 15.0 (0.42) | 5.7 (0.16) |
| 1985 | 39,333 | 1,558 | 3.96 | 41.6 (1.65) | 30.7 (1.22) | 6.5 (0.26) | 3.6 (0.14) | 0.8 (0.03) | 11.0 (0.44) | 5.7 (0.23) |
| 1989 | 37,557 | 1,672 | 4.45 | 42.3 (1.89) | 30.1 (1.34) | 5.4 (0.24) | 3.6 (0.16) | 0.2 (0.01) | 13.0 (0.58) | 5.1 (0.23) |
| 1990 | 37,399 | 1,743 | 4.66 | 33.6 (1.57) | 33.2 (1.55) | 4.8 (0.22) | 3.5 (0.16) | 0.1 (0.00) | 20.7 (0.96) | 4.1 (0.19) |
| 1991 | 35,618 | 1,350 | 3.79 | 33.7 (1.28) | 34.7 (1.32) | 4.7 (0.18) | 3.6 (0.13) | 0.0 (0.00) | 19.2 (0.73) | 4.1 (0.16) |
| 1992 | 33,201 | 1,177 | 3.55 | 36.3 (1.29) | 37.3 (1.32) | 5.4 (0.19) | 4.2 (0.15) | 0.0 (0.00) | 12.3 (0.44) | 4.4 (0.16) |
| 1993 | 31,207 | 1,136 | 3.66 | 37.2 (1.36) | 36.5 (1.34) | 4.5 (0.16) | 3.4 (0.12) | 0.3 (0.01) | 14.5 (0.53) | 3.5 (0.13) |
| 1994 | 27,827 | 882 | 3.17 | 35.5 (1.12) | 40.7 (1.29) | 6.1 (0.19) | 3.2 (0.10) | 0.1 (0.00) | 10.4 (0.33) | 4.0 (0.13) |
An unidentified fungus was observed in the infected organ.
Mixed infection with more than two kinds of fungi in the infected organ.
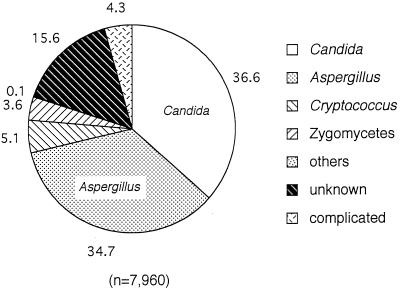
A comparison of the causative agents of mycoses over the 6 years preceding 1994 showed that Candida was the most common causative agent, with 36.6% of the total of 7,960 cases, followed in order by Aspergillus (34.7%), Cryptococcus (5.1%), and Zygomycetes (3.6%) (Fig. 2). Table 2 lists the organ distribution of these causative agents. In candidiasis, the lung and bronchial system constituted the most frequently involved site, with 34.7% of the total candidiasis cases, followed by the kidney (23.3%). Esophagus, heart, and stomach infections also occurred at high frequencies (15.9, 13.4, and 11.1%, respectively). High rates of systemic candidiasis (16.7%) and candidemia (13.7%) were also observed. For aspergillosis, the lung and bronchia comprised the most commonly infected organ system (83.9%); other organs were not involved at a high rate. Cryptococcus also infected the lungs and bronchia most frequently (64%), followed by the brain and meninx (21.5%). Zygomycosis was also observed most commonly in the lung and bronchia (69.4%), followed by the liver (9.6%) and kidney (9.1%).
FIG. 2.
Causative agents for visceral mycoses in autopsy cases. Data were from references 32 to 37. Each agent is reported as a percentage of the total mycoses (7,960 cases).
TABLE 2.
Distribution of causative agents of mycoses by organa
| Infection type or organ | % of infections
caused by:
|
|||
|---|---|---|---|---|
| Candida(n = 2,172) | Aspergillus(n = 1,967) | Cryptococcus(n = 289) | Zygomycetes (n = 209) | |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| Systemic | 16.7 | 8.6 | 13.5 | 9.6 |
| Fungemia | 13.7 | 4.3 | 4.8 | 5.3 |
| Brain + meninx | 4.1 | 3.3 | 21.5 | 5.3 |
| Mouth + tongue | 2.2 | 0.3 | 0.0 | 0.0 |
| Esophagus | 15.9 | 1.3 | 0.7 | 0.5 |
| Stomach | 11.1 | 2.9 | 0.3 | 7.2 |
| Intestine | 8.0 | 2.6 | 0.0 | 4.8 |
| Liver | 8.1 | 3.6 | 3.5 | 9.6 |
| Larynx + pharynx | 1.2 | 0.3 | 0.0 | 0.0 |
| Lung + bronchia | 34.7 | 83.9 | 64.0 | 69.4 |
| Heart | 13.4 | 7.4 | 3.8 | 7.7 |
| Kidney | 23.3 | 7.3 | 9.7 | 9.1 |
| Bladder | 3.5 | 0.5 | 0.0 | 0.0 |
| Thyroid | 3.5 | 3.5 | 2.4 | 3.3 |
| Spleen | 3.7 | 2.2 | 6.2 | 6.2 |
| Other | 11.9 | 5.8 | 10.0 | 14.4 |
When the data on diseases underlying the visceral mycosis cases for the years from 1989 to 1991 and 1993 were compiled, among a total of 5,901 cases, leukemia and myelodysplastic syndrome (MDS) were the major diseases (25.5%), followed by solid cancers (25.1%) and other blood and hematopoietic system diseases not including leukemia (15.5%). When the total number of cases of blood and hematopoietic system diseases, including leukemia, malignant lymphoma, aplastic anemia, and multiple myeloma, etc., was compared with that of solid cancers, they were found to comprise over 40% of all underlying diseases and to be 1.6 times more frequent than solid cancers.
Furthermore, when the frequencies of mycoses in each of these major underlying diseases were compared in more detail over the same periods, higher frequencies were observed in pharyngeal (2.7%), ovarian (2.6%), bladder (2.6%), and lung (2.4%) cancers among the patients with solid cancers (Table 3). Among the patients with blood and hematopoietic system diseases, the highest frequency of mycosis was observed for chronic myeloid leukemia (43.6%), followed by acute lymphatic leukemia (42.8%) and acute myeloid leukemia (35.4%) (Table 3). Mycotic infections occurred at a more-than-10-times-higher frequency in leukemia patients than in solid-cancer patients (24.8 and 1.7%, respectively). Among AIDS patients, about 23% had suffered from mycoses (Table 3). In the comparison of causative mycotic agents for the four major underlying diseases and solid-organ transplantation cases (Table 4), aspergillosis was the most predominant, followed in order by candidiasis, zygomycosis, and cryptococcosis, for leukemia; however, for the others, candidiasis was the most frequent disease, followed by aspergillosis and cryptococcosis. In contrast, mycoses among organ transplant patients were reported in only 10 cases during this period.
TABLE 3.
Details for each category of underlying diseasea
| Disease type | Site or diagnosisb | Total no. of patients | No. (%) with mycosis |
|---|---|---|---|
| Solid cancers | Lung | 15,340 | 369 (2.4) |
| Stomach | 12,719 | 227 (1.8) | |
| Liver | 13,985 | 167 (1.2) | |
| Pancreas | 5,046 | 100 (2.0) | |
| Colon | 7,140 | 82 (1.1) | |
| Esophagus | 3,124 | 58 (1.9) | |
| Gall bladder | 3,478 | 67 (1.9) | |
| Breast | 2,274 | 37 (1.6) | |
| Uterus | 2,068 | 40 (1.9) | |
| Prostate | 3,278 | 29 (0.9) | |
| Ovary | 1,248 | 32 (2.6) | |
| Kidney | 2,110 | 38 (1.8) | |
| Bladder | 1,590 | 41 (2.6) | |
| Brain | 1,824 | 37 (2.0) | |
| Thyroid | 3,005 | 15 (0.5) | |
| Pharynx | 752 | 20 (2.7) | |
| Total | 78,981 | 1,359 (1.7) | |
| Blood and hematopoietic system diseases | AML | 1,753 | 620 (35.4) |
| CML | 424 | 185 (43.6) | |
| ALL | 745 | 319 (42.8) | |
| CLL | 119 | 17 (14.3) | |
| MoL | 278 | 49 (17.6) | |
| MDS | 473 | 105 (22.2) | |
| ATL | 535 | 116 (21.7) | |
| Other leukemia | 1,740 | 95 (5.5) | |
| Total for leukemia and MDS | 6,067 | 1,506 (24.8) | |
| Malignant lymphoma | 4,649 | 487 (10.5) | |
| Multiple myeloma | 1,834 | 170 (9.3) | |
| Aplastic anemia | 388 | 103 (26.5) | |
| DIC | 1,761 | 18 (1.0) | |
| Purpura | 130 | 12 (9.2) | |
| Immunological disorders | 52 | 10 (19.2) | |
| AIDS | 109 | 25 (22.9) | |
| Total for other blood and hematopoietic system diseases | 8,923 | 825 (9.2) | |
| Total | 14,990 | 2,331 (15.6) |
These data were taken from references 32, 33, 34, and 36. Among 78,981 patients with solid cancers, 1,359 patients suffered from mycoses. The mycosis rate for the solid-cancer patients was 1.7%. For blood and hematopoietic system diseases, 1,506 mycosis patients were among 6,067 leukemia and MDS patients (24.8%), and 825 patients, excluding leukemia and MDS patients, suffered from mycoses among 8,923 other patients with blood and hematopoietic system diseases (9.2%). In total, 15.6% of patients with blood and hematopoietic diseases suffered from mycoses.
AML, acute myeloid leukemia; CML, chronic myeloid leukemia; ALL, acute lymphatic leukemia; CLL, chronic lymphatic leukemia; MoL, monocystic leukemia; ATL, adult T-cell leukemia; DIC, disseminated intravascular coagulation syndrome; Purpura, idiopathic thrombocytopenic purpura or thrombotic thrombocytopenic purpura.
TABLE 4.
Causative agents of mycoses with the major underlying diseasesa
| Agent | Leukemia and
MDS
|
Solid
cancersb
|
Lymphoma
|
Myeloma
|
Organ
transplantation
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Candida | 397 | 26.4 | 713 | 48.0 | 178 | 36.6 | 64 | 37.6 | 3 | 30.0 |
| Aspergillus | 564 | 37.5 | 377 | 25.4 | 151 | 31.0 | 50 | 29.4 | 2 | 20.0 |
| Cryptococcus | 25 | 1.7 | 90 | 6.1 | 19 | 3.9 | 13 | 7.6 | 2 | 20.0 |
| Zygomycetes | 101 | 6.7 | 23 | 1.5 | 17 | 3.5 | 12 | 7.1 | 0 | 0.0 |
| Other | 5 | 0.3 | 2 | 0.1 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 |
| Unknown | 296 | 19.7 | 246 | 16.6 | 102 | 20.9 | 23 | 13.5 | 2 | 20.0 |
| Complicated | 118 | 7.8 | 34 | 2.3 | 19 | 3.9 | 8 | 4.7 | 1 | 10.0 |
| Total | 1,506 | 100.0 | 1,485 | 100.0 | 487 | 100.0 | 170 | 100.0 | 10 | 100.0 |
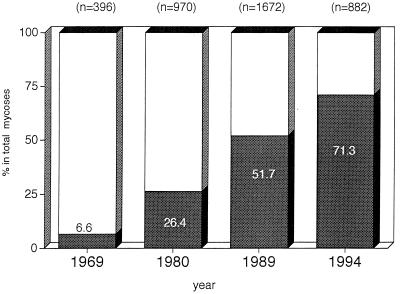
Most systemic or multiorgan mycotic infections caused patients to die in a short period, responded poorly to currently available antifungal agents, or required a long period of treatment. The frequency of such severe mycotic infections has increased dramatically over the 25-year study period, from 6.6% of the total visceral mycosis cases in 1969 to 71.3% in 1994 (Fig. 3).
FIG. 3.
Increase in the proportions of severe infections for all mycoses. (Data are from references 18, 29, 32, and 37.) Frequencies of severe mycoses for the 25-year period were compared. ▩ severe infection; □, nonsevere infection. Severe mycoses increased dramatically, from 6.6% in 1969 to 71.3% in 1994.
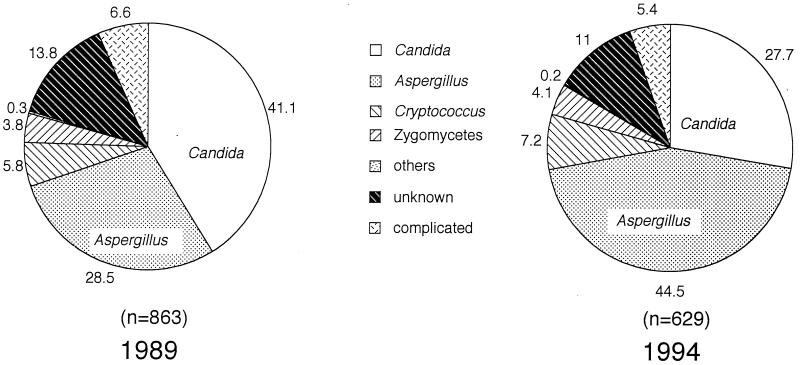
When the proportions of causative agents for the severe mycoses were compared for the years 1989 and 1994, candidiasis (41.1%) was found to be the most frequent mycosis, followed by aspergillosis (28.5%), in 1989, but aspergillosis (44.5%) surpassed candidiasis (27.7%) in 1994 (Fig. 4).
FIG. 4.
Comparison of the proportions of causative agents for severe mycoses in 1989 and in 1994. Each agent is reported as a percentage of total mycoses. Severe candidiasis cases decreased but aspergillosis cases increased in 1994.
Analysis of the frequency of severe infections for each causative agent showed that 93% of zygomycosis, 83% of cryptococcosis, 78% of aspergillosis, and 56% of candidiasis cases were found among the severe cases in 1994. That rate for each agent was almost exactly 50% in 1989.
By age, the highest frequency of mycoses was observed in patients in their 20s, whereas the highest incidence was observed in those in their 60s and 70s. In 1993 and 1994, neonatal babies showed a tendency to suffer from candidiasis, which might be caused by endogenous pathogens (Table 5).
TABLE 5.
Frequencies and incidences of the visceral mycoses by agea
| Age (yr) | Total no. of autopsy cases | Total
mycoses
|
Candida
|
Aspergillus
|
Cryptococcus
|
Zygomycetes
|
Other
|
Unknown
|
Complicated
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Neonatal babies | 1,491 | 23 | 1.54 | 18 | 1.21 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 5 | 0.34 | 0 | 0.00 |
| 0–9 | 1,324 | 40 | 3.02 | 17 | 1.28 | 12 | 0.91 | 0 | 0.00 | 2 | 0.15 | 0 | 0.00 | 7 | 0.53 | 2 | 0.15 |
| 10–19 | 532 | 52 | 9.77 | 14 | 2.63 | 25 | 4.70 | 0 | 0.00 | 4 | 0.75 | 0 | 0.00 | 6 | 1.13 | 3 | 0.56 |
| 20–29 | 836 | 84 | 10.05 | 36 | 4.31 | 34 | 4.07 | 2 | 0.24 | 5 | 0.60 | 0 | 0.00 | 6 | 0.72 | 1 | 0.12 |
| 30–39 | 1,203 | 84 | 6.98 | 29 | 2.41 | 31 | 2.58 | 5 | 0.42 | 5 | 0.42 | 0 | 0.00 | 11 | 0.91 | 3 | 0.25 |
| 40–49 | 3,942 | 156 | 3.96 | 39 | 0.99 | 75 | 1.90 | 10 | 0.25 | 5 | 0.13 | 0 | 0.00 | 20 | 0.51 | 7 | 0.18 |
| 50–59 | 8,169 | 312 | 3.82 | 105 | 1.29 | 120 | 1.47 | 14 | 0.17 | 10 | 0.12 | 2 | 0.02 | 47 | 0.58 | 14 | 0.17 |
| 60–69 | 16,385 | 544 | 3.32 | 192 | 1.17 | 209 | 1.28 | 27 | 0.16 | 18 | 0.11 | 1 | 0.01 | 75 | 0.46 | 22 | 0.13 |
| 70–79 | 15,561 | 537 | 3.45 | 194 | 1.25 | 220 | 1.41 | 30 | 0.19 | 11 | 0.07 | 1 | 0.01 | 62 | 0.40 | 19 | 0.12 |
| ≥80 | 9,567 | 186 | 1.94 | 92 | 0.96 | 48 | 0.50 | 17 | 0.18 | 7 | 0.07 | 0 | 0.00 | 18 | 0.19 | 4 | 0.04 |
| Total | 59,010 | 2,018 | 3.42 | 736 | 1.25 | 774 | 1.31 | 105 | 0.18 | 67 | 0.11 | 4 | 0.01 | 257 | 0.44 | 75 | 0.13 |
There was no remarkable difference between the sexes in the frequency of visceral mycoses in total autopsies as determined from the 1993 and 1994 compiled data (36, 37). There was little difference in candidiasis between men and women (1.21 and 1.31%, respectively); cryptococcosis was found at a higher frequency in women than in men (0.29 and 0.13%, respectively) in total autopsy cases, whereas aspergillosis was found slightly more frequently in men (1.39%) than in women (1.18%). The frequencies of mycoses in both sexes relative to total autopsies were almost the same (3.4%).
DISCUSSION
Candidiasis is the most common mycotic disease in Japan, requiring the treatment of 73,000 patients (ca. 6,000 male and ca. 68,000 female) who received medical treatment in hospitals or clinics in 1993 (51). The most frequently presented symptoms were of Candida vaginitis in women in their 20s and 30s; however, very few of the visceral-candidiasis patients succumbed to the disease. Figure 1 shows the frequency of candidiasis that had been certified as a direct cause of death. It appears that even if a patient suffers from a mycosis and dies from that mycosis, in the presence of an underlying disease, the direct cause of death might be certified as being the major cause because of difficulty in making the diagnosis.
In this epidemiological and etiological study, the retrospective autopsy data that we used were compiled by the Japanese Society of Pathology from data gathered from university hospitals, public hospitals, and large private hospitals all over Japan. Visceral mycoses continued to increase up to 1990; however, their frequency has been decreasing since 1991. Especially, candidiasis cases have tended to decrease after 1989. In contrast, aspergillosis cases had increased by 1990, and that frequency was constant at 1.3% of total autopsy cases from 1990 to 1994. Groll et al. had also reported almost the same trend for the increase of aspergillosis in their pathological autopsy data obtained in Frankfurt, Germany (14, 15). The major reason for this turnaround in our data is thought to be the introduction of fluconazole in Japan. Fluconazole treatment has likely decreased the cases of both severe and nonsevere Candida infections; however, its activity against aspergillosis should be limited (1, 6, 41). Kujath and Lerch reported their clinical experience in treating Aspergillus infections of multiple soft-tissue injuries, which did not improve under treatment with fluconazole (300 mg daily for 16 days) (41). Anaissie et al. also reported that one patient with pneumonia due to Aspergillus glaucus responded partially to fluconazole at 2,000 mg/day but that three patients did not respond to high-dose fluconazole treatment (more than 800 mg/day) (1). Thus, they concluded that the activity of fluconazole was limited in severe infection with Aspergillus species and other molds.
The introduction of fluconazole in the middle of 1989 was a kind of turning point against candidiasis (11, 17, 44) but not against other severe mycoses such as invasive aspergillosis. Itraconazole was introduced in 1993; however, the effect of itraconazole on clinical outcomes might not be evident within this surveyed period. When the causative agents for 1989 and 1994 were compared, we could see that the predominant causative agent for severe mycotic infection had shifted from Candida to Aspergillus (Fig. 4), and the proportion of severe infections among the total mycoses had increased from 6.6% in 1969 to 71.3% in 1994 (the most recent data) (Fig. 3).
We can guess that the reasons for this decrease of candidiasis combined with an increase of aspergillosis or of other severe mycotic infections might be that (i) antifungal-responsive (not disseminated) infections were excluded from the case totals, because they have become controllable by antifungal drugs such as fluconazole (launched in the middle of 1989 in Japan); (ii) an empirical therapy was commonly given to prevent immunocompromised patients from acquiring primary mycoses; (iii) available antifungal drugs were partially efficacious for severe infections; (iv) diagnostic techniques for both candidiasis and aspergillosis were inadequate and not yet fully developed (12, 40, 52, 53, 55); or (v) the number of patients living longer in an immunocompromised state increased as a result of developments in chemotherapy, organ transplantation, and bone marrow transplantation (BMT).
Until 1994, kidney transplantation was not uncommon, even in Japan, but liver transplantation was only starting to be done. BMT was very rare at that time. Therefore, we could not derive the number of mycoses after BMT from available reports of pathological autopsy cases up to 1994.
Even though the number of solid organ transplantation cases is increasing, BMT is still not a common procedure in Japan. Also, AIDS patients were rare in Japan more than 5 years ago. In contrast, many AIDS patients have been diagnosed with mycoses in the United States and European countries (9, 13, 15). In fact, 20% of patients suffering from AIDS have been reported to have mycotic infection as one complication (Table 3). Even now, cases of severe visceral mycoses are essentially uncontrollable, and their numbers are still increasing (4, 8, 15, 54). Therefore, there is an urgent, unmet medical need for drugs that are highly potent against visceral mycoses and have efficacy against aspergillosis, azole-resistant Candida stains, or non-C. albicans Candida species as well as other mycoses.
With respect to the affected-organ distribution data (Table 2), the rate of candidemia was higher than that of fungemia from Aspergillus, Cryptococcus, or Zygomycetes. The increasing use of indwelling intravenous catheters might be one major infection route for fungemia, and Candida as an endogenous pathogen is more easily infective than other pathogens. Unfortunately, we could not find any available data on the incidence of candidemia to be compared with data for autopsy trends.
We observed a difference in the organ distribution among the causative agents: the lung and bronchial system were most frequently involved regardless of the pathogen species. This suggests that the lung and bronchia are at the highest risk of being exposed to not only exogenous pathogens, such as Aspergillus, Cryptococcus, or Zygomycetes, but also Candida species. Although Candida species are normally found commensally in the digestive tract, it is possible for them to be a major causative agent of systemic infection in immunocompromised patients and of topical infections in healthy individuals. Further, such Candida is known to be involved in nosocomial transmission (2). That might explain why the lung and bronchia are found to be the organs predominantly infected by Candida. Reasonably, Candida infections were also observed more commonly than those of the other three major species in the esophagus and stomach.
The data from autopsy surveys include both patients of antemortem-diagnosed cases and those recognized by postmortem necropsy. The former cases include those not completely cured at the time that death occurred from the underlying disease or the mycosis. In Japan, only about 3% of bodies are subjected to pathological autopsy. We assume that even if we were able to analyze all patients who died from disease, the frequency of mycotic infection would probably not change much statistically. If we could count all mycosis patients regardless of their good or bad convalescence, however, the rate of mycoses would probably be greatly different from the result that we arrived at in this study. This is because most of the data on mycoses obtained at autopsy must be biased toward those patients who had died from malignant diseases.
Almost all systemic fungal infections, especially those caused by Aspergillus or Zygomycetes, in immunocompromised patients are refractory to all antifungal agents currently available, even though effective drugs do exist for less severe or locally limited infections. We could expect, however, that many such patients could be cured of or prevented from contracting systemic mycoses with aggressive effective treatments.
Despite the limitations inherent in individual autopsy data in Japan, they are still worthy of use to gain much epidemiological and etiological information. Nevertheless, further information could be derived by using newer data, as they become available, and other means of analysis.
ACKNOWLEDGMENT
We are grateful to S. B. Miwa, Nippon Roche Research Center, for critical reading of the manuscript.
REFERENCES
- 1.Anaissie E J, Kontoyiannis D P, Huls C, Vartivarian S E, Karl C, Prince R A, Bosso J, Bodey G P. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis. 1995;172:599–602. doi: 10.1093/infdis/172.2.599. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee S N, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards T R, Tolson J, Henderson T, Martone W J The National Nosocomial Infections Surveillance System. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am J Med. 1991;91(Suppl. 3B):86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 3.Boydey G, Bueltmann B, Duguid W, Gibbs D, Hanak H, Hotchi M, Mall G, Martino P, Meunier F, Milliken S, Naoe S, Okudaira M, Scevola D, van’t Wout J. Fungal infections in cancer patients. Eur J Clin Microbiol Infect Dis. 1992;11:99–109. doi: 10.1007/BF01967060. [DOI] [PubMed] [Google Scholar]
- 4.Castagnola E, Bucci B, Montinaro E, Viscoli C. Fungal infections in patients undergoing bone marrow transplantation: an approach to a rational management protocol. Bone Marrow Transplant. 1996;18(Suppl. 2):97–106. [PubMed] [Google Scholar]
- 5.Chakrabarti A, Ghosh A, Batra R, Kaushal A, Singh H. Antifungal susceptibility pattern of non-albicans Candidaspecies & distribution of species isolated from candidemia cases over 5 year period. Indian J Med Res. 1996;104:171–176. [PubMed] [Google Scholar]
- 6.Como J A, Dismukes W E. Oral azole drug as systemic antifungal therapy. N Engl J Med. 1994;330:263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 7.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 8.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dronda F, Alonso-Sanz M, Lauguna F, Chaves F, Martinez-Saurez J V, Rodriguez-Tudela J L, Gonzalez-Lopez A, Valencia E. Mixed oropharyngeal candidiasis due to Candida albicans and non-albicans Candidastrains in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1996;15:446–452. doi: 10.1007/BF01691310. [DOI] [PubMed] [Google Scholar]
- 10.Elgart M L. Zygomycosis. Dermatol Clin. 1996;14:141–146. doi: 10.1016/s0733-8635(05)70334-x. [DOI] [PubMed] [Google Scholar]
- 11.Fujii R, Matsumoto S, Sakiyama Y, Ishikawa Y, Takeda T, Hatae Y, Takase A, Sunakawa K, Yokota T, Kobayashi M. A clinical study of fluconazole-granules and injectable in pediatric patients with deep-seated mycoses. Jpn J Antibiot. 1993;46:654–685. . (In Japanese.) [PubMed] [Google Scholar]
- 12.Fussle R. Diagnosis of fungal infections. Mycoses. 1997;40(Suppl. 2):13–15. doi: 10.1111/j.1439-0507.1997.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 13.Graybill J R. Treatment of systemic mycoses in patients with AIDS. Arch Med Res. 1993;24:403–412. [PubMed] [Google Scholar]
- 14.Groll A, Shah P M, Menzel C, Just G, Schneider M, Hübner K. Invasive mycosis in post-mortem findings. J Infect. 1994;28(Suppl. I):57. [Google Scholar]
- 15.Groll A H, Shah P M, Menzel C, Schneider M, Just-Muebling G, Hübner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996;33:23–32. doi: 10.1016/s0163-4453(96)92700-0. [DOI] [PubMed] [Google Scholar]
- 16.Hotchi M, Okada M, Nasu T. Present state of fungal infections in autopsy cases in Japan. Am J Clin Pathol. 1980;74:410–416. doi: 10.1093/ajcp/74.4.410. [DOI] [PubMed] [Google Scholar]
- 17.Ikemoto H. A clinical study of fluconazole for the treatment of deep mycoses. Diagn Microbiol Infect Dis. 1989;12(Suppl. 4):239S–247S. doi: 10.1016/0732-8893(89)90143-0. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 12. Tokyo, Japan: Japanese Society of Pathology; 1970. . (In Japanese.) [Google Scholar]
- 19.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 13. Tokyo, Japan: Japanese Society of Pathology; 1971. . (In Japanese.) [Google Scholar]
- 20.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 14. Tokyo, Japan: Japanese Society of Pathology; 1972. . (In Japanese.) [Google Scholar]
- 21.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 15. Tokyo, Japan: Japanese Society of Pathology; 1973. . (In Japanese.) [Google Scholar]
- 22.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 16. Tokyo, Japan: Japanese Society of Pathology; 1974. . (In Japanese.) [Google Scholar]
- 23.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 17. Tokyo, Japan: Japanese Society of Pathology; 1975. . (In Japanese.) [Google Scholar]
- 24.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 18. Tokyo, Japan: Japanese Society of Pathology; 1976. . (In Japanese.) [Google Scholar]
- 25.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 19. Tokyo, Japan: Japanese Society of Pathology; 1977. . (In Japanese.) [Google Scholar]
- 26.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 20. Tokyo, Japan: Japanese Society of Pathology; 1978. . (In Japanese.) [Google Scholar]
- 27.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 21. Tokyo, Japan: Japanese Society of Pathology; 1979. . (In Japanese.) [Google Scholar]
- 28.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 22. Tokyo, Japan: Japanese Society of Pathology; 1980. . (In Japanese.) [Google Scholar]
- 29.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 23. Tokyo, Japan: Japanese Society of Pathology; 1981. . (In Japanese.) [Google Scholar]
- 30.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 24. Tokyo, Japan: Japanese Society of Pathology; 1982. . (In Japanese.) [Google Scholar]
- 31.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 28. Tokyo, Japan: Japanese Society of Pathology; 1986. . (In Japanese.) [Google Scholar]
- 32.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 32. Tokyo, Japan: Japanese Society of Pathology; 1990. . (In Japanese.) [Google Scholar]
- 33.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 33. Tokyo, Japan: Japanese Society of Pathology; 1991. . (In Japanese.) [Google Scholar]
- 34.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 34. Tokyo, Japan: Japanese Society of Pathology; 1992. . (In Japanese.) [Google Scholar]
- 35.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 35. Tokyo, Japan: Japanese Society of Pathology; 1993. . (In Japanese.) [Google Scholar]
- 36.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 36. Tokyo, Japan: Japanese Society of Pathology; 1994. . (In Japanese.) [Google Scholar]
- 37.Japanese Society of Pathology. Annual of the pathological autopsy cases in Japan. Vol. 37. Tokyo, Japan: Japanese Society of Pathology; 1995. . (In Japanese.) [Google Scholar]
- 38.Kanda M, Moriyama M, Ikeda M, Kojima S, Tokunaga M, Watanabe G. A statistical survey of deep mycoses in Japan, with particular reference to autopsy cases of cryptococcosis. Acta Pathol Jpn. 1974;24:595–609. doi: 10.1111/j.1440-1827.1974.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 39.Kappe R, Jacob P, Kim N, Sonntag H-G. 13th Congress of the International Society for Human and Animal Mycology. 1997. Therapy analysis of patients with proven invasive aspergillosis (IA) in Heidelberg 1990 to 1996, abstract O-88. [Google Scholar]
- 40.Kretschmer M, Nichterlein T, Kurtz P, Hof H. Rapid detection of susceptibility to fluconazole in Candidaspecies by a bioluminescence assay of intracellular ATP. Diagn Microbiol Infect Dis. 1996;25:117–121. doi: 10.1016/s0732-8893(96)00130-7. [DOI] [PubMed] [Google Scholar]
- 41.Kujath P, Lerch K. New antimicrobial agents under clinical investigation. Secondary mycosis in surgery: treatment with fluconazole. Infection. 1989;17:111–117. doi: 10.1007/BF01646895. [DOI] [PubMed] [Google Scholar]
- 42.Kume H, Abe M, Tsukamoto H, Funaoka M, Matsumoto T, Miyazawa S, Murase S, Muramatsu H, Mochizuki M, Yamazaki T, Yamashita E. The manual of treatment for visceral mycoses. 1994. pp. 160–179. . Kamawanu Syoboh, Tokyo, Japan. (In Japanese.) [Google Scholar]
- 43.Kume H, Yamazaki T, Mochizuki M, Funaoka M, Tsukamoto H, Abe M, Yamashita E. Candidiasis. Tokyo, Japan: Kyohwa Kikaku Tsushin Press; 1994. pp. 44–52. . (In Japanese.) [Google Scholar]
- 44.Matsushima T, Ikeda H, Tomizawa S, Nakamura J, Adachi M, Kawanishi M, Tanabe J, Tano Y. Clinical evaluation of fluconazole in patients with mycotic infection. Jpn J Antibiot. 1989;42:153–163. [PubMed] [Google Scholar]
- 45.Miyake M, Okudaira M. A statistical survey of deep fungus infections in Japan. Acta Pathol Jpn. 1967;17:401–415. doi: 10.1111/j.1440-1827.1967.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 46.Okudaira M. Pathology of opportunistic fungus infection. Trans Soc Pathol Jpn. 1985;71:61–91. . (In Japanese.) [Google Scholar]
- 47.Okudaira M, Kume H, Kurata H, Sakabe F. Recent statistical survey of visceral aspergillosis in Japan, and experimental studies on the pathogenicity of Aspergillus fumigatusin rabbits. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;261:529–538. doi: 10.1016/s0176-6724(86)80087-6. [DOI] [PubMed] [Google Scholar]
- 48.Rabodonirina M, Piens M A, Monier M F, Gueho E, Fiere D, Mojon M. Fusariuminfections in immunocompromised patients: case reports and literature review. Eur J Clin Microbiol Infect Dis. 1994;13:152–161. doi: 10.1007/BF01982190. [DOI] [PubMed] [Google Scholar]
- 49.Sandin R L, Meier C S, Crowder M L, Greene J N. Concurrent isolation of Candida krusei and Candida tropicalisfrom multiple blood cultures in a patient with acute leukemia. Arch Pathol Lab Med. 1993;117:521–523. [PubMed] [Google Scholar]
- 50.Statistics and Information Department, Minister’s Secretariat, Ministry of Health and Welfare. 1969–1996. Vital statistics of Japan. Minister’s Secretariat, Ministry of Health and Welfare of Japan, Tokyo. (In Japanese.)
- 51.Statistics and Information Department, Minister’s Secretariat, Ministry of Health and Welfare. Candidiasis. The data book on the number of patients with respective diseases in Japan. Tokyo: Minister’s Secretariat Ministry of Health and Welfare of Japan; 1995. p. 28. . (In Japanese.) [Google Scholar]
- 52.Verweiji P E, Denning D W. Diagnostic and therapeutic strategies for invasive aspergillosis. Semin Respir Clin Care Med. 1997;18:203–215. [Google Scholar]
- 53.Walsh T J, Halthorn J W, Sobel J D, Merz W G, Sanchez V, Maret S M, Buckley H R, Pfaller M A, Schaufele R, Slive C, et al. Detection of circulating Candidaenolase by immunoassay in patients with cancer and invasive candidiasis. N Engl J Med. 1991;324:1026–1031. doi: 10.1056/NEJM199104113241504. [DOI] [PubMed] [Google Scholar]
- 54.Weinberger M, Sacks T, Sulkes J, Shapiro M, Polacheck I. Increasing fungal isolation from clinical specimens: experience in a university hospital over a decade. J Hosp Infect. 1997;35:185–195. doi: 10.1016/s0195-6701(97)90206-1. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi H. Recent progress in molecular diagnostic technology in clinical mycology. Nippon Rinsho. 1996;54:2600–2613. . (In Japanese.) [PubMed] [Google Scholar]